Abstract
Echium amoenum (Boraginaceae) is an important remedy used for various illnesses. In this study, we investigated the anti-inflammatory effects of E. amoenum in the J774.1A macrophage cell line. We prepared ethyl acetate, dichloromethane and hexane extracts from E. amoenum flowers and examined their possible cytotoxic effects using MTT assay. Lipopolysaccharide (LPS)-stimulated macrophages were treated with the extracts after which we measured nitric oxide (NO) production by Griess method. Inducible NO synthase (iNOS), cyclooxygenase-2 (COX2), tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 gene expressions were examined by real time-PCR. IL-1β and IL-6 cytokine levels were measured by enzyme-linked immunosorbent assay (ELISA). The hexane extract with a half maximal inhibitory concentration (IC50) of 39.8 μg/mL most effectively reduced NO production. Real time-PCR analysis indicated reduced levels of iNOS ((0.05 ± 0.006 relative fold change (RFC)) and COX2 (0.06 ± 0.002 RFC) gene expressions with the 100 μg/mL hexane extract (P < 0.001). IL1-β, TNF-α, and IL-6 gene expression levels decreased at all concentrations of the extract (less than ≈ 0.28 RFC). Treatment of LPS-stimulated cells with 100 μg/mL of the extract reduced IL-1β secretion to 27.9 ± 0.21 pg/mL and IL-6 to 555 ± 166 pg/mL. In conclusion, E. amoenum hexane extract showed the greatest reduction in macrophage NO secretion compared to other extracts. This extract could modulate the inflammatory mode of the macrophages by causing reductions in iNOS and COX2 enzymes as well as IL-1β, IL-6, and TNF-α cytokine levels. The results of this study have shown the anti-inflammatory effects of this plant. Further studies regarding its therapeutic potential in inflammatory disorders are recommended.
Keywords: Echium amoenum, Macrophages, Anti-inflammatory
INTRODUCTION
Vascular and cellular components play the main role in inflammation. The circulating cellular components include neutrophils, monocytes, macrophages, lymphocytes, dendritic cells, and mast cells (1). The role of macrophages in inflammation is indisputable. These cells are key players in initiation, development, and termination of inflammatory processes (1). They regulate inflammation by production of various inflammatory mediators such as prostaglandins, nitric oxide (NO), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and IL-1β (2). Prostaglandins are formed after the release of arachidonic acid from the plasma membrane and metabolized by the sequential actions of cyclooxygenase (COX) and relevant synthases. The COX-2 enzyme is mainly induced by inflammatory stimuli and cytokines. Hence, it is an important source of prostanoid formation in inflammatory conditions (3). Another important enzyme in inflammatory responses is inducible NO synthase (iNOS). iNOS is responsible for NO synthesis as one of the main mediators involved in inflammation. NO production due to cytokine-induced expression of iNOS is largely involved in the pathophysiology of various inflammatory diseases (4).
There are various drugs used to treat inflammatory-based diseases. Steroidal and non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used for the treatment of pain associated with inflammation in diseases such as rheumatoid arthritis (5). Despite their effectiveness, they have various adverse effects (5). Therefore, researchers are searching for alternative drugs with lower adverse effects and increased efficacy.
Herbal medicines have been used for centuries to treat different illnesses (6). Numerous medicinal plants and their derivatives are used as the treatment of inflammatory diseases (7,8,9). Examples include Eucalyptus globules, Thymus vulgaris (7), Mentha longifolia (8) and Pycnocycla spinosa (9).
Echium amoenum (borage), is a wild annual herb that belongs to the Boraginaceae family. This plant is called “gole-gavzaban” (ox-tongue) in Persian and grows mainly in the northern mountains of Iran (10). Worldwide, the flowers and leaves of E. amoenum are used for medicinal purposes for treatment of stress, circulatory heart diseases, and pulmonary complaints (10). The antibacterial, antioxidant, and antiviral properties of this plant have been shown in various studies (11). In our previous study, we reported the immunomodulatory effects of the hydroalcoholic extract of E. amoenum on human lymphocyte proliferation and antibody synthesis (12). However, to the best of our knowledge, the anti-inflammatory effects of this plant on macrophages have not been studied. In our preliminary study on the methanol extract of E. amoenum, we observed inhibition of NO production by J774.1A macrophages. In this investigation, we partitioned the E. amoenum methanol extract into hexane, ethyl acetate and dichloromethane extracts and analyzed for their possible anti-inflammatory activity on macrophages as one of the most important cells involved in inflammatory processes.
