Abstract
Objective
To increase understanding of the biological mechanisms underlying the association, we investigated the individual relations to cognitive decline of the primary nutrients and bioactives in green leafy vegetables, including vitamin K (phylloquinone), lutein, β-carotene, nitrate, folate, kaempferol, and α-tocopherol.
Methods
This was a prospective study of 960 participants of the Memory and Aging Project, ages 58–99 years, who completed a food frequency questionnaire and had ≥2 cognitive assessments over a mean 4.7 years.
Results
In a linear mixed model adjusted for age, sex, education, participation in cognitive activities, physical activities, smoking, and seafood and alcohol consumption, consumption of green leafy vegetables was associated with slower cognitive decline; the decline rate for those in the highest quintile of intake (median 1.3 servings/d) was slower by β = 0.05 standardized units (p = 0.0001) or the equivalent of being 11 years younger in age. Higher intakes of each of the nutrients and bioactives except β-carotene were individually associated with slower cognitive decline. In the adjusted models, the rates for the highest vs the lowest quintiles of intake were β = 0.02, p = 0.002 for phylloquinone; β = 0.04, p = 0.002 for lutein; β = 0.05, p < 0.001 for folate; β = 0.03, p = 0.02 for α-tocopherol; β = 0.04, p = 0.002 for nitrate; β = 0.04, p = 0.003 for kaempferol; and β = 0.02, p = 0.08 for β-carotene.
Conclusions
Consumption of approximately 1 serving per day of green leafy vegetables and foods rich in phylloquinone, lutein, nitrate, folate, α-tocopherol, and kaempferol may help to slow cognitive decline with aging.
Population projections indicate sharp increases in the prevalence of dementia worldwide as the oldest age groups continue to grow in number.1 Decline in cognitive abilities, the central feature of dementia, is also one of the more feared conditions of aging. The identification of effective prevention strategies for dementia is critical to staving off a public health crisis and for meeting demand for this kind of information, particularly around diet. Among all of the different types of vegetables, green leafy vegetables have been identified as having the strongest protective relations against cognitive decline.2,3 We examined this relation in a prospective cohort study, and investigated the individual relations to cognitive decline of a number of nutrients and bioactives that are rich in green leafy vegetables: lutein, vitamin K (phylloquinone), nitrate, folate, α-tocopherol, β-carotene, and the flavonoid kaempferol.
Methods
Study population
The analytic sample is drawn from the Rush Memory and Aging Project (MAP), a study of volunteers from more than 40 retirement communities, senior public housing units, and churches and senior centers in the Chicago area. As previously described,4 MAP is an ongoing open cohort study that began in 1997. Participants are free of dementia at enrollment based on structured clinical neurologic examination5 and agree to annual clinical evaluations and organ donation after death. Food frequency questionnaires (FFQ) were incorporated into the study beginning in February 2004, at which point 1,545 persons had enrolled in the study, 90 had died, and 149 withdrew, leaving 1,306 participants eligible for these analyses. Of these, 1,068 completed the FFQ and 960 had at least 2 cognitive assessments for the analyses of cognitive change. There were no material differences in characteristics between the larger cohort and the analyzed sample. For example, on average, both were 80 years of age, had 14 years of education, physical activity score of 3, and body mass index (BMI) of 27; 13% had history of myocardial infarction, 74% hypertension, 18% and 19%, respectively, diabetes, and 11% and 10%, stroke. Participants meeting criteria for mild cognitive impairment6 (n = 220) were not excluded except in secondary analyses. The analytic sample was 95% white and 98.5% non-Hispanic.
Standard protocol approvals, registrations, and patient consents
The institutional review board of Rush University Medical Center approved the study and all participants gave written informed consent.
Cognitive assessments
The annual structured clinical evaluations included a battery of cognitive tests administered by trained and certified technicians. Composite scores from 19 of these tests were used to characterize cognition in 5 cognitive domains (episodic memory, working memory, semantic memory, visuospatial ability, and perceptual speed) as described previously.6 The composite scores were computed for each cognitive domain and for a global measure of all 19 tests; raw scores for each test were standardized using the baseline population mean and standard deviation, and the standardized scores averaged. The number of annual cognitive assessments for analyzed participants ranged from 2 to 10; 52% of the sample participants had 5 or more cognitive assessments.
