Abstract
SERAPHIN was a double-blind, placebo-controlled, event-driven phase III trial that evaluated the effects of long-term treatment with macitentan, an oral endothelin receptor antagonist, in patients with pulmonary arterial hypertension (PAH). The majority of patients were receiving PAH therapy at enrollment, providing the opportunity to evaluate the efficacy and safety of macitentan in combination with other PAH therapies (predominantly phosphodiesterase type 5 inhibitors [PDE-5i]). In patients receiving background therapy, macitentan reduced the risk of morbidity/mortality by 38% compared with placebo (hazard ratio [HR] 0.62; 95% confidence level [CL] 0.43–0.89; p = 0.009). Furthermore, patients receiving macitentan and background therapy had a 37% reduction in the risk of being hospitalized for PAH (HR 0.63; 95% CL 0.41–0.96) compared with patients receiving background therapy only (placebo arm). Macitentan treatment in combination with background therapy was also associated with improvements in exercise capacity, functional class, cardiopulmonary hemodynamics, and health-related quality of life compared with background therapy alone. The safety profile of macitentan as part of a combination therapy regimen was consistent with that of macitentan in the overall SERAPHIN population. The SERAPHIN study has provided evidence that combination therapy with macitentan and a PDE-5i is effective and well tolerated in the management of PAH. Based on these data, and those from subsequent long-term trials, combination therapy is increasingly recognized as an important treatment option for improving long-term outcomes in PAH.
Clinical trial registration number: NCT00660179
Key Points
| Use of combination therapy for pulmonary arterial hypertension (PAH) is on the rise, but—until recently—evidence of its benefits from long-term clinical studies was lacking. |
| The SERAPHIN study, which investigated the effects of long-term treatment with macitentan compared with placebo, enrolled a large number of patients who were taking background PAH therapy (primarily phosphodiesterase type 5 inhibitors [PDE-5i]), allowing examination of the efficacy and safety of macitentan in combination therapy. |
| This review highlights the combination therapy data with macitentan in SERAPHIN, including a reduction in the risk of morbidity/mortality and hospitalization and improvements in functional parameters, cardiopulmonary hemodynamics, and health-related quality of life in the context of their significant contribution to the recent trend towards increasing use of combination therapy to improve outcomes in patients with PAH. |
Introduction
Pulmonary arterial hypertension (PAH) remains a progressive and ultimately fatal disease despite the availability of a number of new therapies over the past couple of decades. These therapies individually target three signaling pathways known to be involved in the pathogenesis of PAH: the endothelin (ET), nitric oxide, and prostacyclin pathways [1–3]. The progressive nature of PAH provides a rationale for early and intensive management and the use of combination therapy to target multiple pathways simultaneously. Combination therapy is an important component of current PAH management strategies, which aim for patients to reach or maintain a low risk for mortality, the defined treatment goal in PAH [2, 3].
Until recently, clinical data to support combination therapy were inconsistent and limited to short-term studies, mostly with primary endpoints of change in 6-minute walk distance (6MWD) (reviewed recently by Sitbon and Gaine [4]). However, more robust clinical evidence from long-term randomized controlled trials now exists. The landmark SERAPHIN study (NCT00660179), which evaluated the effect of macitentan, an ET receptor antagonist (ERA), on morbidity and mortality in patients with PAH, was the first study to demonstrate that a PAH therapy could provide clinically meaningful long-term benefits [5]. As the majority of patients in SERAPHIN were receiving background therapy, predominantly a phosphodiesterase type 5 inhibitor (PDE-5i), this study also provided the first evidence from a randomized controlled trial to support the long-term benefits of combination therapy in PAH. Based on the results from SERAPHIN, macitentan 10 mg once daily is approved in more than 55 countries for the long-term treatment of patients with PAH as monotherapy or in combination with other PAH therapies.
The purpose of this review was to comprehensively examine the evidence from the SERAPHIN study, specifically with respect to macitentan 10 mg in patients already receiving a PAH therapy at baseline. In addition, we discuss the combination therapy data from SERAPHIN in the context of other recently completed trials, clinical practice, and PAH treatment guidelines.
Macitentan
The ET pathway has a well-established role in the pathogenesis of PAH [6–8], and the deleterious effects of ET-1 are mediated by both the ETA and the ETB receptors [9]. ET receptors can be targeted with ERAs, and three ERAs are currently approved for the treatment of PAH (macitentan, bosentan, and ambrisentan) [2, 3]. Macitentan is an oral ERA targeting both ET receptors and is indicated for the treatment of PAH to delay disease progression [10, 11]. In pre-clinical studies, macitentan exhibited high binding affinity to both ET receptors, sustained receptor binding, and high tissue penetration [12–14]. Furthermore, pharmacokinetic data have demonstrated that macitentan and its active metabolite both have a long elimination half-life of approximately 16 and 48 h, respectively, which supports a once-daily dosing regimen [15]. The safety and efficacy of macitentan in patients with PAH have been demonstrated in the SERAPHIN study and its open-label extension [5].
The SERAPHIN Study
SERAPHIN was a double-blind, event-driven phase III randomized controlled trial designed to investigate the effects of long-term treatment with macitentan compared with placebo in patients with PAH using a composite primary endpoint of morbidity and mortality [5]. The trial enrolled 742 patients with PAH, making it the largest trial conducted in PAH at the time of its completion. The study enrolled patients aged ≥ 12 years, who were randomized to receive macitentan 3 mg (n = 250), macitentan 10 mg (n = 242), or placebo (n = 250) once daily. The baseline demographics showed that the majority were prevalent patients (i.e., they had been diagnosed with PAH at least 6 months prior to baseline), with a mean time since PAH diagnosis of 2.7 years. The majority of patients (63.7%) were receiving a PDE-5i and/or an oral/inhaled prostanoid at enrollment. Patients who were receiving background PAH therapy were required to have been receiving stable doses for 3 months prior to enrollment. As SERAPHIN was a large study, including both treatment-naive patients and patients previously treated with PAH-specific therapy, it provided an opportunity to evaluate the long-term efficacy and safety of macitentan as monotherapy and combination therapy (mostly with a PDE-5i). Furthermore, patients with and without background therapy were pre-specified subgroups for analysis.
