Abstract
Chronic obstructive pulmonary disease (COPD) is a major health problem which had not received the attention commensurate with the magnitude of its global burden. This is finally changing with the help of a vibrant community of health-care professionals, public officials, and academic researchers. Advances in characterization of the disease, treatment options, imaging modalities, and better understanding of the comorbidities promise to revolutionize how the disease is managed. COPD should no longer augur despair among physicians and patients.
Keywords: Cardiovascular disease, chronic obstructive pulmonary disease, imaging, oxygen therapy
Chronic obstructive pulmonary disease (COPD) is the most prevalent chronic respiratory ailment in the world. In the United States, it is the third leading cause of death after heart disease and cancer, accounting for nearly $50 billion of direct and indirect health care expenditure. Over the past 2 decades, there have been remarkable advances in our understanding of the epidemiology, clinical features and presentation, associated comorbidities, treatment and imaging findings in COPD. The following commentary attempts to capture some of these new insights and clinical implications. The manuscript is based on a presentation delivered at the GulfThoracic Conference in Dubai on March 18, 2017.
Epidemiology
While the causal relationship of smoking in the pathogenesis of COPD is unequivocal, the magnitude of the impact may have been underestimated. In the Copenhagen City Heart Study,[1] 8045 participants aged 30–60 years with normal spirometry at baseline were followed for 25 years. Among continuous smokers, who started out with normal lung function, cumulative incidence of airflow obstruction was approximately 35%, much higher than previous estimates. The proportion of patients who developed airflow obstruction was related to the extent of smoking exposure. Notably, never smokers developed airflow obstruction ~8% of the time which confirmed environmental and genetic influences in disease pathogenesis.
Kohansal et al., recent report[2] challenged the classical Fletcher-Peto diagram which stipulated reversal of rate of lung function decline to that of normal after smoking cessation at any point in time. In this study, longitudinal spirometric data from 4391 individuals followed in the Framingham cohort for 26 years were utilized to create lung function curves. Continuous smokers displayed a significantly higher rate of forced expiratory volume 1 s (FEV1) decline (38.2 mL/year in males and 23.9 mL/year in females) compared to nonsmokers (19.6 mL/year for males and 17.6 mL/year for females). Interestingly, among patients who quit smoking before age 40, the rate of lung function decline mirrored that of nonsmokers. Among those who quit after age 40, however, the rate of decline was significantly was faster compared to those who quit earlier or never smoked. Therefore, quitting as early as possible is paramount.
Pitfalls in Diagnosis and Classification
While patients with current or former smoking history may experience symptoms and even exacerbations without overt spirometric abnormality,[3] documentation of airflow obstruction remains sine qua non for COPD diagnosis. GOLD Guidelines propose that the diagnosis of COPD be made based on a fixed FEV1/forced vital capacity (FVC) ratio of <0.7.[4] Since FEV1/FVC ratio tends to fall with age, a fixed threshold may be fraught with diagnostic inaccuracy. To this end, in the 2001 Health Survey for England, utilizing a fixed ratio versus one that is based on demographic data tended to overdiagnose obstruction in the elderly (one-third false-positive) and underdiagnose in the young (one in eight false negative).[5] Consequences of misdiagnosis may include surreptitious bronchodilator and inhaled corticosteroid prescription, misattribution of symptoms to COPD, and squandering an opportunity for early detection of an obstruction.[5]
The realization that COPD exacerbations play a significant role, a very negative one, has produced a new paradigm for evaluation of COPD patients. [4] Accordingly, the patients are categorized into 4 different groups according to the presence of symptoms and increasing risk for exacerbations. The latter is conferred by a history of exacerbations (≥2 during the past year or one hospitalization/emergency room visit). Symptom burden is assessed by the modified Medical Research Council (mMRC) or COPD assessment test (CAT) scores. Such duality in assessing symptom burden may be problematic. In an analysis by Jones et al., the classification of patients differed significantly depending on the tool used for determining symptom burden.[6] When the currently proposed thresholds for symptom burden were used (mMRC ≥2 or CAT >10), almost 40% of the 1817 patients in this study were reclassified. When the authors used mMRC ≥1 threshold instead of 2, there was much better agreement and classification. Since group assignments may have therapeutic[4] and prognostic implications,[7] standardizing clinical assessment is a relevant issue.