Diet assessment
FFQs were collected at each annual clinical evaluation. However, because we wanted to relate estimated dietary effects on cognitive change prospectively, we limited our analysis to data from the first obtained FFQ. Daily servings of green leafy vegetables and nutrient intake levels were computed from responses to a modified Harvard semiquantitative FFQ that was shown to be a valid and reliable measure of dietary intake in older Chicago community residents.7 The FFQ ascertained usual frequency of intake of 144 food items over the previous 12 months. The FFQ describes natural portion sizes (e.g., 1 banana) for many food items. For other foods (e.g., fish as main dish), the serving sizes were based on sex-specific mean portion sizes reported by the oldest men and women of national surveys. The 3 green leafy vegetable items and their serving sizes on the FFQ included (1) spinach (1/2 cup cooked), (2) kale/collards/greens (1/2 cup cooked), and (3) lettuce salad (1 cup raw). Dietary intake levels of the nutrients of interest were estimated from responses to all FFQ food items. Supplement intake levels were not analyzed due to the focus on food-derived nutrients and bioactives. Few randomized trials or epidemiologic studies find protective benefit of vitamin supplements on cognitive decline. To compute daily nutrient intake, nutrient levels for the food item portion sizes were multiplied by the frequency of intake and summed over all food items. All nutrients were calorie-adjusted separately for men and women using the regression residual method. The correlation between the baseline level of green leafy vegetable intake and the level on the last obtained FFQ, on average 4.4 years later, was 0.48.
Covariates
Dietary intake levels of total energy (kcal), ratio of unsaturated (g/d) to saturated fats (g/d), alcohol consumption (g/d of beer, wine, and liquor), and seafood consumption (weekly consumption vs less of tuna sandwich, fish sandwich, fish as a main dish, shrimp/lobster/crab) were based on responses to the baseline FFQ. Nondietary variables were obtained from structured interview questions and measurements at the participants' annual clinical evaluations. Age (in years) was computed from self-reported birth date and date of the first cognitive assessment in this analysis. Education was based on self-reported years of regular schooling. APOE genotyping was performed using high throughput sequencing as previously described.4 All other covariates were based on data collected at the time of each cognitive assessment and were modeled as time-varying covariates to represent updated information from participants' previous evaluations. A variable for frequency of participation in cognitively stimulating activities was computed as the average frequency rating, based on a 5-point scale, of different activities (e.g., reading, playing games, writing letters, visiting the library).4,8 Hours per week of physical activity was computed based on the sum of self-reported minutes spent over the previous 2 weeks on 5 activities (walking for exercise, yard work, calisthenics, biking, and water exercise).9 Smoking history was categorized as never, past, and current smoker. Number of depressive symptoms was assessed by a modified 10-item version of the Center for Epidemiologic Studies–Depression scale.10 BMI (weight in kg/height in m2) was computed from measured weight and height and modeled as 2 indicator variables, BMI ≤20 and BMI ≥30, with referent of 20 < BMI < 30. Hypertension history was determined by self-reported medical diagnosis, measured blood pressure (average of 2 measurements ≥160 mm Hg systolic or ≥90 mm Hg diastolic), or current use of hypertensive medications. Myocardial infarction history was based on self-reported medical diagnosis or interviewer-recorded use of cardiac glycosides (e.g., lanoxin, digitoxin). Diabetes history was determined by self-reported medical diagnosis or current use of diabetes medications. Interviewers inspected and recorded data on all medications taken within the previous 2 weeks. Clinical diagnosis of stroke was based on neurologic examination and medical history.11
Statistical methods
To examine the relations of green leafy vegetables and nutrients to change in cognitive scores, we used separate linear mixed models with random effects in SAS version 9.3 (SAS Institute, Cary, NC). We modeled the nutrient variables obtained from the first FFQ in quintiles with the lowest quintile as the referent category. A test for linear trend was also examined for each nutrient by assigning the median quintile intake level to those in a given quintile and modeling these values as a single categorical variable. The basic-adjusted model included terms for age, sex, education, smoking history, physical activity, participation in cognitive activities, alcohol consumption, total energy intake (green leafy model only), seafood consumption (green leafy model only), the dietary variable of interest, a variable for time, and multiplicative terms between time and each model covariate, the latter providing the covariate estimated effect on cognitive change.
Nonstatic covariates (e.g., cognitive and physical activities, BMI, depressive symptoms, and cardiovascular conditions) were modeled as time-varying variables except in analyses in which they were being investigated as potential effect modifiers in which case only the baseline measure for that covariate was modeled. To account for time-varying covariates, the SAS modeling was conducted on a dataset that contained updated values for all variables at each timepoint. Tests for statistical interaction by potential effect modifiers were computed in the basic-adjusted model by modeling 2-way and 3-way multiplicative terms among the nutrient, time, and the effect modifier, with the 3-way multiplicative term test for interaction set at p ≤ 0.05. We characterized the estimated effect of being in the highest vs lowest quintiles of green leafy vegetable intake as the equivalent age difference in years by computing the ratio of the β coefficients (β [time×age]/β [time×quintile 5]) in the basic-adjusted model.