The SERAPHIN trial was the first completed long-term trial in PAH and the first trial to use a clinically relevant composite primary endpoint as a measure of PAH progression. The composite endpoint was the time to the first morbidity event related to PAH or death from any cause, the former defined as worsening of PAH, initiation of intravenous or subcutaneous prostanoid therapy, lung transplantation, or atrial septostomy. Worsening of PAH was defined as the occurrence of all three of the following: a decrease in the 6MWD of ≥ 15% from baseline, worsening of PAH symptoms, and the need for additional treatment for PAH. All primary endpoint events were adjudicated by an independent clinical event committee in a blinded fashion. The study showed that macitentan 10 mg reduced the risk of a morbidity/mortality event by 45% versus placebo (97.5% confidence level [CL] 0.39–0.76; p < 0.001). Worsening of PAH was the most frequent primary endpoint event, occurring in 24% of patients treated with macitentan 10 mg and 37% of placebo-treated patients. Changes in exercise capacity, World Health Organization (WHO) functional class (FC), PAH-related death or hospitalization, and health-related quality of life were examined as secondary endpoints. Hemodynamic parameters at baseline and month 6 were analyzed in a sub-study of patients who underwent an additional right heart catheterization (RHC) at month 6 [16]. The main results from SERAPHIN are summarized in Table 1.
Table 1.
Summary of the main results from the SERAPHIN trial in the overall study population
| Variable | Macitentan 10 mg (N = 242) | Placebo (N = 250) | Treatment effect (Macitentan 10 mg vs. Placebo) | |
|---|---|---|---|---|
| Morbidity event or death as the first eventa [5] | 76 (31.4) | 116 (46.4) | HR 0.55 (97.5% CL 0.39–0.76) | p < 0.001 |
| PAH-related death or hospitalizationa [5] | 50 (20.7) | 84 (33.6) | HR 0.50 (97.5% CL 0.34–0.75) | p < 0.001 |
| PAH-related hospitalizationa,b [20] | 46 (19.0) | 80 (32.0) | HR 0.48 (95% CL 0.34–0.70) | p < 0.0001 |
| Change in 6MWD from BL to month 6, m [5] | 12.5 ± 83.5 | − 9.4 ± 100.6 | LSMD 22.8 (97.5% CL 4.0–41.5) | p = 0.008 |
| Patients improving in WHO FC from BL to month 6 [5] | 54 (22) | 32 (13) | OR 1.95 (95% CL 1.21–3.14) | p = 0.006 |
| HRQoLc [34]d | ||||
| Change from BL to month 6 in SF-36 PCS scores | 2.3 ± 7.8 | − 0.7 ± 8.7 | Mean placebo-corrected change from BL 3.0 (97.5% CL 1.3–4.7) | – |
| Change from BL to month 6 in SF-36 MCS scores | 1.3 ± 11.3 | − 2.1 ± 12.6 | Mean placebo-corrected change from BL 3.4 (97.5% CL 0.9–5.9) | – |
| Change in cardiopulmonary hemodynamic parameters and NT-proBNPe,f [16] | ||||
| Cardiac index, l/min/m2 | 0.30 ± 0.85 | − 0.33 ± 0.65 | 0.63 (95% CL 0.33, 0.93) | p < 0.05 |
| PVR, % | 71.3 (62.4, 81.4) | 115.8 (104.7, 128.1) | − 38.5 (95% CL − 47.8, − 27.5) | p < 0.05 |
| mPAP, mmHg | − 5.3 ± 11.4 | 1.0 ± 7.4 | − 6.4 (95% CL − 10.2, − 2.5) | p < 0.05 |
| NT-proBNP, fmol/ml | − 109 ± 552 | 194 ± 575 | − 303 (95% CL − 533, − 73) | p < 0.05 |
Data are presented as n (%) or mean ± SD
6MWD 6-minute walk distance, BL baseline, CL confidence limit, HR hazard ratio, HRQoL health-related quality of life, LSMD least-squares mean difference, MCS mental component summary, mPAP mean pulmonary arterial pressure, NT-proBNP N-terminal pro-brain natriuretic peptide, OR odds ratio, PAH pulmonary arterial hypertension, PCS physical component summary, PVR pulmonary vascular resistance, SD standard deviation, SF-36 36-Item Short-Form survey, WHO FC World Health Organization functional class
aEndpoints evaluated up to the end of the double-blind treatment period (median duration of treatment, 115 weeks)
bPost hoc analysis
cA higher SF-36 score denotes better HRQoL (scale, 0–100). Ten patients in the placebo group and eight patients in the macitentan 10 mg group were excluded as they were either aged < 14 years or did not have a complete HRQoL assessment at baseline (i.e., they did not have baseline data for all eight health domains, a PCS score and an MCS score). In addition, one patient in the placebo arm did not receive any treatment and was therefore excluded from the analysis
dActelion unpublished data
eFor the change from BL to month 6, PVR data are the geometric mean of month 6/baseline (%) (95% CL), data for all other hemodynamic variables are mean ± SD. For the treatment effect, PVR data are expressed as a percent change (%) between macitentan and placebo: (ratio of geometric means − 1) × 100; data for all other variables are the placebo-corrected mean ± SD
fShown only for the patients in the hemodynamic sub-study with non-missing values for change from BL to month 6, as follows: for cardiac index n = 48 for macitentan, n = 50 for placebo; for PVR n = 48 for macitentan, n = 50 for placebo; for mPAP n = 49 for macitentan, n = 50 for placebo; for NT-proBNP n = 46 for macitentan, n = 49 for placebo
Macitentan in Combination Therapy: Evidence from the SERAPHIN Study
Baseline Characteristics of Patients Receiving Background Therapy
In total, 154 patients randomized to macitentan 10 mg and 154 randomized to placebo were receiving background PAH therapy [5]. Background therapy consisted primarily of a PDE-5i (97.4%) in both macitentan and placebo groups; overall, 5.4% of patients were receiving an inhaled or oral prostacyclin. Patients were not permitted to receive intravenous prostacyclin therapy and, at the time that SERAPHIN was designed, soluble guanylate cyclase stimulators were not approved as a PAH therapy. The baseline characteristics of these populations were similar to those of the overall SERAPHIN population and are shown in Table 2.