MATERIALS AND METHODS
Cell culture
The J774.1A, a murine macrophage cell line, was cultured in Dulbecco’s modified eagle medium (DMEM) (GibcoBRL, USA) containing 2 mM L-glutamine, 100 U penicillin/mL, 0.1 mg streptomycin/mL, and 10% heat-inactivated FBS (GibcoBRL) and incubated in a humidified CO2 incubator until reaching confluence. Cells were then counted and viability was determined by trypan blue dye exclusion test. The cells with 90-95% viability were used for the experiments.
Preparation of the plant materials
Flowers of E. amoenum were collected in June from North of Iran and identified by by Mrs Sedigheh Khademian from Faculty of Pharmacy, Shiraz University of Medical Sciences and a voucher specimen (PM 861) was deposited there. The plant materials (300 g) were dried and extracted with 1000 mL methanol for 3 days by maceration method. The extract was concentrated in a vacuum using a rotary evaporator, then lyophilized (yield, 1% (w/w)) and stored at -20 °C until used. Two g of this extract was dissolved in water and then partitioned into hexane, ethyl acetate and dichloromethane extracts. The extracts were concentrated using rotary evaporator and the obtained residues stored at -20 °C until tested. The yields of ethyl acetate, hexane and dichloromethane extracts obtained from crude methanol extract were within the range of 15.7-19.7% (w/w). Each extract was weighed and dissolved in dimethyl sulfoxide (DMSO, Sigma, USA) to give a 20 mg/mL solution.
Cell viability assay
The effect of E. amoenum extracts on the viability of J774.1A cells was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Predetermined number of cells (7500 cells per well) were incubated with 1-200 μg/mL of E. amoenum extracts for 24 h at 37 °C in a humidified 5% CO2 incubator. A series of triplicate wells containing only DMSO as the solvent at the highest concentration used in tests (0.1%) was considered as negative control. Thereafter, 10 μL of MTT (Sigma) (5 mg/mL) was added to each well and cells incubated for 4 h at 37 °C. The medium was then removed from the wells and 150 mL DMSO was added to dissolve the formazan produced in the cells. The optical density (OD) in the wells was measured with a microplate reader (BioTek, USA) at 570 nm with a background subtraction at 630 nm. The OD of negative control wells was accepted as 100% viability.
Quantification of nitric oxide production
J774.1A macrophages at concentration of 1 × 105/100 μL were seeded in 96-well tissue culture plates. Cells were treated with 1 μL lipopolysaccharide (LPS, Sigma) (1 μg/mL), and various extracts at final concentrations of 1-200 μg/mL for 24 h. Two series of triplicate wells were considered as positive control (cells treated with LPS and 0.1%DMSO (LPS-only treated cells)) and negative control (cells treated only with 0.1% DMSO). NG-nitro-L-arginine-methyl ester (L-NAME, Sigma) (1 mmol/mL) was used as an iNOS inhibitor. After 24 h culture, the supernatants were collected and NO production was determined by adding Griess reagent for 15 min at room temperature (RT) and then the absorbance was read at 550 nm in a microplate reader (BioTek). The nitrite concentration was calculated using a sodium nitrite standard curve. The percentage of NO inhibition in the treated cells was compared to LPS-only treated cells (100%) and the half NO inhibitory concentration (IC50) for each extract was determined.
RNA extraction and cDNA synthesis
J774.1A macrophages at concentration of 5 × 105/mL were seeded in 24-well tissue culture plates. Cells were treated with 1 μL LPS and the hexane extract at concentrations of 25-100 μg/mL. Cells treated with LPS and 0.1% DMSO (LPS-only treated cells) and those treated only with 0.1% DMSO were considered as positive and negative controls, respectively. After 24 h of culture, the supernatants were collected and stored at -80 °C for cytokine assay, and the cells were subjected to RNA extraction for gene expressions changes by real-time PCR. Total RNA was extracted using RNX-plus buffer according to the manufacturer protocols (Pars Tous, Iran). Briefly, 2 × 106 cells from treated and untreated conditions were transferred to 1 mL of RNX-plus buffer, then 200 μL chloroform was added and the mixture centrifuged. The supernatant was collected to mix with equal volume of isopropanol. The purified RNA was quantified by a NanoDrop (Thermo scientific, USA). A sample of RNA (5 μg) was used for cDNA synthesis using high-capacity cDNA reverse transcription kit from ABI (USA) at 37 °C for 120 min in the presence of dNTP mix, reverse transcriptase and random hexamers. Quality of RNA was examined by electrophoresis and observation of intact 28S and 18S rRNA bands in agarose gel.