Results
The sample of 960 MAP participants was on average 81 years of age, 74% female, and had a mean educational level of 14.9 years. Participants had a mean of 4.7 years (range 2–10) of follow-up. Green leafy vegetable intake ranged from a mean 0.09 servings per day for those in the lowest quintile of intake to a mean 1.3 servings per day for the highest quintile. Compared to those participants in the lowest quintile, those in the highest were more likely to be higher educated, male, to participate more frequently in cognitive and physical activities, and to have fewer cardiovascular conditions and depressive symptoms (table 1). Consumption of green leafy vegetables highly correlated with food intakes of nitrate (r = 0.78), lutein and phylloquinone (r = 0.67 for each), and β-carotene (r = 0.60), and moderately correlated with folate (r = 0.47) and kaempferol (r = 0.44). Dietary intakes of lutein, phylloquinone, folate, and β-carotene were also highly to moderately correlated with each other (table 2).
Table 1.
Baseline characteristics by quintile of green leafy vegetable consumption among 960 Rush Memory and Aging Project participants, 2004–2012
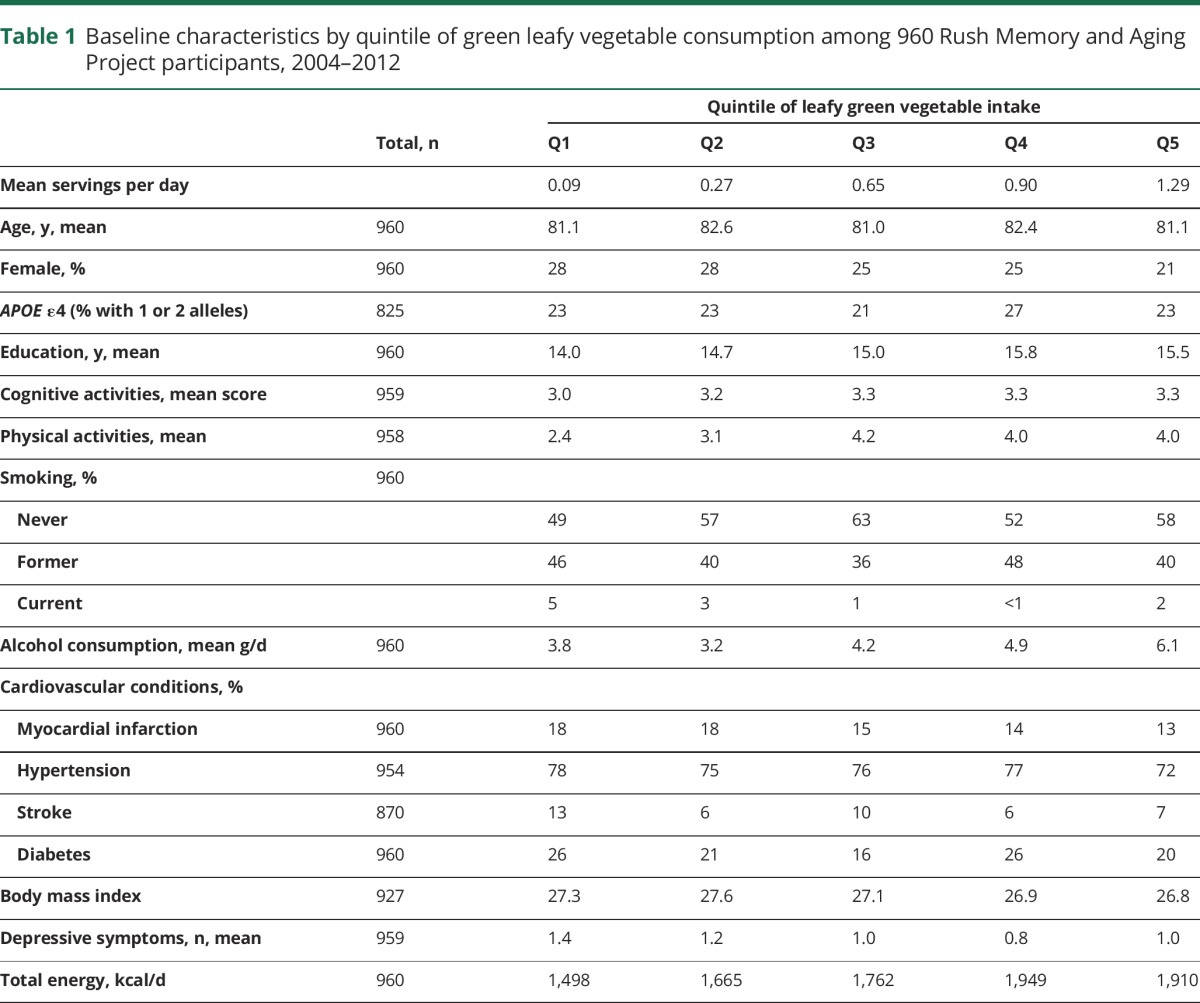
Table 2.
