ABSTRACT
Thermoanaerobacter kivui is one of the very few thermophilic acetogenic microorganisms. It grows optimally at 66°C on sugars but also lithotrophically with H2 + CO2 or with CO, producing acetate as the major product. While a genome-derived model of acetogenesis has been developed, only a few physiological or biochemical experiments regarding the function of important enzymes in carbon and energy metabolism have been carried out. To address this issue, we developed a method for targeted markerless gene deletions and for integration of genes into the genome of T. kivui. The strain naturally took up plasmid DNA in the exponential growth phase, with a transformation frequency of up to 3.9 × 10−6. A nonreplicating plasmid and selection with 5-fluoroorotate was used to delete the gene encoding the orotate phosphoribosyltransferase (pyrE), resulting in a ΔpyrE uracil-auxotrophic strain, TKV002. Reintroduction of pyrE on a plasmid or insertion of pyrE into different loci within the genome restored growth without uracil. We subsequently studied fructose metabolism in T. kivui. The gene fruK (TKV_c23150) encoding 1-phosphofructosekinase (1-PFK) was deleted, using pyrE as a selective marker via two single homologous recombination events. The resulting ΔfruK strain, TKV003, did not grow on fructose; however, growth on glucose (or on mannose) was unaffected. The combination of pyrE as a selective marker and the natural competence of the strain for DNA uptake will be the basis for future studies on CO2 reduction and energy conservation and their regulation in this thermophilic acetogenic bacterium.
IMPORTANCE Acetogenic bacteria are currently the focus of research toward biotechnological applications due to their potential for de novo synthesis of carbon compounds such as acetate, butyrate, or ethanol from H2 + CO2 or from synthesis gas. Based on available genome sequences and on biochemical experiments, acetogens differ in their energy metabolism. Thus, there is an urgent need to understand the carbon and electron flows through the Wood–Ljungdahl pathway and their links to energy conservation, which requires genetic manipulations such as deletion or overexpression of genes encoding putative key enzymes. Unfortunately, genetic systems have been reported for only a few acetogenic bacteria. Here, we demonstrate proof of concept for the genetic modification of the thermophilic acetogenic species Thermoanaerobacter kivui. The genetic system will be used to study genes involved in biosynthesis and energy metabolism, and may further be applied to metabolically engineer T. kivui to produce fuels and chemicals.
KEYWORDS: DNA uptake, Thermoanaerobacter kivui, acetogenesis, fructose metabolism, genetic system
INTRODUCTION
Acetogenic microorganisms are a widespread ecophysiological guild of microorganisms that fulfill important functions in anoxic environments (1). To date, all isolated acetogens belong to the domain Bacteria, and within that domain many species, including the model organisms Acetobacterium woodii, Moorella thermoacetica, and Clostridium ljungdahlii, are associated with the phylum Firmicutes (2). Recently, a metagenome study from a deep biosphere environment revealed the potential for acetogenesis in an uncharacterized archaeon (3). Many acetogens are metabolically versatile, able to degrade sugars, products of primary fermentations such as alcohols or C1 compounds (methanol, formate, and CO), and methylated nitrogen compounds such as glycine betaine (4). An important and eponymous characteristic of acetogenic bacteria is that they thrive on the production of acetic acid from H2 + CO2 (ΔG0′ = −95 kJ · mol−1). Thus, they represent an important link in the anoxic food chain between primary fermenters that produce H2 and CO2 as some of their main products and acetoclastic methanogens (5). Acetate is formed from H2 + CO2 through the Wood-Ljungdahl pathway (6, 7). Notably, this pathway is likely the oldest CO2-fixing pathway in nature (8). Moreover, it is also the only one that does not require a net investment of ATP. While the biochemistry of the Wood-Ljungdahl pathway (WLP) has been studied for decades, the mechanism of how an acetogen, the mesophilic bacterium A. woodii, conserves energy during growth on H2 + CO2 has only recently been found. Interestingly, in A. woodii, reduction of CO2 is catalyzed by soluble enzymes, including the hydrogen-dependent carbon dioxide reductase (9). Energy is conserved by a membrane-bound ferredoxin:NAD+ oxidoreductase, the Rnf complex (10). Genomes of other acetogens lack rnf genes but contain genes encoding all subunits of energy-converting hydrogenases (11), which led to a novel classification into Ech- or Rnf-containing acetogens based on the acetogen's mode of energy conservation (2). However, biochemical evidence for this notion is lacking. The thermophilic acetogenic species Thermoanaerobacter kivui is considered a model for the Ech-containing acetogens and thus a candidate to address this hypothesis.
Here, we describe the development and application of a genetic toolkit for the thermophilic acetogenic bacterium T. kivui. This bacterium also belongs to the phylum Firmicutes, and it grows optimally at 66°C, being therefore the most thermophilic acetogenic bacterium characterized yet (12). Comparably short doubling times of ∼2.5 h during growth on H2 + CO2, the lack of requirement for vitamins (12), and the recent discovery that the organism can be adapted to grow on CO (13) make the bacterium a potential candidate for bioengineering approaches toward synthesis gas fermentation. Analysis of the genome sequence revealed that energy conservation during autotrophic growth is different than that in the mesophilic acetogenic species A. woodii and C. ljungdahlii. While the genome contains the Wood-Ljungdahl pathway for CO2 fixation, genes encoding the Rnf complex are absent (14); however, energy may instead be conserved through one of two Ech hydrogenase complexes. Interestingly, the substrate range of T. kivui is rather restricted compared to that of other, nonacetogenic species within the genus Thermoanaerobacter (15) but also compared to that of other acetogens. For example, unlike the thermophilic species M. thermoacetica, T. kivui is not able to metabolize alcohols, and unlike mesophilic Sporomusa species or A. woodii, it cannot use methylated compounds such as methanol, methylamines, or glycine betaine (4). Besides H2 + CO2 and CO, T. kivui has been described to only use pyruvate, formate, and the sugars glucose, fructose, and mannose to mainly produce acetate (12). These observations indicate that the pathways of substrate oxidation, acetate formation from H2 + CO2, and energy conservation in T. kivui differ from those in other acetogens. This prompted us to develop genetic tools to study its metabolism. In this article, we describe the ability of T. kivui for DNA uptake, the generation of a uracil-auxotrophic pyrE deletion mutant strain, and the application of pyrE as a selective marker for markerless deletions on the chromosome of T. kivui.