Quantitative real-time PCR
For analysis of iNOS, COX2, IL-6, IL-1β and TNF-α gene expression levels, real time-PCR was performed in a final volume of 20 mL containing cDNA, SYBR PremixExTaq II (TAKARA, USA), ROX reference dye-2, PCR forward and reverse primers (10 pM). The primers were used as described previously (13): GAPDH F, 5′-CGGTGTGAACGGATTTGGC, R,5′-GTGAGTGGAGTCATACTGGAAC, TNF-α F, 5′-GTC-TCAGCC-TCTTCTCATTC-3, R, 5′-GGAACTTCTCATCCCTTTGG, IL-1β F, 5′-GAAGAAGAGCCCATCCTC, R, 5′-GTTCATCTCGGAGC-CTGTAG, IL-6 F, 5′-ACCTGTCTATACCACTTCAC, R, 5′-GCATCATCGTTGTTCATAC, iNOS F, 5′-CTGGAGGTTCTGGATGAG, R, 5′-CTGAGGGCTGACACAAGG, COX2 F, 5′-CAGCACTTCACCCATCAG, R, 5′-GATACACCTCTCCACCAATG. Real-time PCR was performed in an Applied Biosystems Step One system (USA). The PCR conditions were as follows; one cycle at 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s, 53.3 °C (TNF-α, COX2 and IL-1β), or 56.3 °C (GAPDH, IL-6 and iNOS) for 18 s and 72 °C for 30 s. GAPDH served as an endogenous control. Results of target mRNA levels were normalized against GAPDH mRNA in each sample and presented as relative fold change (RFC) of LPS-only treated cells.
Cytokine assay
The supernatant of cells cultured as mentioned above was used for measuring of IL-1β and IL-6 cytokines using commercially available enzyme-linked immunosorbent assay (ELISA) kits from eBioscience (USA) according to the manufacturer’s protocols. The sensitivity of the IL-1β and IL-6 ELISA kits were 8 pg/mL and 4 pg/mL, respectively.
Statistical analysis
Data were analyzed using one-way ANOVA and Student’s t-test using GraphPad/Prism software (USA). All values expressed as means ± SD of at least three independent experiments and the level of significance was set at P < 0.05.
RESULTS
Effects of E. amoenum extracts on cell viability
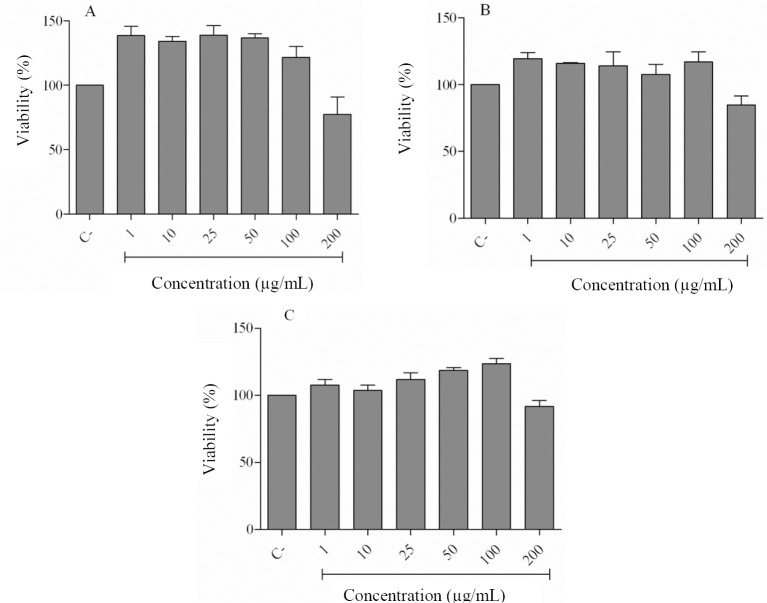
We used the MTT colorimetric assay to study the effects of different concentrations of E. amoenum extracts on the growth of J774.1A cells. Extracts of hexane (Fig. 1A), dichloromethane (Fig. 1B), and ethyl acetate (Fig. 1C) showed no significant cytotoxic effects on the cells at concentrations of 1-100 μg/mL.