Correlations of baseline dietary intake levels of green leafy vegetables, phylloquinone, folate, lutein, β-carotene, α-tocopherol, nitrate, and kaempferol among 960 Rush Memory and Aging Project participants, 2004–2014

On average, the sample declined in global cognitive scores over time at a rate of 0.08 standardized units/y. In age-adjusted models, consumption of green leafy vegetables was positively and significantly associated with slower rate of cognitive decline (table 3). The estimated effect was not changed materially with further adjustment for education, participation in cognitive and physical activities, smoking, alcohol consumption, and seafood consumption in the basic model (figure). When compared to the estimated effect of age on cognitive decline, the top quintile for green leafy vegetable consumption (median of 1.3 servings/d) vs the lowest quintile (median of 0.09 servings/d) was the equivalent of about 11 years younger. There was no evidence that the association was mediated by cardiovascular conditions (table 3). In further analyses, we added variables to the basic-adjusted model for depressive symptoms, low weight (BMI ≤ 20), and obesity (BMI ≥ 30), conditions that may be both cause and effects of dementia processes. However, the estimate of effect remained unchanged and statistically significant (β for Q5 vs Q1 = 0.04, p < 0.001).
Table 3.
Estimated effects (β, 95% confidence interval) of consumption of green leafy vegetables and nutrients on global cognitive decline over a mean 4.5 years among 960 participants of the Rush Memory and Aging Project, 2004–2014

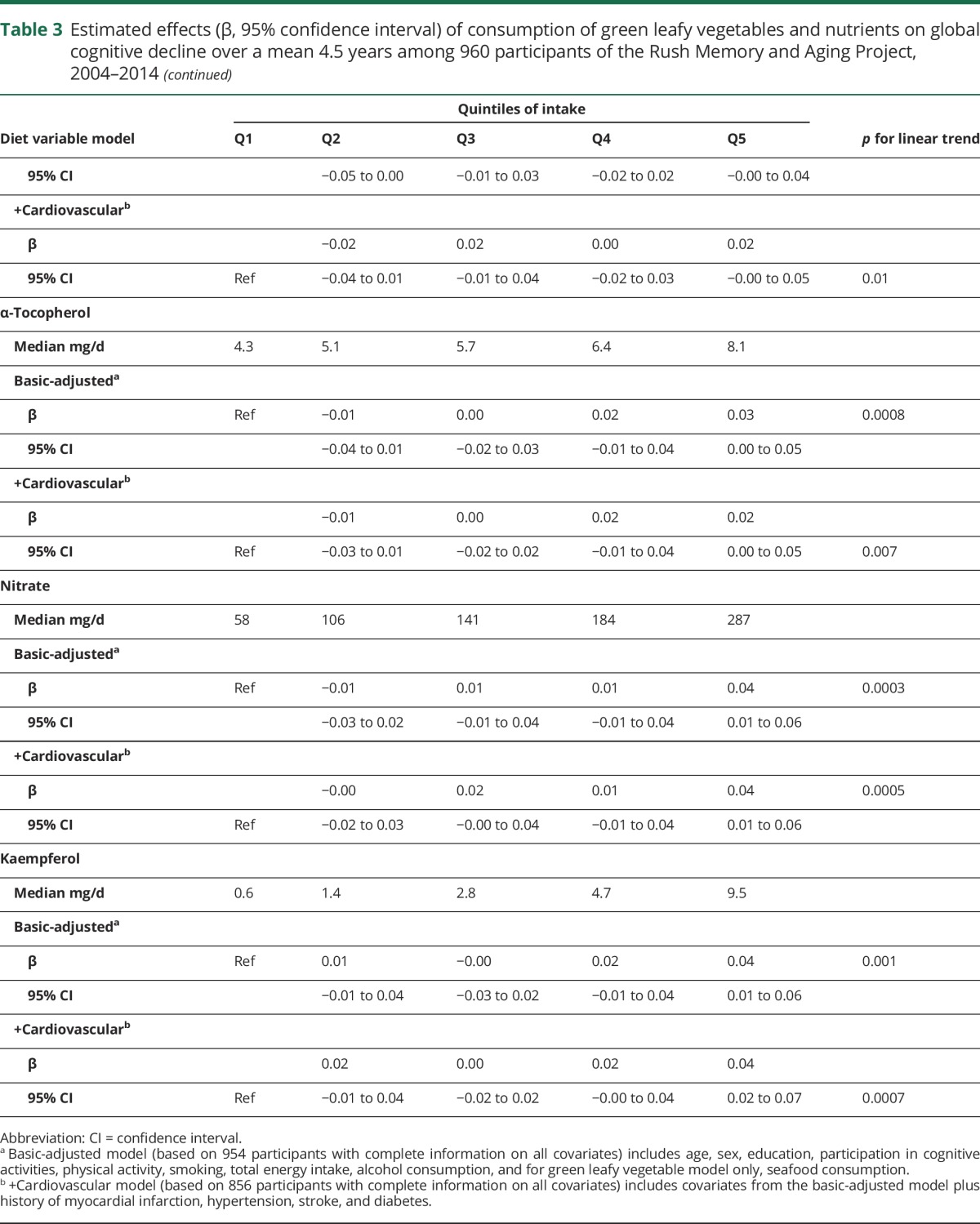
Figure. Rate of decline in global cognitive score for the top and the lowest quintile of intake of green leafy vegetables.