RESULTS AND DISCUSSION
T. kivui is naturally competent for DNA uptake.
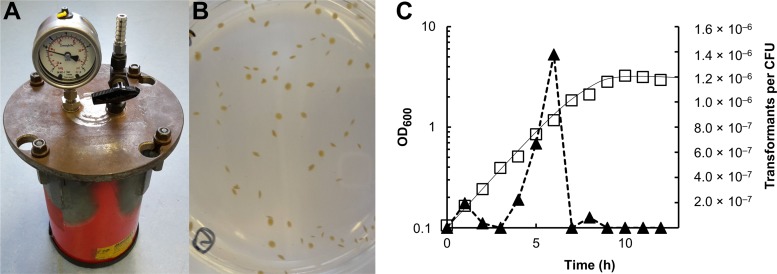
Our aim was to develop methods for genetic modification of the thermophilic acetogenic bacterium T. kivui, including both the uptake and replication of plasmids and the generation of mutations on the chromosome. Initially, we developed a protocol to grow T. kivui on solid media, enabling the selection of single colonies, which was complicated by the requirement for anoxic conditions at elevated temperatures. To address this requirement, custom-made jars made from fire extinguishers already available to the lab were filled with an oxygen-free atmosphere (approximately 1% H2 in N2/CO2 [80/20, vol/vol]) and proved to keep anoxic conditions during incubation at 65°C (Fig. 1A). Plating of T. kivui was initially performed inside an anoxic glove box; however, we found that T. kivui tolerates short exposure to oxygen at room temperature (∼22°C), likely because the strain is thermophilic, with no growth or metabolic activity much below 50°C (12). Maintaining sterile conditions and general handling are much easier outside the anoxic glove box; therefore, we decided to routinely pour the plates at ambient temperature and air and subsequently transferred them to an anoxic glove box, allowing the agar medium to solidify before incubation in the jars. T. kivui formed yellowish to brownish convex colonies when embedded in the agar medium (Fig. 1B), as observed by Leigh et al. (12). Regularly, 5% to 10% of the cells from the liquid medium formed colonies, with a maximal observed plating efficiency of ∼70%. Streaking a culture on the surface of solidified medium, as performed with aerobes, also worked for T. kivui as described before (12), but colonies were less defined, and the plating efficiency was lower.
FIG 1.
Growth of T. kivui in solid medium and natural competence for DNA uptake. (A) Sealed metal jar for anaerobic incubation of T. kivui at 65°C. (B) T. kivui embedded in agar medium formed yellow to brownish disc-like colonies. (C) Natural competence of T. kivui during growth on complex medium with glucose at 65°C. One-ml subsamples of two growing cultures were incubated with 250 ng of plasmid pMU131 (16), and then embedded in complex medium with or without 200 μg · ml−1 kanamycin. Squares, representative growth curve; triangles, corresponding transformants per CFU.
Having established a method for growth on solid medium, the next step was to find a method to insert foreign DNA into the cells. The ability to take up plasmid DNA by natural competence has been described for several Thermoanaerobacter species and for the related Thermoanaerobacterium saccharolyticum (16). Therefore, we decided to obtain a plasmid, pMU131, that was used by Shaw and colleagues (16) and that contains a native origin of replication for Thermoanaerobacterium, a PUC origin for replication in Escherichia coli, and Ampr for ampicillin selection in Escherichia coli. The plasmid also contained a thermostable kanamycin resistance cassette from Staphylococcus aureus (Kanr) that obviously conferred resistance to higher concentrations of kanamycin (>100 μg · ml−1) in several Thermoanaerobacter species (16). That observation was valuable to us, as thermostable antibiotics and proteins conferring mediating resistance to antibiotics at higher temperatures are scarce, and only a few selectable markers for kanamycin, hygromycin, bleomycin (17, 18), and thiamphenicol (18) resistance are available for the genetic manipulation of thermophilic bacteria. The MIC of kanamycin that did not allow growth of T. kivui in liquid and solid agar medium was determined to be 200 μg · ml−1, which is in the range of reported values for other Thermoanaerobacter species (16). Subsequently, growing cultures of T. kivui were incubated in the presence of plasmid pMU131 (0.5 μg · ml−1) until the stationary phase was reached (optical density at 600 nm [OD600], ≥2.5), and then plated. As observed for other Thermoanaerobacter species, after 2 to 3 days of anoxic incubation at 65°C, kanamycin-resistant colonies only occurred on plates with T. kivui that had been grown previously in the presence of plasmid pMU131. Colonies were transferred to liquid medium containing 200 μg · ml−1 kanamycin, and after growth had been observed, the presence of the plasmid in the kanamycin resistant isolates was verified by reisolation of the plasmid, followed by PCR or by restriction digest analysis (data not shown). This showed that T. kivui is naturally competent for DNA uptake, as reported for other Thermoanaerobacter species (16). Unlike in the related species Thermoanaerobacterium saccharolyticum, where the highest transformation frequencies were observed in the early exponential growth phase (16), DNA uptake in T. kivui mainly occurred in the mid-exponential growth phase (Fig. 1C). Accordingly, transcript levels of genes annotated as comEA (TKV_RS04530), comEC1 (TKV_RS04705), comEC2 (TKV_RS11745), and recA (TKV_RS06255), which are involved in natural competence for DNA uptake in other species, e.g., in the related Gram-positive Bacillus subtilis (19) or in T. saccharolyticum (16), were increased by factors of ∼ 2.5 ± 0.4, 2.1 ± 0.4, 2.4 ± 0.8, and 2.1 ± 1.0 in the mid-exponential (OD600 = 1.3) versus the early exponential (OD600 ∼ 0.3) growth phases. Yet, the molecular mechanism of DNA uptake in T. kivui has not been studied, and may or may not be similar to what has been published for other Gram-positive anaerobes. A transformation frequency—the fraction of living cells that took up plasmid pMU131—of up to 3.9 × 10−6 was observed, which is in the range of that reported for other Thermoanaerobacter species (16). In the mid-exponential growth phase (∼4 × 108 cells · ml−1), about 103 cells were transformed per μg plasmid DNA (9.1 × 102 μg−1, 0.25 μg DNA provided per ml culture). A high transformation efficiency was also observed at a DNA concentration of 0.8 μg · ml−1 (7.1 × 102 μg−1), while the transformation efficiencies slightly decreased if 1.0 μg · ml−1 or more DNA was provided (≤3.3 × 102 μg−1). Taken together, we demonstrated that T. kivui is naturally competent for DNA uptake, and a thermostable kanamycin resistance cassette, Kanr, can be used as selective marker.
