Abstract
New generations of biorefinery combine innovative biomass waste resources from different origins, chemical extraction and/or synthesis of biomaterials, biofuels, and bioenergy via green and sustainable processes. From the very beginning, identifying and evaluating all potentially high value-added chemicals that could be removed from available renewable feedstocks requires robust, efficient, selective, reproducible, and benign analytical approaches. With this in mind, green and sustainable separation of natural products from agro-industrial waste is clearly attractive considering both socio-environmental and economic aspects. In this paper, the concepts of green and sustainable separation of natural products will be discussed, highlighting the main studies conducted on this topic over the last 10 years. The principal analytical techniques (such as solvent, microwave, ultrasound, and supercritical treatments), by-products (e.g., citrus, coffee, corn, and sugarcane waste) and target compounds (polyphenols, proteins, essential oils, etc.) will be presented, including the emerging green and sustainable separation approaches towards bioeconomy and circular economy contexts.
Keywords: Green and sustainable extraction, Sustainable separation, Green analytical techniques, Biomass waste, Biorefinery, Bioeconomy and circular economy
Introduction
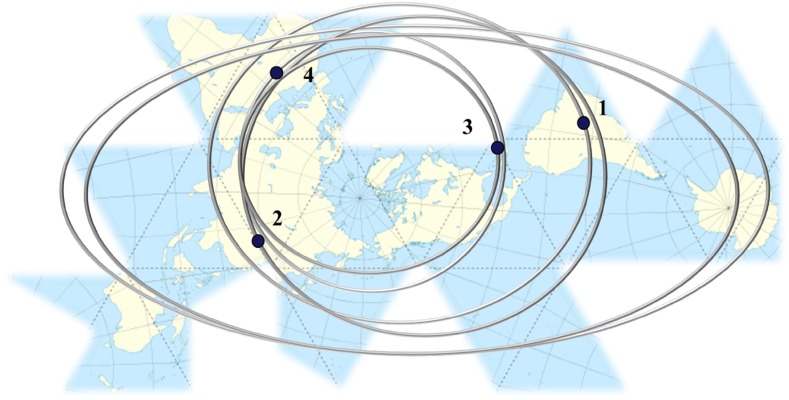
Currently, it can be observed that global sustainability challenges are all closely interconnected, such as pollution, climate change, biodiversity loss, poverty, energy, and food security. As stated by Liu et al. [1], only holistic and disruptive approaches integrating various components of human and natural systems are effective in identifying and proposing suitable solutions for these challenges, especially those related to research, development, and innovation (RD&I) in interdisciplinary and transdisciplinary studies. To exemplify this systemic view, Fig. 1 illustrates the Earth surface that, based on the “Dymaxion map” (the Fuller Projection Map), shows the planet as a continuum without splitting any continents, seas, and oceans, where cycles are integrated through flows of matter, energy, and information [1, 2]. Here, Brazil, China, the Caribbean, and Africa interact across space, time, and organizational levels in many ways. For instance, the expansion of soybean production aggravates deforestation in Brazil, but also provides food and feedstock to China. The food trade between both countries also affects other areas, including the Caribbean and Africa. Dust particles from the Sahara Desert, also increased due to unbalanced agricultural practices, can reach the Caribbean and have an impact on coral reefs and soil fertility, diminishing tourism in this region. In addition, nutrient-rich particles from Africa can reach Brazil, improving its forest productivity.
Fig. 1.
Representation of an integrated planetary flow system based on the Dymaxion map, emphasizing some coupled cycles related to food production and socio-environmental impacts among (1) Brazil, (2) China, (3) the Caribbean, and (4) the Sahara Desert.
Adapted from [1]
According to the Director-General of the Food and Agriculture Organization (FAO) of the United Nations [3], after years of progress, world hunger has increased since 2015. Around 60% of the world’s starving people are from countries affected by conflict and climate change, including northeast Nigeria, Somalia, South Sudan, and Yemen with 20 million people, often suffering extreme climatic events such as droughts and floods. Not surprisingly, some of the FAO’s top priorities for the next 2 years include topics such as sustainable agriculture, climate change mitigation and adaptation, water scarcity and support of subsistence rural practices, and fisheries and forestry [3, 4]. The challenges related to this demanding context can be intensified and better understood when taking into account that the world population is expected to increase by about 30% over the next 35 years, reaching more than 9.5 billion people in 2050 and 11.2 billion in 2100 [5].
As pointed out by Xia et al. [6], the global food waste of approximately 1.3 billion tons per year is shocking in this context and, although it should be avoided or minimized, it cannot be completely prevented nowadays. Primary and secondary processing generates unpreventable food supply chain waste. This can be due to a number of factors along the supply chain, differing by the commodity and country in question. In general terms, developing countries such as some African countries suffer the greatest loss during the early, upstream part of the primary processing, corresponding to 75% of food losses during production and postharvest. Various initiatives, e.g., building better infrastructure through knowledge transfer (more efficient storage and transport technologies) and improving collaboration and market opportunities in the food supply chain could have a positive role. In industrialized countries, waste occurs especially in the consumption stage, accounting for 50% of overall loss of crops in some countries of North America, Europe, and Oceania. In this case, together with educational and cultural actions, other aspects such as developing legislation to make date labels more user-friendly for consumers (sell-by, best-before, and consume-by), redesigning packaging characteristics (avoiding the “buy 1 get 2” offers) and retailer marketing strategies should be considered [7].
It is estimated that around 140 billion tons of biomass from the agricultural sector are generated every year in the world [8, 9], and a considerable part is recognized as waste and not conflicting with food availability, e.g., leaves, roots, stalks, bark, bagasse, straw residues, seeds, wood and animal residues. Using alternative strategies to avoid additional losses and produce several high value-added chemicals could minimize the volume of non-renewable materials used today (i.e., roughly 50 billion tons of fossil fuels), enough to greatly reduce greenhouse gas emissions and dependence on non-sustainable resources. Therefore, considering their available volume and practically low costs locally and globally, associated to rich function, structure and chemical heterogeneity, all agro-industrial waste should also be considered for their chemical and material potential, as well as a source of energy [10–13].
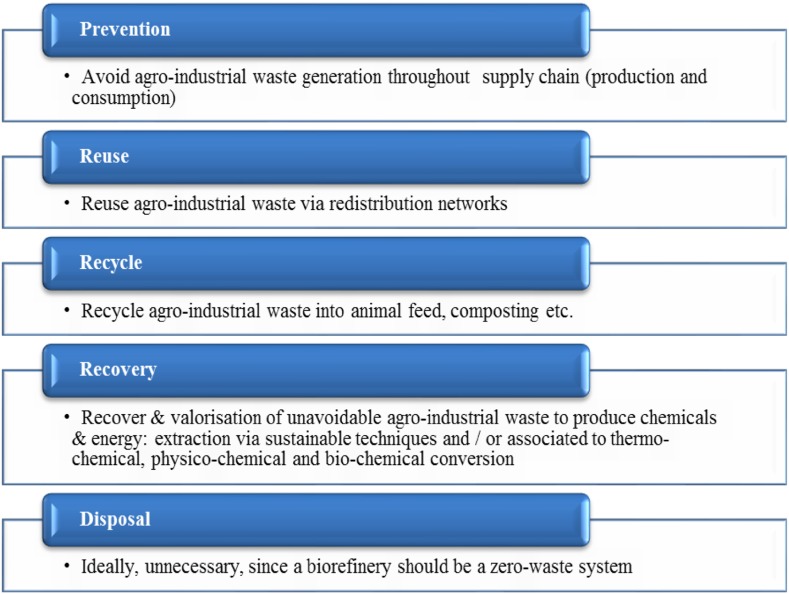
An important proposal related to waste hierarchy as a framework for residue management can be seen in Fig. 2 [14, 15], which was reformulated to include agro-industrial waste. In this case, the agro-industrial waste hierarchy has a different meaning from top to bottom, since all biomass is valued as raw material. ‘Prevention’ is an intrinsic part of optimized processes, avoiding overproduction. Therefore, the least probable option is ‘disposal’ as the supply chain is designed to attend sustainable consumption, using all bio-based material generated. Here, sustainable production also includes eco-efficiency, cleaner and green productivity, whereas sustainable consumption allows greener choices to be made by individuals based on eco-procurement, supply chain management, waste minimization, recycling, and resource efficiency measures. Both sustainable production and consumption comprises ‘life-cycle thinking’, aiming at preventing problems shifting from one life-cycle stage to another, one geographical area or environmental compartment to another.
Fig. 2.
The agro-industrial waste hierarchy modified from [15]. The main idea is to promote sustainable production and consumption systems through zero-waste biorefinery
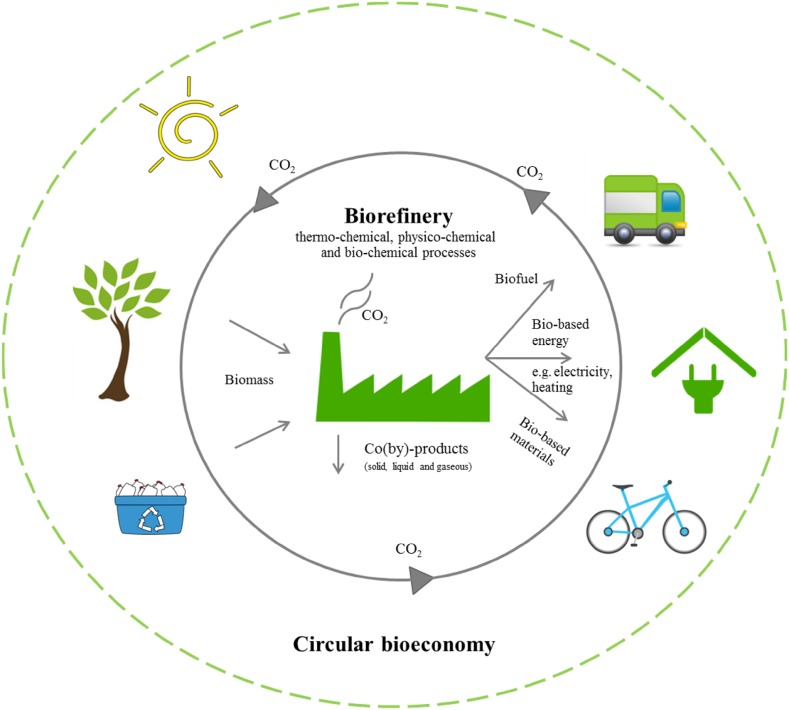
One of the most important and cited references highlighting the advances in genetics, biotechnology, process chemistry, and engineering that has helped establish a new manufacturing concept to convert renewable biomass into valuable fuels and products, known as biorefinery, was published by Ragauskas and collaborators in the mid-2000s [16]. According to these authors and other researchers [16, 17], integrating biomass and biorefinery technologies has the potential to develop sustainable bio-based energy and materials leading to a new manufacturing paradigm (Fig. 3).
Fig. 3.
Holistic biorefinery model integrating biomass, biofuel, biomaterials and bioenergy cycle, based on green and sustainable technologies in the scope of bioeconomy and circular economy. Updated and expanded from [16, 17]
In fact, this paradigm is currently connected to other strong concepts, i.e., bioeconomy and circular economy; the latter is described as an industrial system that is restorative by intention and design. This idea replaces the end-of-life notion with regeneration, focusing on the use of renewable energy, elimination of toxic chemicals, reutilization, return and eradication of “waste through the superior design of materials, products, systems, and business models” [18, 19].
As can be noted, new generations of biorefinery combine innovative biomass resources from different origins, chemical extraction and purification and/or synthesis of biomaterials, biofuels and bioenergy via benign processes. From the very beginning, the identification and quantification of all potentially high value-added compounds that could be removed from the available renewable feedstocks requires another analytical approach, also connected to green chemistry [20, 21].
From Green to Sustainable Separation: Towards Holistic, Flexible, and Zero-Waste Biorefineries
More recently, green extraction and purification have been presented as methods based on establishing processes that reduce energy consumption, using solvents and renewable materials, as well as ensuring a safe and high-quality fraction/product [22]. The aim of their application is to obtain natural products from industrial waste, which is considered a highly attractive initiative [23].
However, a more adequate term for such extraction and purification processes towards vanguard biorefineries could be sustainable separation, adding to the previous green definition, the notion of innovation across all sectors that allows for increased value in a wide sense, enhancing human and environment benefits and providing economically accessible technologies also advantageous to industry and large scale processing systems. It includes another dimension related to the generation of more creative and healthy jobs, contributing to the construction of a positive long-term sustainability agenda, encompassing bio-circular economy, environmental and social justice [24–27].
Sustainable separation can be defined as a holistic approach grounded on the circular and flexible design and application of renewable benign materials and auxiliaries (including bio-derived solvents, solid phases, membranes) and processes [rooted on green analytical techniques and sustainability metrics and indices, e.g., life cycle analysis (LCA), chemometrics, and other interdisciplinary indicators]. The aim is to optimize the tuneable use of energy, time, reagents, devices, scale, yield and number of steps to extract, fractionate, purify or even modify the components of interest from bio-derived waste during these in situ processes, ensuring analytical reproducibility, efficiency, selectivity robustness and scalability, with online evaluation regarding measurable objectives to create safer, healthier, and more efficient products, processes, and services under fair conditions, commercially available at accessible and just prices [28–30].
Natural products are among the most attractive value-added chemicals to be considered, which can be classified as organic compounds formed by living systems divided into three main categories: (1) compounds that occur in all cells and have a central role in their metabolism and reproduction (nucleic acids, amino acids, and sugars), also known as primary metabolites; (2) high-molecular polymeric materials which form cellular structures (cellulose, lignins, and proteins) and; (3) chemicals which are characteristic of a limited number of species, called secondary metabolites [22, 30]. Many of these bioactive compounds (e.g., alkaloids, terpenoids, and phenols) have been extensively used as medicine, nutraceuticals, flavors, fragrances, cosmetics, food additives, antimicrobials, bio-pesticides, etc. However, among the biggest challenges for biomass utilization is establishing benign methods to separate, purify and modify it into chemicals, fuels, and new materials. This is partially due to, with rare exceptions, the small amounts which are lower than 0.01% of the dry weight of vegetal, associated to possible product inhibition issues, large raw material variability, feed detoxification (when necessary), instability of the target compound (or fractions) and its presence in a complex mixture [23, 30].
It is well known that the separation steps, especially extraction, correspond up to 40–80% of the total costs of most common chemical processes currently used. From the point of view of a holistic biorefinery, separation has attracted more and more attention [31]. For instance, for natural products, solvent-based extraction is one of the best options nowadays considering the nature of many bio-based chemicals and matrices, and also the fact that other separation methods, such as those based on chromatography or membranes, do not have the same advantages taking into account commercial scales [32].
It is expected that high value-added components from biomass waste such as essential oils, polyphenols, and other food or medicinal-related products are extracted first, followed by polysaccharides, lignocelluloses or waxes via advanced separation and depolymerization processes. Among them, green solvents in general, supercritical CO2, subcritical water, microwave (MW)-assisted acidolysis and gas-expanded liquids have been mentioned [33]. Green solvents offer important separation advantages, including near-supercritical or supercritical fluids, which have outstanding mass transport properties, polarity, and easiness of solvent removal after extracting the compound of interest [34]. Another interesting solvent is water, but the range of compounds that are soluble in this medium is quite limited. Nevertheless, the use of subcritical water has been demonstrated to be advantageous for organic modification to depolymerize, hydrolyze, gasify, and carbonize biomass to produce bioactive compounds, sugars, biogas, and other valuable solids [16, 35].
Integrating two or more green techniques combining different strategies has played an important role in overcoming the main drawbacks of a single technique towards sustainable separation. For instance, for high-pressure solvent extraction in which the extractants do not reach supercritical conditions, the temperature, time, and solvent consumed can be dramatically reduced associating ultrasound-assisted treatment [28, 36]. In fact, more attention has been paid to green extraction, purification, or modification of natural products derived from agro-industrial waste nowadays, opening up new opportunities for sustainable approaches designed for bioeconomy and circular economy models. The aim of this paper is to present an overview of the design and application of green and sustainable separation of natural products for vanguard zero-waste biorefineries. The main analytical techniques and procedures described over the last 10 years will be described in detail, showing the potentialities, challenges, and perspectives in this topical and emergent scenario.
High Value-Added Approaches for Green and Sustainable Separation of Natural Products from Waste: What can be Observed from the Literature?
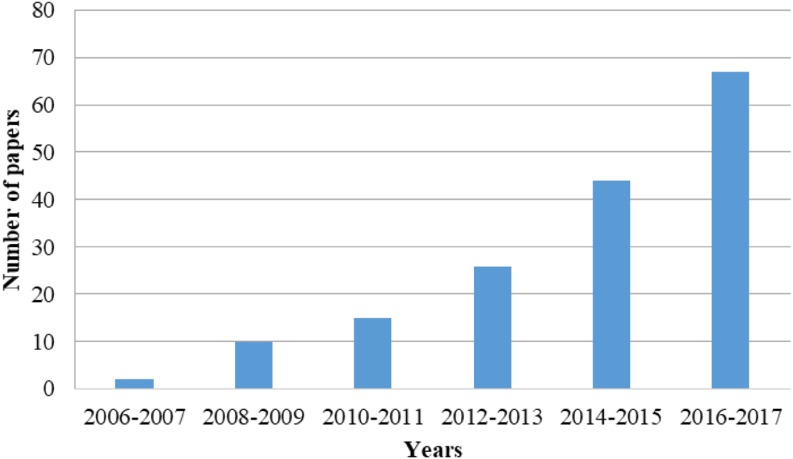
More recently, trends in green and sustainable extraction, fractionation and purification techniques have largely focused on minimizing the use of solvents, energy and materials that are intrinsically benign to human health and the environment [37]. In order to analyze the status quo and perspectives related to natural product separation from waste, a systematic literature review was conducted using the ISIS Web of Knowledge platform (reviews and papers) from 2006 to 2017, combining the descriptors “natural product” and “green extraction/separation” (or “sustainable extraction/separation” or “eco-friendly extraction/separation”) and “waste” (or “residue”). Figure 4 shows the number of publications during this period. There were more than 160 research papers and reviews that, to the best of our knowledge, are reasonably representative to show the strongest tendencies in this field over the last decade. It can be clearly observed that there has been an increase in the number of manuscripts over the last 10 years, covering the principles, advances, and applications of these green methods.
Fig. 4.
Number of publications per year focusing on green and sustainable separation (extraction, fractionation and purification) of natural products from waste (ISIS Web of Knowledge, January 2006 to December 2017)
The obtained data reflect the growing interest and potential of green and sustainable methods to separate natural products from waste. One tendency observed in particular was the innovative ways to remove (integrating extraction, purification and/or modification in the same integrated system) and use such compounds in more contemporary sectors, promoting human and environmental health instead of general and old-fashioned remediation [19, 38]. As a result, new applications for food, nutraceutical, and agricultural sectors have been further explored, based on their advantageous properties as natural colorants, flavors, aromas, antioxidants, antifungals, bioformulations (bio-pesticides) or simply their use as precursors to generate other compounds for similar uses. Some details related to patents, (non-) clinical trials, sustainable indicators, scaling-up, regulatory, agro-industrial variability and availability, traceability, seasonality, good laboratory and manufacturing practices, additional economical and marketing issues have also been discussed.