Fig. 1.
Effects of Echium amoenum extracts on the viability of J774.1A cell line performed by MTT assay. The cells were treated with different concentrations of (A) hexane, (B) dichloromethane and (C) ethyl acetate extracts for 24 h. (C-), negative control values were obtained in the absence of components. Data represent means ± SD of three independent experiments. None of the extracts showed a significant reducing effect at different concentrations.
The 200 μg/mL concentration slightly decreased cell viability. Therefore, we used the 1-100 μg/mL concentrations of the extracts for further experiments on this cell line.
Effect of E. amoenum extracts on nitric oxide secretion
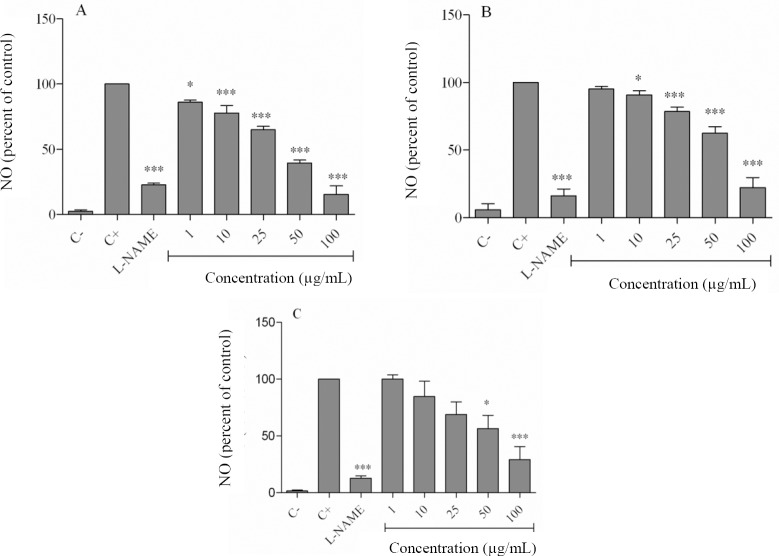
Cells were stimulated with LPS in the presence and absence of the extracts. Treatment of cells with LPS increased the level of NO from 2.2 ± 1.3 μmol/L in negative control to 91.1 ± 1.6 μmol/L (P < 0.001, Fig. 2). L-NAME, as expected, decreased NO production to 19.4 ± 3.8% of the LPS-only treated cells (P < 0.001). Hexane extract at concentrations of 1-100 μg/mL significantly decreased NO secretion from 86.1 ± 2.3% to 15.4 ± 8.9% (Fig. 2A). The dichloromethane extract also dose-dependently decreased NO production from 90.76 ± 5.5% at 10 μg/mL to 22.3 ± 12.7% at 100 μg/mL (Fig. 2B). Fig. 2C shows the significant NO inhibitory effects of the ethyl acetate extract at the 50 μg/mL (56.4 ± 20.1%, P < 0.05) and 100 μg/mL (29.2 ± 19.9%, P < 0.001) concentrations.
Fig. 2.
Effects of Echium amoenum extracts on nitric oxide production in lipopolysaccharide-stimulated macrophages. Cells were treated with lipopolysaccharide and different concentrations of (A) hexane, (B) dichloromethane and (C) ethyl acetate for 24 h. The supernatant of cultured cells were examined for NO levels by Griess method. (C-), negative control values were obtained in the absence of lipopolysaccharide and the extracts. (C+), positive control was lipopolysaccharide-only treated cells. *P < 0.05, **P < 0.01, ***P < 0.001 vs positive control. L-NAME, NG-nitro-L-arginine-methyl ester; NO, nitric oxide.
Effect of E. amoenum hexane extract on lipopolysaccharide-induced inflammatory gene expressions
We compared the half maximal inhibitory (IC50) values for the NO inhibitory effects of the extracts and observed the following values: hexane (38.9 μg/mL), dichloromethane (83.1 μg/mL), and ethyl acetate (91.2 μg/mL). We chose the hexane extract which had a greater inhibitory effect on NO production to assess COX-2, iNOS, IL-1β, TNF-α, and IL-6 expressions.
