Abstract
Brain activity levels are tightly regulated to minimize imbalances in activity state. Deviations from the normal range of activity are deleterious and often associated with neurological disorders. To maintain optimal levels of activity, regulatory mechanisms termed homeostatic synaptic plasticity establish desired ‘set points’ for neural activity, monitor the network for deviations from the set point and initiate compensatory responses to return activity to the appropriate level that permits physiological function [1,2]. We speculate that impaired homeostatic control may contribute to the etiology of various neurological disorders including epilepsy and Alzheimer's disease, two disorders that exhibit hyperexcitability as a key feature during pathogenesis. Here, we will focus on recent progress in developing homeostatic regulation of neural activity as a therapeutic tool.
Keywords: : Alzheimer's disease, antiseizure drugs, CA3, dentate gyrus, epilepsy, homeostatic synaptic plasticity, kappa opioid receptors, mossy fiber, Plk2, synaptoporin
Homeostatic synaptic plasticity in epilepsy: too much of a good thing?
Epilepsy is characterized by repeated and spontaneously occurring seizures [3,4], but we still do not fully understand the mechanisms underlying the development of epilepsy (epileptogenesis). The temporal lobe is particularly vulnerable, with mesial temporal lobe epilepsy (mTLE; affecting hippocampus, parahippocampal gyrus and amygdala) being the most prevalent seizure condition in adults [3]. Classically, the view has been that an initial insult (or first seizure episode in some animal models) leads to a latent period that can induce a prolonged period of hypoactivity, followed weeks later by the emergence of epileptiform activity. For example, transient hypoactivity has been reported after epileptogenic insults such as traumatic brain injury [5,6]. Indeed, even after acute generalized seizures, postictal depression, in which the patient experiences sedation and confusion, is a common finding, and has been associated with profound release of opioid peptides [7]. Todd's paresis, a temporary loss of function in a region impacted by a seizure, may also occur and can last for hours to days before resolving. Persistent changes in neuronal activity induce homeostatic compensation, which can manifest in numerous ways depending on the particular context. These mechanisms include alterations in the number, size and strength of synapses (homeostatic synaptic plasticity [HSP]) as well as a variety of other forms of regulating excitability [1,2]. Chronic hypoactivity following seizures is predicted to induce homeostatic strengthening of excitatory synapses in order to restore network activity; a phenomenon that has been observed in some rodent seizure models [8]. Thus, aberrant or dysfunctional homeostatic compensation could contribute to the emergence of spontaneous recurrent seizures through an overshooting ‘rebound’ effect. Homeostatic strategies, described below, may be able to rescue hypoactivity during the latent period or dampen the ensuing rebound effect, and thereby potentially alter the course of epileptogenesis.
Acute HSP strategies for epilepsy: closing the loop
Closed-loop control is a form of homeostatic regulation, often employed in man-made engineering processes, in which a system output is monitored, compared against a desired set point value and any deviations are corrected by a compensatory adjustment [2]. This type of bioengineering approach has been successfully employed to combat seizures by combining EEG recordings (the sensor) with a regulator. Electrical stimulation has been used as a regulator in both animal models and in human patients [9,10]. More recently, cell-type-specific expression of light-gated cation channels (i.e., optogenetics) in GABAergic neurons have been used as regulators in animal models [11]. In these ‘closed loop’ or ‘responsive neurostimulation’ paradigms, EEG detection of seizure activity automatically activates light delivery via a fiber optic cable, immediately terminating seizures in freely moving mice [11]. This strategy has now been used in a wide variety of brain regions (e.g., hippocampus, cerebellum, thalamus) [11–14] demonstrating that diverse components of seizure circuits may be harnessed to suppress pathological activity. A drawback to this approach is the invasive nature of the procedure using fiber optics [15]); however, with advances in optogenetics using far-red excitation wavelengths, it may be possible theoretically to activate interneurons by light transmitted through the intact cranium [16]. Another caveat is that these procedures use operational definitions of seizure (aberrant activity in EEG) for detection and termination, and therefore may miss some important aspects of pathogenesis or cause unforeseen ramifications. The degree to which closed-loop interventions (electrical, optogenetic or other) may be harnessed to suppress epileptogenesis, as compared with suppression of seizures after they have already emerged, remains an open and interesting question.