Generation of a pyrE deletion mutant.
Only a few genetic systems for markerless mutants have been reported for anaerobic thermophiles. The most commonly used selection mechanism is based on the requirement for uracil for the biosynthesis of pyrimidines (17). On the one hand, incubation with 5-fluoroorotate (5-FOA) allows selection against the presence of either gene (pyrE or pyrF) encoding orotate phosphoribosyltransferase and orotidine 5′-phosphate decarboxylase, respectively, as 5-fluoroorotic acid is converted to toxic 5-fluorouracil in the presence of both enzymes. On the other hand, reintroduction of the respective gene complements the uracil auxotrophy, again leading to a 5-FOA-sensitive phenotype. Genetic systems based on uracil auxotrophy and sensitivity to 5-FOA have been developed, e.g., for the hyperthermophilic archaeon Thermococcus kodakarensis (20) and for the extreme thermophilic Thermoanaerobacter-related plant biomass degrader Caldicellulosiruptor bescii (21, 22). Many Thermoanaerobacter species, however, require the addition of yeast extract, which contains uracil, to the medium. Hence, for some species, kanamycin resistance cassettes have been integrated to disrupt genes (23), or alternative genetic markers such as thymidine kinase have been used (24).
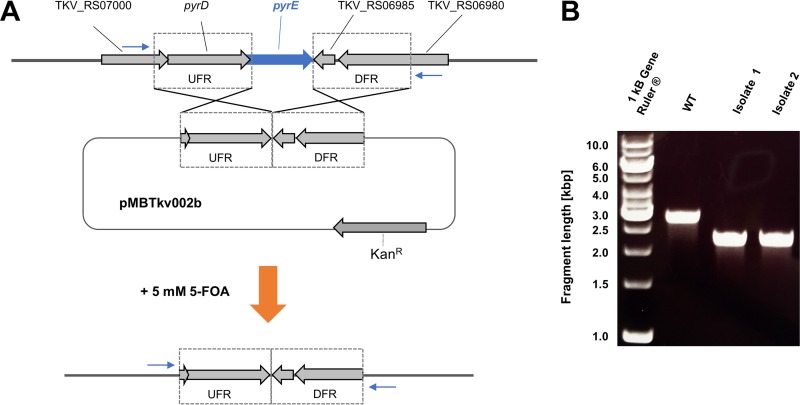
As mentioned above, T. kivui does not depend on the addition of yeast extract or even on vitamins (12), enabling us to use pyrE as a genetic marker. This was fortunate, as our goal was the development of a markerless mutagenesis system in T. kivui. Initially, to enforce integration of DNA into the T. kivui genome, a basic plasmid (pMBTkv001) was generated that contained Kanr but not the putative Thermoanaerobacterium origin of replication. As expected, transformation with pMBTkv001 per se did not result in any kanamycin-resistant colonies. We then fused ∼1-kbp upstream and downstream regions (UFR and DFR, respectively) flanking the pyrE gene of T. kivui into the multiple cloning site of pMBTkv001, resulting in plasmid pMBTkv002b (Fig. 2A). This enabled homologous recombination at the UFR or DFR site, and integration of pMBTkv002b into the genome resulted in kanamycin-resistant colonies of T. kivui. When we isolated DNA from these colonies and verified by PCR the integration of the plasmid into the genome, we also observed an additional smaller DNA fragment that already indicated loss of pyrE in a subpopulation. Therefore, we concluded that plasmid DNA does not only integrate into the genome by a single homologous recombination event but also via double homologous recombination (see also Fig. 4B). This prompted us to switch our strategy. T. kivui was then transformed with plasmid pMBTkv002b and immediately subjected to 5-FOA in the presence of uracil to select for the loss of pyrE. Seven isolates were obtained that were resistant to 5-FOA but dependent on uracil, and which lacked pyrE, as verified by PCR (Fig. 2B). We selected one of them and verified the clean and markerless deletion of pyrE by sequencing the PCR product. Unlike the wild-type strain, the respective T. kivui ΔpyrE isolate, TKV002, no longer grew in the absence of uracil in minimal medium (Fig. 3A). However, uptake of plasmid pKOM1 and pKOM2 containing pyrE either under the control of the promoter of the Kanr cassette employed, Pkan (Fig. 3A), or under the control of the gyrase promoter from Thermoanaerobacter sp. strain X514, PgyrX514, respectively, fully restored growth of T. kivui TKV002 in minimal medium without uracil.
FIG 2.
Deletion of pyrE. (A) T. kivui was transformed with plasmid pMBTkv002b, which was constructed for the deletion of the pyrE gene via double homologous recombination using 1-kbp upstream and downstream flanking regions (UFR and DFR). After transformation, T. kivui was plated in the presence of 5 mM 5-fluoroorotic acid (5-FOA). (B) The loss of pyrE (573 bp) was verified by PCR using primers binding outside the flanking regions (blue arrows in panel A). Shown is the electrophoretic separation of the DNA fragments from the PCRs using genomic DNA of T. kivui DSM2030 (wild type, WT) and of two 5-FOA-resistant isolates.