Table 1 presents the research papers and reviews published during this period, highlighting their main focus, the green or sustainable techniques/approaches adopted, raw materials (mostly agro-industrial waste) and target compounds studied. The most common raw materials described as chemical feedstocks were waste derived from plants, for instance, food, mainly fruits (citrus, mango, papaya, grape, passiflora, banana, tomato, olive), grains (corn, soybean, sunflower, coffee) and other abundant materials (sugarcane bagasse, tea, wood bark, rice and wheat straw). Additional issues that affect the quality of the final products were also discussed, namely the procedure used for waste collection, selection, storage, drying, matrix characteristics (particle size, shape, specific surface area and porosity). The latter aspects play an important role in extraction efficiency due to the mass and heat transfer processes. Understanding the nature of raw material is crucial to avoid negative influences impacting the quality and yield during the removal of the target compounds, e.g., caused by co-extracted contaminants or due to the presence of some components in these matrices, such as water or high molecular weight compounds [39].
Table 1.
Research papers and reviews focusing on green and sustainable separation of natural products from agro-industrial waste published from January 2006 to December 2017 (ISIS Web of Knowledge)
| Year | Crop | Waste stream | Target compounds | Geographical location | Green or sustainable separation approach | References |
|---|---|---|---|---|---|---|
| 2017 | Olives | Olive kernels | Phenolic compounds and oil | France and Spain | Aqueous liquid solid extraction (LSE), mechanical expression (ME), supercritical CO2 (SC-CO2) and gas-assisted mechanical expression (GAME) | Gas-assisted mechanical expression (GAME) for the selective recovery of lipophilic and hydrophilic compounds from olive kernel [145] |
| 2017 | Figs | Leaves | Bioactive compounds | China | Deep eutectic solvent with microwave and ultrasound extraction Time: 10 min (MW) and 60 min (US) Temperature: 40–80 °C Power: 250 W (MW) and 700 W (US) |
Enhanced and green extraction polyphenols and furanocoumarins from Fig (Ficus carica L.) leaves using deep eutectic solvents [136] |
| 2017 | Polygonum multiflorum | Herbal raw materials | Stilbene glycoside and anthraquinones | China | Ionic liquids with ultrasonic extractor Time: 1–120 min Power: 40–120 W |
Sequential extraction and separation using ionic liquids for stilbene glycoside and anthraquinones in Polygonum multiflorum [131] |
| 2017 | Several sources | Not defined | Mostly bioactive compounds | Spain | Review Critical overview about the greenness of water as extraction solvent |
Water as green extraction solvent: Principles and reasons for its use [146] |
| 2017 | Pomelo | Flavedo | Essential oil | China | Microwave irradiation Power: 240–700 W Time: 24 min |
A process to preserve valuable compounds and acquire essential oils from pomelo flavedo using a microwave irradiation treatment [52] |
| 2017 | Selaginella doederleinii | Not defined | Biflavonoids | China | Ionic liquids and microwave-assisted extraction Power: 300–700 W Time: 30–50 min Temperature: 40–60 °C |
Optimization of ionic liquid-assisted extraction of biflavonoids from Selaginella doederleinii and evaluation of its antioxidant and antitumor activity [132] |
| 2017 | Pogostemon cablin | Leaves | Essential oils | Indonesia | Microwave-assisted hydrodistillation (MAHD) and solvent-free microwave extraction (SFME) Power: 600 W (MAHD) and 264 W (SFME) Time: 66 min (MAHD) and 45 min (SFME); solvent: water |
Comparison of conventional and microwave-assisted distillation of essential oil from Pogostemon cablin leaves: analysis and modeling of heat and mass transfer [147] |
| 2017 | Juglans regia L. | Fresh male flowers and unripe walnut seeds | Phenolic content and water-soluble polyphenols | Italy | Microwave-assisted extraction Frequency: 2.45 GHz Max. power: 500 W Solvent: ethanol/water Temperature: 60–100 °C Time: 6–30 min |
Process intensification by experimental design application to microwave-assisted extraction of phenolic compounds from Juglans regia L. [148] |
| 2017 | Walnuts | Walnut de-pellicle | Flavonoids | China | Macroporous resins Pretreated with 5% HCl and 5% NaOH solutions |
Recovery of flavonoids from walnuts de-pellicle wastewater with macroporous resins and evaluation of antioxidant activities in vitro [149] |
| 2017 | Ginseng | Roots | Bioactive compounds | Brazil | Sequential extraction system using ethanol followed by water Temperature: 333 K Time: 5–240 min |
Techno-economic evaluation of obtaining Brazilian ginseng extracts in potential production scenarios [150] |
| 2017 | Food ingredients and natural products | Not defined | Nutraceutics, cosmetic, pharmaceutical, and bioenergy applications | France | Review current knowledge on ultrasound-assisted extraction |
Ultrasound-assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review [151] |
| 2017 | Coffee | Coffee chaff | Antioxidants | Portugal | Solid–liquid extraction and multi-frequency multimode modulated (MMM) Frequency: 19.8 kHz Power: 250 and 500 W Time: 60–600 s |
Multi-frequency multimode modulated technology as a clean, fast, and sustainable process to recover antioxidants from a coffee by-product [152] |
| 2017 | Apples | Wild apple fruit dust | Bioactive compounds, polyphenolic antioxidants | Serbia | Microwave-assisted extraction Time: 15–35 min Ethanol conc.: 40–80% Irradiation power: 400–800 W |
Microwave-assisted extraction of wild apple fruit dust production of polyphenol-rich extracts from filter tea factory by-products [153] |
| 2017 | Wood | Wood biomass | Lignin oligomers | China | Microwave-assisted treatment with deep eutectic solvent Solvent: choline chloride and oxalic acid dehydrate Temperature: 80 °C Power: 800 W Time: 3 min |
Efficient cleavage of lignin-carbohydrate complexes and ultrafast extraction of lignin oligomers from wood biomass by microwave-assisted treatment with deep eutectic solvent [137] |
| 2017 | Wood | Oak wood from cooperage by-products | Furanic compounds, cis- and trans- B-methyl-y-octalactones, terpenes and norisoprenoids, benzenic compounds | Spain | Pressurized liquid extraction Solvent: water, ethanol/water (80:20) and ethyl lactate Temperature: 60–120 °C Pressure: 10.34 MPa Flush volume: 60% Purging time: 80 s |
Extraction of natural flavorings with antioxidant capacity from cooperage by-products by green extraction procedure with subcritical fluids [154] |
| 2017 | P. armeniaca, P. persica, P. domestica, Triticum aesativum | Fruit and vegetables seeds and peels | Phenolic compounds | Pakistan | Ultrasonic water bath Solvent: 65% (v/v) ethanol (methanol and acetone) Extraction time: 30 min Temperature: 50 °C |
Extraction and quantification of phenolic compounds from Prunus armeniaca seed and their role in biotransformation of xenobiotic compounds [71] |
| 2017 | Lignocellulose materials | Lignocellulosic biomass such as crops or forestry residues | High value-added bio-based products (e.g., bioethanol, biogas, acetic acid, acetic acid, or activated carbon) | Mexico and Pakistan | Review Focus on transformation based on syngas platform (thermochemical platform) and sugar platform (biochemical platform) |
Lignocellulose: a sustainable material to produce value-added products with zero-waste approach [155] |
| 2017 | Olives | Olive by-product (paté) | Fatty acids and phenolic compounds | Spain and Italy | Soxhlet extraction (percolation with petroleum ether, under reflux) | Macro and micro functional components of a spreadable olive by-product (pate) generated by new concept of two-phase decanter [156] |
| 2017 | Tucumã palm fruit | Tucumã’s endocarp | Cellulose | Brazil and USA | Alkaline extraction (135 °C, autoclave, 2 bar, 2 min, 20% of aqueous NaOH, 1:30 straw to liquor (g/ml), 30 min) | New approach for extraction of cellulose from tucuma’s endocarp and its structural characterization [115] |
| 2017 | Grapes | Seeds | Resveratrol | China | Subcritical water extraction Pressure: 0.5–1.5 MPa Time: 20–30 min Temperature: 130–170 °C |
Optimization of subcritical water extraction of resveratrol from grape seeds by response surface methodology [100] |
| 2017 | Mango, rambutan, santol | Peels | Antioxidant activity | Thailand | Solid–liquid extraction Ethanol (95%) |
Study effect of natural extracts on the antioxidant activity in pork balls [157] |
| 2017 | Tomatoes | Pericarps without seeds | Nutrient-rich antioxidant ingredients | Portugal, Spain, Ireland | Microwave extraction (600 rpm, 200 W) Time: 0–20 min Temperature: 60–180 °C Ethanol conc.: 0–100% Solid/liquid ratio: 5–45 g/l |
Valorization of tomato wastes for development of nutrient-rich antioxidant ingredients: a sustainable approach towards the needs of today’s society [158] |
| 2017 | Citrus latifolia, Rubus sp., Origanum vulgare and Heterotheca inuloides | Peel and broken down vegetable material | Fatty acids and antioxidants compounds | Mexico, Belgium | SC-CO2 Extraction time: 1 h Flow: 25 g/min Pressure: 10–40 MPa Temperature: 35–60 °C Co-sol.: 0–8 g/min Percent flow: 0–32% |
Thermodynamics and statistical correlation between supercritical CO2 fluid extraction and bioactivity profile of locally available Mexican plant extracts [159] |
| 2017 | Pomegranates | Peels | Carotenoids | Greece | Ultrasound-assisted extraction (139 W, 20 kHz); solvents: vegetable oils Extraction time: 10–60 min Temperature: 20–60 °C |
Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils [72] |
| 2017 | Pomegranates | Both edible and non-edible parts | Polyphenols | Greece | Semi-automatic extractor Solvents: H2O, β-CD, HP-β-CD Extraction time: 363 min Temperature: 25 °C |
Green extraction of polyphenols from whole pomegranate fruit using cyclodextrins [121] |
| 2016 | Quince | Leaves | Natural dyes and bioactive compounds | Romania | Aqueous extraction Extraction time: 60–240 min Temperature: 4–100 °C |
Dyeing and antibacterial properties of aqueous extracts from quince (Cydonia oblonga) leaves [160] |
| 2016 | Corn | Steep liquor | Vanillic acid, p-coumaric acid, ferulic acid, sinapic acid and quercetin | Spain, Portugal, and Italy | Liquid–liquid extraction Solvents: chloroform (56 °C, 60 min) Ethyl acetate (25 °C, 45 min) |
A multifunctional extract from corn steep liquor: antioxidant and surfactant activities [161] |
| 2016 | Palm | Oil palm empty fruit bunches | Cellulose with polypropylene as biocomposite material | Malaysia, Pakistan | Ultrasonic treatment (40 kHz) solvent: hydrogen peroxide Extraction time: 1–3 h Room temperature |
Autoclave and ultra-sonication treatments of oil palm empty fruit bunch fibers for cellulose extraction and its polypropylene composite properties [73] |
| 2016 | Tomatoes | Seeds and peels | Carotenoids/proteins | Tunisia and Germany | Supercritical CO2 extraction 80 °C, 400 bar, 4 g CO2/min for 2 h |
Biorefinery cascade processing for creating added value on tomato industrial by-products from Tunisia [82] |
| 2016 | Black tea | Black tea processing waste | Antioxidant and antimicrobial phenolic compounds | Turkey and USA | Solvent extraction Solvents: H2O, ethanol Extraction time: 2 h Temperature: 70 °C |
Black tea processing waste as a source of antioxidant and antimicrobial phenolic compounds [46] |
| 2016 | Rapeseed | Rapeseed oil cakes | Protein- and lignin-rich fractions | France | Ultrafine miffing and electrostatic separation Solvents: NaOH, diethylether, hexane Extraction time: 5 h Temperature: 60 °C |
Chemical- and solvent-free mechanophysical fractionation of biomass induced by tribo-electrostatic charging: separation of proteins and lignin [139] |
| 2016 | Sunflower | Seeds | Sunflower protein-based ingredients | USA | Review Green pigmentation associated with the interaction of sunflower protein and oxidized chlorogenic acid (CGA) by outlining the sunflower oil and protein meal market, CGA reactions contributing to greening, methods for CGA extraction, and the effect of processing on sunflower protein quality and the greening reaction |
Chlorogenic acid oxidation and its reaction with sunflower proteins to form green-colored complexes [162] |
| 2016 | Passion fruit | Peels | Pectin | Malaysia | Acidic and enzymatic extraction Citric solution, celluclast Extraction time: 30–120 min Temperature: 35–85 °C |
Comparison of acidic and enzymatic pectin extraction from passion fruit peels and its gel properties [107] |
| 2016 | Red grape | Pomace | Polyphenols and anthocyanin pigments | Greece | Ultrasound-assisted extraction (140 W, 37 kHz) Solvent: aqueous glycerol Extraction time: 60 min Temperature: 45 °C |
Development of a green process for the preparation of antioxidant and pigment-enriched extracts from winery solid wastes using response surface methodology and kinetics [74] |
| 2016 | Orange and lemon | Fresh and waste peel | Pectin and d-limonene | Portugal and Italy | Microwave Solvent: water Extraction time: 1 h Temperature: 80 °C |
Eco-friendly extraction of pectin and essential oils from orange and lemon peels [53] |
| 2016 | Coffee | Spent coffee grounds | Oil | China | Ultrasonication extraction Solvent: hexane Extraction time: 15–75 min |
Effect of oil extraction on properties of spent coffee grounds-plastic composites [98] |
| 2016 | Tomato | Waste of tomato paste plants | Lycopene | Iran and Canada | Microemulsion technique (MET) Solvents: water, saponin: glycerol, surfactant: lycopene Extraction time: 30 min Temperature: 25 °C |
Enhanced lycopene extraction from tomato industrial waste using microemulsion technique: optimization of enzymatic and ultrasound pre-treatments [163] |
| 2016 | Red capsicum (Capsicum annuum) | Processing residue | Carotenoids | India | Enzymatic liquefaction Pectinase, viscozyme L, cellulose extraction Time: 1 h Temperature: 60 °C |
Enzyme-assisted extraction of carotenoid-rich extract from red capsicum (Capsicum annuum) [108] |
| 2016 | Rice | Husk | Cellulose | India | Eco-friendly method montmorillonite, LiOH, H2O2 Extraction time: 6 h Temperature: 80 °C |
Extraction of cellulose from agricultural waste using montmorillonite K-10/LiOH and its conversion to renewable energy: biofuel by using Myrothecium gramineum [122] |
| 2016 | Tea (yarrow and rose hip) | By-products from filter-tea factory | Chlorophylls and carotenoids | Serbia | Supercritical fluid extraction Extraction time: 5 h Temperature: 40 and 60 °C Pressure: 100–300 bar CO2 flow rate: 0.194 hk/h |
Extraction of minor compounds (chlorophylls and carotenoids) from yarrow-rose hip mixtures by traditional versus green technique [83] |
| 2016 | Corn, sugarcane, sorghum, pearl millet, green gram, groundnut sesame | Bagasse, stover, stalk and shell | Para-coumaric acid (pCA) | India and USA | Alkaline hydrolysis pH 3, alkali conc.: 0.5–4 M Hydrolysis duration: 4–24 h Sugaring-out for separation of pCA from hydrolysate |
Extraction of p-coumaric acid from agricultural residues and separation using ‘sugaring out’ [116] |
| 2016 | Winery | Grape wastes and by-products | Antioxidant compounds and polyphenols | Denmark, China, France and Brazil | Review Conventional (solid liquid extraction, heating, grinding, etc.) and non-conventional (pulsed electric fields, high voltage electrical discharges, pulsed ohmic heating, ultrasounds, microwave-assisted extractions, sub- and supercritical fluid extractions, as well as pressurized liquid extraction) methods |
Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: a review [164] |
| 2016 | 1st to 3rd generation biodiesel feedstocks | Mostly microalgae | Biodiesel | Malaysia and Japan | Review Integration of enzymatic reactors with supercritical fluid technology |
Green biodiesel production: a review on feedstock, catalyst, monolithic reactor, and supercritical fluid technology [84] |
| 2016 | Jatropha curcas, oil palm | Seeds, empty fruit bunch | Bio-oil | Malaysia | Microwave extraction Solvent: water Extraction time: 60–140 min Power: 200–700 W |
Green bio-oil extraction for oil crops [54] |
| 2016 | Green tea | Green tea residue | Protein | The Netherlands | Alkaline protein extraction Solvent: NaOH Extraction time: 2 h Temperature: 95 °C |
Improving yield and composition of protein concentrates from green tea residue in an agri-food supply chain: effect of pre-treatment [117] |
| 2016 | Eucalyptus wood | Eucalyptus chips | Hemicelluloses | Uruguay | Green liquor extraction Solvents: water and green liquor (Na2CO3, Na2S, and NaOH) extraction time: 30–150 min temperature: 100–160 °C |
Integrated forest biorefineries: green liquor extraction in eucalyptus wood prior to kraft pulping [123] |
| 2016 | Watermelons | Juice | Lycopene | Brazil | Microfiltration, diafiltration, reverse osmosis α-Al2O3 membranes T1-70 (35 °C) Polyamide composite membranes (35 °C, 60 bar) |
Integrated membrane separation processes aiming to concentrate and purify lycopene from watermelon juice [140] |
| 2016 | Larch wood | Sapwood, heartwood, bark and branches | Phenolic compounds | Slovenia | Pressurized hot water Extraction time: 30 min Temperature: 100 °C |
Isolation of phenolic compounds from larch wood waste using pressurized hot water: extraction, analysis and economic evaluation [165] |
| 2016 | Tomatoes | Pomace | Lycopene | Iran | Microemulsion technique H2O and surfactants Extraction time: 30 min Temperature: 35 °C |
Microemulsion-based lycopene extraction: effect of surfactants, co-surfactants, and pretreatments [166] |
| 2016 | Melons | Rind | Carbohydrates, phenolic compounds, and fatty acids | Spain | Solvent extraction Solvent: cyclohexane, ethanol Extraction time: 2 h Microwave radiation: 190 °C, 20 min, 200 W |
Microwave heating for the catalytic conversion of melon rind waste into biofuel precursors [167] |
| 2016 | Tomatoes, fungus Blakeslea trispora |
Processing waste | Lycopene | Greece | Review Emphasis on final product safety and ecofriendly processing (solvent extraction, SFE, MAE, high-pressure processing, ultrasound, electrical methods) |
Natural origin lycopene and its “green” downstream processing [168] |
| 2016 | Oranges | Peel | Pectin | Italy | Conventional hydrodistillation, MAE, US Solvents: water Extraction time: 5–155 min Temperature: 90–333 °C |
Novel configurations for a citrus waste based biorefinery: from solventless to simultaneous ultrasound and microwave-assisted extraction [55] |
| 2016 | Lemons, olives, onion, red grape, coffee, and wheat | Peel, leaves, solid wastes, pomace, spent filter and bran | Polyphenolic compounds | Greece | Ultrasound extraction (140 W, 37 kHz) eutectic mixtures Extraction time: 90 min Temperature: 80 °C |
Novel glycerol-based natural eutectic mixtures and their efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from agri-food waste biomass [75] |
| 2016 | Potatoes | Peels | Polyphenolic antioxidants | Greece | Ultrasound extraction (140 W, 37 kHz) Solvents: ethanol and glycerol Extraction time: 90 min Extraction temperature: 50–80 °C |
Optimization of a green ultrasound-assisted extraction process for potato peel (Solanum tuberosum) polyphenols using bio-solvents and response surface methodology [76] |