Chronic HSP strategies for epilepsy: fighting fire with fire
The most commonly used antiseizure drugs (ASDs) currently all operate on the same general principle: to decrease neuronal firing either directly or indirectly, through blockade of cation channels or enhancement of GABA-mediated inhibition. Thus, acute administration of ASDs acts in a powerfully anticonvulsant manner. However, a third of epilepsy patients are not satisfactorily responsive to ASDs, and these medications do not alter the progression of epileptogenesis or modify disease course once epilepsy has developed [3,17]. Another important caveat from a homeostatic standpoint is that chronic inhibition of neuronal activity produces a compensatory increase in excitatory synaptic strength on hippocampal pyramidal neurons [2,18,19]. The prediction from HSP is that chronic, long-term administration of ASDs would eventually increase excitatory drive in neurons, which would manifest upon drug removal, again, leading to an opposite rebound effect that may explain the refractory nature of some patients to ASD treatment. Along these lines, experimental manipulations that suppress neuronal activity (e.g., lidocaine administration) have been shown to produce a kindling phenomenon [20]. Similarly, rebound seizures after precipitous withdrawal of benzodiazepines have typically been attributed to homeostatic changes at the level of GABAA receptor expression; however, chronic diazepam also induces HSP upregulation of the size and strength of MF-CA3 synapses in vivo [18], suggesting that this may also play into the emergence of seizures after drug withdrawal.
From homeostatic principles, it may be that rather than chronically inhibiting activity, an alternative approach in some cases may be to persistently increase activity, as chronic hyperexcitation is known to cause compensatory weakening of excitatory synapses [21]. Thus, moderate overexcitation could be used to counter seizures, to fight fire with fire. This counterintuitive strategy has garnered some experimental support. For instance, vagus nerve stimulation is used as a treatment of epilepsy [22]. We hypothesize that this action may be due to repeated mild stimulation of projections to the hippocampus and amygdala areas implicated in the generation of seizure activity, thereby activating endogenous homeostatic downregulation of neurons in those target regions. It is interesting to note that even acute, but subconvulsant hyperactivity within the hippocampus may exert anticonvulsant effects. For example, intra-hippocampal microinjection of GABA antagonist or glutamate agonists seem to potently suppress evoked seizures, suggesting that circuit level closed-loop mechanisms may also exist within the hippocampus to restrain pathological overactivity (author's unpublished observations). The effect of long-term implementation of these stimulation protocols on altering disease progression remains to be determined.
Engaging the hippocampal ‘homeostatic brake’
Global excitation or inhibition of the brain may be effective in controlling seizures, but not optimal in terms of preserving normal brain functioning. Indeed, current ASDs have significant adverse effects on the ability of patients to perform daily activities of life. A more surgical approach would be to identify and target specific systems that regulate homeostatic adaptation, which could lead to fewer side effects. Toward this goal, our recent work has shown that specific neurons and specific subsets of synapses, are more homeostatic than others. In other words, some neurons initiate strong HSP mechanisms at excitatory synapses following perturbations in activity, whereas other neuron types seem to respond less robustly [18]. One prominent regulatory locus lies at the synapses between mossy fibers (MF) of the dentate gyrus (DG) and pyramidal neurons of area CA3. We found that chronic changes in network activity caused homeostatic adaptation specifically in the MF-CA3 synapse, when analyzed both morphologically and functionally [18]. Thus, this synapse performs a designated role as a homeostatic ‘gain control’ mechanism for hippocampal circuits.
The MF-CA3 synapse has properties that make it particularly attractive as a homeostatic control point. The CA3 network is a highly interconnected system which functions to associate cortical inputs, but which by its recurrent nature is also highly prone to seizure activity [23]. Thus, inputs to CA3 from DG must be strictly gated to prevent runaway excitation of the CA3 autoassociative network. Additionally, MF-CA3 connections are very powerful, often comprised of many synapses onto the same site, and could effectively gate inputs from DG to CA3 to prevent seizure generation in CA3 recurrent networks [24]. MFs also innervate interneuron populations which control CA3 networks, some of which are selectively lost in epilepsy [25]. Thus, dysregulation of the MF inputs could lead to dramatic alterations in network activity, but if harnessed could also provide a powerful means to control activity, acting as a ‘homeostatic brake’.