Phenotypes and Endotypes: Focus on Asthma Chronic Obstructive Pulmonary Disease Overlap
It has become clear that COPD is not a single lung disease but a condition consequent to various pathophysiological mechanisms which result in the same physiological abnormality, i.e., persistent airflow obstruction. Thus, we now consider various COPD phenotypes (groups of patients who share common clinical features, prognosis, and therapeutic needs) and COPD “endotypes” which refer to established common pathophysiology.[8] As expected, phenotypes may also share similar pathophysiology. Asthma COPD overlap syndrome (ACOS) has attracted particular attention since it comprises up to 15% of patients with COPD. Although ACOS is characterized by persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD, a precise definition has been elusive. Spanish guidelines provided the first proposed set of diagnostic criteria for ACOS.[9] Accordingly, having a history of asthma, a very positive bronchodilator response (FEV1 improvement >400 mL and >15%) and sputum eosinophilia constituted major criteria for the diagnosis. Minor criteria included elevated total serum IgE, previous history of atopy or a less positive bronchodilator response (FEV1 improvement >200 mL and >12%). The presence of 2 major criteria or one major plus 2 minor criteria defined ACOS.
In a multicenter observational cross-sectional study performed in 3125 COPD patients, 15.9% of patients had a history of asthma before age 40.[10] One-third of the patients fulfilled the more stringent criteria for ACOS by the Spanish guidelines. When the group determined by Spanish guidelines was compared with the rest of patients who only had a history of asthma before age 40, there was no difference in gender, body mass index, smoking exposure, activity levels, and symptom burden. Patients with strictly defined ACOS tended to be younger and have more frequent exacerbations with slightly better lung function. Along these lines, the presence of a history of asthma in a smoker (≥10 pack-years) with persistent airflow obstruction is currently considered a diagnostic criterion for ACOS.[11] Based on the accumulating data on blood eosinophils as a marker for corticosteroid responsiveness, blood eosinophil blood count ≥300 cells/μl has been suggested as a replacement for sputum eosinophilia as an ACOS inclusion criterion.[11]
Chronic Obstructive Pulmonary Disease and Comorbidities
Patients with COPD suffer from a multitude of diseases that tend to occur with a higher prevalence in COPD than the general population. Comorbidities impact the quality of life and prognosis. Consequently, the management of COPD cannot solely focus on treatment of airflow obstruction. A seminal study by Divo et al., brought the impact of comorbidities in COPD to clear focus.[12] These investigators followed 1664 patients with COPD for a median of 51 months. Using 12 comorbidities that differed in prevalence between survivors and nonsurvivors, they developed a COPD-specific comorbidity test (COTE) score. A COTE score ≥4 points increased the risk of death (hazard ratio 2.26–2.68, P = 0.001) in all BODE (an established prognostic index comprising physiological parameters) quartiles. The comorbidities that contributed to the risk of death were elegantly displayed in the “COPD comorbidome” [Figure 1].
Figure 1.