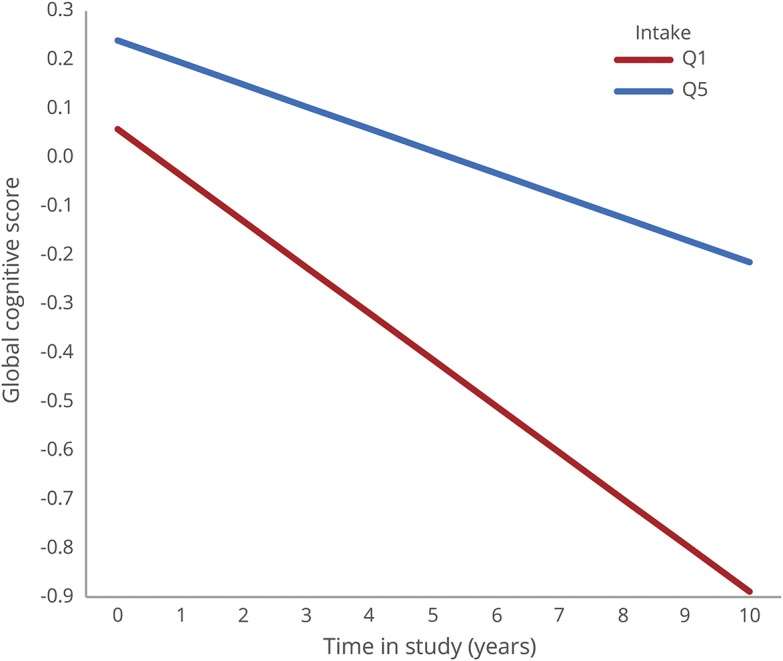
Rate of decline in global cognitive score for the top quintile of intake of green leafy vegetables (median 1.3 servings/d) and the lowest quintile of intake (median 0.09 servings/d) based on mixed models adjusted for age, sex, education, energy intake, participation in cognitive activities, physical activity, smoking, and alcohol consumption in 960 Rush Memory and Aging Project participants, 2004–2014.
We next investigated the relations to cognitive change of food sources of folate, phylloquinone, lutein, nitrate, α-tocopherol, and kaempferol. Food intakes of these nutrients and bioactives were each positively and significantly associated with slower rates of cognitive decline. The effect estimates for these nutrients and bioactives did not change appreciably in models adjusted for the primary risk factors of cognitive change, nor did they appear to be mediated by cardiovascular conditions (table 3). The effect estimates also did not change in analyses with additional adjustment for depressive symptoms, low weight, and obesity in the basic-adjusted model (β, p value for Q5 vs Q1 were folate β = 0.04, p = 0.003; phylloquinone β = 0.04, p = 0.004; lutein β = 0.04, p = 0.004; α-tocopherol β = 0.03, p = 0.02; nitrate β = 0.04, p = 0.006; kaempferol β = 0.03, p = 0.01). The exception was β-carotene, which was modified and no longer statistically significant (β = 0.01, p = 0.24). We also considered whether these observed associations could be due to confounding by other nutrients shown in previous studies to protect against cognitive decline. For all of these nutrients and bioactives, except β-carotene, which was no longer statistically significant (0.02, p = 0.16), the effect estimates changed little with additional adjustment for dietary intakes of α-tocopherol and the ratio of unsaturated to saturated fats in the basic-adjusted models (Q5 vs Q1 were folate β = 0.04, p = 0.002; phylloquinone β = 0.03, p = 0.006; lutein β = 0.03, p = 0.005).
Next, we examined whether the strong protective relation of green leafy vegetable consumption to cognitive decline could be accounted for by dietary intakes of the nutrients and bioactives in separate basic-adjusted models. The estimate of the green leafy vegetable effect was modified and no longer statistically significant with inclusion of phylloquinone (β = 0.02, p = 0.13), lutein (β = 0.01, p = 0.22), or folate (β = 0.02, p = 0.10), suggesting that these nutrients were the source of the effect on cognitive decline.