To further test the role of the MF-CA3 synapses in regulating epileptogenesis, we examined the role of opioid receptors. The MF tract is highly enriched in endogenous opioid peptides, the levels of which are markedly regulated by neuronal activity [26]. By testing various agonists and antagonists of different classical opioid receptors, we found that kappa opioid receptor (KOR) signaling was both necessary and sufficient to induce HSP in cultured hippocampal neurons in vitro. Furthermore, chronic administration of low dose KOR antagonist norbinaltorphimine (nor-BNI) dampened seizure development in electrical and chemical kindling paradigms [27]. This finding is interesting in two ways. First, KOR antagonists are known to exert acute excitatory actions; thus this antiseizure effect of chronic nor-BNI is another example of ‘fighting fire with fire’. Second, the nor-BNI treatment was given after each kindling episode, suggesting that the therapy could be administered even after an initial insult during the latent period to potentially modify disease progression.
Future studies will test whether similar approaches may be fruitful in epilepsy models featuring spontaneous development of seizures. This MF-CA3 ‘gain control’ synapse may also offer additional therapeutic targets; for instance, the presynaptic protein synaptoporin, which is selectively enriched in MF terminals, is necessary to induce homeostatic upregulation of MF-CA3 synapses in vitro in response to chronic action potential blockade by tetrodotoxin [18]. Reducing the expression of synaptoporin in vivo may prevent the ‘rebound’ strengthening of neuronal activity and hence reduce the propensity to develop seizures in the first place.
Hyperexcitability in Alzheimer's disease: an epilepsy connection?
Alzheimer's disease (AD) is a neurodegenerative disorder characterized by dementia and progressive memory loss, and is associated with the accumulation of hallmark Aβ plaques and neurofibrillary tangles (NFTs) in the brains of AD patients [28,29]. Aβ is produced from the cleavage of the amyloid precursor protein (APP) by β- and γ-secretases, whereas NFTs are comprised chiefly of hyperphosphorylated tau, a microtubule-associated protein. The mechanisms underlying the disease process are still not well understood and are intensely under investigation, but it is generally thought that aggregation of Aβ initiates a cascade of events that causes dysfunction in tau and eventually culminates in profound neurodegeneration in the late stages of AD [28,29]. It is noteworthy that AD patients have an increased incidence of epileptic seizures, especially in those with early onset familial AD mutations [30–33], with an estimated 10–22% of AD patients developing epilepsy. In this regard, it has been observed that hyperactive neurons are prevalent in presymptomatic AD patients and transgenic mouse models [34–41], which may contribute to increased seizure susceptibility [37,42].
The extent of seizures may actually be underappreciated in AD patients due to the phenomenon of ‘silent’ seizures. Overt convulsive events are infrequent in AD, and typical AD symptoms may interfere with the detection of nonconvulsive seizures [31,43,44]. In this regard, a recent study described two AD patients for which no seizure activity could be detected by EEG, but intracranial electrodes were able to detect significant subclinical seizure activity originating from the mesial temporal lobe [45]. Treatment of one of these patients with levetiracetam, a widely used ASD that is orally available and readily crosses the blood–brain barrier [46], reduced temporal lobe seizure activity and almost completely abrogated episodes of extreme confusion for at least 1 year [45]. Although this study is limited by an extremely small sample size, levetiracetam also has been shown by others to dampen abnormal hyperactivity in the hippocampal DG and CA3 regions, rescue cognitive deficits in AD models and improve memory in patients with mild cognitive impairment, the earliest diagnosed stage of AD [47–49]. Taken together, these data suggest that seizure in AD not only contributes to pathological symptoms, but also that common antiepileptic drugs may have significantly positive effects in some cases. Additional research is required to elucidate the mechanism of action of levetiracetam and related compounds in AD, and further investigation with clinical trials will be needed to explore the long-term benefits of ASDs for AD patient populations [50].