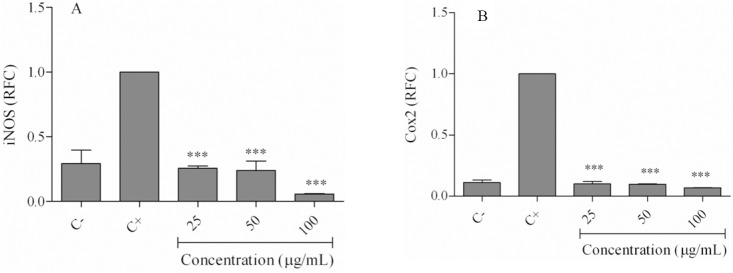
The cells were incubated with LPS and different concentrations of the hexane extract for 24 h, followed by RNA extraction and DNA synthesis. Real-time PCR results indicated that LPS strongly increased mRNA expressions of COX-2, iNOS, TNF-α, IL-1β, and IL-6 compared to the negative control (Figs. 3, 4).
Fig. 3.
Effects of the hexane extract on lipopolysaccharide-induced inflammatory gene expressions. Cells were treated with 25-100 μg/mL of the hexane extract in the presence of LPS for 24 h. Expression of (A) inducible NO synthase, and (B) cyclooxygenase-2 was evaluated using real time-PCR. (C-) negative control values were obtained in the absence of lipopolysaccharide and the extract; (C+) positive control was lipopolysaccharide-only treated cells. ***P< 0.001 vs positive control. RFC, relative fold-change; iNOS, inducible NO synthase; COX2, cyclooxygenase-2.
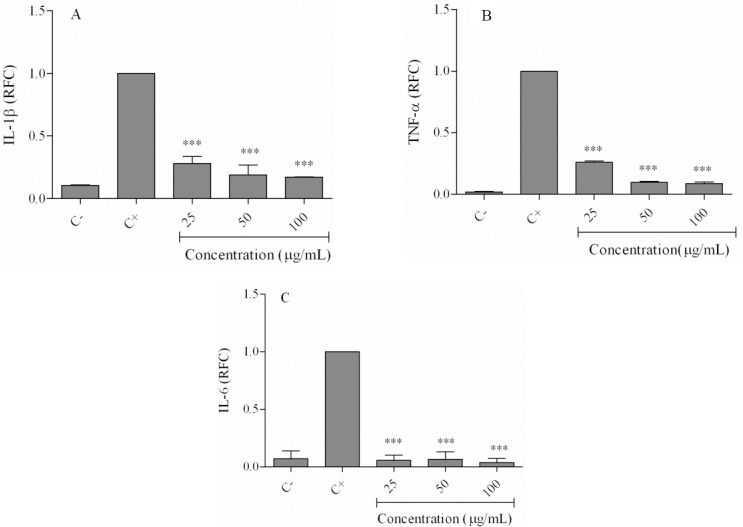
Fig. 4.
Effects of hexane extract on lipopolysaccharide-induced inflammatory cytokines gene expressions. Cells were treated with 25-100 μg/mL of the hexane extract in the presence of lipopolysaccharide for 24 h. Expression of (A) IL-1β, (B) TNF-α and (C) IL-6 was evaluated using real time-PCR. (C-) negative control values were obtained in the absence of lipopolysaccharide and the extract; (C+) positive control was lipopolysaccharide-only treated cells. ***P < 0.001 vs positive control. RFC; relative fold-change. IL, interleukin; TNF, tumor necrosis factor.
As shown in Fig. 3A, iNOS expression levels in LPS-only treated cells decreased to 0.25 ± 0.03 relative fold change (RFC) at 25 μg/mL, 0.23 ± 0.12 RFC at 50 μg/mL, and 0.05 ± 0.006 at 100 μg/mL of the extract (P < 0.001). Similarly, COX-2 expression decreased from 0.099 ± 0.02 RFC (25 μg/mL) to 0.097 ± 0.002 RFC (50 μg/mL) and 0.06 ± 0.002 RFC (100 μg/mL; P < 0.001; (Fig. 3B). Treatment of cells with hexane extract reduced IL-1β gene expression from 0.28 ± 0.07 RFC at 25 μg/mL to 0.16 ± 0.004 RFC at 100 μg/mL (Fig. 4A). TNF-α expression levels in the extract treated cells decreased from 0.25 ± 0.01 RFC at 25 μg/mL to 0.08 ± 0.01 RFC at 100 μg/mL of the extract (Fig. 4B). The gene expression level of IL-6 in cells treated with 25-100 μg/mL of the extract decreased to less than 0.05 RFC (Fig. 4C).