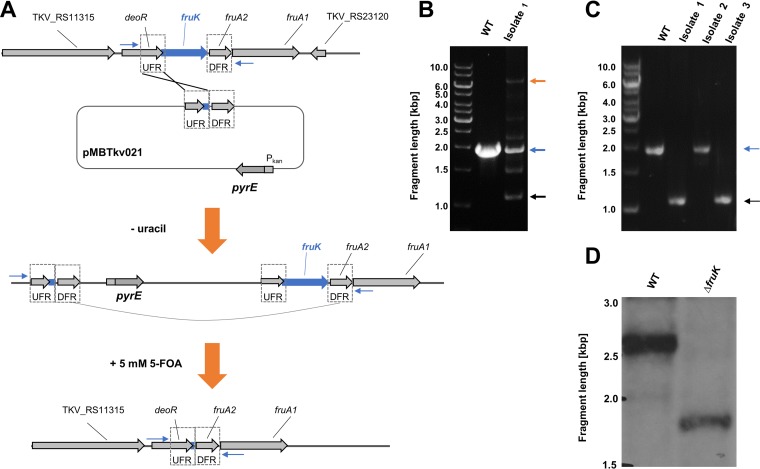
FIG 4.
Deletion of fruK. (A) Strategy for deletion of fruK using plasmid pMBTkv021 via two independent homologous recombination events. (B) DNA fragments separated by agarose gel electrophoresis after PCR amplification of the fruK locus from genomic DNA of wild type (WT) T. kivui and of uracil-auxotrophic isolates after selection against uracil auxotrophs in the first round of selection and (C) 5-FOA resistant isolates after the second round of selection. Putative plasmid integration, orange arrow; wild type allele, blue arrow; fruK deletion (black arrow). (D) Southern blot analysis of the fruK locus with NsiI-digested DNA from T. kivui DSM2030 (wild type, WT; expected fragment size, 2,668 bp) and the ΔfruK strain (expected fragment size, 1,735 bp).
FIG 3.
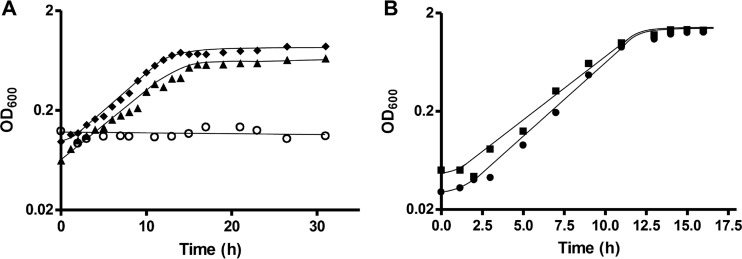
Physiology of the T. kivui ΔpyrE strain TKV002. (A) Growth of the wild type (DSM 2030, filled triangles), T. kivui TKV002 (open circles), and T. kivui TKV002 containing plasmid pKOM1 encoding pyrE under the control of Pkan (filled diamonds), without the addition of uracil. (B) Growth of T. kivui TKV002 with Pkan-pyrE reinserted into the genome in between open reading frames (ORFs) TKV_c24500 and TKV_c24520 (filled squares), and growth of T. kivui TKV002 in the presence of 40 μM uracil (closed circles). All experiments were performed in minimal medium with glucose (25 mM) at 65°C. Shown is one representative experiment out of three independent biological replicates.
Subsequently, we aimed to complement the uracil auxotrophy of strain TKV002 by reintroducing pyrE into the genome. Purposely, to avoid polar effects, this was not performed at its original locus. Instead, we found a region between the convergent genes TKV_c24500 and TKV_c24520 that may not be transcribed. Subsequently, plasmid pMBTkv007 was manufactured for the reintegration of the Pkan-pyrE cassette into the genome between these two genes. The resulting strain with Pkan-pyrE integrated between the genes TKV_c24500 and TKV_c24520 was indeed able to grow again on minimal medium without uracil (Fig. 3B). One the one hand, this enabled us to use pyrE as a genetic marker for markerless deletions. On the other hand, as we did not observe adverse effects on growth of the resulting strain, the region between the convergent genes TKV_c24500 and TKV_c24520 may be useful for integration of genes or metabolic pathways in bioengineering approaches. For example, similarly, genes for a metabolic pathway to produce 3-hydroxypropionate have been inserted into a region with little transcriptional activity in the genome of the hyperthermophilic archaeon Pyrococcus furiosus (25).
fruK deletion mutant.
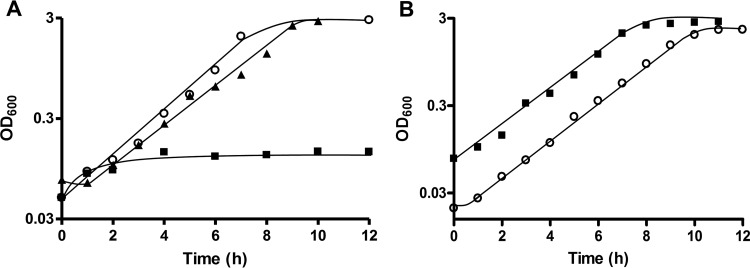
As described above, T. kivui grows with fructose as sole carbon and energy source, with an observed approximate doubling time of 1.3 h (see also Fig. 5A). The genome of T. kivui contains a gene cluster encoding a putative phosphotransferase uptake system (genes fruA1 and fruA2) and a putative 1-phosphofructokinase (14). Additionally, the gene cluster includes a gene for a deoR-type regulator (Fig. 4A). The organization of the gene cluster is similar to what has been described in Lactococcus lactis (26), where fructose-1-phosphate has been demonstrated to accumulate in the cell and to act as an effector molecule. While a detailed study of fructose metabolism and its regulation in T. kivui was not the primary subject of this study, we aimed for a markerless deletion of fruK to demonstrate the use of pyrE as selective marker. T. kivui TKV002 was transformed with plasmid pMBTkv021, which contained pyrE and the UFR and DFR of fruK. In the first round of selection, we obtained uracil-prototroph colonies that contained the plasmid (Fig. 4B) and that were subjected to 5 mM 5-FOA in the second round of selection (Fig. 4A). 5-FOA-resistant colonies that did not contain pyrE anymore partly reverted to the parent strain TKV002, but some did not contain fruK (Fig. 4C) anymore. The clean and markerless deletion of fruK was verified by PCR, by sequencing, and by Southern blot analysis (Fig. 4D). The resulting uracil-dependent ΔfruK strain, TKV003, did not grow anymore on complex medium with fructose as sole source of energy, carbon, and electrons (Fig. 5A), even after prolonged incubation time (>3 days), while its growth with glucose (Fig. 5B) or mannose remained unaffected. Finally, the uptake of a plasmid (pKOM3) containing fruK under the control of Pkan restored the growth of strain TKV003 on fructose (doubling time [tD], 1.7 h). While biochemical evidence that T. kivui fruK encodes a 1-phosphofructokinase is pending, the presented phenotype clearly demonstrated the essential role of the gene product of fruK in fructose metabolism. Moreover, the deletion of fruK and its complementation represents a promising proof-of-concept for future genetic manipulations of the thermophilic acetogenic bacterium Thermoanaerobacter kivui, toward understanding of its energy metabolism, and potentially toward its biotechnological application.