Homeostatic synaptic plasticity & AD
A caveat to the chronic use of levetiracetam for AD is that this approach may lead to homeostatic synaptic strengthening and an opposite rebound effect that could exacerbate hyperactivity, as we discussed for other ASDs in epilepsy. However, levetiracetam is atypical in mechanism as it modulates neurotransmitter release rather than directly impacting neuronal excitability. Examination of levetiracetam in vitro and in vivo for the ability to induce HSP is thus of particular interest. Even in the absence of therapies, hyperactive neurons in early AD would be expected to trigger endogenous homeostatic synaptic downregulation as a protective mechanism to reduce hyperexcitability and attendant excitotoxicity. One way in which such endogenous closed-loop control could be manifested is through excitatory synapse loss. Indeed, glutamatergic synapse loss is a hallmark feature of early AD which occurs prior to the development of Aβ plaques and NFTs, and which more closely correlates with symptomatic progression in patients [51–55]. These observations are consistent with compensatory HSP mechanisms as an attempt to bring balance to the network.
Excitatory synapse loss may be related to the production of Aβ itself, which has properties of a homeostatic regulator. Neuronal activity has been shown to upregulate β-secretase processing of APP and subsequent generation and secretion of extracellular Aβ [56–60]. Aβ levels seem to have a hormetic effect on neuronal transmission resulting in both inhibitory and excitatory effects depending on its extrasynaptic concentration [55,61,62]. Low concentrations of extracellular Aβ in the picomolar range lead to increased neuronal potentiation and network excitability, whereas higher Aβ concentrations (nanomolar to micromolar range) lead to decreased potentiation, as well as long-term depression (LTD) and loss of dendritic spines [37,57,61]. This bidirectional characteristic is ideal for a homeostatic regulator of synaptic activity, during hypoactivity (and hence low Aβ concentrations), Aβ has a stimulatory effect, while strong neuronal activity induces more Aβ, which then acts to restrain excitation. Hyperexcitation during AD may initially be compensated for by adjustments in synaptic strength, followed by synapse loss only after significant synaptic remodeling has occurred [28].
During AD, the physiological effects of Aβ may become subverted due to its aggregation into pathological soluble oligomers, which have been shown to induce hyperexcitability in the vicinity of amyloid plaques [34,63]. Thus, increases in neuronal activity trigger Aβ production and, in turn excess oligomeric Aβ further promotes excitation. In contrast to the typical homeostatic closed-loop negative feedback control, this dysregulation leads to a positive feedback loop, which would be predicted to become self-perpetuating and may drive early stage pathogenesis.
Role of Polo-like kinase 2 in APP processing
To interrupt the putative positive feedback loop of hyperexcitation in AD, one strategy is to decipher and modulate the mechanisms that control the activity-dependent formation of Aβ. A potential molecular link between neuronal activity and APP β-processing is the homeostatic regulator Polo-like kinase 2 (Plk2). Similar to Aβ, Plk2 is induced in response to sustained network overactivity and functions to weaken excitatory synapses [8,21,64,65], leading to the hypothesis that Plk2 might be involved in Aβ production and/or participate in shared functions. We recently demonstrated that Plk2 is required for neuronal activity-induced production of Aβ, mediated by direct phosphorylation of APP by Plk2 at two sites, threonine-668 and serine-675 [58]. Importantly, both sites must be blocked in order to see a change in APP surface expression and β-secretase processing, providing an explanation for the lack of effect of the single threonine-668 to alanine mutation using knock-in mice [66]. Thus, combinatorial phosphorylation of APP is triggered by neuronal hyperexcitation through Plk2, leading to APP endocytosis, cleavage, and subsequent Aβ secretion. These data further implicate APP/Aβ as potential components of the HSP machinery as part of their normal physiological functions, and present Plk2 as an attractive novel target for AD drug therapy.
Future perspective
Although it is still unknown whether HSP mechanisms are related to the etiology of neurological disorders including epilepsy and AD, tantalizing clues are beginning to emerge that suggest this may be the case (Figure 1). If so, understanding these systems may allow us to harness the power of endogenous hippocampal gain control to reduce hyperexcitability and alter disease progression. Within the next decade, we may begin to see some of these ideas reaching the clinic, for example, implantable closed-loop control systems or drugs targeting putative homeostatic components such as KORs, synaptoporin or Plk2. These principles may also be useful for mitigating other neurological and neurodegenerative disorders, including Parkinson's and Huntington's diseases as well as primary tauopathies.