In secondary analyses, we investigated whether the observed relations of green leafy vegetable consumption and dietary intakes of folate, phylloquinone, and lutein were modified by age (>80 y/≤80 y), sex, APOE ε4 status, or presence/absence of mild cognitive impairment. However, there was no evidence (all tests of statistical interaction p > 0.10) that the observed relations differed by level of any of these factors.
We also examined whether any of the observed associations could be due to inaccurate reporting of dietary intake by those who had cognitive impairment at baseline. We reanalyzed the basic-adjusted models for green leafy vegetable consumption and intakes of each nutrient after eliminating 220 individuals who were clinically assessed as having mild cognitive impairment at baseline. The effect estimates changed minimally and remained statistically significant (β, p value for Q5 vs Q1 were green leafy vegetables, β = 0.06, p < 0.001; folate β = 0.05, p = 0.001; phylloquinone β = 0.03, p = 0.005; lutein β = 0.04, p = 0.003; β-carotene β = 0.03, p = 0.04). In further analyses, we reanalyzed the data after excluding 144 participants whose green leafy vegetable consumption either increased (top 10%) or decreased (bottom 10%) over the study period. Restricting the sample to those with more stable consumption patterns over time had minimal effect on the observed associations (β, p value for Q5 vs Q1 in the basic-adjusted models were green leafy vegetables, β = 0.05, p < 0.001; folate β = 0.04, p = 0.005; phylloquinone β = 0.04, p = 0.003; lutein β = 0.03, p = 0.01).
Discussion
In this prospective study of an older US community population, the consumption of green leafy vegetables was linearly associated with slower cognitive decline. The rate of decline among those who consumed 1–2 servings per day was the equivalent of being 11 years younger compared with those who rarely or never consumed green leafy vegetables. Investigation of the nutrients for which green leafy vegetables are a rich or primary source indicated that higher food intakes of folate, phylloquinone, and lutein were each linearly associated with slower cognitive decline and appeared to account for the protective relation of green leafy vegetables to cognitive change. These associations were independent of a number of demographic, behavioral, health, and nutrient risk factors as well as the presence of cognitive impairment. A weaker association was observed with dietary intake of β-carotene.
The study findings are supported by 2 large prospective studies2,3 that examined the relations of different types of vegetables on cognitive decline. In both studies, the consumption of green leafy vegetables, including spinach, kale, collards, and lettuce, had the strongest association with slowed cognitive decline. In this study, we attempted to identify what individual dietary components in green leafy vegetables may be the underlying protective mechanisms. Many prospective studies report protective relations against dementia with dietary intakes of folate12–16 and β-carotene,17–20 although the evidence is by no means consistent for either folate21–24 or β-carotene.17,25–27 One randomized trial of 3-year supplementation with folic acid in individuals with biochemical evidence of marginal folate status found significantly slower cognitive decline compared with placebo.28 The effect of β-carotene supplementation on cognitive decline was also tested in randomized trials,29,30 with protective benefit demonstrated only after 18 years of supplementation.29 There has been limited investigation of lutein and phylloquinone. One cross-sectional study reported a positive correlation between dietary intake levels of phylloquinone and Mini-Mental State Examination scores.31 Plasma levels of lutein were associated with decreased risk of incident dementia in the 3-City Bordeaux study,32 and a small cross-sectional study of octogenarians and centenarians found that brain and serum concentrations of lutein were associated with higher cognitive scores.33 However, no effect of lutein (10 mg)/zeaxanthin (2 mg) supplementation on cognitive function was found in the Age-Related Eye Disease Study 2 (AREDS2) trial of older persons at risk of late age-related macular degeneration.34 The negative trial results should not be interpreted as being inconsistent with the epidemiologic studies because the trial was not designed to test whether lutein supplementation is beneficial for cognition in individuals who have low or marginal lutein status.
These nutrients for which green leafy vegetables are a rich source may have independent mechanisms of action that synergistically protect the brain. Serum carotenoid levels were associated with less severe periventricular white matter lesions, particularly in older smokers.35 In addition, lutein has been shown to reduce phospholipid peroxidation in human erythrocytes,36 and to attenuate oxidative stress and mitochondrial dysfunction37 and neuroinflammation.38 Folate was reported to inhibit tau phosphorylation and APP, PS1, and Aβ protein levels that underlie Alzheimer disease pathogenesis, and to increase methylation potential and DNA methyltransferase activity.39
Confidence in the validity of the study findings is supported by a number of strengths. The prospective study design and high follow-up rates decrease the potential for biased results. The outcome of change in cognitive abilities was measured by in-person administration of a large battery of tests conducted annually for up to 10 years, thus providing a sensitive measure of decline that is unlikely to be biased by baseline levels of attained cognitive ability.40 Dietary intake levels were assessed by a validated comprehensive FFQ shown to be unbiased by age and cognitive abilities.7 Further, the results did not change when cognitively impaired individuals were excluded from the analyses and there was no evidence of effect modification by age.