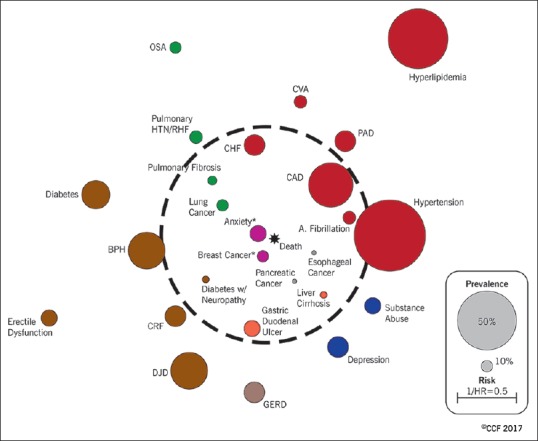
Chronic obstructive pulmonary disease comorbidome: In the graphic expression of this concept, the size of the circle represents the prevalence of the comorbidity in the cohort and the proximity to the center is proportional to the strength of the association between the comorbidity and mortality. Reprinted with permission of the American Thoracic Society. Copyright© 2018 American Thoracic Society. From Divo et al.[12] The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society
Cardiovascular Risk during Chronic Obstructive Pulmonary Disease Exacerbations and β-blockers
Cardiovascular disease is more prevalent among patients with COPD. Epidemiological studies suggest an increased risk of cardiovascular events during exacerbations of COPD. Patel et al., prospectively evaluated arterial stiffness, an index strongly related to cardiovascular events, and cardiac biomarkers in COPD patients.[13] Measurements of aortic pulse wave velocity, a surrogate for arterial stiffness were performed in 55 patients at baseline and during exacerbation at day 3, 7, 14, and 35 (recovery phase). A higher aortic pulse with velocity indicated reduced arterial distensibility, i.e., increased susceptibility to cardiovascular events. Arterial stiffness rose acutely during COPD exacerbations and was slow to recover [Figure 2]. In fact, aortic pulse-wave velocity had not returned to baseline levels at day 35 after the onset of an exacerbation. The magnitude of rise was greater in those with documented infection suggesting a role for inflammation. An increased procoagulant state and sympathetic activation were other putative mediators of vascular dysfunction.
Figure 2.
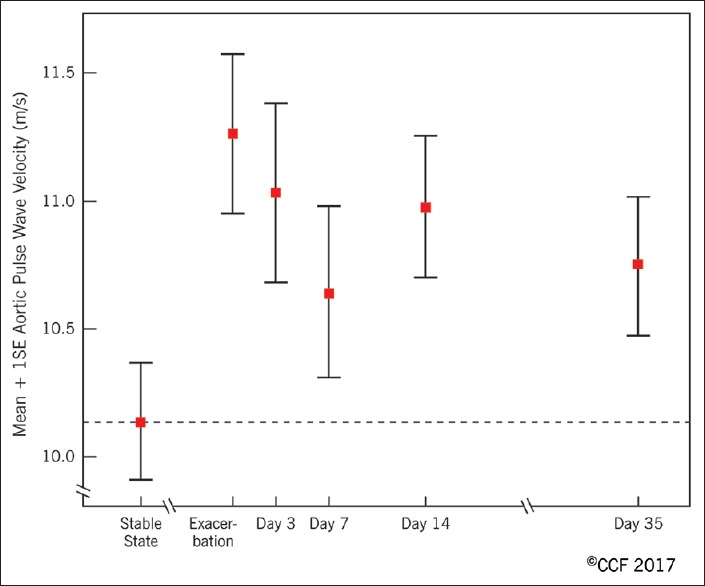
Aortic pulse wave velocity at baseline and around the time of a chronic obstructive pulmonary disease exacerbation. Reprinted with permission of the American Thoracic Society. Copyright© 2018 American Thoracic Society. From Patel et al.[13] The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society
Increased incidence of cardiovascular events during exacerbations begets the question of β-blocker use, cornerstone of cardiovascular disease management, in patients with COPD. For years, practitioners avoided β-blocker prescription for COPD patients due to the concern of worsening bronchomotor tone with β2-receptor blockade. Stefan et al. reported no increase in mortality, readmissions or late mechanical ventilation when patients with a history of cardiovascular disease continued to receive β1-selective blockers during hospitalization for COPD exacerbation.[14] However, a prospective study from Sweden found increased risk of mortality associated with cardioselective β-blockers among patients with severe COPD starting oxygen therapy, raising caution.[15] Recently, Bhatt et al. published the analysis from 3464 subjects in COPD gene cohort, prospectively followed for 2.1 years.[16] When comparing patients who received cardioselective β-blockers with those who did not, β-blocker use was associated with lower rate of exacerbations. The association persisted after adjusting for history of exacerbations, age, gender, race, smoking burden, body mass index, severity of obstruction, degree of emphysema on computed tomography (CT) imaging, coronary artery calcification, presence of congestive heart failure and coronary artery disease, long-acting respiratory medications and for the propensity to prescribe β-blockers. Contrary to the Swedish study, there was no association between β-blockers and mortality including among those patients with severe COPD on home O2. A randomized control trial which aims to assess the efficacy of beta-blockers in preventing COPD exacerbations is currently recruiting subjects in the United States (NCT02587351). While awaiting results of further inquiry in the role of β-blockers in COPD, the author does not hold prescription of cardioselective β-blockers during COPD exacerbation, but exercises caution when starting β-blockers for patients with severe COPD who have FEV1 < 50% and/or evidence of ventilatory failure.