Effect of E. amoenum hexane extract on lipopolysaccharide-induced cytokine secretion
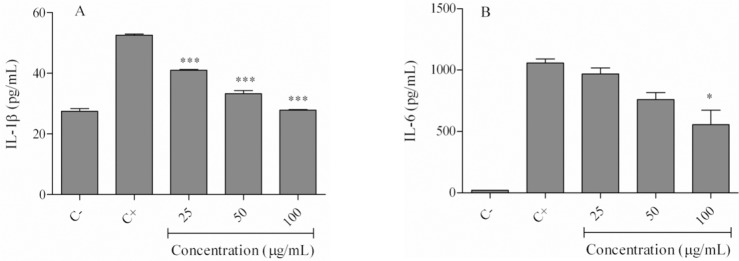
We used the ELISA technique to examine effects of the hexane extract on IL-1β and IL-6 cytokine secretion in LPS-stimulated cells. As shown in Fig. 5A, LPS-treated cells had increased IL-1β levels (52.5 ± 0.53 pg/mL) compared with the negative control (27.4 ± 1.27 pg/mL). IL-6 levels increased from 21.3 ± 0.54 pg/mL in the negative control to 1058 ± 47.01 pg/mL in LPS-only treated cells (Fig. 5B). Treatment of cells with various concentrations (25-100 μg/mL) of the hexane extract significantly reduced IL-1β levels to 41.02 ± 0.31 pg/mL (25μg/mL), 33.3 ± 1.48 pg/mL (50 μg/mL), and 27.9 ± 0.21 pg/mL (100 μg/mL; P < 0.001; Fig. 5A). Cells treated with the hexane extract also showed decreased IL-6 secretion. This was significant (P < 0.05) only at the100 μg/mL amount of hexane extract (555 ± 166 pg/mL; Fig. 5B).
Fig. 5.
Effects of the Echium amoenum hexane extract on lipopolysaccharide-induced inflammatory cytokine levels. Cells were treated with 25-100 μg/mL of the hexane extract in the presence of lipopolysaccharide for 24 h. (A) IL-1β, and (B) IL-6 (B) cytokine levels were determined using ELISA. (C-) negative control values were obtained in the absence of lipopolysaccharide and the extract; (C+) positive control was lipopolysaccharide-only treated cells. *P < 0.05, ***P < 0.001 vs positive control; IL, interleukin.
DISCUSSION
Macrophages are important cells in inflammatory responses because of their antigen presentation, phagocytosis, and immunomodulation properties. The central role of macrophages in the inflammatory process is secretion of various cytokines and mediators (1). Suppression of these cells or their derived mediators is a key approach by which the host permits repair of tissue damages that occur during inflammation. Certain medicinal plants are used to relieve inflammation in traditional medicine (7,8,9). E. amoenum is one of these plants used for its anti-inflammatory and analgesic properties in Iranian folk medicine, particularly for the common cold (14). In the present study we investigated the effects of E. amoenum extracts on inflammatory mediators in LPS-stimulated macrophages. We first examined the extracts for their effects on J774.1A macrophage cell viability in an attempt to exclude any probable cytotoxic effects. The extracts at the 1-100 μg/mL concentrations did not show any cytotoxic effects on the cells. Next, we examined the extracts to determine the effects on production of NO as a chemical indicator of inflammation (16). NO is an important biological mediator and regulator of inflammation (16).
When NO is released, within a few seconds it is oxidized to nitrites or nitrates. These products are measured by chemical reactions. The results of the current study have shown that the hexane, dichloromethane, and ethyl acetate extracts of E. amoenum significantly decreased NO production. The hexane extract had the strongest effects and subsequently we selected this extract for additional experimentation.