Table 2.
Baseline characteristics
| Characteristic | Overall SERAPHIN population [5] (N = 742) | Combination therapy: macitentan 10 mg + background therapya (N = 154) | Monotherapy: placebo + background therapya (N = 154) |
|---|---|---|---|
| Female sex | 565 (77) | 117 (76) | 113 (73) |
| Age, years | 46 ± 16 | 45 ± 15 | 46 ± 17 |
| Race | |||
| White | 403 (54.5) | 71 (46.1) | 73 (47.4) |
| Black | 19 (2.6) | 4 (2.6) | 5 (3.2) |
| Asian | 205 (27.7) | 50 (32.5) | 51 (33.1) |
| Hispanic | 109 (14.7) | 29 (18.8) | 24 (15.6) |
| Other | 3 (0.4) | – | 1 (0.6) |
| Year from diagnosis of PAHb | 2.7 ± 4.0 | 2.6 ± 3.4 | 2.7 ± 4.1 |
| PAH classificationb | |||
| Idiopathic PAH | 404 (55.0) | 91 (59.1) | 80 (52.3) |
| Hereditary PAH | 13 (1.8) | 1 (0.6) | 2 (1.3) |
| Associated with CTD | 224 (30.5) | 43 (27.9) | 49 (32.0) |
| Associated with CHD | 62 (8.4) | 12 (7.8) | 13 (8.5) |
| Associated with HIV | 10 (1.4) | 4 (2.6) | 2 (1.3) |
| Associated with drug use or toxin exposure | 22 (3.0) | 3 (1.9) | 7 (4.6) |
| 6MWD, m | 360 ± 100 | 364 ± 97 | 360 ± 111 |
| WHO FC | |||
| Ic | 1 (0.1) | – | – |
| II | 387 (52.4) | 73 (47.4) | 78 (50.6) |
| III | 337 (45.6) | 77 (50.0) | 74 (48.1) |
| IV | 14 (1.9) | 4 (2.6) | 2 (1.3) |
| Background therapy | |||
| Patients receiving background therapyd | 471 (63.7) | 154 (100.0) | 154 (100.0) |
| PDE-5i | 454 (96.4) | 150 (97.4) | 150 (97.4) |
| Oral or inhaled prostanoid | 40 (5.4) | 16 (10.4) | 7 (4.5) |
Data are presented as n (%) or mean ± SD
6MWD 6-minute walk distance, CHD congenital heart disease, CTD connective tissue disease, HIV human immunodeficiency virus, PAH pulmonary arterial hypertension, PDE-5i phosphodiesterase type 5 inhibitor, SD standard deviation, WHO FC World Health Organization functional class
aActelion unpublished data
b N = 153 for the placebo group
cOne patient in WHO FC I was incorrectly included in the study
dPatients could have received more than one background PAH therapy
Effect of Combination Therapy with Macitentan on Long-Term Outcomes
Effect of Combination Therapy with Macitentan on the Risk of Morbidity/Mortality
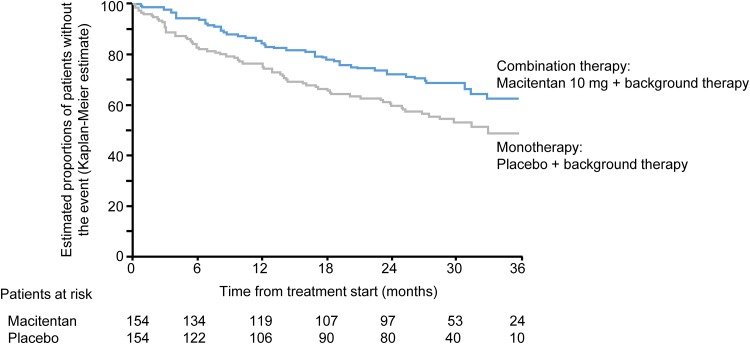
To investigate the efficacy of a combination therapy regimen with macitentan, the composite primary endpoint of time to first morbidity/mortality event was examined in the subgroup of patients receiving background PAH therapy [5]. The risk of morbidity/mortality was reduced by 38% in patients who received macitentan and background therapy compared with those receiving background therapy alone (placebo) (hazard ratio [HR] 0.62; 95% CL 0.43–0.89; p = 0.009) (Fig. 1) [5]. These data were the first randomized controlled trial-based evidence demonstrating that combination therapy improves long-term outcomes in PAH.
Fig. 1.