FIG 5.
FruK is essential for fructose metabolism. (A) Growth of the T. kivui strain ΔfruK strain TKV003 (closed squares) and the wild-type strain DSM 2030 (open circles) in the presence of 40 μM uracil. Growth of T. kivui TKV003 with plasmid pKOM3 (with fruK under the control of Pkan) in the absence of uracil (closed triangles). All experiments were performed on complex medium with 25 mM fructose at 65°C. (B) Growth of T. kivui TKV003 (closed squares) in the presence of uracil and the wild-type strain DSM 2030 (open circles) on complex medium with 25 mM glucose. Shown are data from one representative experiment out of three independent biological replicates.
MATERIALS AND METHODS
Growth conditions.
T. kivui strain LKT-1 (DSM2030), referred to as wild type, and all derived mutant strains (Table 1) were routinely cultivated under strict anoxic conditions at 65°C. Complex medium contained 2 g · liter−1 yeast extract and was prepared as described previously (13). Defined minimal medium was prepared as described previously (13), with the following slight modifications. It contained 60 mM NaHCO3, 3.7 mM KH2PO4, 7.5 mM NH4Cl, 1.6 mM MgCl2 · 6H2O, 6.8 mM NaCl, 0.34 mM CaCl2 · 2H2O, 3 mM cysteine-HCl, 10 ml · liter−1 trace element solution DSM141, 10 ml · liter−1 vitamin solution DSM141, and 4 μM resazurin. The medium was flushed with N2/CO2 (80/20 vol/vol) before autoclaving. All gases, including the N2/CO2 and H2/CO2 gas mixtures were purchased from Praxair Deutschland GmbH (Düsseldorf, Germany). Unless denoted otherwise, glucose from a sterile anoxic stock solution was added as growth substrate to a final concentration of 25 mM after autoclaving.
TABLE 1.
Strains used in this study
| Strain | Deleted gene(s) (locus) | Parent strain | Reference or source |
|---|---|---|---|
| DSM 2030 | NAa | NA | 12 |
| TKV002 | pyrE (TKV_c14380) | DSM 2030 | This study |
| TKV003 | pyrE, fruK (TKV_c23150) | TKV002 | This study |
NA, not applicable.
All growth experiments were carried out under strict anoxic conditions using 20-ml Hungate glass tubes or in 100-ml serum bottles filled with 10 ml or 50 ml medium, respectively, and sealed with butyl rubber stoppers. The gas phase contained a mixture of N2 and CO2 (80/20, vol/vol; at 110 kPa). Growth in liquid medium was monitored by measuring the optical density at 600 nm.
Agar medium was supplemented with 1.5% Bacto agar (BD Difco, BD Life Sciences, Heidelberg, Germany). After autoclaving, the medium was allowed to cool to below 60°C before addition of substrates, kanamycin, uracil, or 5-fluoroorotic acid (5-FOA), if needed. Routinely, cell suspensions (100 μl to 650 μl) were mixed with 8 to 25 ml agar-containing medium and poured onto petri dishes, which were immediately transferred to an anoxic glove box (Coy Laboratory Products, Grass Lake, MI) and allowed to solidify at ambient temperature. Alternatively, cells were also transferred by an inoculation loop onto the surface of agar-containing medium which had been allowed to solidify at ambient air. The solid agar plates were subsequently transferred to a custom-made jar (Fig. 1A) containing palladium catalyst (Oxoid, Hampshire, England). The jar was sealed inside the anoxic glove box and the gas atmosphere inside the jar was adjusted so that it finally contained approximately 1% H2 in N2/CO2 (80/20, vol/vol) at 50 kPa overpressure. The jar containing the plates was then transferred to incubation at 65°C for 2 to 3 days. Before the jar was opened to evaluate growth on solid agar medium, it was allowed to cool to room temperature. Screening for colonies and transfer of colonies to liquid medium was regularly performed under oxic conditions at ambient temperature.
Chemical analyses.
Fructose was determined by high-performance liquid chromatography (HPLC) (P680 pump, ASI-100 autosampler, TCC-100 column compartment; Dionex, Idstein, Germany). Samples were centrifuged and the supernatant was passed through a 0.45-μm filter to remove particles. Sugars were separated on a HyperREZ XP Carbohydrate Ca2+ column at 80°C (ThermoFisher Scientific, Waltham, USA) using degassed and filtered water as eluent at a flow rate of 0.6 ml · min−1. Compounds were detected using a RefractoMax 512 refractive index detector (ThermoFisher Scientific, Waltham, USA). Organic acids, including acetate and short-chain alcohols such as ethanol, were detected by gas chromatography using a Clarus 580 GC gas chromatograph (PerkinElmer, Waltham, MA, USA) as described previously (13).
Transformation of T. kivui.
DNA uptake by T. kivui was routinely achieved by natural transformation. Therefore, 1 to 2.5 μg of plasmid DNA was added to a freshly inoculated 5-ml culture of T. kivui. Cells were incubated at 65°C overnight (∼16 h) and subsequently, 100 to 500 μl of the dense culture (OD600 ≥2.5 for complex medium, OD600 ≥0.8 for defined medium) was embedded in agar medium as described above. Transformation efficiency was studied using plasmid pMU131 (16), which confers resistance to kanamycin (200 μg · ml−1). To test growth-phase dependency of plasmid DNA uptake, 1-ml subsamples of a growing T. kivui culture were taken and incubated with 250 ng plasmid pMU131 for a period of 1 h at 65°C and then embedded in agar medium containing kanamycin. As no DNA uptake was observed in agar medium, no DNase treatment was necessary to remove excess DNA before plating.