| 2016 | Grapes | Seeds | Grape seed oil | Croatia | Supercritical CO2 Extraction time: 90 min Temperature: 35–64 °C Pressure: 158–441 bar CO2 flow rate: 1.94 kg/h |
Optimization of supercritical CO2 extraction of grape seed oil using response surface methodology [85] |
| 2016 | Crocus sativus | Petals (underutilized bulk agro-waste) | Phenolic compounds | Iran | Subcritical water extraction Extraction time: 20–60 min Temperature: 120–160 °C |
Optimization of the subcritical water extraction of phenolic antioxidants from Crocus sativus petals of saffron industry residues: Box–Behnken design and principal component analysis [101] |
| 2016 | Bananas | Peels | Antioxidants | Malaysia and Turkey | Solvent extraction Solvents: acetone, ethanol, hexane, methanol, H2O Extraction time: 1–5 h |
Optimization of extraction parameters on the antioxidant properties of banana waste [47] |
| 2016 | Pea vine | Pea vine waste | Potential platform molecules (5-hydroxy furfural; ethanoic acid); sugars (levoglucosenone, rhamnose, xylose, fructose); biopolymer with pectinaceous and starch-like characteristics | United Kingdom | Pseudo-subcritical water extraction Temperature: 125–175 °C Pressure: 20–60 bar Flow rate: 1–5 ml/min |
Potential utilization of unavoidable food supply chain wastes-valorization of pea vine wastes [6] |
| 2016 | Keratin-containing products stored in large waste deposits | Processing waste | Keratin | Romania | Review Keratins solubilization (protected and unprotected methods) followed by dehydro-thermal, physical-type bonding or chemical treatments |
Practical ways of extracting keratin from keratinous wastes and by-products: a review [169] |
| 2016 | Taxus baccata L. | Case study based on European yew | 10-deacetylbaccatin III (10-DAB) | Germany | Review Theoretical approach in thermodynamics and process modelling as an alternative process design |
Process design for integration of extraction, purification and formulation with alternative solvent concepts [170] |
| 2016 | Olives | Olive mill waste water | Biophenols (hydroxytyrosol and tyrosol) | Italy | Liquid–liquid extraction Solvents: n-hexane, EtOAc |
Quick assessment of the economic value of olive mill waste water [171] |
| 2016 | Olives | Olive mill waste water | Tyrosol | Spain, United Kingdom and Spain | Hydrophobic ionic liquids Solvents: ILs Extraction time: 2 h Temperature: 303–323 K |
Recovery of tyrosol from aqueous streams using hydrophobic ionic liquids: a first step towards developing sustainable processes for olive mill wastewater (OMW) management [133] |
| 2016 | Cupuassu | Seeds | Cupuassu butter (phenolic content/tocopherols/fatty acids) | Brazil | Supercritical CO2 extraction Temperature: 50 and 70 °C Pressures: 20–40 MPa |
Supercritical CO2 extraction of cupuassu butter from defatted seed residue: experimental data, mathematical modeling and cost of manufacturing [86] |
| 2016 | Coffee | Spent coffee grounds | Oil fraction | Portugal, Brazil, Portugal | Supercritical CO2 Extraction time: 1 h Temperature: 55 °C Pressure: 250 bar Flow rate: 15 kg/h |
The green generation of sunscreens: using coffee industrial sub-products [87] |
| 2016 | Ginger | Not defined | Essential oil, phenolics, fibers and phenolic acids | France | Microwave hydrodiffusion and gravity processing (MHG) and UAE Solvents: water Extraction time: 83 and 90 min Temperature: up to 100 and 50 °C |
Towards a “dry” bio-refinery without solvents or added water using microwaves and ultrasound for total valorization of fruit and vegetable by-products [56] |
| 2016 | Passion fruit | Passion fruit seeds and passion fruit seed cake (the residue from the seed oil production by cold pressing) | Oil and extract with promising antioxidant and antimicrobial activities | Brazil and USA | SFE, LPE, MAC, UE Solvents: sCO2, hexane, ethyl acetate, ethanol, H2O Extraction time: 45 min–7 days temperature: room temp.− 50 °C |
Valorization of passion fruit (Passiflora edulis sp.) by-products: sustainable recovery and biological activities [88] |
| 2016 | Wood | Broken pallets, crates, and waste timber from building and demolition works | Renewable energy source | Romania | Review Overview of the technical and economic opportunity of using wood waste as a renewable energy source |
Wood waste as a renewable source of energy [172] |
| 2015 | Plants of spontaneous flora, cultivated plant, and wastes resulted in agricultural and food industry | General bio-derived materials | Polyphenols | Romania | Review Microwave-assisted extraction (MAE), supercritical fluid extraction (SFE), and ultrasound-assisted extraction (UAE) |
A comparative analysis of the ‘green’ techniques applied for polyphenols extraction from bioresources [173] |
| 2015 | Onion | Onion solid wastes | Polyphenol- and pigment-enriched extracts with antioxidant activity | Greece | Ultrasound extraction (140 W, 37 kHz) Extraction time: 60 min Temperature: 45 °C |
A green ultrasound-assisted extraction process for the recovery of antioxidant polyphenols and pigments from onion solid wastes using Box–Behnken experimental design and kinetics [174] |
| 2015 | Six types of plant fibers (bast, leaf, seed, straw, grass, and wood) and animal fibers and regenerated cellulose fibers | Seed (coir) and animals (chicken feather) as they are secondary or made from waste products | Fibers | Sweden | Review Dew, stand, cold and warm water, steam, enzyme, mechanical, ultrasound chemical and Surfactant retting |
A review of natural fibers used in biocomposites: plant, animal and regenerated cellulose fibers [175] |
| 2015 | Non edible vegetables | Seeds | Biodiesel | Egypt | Review | A review on green trend for oil extraction using subcritical water technology and biodiesel production [102] |
| 2015 | Neem | Neem seed cake (NSC) | Neem Protein (NP) | USA | Alkaline extraction Solvents: H2O and NaOH Extraction time: 60 min Temperature: 75 °C |
Bio-based polymeric resin from agricultural waste, neem (Azadirachta indica) seed cake, for green composites [118] |
| 2015 | Oranges | Peel | Essential oil, polyphenols and pectin | Algeria and France | MHG, UAE, MAE Solvents: “in situ” water Extraction time: 25 and 3 min Temperature: 59 °C |
Bio-refinery of orange peels waste: a new concept based on integrated green and solvent free extraction processes using ultrasound and microwave techniques to obtain essential oil, polyphenols and pectin [57] |
| 2015 | Corn, sugarcane, sorghum, soybean, rice, barley, potato, other lignocellulose, vegetable oils, oilseed | By-products (bagasse, straw, cobs, stalks, stover, grass etc.) | Biofuel, 1,3-propanediol, succinic acid, adhesives, solvents, surfactants, ethyl lactate, erucic acid, amylose ethers, among others | Denmark | Review Focus on integrating sustainability assessment procedures and tools (LCA and evaluation approaches) |
Biorefining in the prevailing energy and materials crisis: a review of sustainable pathways for biorefinery value chains and sustainability assessment methodologies [144] |
| 2015 | Agro-industrial products | Agro-industrial co-products | Phenolic compounds | Brazil | Solid-state fermentation, even as friendly enzyme-assisted extractions | Biotransformation and bioconversion of phenolic compounds obtainment: an overview [176] |
| 2015 | Cashew-nut | Husk | Natural dyes | India | Enzyme-assisted extraction cellulase and pectinase Solvent: water Extraction time: 60–180 min pH 9.5 |
Cashew-nut husk natural dye extraction using Taguchi optimization: green chemistry approach [109] |
| 2015 | Beet | Sugar beet pulp | Monosaccharides present in hydrolyzed SBP pectin: l-rhamnose, l-arabinose, d-galactose and d-galacturonic acid | United Kingdom | Centrifugal partition chromatography ascending mode, 1000 rpm Mobile phase flow rate: 8 ml/min |
Centrifugal partition chromatography in a biorefinery context: separation of monosaccharides from hydrolyzed sugar beet pulp [141] |
| 2015 | Mangoes (Mangifera indica L.) and rye grains (Secale cereals L.) | Peels and grains | Alk(en)ylresorcinols (ARs) | Germany | Ultrasound-assisted extraction Solvent: dichloromethane Extraction time: 15 s cooled in ice bath |
Development and validation of an HPLC method for the determination of alk(en)ylresorcinols using rapid ultrasound-assisted extraction of mango peels and rye grains [78] |
| 2015 | Olives | Waste from olive oil production | High-added value compounds (polyphenols, fatty acids, coloring pigments (chlorophylls and carotenoids), tocopherols, phytosterols, squalene, volatile and aromatic compounds) | Spain, France, Morocco and Portugal | Review Conventional (solvent, heat, grinding) and non-conventional methodologies (ultrasounds, microwaves, sub- and supercritical fluid extractions, pressurized liquid extraction, pulsed electric fields and high voltage electrical discharges) |
Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: non-conventional methods for the recovery of high-added value compounds [142] |
| 2015 | Asparagus | Dried segments (residues) | Antioxidant compounds | China | Solid–liquid extraction Solvents: acetone, methanol or ethanol Extraction time: 2 h Temperature: 70 °C |
Extraction and analysis of antioxidant compounds from the residues of Asparagus officinalis L. [177] |
| 2015 | Grapes | Skin | Anthocyanins | Korea | Deep eutectic solvents (DESs) Extraction time: 45 min room temperature |
Highly efficient extraction of anthocyanins from grape skin using deep eutectic solvents as green and tunable media [138] |
| 2015 | Green tea | Green tea leaf residue | HG pectin, RGII pectin, organic acids, cellulose and hemi-cellulose | The Netherlands | Alkaline extraction Solvents: 0.1 M NaOH Extraction time: 2 h (protein), 5 min–24 h (carbohydrates or lignin) Temperature: 95 °C |
How does alkali aid protein extraction in green tea leaf residue: a basis for integrated biorefinery of leaves [119] |
| 2015 | Papaya (Carica papaya L.) | Processing waste | Lycopene | China | Ultrasound extraction (600 W, 40 kHz) Solvents: ethanol/ethyl acetate Extraction time: 15–40 min Temperature: 20–70 °C |
Optimization of ultrasound-assisted extraction of lycopene from papaya processing waste by response surface methodology [77] |
| 2015 | Carrots, green beans, leeks and celeriac | Vegetable waste streams (rejected carrots, carrot steam peels, green beans cutting waste, leek cutting waste and celeriac steam peels) | Pectin | Belgium | Alcohol insoluble residue Solvents: ethanol and acetone |
Pectin characterization in vegetable waste streams: a starting point for waste valorization in the food industry [178] |
| 2015 | Berries of A. melanocarpa | Black chokeberry wastes | Antioxidants | France | Extraction-adsorption process Extraction time: 2–8 h Temperature: 22 °C |
Pilot scale demonstration of integrated extraction-adsorption eco-process for selective recovery of antioxidants from berries wastes [179] |
| 2015 | Cashew nuts (CNS) | Shells | Anacardic acid | Tanzania | Review Focus on natural anacardic acids from CNS and other plants and their semi-synthetic derivatives as possible lead compounds in medicine |
Potential biological applications of bio-based anacardic acids and their derivatives [180] |
| 2015 | Soy, sugarcane, tea | Soy sauce residues, sugarcane bagasse and tea dregs | Hemicelluloses | China | Ionic liquid Solvents: ionic liquids Extraction time: 1–5 h Temperature: 70–100 °C |
Quantitative industrial analysis of lignocellulosic composition in typical agro-residues and extraction of inner hemicelluloses with ionic liquid [134] |
| 2015 | Tomatoes | Processing tomato | Nutritional bioactive compounds, lycopene | Italy | Biocompatible technology extraction | Recovery of tomato bioactive compounds through a biocompatible and eco-sustainable new technology for the production of enriched “nutraceutical tomato products” [181] |
| 2015 | Citrus sinensis (Hamlin, Valencia, Pera riu and Pera Natal) | Albedo and flavedo | Flavanone | Brazil | Enzymatic process tannase, pectinase and cellulase Extraction time: 30 h Temperature: 40 °C pH 5 |
Simultaneous extraction and biotransformation process to obtain high bioactivity phenolic compounds from Brazilian citrus residues [110] |
| 2015 | Sunflower | Seeds | Oil- (fatty acids and their antioxidant capacities) and water-soluble phase (proteins, carbohydrates and phenolics) | Slovenia | Subcritical water extraction Extraction time: 5–120 min Temperature: 60–160 °C Pressure: 30 bar |
Simultaneous extraction of oil- and water-soluble phase from sunflower seeds with subcritical water [103] |
| 2015 | Cereals, root crops, fruits, vegetables, oilseeds, meat, dairy products |
Food waste | Nutritionally interesting compounds, chemicals and biofuels | Brazil | Review Sub- and supercritical technologies |
Sub- and supercritical fluid technology applied to food waste processing [89] |
| 2015 | Agricultural biomass | By-products such as durian peel, mango peel, corn straw, rice bran, corn shell and potato peel | Bio-fuel, water soluble sugars and phenolic compounds | Malaysia and Nigeria | Review Sub-critical water |
Sub-critical water as a green solvent for production of valuable materials from agricultural waste biomass: a review of recent work [182] |
| 2015 | Sugarcane | Sugarcane waste (rind, leaf and bagasse) | Wax/long-chain aldehydes and n-policosanols (nutraceutical compounds) triterpenoids | UK and Brazil | Supercritical CO2 (scCO2) Extraction time: 4 h Temperature: 50 °C Pressure: 350 bar Flow rate: 40 g/min |
Sugarcane waste as a valuable source of lipophilic molecules [183] |
| 2015 | Mangoes | Peel | Pectin | Germany and Saudi Arabia | Hot-acid extraction Extraction time: 90 min pH 1.5 |
The arabinogalactan of dried mango exudate and its co-extraction during pectin recovery from mango peel [184] |
| 2015 | Coffee | Spent coffee grounds | Tannin compounds | Malaysia | Alkaline extraction Solvent: NaOH Extraction time: 30–90 min Temperature: 60–100 °C |
The influence of extraction parameters on spent coffee grounds as a renewable tannin resource [185] |
| 2014 | Eucalyptus globulus wood | Trimmings of Eucalyptus globulus wood veneers | Phenolic compounds | Spain | Aqueous two-phase extraction PEG 2000 and ammonium sulphate Extraction time: 30–390 min Temperature: 25–65 °C |
Aqueous two-phase systems for the extraction of phenolic compounds from eucalyptus (Eucalyptus globulus) wood industrial wastes [124] |
| 2014 | Pomegranates | By-products after winemaking of pomegranate | (poly)phenolic compounds | Spain, Mexico and Italy | Extraction with MeOH 70% (v/v) and sonication | Assessment of pomegranate wine lees as a valuable source for the recovery of (poly)phenolic compounds [186] |
| 2014 | Citrus | Peel, pulp and seeds | Several value-added products, such as essential oils, pectin, enzymes, single cell protein, natural antioxidants, ethanol, organic acids, and prebiotics | Greece and Sweden | Review | Biotransformation of citrus by-products into value added products [187] |
| 2014 | Olives | Olive solid waste | Natural dye | Tunisia | Aqueous extraction in closed flasks Solvent: NaOH Extraction time: 15–120 min Temperature: 30–90 °C |
Development and optimisation of a non-conventional extraction process of natural dye from olive solid waste using response surface methodology (RSM) [125] |
| 2014 | Coffee | Waste coffee grounds | Biodiesel production | United Kingdom | Suspended in fresh heptane room temperature | Effect of the type of bean, processing, and geographical location on the biodiesel produced from waste coffee grounds [188] |
| 2014 | Grapevine and hazelnut | Grapevine waste and hazelnut skins | Polyphenols content | Italy and France | UAE and MAE Solvents: ethanol, methanol, acetone, butanone, β-cyclodextrin Extraction time: 5–40 min Temperature: 20–60 °C |
Efficient green extraction of polyphenols from post-harvested agro-industry vegetal sources in Piedmont [58] |
| 2014 | Bamboo | Raw bamboo culm | Lignin | Malaysia | Review Chemical and steam explosion methods |
Extraction and preparation of bamboo fibre-reinforced composites [189] |
| 2014 | Spruce | Spruce sawdust | Carboxylic acids | Finland | Alkaline extraction Solvents: Na2CO3 or Na2S.9H2O Extraction time: 30 min + 30 min; Temperature: 80 °C up to 160 °C and 210 °C |
Production of carboxylic acids from alkaline pretreatment byproduct of softwood [120] |
| 2014 | Variety of biomass sources (rapeseed, soybean, palm oil and nonedible feedstocks) | Preferably 2nd–4th generation feedstock (non-edible materials as bagasse, oil waste, microalgae, cyanobacteria and microbes) | Biodiesel | Malaysia | Review Supercritical fluid process and catalytic in situ or reactive extraction process |
Integration of reactive extraction with supercritical fluids for process intensification of biodiesel production: prospects and recent advances [90] |
| 2014 | Cherries | Cherry seeds | Total phenolic content | Brazil and France | Pressurized fluid extraction (PFE) Solvent: anhydrous ethanol Extraction time: 2–10 min Temperature: 40–80 °C |
Isolation by pressurized fluid extraction (PFE) and identification using CPC and HPLC/ESI/MS of phenolic compounds from Brazilian cherry seeds (Eugenia uniflora L.) [190] |
| 2014 | Corn | Corn stover | Lignin | USA | Protic ionic liquid (PIL) Extraction time: 24 h Temperature: 90 °C |
Lignin extraction from biomass with protic ionic liquids [135] |
| 2014 | Oranges | Peel | d-limonene | United Kingdom | Microwave-assisted extraction 200 W, closed vessel Solvent: hexane Temperature: 70–110 °C |
Microwave-assisted extraction as an important technology for valorising orange waste [59] |
| 2014 | Sweet Limes | Peel | Antioxidant phenolics | Pakistan | Enzymatic treatment Incubation time: 30–120 min Temperature: 30–75 °C pH 5 to 8 |
Optimization of enzyme-assisted revalorization of sweet lime (Citrus limetta Risso) peel into phenolic antioxidants [111] |
| 2014 | Artichoke | Artichoke scraps | Phenolic compounds | Italy | Ultrasound-assisted extraction (UAE) Time: 60 min Solvent: water |
Phenols and antioxidant activity in vitro and in vivo of aqueous extracts obtained by ultrasound-assisted extraction from artichoke by-products [79] |
| 2014 | Cachrys pungens Jan (Umbelliferae) | Aerial parts of Cachrys pungens Jan (Umbelliferae) | Bioactive compounds | Italy | Solvent extraction Solvents: methanol Extraction time: 72 h room temperature dark conditions |
Phytotoxic activity of Cachrys pungens Jan, a Mediterranean species: separation, identification and quantification of potential allelochemicals [191] |
| 2014 | Wheat | Wheat straw | Major organic components (e.g., N-heterocycles, fatty acids, phenols and lignins) | Canada | Fast pyrolysis steel shots 475 °C | Wheat straw biomass: a resource for high-value chemicals [192] |
| 2013 | Cranberries | Cranberry juice and pomace | Polyphenolics | Canada and Mexico | Pilot scale methods Solvents: ethanol Extraction time: 24 h |
Bioactivities of pilot-scale extracted cranberry juice and pomace [48] |
| 2013 | Fruits, vegetables, eggs, shrimp | Plant residues, industrial and post-harvest materials | Carotenoids | Mexico | Review Novel environmentally friendly solvents (e.g., ethyl lactate, bioethanol, vegetal oil, commercial enzymes) |
Carotenoids extraction and quantification: a review [193] |