Effect of macitentan on morbidity or mortality as a first event in patients receiving background therapy [5]. The hazard ratio for macitentan vs. placebo was 0.62 (95% CL 0.43–0.89; log-rank p value 0.009). The Kaplan–Meier curves are displayed up to 36 months. The analysis (conducted on the all-randomized set) takes into account all available data
Effect of Combination Therapy with Macitentan on Pulmonary Arterial Hypertension (PAH)-Related Hospitalization
The long-term data from SERAPHIN provided an opportunity to assess how combination therapy affects rates of hospitalization due to PAH. This is clinically relevant as many patients with PAH require hospitalization at some point in the course of their disease, for reasons such as worsening symptoms, escalation of treatment to address disease progression, or management of therapy-related adverse events (AEs) [17]. Hospitalization both affects a patient’s quality of life and places a financial burden on the healthcare system [18]. Hospitalization due to PAH is recognized as an indicator of disease progression and has been included in a number of randomized controlled trials [19]. In a subgroup analysis of time to first PAH-related hospitalization in SERAPHIN, patients receiving macitentan and background therapy had a reduction in the risk of being hospitalized for PAH of 37.4% (HR 0.63; 95% CL 0.41–0.96) compared with patients receiving background therapy only (placebo arm) [20]. These results were consistent with the findings in the overall SERAPHIN population (macitentan 10 mg vs. placebo) (HR 0.48; 95% CL 0.34–0.70; p < 0.0001) [20]. These data show that macitentan reduces PAH-related hospitalizations and provide further evidence of the long-term clinical benefits of macitentan when used in combination with other PAH therapies.
Effect of Combination Therapy with Macitentan on Short-Term Endpoints
In addition to the long-term outcome data, the SERAPHIN study also provided an opportunity to examine the effect of combination therapy with macitentan on short-term endpoints, including those measuring functional, symptomatic, and hemodynamic changes.
Effect of Combination Therapy with Macitentan on 6-Minute Walk Distance
6MWD is an important measure in clinical practice and is a useful indicator of a patient’s clinical condition. As such, change from baseline in 6MWD was a secondary endpoint in the three largest randomized controlled trials in PAH, including the SERAPHIN trial [5, 21, 22]. For patients receiving macitentan and background PAH therapy in SERAPHIN, 6MWD increased by a mean (standard deviation [SD]) of 17.9 (82.3) m from baseline to month 6. For those receiving background PAH therapy alone, 6MWD decreased by a mean (SD) of 7.8 (84.8) m. The treatment effect of macitentan versus placebo was 25.9 m (97.5% CL 4.5–47.3; p = 0.007) [5]. This treatment response was similar to that observed in the overall population (least-squares mean difference 22.8 m; 97.5% CL 4.0–41.5; p = 0.007 for macitentan vs. placebo) [5] and is within the range of that observed in PAH trials that enrolled patients already receiving one or more PAH therapies at baseline [21–28].
The observed deterioration in exercise capacity over time in patients receiving background PAH therapy alone suggests that these patients may have been undertreated. Data from the REVEAL registry indicated that worsening of 6MWD over time of at least 15% of baseline was associated with poor prognosis [29]. The initiation of macitentan in patients already receiving PAH therapy not only prevented the worsening in exercise capacity that occurred in patients receiving only a PDE-5i but also improved functional capacity from baseline.
Effect of Combination Therapy with Macitentan on World Health Organization Functional Class
WHO FC is an important indicator of the clinical severity of PAH and is a key part of the risk assessment process recommended in the European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines [2, 3, 30]. The association between WHO FC and survival is well established, with a lower WHO FC associated with better survival [31]. The proportion of patients with an improved WHO FC from baseline to month 6 was examined in the subgroup of patients receiving background therapy in SERAPHIN. Patients who received macitentan plus background therapy were twice as likely to show an improvement in WHO FC than those receiving placebo and background therapy (odds ratio [OR] 2.25; 95% CL 1.23–4.12; Actelion, unpublished data). The treatment effect of macitentan on WHO FC was consistent with that observed in the overall SERAPHIN population (OR 1.95; 95% CL 1.21–3.14; p = 0.006; Actelion, unpublished data). These data suggest that macitentan, including in combination with other PAH therapies, improves functional capacity and symptoms, further supporting the role of combination therapy in the management of PAH.
Effect of Combination Therapy with Macitentan on Quality of Life
Patients with PAH are likely to experience an increasing symptom burden, which may lead to incapacitation. This can affect the patient’s quality of life, including physical, social, and emotional well-being [32, 33]. The importance of improving quality of life for patients with PAH has increasingly been recognized in recent years. The most recent PAH guidelines recommended that the psychological, social, emotional, and spiritual needs of patients should be addressed by the health provider teams managing their care [2, 3]. In SERAPHIN, the change in 36-Item Short Form (SF-36) survey scores from baseline to month 6 was evaluated as a secondary endpoint. At month 6, macitentan significantly improved seven of eight domains in the SF-36, including those evaluating physical and mental health, providing the first clinical evidence of a benefit of PAH therapy in the majority of the SF-36 domains [34]. The treatment effect in the patients receiving combination therapy was consistent with the overall population for the physical and mental component scores (Table 3; Actelion, unpublished data). Improving quality of life is an important consideration in the treatment of patients with PAH [2, 3], and these data support the use of macitentan as part of a combination therapy regimen to help patients reach this goal.
Table 3.
Change from baseline to month 6 in SF-36 physical and mental component summary scores
| Variable | Combination therapy: macitentan 10 mg + background therapy | Monotherapy: placebo + background therapy | Mean placebo-corrected change in scores |
|---|---|---|---|
| Change in PCS | 2.8 ± 7.6 | 0.1 ± 7.9 | 2.8 (1.0–4.5) |
| Change in MCS | 1.0 ± 11.5 | − 2.1 ± 12.8 | 3.1 (0.3–5.8) |
Data are presented as mean ± SD deviation or mean (95% confidence limit). Actelion unpublished data. A higher SF-36 score denotes better health-related quality of life (scale, 0–100)
CL confidence limit, MCS mental component summary score, PCS physical component summary score, SF-36 36-Item Short-Form survey
Effect of Combination Therapy with Macitentan on Cardiopulmonary Hemodynamic Parameters and NT-proBNP
A sub-study of SERAPHIN analyzed hemodynamic parameters in a subgroup of patients who had a baseline RHC assessment within 3 months before randomization and a further RHC at month 6 [16]. The sub-study also analyzed changes from baseline to month 6 in N-terminal pro-brain natriuretic peptide (NT-proBNP) levels. In patients receiving background PAH therapy (macitentan n = 24; placebo n = 34), a significant treatment effect of macitentan versus placebo was observed for cardiac index, pulmonary vascular resistance, mean pulmonary arterial pressure, and levels of NT-proBNP (Table 4). These effects were consistent with those observed for macitentan 10 mg in the overall SERAPHIN population [16]. Given that these parameters are indicators of or have an impact on right ventricular (RV) function [16, 35, 36], the hemodynamic findings from SERAPHIN suggest that combination therapy with macitentan may lead to improvements in the functioning of the RV. As RV function is a critical determinant of functional capacity and survival in PAH [37, 38], preserving or achieving good RV function is an important aspect of patient management and is required to achieve a low risk status [2, 3].