Oxygen Use in Chronic Obstructive Pulmonary Disease
The data that support mortality benefit of oxygen originate from 2 randomized trials conducted in the 1970's.[17] In the United Kingdom Medical Research Council trial randomized 87 patients with severe obstruction, hypoxemia (PaO2 49–52), hypercapnia (PCO2 56–59), and mild pulmonary hypertension to receive domiciliary oxygen to increase PaO2 >60 versus no oxygen.[18] Improved survival (55% vs. 33%) was reported in the oxygen supplementation group which received O2 for 15 h a day.
In the second trial (Nocturnal Oxygen Therapy Trial) sponsored by National Institute of Health, 203 patients who had COPD and severe resting hypoxemia (PaO2 ≤55 or PaO2 ≤59 with edema or polycythemia −≥55% or P pulmonale were randomized to receive continuous oxygen versus nocturnal oxygen.[19] Continuous oxygen (18 h a day) conferred a significant survival benefit compared to nocturnal oxygen only (12 h a day) over a 3-year follow-up (relative risk [death] =1.94 with nocturnal oxygen only). In essence, these seminal trials showed that some oxygen is better than none and more is better than some among patients with resting oxygen desaturation or clinical signs of hypoxia.
Nonetheless, practitioners did not have guidance on oxygen therapy for COPD patients who have less severe or exercise-induced desaturation until recently. Long-term Oxygen Treatment Trial (LOTT) randomized 738 patients with moderate resting (SpO2 89%–93%) or exercise-induced desaturation to supplemental versus no oxygen.[20] Moderate exercise-induced desaturation was measured during the 6 min walk test and was defined as SpO2 ≥80% for ≥5 min and <90% for ≥10 s. Supplemental oxygen group received an average of 13.6 h of oxygen/day. There was no difference between the groups in the primary outcome of time to death or hospitalization. Furthermore, the study found no consistent differences between the groups in measures of quality of life, anxiety, depression, or functional status. The limitations of the study include selection bias (severely symptomatic patients may have avoided participation), unblinded study design and relatively short duration of daily oxygen exposure in the intervention group. In the wake of the LOTT, the author routinely discusses the study findings with patients with moderate resting or exertional desaturation. Unless the patient wishes otherwise, a trial of domiciliary oxygen therapy is begun to assess the impact on symptom burden. After 1–2 months of supplementation, continued use of oxygen is readdressed with the patient. Oxygen may be discontinued in the absence of subjective benefit. The author continues to prescribe oxygen for nocturnal desaturation associated with COPD.
Advances in Imaging and Lung Volume Reduction
Emphysema is a distinct COPD phenotype characterized by permanent enlargement of the airspaces distal to the terminal bronchioles due to the destruction of alveolar walls. The end result of emphysema is worsening of gas exchange and reduction in elastic lung recoil with consequent air trapping or hyperinflation. Lung volume reduction, which can be achieved by surgical[21] or bronchoscopic[22] means, aims to reduce the size of the lungs by resection, deflation or scarring of lung tissue. Consequently, the optimal length-tension relationship of respiratory muscles is restored with improvement in dyspnea. Reduction of intrathoracic pressure- and exercise-induced hyperinflation contributes to improved patient outcomes.