| 2013 | Tomatoes | Peels | Lycopene | Italy | Enzymatic-assisted extraction Temperature: 45 and 60 °C pH 4–5 and 9–10.5 |
Environmentally friendly lycopene purification from tomato peel waste: enzymatic-assisted aqueous extraction [112] |
| 2013 | Coffee | Coffee residue left after the preparation of the brew (spent coffee grounds—SCG) | Polysaccharides | Portugal | Alkali extraction Solvent: H2O and 4 M NaOH Extraction time: 3 h Temperature: 20–120 °C |
Extractability and structure of spent coffee ground polysaccharides by roasting pre-treatments [194] |
| 2013 | Coffee | Spent coffee grounds | Lipids, oil | Iran | Soxhlet, UAE, MAE, SFE Solvents: petroleum benzene and n-hexane Soxhlet: 6 h, boiling temperature UAE: 45 min, ambient conditions MAE: 30 s, 200 and 800 W SFE: 200–250 bar, 40–60 °C, modifier (water, ethanol, hexane) |
Extraction of lipids from spent coffee grounds using organic solvents and supercritical carbon dioxide [60] |
| 2013 | Forest Industry | Forest residues, including bark | Bioactive molecules | Canada | Review Green alternatives for the design, formulation, and manufacture of new products with applications in various markets (cosmetics, natural health products, biocides, adhesives, coatings) |
Forest extractives, the 4th pathway of the forest biorefinery concept [195] |
| 2013 | Coffee | Spent coffee grounds (SCG) | Lipid fraction | Portugal and Brazil | Supercritical carbon dioxide Extraction time: 1 h Temperature: 55 °C Pressure: 250 bar CO2 flow rate: 15 kg/h |
From coffee industry waste materials to skin-friendly products with improved skin fat levels [91] |
| 2013 | Walnuts | Green husk | Natural compounds with antioxidant and antimicrobial properties | Spain and Portugal | Solvent extraction Solvents: water, methanol, ethanol Extraction time: 45 min room temperature |
Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts [49] |
| 2013 | Coffee | Spent coffee | Antioxidants | Spain | Soxhlet, SPE, filter coffeemaker Solvents: water, ethanol, methanol Extraction time: 6–165 min Temperature: 80–100 °C |
Influence of extraction process on antioxidant capacity of spent coffee [50] |
| 2013 | Tomatoes | Peel | Fatty acids | France | Depolymerization 1.5 M KOMe overnight treatment at room temperature | Interfacial properties of functionalized assemblies of hydroxy-fatty acid salts isolated from fruit tomato peels [196] |
| 2013 | Coffee | Spent coffee grounds (SCG) | Polysaccharides | Portugal | Microwave superheated water extraction Extraction time: 5 min Temperature: 200 °C |
Microwave superheated water extraction of polysaccharides from spent coffee grounds [61] |
| 2013 | Turkish red pine timber | Waste barks | Natural dye | Turkey | Natural dyestuff extraction machine Solvents: water and ethanol Extraction time: 24 h (osmosis) |
Natural dye extraction from waste barks of Turkish red pine (Pinus brutia Ten.) Timber and eco-friendly natural dyeing of various textile fibers [126] |
| 2013 | Cotton, jute, flax, hemp, ramie and natural colorants | Wastes and manufacturing by-products | Fibres, polysaccharides, dyes and pigments, polyphenols, oils and other biologically active compounds | India | Review Conventional maceration, soxhlet, MAE, SFE, ultrasonic extraction |
Perspectives for natural product based agents derived from industrial plants in textile applications: a review [197] |
| 2013 | Coffee | Spent coffee grounds | Natural antioxidants | Italy | Solvent extraction Solvents: H2O, ethanol, Extraction time: 30 min Temperature: 60 °C |
Recovery of natural antioxidants from spent coffee grounds [198] |
| 2013 | Feijoa fruits | Primarily skin and some flesh | Total soluble solids (TSS), pectin fibre content, total extractable PP content (TEPC) and total antioxidant activity | New Zealand | Accelerated solvent extraction Solvents: (acidified) water, ethanol Temperature: 20 or 50 °C |
Utilisation potential of feijoa fruit wastes as ingredients for functional foods [127] |
| 2012 | Green tea | Green tea waste | Noncaffeine tea polyphenols | China | Water bath 20 min 90 °C |
A novel way of separation and preparation non-caffeine tea polyphenols from green tea waste [199] |
| 2012 | Larch | Larch wood-derived lignocellulosic residue | Arabinogalactan, pectin, and crystalline glucose | Russia | Water extraction Extraction time: 2–3 h Temperature: 60–80 °C |
An eco-friendly technology for polysaccharide production from logging and sawing waste [128] |
| 2012 | Olives | Olive leaves | Oleuropein | Greece | SFE and PLE SFE: 30 MPa, 50 °C, 9.6 kg/h PLE: 10.34 MPa, 10 min, 40–150 °C Solvents: H2O and EtOH |
Development of a green extraction procedure with super/subcritical fluids to produce extracts enriched in oleuropein from olive leaves [92] |
| 2012 | Wood | Wood barks, obtained from pulp mills as industrial wastes | Natural phenolic polymers of tannins and lignin | France | Aqueous extraction urea and sulfite used as water-additives Extraction time: 1 h under reflux Temperature: 75 °C |
Development of green adhesives for fibreboard manufacturing, using tannins and lignin from pulp mill residues [129] |
| 2012 | Wheat | Wheat milling by-products | High quality oil and vitamin E | Italy | Review Solvent extraction, mechanical pressing or the eco-friendly supercritical carbon dioxide (SC-CO2) extraction technology |
Durum wheat by-products as natural sources of valuable nutrients [200] |
| 2012 | Tree bark | Waste product from paper pulp industries | Antioxidants | Sweden | SFE, PFE, SLE Solvents: scCO2, ethanol, H2O Extraction time: 30 min–24 h Temperature: 70–180 °C |
Extraction of antioxidants from spruce (Picea abies) bark using eco-friendly solvents [93] |
| 2012 | Timber | Empty fruit bunches | Fiber | Malaysia | Perspective paper | Fiber resin matrix composites: nature’s gift [201] |
| 2012 | Oranges | Peel | Essential oil | United Kingdom | Steam distillation and microwave irradiation SD: water, 1 h MW: 12.5 min, 200 °C, power gradient from 400 to 1200 W |
p-cymenesulphonic acid: an organic acid synthesized from citrus waste [202] |
| 2012 | Black tea | Black tea wastes | Pancreatic lipase-inhibiting polyphenols | Japan | Hot-compressed water (HCW) ion-exchange water extraction temperature: 100–200 °C | Polyphenols extracted from black tea (Camellia sinensis) residue by hot-compressed water and their inhibitory effect on pancreatic lipase in vitro [203] |
| 2012 | Green tea | Green tea waste | Polyphenols | China | Liquid–liquid extraction Solvents: H2O, glyceryl, triacetate, n-butanol, ethyl acetate Extraction time: 12 h + 2 h |
Recovery of tea polyphenols from green tea waste by liquid–liquid extraction [204] |
| 2012 | Citrus | Peels | Polymethoxy flavonoids | China | Solvent extraction Solvents: methanol and ethanol Extraction time: 1–3 h Temperature: 65–85 °C |
Study on the extraction technique of poly-methoxyflavonoids from citrus peels by using response surface methodology [205] |
| 2011 | Coffee | Husks | Caffeine | Spain | Supercritical CO2 Extraction time: 20 min Temperature: 323 K Pressure: 60 bar CO2 flow rate: 2–3 g/min |
Extraction of caffeine from Robusta coffee (Coffea canephora var. Robusta) husks using supercritical carbon dioxide [94] |
| 2011 | Oranges | Peel | Essential oils | France and Tunisia | Microwave steam diffusion (MSDf) Extraction time: 12 min Temperature:100 °C |
Microwave steam diffusion for extraction of essential oil from orange peel: kinetic data, extract’s global yield and mechanism [62] |
| 2011 | Grape | Skins | Anthocyanins | Spain | Microwave-assisted extraction Solvents: H2O, methanol Extraction time: 5–20 min Temperature: 50–100 °C |
Microwave-assisted extraction of anthocyanins from grape skins [63] |
| 2011 | Tea (green, oolong and black) | Tea residues (green, oolong and black tea residues) | Phenolic compounds | Japan | Microwave-assisted extraction water under autohydrolytic conditions Extraction time: 2 min Temperature: 110–230 °C |
Microwave-assisted extraction of phenolic compounds from tea residues under autohydrolytic conditions [64] |
| 2011 | Sea Buckthorn (Hippophae rhamnoides | By-Products of juice production | Flavonoids | France | Solvent-free microwave hydrodiffusion and gravity (MHG) without addition of solvent or water atmospheric pressure | Solvent free microwave-assisted extraction of antioxidants from sea buckthorn (Hippophae rhamnoides) food by-products [206] |
| 2011 | Wheat | Wheat straw | Energy and CO2 secondary metabolites including fatty acids, wax esters and fatty alcohols | England | Supercritical CO2 extraction Temperature: 40–100 °C Pressure: 100–300 bar CO2 flow rate: 40 g/min |
Use of green chemical technologies in an integrated biorefinery [95] |
| 2011 | Olives | By-products generated during storage of extra virgin olive oil | Phenolic compounds, hydroxytyrosol, tyrosol, decarboxymethyl oleuropein aglycone, and luteolin | Italy and Spain | Solid–liquid and liquid–liquid extraction Solvents: n-hexane, methanol, H2O Extraction time: 1 h |
Wastes generated during the storage of extra virgin olive oil as a natural source of phenolic compounds [207] |
| 2010 | Tomatoes | Ground tomatoes without seeds | Lycopene | France and Algeria | Solvent extraction Solvent: d-limonene |
Carotenoid extraction from tomato using a green solvent resulting from orange processing waste [208] |
| 2010 | Tea plant | Tea stalk and fiber wastes | Caffeine | Turkey | Supercritical CO2 ethanol as co-solvent Extraction time: 1–5 h Temperature: 50–70 °C Pressure: 250 bar semi-continuous flow |
Effect of ethanol content on supercritical carbon dioxide extraction of caffeine from tea stalk and fiber wastes [96] |
| 2010 | Portuguese elderberry | Pomace | Anthocyanins | Portugal | Supercritical CO2 extraction Solvents: CO2, water, ethanol Extraction time: 40 min Temperature: 313 K |
Effect of solvent (CO2/ethanol/H2O) on the fractionated enhanced solvent extraction of anthocyanins from elderberry pomace [97] |
| 2010 | Green tea | Green tea waste | Polyphenols, total catechins, and reducing sugars | South Korea and USA | Solvents: cold water (25 °C), hot water (90 °C), sulfuric acid, hydrochloric acid and methanol Extraction time: 20 min 250 rpm |
Effects of cellulase from Aspergillus niger and solvent pretreatments on the extractability of organic green tea waste [130] |
| 2010 | Tea | Tea waste | Caffeine | Iran | Subcritical water extraction Temperature: 100–200 °C Pressure: 20–40 bar water flow rate: 1–4 g/min |
Isolation of caffeine from tea waste using subcritical water extraction [104] |
| 2010 | Citrus sudachi | Peels | Flavones | Japan | Microwave-assisted extraction Solvents: methanol extraction time: 10 to 12 min |
Microwave-assisted extraction and methylation of useful flavones from waste peels of Citrus sudachi [209] |
| 2010 | Mate (Ilex paraguariensis) | Mate residue | Compounds with antioxidant properties, such as phenolic acids and methylxanthines, such as caffeine | Brazil | Solvent extraction Solvent: methanol, H2O, ethanol sonication for 15 min room temperature |
Phenolic acids and methylxanthines composition and antioxidant properties of mate (Ilex paraguariensis) residue [210] |
| 2010 | Rice | Rice bran | Phenolic compounds as well as other valuable materials | Japan | Subcritical water Preheated oil: 100–180 °C, 10 min Preheated water bath: 180–360 °C, 10 min and 220 °C for 2–30 min |
Production of phenolic compounds from rice bran biomass under subcritical water conditions [105] |
| 2009 | Citrus | Peels | Essential oil | France and Algeria | Microwave hydrodiffusion gravity Extraction time: 15 min atmospheric pressure 500 W |
A new process for extraction of essential oil from citrus peels: microwave hydrodiffusion and gravity [65] |
| 2009 | Kiwifruit | By-products derived from kiwifruit processing | Phenolics and pectin polysaccharides | New Zealand | Solvent extraction Solvents: water, ethanol Extraction time: 1 h room temperature |
Evaluation of the extraction efficiency for polyphenol extracts from by-products of green kiwifruit juicing [211] |
| 2009 | Palm | Black liquor of oil palm waste | Lignin | Malaysia | Solvent extraction Chemical extractions: di-ethyl ether, alcohol-benzene mixture treatment with H2SO4 for 30–45 min |
Exploring the antioxidant potential of lignin isolated from black liquor of oil palm waste [212] |
| 2009 | Turkish tea plants | Tea stalk and fiber wastes | Caffeine | Turkey | Supercritical carbon dioxide Extraction time: 1–10 h Temperature: 55–75 °C increasing pressure up to 250 bar semi-continuous flow |
Extraction of caffeine from tea stalk and fiber wastes using supercritical carbon dioxide [99] |
| 2009 | Rice | Rice bran | Oil (value-added materials such as amino acids, organic acids, and water-soluble saccharides) | Japan | Subcritical water preheated oil bath: 100–180 °C Preheated salt bath: 200–360 °C Reaction time: 5 min |
Sub-critical water treatment of rice bran to produce valuable materials [106] |
| 2009 | Several biomass | Residues rich in lignocellulosics | Bio-based chemicals (e.g., succinic, lactic, fumaric l-malic, l-aspartic acids) | England | Review Focus on green chemical conversion of lignin into higher value chemicals |
The integration of green chemistry into future biorefineries [21] |
| 2009 | Apple | Industrially generated apple pomace | Antioxidants and polyphenols | Ireland | Pressurized liquid extraction accelerated solvent extractor static extraction of 5 min Temperature: 75–193 °C |
The optimization of extraction of antioxidants from apple pomace by pressurized liquids [213] |
| 2008 | Chicory, citrus, cauliflower, endive, and sugar beet | Plant by-products (chicory roots, citrus peel, cauliflower florets and leaves, endive, and sugar beet pulps) | Pectins | France and Finland | Enzymatic extraction Extraction time: 4 h Temperature: 50 °C |
Extraction of green labeled pectins and pectic oligosaccharides from plant by-products [113] |
| 2008 | Tea (green, oolong, and black) | Green, oolong, and black tea residues | Polysaccharides, polyphenols, arabinose, galactose, xylose, catechins | Japan | Microwave heating Solvent: water Temperature: 110–230 °C |
Microwave heating of tea residue yields polysaccharides, polyphenols, and plant biopolyester [66] |
| 2008 | Plant lipids | Plant oils and other natural lipidic phases | Phytosterols, vitamins | Czech Republic | Review Enzymes as efficient natural catalysts |
Plant products for pharmacology: application of enzymes in their transformations [114] |
| 2007 | Broccoli | Broccoli seeds | Natural sulforaphane | China and Australia | Liquid–liquid and solid-phase extraction Solvents: ethanol, hexane, ethyl acetate |
Separation and purification of sulforaphane from broccoli seeds by solid phase extraction and preparative high-performance liquid chromatography [214] |
| 2006 | Tea | Tea waste | Caffeine | Turkey | Solid–liquid extraction solvents: hot water and chloroform Temperature: 370 K and 293 K |
Solid–liquid extraction of caffeine from tea waste using battery type extractor: process optimization [215] |
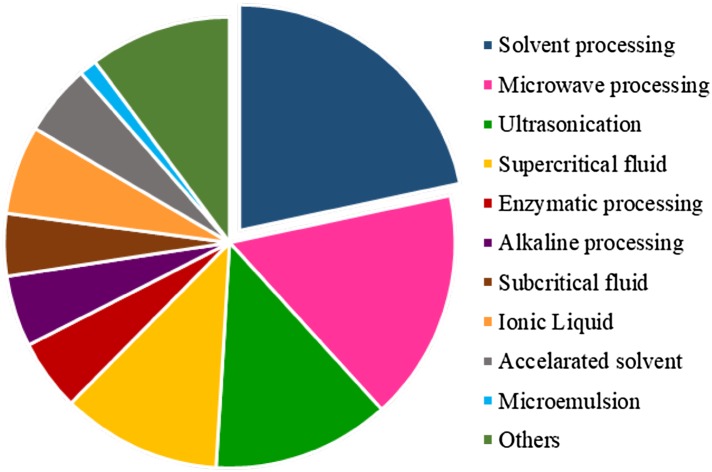
The decision concerning the best method to separate the compounds of interest from the raw material is dependent on several aspects, such as the characteristics of the target extracts and raw material (physical–chemical properties), available technology, required purity, selectivity, stability and, more importantly here, the greenness of the whole process. As can be seen in Fig. 5, the most cited techniques in these research papers were based on solvent/maceration (25% of the total), microwave (19%), ultrasonication (14.7%) and supercritical fluid processing (13%), followed by methods using ionic liquids (7%), enzymatic and subcritical fluid treatment (6%), as well as the association of two or more techniques.
Fig. 5.
Main green and sustainable techniques used to separate natural products from waste described in research papers (ISIS Web of Knowledge, January 2006 to December 2017)
According to the literature, the most widespread approaches for separating natural products from a number of matrices are based on liquid–liquid or solid–liquid extraction (LLE and SLE). Several greener alternatives have been proposed by replacing toxic or non-renewable organic solvents, as well as the extraction times. In some cases, solid-phase extractions (SPE) were also carried out and decreased both the amount of solvent and the number of extraction cycles, offering high enrichment factors [39, 40]. Actually, the mass transfer enhancement for SLE has been largely studied and applied, contributing to technology innovation, process intensification and integration, and energy saving, especially important for microwave, ultrasound, and high-pressure processing, for instance [41]. An overview of these techniques and related examples will be discussed in this section.
From Conventional Solvent Separation to Enhancement Processing Approaches Over the Last 10 Years
Solvent processing is one of the most traditional methods to remove natural products from bio-derived materials. In this extraction approach, the raw material in adequate size is exposed to different solvents, mostly organic, which remove soluble components of interest. The samples are then usually centrifuged and filtered to separate the solid residue, and the extract is used in this way (as a food supplement or for preparing functional foods, for example) or treated after this step. Solvent extraction is attractive compared to other methods due to low cost and simplicity. However, this method does not always use benign solvents; it frequently requires an evaporation/concentration step for recovery, it usually demands large amounts of solvent and needs a long time to be carried out. Additionally, the possibility of thermal degradation of natural bioactive components is also possible due to the high temperatures used during the extraction process [42]. Despite this, it is largely used in industries, where solvent reuse is of great economic importance. In general, the raw material (in its liquid or solid form) is mixed with a solvent, and the separation kinetic of the target compounds is influenced by parameters such as the solvent ratio, pH, and temperature and, for SLE, the particle size. The solvent should be atoxic, non-flammable and stable at working conditions, ideally renewable and cheap, with low viscosity and an adequate boiling point, allowing for easier solvent removal from the extract/fraction [43]. Recently, several models have been proposed to predict the best solvents to be used in a specific case, which do not only take into account physical descriptors, such as enthalpy of vaporization, dielectric constant, refractive index, boiling point, etc., but also empirical descriptors to evaluate, for instance, intermolecular forces (specific and non-specific solute–solvent interactions, e.g., hydrogen bond donor and/or hydrogen bond acceptor, Van der Waals and ion/dipole forces). Purely theoretical descriptors have been also introduced, offering the most important advantage of not requiring any experiments, as is the case of the model known as quantitative structure property relationship (QSPR), able to predict 127 polarity scales for more than 700 solvents [44].