Table 4.
Changes from baseline to month 6 for hemodynamic parameters and NT-proBNP [16]
| Variable | Combination therapy: macitentan 10 mg + background therapy (N = 24) | Monotherapy: placebo + background therapy (N = 34) | Mean treatment effect vs. placebo (95% CL)a | ||
|---|---|---|---|---|---|
| n | Mean change ± SDb | n | Mean change ± SDb | ||
| Cardiac index, l/min/m2 | 22 | 0.28 ± 0.79 | 29 | − 0.34 ± 0.52 | 0.61 (0.24, 0.98)c |
| PVR, % | 22 | 75.5 (63.7–89.6) | 29 | 119.7 (105.4–135.8) | − 36.9 (− 48.5 to − 22.7)c |
| mPAP, mmHg | 22 | − 3.3 ± 7.9 | 29 | 1.1 ± 6.7 | − 4.4 (− 8.5, − 0.3)c |
| NT-proBNP, fmol/ml | 21 | − 228.8 ± 501 | 28 | 37.5 ± 321.9 | − 266 (− 503, − 29)c |
N represents the number of patients in the hemodynamic sub-study at baseline who were receiving background PAH therapy; n represents the number of patients receiving background PAH therapy with non-missing values for the change from baseline to month 6. Results are based on observed data with no imputation rules applied for missing values
CL confidence limit, mPAP mean pulmonary arterial pressure, NT-proBNP N-terminal pro-brain natriuretic peptide, PVR pulmonary vascular resistance, SD standard deviation
aPVR data are expressed as a percent change (%) between macitentan and placebo: (ratio of geometric means − 1) × 100, data for all other variables are the placebo corrected mean ± SD
bPVR data are the geometric mean of month 6/baseline (%) (95% CL), data for all other variables are mean ± SD
c P value < 0.05 for the comparison between macitentan and placebo
Safety and Tolerability of Combination Therapy with Macitentan
Even if PAH monotherapy is well tolerated, it is possible that additive AEs may occur when two or more PAH therapies are used together and may limit the utilization of combination regimens. It is therefore important to assess the safety and tolerability of PAH treatments as combination therapy. The large number of patients receiving background therapy in SERAPHIN allowed assessment of the safety of macitentan in combination with other PAH therapies, primarily a PDE-5i [5]. AEs experienced by patients receiving macitentan plus background therapy were similar to those recorded for patients receiving background therapy alone. The percentages of patients receiving background therapy plus macitentan or placebo who experienced at least one AE were 93.5 and 97.4%, respectively (Table 5; Actelion, unpublished data). A higher proportion of patients receiving macitentan plus background therapy than those receiving background therapy alone experienced anemia and bronchitis (anemia 16.2 and 4.6%; bronchitis 11.0 and 5.9%, respectively) (Table 5; Actelion, unpublished data). The incidence of peripheral edema, a known ERA-related AE, was similar in macitentan- and placebo-treated patients receiving background therapy (19.5 and 23.5%, respectively; Actelion, unpublished data). Treatment discontinuations due to AEs in patients receiving background therapy were similar between those receiving macitentan and those receiving placebo (9.1 vs. 11.8%, respectively; Actelion, unpublished data). Therefore, the safety profile of macitentan as part of a combination therapy regimen was consistent with that of macitentan in the overall SERAPHIN population, suggesting that—overall—combination treatment with macitentan and a PDE-5i does not adversely affect tolerability compared with monotherapy, predominantly with a PDE-5i [5].
Table 5.