The study findings were based on an observational study and as such, confounding bias can never be ruled out as an alternative explanation of the observed relations. Further, results of the study may not be generalizable to younger adults or to nonwhite or Hispanic populations.
Consumption of green leafy vegetables may help to slow decline in cognitive abilities with older age, perhaps due to the neuroprotective actions of lutein, folate, β-carotene, and phylloquinone. The addition of a daily serving of green leafy vegetables to one's diet may be a simple way to contribute to brain health.
Glossary
- BMI
body mass index
- FFQ
Food frequency questionnaires
- MAP
Rush Memory and Aging Project
Author contributions
All authors participated in the design (M.C.M., D.A.B.), funding (M.C.M., D.A.B.), data collection (M.C.M., L.L.B., D.A.B.), statistical analyses (M.C.M., Y.W., D.A.B., L.L.B., B.D.-H., S.L.B.), writing (M.C.M., D.A.B., L.L.B., B.D.-H., S.L.B.), or final approval of the manuscript (M.C.M., Y.W., L.L.B., D.A.B., B.D.-H., S.L.B.).
Study funding
Supported by grants R01 AG031553 and R01 AG17917 and the USDA Agricultural Research Service cooperative agreement 58-1950-7-707. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the USDA.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366:2112–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang JH, Ascherio A, Grodstein F. Fruit and vegetable consumption and cognitive decline in aging women. Ann Neurol 2005;57:713–720. [DOI] [PubMed] [Google Scholar]
- 3.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology 2006;67:1370–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res 2012;9:646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology 2006;27:169–176. [DOI] [PubMed] [Google Scholar]
- 6.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology 2002;59:198–205. [DOI] [PubMed] [Google Scholar]
- 7.Morris MC, Tangney CC, Bienias JL, Evans DA, Wilson RS. Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am J Epidemiol 2003;158:1213–1217. [DOI] [PubMed] [Google Scholar]
- 8.Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc 2005;11:400–407. [PubMed] [Google Scholar]
- 9.Buchman AS, Boyle PA, Wilson RS, Bienias JL, Bennett DA. Physical activity and motor decline in older persons. Muscle Nerve 2007;35:354–362. [DOI] [PubMed] [Google Scholar]
- 10.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. J Aging Health 1993;5:179–193. [DOI] [PubMed] [Google Scholar]
- 11.Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement 2015;11:1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luchsinger JA, Tang MX, Miller J, Green R, Mayeux R. Higher folate intake is related to lower risk of Alzheimer's disease in the elderly. J Nutr Health Aging 2008;12:648–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravaglia G, Forti P, Maioli F, et al. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am J Clin Nutr 2005;82:636–643. [DOI] [PubMed] [Google Scholar]
- 14.Corrada MM, Kawas CH, Hallfrisch J, Muller D, Brookmeyer R. Reduced risk of Alzheimer's disease with high folate intake: the Baltimore Longitudinal Study of Aging. Alzheimers Dement 2005;1:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang HX, Wahlin A, Basun H, Fastbom J, Winblad B, Fratiglioni L. Vitamin B(12) and folate in relation to the development of Alzheimer's disease. Neurology 2001;56:1188–1194. [DOI] [PubMed] [Google Scholar]
- 16.Agnew-Blais JC, Wassertheil-Smoller S, Kang JH, et al. Folate, vitamin B-6, and vitamin B-12 intake and mild cognitive impairment and probable dementia in the Women's Health Initiative Memory Study. J Acad Nutr Diet 2015;115:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devore EE, Kang JH, Stampfer MJ, Grodstein F. The association of antioxidants and cognition in the Nurses' Health Study. Am J Epidemiol 2013;177:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wengreen HJ, Munger RG, Corcoran CD, et al. Antioxidant intake and cognitive function of elderly men and women: the Cache County Study. J Nutr Health Aging 2007;11:230–237. [PubMed] [Google Scholar]
- 19.Engelhart MJ, Geerlings MI, Ruitenberg A, et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA 2002;287:3223–3229. [DOI] [PubMed] [Google Scholar]