To the best of our knowledge, no other research has evaluated the production of NO in macrophages exposed to E. amoenum extract. There are various studies that report NO reducing activities of the extracts from other medicinal plants.
Examples include extracts from Eucalyptus globules and Thymus vulgaris (7) which have decreased NO production in J774.1A cells stimulated with LPS and interferon (IFN)-γ. Other studies reported the inhibitory effects of Andrographis paniculata (9) and Echinacea (16) on NO production and iNOS expression in LPS-activated macrophages. Mentha longifolia has also reduced NO in LPS-stimulated J774.1A cells (8).
The methanol extract of this plant decreased LPS-induced iNOS mRNA. Bacterial pathogens or cytokine-induced expression of iNOS is largely involved in the pathophysiology of inflammation. In the present study, we have observed decreased NO secretion associated with reduced iNOS gene expression which suggested that the reduction in NO levels might be attributed to the inhibition of iNOS expression by the hexane extract.
COX2 also plays an important role in inflammation (17). This important enzyme is required for the conversion of arachidonic acid to prostaglandins. The ability of NSAIDs to suppress inflammation is partly due to their inhibition of the COX enzyme (18). Inhibited gene expression of this enzyme in the J774.1A macrophage cell line by the hexane extract is further proof of the anti-inflammatory effects of the E. amoenum plant.
Our study has demonstrated that the extract had the capacity to strongly reduce IL-6, IL-1β, and TNF-α gene expressions. TNF-α and IL-1β are two major cytokines involved in inflammation.
TNF-α is mainly produced by macrophages and is involved in the acute phase of inflammation by recruitment of neutrophils and activation of arachidonic acid metabolism (19).
IL-1β plays a key role in inflammatory responses due to infections and immune-mediated diseases. Inhibitors of IL-1β are used to relieve inflammatory arthritis in experimental models (19). IL-6 is another proinflammatory cytokine involved in the generation and propagation of inflammation (20). The results of the present study have shown that the hexane extract of E. amoenum dose-dependently reduced IL-6 in macrophages at both the gene and protein expression levels.
Various studies have investigated the effects of a number of medicinal plants on the expressions of these cytokines. Examples of these plants that reduce TNF-α, IL-6, and IL-1β mRNA expressions in macrophages include Mentha longifolia (8), and Echinacea (16). The results of the current study on cytokine expressions support the results of the inhibitory effects of the hexane extract on iNOS and COX2 expression and the antiinflammatory effects of E. amoenum.
Study of the chemical composition and secondary metabolites of the E. amoenum have shown the presence of various flavonoid aglycans, anthocyanidine, rhosmarinic acid, γ-linolenic acid and low levels of alkaloids (11).
As the major effects of the E. amoenum was observed in the hexane extract, the antiinflammatory effects are likely due to low or non-polar hydrophobic compounds with high lipophilicity. Further experiments are needed to identify the responsible compounds.
CONCLUSION
Various extracts of E. amoenum reduced NO production by macrophages, among which the hexane extract was the most effective. This extract has the capacity to modulate the inflammatory mode of macrophages via reductions in iNOS and COX2 enzyme levels as well as IL-1β, IL-6, and TNF-α cytokines. These data may suggest additional investigation of E. amoenum in terms of its therapeutic potential in inflammatory disorders and identification of responsible components.
ACKNOWLEDGEMENTS
The content of this paper is extracted from a MSc thesis (Grant No. 9840) submitted by Najmeh Nasseri which was financially supported by the Shiraz University of Medical Sciences, Shiraz, Iran.