Most frequent adverse events in patients with background therapy
| Combination therapy: macitentan 10 mg + background therapy (N = 154) | Monotherapy: placebo + background therapy (N = 153a) | |
|---|---|---|
| Patients with at least one AE | 144 (93.5) | 149 (97.4) |
| Number (%) of patients with an AEb | ||
| Worsening of PAH | 35 (22.7) | 57 (37.3) |
| Edema peripheral | 30 (19.5) | 36 (23.5) |
| Anemia | 25 (16.2) | 7 (4.6) |
| URTI | 24 (15.6) | 27 (17.6) |
| RV failure | 22 (14.3) | 38 (24.8) |
| Headache | 21 (13.6) | 16 (10.5) |
| Diarrhea | 20 (13.0) | 15 (9.8) |
| Nasopharyngitis | 17 (11.0) | 16 (10.5) |
| Dizziness | 17 (11.0) | 22 (14.4) |
| Bronchitis | 17 (11.0) | 9 (5.9) |
| Cough | 12 (7.8) | 21 (13.7) |
| Dyspnea | 12 (7.8) | 16 (10.5) |
| Back pain | 6 (3.9) | 16 (10.5) |
Data are presented as n (%) Actelion unpublished data
For each preferred term, a patient is counted once if the patient reported one or more events in that category
AE adverse event, PAH pulmonary arterial hypertension, RV right ventricular, URTI upper respiratory tract infection
aOne patient randomized to placebo did not receive treatment
bAEs listed are those that occurred in more than 10% of the patients in either study group and are presented in descending order based on the frequency count in the “Combination therapy: macitentan + background therapy” group
Combination Therapy in PAH: From Clinical Trials to Clinical Practice
As discussed in this review, the SERAPHIN study has provided robust evidence that combination therapy with macitentan and a PDE-5i is beneficial, and it was the first trial to provide evidence that combination therapy improves long-term outcomes in PAH [5]. Since the completion of SERAPHIN, three additional long-term randomized controlled trials have reported results corroborating the importance of combination therapy: AMBITION, COMPASS-2, and GRIPHON ([21, 22, 39] and reviewed by Sitbon and Gaine [4]). Consistent results have been reported in AMBITION, which compared the treatment strategies of initial double combination therapy and monotherapy. Initial combination therapy with ambrisentan and tadalafil resulted in a significantly lower risk of clinical failure events than either drug as monotherapy in a population of treatment-naive newly diagnosed patients (HR 0.50; 95% CL 0.35–0.72; p < 0.001) [21]. The COMPASS-2 trial evaluated the effect of administering bosentan or placebo to patients already receiving stable doses of sildenafil. While the study showed a reduction in the risk of a morbidity/mortality event in patients receiving combination therapy compared with monotherapy, the treatment effect was not significant. However, addition of bosentan did significantly improve 6MWD compared with placebo (+ 21.8 m, 95% CL 5.9–37.8; p = 0.0106), and NT-proBNP levels were stable in patients receiving bosentan but increased in those receiving placebo (p = 0.0003) [39]. Long-term benefits of double and triple combination therapy with the oral selective IP prostacyclin receptor agonist selexipag in combination with an ERA, a PDE-5i, or both, were recently reported in the event-driven GRIPHON trial [22]. This study included patients who were predominantly in WHO FC II or III and were treatment-naive (20.4%) or receiving background therapy at enrollment (79.6%). All patients in GRIPHON who were receiving a PAH therapy at baseline had to have been receiving a stable dose for at least 3 months before enrollment. In the overall study population, selexipag reduced the risk of the morbidity/mortality primary endpoint by 40% (HR 0.60; 99% CL 0.46–0.78; p < 0.001). Pre-specified subgroup analyses by background therapy showed that the treatment effect for patients treated with double and triple combination therapy was consistent with the results in the overall population (PDE-5i background therapy HR 0.58, 99% CL 0.37–0.91; ERA background therapy HR 0.66, 99% CL 0.32–1.35; PDE-5i and ERA background therapy HR 0.63, 99% CL 0.39–1.01) [22]. This study provided the first randomized controlled trial data on the benefits of triple combination therapy in patients with PAH. Together, results from these trials have demonstrated that combination therapy can improve outcomes for patients with PAH and have paved the way for a paradigm shift in the management of PAH. Triple combination therapy is being investigated further in the ongoing TRITON study (NCT02558231), which will assess the efficacy and safety of an initial triple oral combination regimen (macitentan, tadalafil, and selexipag) versus an initial double oral treatment regimen (macitentan and tadalafil).
Disease registries can be helpful in understanding how clinical trial evidence supporting combination therapy has impacted upon clinical practice because they provide insights into the real-world use of combination therapy in PAH. Historically, the use of combination therapy for PAH was not widespread. This is evident from REVEAL, a large multicenter observational registry that enrolled patients with PAH between 2006 and 2009 in the USA, which showed that only 46% of previously diagnosed patients were receiving double combination therapy at enrollment and only 9% were receiving triple combination therapy [40]. More recently, data from an ongoing large US-based registry of patients with PAH who were newly treated with macitentan (the OPUS registry) have suggested that use of combination therapy has increased; 68% of patients in this registry were receiving at least one other PAH therapy at follow-up, with the majority of these patients receiving a PDE-5i (84.8%) [41]. However, a comparison of other recent registries has indicated that the use of initial combination therapy in clinical practice still varies. For example, among newly diagnosed patients, 43% received combination therapy within 3 months of diagnosis in the French registry [30]; in contrast, this figure was only 19% in the COMPERA registry [42]. These data suggest that combination therapy may sometimes still be underutilized in clinical practice.
To ensure that the robust clinical trial data supporting combination therapy translates into changes in clinical practice, it is essential that clear guidance is available on how and when to implement combination therapy. As outlined in the ESC/ERS guidelines, the decision on when to combine therapies must be based on the patient’s risk profile, as determined by comprehensive multi-parameter risk assessments, which should be conducted at baseline and regularly during treatment. An estimated 1-year mortality rate for patients with a low risk status is < 5%, whereas for those with an intermediate or high risk status it is 5–10 or > 10%, respectively. The guidelines recommend treating to achieve a low risk profile, which includes, among others, WHO FC I or II and near-normalization of resting cardiac index and/or NT-proBNP plasma levels [2, 3]. Recent evidence has shown that patients reaching targets associated with low risk status have better prognosis and long-term outcomes than those who do not [16, 30, 42, 43]. Given the importance of reaching a low risk profile, the ESC/ERS guidelines strongly recommend combination therapy [2, 3]. Timely initiation of combination therapy has the potential to increase the likelihood of achieving a low risk status in PAH and thereby improve patient outcomes.
Conclusions
Combination therapy with macitentan is effective and well tolerated in the management of PAH. Efficacy of combination treatment with macitentan and other PAH therapies, primarily a PDE-5i, was demonstrated in the SERAPHIN study for a number of its endpoints [5]. Compared with background therapy alone, macitentan was associated with a reduction in the risk of clinically relevant long-term outcomes, such as morbidity/mortality and hospitalization, as well as with improvement of functional parameters of exercise capacity and functional class, and in cardiopulmonary hemodynamics and health-related quality of life [5, 16, 20, 34]. SERAPHIN contributes long-term data on combination therapy with macitentan to the growing body of evidence for treatment of patients with PAH with multiple treatment modalities. Based on recent evidence from long-term randomized controlled trials and recommendations from the ESC/ERS guidelines for PAH, in the current treatment era, combination therapy regimens, such as those including macitentan and a PDE-5i, are an important treatment option for patients with PAH.