For the determination of the optimal amount of DNA for uptake, 1-ml subsamples of a growing T. kivui culture from the mid-exponential phase were incubated with different amounts of plasmid pMU131 for a period of 1 h and then plated. Plasmid DNA was routinely isolated from kanamycin-resistant T. kivui isolates, and the presence of pMU131 was verified by PCR or by restriction analysis. Plasmid DNA for transformation of T. kivui for sequencing and restriction analysis was isolated with the GenElute miniprep kit (Sigma-Aldrich, Munich, Germany).
Gene expression analysis.
The 10-ml cultures were rapidly cooled to 20°C or lower in an ice-water bath. Cells were pelleted by centrifugation and subsequently resuspended in 1 to 2 ml 1× salt solution containing 8.9 g · liter−1 Na2HPO4 · 2H2O, 7.8 g · liter−1 NaH2PO4 · 2H2O, 0.22 g · liter−1 KH2PO4, 0.22 g · liter−1 K2HPO4, 0.31 g · liter−1 (NH4)Cl, 0.22 g · liter−1 (NH4)2SO4, 0.45 g · liter−1 NaCl, 0.09 g · liter−1 MgSO4, 0.006 g · liter−1 NaCl, 0.002 g · liter−1 FeSO4. Lysozyme (3 mg · ml−1) was added, and the suspension was incubated for 30 min at room temperature. Afterwards, approximately 30 glass beads, 350 μl Lysis Solution R (InviTrap Spin Cell RNA minikit 0113; Stratec Molecular GmbH, Berlin, Germany), and 5 μl mercaptoethanol were added to the suspension, and cells were lysed in a cell disruptor (MM301; Retsch, Haan, Germany) for 5 min at 50 Hz. After separation of the cell debris by centrifugation (14,000 × g, 5 min, 4°C), RNA was isolated from the supernatant using the InviTrap Spin Cell RNA minikit according to the manufacturer's protocol. Ten μg of eluted RNA was subjected to a DNase treatment with TurboDNAse (Ambion Thermo Fisher Scientific, Waltham, USA). RNA was subsequently precipitated with 2.5 volumes of ethanol in the presence of 0.3 M sodium acetate at −20°C, pelleted by centrifugation, washed with 70% ethanol, and subsequently eluted in RNase-free water. cDNA synthesis was carried out with 1 μg of DNA-free RNA using murine leukemia virus (M-MLV) reverse transcriptase according to the manufacturer's protocol (Promega, Madison, USA). Relative transcript analysis was performed with cDNA from three independent biological replicates in a Rotor Gene RG-3000 quantitative PCR (qPCR) cycler (Corbett Research, Cambridge, UK) using SYBR green qPCR Kits (Fisher Scientific GmbH, Schwerte, Germany) according to the manufacturer's protocol. Primers used for amplification were MB_IG_0007 and MB_IG_0008 (comEA, TKV_c09400/TKV_RS04530), MB_IG_0033 and MB_IG_0034 (comEC1, TKV_c09780/TKV_RS04705), MB_IG_0011 and MB_IG_0012 (comEC2, TKV_c24050/TKV_RS11745), and MB_IG_0013 and MB_IG_0014 (recA, TKV_c12920/TKV_RS06255). Relative transcript levels were calculated using the housekeeping gene gyrase (TKV_c00100TKV_RS00050) amplified with primers gyrB_for_qRT/gyrB_rev_qRT as reference. Sequences of all primers used in this study are listed in Table 2.
TABLE 2.
Primers used in this study
Deletion of pyrE.
Genomic DNA (gDNA) was extracted after Zhou et al. (27). All molecular cloning procedures, transformation in E. coli DH5α, and colony screening were performed according to standard protocols (28). Initially, a suicide plasmid lacking a functional origin of replication for T. kivui, pMBTkv001, was developed. Therefore, the kanamycin resistance cassette Kanr of plasmid pMU31 was amplified by PCR using primers MB_1001 and MB_1002, and then inserted into the multiple cloning site of plasmid PUC19 using SphI and HindIII. For the development of plasmid pMBTkv002b for the deletion of pyrE, initially, 1-kb upstream (UFR) and downstream (DFR) regions flanking pyrE (TKV_c14380; TKV_RS06990) were amplified by PCR using primer pairs MB_1003/MB_IG_0029 and MB_IG_0030/MB_1006, respectively. Subsequently, the UFR and DFR DNA fragments were fused by overlap extension PCR using primers MB_1003/MB_1006, and plasmid pMBTkv_001 was amplified using primers MBIG_0031 and MB_IG_0032. Finally, the UFR-DFR fusion was inserted into pMBTkv_001 using a Gibson Assembly Mastermix (NEB, Frankfurt am Main, Germany), and E. coli DH5α was transformed with the reaction mix. The plasmid was retrieved from E. coli DH5α and transformed into T. kivui. Colonies resistant to kanamycin (200 μg · ml−1) were transferred to liquid complex medium containing kanamycin (200 μg · ml−1). In the second round, selection for loss of the plasmid and of pyrE was performed using 5 mM 5-fluoroorotic acid, (5-FOA), while the medium contained 40 μM uracil to complement auxotrophic pyrE mutants. Alternatively, selection for the loss of pyrE without integration of the whole plasmid was directly performed using 5 mM 5-FOA and 40 μM uracil. Integration into the pyrE locus and loss of pyrE was verified by PCR using primers MB_IG_0006 and MB_IG_0005. Deletion of pyrE was additionally verified by sequencing the locus.