The efficacy and safety of lung volume reduction surgery (LVRS) were investigated in the National Emphysema Treatment Trial (NETT).[21] The main finding was that LVRS benefited a subset of patients with emphysema who had predominantly upper lobe emphysema and low exercise capacity before the operation. LVRS was associated with worse survival in patients with severe disease and homogeneous emphysema with high baseline exercise capacity. Distribution of emphysema weighed heavily in determining the efficacy of the intervention. Similar observations have been made for bronchoscopic interventions where patients with heterogeneous, upper lobe predominant emphysema, tend to have more favorable outcomes than those with homogeneous distribution.[22]
Given the importance of emphysema distribution in predicting outcomes, a standardized and accurate approach to assessment is needed. In the NETT, visual grading of emphysema in 3 lung zones, upper, middle, and lower was carried out by trained thoracic radiologists. Accordingly, emphysema severity was divided into four grades which corresponded to 0%–25%, 26%–50%, 51%–75% and 76%–100% of lung tissue involved with emphysema in the scored images. Heterogeneity was defined as a difference of 1 grade in between the neighboring zones. In a study which involved 2 thoracic radiologists and 3 pulmonologists, overall agreement in the assessment of emphysema distribution using the NETT paradigm was 75%.[23]
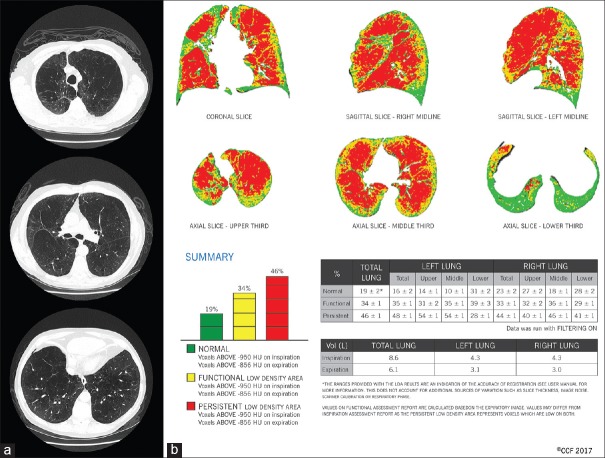
The recent advances in quantitative CT imaging may fulfill the need for standardization in the assessment of emphysema distribution. For instance, parametric response mapping is a novel image processing method that coregisters CT data points voxel by voxel which allows comparison of changes in attenuation between inspiration and exhalation.[24] The change in lung density during the respiratory cycle allows discrimination between normal lung tissue, emphysema and hyperinflation due to functional small airway dysfunction (fSAD) [Figure 3]. One could surmise that the latter two categories of hypoattenuation may pose particular difficulty to the human eye. Accordingly, normal lung tissue attenuation remains >−856 Hounsfield units (HU) during inspiration and expiration. Emphysematous tissue is <−950 HU during inspiration and remains <−856 HU during exhalation. FSAD affected alveolar units are <−856 HU but remain >−950 during inspiration [Figure 4]. In assessing individual patients, visual grading and quantitative CT imaging may potentially reveal discordant findings as illustrated in a patient evaluated in the COPD Center at the Cleveland Clinic [Figure 4].
Figure 3.