Acknowledgements
The authors would like to thank Mariana Thomson, PhD, from nspm ltd (Meggen, Switzerland) for medical writing assistance, funded by Actelion Pharmaceuticals Ltd (Allschwil, Switzerland).
Compliance with Ethical Standards
Conflict of interest
Pavel Jansa has received fees and grants from Actelion Pharmaceuticals Ltd, United Therapeutics, AOP Orphan, Bayer HealthCare, MSD, and GlaxoSmithKline. He has served on advisory boards for Actelion Pharmaceuticals Ltd, Bayer HealthCare, and MSD. Tomás Pulido’s institution has received research grants from Actelion Pharmaceuticals Ltd, United Therapeutics, GlaxoSmithKline, Bayer HealthCare, and Reata Pharmaceuticals. He has served as a consultant and received honoraria for lectures from Actelion Pharmaceuticals Ltd, Bayer HealthCare, and GlaxoSmithKline. He has participated as a principal investigator in trials sponsored by Actelion Pharmaceuticals Ltd.
References
- 1.Humbert M, Lau EM, Montani D, Jais X, Sitbon O, Simonneau G. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation. 2014;130:2189–2208. doi: 10.1161/CIRCULATIONAHA.114.006974. [DOI] [PubMed] [Google Scholar]
- 2.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 3.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 4.Sitbon O, Gaine S. Beyond a single pathway: combination therapy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:408–417. doi: 10.1183/16000617.0085-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pulido T, Adzerikho I, Channick RN, Delcroix M, Galie N, Ghofrani HA, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809–818. doi: 10.1056/NEJMoa1213917. [DOI] [PubMed] [Google Scholar]
- 6.Stewart DJ, Levy RD, Cernacek P, Langleben D. Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med. 1991;114:464–469. doi: 10.7326/0003-4819-114-6-464. [DOI] [PubMed] [Google Scholar]
- 7.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 8.Rubens C, Ewert R, Halank M, Wensel R, Orzechowski HD, Schultheiss HP, et al. Big endothelin-1 and endothelin-1 plasma levels are correlated with the severity of primary pulmonary hypertension. Chest. 2001;120:1562–1569. doi: 10.1378/chest.120.5.1562. [DOI] [PubMed] [Google Scholar]
- 9.Dupuis J, Hoeper MM. Endothelin receptor antagonists in pulmonary arterial hypertension. Eur Respir J. 2008;31:407–415. doi: 10.1183/09031936.00078207. [DOI] [PubMed] [Google Scholar]
- 10.Opsumit® (macitentan). Summary of Product Characteristics; 2017.
- 11.Opsumit® (macitentan). US Prescribing Information; 2016.
- 12.Iglarz M, Binkert C, Morrison K, Fischli W, Gatfield J, Treiber A, et al. Pharmacology of macitentan, an orally active tissue targeting dual endothelin receptor antagonist. J Pharmacol Exp Ther. 2008;327:736–745. doi: 10.1124/jpet.108.142976. [DOI] [PubMed] [Google Scholar]
- 13.Gatfield J, Mueller GC, Sasse T, Clozel M, Nayler O. Slow receptor dissociation kinetics differentiate macitentan from other endothelin receptor antagonists in pulmonary arterial smooth muscle cells. PLoS One. 2012;7:e47662. doi: 10.1371/journal.pone.0047662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iglarz M, Landskroner K, Bauer Y, Vercauteren M, Rey M, Renault B, et al. Comparison of macitentan and bosentan on right ventricular remodeling in a rat model of non-vasoreactive pulmonary hypertension. J Cardiovasc Pharmacol. 2015;66:457–467. doi: 10.1097/FJC.0000000000000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruderer S, Hopfgartner G, Seiberling M, Wank J, Sidharta PN, Treiber A, et al. Absorption, distribution, metabolism, and excretion of macitentan, a dual endothelin receptor antagonist, in humans. Xenobiotica. 2012;42:901–910. doi: 10.3109/00498254.2012.664665. [DOI] [PubMed] [Google Scholar]
- 16.Galie N, Jansa P, Pulido T, Channick RN, Delcroix M, Ghofrani HA, et al. SERAPHIN haemodynamic sub-study: the effect of the dual endothelin receptor antagonist macitentan on haemodynamic parameters and NT-proBNP levels and their association with disease progression in patients with pulmonary arterial hypertension. Eur Heart J. 2017;38:1147–1155. doi: 10.1093/eurheartj/ehx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burger CD, Long PK, Shah MR, McGoon MD, Miller DP, Romero AJ, et al. Characterization of first-time hospitalizations in patients with newly diagnosed pulmonary arterial hypertension in the REVEAL registry. Chest. 2014;146:1263–1273. doi: 10.1378/chest.14-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirson NY, Birnbaum HG, Ivanova JI, Waldman T, Joish V, Williamson T. Excess costs associated with patients with pulmonary arterial hypertension in a US privately insured population. Appl Health Econ Health Policy. 2011;9:293–303. doi: 10.2165/11592430-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Galiè N, Simonneau G, Barst RJ, Badesch D, Rubin L. Clinical worsening in trials of pulmonary arterial hypertension: results and implications. Curr Opin Pulm Med. 2010;16:S11–S19. doi: 10.1097/01.mcp.0000370206.61003.7e. [DOI] [PubMed] [Google Scholar]
- 20.Channick RN, Delcroix M, Ghofrani HA, Hunsche E, Jansa P, Le Brun FO, et al. Effect of macitentan on hospitalizations: results from the SERAPHIN Trial. JACC Heart Fail. 2015;3:1–8. doi: 10.1016/j.jchf.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Galiè N, Barbera JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373:834–844. doi: 10.1056/NEJMoa1413687. [DOI] [PubMed] [Google Scholar]