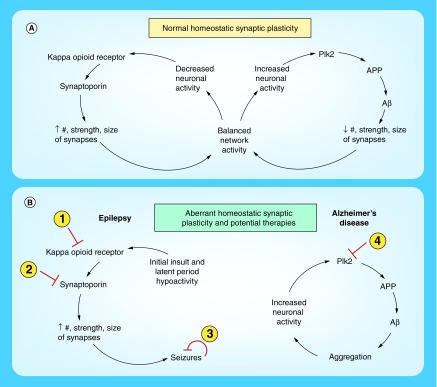
Figure 1. . Mechanisms of homeostatic synaptic plasticity that are discussed in the text.
(A) Normal homeostatic synaptic plasticity. On the left are pathways involved in homeostatic upregulation in response to chronic hypoactivity and on the right are those involved in downregulation following persistent hyperactivity. Negative feedback compensation alters synaptic number, strength or size in order to return network activity to an optimal, balanced level. Multiple homeostatic mechanisms have been identified and only a subset is depicted here. (B) Proposed mechanisms of aberrant homeostatic plasticity that may contribute to neurological disorders. On the left is a hypothetical pathway that may promote development of epilepsy, in which an initial insult leads to a latent period of hypoactivity, triggering overcompensation and excessive synaptic strengthening due to dysfunctional homeostatic machinery that ultimately leads to seizures. Therapeutic strategies for epilepsy could be to inhibit: kappa opioid receptors or synaptoporin function to prevent development of seizures or perform closed-loop bioengineering control to terminate seizures acutely. On the right is a proposed model for pathogenesis of Alzheimer's disease in which aggregation of Aβ leads to hyperactivity of neurons rather than downregulation as in the normal condition. Hyperexcitation leads to further production of Aβ via Plk2, forming a positive feedback, amplifying loop instead of a negative feedback control system. A therapeutic approach to address this model could be to inhibit Plk2 activity and interrupt the vicious cycle.
Conclusion
HSP mechanisms seek to maintain brain activity levels in an optimal and balanced state that allows for optimal physiological function. Further studies are needed to elucidate the myriad of homeostatic pathways that govern neuronal circuits and begin to appreciate the consequences of perturbing these systems. Deepening our understanding of these mechanisms may shed light on a host of neurological disorders that feature imbalanced neural activity. However, it is important to proceed cautiously, keeping in mind that for every action there is an opposite reaction, which, especially in the context of HSP, can lead to unintended consequences due to chronic administration of therapeutics.
Executive summary.
Homeostatic synaptic plasticity mechanisms maintain brain activity within a ‘normal’ range of activity
These mechanisms can include changes in the number, size and strength of synapses.
Failure of homeostatic synaptic plasticity mechanisms may result in network activity dysregulation after seizures
Periods of hypoactivity in brain regions affected by a seizure can lead to synaptic strengthening and subsequent increase in general network activity that may contribute to the development of epileptiform activity.
Harnessing homeostatic regulation could be a promising avenue of research for the treatment of epilepsy
Closed-loop control using electrical stimulation to correct aberrant network activity can decrease seizures in some patients.
To engage homeostatic synaptic plasticity (HSP) mechanisms, mild persistent overexcitation leading to synaptic weakening may be effective for long-term seizure reductions.
Engagement of HSP mechanisms in synapses that have been shown to demonstrate significant homeostatic remodeling, rather than attempting to modulate activity in the entire brain could be a more effective treatment paradigm.
Dysregulation of network activity & HSP mechanisms may also be seen in Alzheimer's disease
Increased clinical and subclinical seizure activity that could cause exacerbated symptoms has been observed in some Alzheimer's disease patients.
Extracellular Aβ concentrations, which are regulated by neuronal activity, have been shown to cause hyperexcitability at low concentrations and synaptic depression and spine loss at high concentrations.
Regulating production of Aβ may be an effective way to harness this potentially aberrant HSP mechanism.