The solvent selection also depends on the physical–chemical proprieties of the compounds of interest, considering principally the selectivity and greenness degree of the process, aiming at obtaining high recoveries and the integrity of the target compounds. In general, the raw material stays in contact with the solvent for a certain period (from minutes to days), when the soluble compounds are transferred from the matrix to the extractor phase, usually by shaking the system. For SLE, the dispersion of the particles in the solvent is facilitated agitating them, optimizing their contact and accelerating the separation process. Traditionally, solvent treatment is performed at room temperature, although heating can promote higher recoveries to these compounds that are not thermosensitive. In some cases, LLE and SLE can be time-consuming, demanding further purification and concentration steps, which are their main drawbacks [41, 45].
Maceration using green and non-toxic solvents for the separation of natural products from plant-derived waste has been described over the last years (e.g., to remove dyes from quince leaves or catechins, theaflavins, gallic acid, and antioxidants in general from walnut green husk, cranberry pomace, black tea and banana processing waste). According to these studies, using water, methanol, ethanol or a mixture of them at 70–100 °C can be a low-cost, benign alternative for the recovery of high added-value compounds derived from residual biomass [46–49]. Scaling-up was also studied, whose results showed to be useful in determining industrial process feasibility and the economic value of polyphenols for commercial use, increasing the overall profitability of the cranberry industry [48].
Whenever possible, higher temperatures allow for higher mass transfer in a shorter time with lower energy consumption in general, resulting in better recovery efficiency than conventional systems [50]. As observed in Fig. 5, the second most cited green and sustainable separation process is based on microwave heating and can be considered a non-conventional technique nowadays. Heating is based on non-ionizing electromagnetic waves. Those between 0.915 and 2.45 GHz are used for industrial, scientific and medical applications. The overall principle of heating is rooted in its direct impact with polar materials/solvents and is dependent on ionic conduction and dipole rotation, occurring simultaneously in most cases. The increased temperature can overcome the natural product-matrix interaction caused by Van der Waals forces, dipole attraction, hydrogen bonding of the compounds of interest and active sites in the matrix. Therefore, thermal energy can disrupt both solute–solute and solute–matrix interactions, providing the activation energy required for the desorption process. The mass transfer of the compounds from the raw material to the solvent is also accomplished by convection and diffusion mechanisms, causing the explosion of plant cells and releasing their content into the liquid phase [51].
The eco-friendly removal of essential oils, pectin and polyphenols from a number of plant raw materials mediated by microwave irradiation has been described over the last years, paying special attention to citrus waste [52–66]. In fact, the orange juice processing industry can be considered more than a good case study. This sector is highly wasteful, generating 50% of waste from the total fruit/starting material (e.g., peel, bagasse, seeds and yellow water). Around 20 million tonnes of orange peel per year are produced worldwide, which consist of water (80%) and sugars, cellulose, hemicellulose, pectin and d-limonene (20%). Recently, it was shown using a mathematical model that d-limonene extraction consisted of a two stage diffusion process for a microwave (MW) heating approach: initial extraction from the exterior of cells followed by trans-membrane diffusion. Compared to other conventional extraction methods, it was found that the microwave treatment was more efficient, resulting in a higher overall yield due to the access to a higher amount of d-limonene [59].
The successful microwave-assisted solvent-free modification of pectin derived from citrus waste has also been reported [53]. These approaches not only allow for the separation of the major components of citrus peel, but they also add further value through the production of other high value-added products, such as pectin, d-limonene and a rare form of mesoporous cellulose which are produced in a single step, without added acid [67]. Along these lines, the concept of dry-biorefinery is gaining momentum, since valuable products can be recovered from plant by-products without adding solvents or water, using green processes such as MW [56]. Innovation relies on the separation of the target compounds from raw materials, which are rich in water, achieved without adding solvents or water, illustrating a circular systemic process; i.e., all materials and resources could be reintegrated into the integrated and zero-waste biorefinery [19]. Although very attractive, as expected, the design and use of real MW industrial scale equipment requires additional studies related to safety, corrosion and maintenance intervals [68].
The combination of two or more extraction/concentration methods is quite common in the literature (Table 1). As described by Boukroufa et al. [56], the removal of essential oil, polyphenols and pectin from orange waste was conducted using microwave and ultrasound technology, without adding any solvents. Essential oil separation was performed by Microwave Hydrodiffusion and Gravity (MHG), and thereafter the remaining water of this process was used as a solvent for the subsequent extraction of flavonoids and pectin. For polyphenol separation, ultrasound-assisted extraction (UAE) was used, and response surface methodology (RSM) using the central composite design (CCD) approach was used to investigate the influence of some variables. The CCD revealed that the optimized conditions of ultrasound power and temperature were 0.956 W/cm2 and 59.83 °C giving a polyphenol yield of 50.02 mg GA/100 g dm, which, compared to conventional extraction, promoted an increase of 30% in the yield. Pectin was extracted by microwave-assisted extraction, resulting in a maximal yield of 24.2% for microwave power of 500 W (3 min), whereas traditional extraction provides18.32% (120 min). As can be seen, the combination of microwave, ultrasound and recycled water resulted in higher recoveries of the compounds of interest in a shorter time, so that a systemic loop/cycle could be closed using only the resources generated in the plant. This makes the whole process optimized in terms of time, energy savings, cleanliness and reduced amount of waste.
As can be noted, ultrasound has been widely utilized for helping to extract target components from waste plant-derived sources, reducing separation time, solvents, energy consumption and improving the product quality. The effectiveness of ultrasound is attributed to the cavitation phenomenon, assisting the solubilization of the compounds of interest into the solvent, enhancing their removal from the bulk raw material [69]. According to Chemat [70], the ultrasound waves (from 20 kHz to 10 MHz) pass through an elastic medium, inducing a longitudinal displacement of particles resulting in a succession of compression and rarefaction phases in this medium. Every medium has a critical molecular distance and, below this critical point, the liquid remains intact. However, above this distance, the liquid would break down, creating voids (cavitation bubbles) in the liquid. When the size of these bubbles reaches a critical point they collapse, releasing a large amount of energy. The estimated temperature and pressure at this time are estimated at 5000 and 2000 K atmospheres. This creates hotspots that accelerate the chemical reactivity into the medium, generating microjets directed towards the solid surface, also responsible for the general higher effectiveness of this technique, as the high pressure and temperature involved in the process destroy the cell walls of the plant matrices and their content can be released into the medium more easily.
Some new process aiming at agro-industrial waste application in food industries based on ultrasound-assisted extraction of natural products have been reported [71–79], as is the case of carotenoid separation from pomegranate peels using different vegetable oils as solvents [72]. Sunflower and soybean oils were used as solvents and parameters such as time, temperature, solid/oil ratio used were analyzed considering the yield. It was found that the optimum mild operating conditions were: extraction temperature, 51.5 °C; peel/solvent ratio, 0.10; amplitude level, 58.8%; solvent, sunflower oil. Additionally, a subsequent separation of oil and carotenoids was not necessary, since the pigmented oil can be used as a carotenoid source in different commercial products in this format.
The green recovery of cellulose from oil palm bunches by autoclave-based and ultrasonication pre-treatments were successfully developed to replace the non-green chlorite method [73]. An ultrasonic process with hydrogen peroxide yielded 49% cellulose with 9.13% alpha-cellulose content and 68.7% crystallinity, as compared to 64% cellulose with an autoclave treatment. The cellulose/polypropylene composites generated with high tensile strength, high thermal stability, and low water and diesel sorption showed great potentials for conversion into eco-composite products such as polymeric material insulated cables for high voltage engineering, automotive parts, sports tools and other household or office items.
Another highly cited green and sustainable technique to isolate organic compounds from bio-based waste is based on supercritical fluid processing (Fig. 5). It is widely known that substances at temperatures and pressures near or above their critical points have exceptional solvent characteristics for analytical purposes. These supercritical fluids possess liquid-like solvating and gas-like diffusivity power, and other tuneable properties that can be adjusted varying temperature, pressure and the addition of other components acting as a modifier. Due to its gas-like low viscosity and high diffusivity, the supercritical fluid can easily penetrate into plant materials with a fast mass transfer rate. Possibly, the most important property of supercritical fluids for separation processes is diffusion, obtaining solubility and diffusion good enough to provide quantitative extraction yield [80, 81]. Carbon dioxide (scCO2) is the fluid most widely used for extractions, with critical parameters of 31.1 °C and 73 atm (7.39 MPa), at relatively low operating conditions. It behaves as a nonpolar or polarizable solvent and low molar mass alcohols (co-solvents) are often added in small quantities to modify the solvent polarity. Because carbon dioxide can be depressurized to the gaseous state, the solvent is easily removed and supercritical fluid-based separation methods are easily coupled with subsequent analysis. Therefore, scCO2 provides miscibility to the majority of natural products, availability and low cost, reliably high purity, negligible toxicity, facility for removal and reuse, resulting in many advantages for downstream processing in terms of product purification and/or catalyst recycling [80].
The approach using scCO2 has been widely used for isolation and purification of chlorophylls, carotenoids, lipids, alkaloids, antioxidants from matrices such as filter tea, spruce bark, tomato and elderberry pomace, grape, passiflora, coffee and cupuassu seed waste [82–99]. In addition to the optimization of the separation process, some studies also aim to evaluate the techno-economic viability of large-scale commercial production, for example, to obtain cupuassu butter from cold-pressed seed residues, also evaluating the influence of thermodynamic and kinetic variables of yield, chemical composition and production costs of the extracts [86]. Optimal conditions related to extraction kinetics, chemical composition and production costs were 30–35 MPa and 50 °C. It was shown that the phenolic content (0.47–2.82 mg/g) was lower than those commonly found using other methods (20–23 mg/g). The high contents of tocopherols, as well as the unsaturated fatty acids (48%) compared to the saturated fatty acids (52%) present in the butter obtained by scCO2 demonstrated its great potential as an ingredient in food, pharmaceutical and cosmetic industries. In addition, process intensification for biodiesel production involving supercritical fluids has been reported [84, 90]. Such approaches can allow biodiesel production without any addition of catalyst, or via catalytic in situ or reactive extraction process, combining the extraction and reaction phase together in a single operation unit. These studies also discuss both processes towards the future bio-refinery setup and more efficient use of all waste produced.
The use of fluids different to CO2 has been described in the literature, but as they are usually organic solvents, they do not show any distinct advantages and often have high critical temperatures. Despite having a very high critical temperature, water shows unique properties in the subcritical region (200–300 °C), as a reduction in dielectric constant (20–30) and density (0.7–0.8 g/cm3) compared to water at room temperature, improving its ability to dissolve nonpolar organic and inorganic compounds. Under these conditions, the water dissociation constant into hydroxide and hydrogen ions are more than three orders of magnitude higher, so that near-critical water acts as a self-neutralizing acid or base catalyst, avoiding salt waste generation. Moreover, using subcritical and supercritical water conditions greatly simplifies the product purification step in some cases, since nonpolar products are insoluble in water in lower temperatures [80, 100–106].
Other potential scalable approaches have been described, such as enzymatic [107–114], alkaline [115–120] and based on different types of aqueous media (e.g., cyclodextrins, montmorillonite K-10/LiOH, green liquor) [121–130]; ionic liquids [131–135], deep eutectic solvents [136–138], constituting alternative methods for the recovery of high added-value compounds from agro-industrial waste aiming at obtaining the best analytical, economical and socio-environmental compromise [139–142].
Based on the investigated literature [143], Table 2 summarizes the advantages and disadvantages of the four most cited green and sustainable techniques.
Table 2.
Advantages and disadvantages of different technologies that were most cited as green and sustainable techniques over the last 10 years
| Advantages | Disadvantages | |
|---|---|---|
| Solvent processing | Inexpensive and simplicity; allows for solvent reuse | Does not always uses benign solvents; frequently requires an evaporation/concentration step for recovery; usually demands large amounts of solvent and long extraction time; possibility of thermal degradation |
| Microwave processing | Reduced extraction time; reduced solvent usage; improved extraction yield; simple and inexpensive | Not good when either target compounds or solvents are non-polar or volatiles |
| Ultrasonication | Inexpensive, simple and efficient; can reduce the operating temperature (good for thermolabile compounds); can be used with any solvent | Its efficiency may be linked to the nature of plant matrix; the active part of ultrasound inside the extractor is restricted to a zone located in the vicinity of the ultrasonic emitter |
| Supercritical fluid | Moderate extraction temperature (good for thermolabile compounds); rapid mass transfer (larger extraction rate); solubility of a chemical in a supercritical fluid can be manipulated; can eliminate concentration process; the solutes can be separated from supercritical fluids without losing volatiles due to its extreme volatility; additional filtration or centrifugation to remove solid residue is not necessary | Onerous operating conditions |
Conclusions
The establishment of vanguard biorefineries for bioeconomy and circular economy urgently demands innovation in green and sustainable separation for the recovery of natural products from agro-industrial by-products all over the world. Sustainable separation includes the idea of integrated valorization not only in an economic sense, but also strengthens other social and environmental dimensions, from small to large producing scales. According to the literature over the last decade, the number of studies in this field has grown significantly in recent years. New approaches incorporating holistic extraction and/or purification techniques, also integrating systemic chemical transformation through the design and use of renewable materials and optimized processes should combine the best green analytical figures of merit with online evaluation of the whole production chain. These approaches should generate healthier and more efficient products, methods and processes at an affordable and fair cost.
Overall, solvent processing and its modification towards the enhancement of mass transfer to remove the compounds of interest from selected waste have been widely used (25%), also on industrial scales. Alternative extraction or purification methods have shown increasingly more applications, such as for microwave, ultrasonication and supercritical fluid processing. It was shown that a wide range of natural products and their derivatives are used mainly in food (as dyes, aromas, flavors) in medicines or green formulations in agriculture. According to the data available, one paradigmatic case largely studied is the valorization of citrus waste, representing more than 10% of all residues considered in the research papers.
Moreover, an emergent challenging topic is to evaluate biorefinery processing alternatives, i.e., sustainability assessment tools, for example LCA, which include parameters such as feedstock supply (to verify the suitability and adequacy of a potential biomass feedstock for the separation or transformation treatment), process performance (to assess the input–output balance of material and energy flows) and bio-based chemical production [144]. Therefore, the decision about the best separation approach takes into account various fundamental aspects and is based on green and sustainable assessment tools, considering the type of agro-industrial waste (e.g., quantity, periodicity, chemical variability, water amount, distance to the processing unit), the natural target products (chemical quality, purity, humidity, costs etc.) and available technologies.
Using sustainability indicators and tools will be increasingly demanded in this field, contributing to the greenness or sustainability of the whole processing system. The development of a sustainable separation method which provides better recovery efficiency will not only add value to the agro-industrial waste, reducing the overall manufacturing costs and the use of synthetic chemicals, but will also aggregate value to the whole production chain, including its final products. The emergence of bio-based industries is changing the current status of the producing systems, contributing to the current biomass residual losses. Based on the literature, the scenario for future research and innovation in green and sustainable separation for the recovery of agro-industrial waste is truly beginning, bringing together various areas and sectors towards more efficient and circular systems.
Acknowledgements
The authors wish to thank FAPESP (13/12052-5, 14/50827-1), Capes (2032/2014-07), EPSRC-UK (EP/M028763/1) and Mateus Segatto.
Footnotes
This article is part of the Topical Collection “Chemistry and Chemical Technologies in Waste Valorization”; edited by Carol Sze Ki Lin.