- 22.Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galie N, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373:2522–2533. doi: 10.1056/NEJMoa1503184. [DOI] [PubMed] [Google Scholar]
- 23.Tapson VF, Jing ZC, Xu KF, Pan L, Feldman J, Kiely DG, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest. 2013;144:952–958. doi: 10.1378/chest.12-2875. [DOI] [PubMed] [Google Scholar]
- 24.Tapson VF, Torres F, Kermeen F, Keogh AM, Allen RP, Frantz RP, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (The FREEDOM-C Study): a randomized controlled trial. Chest. 2012;142:1383–1390. doi: 10.1378/chest.11-2212. [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin VV, Benza RL, Rubin LJ, Channick RN, Voswinckel R, Tapson VF, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol. 2010;55:1915–1922. doi: 10.1016/j.jacc.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 26.McLaughlin VV, Oudiz RJ, Frost A, Tapson VF, Murali S, Channick RN, et al. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;174:1257–1263. doi: 10.1164/rccm.200603-358OC. [DOI] [PubMed] [Google Scholar]
- 27.Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 28.Ghofrani HA, Galiè N, Grimminger F, Grunig E, Humbert M, Jing ZC, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330–340. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 29.Farber HW, Miller DP, McGoon MD, Frost AE, Benton WW, Benza RL. Predicting outcomes in pulmonary arterial hypertension based on the 6-minute walk distance. J Heart Lung Transplant. 2015;34:362–368. doi: 10.1016/j.healun.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 30.Boucly A, Weatherald J, Savale L, Jais X, Cottin V, Prevot G, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50:1700889. doi: 10.1183/13993003.00889-2017. [DOI] [PubMed] [Google Scholar]
- 31.Farber HW, Miller DP, Poms AD, Badesch DB, Frost AE, Muros-Le Rouzic E, et al. Five-year outcomes of patients enrolled in the REVEAL registry. Chest. 2015;148:1043–1054. doi: 10.1378/chest.15-0300. [DOI] [PubMed] [Google Scholar]
- 32.Guillevin L, Armstrong I, Aldrighetti R, Howard LS, Ryftenius H, Fischer A, et al. Understanding the impact of pulmonary arterial hypertension on patients’ and carers’ lives. Eur Respir Rev. 2013;22:535–542. doi: 10.1183/09059180.00005713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taichman DB, Shin J, Hud L, Archer-Chicko C, Kaplan S, Sager JS, et al. Health-related quality of life in patients with pulmonary arterial hypertension. Respir Res. 2005;6:92. doi: 10.1186/1465-9921-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta S, Sastry BK, Souza R, Torbicki A, Ghofrani HA, Channick RN, et al. Macitentan improves health-related quality of life for patients with pulmonary arterial hypertension: results from the randomized controlled SERAPHIN trial. Chest. 2017;151:106–118. doi: 10.1016/j.chest.2016.08.1473. [DOI] [PubMed] [Google Scholar]
- 35.Chin KM, Kim NH, Rubin LJ. The right ventricle in pulmonary hypertension. Coron Artery Dis. 2005;16:13–18. doi: 10.1097/00019501-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Gan CT, McCann GP, Marcus JT, van Wolferen SA, Twisk JW, Boonstra A, et al. NT-proBNP reflects right ventricular structure and function in pulmonary hypertension. Eur Respir J. 2006;28:1190–1194. doi: 10.1183/09031936.00016006. [DOI] [PubMed] [Google Scholar]
- 37.Grunig E, Tiede H, Enyimayew EO, Ehlken N, Seyfarth HJ, Bossone E, et al. Assessment and prognostic relevance of right ventricular contractile reserve in patients with severe pulmonary hypertension. Circulation. 2013;128:2005–2015. doi: 10.1161/CIRCULATIONAHA.113.001573. [DOI] [PubMed] [Google Scholar]
- 38.Tatebe S, Fukumoto Y, Oikawa-Wakayama M, Sugimura K, Satoh K, Miura Y, et al. Enhanced [18F]fluorodeoxyglucose accumulation in the right ventricular free wall predicts long-term prognosis of patients with pulmonary hypertension: a preliminary observational study. Eur Heart J Cardiovasc Imaging. 2014;15:666–672. doi: 10.1093/ehjci/jet276. [DOI] [PubMed] [Google Scholar]
- 39.McLaughlin VV, Channick R, Ghofrani HA, Lemarie JC, Naeije R, Packer M, et al. Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. Eur Respir J. 2015;46:405–413. doi: 10.1183/13993003.02044-2014. [DOI] [PubMed] [Google Scholar]
- 40.McGoon MD, Miller DP. REVEAL: a contemporary US pulmonary arterial hypertension registry. Eur Respir Rev. 2012;21:8–18. doi: 10.1183/09059180.00008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chin KM, Channick R, Kim NH, Muros-Le Rouzic E, Selej M, McLaughlin VV. OPUS registry: treatment patterns with macitentan in patients with pulmonary arterial hypertension. Am J Crit Care Med. 2017;195:A2299. [Google Scholar]
- 42.Hoeper MM, Kramer T, Pan Z, Eichstaedt CA, Spiesshoefer J, Benjamin N, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J. 2017;50:1700740. doi: 10.1183/13993003.00740-2017. [DOI] [PubMed] [Google Scholar]
- 43.Kylhammar D, Kjellstrom B, Hjalmarsson C, Jansson K, Nisell M, Soderberg S, et al. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J. 2017 doi: 10.1093/eurheartj/ehx257. [DOI] [PubMed] [Google Scholar]