Footnotes
Financial & competing interests disclosure
The authors have received grants from the NIH (R01NS097762 to PAF; R03AG052730 and RF1AG056603 to DTSP). The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was used in the production of this manuscript.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Queenan BN, Lee KJ, Pak DTS. Wherefore art thou, homeo(stasis)? Functional diversity in homeostatic synaptic plasticity. Neural Plast. 2012;2012:1–12. doi: 10.1155/2012/718203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang BS, Lowenstein DH. Epilepsy. N. Engl. J. Med. 2003;349:1257–1266. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- 4.Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 5.Johnstone VPA, Shultz SR, Yan EB, O'brien TJ, Rajan R. The acute phase of mild traumatic brain injury is characterized by a distance-dependent neuronal hypoactivity. J. Neurotrauma. 2014;31:1881–1895. doi: 10.1089/neu.2014.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ping X, Jin X. Transition from initial hypoactivity to hyperactivity in cortical layer v pyramidal neurons after traumatic brain injury in vivo . J. Neurotrauma. 2016;33:354–361. doi: 10.1089/neu.2015.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tortella FC, Cowan A, Belenky GL, Holaday JW. Opiate-like electroencephalographic and behavioral effects of electroconvulsive shock in rats. Eur. J. Pharmacol. 1981;76:121–128. doi: 10.1016/0014-2999(81)90493-3. [DOI] [PubMed] [Google Scholar]
- 8.Sun H, Kosaras B, Klein PM, Jensen FE. Mammalian target of rapamycin complex 1 activation negatively regulates Polo-like kinase 2-mediated homeostatic compensation following neonatal seizures. Proc. Natl Acad. Sci. 2013;110:5199–5204. doi: 10.1073/pnas.1208010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrell MJ, Group RSIES. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 10.Berényi A, Belluscio M, Mao D, Buzsáki G. Closed-loop control of epilepsy by transcranial electrical stimulation. Science. 2012;337:735–737. doi: 10.1126/science.1223154. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The first manuscript reporting closed-loop homeostatic control of seizures.
- 11.Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat. Commun. 2013;4:1376. doi: 10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The first to demonstrate closed-loop seizure control using optogenetics.
- 12.Paz JT, Davidson TJ, Frechette ES, et al. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat. Neurosci. 2013;16:64–70. doi: 10.1038/nn.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Pioneered the use of optogenetics for closed-loop control of seizures.
- 13.Kros L, Eelkman Rooda OHJ, Spanke JK, et al. Cerebellar output controls generalized spike-and-wave discharge occurrence. Ann. Neurol. 2015;77:1027–1049. doi: 10.1002/ana.24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M, Soltesz I. Cerebellar directed optogenetic intervention inhibits spontaneous hippocampal seizures in a mouse model of temporal lobe epilepsy. eNeuro. 2014;1 doi: 10.1523/ENEURO.0005-14.2014. Article ID: 1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forcelli PA. Applications of optogenetic and chemogenetic methods to seizure circuits: where to go next? J. Neurosci. Res. 2017 doi: 10.1002/jnr.24135. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klapoetke NC, Murata Y, Kim SS, et al. Independent optical excitation of distinct neural populations. Nat. methods. 2014;11:338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N. Engl. J. Med. 2011;365:919–926. doi: 10.1056/NEJMra1004418. [DOI] [PubMed] [Google Scholar]
- 18.Lee KJ, Queenan BN, Rozeboom AM, et al. Mossy fiber-CA3 synapses mediate homeostatic plasticity in mature hippocampal neurons. Neuron. 2013;77:99–114. doi: 10.1016/j.neuron.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrated that CA3 neurons play a special role in hippocampal homeostatic regulation.