- 20.Hu P, Bretsky P, Crimmins EM, Guralnik JM, Reuben DB, Seeman TE. Association between serum beta-carotene levels and decline of cognitive function in high-functioning older persons with or without apolipoprotein E 4 alleles: MacArthur Studies of Successful Aging. J Gerontol A Biol Sci Med Sci 2006;61:616–620. [DOI] [PubMed] [Google Scholar]
- 21.Clarke R, Birks J, Nexo E, et al. Low vitamin B-12 status and risk of cognitive decline in older adults. Am J Clin Nutr 2007;86:1384–1391. [DOI] [PubMed] [Google Scholar]
- 22.Kang JH, Irizarry MC, Grodstein F. Prospective study of plasma folate, vitamin B12, and cognitive function and decline. Epidemiology 2006;17:650–657. [DOI] [PubMed] [Google Scholar]
- 23.Morris MC, Evans DA, Bienias JL, et al. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Arch Neurol 2005;62:641–645. [DOI] [PubMed] [Google Scholar]
- 24.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor dementia and Alzheimer's disease. NEJM 2002;346:476–483. [DOI] [PubMed] [Google Scholar]
- 25.Morris MC, Evans DA, Bienias JL, et al. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer's disease in a biracial community study. JAMA 2002;287:3230–3237. [DOI] [PubMed] [Google Scholar]
- 26.Kang JH, Grodstein F. Plasma carotenoids and tocopherols and cognitive function: a prospective study. Neurobiol Aging 2008;29:1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luchsinger JA, Tang MX, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer's disease. Arch Neurol 2003;60:203–208. [DOI] [PubMed] [Google Scholar]
- 28.Durga J, van Boxtel MP, Schouten EG, et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet 2007;369:208–216. [DOI] [PubMed] [Google Scholar]
- 29.Grodstein F, Kang JH, Glynn RJ, Cook NR, Gaziano JM. A randomized trial of beta carotene supplementation and cognitive function in men: the Physicians' Health Study II. Arch Intern Med 2007;167:2184–2190. [DOI] [PubMed] [Google Scholar]
- 30.Kang JH, Cook NR, Manson JE, Buring JE, Albert CM, Grodstein F. Vitamin E, vitamin C, beta carotene, and cognitive function among women with or at risk of cardiovascular disease: the Women's Antioxidant and Cardiovascular Study. Circulation 2009;119:2772–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chouet J, Ferland G, Feart C, et al. Dietary vitamin K intake is associated with cognition and behaviour among geriatric patients: the CLIP Study. Nutrients 2015;7:6739–6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feart C, Letenneur L, Helmer C, et al. Plasma carotenoids are inversely associated with dementia risk in an elderly French cohort. J Gerontol A Biol Sci Med Sci 2016;71:683–688. [DOI] [PubMed] [Google Scholar]
- 33.Johnson EJ, Vishwanathan R, Johnson MA, et al. Relationship between serum and brain carotenoids, alpha-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the Georgia Centenarian Study. J Aging Res 2013;2013:951786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chew EY, Clemons TE, Agron E, Launer LJ, Grodstein F, Bernstein PS. Effect of Omega-3 fatty acids, lutein/zeaxanthin, or other nutrient supplementation on cognitive function: the AREDS2 randomized clinical trial. JAMA 2015;314:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Den HT, Launer LJ, De Groot JC, et al. Serum carotenoids and cerebral white matter lesions: the Rotterdam Scan Study. J Am Geriatr Soc 2001;49:642–646. [DOI] [PubMed] [Google Scholar]
- 36.Nakagawa K, Kiko T, Hatade K, Sookwong P, Arai H, Miyazawa T. Antioxidant effect of lutein towards phospholipid hydroperoxidation in human erythrocytes. Br J Nutr 2009;102:1280–1284. [DOI] [PubMed] [Google Scholar]
- 37.Binawade Y, Jagtap A. Neuroprotective effect of lutein against 3-nitropropionic acid-induced Huntington's disease-like symptoms: possible behavioral, biochemical, and cellular alterations. J Med Food 2013;16:934–943. [DOI] [PubMed] [Google Scholar]
- 38.Wu W, Li Y, Wu Y, Zhang Y, Wang Z, Liu X. Lutein suppresses inflammatory responses through Nrf2 activation and NF-kappaB inactivation in lipopolysaccharide-stimulated BV-2 microglia. Mol Nutr Food Res 2015;59:1663–1673. [DOI] [PubMed] [Google Scholar]
- 39.Li W, Liu H, Yu M, et al. Folic acid administration inhibits amyloid beta-peptide accumulation in APP/PS1 transgenic mice. J Nutr Biochem 2015;26:883–891. [DOI] [PubMed] [Google Scholar]
- 40.Morris MC, Evans DA, Hebert LE, Bienias JL. Methodological issues in the study of cognitive decline. Am J Epidemiol 1999;149:789–793. [DOI] [PubMed] [Google Scholar]