To generate replicating plasmids pKOM1 and pKOM2 for complementation of the uracil auxotrophy of strain TKV002, the kanamycin resistance cassette in plasmid pMU131was replaced with pyrE. For plasmid pKOM1 the plasmid backbone of pMU131, excluding Kanr but including the promoter Pkan, was amplified by PCR using primers MB_IG_0024 and MB_IG_0028 and fused to pyrE of T. kivui, which was amplified by primers MB_IG_0018 and MB_IG_0027 using the Gibson Assembly Mastermix. For the construction of plasmid pKOM2, the promoter of gyrase PgyrX514 from Thermoanaerobacter sp. strain X514 was amplified using primers MB_0029 and MB0032. The pyrE gene fragment was amplified as for plasmid pKOM1, and the plasmid backbone of pMU131 was amplified using primers MB_0028 and MB_IG_024. All three products were fused using the Gibson Assembly Mastermix to generate plasmid pKOM2. pKOM1 and pKOM2 were transformed into T. kivui as described above, although minimal medium without uracil was used to allow for selection of uracil prototrophs.
Plasmid pMBTkv007 was constructed to reintroduce pyrE into the genome between the convergent genes TKV_c24500 and TKV_c24520. The UFR and DFR (∼1000 bp each) of that genome region were amplified by PCR using primer pairs LH013/LH024 and LH017/LH018, respectively. PgyrX514 from Thermoanaerobacter species strain X514 and pyrE from T. kivui genomic DNA were amplified by PCR using primer pairs LH025/MB_0032 and MB_IG_0027/LH016, respectively. The four PCR products were fused by PCR, digested with BamHI and XbaI, and ligated into plasmid pMBTkv001. T. kivui was transformed with plasmid pMBTkv007 and selection for transformants with pyrE was performed using minimal medium without uracil. Integration of pyrE was verified by PCR, with primers LH029 and LH028 binding outside the locus, and by subsequent sequencing of the PCR product.
Deletion of fruK.
Initially, a basic plasmid, pMBTkv005, was constructed for generating markerless deletions in the genome of T. kivui. pMBTkv005 is derived from pMBTkv001, except that Kanr was replaced with T. kivui pyrE, amplified by PCR using primers LH009 and LH010, and fused to the pMBTkv001 backbone (which was amplified by PCR using primers MB_IG_0024 and MB_IG_0028) by Gibson Assembly Mastermix. pMBTkv005 lacked an origin of replication that was functional in T. kivui.
Plasmid pMBTkv021 for the deletion of fruK (TKV_RS11305; old locus tag TKV_c23150) was generated from plasmid pMBTkv005. Initially, 0.5-kbp upstream (UFR) and downstream (DFR) regions flanking fruK were amplified using the primer pairs MB_1007/MB_1008 and MB_1009/MB_1010, respectively. The flanking regions were fused by overlap extension PCR using primers MB_1007 and MB_1010, digested with BamHI and XbaI, and ligated into the multiple cloning site of pMBTkv005. The resulting plasmid pMBTkv021 was transformed into T. kivui. Selection for transformants containing the plasmid integrated into the genome was performed using minimal medium without uracil. Integration of the plasmid was verified by PCR using primer LH038 and LH039. In the second round, selection for loss of the plasmid and of pyrE was performed using 5 mM 5-fluoroorotic acid, while the minimal medium contained 40 μM uracil. Uracil-auxotrophic isolates were screened by PCR for the loss of fruK using primers LH038 and LH039. Deletion of pyrE was additionally verified by sequencing the locus and by Southern blot analysis (see below).
Replicating plasmid pKOM3 was constructed to complement the deficiency to grow on fructose. Therefore, fruK was amplified by PCR using primer pair LH056/LH057 and the backbone of plasmid pKOM1 was amplified by PCR using primer pair LH058/LH059, and both fragments were fused using the Gibson Assembly Mastermix.
Southern blot analysis.
The genotypes of wild type and mutants in the fru gene cluster were analyzed by hybridization with digoxigenin (DIG)-labeled DNA according to standard procedures (28), with the following modifications. A 506-bp-long probe for the fruK locus was generated using primers LH054 and LH055, in a PCR with 20 μM labeled UTP and a deoxynucleoside triphosphate (dNTP) mixture (0.24 mM dGTP, 0.24 mM dCTP, 0.16 mM dATP, and 0.03 mM dTTP). T. kivui genomic DNA was extracted using the DNeasy blood and tissue kit (Qiagen, Hilden, Germany). One μg of genomic DNA was digested with NsiI. DNA fragments were separated on a 1% agarose gel and subsequently transferred to a charged nylon membrane (Genescreen Plus hybridization transfer membrane; PerkinElmer, Waltham, MA, USA) by capillary transfer. The membrane was hybridized with the probe at 50°C overnight. Binding of the probe to the anti-DIG antibody (Roche, Basel, Switzerland) and detection of DIG-labeled DNA fragments with CDP-Star (Roche) was performed according to the manufacturer's recommendations.
ACKNOWLEDGMENTS
We thank Dan Olson and Lee Lynd (Dartmouth College, NH, USA) for providing us with plasmid pMU131. We are indebted to Michael Rother (TU Dresden, Germany) for the development of the metal jars used for the anaerobic incubation of T. kivui on solid medium.
We are grateful to Deutsche Forschungsgemeinschaft (DFG) for financial support.