- 19.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 20.Post RM, Kopanda RT, Lee A. Progressive behavioral changes during chronic lidocaine administration: relationship to kindling. Life Sci. 1975;17:943–950. doi: 10.1016/0024-3205(75)90447-6. [DOI] [PubMed] [Google Scholar]
- 21.Pak DT, Sheng M. Targeted protein degradation and synapse remodeling by an inducible protein kinase. Science. 2003;302(5649):1368–1373. doi: 10.1126/science.1082475. [DOI] [PubMed] [Google Scholar]
- 22.Fisher RS. Therapeutic devices for epilepsy. Ann. Neurol. 2012;71:157–168. doi: 10.1002/ana.22621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ang CW, Carlson GC, Coulter DA. Massive and specific dysregulation of direct cortical input to the hippocampus in temporal lobe epilepsy. J. Neurosci. 2006;26:11850–11856. doi: 10.1523/JNEUROSCI.2354-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawrence JJ, Mcbain CJ. Interneuron diversity series: containing the detonation: feed forward inhibition in the CA3 hippocampus. Trends Neurosci. 2003;26:631–640. doi: 10.1016/j.tins.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- 26.Gall C. Seizures induce dramatic and distinctly different changes in enkephalin, dynorphin, and CCK immunoreactivities in mouse hippocampal mossy fibers. J. Neurosci. 1988;8:1852–1862. doi: 10.1523/JNEUROSCI.08-06-01852.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Queenan BN, Dunn RL, Santos VR, et al. Kappa opioid receptors regulate hippocampal synaptic homeostasis and epileptogenesis. Epilepsia. 2017 doi: 10.1111/epi.13941. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overk C, Masliah E. Pathogenesis of synaptic degeneration in Alzheimer's disease and Lewy body disease. Biochem. Pharmacol. 2014;88:508–516. doi: 10.1016/j.bcp.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang SS, Chung HJ. Emerging link between Alzheimer's disease and homeostatic synaptic plasticity. Neural Plast. 2016 doi: 10.1155/2016/7969272. Article ID: 7969272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicastro N, Assal F, Seeck M. From here to epilepsy: the risk of seizure in patients with Alzheimer's disease. Epileptic Disord. 2016;18(1):1–12. doi: 10.1684/epd.2016.0808. [DOI] [PubMed] [Google Scholar]
- 31.Chin J, Scharfman HE. Shared cognitive and behavioral impairments in epilepsy and Alzheimer's disease and potential underlying mechanisms. Epilepsy Behav. 2013;26:343–351. doi: 10.1016/j.yebeh.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabrejo L, Guyant-Maréchal L, Laquerrière A, et al. Phenotype associated with APP duplication in five families. Brain. 2006;129:2966–2976. doi: 10.1093/brain/awl237. [DOI] [PubMed] [Google Scholar]
- 33.Larner AJ, Doran M. Clinical phenotypic heterogeneity of Alzheimer's disease associated with mutations of the presenilin-1 gene. J. Neurol. 2006;253:139–158. doi: 10.1007/s00415-005-0019-5. [DOI] [PubMed] [Google Scholar]
- 34.Busche MA, Eichhoff G, Adelsberger H, et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]; •• Demonstrated amyloid is associated with neuronal hyperactivity.
- 35.Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu H-Y, Hyman BT, Bacskai BJ. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minkeviciene R, Rheims S, Dobszay MB, et al. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J. Neurosci. 2009;29:3453–3462. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palop JJ, Chin J, Roberson ED, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stargardt A, Swaab DF, Bossers K. The storm before the quiet: neuronal hyperactivity and Aβ in the presymptomatic stages of Alzheimer's disease. Neurobiol. Aging. 2015;36:1–11. doi: 10.1016/j.neurobiolaging.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 39.Amatniek JC, Hauser WA, Delcastillo-Castaneda C, et al. Incidence and predictors of seizures in patients with Alzheimer's disease. Epilepsia. 2006;47:867–872. doi: 10.1111/j.1528-1167.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- 40.Mackenzie IR, Miller LA. Senile plaques in temporal lobe epilepsy. Acta Neuropathol. 1994;87:504–510. doi: 10.1007/BF00294177. [DOI] [PubMed] [Google Scholar]
- 41.Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch. Neurol. 2009;66:435. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan J, Jones NC, Bush AI, O'brien TJ, Kwan P. A mouse model of Alzheimer's disease displays increased susceptibility to kindling and seizure-associated death. Epilepsia. 2015;56:e73–e77. doi: 10.1111/epi.12993. [DOI] [PubMed] [Google Scholar]
- 43.Friedman D, Honig LS, Scarmeas N. Seizures and epilepsy in Alzheimer's disease. CNS Neurosci. Ther. 2012;18:285–294. doi: 10.1111/j.1755-5949.2011.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larner AJ. Epileptic seizures in AD patients. Neuromolecular Med. 2010;12:71–77. doi: 10.1007/s12017-009-8076-z. [DOI] [PubMed] [Google Scholar]
- 45.Lam AD, Deck G, Goldman A, Eskandar EN, Noebels J, Cole AJ. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer's disease. Nat. Med. 2017;23:678–680. doi: 10.1038/nm.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dohenya HC, Ratnaraja N, Whittingtonb MA, Jefferysc JGR, Patsalosa PN. Blood and cerebrospinal fluid pharmacokinetics of the novel anticonvulsant levetiracetam (ucb L059) in the rat. Epilepsy Res. 1999;34:161–168. doi: 10.1016/s0920-1211(98)00104-1. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez PE, Zhu L, Verret L, et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer's disease model. Proc. Natl Acad. Sci. 2012;109:E2895–2903. doi: 10.1073/pnas.1121081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Musaeus CS, Shafi MM, Santarnecchi E, Herman ST, Press DZ. Levetiracetam alters oscillatory connectivity in Alzheimer's disease. J. Alzheimer's Dis. 2017;58:1065–1076. doi: 10.3233/JAD-160742. [DOI] [PubMed] [Google Scholar]
- 49.Bakker A, Krauss GL, Albert MS, et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Documents an improvement in cognitive function in mild cognitive impairment (MCI) patients with an antiepileptic drug.