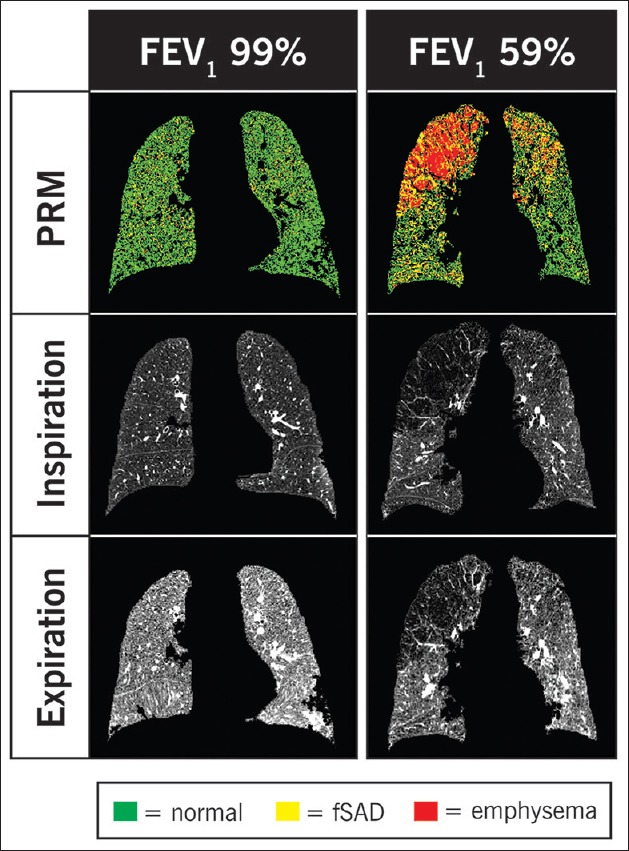
Parametric response mapping coregistered voxels from inspiratory and expiratory computed tomography scans are color-coded green for normal, yellow for functional small airway dysfunction and red for emphysema. On the left, a patient with normal forced expiratory volume 1 s displays the expected increase in lung density (i.e., >–856 HU) during expiration. On the right, a patient with moderately severe chronic obstructive pulmonary disease is displayed. In the paramedic response mapping image, fair amount of emphysema (red) is seen in the right lung whereas the left lung seems to have more functional small airway dysfunction (yellow). Adapted from Galbán et al.[24] with permission
Figure 4.
(a) Visual scoring is the standard for assessing emphysema distribution. In this particular patient, computed tomography scan of the chest was scored in 3 lung zones by a thoracic radiologist in cephalocaudal direction as 3-2-2 on the right and 3-2-1 on the left, suggesting an upper lobe predominant heterogeneity in the distribution of emphysema. (b) When the same patient underwent parametric response mapping analysis, coronal and sagittal views showed a relatively diffuse distribution of emphysema (red voxels across all three regions). Percent distribution of emphysema in each zone supported a homogeneous distribution: 40%, 46% and 41% on the right and 54%, 54% and 28% on the left
Summary
COPD remains a major challenge for healthcare practitioners worldwide. Recent advances derived from multiple venues of research have provided optimism for physicians and patients. Smoking cessation remains the resounding message for COPD patients and the public in general. Benefits are evident whenever smoking cessation is accomplished, but earlier the better. COPD is heterogeneous and consists of phenotypes and endotypes which may demand modifications in management. “COPD comorbidome” should be routinely considered in the management of patients with COPD. Domiciliary oxygen treatment should be considered on a case by case basis among patients with COPD who have moderate- or exercise-induced desaturation. Selection for lung volume reduction candidates may be impacted by advances in quantitative CT scanning.
References
- 1.Løkke A, Lange P, Scharling H, Fabricius P, Vestbo J. Developing COPD: A 25 year follow up study of the general population. Thorax. 2006;61:935–9. doi: 10.1136/thx.2006.062802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kohansal R, Martinez-Camblor P, Agustí A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: An analysis of the Framingham offspring cohort. Am J Respir Crit Care Med. 2009;180:3–10. doi: 10.1164/rccm.200901-0047OC. [DOI] [PubMed] [Google Scholar]
- 3.Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374:1811–21. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. [Last accessed on 2017 May 14]. Available from: http://www.goldcopd.org .