References
- 1.Liu J, Mooney H, Hull V, Davis SJ, Gaskell J, Hertel T, Lubchenco J, Seto KC, Gleick P, Kremen C, Li S. Systems integration for global sustainability. Science. 2015;347:12588320–12588329. doi: 10.1126/science.1258832. [DOI] [PubMed] [Google Scholar]
- 2.Kiser B. Circular economy: getting the circulation going. Nature. 2016;53:443–446. doi: 10.1038/531443a. [DOI] [PubMed] [Google Scholar]
- 3.Food and Agriculture Organization of the United Nations. World hunger on the rise again, reversing years of progress. http://www.fao.org/news/story/en/item/902489/icode/. Accessed 03 Jul 2017
- 4.Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C. Food security: the challenge of feeding 9 billion people. Science. 2010;327(5967):812–818. doi: 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]
- 5.United Nations (2015) World Population prospects: the 2015 revision and key findings and advance tables. United Nations, New York. https://esa.un.org/unpd/wpp/publications/files/key_findings_wpp_2015.pdf. Accessed 20 Oct 2016
- 6.Xia H, Houghton JA, Clark JH, Matharu AS. Potential utilization of unavoidable food supply chain wastes–valorization of pea vine wastes. ACS Sustain Chem Eng. 2016;4(11):6002–6009. doi: 10.1021/acssuschemeng.6b01297. [DOI] [Google Scholar]
- 7.Aschemann-Witzel J. Waste not, want not, emit less. Science. 2016;2978(6284):408–409. doi: 10.1126/science.aaf2978. [DOI] [PubMed] [Google Scholar]
- 8.Perlatti B, Forim MR, Zuin VG. Green chemistry, sustainable agriculture and processing systems: a Brazilian overview. Chem Biol Technol Agric. 2014;1:5–9. doi: 10.1186/s40538-014-0005-1. [DOI] [Google Scholar]
- 9.Foster-Carneiro L, Berni MD, Dorileo IL, Rostagno MA. Biorefinery study of availability of agriculture residues and wastes for integrated biorefineries in Brazil. Resour Conserv Recycl. 2013;77:78–88. doi: 10.1016/j.resconrec.2013.05.007. [DOI] [Google Scholar]
- 10.Tuck CO, Perez E, Horvath IT, Sheldon RA, Poliakoff M. Valorization of biomass: deriving more value from waste. Science. 2012;337:695–699. doi: 10.1126/science.1218930. [DOI] [PubMed] [Google Scholar]
- 11.Lin CSK, Pfaltzgraff LA, Herrero-Davila L, Mubofu EB, Abderrahim S, Clark JH, Koutinas AA, Kopsahelis N, Stamatelatou K, Dickson F, Thankappan S, Mohamed Z, Brocklesby R, Luque R. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ Sci. 2013;6(2):426–464. doi: 10.1039/c2ee23440h. [DOI] [Google Scholar]
- 12.Lin CSK, Koutinas AA, Stamatelatou K, Mubofu EB, Matharu AS, Kopsahelis N, Pfaltzgraff LA, Clark JH, Papanikolaou S, Kwan TH, Luque R. Current and future trends in food waste valorization for the production of chemicals, materials and fuels: a global perspective. Bioprod Biorefin Biofuels. 2014;8(5):686–715. doi: 10.1002/bbb.1506. [DOI] [Google Scholar]
- 13.Lin CSK, Luque R. Renewable resources and biorefineries. Cambridge: Royal Society of Chemistry; 2014. [Google Scholar]
- 14.Papargyropoulou E, Lozano R, Steinberger J, Wright N, Zb Ujang. The food waste hierarchy as a framework for the management of food surplus and food waste. J Clean Prod. 2014;76:106–115. doi: 10.1016/j.jclepro.2014.04.020. [DOI] [Google Scholar]
- 15.European Parliament Council. Directive 2008/1/EC of the European Parliament and of the council of 15 January 2008 concerning integrated pollution prevention and control
- 16.Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, Frederick WJ, Hallett JP, Leak DJ, Liotta CL, Mielenz JR, Murphy R, Templer R, Tschaplinski T. The path forward for biofuels and biomaterials. Science. 2006;311:484–489. doi: 10.1126/science.1114736. [DOI] [PubMed] [Google Scholar]
- 17.Mohan SV, Nikhil GN, Chiranjeevi P, Reddy CN, Rohit MV, Kumar AN, Sarkar O. Waste biorefinery models towards sustainable circular bioeconomy: critical review and future perspectives. Bioresour Technol. 2016;215:2–12. doi: 10.1016/j.biortech.2016.03.130. [DOI] [PubMed] [Google Scholar]
- 18.Clark JH, Deswarte F. Introduction to chemicals from biomass. 2. Chichester: John Wiley and Sons Ltd.; 2015. [Google Scholar]
- 19.Zuin VG. Circularity in green chemical products, processes and services: innovative routes based on integrated eco-design and solution systems. Curr Opin Green Sustain Chem. 2016;2:40–44. doi: 10.1016/j.cogsc.2016.09.008. [DOI] [Google Scholar]
- 20.Anastas PT. Green chemistry and the role of analytical methodology development. Crit Rev Anal Chem. 1999;29:167–175. doi: 10.1080/10408349891199356. [DOI] [Google Scholar]
- 21.Clark JH, Deswarte FEI, Farmer TJ. The integration of green chemistry into future biorefineries. Biofuels Bioprod Biorefin. 2009;3:72–90. doi: 10.1002/bbb.119. [DOI] [Google Scholar]
- 22.Chemat F, Avian M, Cravotto G. Green extraction of natural products: concept and principles. Int J Mol Sci. 2012;13:8615–8627. doi: 10.3390/ijms13078615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark JH, Budarin V, Deswarte FEI, Hardy JJE, Kerton FM, Hunt AJ, Luque R, Macquarrie DJ, Milkowski K, Rodriguez A, Samuel O, Tavener SJ, White RJ, Wilson AJ. Green chemistry and the biorefinery: a partnership for a sustainable future. Green Chem. 2006;8:853–860. doi: 10.1039/b604483m. [DOI] [Google Scholar]
- 24.Sustainable Chemistry (2017) The Organisation for Economic Co-operation and Development (OECD). Paris. http://www.oecd.org/chemicalsafety/risk-management/sustainablechemistry.htm Accessed 07 Jul 2017
- 25.Sustainable chemistry (2017) Umweltbundesamt, Dessau-Roßlau Germany. http://www.umweltbundesamt.de/en/topics/chemicals/chemicals-management/sustainable-chemistry#textpart-1. Accessed 07 Jul 2017
- 26.Campbell SD. The planner’s triangle revisited: sustainability And the evolution of a planning ideal that can’t stand still. JAPA. 2016;82(4):388–397. [Google Scholar]
- 27.Hanson JR. Natural products: the secondary metabolites. Cambridge: RSC; 2003. [Google Scholar]
- 28.Armenta S, Garrigues S, de la Guardia M. The role of green extraction techniques in Green Analytical Chemistry. TrAC Trends Anal Chem. 2015;71:2–8. doi: 10.1016/j.trac.2014.12.011. [DOI] [Google Scholar]
- 29.Zuin VG. Green sample preparation of complex matrices: towards sustainable separations of organic compounds based on the biorefinery concept. Pure Appl Chem. 2016;88:29–36. doi: 10.1515/pac-2015-0904. [DOI] [Google Scholar]
- 30.Zuin VG, Budarin VL, De Bruyn M, Shuttleworth PS, Hunt AJ, Pluciennik C, Borisova A, Dodson JR, Parker H, Clark J. Polysaccharide-derived mesoporous materials (Starbon®) for sustainable separation of complex mixtures. Faraday Discuss. 2017;196:1–9. doi: 10.1039/C7FD90007D. [DOI] [PubMed] [Google Scholar]
- 31.Jessop PG. The use of auxiliary substances (e.g. solvents, separation agents) should be made unnecessary wherever possible and innocuous when used. Green Chem. 2016;18:2577–2578. doi: 10.1039/C6GC90039A. [DOI] [Google Scholar]
- 32.Kiss AA, Lange J-P, Schuur B, Brilman DWF, van der Ham AGJ, Kersten SRA. Separation technology: making a difference in biorefineries. Biomass Bioenergy. 2016;95:296–309. doi: 10.1016/j.biombioe.2016.05.021. [DOI] [Google Scholar]
- 33.Long Z, Budarin V, Jiajun F, Sloan R, MacQuarrie DJ. Efficient method of lignin isolation using microwave-assisted acidolysis and characterisation of the residual lignin. ACS Sustain Chem Eng. 2017;5:3768–3774. doi: 10.1021/acssuschemeng.7b02196. [DOI] [Google Scholar]
- 34.Abou-Shehada S, Clark JH, Paggola G, Sherwood J. Tunable solvents: shades of green. Chem Eng Process. 2016;99:88–96. doi: 10.1016/j.cep.2015.07.005. [DOI] [Google Scholar]
- 35.Lachos-Perez D, Brown AB, Mudhoo A, Martinez J, Timko MT, Rostagno MA, Forster-Carneiro T. Applications of subcritical and supercritical water conditions for extraction, hydrolysis, gasification, and carbonization of biomass: a critical review. Biofuels. 2017;14:611–626. [Google Scholar]
- 36.Filly A, Fabiano-Tixier AS, Louis C, Fernandez X, Chemat F. Water as a green solvent combined with different techniques for extraction of essential oil from lavender flowers. C R Chim. 2016;19:707–717. doi: 10.1016/j.crci.2016.01.018. [DOI] [Google Scholar]
- 37.Chemat F, Strube J. Green extraction of natural products. Weinheim: Wiley; 2015. [Google Scholar]
- 38.Costa ES, Perlatti B, Silva EM, Matos AP, Silva MFGF, Fernandes JB, Zuin VG, Silva CMP, Forim MR. Use of lignins from sugarcane bagasse for assembling microparticles loaded with Azadirachta indica extracts for use as neem-based organic insecticides. J Braz Chem Soc. 2017;28:126–135. [Google Scholar]
- 39.Guardia M, Garrides S. Handbook of green analytical chemistry. New York: Wiley; 2012. [Google Scholar]
- 40.Braga EM, Seabra IJ, Dias AMA, Sousa HC. Recent trends and perspectives for the extraction of natural products. In: Rostagno M, Prado J, editors. Natural product extraction. Cambridge: RSC; 2013. [Google Scholar]
- 41.Both S, Strube J, Cravatto G. Mass transfer enhancement for solid–liquid extractions. In: Chemat F, Strube J, editors. Green extraction of natural products: theory and practice. Weinheim: Wiley; 2015. [Google Scholar]
- 42.Kumar K, Yadav AN, Kumar V, Vyas P, Dhaliwal HS. Food waste: a potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour Bioprocess. 2017;4(1):18. doi: 10.1186/s40643-017-0148-6. [DOI] [Google Scholar]
- 43.Regulation (EC) No 1907/2006 (2006) European Parliament and of the council concerning the registration, evaluation, authorisation and restriction of chemicals (REACH), European Commission, Luxembourg. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02006R1907-20140410 Accessed 21 Jul 2017
- 44.Chemat F, Vian MA. Alternative solvents for natural products extraction. Heidelberg: Springer; 2014. [Google Scholar]
- 45.Ibanez E, Cifuentes A. Green extraction techniques: principles, advances and applications. Amsterdam: Elsevier; 2017. [Google Scholar]
- 46.Güçlü Üstündağ Ö, Erşan S, Özcan E, Özan G, Kayra N, Ekinci FY. Black tea processing waste as a source of antioxidant and antimicrobial phenolic compounds. Eur Food Res Technol. 2016;242:1523–1532. doi: 10.1007/s00217-016-2653-9. [DOI] [Google Scholar]
- 47.Toh PY, Leong FS, Chang SK, Khoo HE, Yim HS. Optimization of extraction parameters on the antioxidant properties of banana waste. Acta Sci Pol Technol Aliment. 2016;15:65–78. doi: 10.17306/J.AFS.2016.1.7. [DOI] [PubMed] [Google Scholar]
- 48.Harrison JE, Oomah BD, Diarra MS, Ibarra-Alvarado C. Bioactivities of pilot-scale extracted cranberry juice and pomace. J Food Process Preserv. 2013;37:356–365. doi: 10.1111/j.1745-4549.2011.00655.x. [DOI] [Google Scholar]
- 49.Fernández-Agulló A, Pereira E, Freire MS, Valentao P, Andrade PB, González-Álvarez J, Pereira JA. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind Crops Prod. 2013;42:126–132. doi: 10.1016/j.indcrop.2012.05.021. [DOI] [Google Scholar]
- 50.Bravo J, Monente C, Juániz I, De Peña MP, Cid C. Influence of extraction process on antioxidant capacity of spent coffee. Food Res Int. 2013;50:610–616. doi: 10.1016/j.foodres.2011.04.026. [DOI] [Google Scholar]
- 51.Clodoveo ML, Dipalmo T, Rizzello CG, Corbo F, Crupi P. Emerging technology to develop novel red winemaking practices: an overview. Innov Food Sci Emerg Technol. 2016;38:41–56. doi: 10.1016/j.ifset.2016.08.020. [DOI] [Google Scholar]
- 52.Liu Z, Zu Y, Yang L. A process to preserve valuable compounds and acquire essential oils from pomelo flavedo using a microwave irradiation treatment. Food Chem. 2017;224:172–180. doi: 10.1016/j.foodchem.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 53.Fidalgo A, Ciriminna R, Carnaroglio D, Tamburino A, Cravotto G, Grillo G, Ilharco L, Pagliaro M. Eco-friendly extraction of pectin and essential oils from orange and lemon peels. ACS Sustain Chem Eng. 2016;4:2243–2251. doi: 10.1021/acssuschemeng.5b01716. [DOI] [Google Scholar]
- 54.Zainab H, Nurfatirah N, Norfaezah A, Othman H. Green bio-oil extraction for oil crops. IOP Conf Ser Mater Sci Eng. 2016;133:12053. doi: 10.1088/1757-899X/133/1/012053. [DOI] [Google Scholar]
- 55.González-Rivera J, Spepi A, Ferrari C, Duce C, Longo I, Falconieri D, Piras A, Tinè MR. Novel configurations for a citrus waste based biorefinery: from solventless to simultaneous ultrasound and microwave-assisted extraction. Green Chem. 2016;18:6482–6492. doi: 10.1039/C6GC02200F. [DOI] [Google Scholar]
- 56.Jacotet-Navarro M, Rombaut N, Deslis S, Fabiano-Tixier AS, Pierre FX, Bily A, Chemat F. Towards a “dry” bio-refinery without solvents or added water using microwaves and ultrasound for total valorization of fruit and vegetable by-products. Green Chem. 2016;18:3106–3115. doi: 10.1039/C5GC02542G. [DOI] [Google Scholar]
- 57.Boukroufa M, Boutekedjiret C, Petigny L, Rakotomanomana N, Chemat F. Bio-refinery of orange peels waste: a new concept based on integrated green and solvent free extraction processes using ultrasound and microwave techniques to obtain essential oil, polyphenols and pectin. Ultrason Sonochem. 2015;24:72–79. doi: 10.1016/j.ultsonch.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 58.Alexandru L, Binello A, Mantegna S, Boffa L, Chemat F, Cravotto G. Efficient green extraction of polyphenols from post-harvested agro-industry vegetal sources in Piedmont. C R Chim. 2014;17:212–217. doi: 10.1016/j.crci.2013.09.012. [DOI] [Google Scholar]
- 59.Attard TM, Watterson B, Budarin VL, Clark JH, Hunt AJ. Microwave-assisted extraction as an important technology for valorising orange waste. N J Chem. 2014;38:2278–2283. doi: 10.1039/C4NJ00043A. [DOI] [Google Scholar]
- 60.Ahangari B, Sargolzaei J. Extraction of lipids from spent coffee grounds using organic solvents and supercritical carbon dioxide. J Food Process Preserv. 2013;37:1014–1021. doi: 10.1111/j.1745-4549.2012.00757.x. [DOI] [Google Scholar]
- 61.Passos CP, Coimbra MA. Microwave superheated water extraction of polysaccharides from spent coffee grounds. Carbohydr Polym. 2013;94:626–633. doi: 10.1016/j.carbpol.2013.01.088. [DOI] [PubMed] [Google Scholar]
- 62.Farhat A, Fabiano-Tixier A-S, El Maataoui M, Maingonnat JF, Romdhane M, Chemat F. Microwave steam diffusion for extraction of essential oil from orange peel: kinetic data, extract’s global yield and mechanism. Food Chem. 2011;125:255–261. doi: 10.1016/j.foodchem.2010.07.110. [DOI] [Google Scholar]
- 63.Liazid A, Guerrero RF, Cantos E, Palma M, Barroso CG. Microwave-assisted extraction of anthocyanins from grape skins. Food Chem. 2011;124:1238–1243. doi: 10.1016/j.foodchem.2010.07.053. [DOI] [Google Scholar]
- 64.Tsubaki S, Sakamoto M, Azuma J. Microwave-assisted extraction of phenolic compounds from tea residues under autohydrolytic conditions. Food Chem. 2010;123:1255–1258. doi: 10.1016/j.foodchem.2010.05.088. [DOI] [Google Scholar]
- 65.Bousbia N, Vian MA, Ferhat MA, Meklati BY, Chemat F. A new process for extraction of essential oil from Citrus peels: microwave hydrodiffusion and gravity. J Food Eng. 2009;90:409–413. doi: 10.1016/j.jfoodeng.2008.06.034. [DOI] [Google Scholar]
- 66.Tsubaki S, Iida H, Sakamoto M, Azuma J. Microwave heating of tea residue yields polysaccharides, polyphenols, and plant biopolyester. J Agric Food Chem. 2008;56:11293–11299. doi: 10.1021/jf802253s. [DOI] [PubMed] [Google Scholar]
- 67.Balu AM, Budarin V, Shuttleworth PS, Pfaltzgraff LA, Waldron K, Luque R, Clark JH. Valorisation of orange peel residues: waste to biochemicals and nanoporous materials. Chemsuschem. 2012;5:1694–1697. doi: 10.1002/cssc.201200381. [DOI] [PubMed] [Google Scholar]
- 68.Chemat F, Cravotto G. Microwave-assisted extraction for bioactive compounds: theory and practice. Heidelberg: Springer; 2013. [Google Scholar]
- 69.Preece KE, Hooshyar N, Krijgsman AJ, Fryer PJ, Zuidam NJ. Pilot-scale ultrasound-assisted extraction of protein from soybean processing materials. J Food Eng. 2017;206:1–12. doi: 10.1016/j.jfoodeng.2017.02.002. [DOI] [Google Scholar]
- 70.Chemat F, Huma Z, Khan MK. Applications of ultrasound in food technology: processing, preservation and extraction. Ultrason Sonochem. 2011;18:813–835. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 71.Bibi I, Sultan A, Kamal S, Nouren S, Safa Y, Jalani K, Sultan M, Atta S, Rehman F. Extraction and quantification of phenolic compounds from Prunus armeniaca seed and their role in biotransformation of xenobiotic compounds. Korean J Chem Eng. 2017;34(2):392–399. doi: 10.1007/s11814-016-0275-3. [DOI] [Google Scholar]
- 72.Goula AM, Ververi M, Adamopoulou A, Kaderides K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason Sonochem. 2017;34:821–830. doi: 10.1016/j.ultsonch.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 73.Abdullah MA, Nazir MS, Raza MR, Wahjoedi BA, Yussof AW. Autoclave and ultra-sonication treatments of oil palm empty fruit bunch fibers for cellulose extraction and its polypropylene composite properties. J Clean Prod. 2016;126:686–697. doi: 10.1016/j.jclepro.2016.03.107. [DOI] [Google Scholar]
- 74.Trasanidou D, Apostolakis A, Makris DP. Development of a green process for the preparation of antioxidant and pigment-enriched extracts from winery solid wastes using response surface methodology and kinetics. Chem Eng Commun. 2016;203:1317–1325. doi: 10.1080/00986445.2016.1189416. [DOI] [Google Scholar]
- 75.Mouratoglou E, Malliou V, Makris DP. Novel glycerol-based natural eutectic mixtures and their efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from agri-food waste biomass. Waste Biomass Valoriz. 2016;7:1377–1387. doi: 10.1007/s12649-016-9539-8. [DOI] [Google Scholar]
- 76.Paleologou I, Vasiliou A, Grigorakis S, Makris DP. Optimisation of a green ultrasound-assisted extraction process for potato peel (Solanum tuberosum) polyphenols using bio-solvents and response surface methodology. Biomass Convers Biorefin. 2016;6:289–299. doi: 10.1007/s13399-015-0181-7. [DOI] [Google Scholar]