- 50.Xiao R. Levetiracetam might act as an efficacious drug to attenuate cognitive deficits of Alzheimer's disease. Curr. Top. Med. Chem. 2016;16:565–573. doi: 10.2174/1568026615666150813144603. [DOI] [PubMed] [Google Scholar]
- 51.Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 52.Dekosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann. Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 53.Scheff SW, Price DA. Synapse loss in the temporal lobe in Alzheimer's disease. Ann. Neurol. 1993;33:190–199. doi: 10.1002/ana.410330209. [DOI] [PubMed] [Google Scholar]
- 54.Scheff SW, Dekosky ST, Price DA. Quantitative assessment of cortical synaptic density in Alzheimer's disease. Neurobiol. Aging. 1990;11:29–37. doi: 10.1016/0197-4580(90)90059-9. [DOI] [PubMed] [Google Scholar]
- 55.Oddo S, Caccamo A, Shepherd JD, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 56.Cirrito JR, Yamada KA, Finn MB, et al. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo . Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 57.Kamenetz F, Tomita T, Hsieh H, et al. app processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]; •• Describes the activity-dependent processing of amyloid precursor protein and production of Abeta.
- 58.Lee Y, Lee JS, Lee KJ, Turner RS, Hoe HS, Pak DTS. Polo-like kinase 2 phosphorylation of amyloid precursor protein regulates activity-dependent amyloidogenic processing. Neuropharmacology. 2017;117:387–400. doi: 10.1016/j.neuropharm.2017.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrates that Plk2 mediates activity-stimulated production of Abeta.
- 59.Bero AW, Yan P, Roh JH, et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat. Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cirrito JR, Kang J-E, Lee J, et al. Endocytosis is required for synaptic activity-dependent release of amyloid-β in vivo . Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Puzzo D, Privitera L, Palmeri A. Hormetic effect of amyloid-beta peptide in synaptic plasticity and memory. Neurobiol. Aging. 2012;33:1484.e1415–1484.e1424. doi: 10.1016/j.neurobiolaging.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 62.Lazarevic V, Fienko S, Andres-Alonso M, et al. Physiological concentrations of amyloid beta regulate recycling of synaptic vesicles via Alpha7 acetylcholine receptor and CDK5/calcineurin signaling. Front. Mol. Neurosci. 2017;10:221. doi: 10.3389/fnmol.2017.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Busche MA, Chen X, Henning HA, et al. Critical role of soluble amyloid for early hippocampal hyperactivity in a mouse model of Alzheimer's disease. Proc. Natl Acad. Sci. 2012;109:8740–8745. doi: 10.1073/pnas.1206171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Evers DM, Matta JA, Hoe H-S, et al. Plk2 attachment to NSF induces homeostatic removal of GluA2 during chronic overexcitation. Nat. Neurosci. 2010;13:1199–1207. doi: 10.1038/nn.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee KJ, Lee Y, Rozeboom A, et al. Requirement for Plk2 in orchestrated ras and rap signaling, homeostatic structural plasticity, and memory. Neuron. 2011;69:957–973. doi: 10.1016/j.neuron.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sano Y, Nakaya T, Pedrini S, et al. Physiological mouse brain Aβ levels are not related to the phosphorylation state of threonine-668 of Alzheimer's APP. PLoS One. 2006;1:e51. doi: 10.1371/journal.pone.0000051. [DOI] [PMC free article] [PubMed] [Google Scholar]