- 5.Miller MR, Levy ML. Chronic obstructive pulmonary disease: Missed diagnosis versus misdiagnosis. BMJ. 2015;351:h3021. doi: 10.1136/bmj.h3021. [DOI] [PubMed] [Google Scholar]
- 6.Jones PW, Adamek L, Nadeau G, Banik N. Comparisons of health status scores with MRC grades in COPD: Implications for the GOLD 2011 classification. Eur Respir J. 2013;42:647–54. doi: 10.1183/09031936.00125612. [DOI] [PubMed] [Google Scholar]
- 7.Lange P, Marott JL, Vestbo J, Olsen KR, Ingebrigtsen TS, Dahl M, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: A study of the general population. Am J Respir Crit Care Med. 2012;186:975–81. doi: 10.1164/rccm.201207-1299OC. [DOI] [PubMed] [Google Scholar]
- 8.Woodruff PG, Agusti A, Roche N, Singh D, Martinez FJ. Current concepts in targeting chronic obstructive pulmonary disease pharmacotherapy: Making progress towards personalised management. Lancet. 2015;385:1789–98. doi: 10.1016/S0140-6736(15)60693-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miravitlles M, Soler-Cataluña JJ, Calle M, Molina J, Almagro P, Quintano JA, et al. Spanish COPD Guidelines (GesEPOC): Pharmacological treatment of stable COPD. Spanish Society of Pulmonology and Thoracic Surgery. Arch Bronconeumol. 2012;48:247–57. doi: 10.1016/j.arbres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Barrecheguren M, Román-Rodríguez M, Miravitlles M. Is a previous diagnosis of asthma a reliable criterion for asthma-COPD overlap syndrome in a patient with COPD? Int J Chron Obstruct Pulmon Dis. 2015;10:1745–52. doi: 10.2147/COPD.S87025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miravitlles M, Alvarez-Gutierrez FJ, Calle M, Casanova C, Cosio BG, Lopez-Vina A, et al. Algorithm for identification of asthma. COPD overlap: Consensus between the Spanish COPD and asthma guidelines. Eur Respir J. 2017;49 doi: 10.1183/13993003.00068-2017. pii: 1700068. [DOI] [PubMed] [Google Scholar]
- 12.Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto-Plata V, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:155–61. doi: 10.1164/rccm.201201-0034OC. [DOI] [PubMed] [Google Scholar]
- 13.Patel AR, Kowlessar BS, Donaldson GC, Mackay AJ, Singh R, George SN, et al. Cardiovascular risk, myocardial injury, and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:1091–9. doi: 10.1164/rccm.201306-1170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefan MS, Rothberg MB, Priya A, Pekow PS, Au DH, Lindenauer PK. Association between ß-blocker therapy and outcomes in patients hospitalised with acute exacerbations of chronic obstructive lung disease with underlying ischaemic heart disease, heart failure or hypertension. Thorax. 2012;67:977–84. doi: 10.1136/thoraxjnl-2012-201945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekström MP, Hermansson AB, Ström KE. Effects of cardiovascular drugs on mortality in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:715–20. doi: 10.1164/rccm.201208-1565OC. [DOI] [PubMed] [Google Scholar]
- 16.Bhatt SP, Wells JM, Kinney GL, Washko GR, Jr, Budoff M, Kim YI, et al. ß-Blockers are associated with a reduction in COPD exacerbations. Thorax. 2016;71:8–14. doi: 10.1136/thoraxjnl-2015-207251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flenley DC. Long-term home oxygen therapy. Chest. 1985;87:99–103. doi: 10.1378/chest.87.1.99. [DOI] [PubMed] [Google Scholar]
- 18.Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981;1:681–6. [PubMed] [Google Scholar]
- 19.Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: A clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93:391–8. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 20.Long-Term Oxygen Treatment Trial Research Group, Albert RK, Au DH, Blackford AL, Casaburi R, Cooper JA., Jr A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375:1617–27. doi: 10.1056/NEJMoa1604344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–73. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 22.Shah PL, Herth FJ, van Geffen WH, Deslee G, Slebos DJ. Lung volume reduction for emphysema. Lancet Respir Med. 2017;5:147–56. doi: 10.1016/S2213-2600(16)30221-1. [DOI] [PubMed] [Google Scholar]
- 23.Hersh CP, Washko GR, Jacobson FL, Gill R, Estepar RS, Reilly JJ, et al. Interobserver variability in the determination of upper lobe-predominant emphysema. Chest. 2007;131:424–31. doi: 10.1378/chest.06-1040. [DOI] [PubMed] [Google Scholar]
- 24.Galbán CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18:1711–5. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]