- 77.Li A-N, Li S, Xu D-P, Xu X-R, Chen Y-M, Ling W-H, Chen F, Li H-B. Optimization of ultrasound-assisted extraction of lycopene from papaya processing waste by response surface methodology. Food Anal Methods. 2015;8:1207–1214. doi: 10.1007/s12161-014-9955-y. [DOI] [Google Scholar]
- 78.Geerkens CH, Matejka AE, Carle R, Schweiggert RM. Development and validation of an HPLC method for the determination of alk(en)ylresorcinols using rapid ultrasound-assisted extraction of mango peels and rye grains. Food Chem. 2015;169:261–269. doi: 10.1016/j.foodchem.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 79.Punzi R, Paradiso A, Fasciano C, Trani A, Faccia M, de Pinto MC, Gambacorta G. Phenols and antioxidant activity in vitro and in vivo of aqueous extracts obtained by ultrasound-assisted extraction from artichoke by-products. Nat Prod Commun. 2014;9:1315–1318. [PubMed] [Google Scholar]
- 80.Guardia M, Garrides S. Challenges in green analytical chemistry. Cambridge: RSC; 2011. [Google Scholar]
- 81.Khaw K-Y, Parat M-O, Shaw PN, Falconer JR. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: a review. Molecules. 2017;22:1186–1208. doi: 10.3390/molecules22071186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kehili M, Schmidt LM, Reynolds W, Zammel A, Zetzl C, Smirnova I, Allouche N, Sayadi S. Biorefinery cascade processing for creating added value on tomato industrial by-products from Tunisia. Biotechnol Biofuels. 2016;9:261. doi: 10.1186/s13068-016-0676-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pavlić B, Ðurković AV, Vladić J, Gavarić A, Zeković Z, Tepić A, Vidović S. Extraction of minor compounds (chlorophylls and carotenoids) from yarrow-rose hip mixtures by traditional versus green technique. J Food Process Eng. 2016;39:418–424. doi: 10.1111/jfpe.12242. [DOI] [Google Scholar]
- 84.Gumba RE, Saallah S, Misson M, Ongkudon CM, Anton A. Green biodiesel production: a review on feedstock, catalyst, monolithic reactor, and supercritical fluid technology. Biofuel Res J. 2016;3:431–447. doi: 10.18331/BRJ2016.3.3.3. [DOI] [Google Scholar]
- 85.Jokić S, Bijuk M, Aladić K, Bilić M, Molnar M. Optimisation of supercritical CO2 extraction of grape seed oil using response surface methodology. Int J Food Sci Technol. 2016;51:403–410. doi: 10.1111/ijfs.12986. [DOI] [Google Scholar]
- 86.Cavalcanti RN, Albuquerque CLC, Meireles MAA. Supercritical CO2 extraction of cupuassu butter from defatted seed residue: experimental data, mathematical modeling and cost of manufacturing. Food Bioprod Process. 2016;97:48–62. doi: 10.1016/j.fbp.2015.10.004. [DOI] [Google Scholar]
- 87.Marto J, Gouveia LF, Chiari BG, Paiva A, Isaac V, Pinto P, Simões P, Almeida AJ, Ribeiro HM. The green generation of sunscreens: using coffee industrial sub-products. Ind Crops Prod. 2016;80:93–100. doi: 10.1016/j.indcrop.2015.11.033. [DOI] [Google Scholar]
- 88.Oliveira DA, Angonese M, Gomes C, Ferreira SRS. Valorization of passion fruit (Passiflora edulis sp.) by-products: sustainable recovery and biological activities. J Supercrit Fluids. 2016;111:55–62. doi: 10.1016/j.supflu.2016.01.010. [DOI] [Google Scholar]
- 89.Viganó J, da Machado APF, Martínez J. Sub- and supercritical fluid technology applied to food waste processing. J Supercrit Fluids. 2015;96:272–286. doi: 10.1016/j.supflu.2014.09.026. [DOI] [Google Scholar]
- 90.Lee KT, Lim S, Pang YL, Ong HC, Chong WT. Integration of reactive extraction with supercritical fluids for process intensification of biodiesel production: prospects and recent advances. Prog Energy Combust Sci. 2014;45:54–78. doi: 10.1016/j.pecs.2014.07.001. [DOI] [Google Scholar]
- 91.Ribeiro H, Marto J, Raposo S, Agapito M, Isaac V, Chiari BG, Lisboa PF, Paiva A, Barreiros S, Simões P. From coffee industry waste materials to skin-friendly products with improved skin fat levels. Eur J Lipid Sci Technol. 2013;115:330–336. doi: 10.1002/ejlt.201200239. [DOI] [Google Scholar]
- 92.Xynos N, Papaefstathiou G, Psychis M, Argyropoulou A, Aligiannis N, Skaltsounis AL. Development of a green extraction procedure with super/subcritical fluids to produce extracts enriched in oleuropein from olive leaves. J Supercrit Fluids. 2012;67:89–93. doi: 10.1016/j.supflu.2012.03.014. [DOI] [Google Scholar]
- 93.Co M, Fagerlund A, Engman L, Sunnerheim K, Sjöberg PJ, Turner C. Extraction of antioxidants from Spruce (Picea abies) bark using eco-friendly solvents. Phytochem Anal. 2012;23:1–11. doi: 10.1002/pca.1316. [DOI] [PubMed] [Google Scholar]
- 94.Tello J, Viguera M, Calvo L. Extraction of caffeine from Robusta coffee (Coffea canephora var. Robusta) husks using supercritical carbon dioxide. J Supercrit Fluids. 2011;59:53–60. doi: 10.1016/j.supflu.2011.07.018. [DOI] [Google Scholar]
- 95.Budarin VL, Shuttleworth PS, Dodson JR, Hunt AJ, Lanigan B, Marriott R, Milkowski KJ, Wilson AJ, Breeden SW, Fan J, Sin EHK, Clark JH. Use of green chemical technologies in an integrated biorefinery. Energy Environ Sci. 2011;4:471–479. doi: 10.1039/C0EE00184H. [DOI] [Google Scholar]
- 96.İçen H, Gürü M. Effect of ethanol content on supercritical carbon dioxide extraction of caffeine from tea stalk and fiber wastes. J Supercrit Fluids. 2010;55:156–160. doi: 10.1016/j.supflu.2010.07.009. [DOI] [Google Scholar]
- 97.Seabra IJ, Braga MEM, Batista MT, de Sousa HC. Effect of solvent (CO2/ethanol/H2O) on the fractionated enhanced solvent extraction of anthocyanins from elderberry pomace. J Supercrit Fluids. 2010;54:145–152. doi: 10.1016/j.supflu.2010.05.001. [DOI] [Google Scholar]
- 98.Wu H, Hu W, Zhang Y, Huang L, Zhang J, Tan S, Cai X, Xal Liao. Effect of oil extraction on properties of spent coffee ground–plastic composites. J Mater Sci. 2016;51:10205–10214. doi: 10.1007/s10853-016-0248-2. [DOI] [Google Scholar]
- 99.İçen H, Gürü M. Extraction of caffeine from tea stalk and fiber wastes using supercritical carbon dioxide. J Supercrit Fluids. 2009;50:225–228. doi: 10.1016/j.supflu.2009.06.014. [DOI] [Google Scholar]
- 100.Tian Y, Wang Y, Ma Y, Zhu P, He J, Lei J. Optimization of subcritical water extraction of resveratrol from grape seeds by response surface methodology. Appl Sci. 2017;7(4):321. doi: 10.3390/app7040321. [DOI] [Google Scholar]
- 101.Ahmadian-Kouchaksaraie Z, Niazmand R, Najafi MN. Optimization of the subcritical water extraction of phenolic antioxidants from Crocus sativus petals of saffron industry residues: Box–Behnken design and principal component analysis. Innov Food Sci Emerg Technol. 2016;36:234–244. doi: 10.1016/j.ifset.2016.07.005. [DOI] [Google Scholar]
- 102.Abdelmoez W, Ashour E, Naguib SM. A review on green trend for oil extraction using subcritical water technology and biodiesel production. J Oleo Sci. 2015;64:467–478. doi: 10.5650/jos.ess14269. [DOI] [PubMed] [Google Scholar]
- 103.Ravber M, Knez Ž, Škerget M. Simultaneous extraction of oil- and water-soluble phase from sunflower seeds with subcritical water. Food Chem. 2015;166:316–323. doi: 10.1016/j.foodchem.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 104.Shalmashi A, Abedi M, Golmohammad F, Eikani MH. Isolation of caffeine from tea waste using subcritical water extraction. J Food Process Eng. 2009;33:701–711. [Google Scholar]
- 105.Pourali O, Asghari FS, Yoshida H. Production of phenolic compounds from rice bran biomass under subcritical water conditions. Chem Eng J. 2010;160:259–266. doi: 10.1016/j.cej.2010.02.057. [DOI] [Google Scholar]
- 106.Pourali O, Asghari FS, Yoshida H. Sub-critical water treatment of rice bran to produce valuable materials. Food Chem. 2009;115:1–7. doi: 10.1016/j.foodchem.2008.11.099. [DOI] [Google Scholar]
- 107.Liew SQ, Chin NL, Yusof YA, Sowndhararajan K. Comparison of acidic and enzymatic pectin extraction from passion fruit peels and its gel properties. J Food Process Eng. 2016;39:501–511. doi: 10.1111/jfpe.12243. [DOI] [Google Scholar]
- 108.Nath P, Kaur C, Rudra SG, Varghese E. Enzyme-assisted extraction of carotenoid-rich extract from red capsicum (Capsicum annuum) Agric Res. 2016;5:193–204. doi: 10.1007/s40003-015-0201-7. [DOI] [Google Scholar]
- 109.Patil PD, Rao CR, Wasif AI, Anekar SV, Nagla JR. Cashew-nut husk natural dye extraction using Taguchi optimization: green chemistry approach. J Sci Ind Res (India) 2015;74:512–517. [Google Scholar]
- 110.Madeira JV, Macedo GA. Simultaneous extraction and biotransformation process to obtain high bioactivity phenolic compounds from Brazilian citrus residues. Biotechnol Prog. 2015;31:1273–1279. doi: 10.1002/btpr.2126. [DOI] [PubMed] [Google Scholar]
- 111.Mushtaq M, Sultana B, Bhatti HN, Asgher M. Optimization of enzyme-assisted revalorization of sweet lime (Citrus limetta Risso) peel into phenolic antioxidants. BioResources. 2014;9:6153–6165. doi: 10.15376/biores.9.4.6153-6165. [DOI] [Google Scholar]
- 112.Cuccolini S, Aldini A, Visai L, Daglia M, Ferrari D. Environmentally friendly lycopene purification from tomato peel waste: enzymatic-assisted aqueous extraction. J Agric Food Chem. 2013;61:1646–1651. doi: 10.1021/jf3027815. [DOI] [PubMed] [Google Scholar]
- 113.Zykwinska A, Boiffard MH, Kontkanen H, Buchert J, Thibault JF, Bonnin E. Extraction of green labeled pectins and pectic oligosaccharides from plant byproducts. J Agric Food Chem. 2008;56:8926–8935. doi: 10.1021/jf801705a. [DOI] [PubMed] [Google Scholar]
- 114.Zarevúcka M, Wimmer Z. Plant products for pharmacology: application of enzymes in their transformations. Int J Mol Sci. 2008;9:2447–2473. doi: 10.3390/ijms9122447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Manzato L, Rabelo LCA, de Souza SM, da Silva CG, Sanches EA, Rabelo D, Mariuba LAM, Simonsen J. New approach for extraction of cellulose from tucumã’s endocarp and its structural characterization. J Mol Struct. 2017;1143:229–234. doi: 10.1016/j.molstruc.2017.04.088. [DOI] [Google Scholar]
- 116.Dhamole PB, Chavan S, Patil RG, Feng H, Bule M, Kinninge P. Extraction of p-coumaric acid from agricultural residues and separation using “sugaring out”. Korean J Chem Eng. 2016;33:1860–1864. doi: 10.1007/s11814-016-0020-y. [DOI] [Google Scholar]
- 117.Zhang C, Van Krimpen MM, Sanders JPM, Bruins ME. Improving yield and composition of protein concentrates from green tea residue in an agri-food supply chain: effect of pre-treatment. Food Bioprod Process. 2016;100:92–101. doi: 10.1016/j.fbp.2016.06.001. [DOI] [Google Scholar]
- 118.Rahman MM, Ho K, Netravali AN. Bio-based polymeric resin from agricultural waste, neem (Azadirachta indica) seed cake, for green composites. J Appl Polym Sci. 2015;132:1–11. doi: 10.1002/app.41291. [DOI] [Google Scholar]
- 119.Zhang C, Sanders JPM, Xiao TT, Bruins ME. How does alkali aid protein extraction in green tea leaf residue: a basis for integrated biorefinery of leaves. PLoS One. 2015;10:e0133046. doi: 10.1371/journal.pone.0133046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mudassar HR, Melin K, Koskinen J. Production of carboxylic acids from alkaline pretreatment byproduct of softwood. Cellul Chem Technol. 2014;48:835–842. [Google Scholar]
- 121.Diamanti AC, Igoumenidis PE, Mourtzinos I, Yannakopoulou K, Karathanos VT. Green extraction of polyphenols from whole pomegranate fruit using cyclodextrins. Food Chem. 2017;214:61–66. doi: 10.1016/j.foodchem.2016.07.072. [DOI] [PubMed] [Google Scholar]
- 122.Das AM, Hazarika MP, Goswami M, Yadav A, Khound P. Extraction of cellulose from agricultural waste using Montmorillonite K-10/LiOH and its conversion to renewable energy: biofuel by using Myrothecium gramineum. Carbohydr Polym. 2016;141:20–27. doi: 10.1016/j.carbpol.2015.12.070. [DOI] [PubMed] [Google Scholar]
- 123.Cabrera MN, Arrosbide MF, Franzoni P, Cassella N. Integrated forest biorefineries: green liquor extraction in eucalyptus wood prior to kraft pulping. Biomass Convers Biorefin. 2016;6:465–474. doi: 10.1007/s13399-016-0203-0. [DOI] [Google Scholar]
- 124.Xavier L, Freire MS, Vidal-Tato I, González-Álvarez J. Aqueous two-phase systems for the extraction of phenolic compounds from eucalyptus (Eucalyptus globulus) wood industrial wastes. J Chem Technol Biotechnol. 2014;89:1772–1778. doi: 10.1002/jctb.4260. [DOI] [Google Scholar]
- 125.Elksibi I, Haddar W, Ticha MB, Gharbi R, Mhenni MF. Development and optimisation of a non conventional extraction process of natural dye from olive solid waste using response surface methodology (RSM) Food Chem. 2014;161:345–352. doi: 10.1016/j.foodchem.2014.03.108. [DOI] [PubMed] [Google Scholar]
- 126.Avinc O, Celik A, Gedik G, Yavas A. Natural dye extraction from waste barks of Turkish red pine (Pinus brutia Ten.) timber and eco-friendly natural dyeing of various textile fibers. Fibers Polym. 2013;14:866–873. doi: 10.1007/s12221-013-0866-0. [DOI] [Google Scholar]
- 127.Sun-Waterhouse D, Wang W, Waterhouse GIN, Wadhwa SS. Utilisation potential of feijoa fruit wastes as ingredients for functional foods. Food Bioprocess Technol. 2013;6:3441–3455. doi: 10.1007/s11947-012-0978-3. [DOI] [Google Scholar]
- 128.Babkin VA, Malkov YA, Medvedeva EN, Trofimova NN, Ivanova NV. An eco-friendly technology for polysaccharide production from logging and sawing waste. Russ J Gen Chem. 2012;82:955–962. doi: 10.1134/S1070363212050283. [DOI] [Google Scholar]
- 129.Bertaud F, Tapin-Lingua S, Pizzi A, Navarrete P, Petit-Conil M. Development of green adhesives for fibreboard manufacturing, using tannins and lignin from pulp mill residues. Cellul Chem Technol. 2012;46:7–8. [Google Scholar]
- 130.Kim JH, Pan JH, Heo W, Lee H, Kwon EG, Lee H-G, Shin DH, Liu RH, Kim YJ. Effects of cellulase from Aspergillus niger and solvent pretreatments on the extractability of organic green tea waste. J Agric Food Chem. 2010;58:10747–10751. doi: 10.1021/jf102346p. [DOI] [PubMed] [Google Scholar]
- 131.Feng X, Song H, Dong B, Yang Y, Yao S. Sequential extraction and separation using ionic liquids for stilbene glycoside and anthraquinones in Polygonum multiflorum. J Mol Liq. 2017;241:27–36. doi: 10.1016/j.molliq.2017.06.012. [DOI] [Google Scholar]
- 132.Li D, Qian Y, Tian YJ, Yuan SM, Wei W, Wang G. Optimization of ionic liquid-assisted extraction of biflavonoids from Selaginella doederleinii and evaluation of its antioxidant and antitumor activity. Molecules. 2017;22(4):586. doi: 10.3390/molecules22040586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Larriba M, Omar S, Navarro P, García J, Rodríguez F, Gonzalez-Miquel M. Recovery of tyrosol from aqueous streams using hydrophobic ionic liquids: a first step towards developing sustainable processes for olive mill wastewater (OMW) management. RSC Adv. 2016;6:18751–18762. doi: 10.1039/C5RA26510J. [DOI] [Google Scholar]
- 134.Yao S, Yang Y-Y, Song H, Wang Y, Wan H-Q. Quantitative industrial analysis of lignocellulosic composition in typical agro-residues and extraction of inner hemicelluloses with ionic liquid. J Sci Ind Res (India) 2015;74:58–63. [Google Scholar]
- 135.Achinivu EC, Howard RM, Li G, Gracz H, Henderson WA. Lignin extraction from biomass with protic ionic liquids. Green Chem. 2014;16:1114–1119. doi: 10.1039/C3GC42306A. [DOI] [Google Scholar]
- 136.Wang T, Jiao J, Gai QY, Wang P, Guo N, Niu LL, Fu YJ. Enhanced and green extraction polyphenols and furanocoumarins from Fig (Ficus carica L.) leaves using deep eutectic solvents. J Pharm Biomed Anal. 2017;145:339–345. doi: 10.1016/j.jpba.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 137.Liu Y, Chen W, Xia Q, Guo B, Wang Q, Liu S, Liu Y, Li J, Yu H. Efficient cleavage of lignin-carbohydrate complexes and ultrafast extraction of lignin oligomers from wood biomass by microwave-assisted treatment with deep eutectic solvent. Chemsuschem. 2017;10(8):1692–1700. doi: 10.1002/cssc.201601795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jeong KM, Zhao J, Jin Y, Heo SR, Han SY, Yoo DE, Lee J. Highly efficient extraction of anthocyanins from grape skin using deep eutectic solvents as green and tunable media. Arch Pharm Res. 2015;38:2143–2152. doi: 10.1007/s12272-015-0678-4. [DOI] [PubMed] [Google Scholar]
- 139.Basset C, Kedidi S, Barakat A. Chemical—and solvent-free mechanophysical fractionation of biomass induced by tribo—electrostatic charging: separation of proteins and lignin. ACS Sustain Chem Eng. 2016;4:4166–4173. doi: 10.1021/acssuschemeng.6b00667. [DOI] [Google Scholar]
- 140.Oliveira CS, Gomes FS, Constant LS, Silva LF, Godoy RL, Tonon RV, Cabral LM. Integrated membrane separation processes aiming to concentrate and purify lycopene from watermelon juice. Innov Food Sci Emerg Technol. 2016;38:149–154. doi: 10.1016/j.ifset.2016.09.025. [DOI] [Google Scholar]
- 141.Ward DP, Cárdenas-Fernández M, Hewitson P, Ignatova S, Lye GJ. Centrifugal partition chromatography in a biorefinery context: separation of monosaccharides from hydrolysed sugar beet pulp. J Chromatogr A. 2015;1411:84–91. doi: 10.1016/j.chroma.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 142.Roselló-Soto E, Koubaa M, Moubarik A, Lopes RP, Saraiva JA, Boussetta N, Grimi N, Barba FJ. Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: non-conventional methods for the recovery of high-added value compounds. Trends Food Sci Technol. 2015;45:296–310. doi: 10.1016/j.tifs.2015.07.003. [DOI] [Google Scholar]
- 143.Wang L, Weller CL. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol. 2006;17(6):300–312. doi: 10.1016/j.tifs.2005.12.004. [DOI] [Google Scholar]
- 144.Parajuli R, Dalgaard T, Jørgensen U, Adamsen APS, Knudsen MT, Birkved M, Gylling M, Schjørring JK. Biorefining in the prevailing energy and materials crisis: a review of sustainable pathways for biorefinery value chains and sustainability assessment methodologies. Renew Sustain Energy Rev. 2015;43:244–263. doi: 10.1016/j.rser.2014.11.041. [DOI] [Google Scholar]
- 145.Koubaa M, Lepreux L, Barba FJ, Mhemdi H, Vorobiev E. Gas-assisted mechanical expression (GAME) for the selective recovery of lipophilic and hydrophilic compounds from olive kernel. J Clean Prod. 2017;166:387–394. doi: 10.1016/j.jclepro.2017.07.253. [DOI] [Google Scholar]