REFERENCES
- 1.Drake HL, Gössner AS, Daniel SL. 2008. Old acetogens, new light. Ann N Y Acad Sci 1125:100–128. doi: 10.1196/annals.1419.016. [DOI] [PubMed] [Google Scholar]
- 2.Schuchmann K, Müller V. 2014. Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria. Nat Rev Microbiol 12:809–821. doi: 10.1038/nrmicro3365. [DOI] [PubMed] [Google Scholar]
- 3.He Y, Li M, Perumal V, Feng X, Fang J, Xie J, Sievert SM, Wang F. 2016. Genomic and enzymatic evidence for acetogenesis among multiple lineages of the archaeal phylum Bathyarchaeota widespread in marine sediments. Nat Microbiol 1:16035. doi: 10.1038/nmicrobiol.2016.35. [DOI] [PubMed] [Google Scholar]
- 4.Schuchmann K, Müller V. 2016. Energetics and application of heterotrophy in acetogenic bacteria. Appl Environ Microbiol 82:4056–4069. doi: 10.1128/AEM.00882-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schink B, Stams A. 2006. Syntrophism among prokaryotes, p 309–336. In Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (ed), The prokaryotes: a handbook on the biology of bacteria, 3rd ed, vol 2 Springer Science+Business Media, Berlin, Germany. [Google Scholar]
- 6.Wood HG, Ragsdale SW, Pezacka E. 1986. The acetyl-CoA pathway of autotrophic growth. FEMS Microbiol Lett 39:345–362. doi: 10.1111/j.1574-6968.1986.tb01865.x. [DOI] [Google Scholar]
- 7.Ljungdahl LG. 1986. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol 40:415–450. doi: 10.1146/annurev.mi.40.100186.002215. [DOI] [PubMed] [Google Scholar]
- 8.Weiss MC, Sousa FL, Mrnjavac N, Neukirchen S, Roettger M, Nelson-Sathi S, Martin WF. 2016. The physiology and habitat of the last universal common ancestor. Nat Microbiol 1:16116. doi: 10.1038/nmicrobiol.2016.116. [DOI] [PubMed] [Google Scholar]
- 9.Schuchmann K, Müller V. 2013. Direct and reversible hydrogenation of CO2 to formate by a bacterial carbon dioxide reductase. Science 342:1382–1385. doi: 10.1126/science.1244758. [DOI] [PubMed] [Google Scholar]
- 10.Biegel E, Müller V. 2010. Bacterial Na+-translocating ferredoxin: NAD+ oxidoreductase. Proc Natl Acad Sci U S A 107:18138–18142. doi: 10.1073/pnas.1010318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedderich R, Forzi L. 2005. Energy-converting [NiFe] hydrogenases: more than just H2 activation. J Mol Microbiol Biotechnol 10:92–104. doi: 10.1159/000091557. [DOI] [PubMed] [Google Scholar]
- 12.Leigh JA, Mayer F, Wolfe RS. 1981. Acetogenium kivui, a new thermophilic hydrogen-oxidizing, acetogenic bacterium. Arch Microbiol 129:275–280. doi: 10.1007/BF00414697. [DOI] [Google Scholar]
- 13.Weghoff MC, Müller V. 2016. CO metabolism in the thermophilic acetogen Thermoanaerobacter kivui. Appl Environ Microbiol 82:2312–2319. doi: 10.1128/AEM.00122-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hess V, Poehlein A, Weghoff MC, Daniel R, Müller V. 2014. A genome-guided analysis of energy conservation in the thermophilic, cytochrome-free acetogenic bacterium Thermoanaerobacter kivui. BMC Genomics 15:1139. doi: 10.1186/1471-2164-15-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onyenwoke RU, Wiegel J. 2015. Thermoanaerobacter. In Whitman WB. (ed), Bergey's manual of systematics of Archaea and Bacteria. John Wiley & Sons, Hoboken, NJ. [Google Scholar]
- 16.Shaw AJ, Hogsett DA, Lynd LR. 2010. Natural competence in Thermoanaerobacter and Thermoanaerobacterium species. Appl Environ Microbiol 76:4713–4719. doi: 10.1128/AEM.00402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeldes BM, Keller MW, Loder AJ, Straub CT, Adams MWW, Kelly RM. 2015. Extremely thermophilic microorganisms as metabolic engineering platforms for production of fuels and industrial chemicals. Front Microbiol 6:1209. doi: 10.3389/fmicb.2015.01209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson DG, Maloney M, Lanahan AA, Hon S, Hauser LJ, Lynd LR. 2015. Identifying promoters for gene expression in Clostridium thermocellum. Metab Eng Commun 2:23–29. doi: 10.1016/j.meteno.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen I, Dubnau D. 2004. DNA uptake during bacterial transformation. Nat Rev Microbiol 2:241–249. doi: 10.1038/nrmicro844. [DOI] [PubMed] [Google Scholar]
- 20.Sato T, Fukui T, Atomi H, Imanaka T. 2003. Targeted gene disruption by homologous recombination in the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J Bacteriol 185:210–220. doi: 10.1128/JB.185.1.210-220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung D, Cha M, Farkas J, Westpheling J. 2013. Construction of a stable replicating shuttle vector for Caldicellulosiruptor species: use for extending genetic methodologies to other members of this genus. PLoS One 8:e62881. doi: 10.1371/journal.pone.0062881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipscomb GL, Conway JM, Blumer-Schuette SE, Kelly RM, Adams MW. 2016. A highly thermostable kanamycin resistance marker expands the tool kit for genetic manipulation of Caldicellulosiruptor bescii. Appl Environ Microbiol 82:4421–4428. doi: 10.1128/AEM.00570-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao S, Mikkelsen MJ. 2010. Identification and overexpression of a bifunctional aldehyde/alcohol dehydrogenase responsible for ethanol production in Thermoanaerobacter mathranii. J Mol Microbiol Biotechnol 19:123–133. doi: 10.1159/000321498. [DOI] [PubMed] [Google Scholar]
- 24.Shao X, Zhou J, Olson DG, Lynd LR. 2016. A markerless gene deletion and integration system for Thermoanaerobacter ethanolicus. Biotechnol Biofuels 9:1–8. doi: 10.1186/s13068-015-0423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller MW, Schut GJ, Lipscomb GL, Menon AL, Iwuchukwu IJ, Leuko TT, Thorgersen MP, Nixon WJ, Hawkins AS, Kelly RM, Adams MWW. 2013. Exploiting microbial hyperthermophilicity to produce an industrial chemical, using hydrogen and carbon dioxide. Proc Natl Acad Sci U S A 110:5840–5845. doi: 10.1073/pnas.1222607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrière C, Veiga-da-Cunha M, Pons N, Guédon E, van Hijum SAFT, Kok J, Kuipers OP, Ehrlich DS, Renault P. 2005. Fructose utilization in Lactococcus lactis as a model for low-GC Gram-positive bacteria: its regulator, signal, and DNA-binding site. J Bacteriol 187:3752–3761. doi: 10.1128/JB.187.11.3752-3761.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou JZ, Fries MR, Cheesanford JC, Tiedje JM. 1995. Phylogenetic analyses of a new group of denitrifiers capable of anaerobic growth on toluene and description of Azoarcus tolulyticus sp. nov. Int J Syst Bacteriol 45:500–506. doi: 10.1099/00207713-45-3-500. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Russell DW (ed). 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]