REFERENCES
- 1.Dunster JL. The macrophage and its role in inflammation and tissue repair: mathematical and systems biology approaches. Wiley Interdiscip Rev Syst Biol Med. 2016;8:87–99. doi: 10.1002/wsbm.1320. [DOI] [PubMed] [Google Scholar]
- 2.Jiang Z, Zhu L. Update on the role of alternatively activated macrophages in asthma. J Asthma Allergy. 2016;9:101–107. doi: 10.2147/JAA.S104508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadeghi-Aliabadi H, Aliasgharluo M, Fattahi A, Mirian M, Ghannadian M. In vitro cytotoxic evaluation of some synthesized COX-2 inhibitor derivatives against a panel of human cancer cell lines. Res Pharm Sci. 2013;8:298–303. [PMC free article] [PubMed] [Google Scholar]
- 4.Vannini F, Kashfi K, Nath N. The dual role of iNOS in cancer. Redox Biol. 2015;6:334–343. doi: 10.1016/j.redox.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suthar SK, Sharma M. Recent developments in chimeric NSAIDs as safer anti-inflammatory agents. Med Res Rev. 2015;35:341–407. doi: 10.1002/med.21331. [DOI] [PubMed] [Google Scholar]
- 6.Amirghofran Z. Herbal medicines for immunosuppression. Iran J Allergy Asthma Immunol. 2012;11:111–119. [PubMed] [Google Scholar]
- 7.Vigo E, Cepeda A, Gualillo O, Perez-Fernandez R. In-vitro anti-inflammatory effect of Eucalyptus globulus and Thymus vulgaris: nitric oxide inhibition in J774A.1 murine macrophages. J Pharm Pharmacol. 2004;56:257–263. doi: 10.1211/0022357022665. [DOI] [PubMed] [Google Scholar]
- 8.Karimian P, Kavoosi G, Amirghofran Z. Anti-inflammatory effect of Mentha longifolia in lipopoly saccharide-stimulated macrophages: reduction of nitric oxide production through inhibition of inducible nitric oxide synthase. J Immunotoxicol. 2013;10:393–400. doi: 10.3109/1547691X.2012.758679. [DOI] [PubMed] [Google Scholar]
- 9.Minaiyan M, Asghari G, Sadraei H, Feili E. Anti-inflammatory effect of Pycnocycla spinosa extract and its component isoacetovanillone on acetic acid induced colitis in rats. Res Pharm Sci. 2015;10:345–355. [PMC free article] [PubMed] [Google Scholar]
- 10.Abolhassani M. Antiviral activity of borage (E. amoenum) Arch Med Sci. 2010;6:366–369. doi: 10.5114/aoms.2010.14256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranjbar A, Khorami S, Safarabadi M, Shahmoradi A, Malekirad AA, Vakilian K, et al. Antioxidant activity of Iranian Echium amoenum Fisch & C.A. Mey flower decoction in humans: A cross-sectional before/after clinical trial. Evid Based Complement Alternat Med. 2006;3:469–473. doi: 10.1093/ecam/nel031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amirghofran Z, Azadbakht M, Keshavarzi F. E. amoenum stimulate of lymphocyte proliferation and inhibit of humoral antibody synthesis. Iranian J Med Sci. 2000;25:119–124. [Google Scholar]
- 13.Gholijani N, Gharagozloo M, Farjadian S, Amirghofran Z. Modulatory effects of thymol and carvacrol on inflammatory transcription factors in lipopolysaccharide-treated macrophages. J Immunotoxicol. 2016;13:157–164. doi: 10.3109/1547691X.2015.1029145. [DOI] [PubMed] [Google Scholar]
- 14.Heidari MR, Azad EM, Mehrabani M. Evaluation of the analgesic effect of Echium amoenum Fisch & C.A. Mey. extract in mice: possible mechanism involved. J Ethnopharmacol. 2006;103:345–349. doi: 10.1016/j.jep.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life sciences. 2004;75:639–353. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 16.Zhai Z, Solco A, Wu L, Wurtele ES, Kohut ML, Murphy PA, Cunnick JE. Echinacea increases arginase activity and has anti-inflammatory properties in RAW 264.7 macrophage cells, indicative of alternative macrophage activation. J Ethnopharmacol. 2009;122:76–85. doi: 10.1016/j.jep.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hugo HJ, Saunders C, Ramsay RG, Thompson EW. New insights on COX-2 in chronic inflammation driving breast cancer growth and metastasis. J Mammary Gland Biol Neoplasia. 2015;20:109–119. doi: 10.1007/s10911-015-9333-4. [DOI] [PubMed] [Google Scholar]
- 18.Buffum M. Buffum JC. Nonsteroidal anti-inflammatory drugs in the elderly. Pain Management Nursing. 2000;1:40–50. doi: 10.1053/jpmn.2000.7779. [DOI] [PubMed] [Google Scholar]
- 19.Dayer JM. Interleukin 1 or tumor necrosis factor-alpha: which is the real target in rheumatoid arthritis. J Rheumatol Suppl? 2002;65:10–15. [PubMed] [Google Scholar]
- 20.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]