- 146.Castro-Puyana M, Marina ML, Plaza M. Water as green extraction solvent: principles and reasons for its use. Curr Opin Green Sustain Chem. 2017;5:31–36. doi: 10.1016/j.cogsc.2017.03.009. [DOI] [Google Scholar]
- 147.Kusuma HS, Mahfud M. Comparison of conventional and microwave-assisted distillation of essential oil from Pogostemon cablin leaves: analysis and modelling of heat and mass transfer. J Appl Res Med Aromat Plants. 2017;4:55–65. [Google Scholar]
- 148.Rosa R, Tassi L, Orteca G, Saladini M, Villa C, Veronesi P, Leonelli C, Ferrari E. Process intensification by experimental design application to microwave-assisted extraction of phenolic compounds from Juglans regia L. Food Anal Methods. 2017;10(3):575–586. doi: 10.1007/s12161-016-0624-1. [DOI] [Google Scholar]
- 149.Zhu Y, Song H, Zhang X, Chen C, Zhao S, Ge F, Liu D. Recovery of flavonoids from walnuts de-pellicle wastewater with macroporous resins and evaluation of antioxidant activities in vitro. J Food Process Eng. 2017;40:1. [Google Scholar]
- 150.Vardanega R, Carvalho PI, Albarelli JQ, Santos DT, Meireles MAA. Techno-economic evaluation of obtaining Brazilian ginseng extracts in potential production scenarios. Food Bioprod Process. 2017;101:45–55. doi: 10.1016/j.fbp.2016.10.010. [DOI] [Google Scholar]
- 151.Chemat F, Rombaut N, Sicaire AG, Meullemiestre A, Fabiano-Tixier AS, Abert-Vian M. Ultrasound-assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason Sonochem. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 152.Puga H, Alves RC, Costa AS, Vinha AF, Oliveira MBP. Multi-frequency multimode modulated technology as a clean, fast, and sustainable process to recover antioxidants from a coffee by-product. J Clean Prod. 2017;168:14–21. doi: 10.1016/j.jclepro.2017.08.231. [DOI] [Google Scholar]
- 153.Pavlić B, Naffati A, Hojan T, Vladić J, Zeković Z, Vidović S. Microwave-assisted extraction of wild apple fruit dust—production of polyphenol-rich extracts from filter tea factory by-products. J Food Process Eng. 2017;40:4. doi: 10.1111/jfpe.12508. [DOI] [Google Scholar]
- 154.Alañón ME, Alarcón M, Marchante L, Díaz-Maroto MC, Pérez-Coello MS. Extraction of natural flavorings with antioxidant capacity from cooperage by-products by green extraction procedure with subcritical fluids. Ind Crops Prod. 2017;103:222–232. doi: 10.1016/j.indcrop.2017.03.050. [DOI] [Google Scholar]
- 155.Arevalo-Gallegos A, Ahmad Z, Asgher M, Parra-Saldivar R, Iqbal HM. Lignocellulose: a sustainable material to produce value-added products with a zero waste approach—a review. Int J Biol Macromol. 2017;99:308–318. doi: 10.1016/j.ijbiomac.2017.02.097. [DOI] [PubMed] [Google Scholar]
- 156.Lozano-Sánchez J, Bendini A, Di Lecce G, Valli E, Gallina Toschi T, Segura-Carretero A. Macro and micro functional components of a spreadable olive by-product (pâté) generated by new concept of two-phase decanter. Eur J Lipid Sci Technol. 2017;119:1. doi: 10.1002/ejlt.201600096. [DOI] [Google Scholar]
- 157.Klinjapo R, Klinjapo R, Areerat K, Areerat K, Sutthirak P, Sutthirak P. Study effect of natural extracts on the antioxidant activity in pork balls. Br Food J. 2017;119(10):2217–2228. doi: 10.1108/BFJ-11-2016-0532. [DOI] [Google Scholar]
- 158.Pinela J, Prieto MA, Barreiro MF, Carvalho AM, Oliveira MBP, Curran TP, Ferreira IC. Valorisation of tomato wastes for development of nutrient-rich antioxidant ingredients: a sustainable approach towards the needs of the today’s society. Innov Food Sci Emerg Technol. 2017;41:160–171. doi: 10.1016/j.ifset.2017.02.004. [DOI] [Google Scholar]
- 159.García-Pérez JS, Robledo-Padilla F, Cuellar-Bermudez SP, Arévalo-Gallegos A, Parra-Saldivar R, Zavala-Yoe R, Ramirez-Mendoza RA, Iqbal HMN. Thermodynamics and statistical correlation between supercritical-CO2 fluid extraction and bioactivity profile of locally available Mexican plants extracts. J Supercrit Fluids. 2017;122:27–34. doi: 10.1016/j.supflu.2016.12.002. [DOI] [Google Scholar]
- 160.Cerempei A, Mureşan EI, Cimpoeşu N, Carp-Cărare C, Rimbu C. Dyeing and antibacterial properties of aqueous extracts from quince (Cydonia oblonga) leaves. Ind Crops Prod. 2016;94:216–225. doi: 10.1016/j.indcrop.2016.08.018. [DOI] [Google Scholar]
- 161.Rodríguez-López L, Vecino X, Barbosa-Pereira L, Moldes AB, Cruz JM. A multifunctional extract from corn steep liquor: antioxidant and surfactant activities. Food Funct. 2016;7:3724–3732. doi: 10.1039/C6FO00979D. [DOI] [PubMed] [Google Scholar]
- 162.Wildermuth SR, Young EE, Were LM. Chlorogenic acid oxidation and its reaction with sunflower proteins to form green-colored complexes. Compr Rev Food Sci Food Saf. 2016;15:829–843. doi: 10.1111/1541-4337.12213. [DOI] [PubMed] [Google Scholar]
- 163.Amiri-Rigi A, Abbasi S, Scanlon MG. Enhanced lycopene extraction from tomato industrial waste using microemulsion technique: optimization of enzymatic and ultrasound pre-treatments. Innov Food Sci Emerg Technol. 2016;35:160–167. doi: 10.1016/j.ifset.2016.05.004. [DOI] [Google Scholar]
- 164.Barba FJ, Zhu Z, Koubaa M, Sant’Ana AS, Orlien V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: a review. Trends Food Sci Technol. 2016;49:96–109. doi: 10.1016/j.tifs.2016.01.006. [DOI] [Google Scholar]
- 165.Ravber M, Knez Ž, Škerget M. Isolation of phenolic compounds from larch wood waste using pressurized hot water: extraction, analysis and economic evaluation. Cellulose. 2015;22:3359–3375. doi: 10.1007/s10570-015-0719-7. [DOI] [Google Scholar]
- 166.Amiri-Rigi A, Abbasi S. Microemulsion-based lycopene extraction: effect of surfactants, co-surfactants and pretreatments. Food Chem. 2016;197:1002–1007. doi: 10.1016/j.foodchem.2015.11.077. [DOI] [PubMed] [Google Scholar]
- 167.Lucas-Torres C, Lorente A, Cabañas B, Moreno A. Microwave heating for the catalytic conversion of melon rind waste into biofuel precursors. J Clean Prod. 2016;138:59–69. doi: 10.1016/j.jclepro.2016.03.122. [DOI] [Google Scholar]
- 168.Papaioannou EH, Liakopoulou-Kyriakides M, Karabelas AJ. Natural origin lycopene and its “green” downstream processing. Crit Rev Food Sci Nutr. 2016;56:686–709. doi: 10.1080/10408398.2013.817381. [DOI] [PubMed] [Google Scholar]
- 169.Rajabinejad H, Bucişcanu I-I, Maier S-S. Practical ways of extracting keratin from keratinous wastes and by-products: a review. Environ Eng Manag J. 2016;15:1131–1147. [Google Scholar]
- 170.Sixt M, Koudous I, Strube J. Process design for integration of extraction, purification and formulation with alternative solvent concepts. C R Chim. 2016;19:733–748. doi: 10.1016/j.crci.2015.12.016. [DOI] [Google Scholar]
- 171.Delisi R, Saiano F, Pagliaro M, Ciriminna R. Quick assessment of the economic value of olive mill waste water. Chem Cent J. 2016;10:63. doi: 10.1186/s13065-016-0207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Suteu D, Zaharia C, Popovici C, Malutan T, Rusu L, Tabacaru L. Wood waste as a renewable source of energy. Environ Eng Manag J. 2016;15:665–673. [Google Scholar]
- 173.Talmaciu AI, Volf I, Popa VI. A comparative analysis of the “green” techniques applied for polyphenols extraction from bioresources. Chem Biodivers. 2015;12:1635–1651. doi: 10.1002/cbdv.201400415. [DOI] [PubMed] [Google Scholar]
- 174.Katsampa P, Valsamedou E, Grigorakis S, Makris DP. A green ultrasound-assisted extraction process for the recovery of antioxidant polyphenols and pigments from onion solid wastes using Box–Behnken experimental design and kinetics. Ind Crops Prod. 2015;77:535–543. doi: 10.1016/j.indcrop.2015.09.039. [DOI] [Google Scholar]
- 175.Ramamoorthy SK, Skrifvars M, Persson A. A review of natural fibers used in biocomposites: plant, animal and regenerated cellulose fibers. Polym Rev. 2015;55:107–162. doi: 10.1080/15583724.2014.971124. [DOI] [Google Scholar]
- 176.Madeira Junior JV, Teixeira CB, Macedo GA. Biotransformation and bioconversion of phenolic compounds obtainment: an overview. Crit Rev Biotechnol. 2015;35:75–81. doi: 10.3109/07388551.2013.803020. [DOI] [PubMed] [Google Scholar]
- 177.Fan R, Yuan F, Wang N, Gao Y, Huang Y. Extraction and analysis of antioxidant compounds from the residues of Asparagus officinalis L. J Food Sci Technol. 2015;52:2690–2700. doi: 10.1007/s13197-014-1360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Christiaens S, Uwibambe D, Uyttebroek M, Van Droogenbroeck B, Van Loey AM, Hendrickx ME. Pectin characterisation in vegetable waste streams: a starting point for waste valorisation in the food industry. LWT Food Sci Technol. 2015;61:275–282. doi: 10.1016/j.lwt.2014.12.054. [DOI] [Google Scholar]
- 179.Vauchel P, Galván D’Alessandro L, Dhulster P, Nikov I, Dimitrov K. Pilot scale demonstration of integrated extraction–adsorption eco-process for selective recovery of antioxidants from berries wastes. J Food Eng. 2015;158:1–7. doi: 10.1016/j.jfoodeng.2015.02.023. [DOI] [Google Scholar]
- 180.Hamad F, Mubofu E. Potential biological applications of bio-based anacardic acids and their derivatives. Int J Mol Sci. 2015;16:8569–8590. doi: 10.3390/ijms16048569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sandei L, Bandini M, Del Rio D. Recovery of tomato bioactive compounds through a biocompatible and eco-sustainable new technology for the production of enriched “nutraceutical tomato products”. Acta Hortic. 2015;1081:345–351. doi: 10.17660/ActaHortic.2015.1081.45. [DOI] [Google Scholar]
- 182.Shitu A, Izhar S, Tahir TM. Sub-critical water as a green solvent for production of valuable materials from agricultural waste biomass: a review of recent work. Glob J Environ Sci Manag. 2015;1:255–264. [Google Scholar]
- 183.Attard TM, McElroy CR, Rezende CA, Polikarpov I, Clark JH, Hunt AJ. Sugarcane waste as a valuable source of lipophilic molecules. Ind Crops Prod. 2015;76:95–103. doi: 10.1016/j.indcrop.2015.05.077. [DOI] [Google Scholar]
- 184.Nagel A, Mix K, Kuebler S, Bogner H, Kienzle S, Elstner P, Carle R, Neidhart S. The arabinogalactan of dried mango exudate and its co-extraction during pectin recovery from mango peel. Food Hydrocoll. 2015;46:134–143. doi: 10.1016/j.foodhyd.2014.11.029. [DOI] [Google Scholar]
- 185.Low JH, Rahman WAWA, Jamaluddin J. The influence of extraction parameters on spent coffee grounds as a renewable tannin resource. J Clean Prod. 2015;101:222–228. doi: 10.1016/j.jclepro.2015.03.094. [DOI] [Google Scholar]
- 186.Mena P, Ascacio-Valdés JA, Gironés-Vilaplana A, Del Rio D, Moreno DA, García-Viguera C. Assessment of pomegranate wine lees as a valuable source for the recovery of (poly) phenolic compounds. Food Chem. 2014;145:327–334. doi: 10.1016/j.foodchem.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 187.Mamma D, Christakopoulos P. Biotransformation of citrus by-products into value added products. Waste Biomass Valoriz. 2014;5:529–549. doi: 10.1007/s12649-013-9250-y. [DOI] [Google Scholar]
- 188.Jenkins RW, Stageman NE, Fortune CM, Chuck CJ. Effect of the type of bean, processing, and geographical location on the biodiesel produced from waste coffee grounds. Energy Fuels. 2014;28:1166–1174. doi: 10.1021/ef4022976. [DOI] [Google Scholar]
- 189.Zakikhani P, Zahari R, Sultan MTH, Majid DL. Extraction and preparation of bamboo fibre-reinforced composites. Mater Des. 2014;63:820–828. doi: 10.1016/j.matdes.2014.06.058. [DOI] [Google Scholar]
- 190.Oliveira AL, Destandau E, Fougère L, Lafosse M. Isolation by pressurised fluid extraction (PFE) and identification using CPC and HPLC/ESI/MS of phenolic compounds from Brazilian cherry seeds (Eugenia uniflora L.) Food Chem. 2014;145:522–529. doi: 10.1016/j.foodchem.2013.08.065. [DOI] [PubMed] [Google Scholar]
- 191.Araniti F, Marrelli M, Lupini A, Mercati F, Statti GA, Abenavoli MR. Phytotoxic activity of Cachrys pungens Jan, a Mediterranean species: separation, identification and quantification of potential allelochemicals. Acta Physiol Plant. 2014;36:1071–1083. doi: 10.1007/s11738-013-1482-8. [DOI] [Google Scholar]
- 192.Schnitzer M, Monreal CM, Powell EE. Wheat straw biomass: a resource for high-value chemicals. J Environ Sci Heal Part B. 2014;49:51–67. doi: 10.1080/03601234.2013.836924. [DOI] [PubMed] [Google Scholar]
- 193.Arvayo-Enríquez H, Mondaca-Fernández I, Gortárez-Moroyoqui P, López-Cervantes J, Rodríguez-Ramírez R. Carotenoids extraction and quantification: a review. Anal Methods. 2013;5:2916. doi: 10.1039/c3ay26295b. [DOI] [Google Scholar]
- 194.Simões J, Nunes FM, Domingues MR, Coimbra MA. Extractability and structure of spent coffee ground polysaccharides by roasting pre-treatments. Carbohydr Polym. 2013;97:81–89. doi: 10.1016/j.carbpol.2013.04.067. [DOI] [PubMed] [Google Scholar]
- 195.Royer M, Kinuani N, Diouf PN. Forest extractives, the 4th pathway of the Forest biorefinery concept. J FOR. 2013;3:32–41. [Google Scholar]
- 196.Fameau A-L, Gaillard C, Marion D, Bakan B. Interfacial properties of functionalized assemblies of hydroxy-fatty acid salts isolated from fruit tomato peels. Green Chem. 2013;15:341–346. doi: 10.1039/C2GC36677K. [DOI] [Google Scholar]
- 197.Shahid-ul-Islam Shahid M, Mohammad F. Perspectives for natural product based agents derived from industrial plants in textile applications—a review. J Clean Prod. 2013;57:2–18. doi: 10.1016/j.jclepro.2013.06.004. [DOI] [Google Scholar]
- 198.Panusa A, Zuorro A, Lavecchia R, Marrosu G, Petrucci R. Recovery of natural antioxidants from spent coffee grounds. J Agric Food Chem. 2013;61:4162–4168. doi: 10.1021/jf4005719. [DOI] [PubMed] [Google Scholar]
- 199.Tan HP, Li HP, Song H, Xu WP, Guan C, Ran LP. A novel way of separation and preparation noncaffeine tea polyphenols from green tea waste. Adv Mater Res. 2012;550–553:1875–1880. doi: 10.4028/www.scientific.net/AMR.550-553.1875. [DOI] [Google Scholar]
- 200.Durante M, Lenucci MS, Rescio L, Mita G, Caretto S. Durum wheat by-products as natural sources of valuable nutrients. Phytochem Rev. 2012;11:255–262. doi: 10.1007/s11101-012-9232-x. [DOI] [Google Scholar]
- 201.Liew MS, Shamsuddin AH, Yew GZ. Fiber resin matrix composites: nature’s gift. WIT Trans Ecol Environ. 2011;148:131–140. doi: 10.2495/RAV110131. [DOI] [Google Scholar]
- 202.Clark JH, Fitzpatrick EM, Macquarrie DJ, Pfaltzgraff LA, Sherwood J. p-Cymenesulphonic acid: an organic acid synthesised from citrus waste. Catal Today. 2012;190:144–149. doi: 10.1016/j.cattod.2011.12.007. [DOI] [Google Scholar]
- 203.Yuda N, Tanaka M, Suzuki M, Asano Y, Ochi H, Iwatsuki K. Polyphenols extracted from black tea (Camellia sinensis) residue by hot-compressed water and their inhibitory effect on pancreatic lipase in vitro. J Food Sci. 2012;77:H254–H261. doi: 10.1111/j.1750-3841.2012.02967.x. [DOI] [PubMed] [Google Scholar]
- 204.Li ZJ, Wei Z, Xiao W, Wang J, Wu FA. Recovery of tea polyphenols from green tea waste by liquid–liquid extraction. Adv Mater Res. 2012;396–398:1592–1595. [Google Scholar]
- 205.Li CP, Wang LL, Jin ZS, Tang L. Study on the extraction technique of poly-methoxyflavonoids from citrus peels by using response surface methodology. Adv Mater Res. 2012;560–561:544–549. [Google Scholar]
- 206.Périno-Issartier S, Zill-e-Huma Abert-Vian M, Chemat F. Solvent free microwave-assisted extraction of antioxidants from sea buckthorn (Hippophae rhamnoides) food by-products. Food Bioprocess Technol. 2011;4:1020–1028. doi: 10.1007/s11947-010-0438-x. [DOI] [Google Scholar]
- 207.Lozano-Sánchez J, Giambanelli E, Quirantes-Piné R, Cerretani L, Bendini A, Segura-Carretero A, Fernández-Gutiérrez A. Wastes generated during the storage of extra virgin olive oil as a natural source of phenolic compounds. J Agric Food Chem. 2011;59:11491–11500. doi: 10.1021/jf202596q. [DOI] [PubMed] [Google Scholar]
- 208.Chemat-Djenni Z, Ferhat MA, Tomao V, Chemat F. Carotenoid extraction from tomato using a green solvent resulting from orange processing waste. J Essent Oil Bear Plants. 2010;13:139–147. doi: 10.1080/0972060X.2010.10643803. [DOI] [Google Scholar]
- 209.Tsukayama M, Sasaki T, Yamamoto K, Kawamura Y, Ichikawa R. Microwave-assisted extraction and methylation of useful flavones from waste peels of citrus sudachi. Nippon Shokuhin Kagaku Kogaku Kaishi. 2010;57:427–433. doi: 10.3136/nskkk.57.427. [DOI] [Google Scholar]
- 210.Vieira MA, Maraschin M, Pagliosa CM, Podestá R, De Simas KN, Rockenbach II, Amboni RDMC, Amante ER. Phenolic acids and methylxanthines composition and antioxidant properties of mate (Ilex paraguariensis) residue. J Food Sci. 2010;75:C280–C285. doi: 10.1111/j.1750-3841.2010.01548.x. [DOI] [PubMed] [Google Scholar]
- 211.Sun-Waterhouse D, Wen I, Wibisono R, Melton LD, Wadhwa S. Evaluation of the extraction efficiency for polyphenol extracts from by-products of green kiwifruit juicing. Int J Food Sci Technol. 2009;44:2644–2652. doi: 10.1111/j.1365-2621.2009.02097.x. [DOI] [Google Scholar]
- 212.Bhat R, Khalil HPSA, Karim AA. Exploring the antioxidant potential of lignin isolated from black liquor of oil palm waste. C R Biol. 2009;332:827–831. doi: 10.1016/j.crvi.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 213.Wijngaard H, Brunton N. The optimization of extraction of antioxidants from apple pomace by pressurized liquids. J Agric Food Chem. 2009;57:10625–10631. doi: 10.1021/jf902498y. [DOI] [PubMed] [Google Scholar]
- 214.Liang H, Li C, Yuan Q, Vriesekoop F. Separation and purification of sulforaphane from broccoli seeds by solid phase extraction and preparative high-performance liquid chromatography. J Agric Food Chem. 2007;55:8047–8053. doi: 10.1021/jf0706833. [DOI] [PubMed] [Google Scholar]
- 215.Senol A, Aydin A. Solid–liquid extraction of caffeine from tea waste using battery type extractor: process optimization. J Food Eng. 2006;75:565–573. doi: 10.1016/j.jfoodeng.2005.04.039. [DOI] [Google Scholar]