ABSTRACT
Among the best-studied interactions between soil phagocytic predators and a human-pathogenic fungus is that of Acanthamoeba castellanii and Cryptococcus neoformans. The experimental conditions used in amoeba-fungus confrontation assays can have major effects on whether the fungus or the protozoan is ascendant in the interaction. In the presence of Mg2+ and Ca2+ in phosphate-buffered saline (PBS), C. neoformans was consistently killed when incubated with A. castellanii. A. castellanii survived better in the presence of Mg2+ and Ca2+, even when incubated with C. neoformans. In the absence of Mg2+ and Ca2+, C. neoformans survived when incubated with A. castellanii, and the percentage of dead amoebae was higher than when incubated without yeast cells. These results show that the presence of Mg2+ and Ca2+ can make a decisive contribution toward tilting the outcome of the interaction in favor of the amoeba. Of the two metals, Mg2+ had a stronger effect than Ca2+. The cations enhanced A. castellanii activity against C. neoformans via enhanced phagocytosis, which is the major mechanism by which amoebae kill fungal cells. We found no evidence that amoebae use extracellular killing mechanisms in their interactions with C. neoformans. In summary, the presence of Mg2+ and Ca2+ enhanced the cell adhesion on the surfaces and the motility of the amoeba, thus increasing the chance for contact with C. neoformans and the frequency of phagocytosis. Our findings imply that the divalent cation concentration in soils could be an important variable for whether amoebae can control C. neoformans in the environment.
IMPORTANCE The grazing of soil organisms by phagocytic predators such as amoebae is thought to select for traits that enable some of them to acquire the capacity for virulence in animals. Consequently, knowledge about the interactions between amoebae and soil microbes, such as pathogenic fungi, is important for understanding how virulence can emerge. We show that the interaction between an amoeba and the pathogenic fungus C. neoformans is influenced by the presence in the assay of magnesium and calcium, which potentiate amoebae. The results may also have practical applications, since enriching soils with divalent cations may reduce C. neoformans numbers in contaminated soils.
KEYWORDS: amoeba, fungi, Cryptococcus neoformans, predation, cations
INTRODUCTION
Cryptococcus neoformans is a soil-dwelling fungus that is a frequent cause of life-threatening meningoencephalitis in individuals with impaired immunity (1). One of the fascinating aspects of C. neoformans biology is that it has the capacity for virulence in very different animal and plant species, and yet this organism has no need for pathogenicity, as it can survive in soils without hosts. To explain this phenomenon, the concept of accidental virulence was proposed, which posits that environmental pressures select for traits that confer upon the microbe the capacity for pathogenicity independent of the final host (2, 3). In the case of C. neoformans, the environmental pressure was proposed to include amoeboid predators such as amoebae (4, 5). Supporting this hypothesis is the fact that many virulence factors function in the same manner during the interaction between macrophages and amoebae (5, 6). Furthermore, the passage of an avirulent strain of C. neoformans in social amoebae restored virulence (7). Recently, the interaction of C. neoformans with Acanthamoeba castellanii was shown to result in increased fungal virulence for Galleria mellonella as a result of amoeba-induced changes in susceptibility to microbicidal oxidants and the cell wall (8). Studies of amoeba interactions with other pathogenic fungi such as Histoplasma capsulatum (9), Sporothrix schenckii (9), Blastomyces dermatitidis (9), Aspergillus fumigatus (10, 11), and entomopathogenic fungi (12) suggest that fungus-amoeba interactions could be important in their virulence for animal hosts.
In recent years, amoebae have emerged as a major system for studying bacterial and fungal host-microbe interactions (13, 14), including C. neoformans biology and virulence (15–19). The realization that amoebae served as reservoirs and training hosts for animal virulence together with the ease that these protozoa can be maintained in the laboratory have popularized the study of their interactions with various microbes. Insights gained with amoebae on mechanisms of intracellular pathogenesis were shown to apply to microbe-macrophage interactions and vice versa. For example, the discovery that phospholipids are a trigger for capsular enlargement in C. neoformans followed the observation that cryptococcal cells enlarged their capsules in the presence of amoebae, and then the same phenomenon was shown in macrophages (20). On the other hand, a Mycobacterium avium pathogenicity island important for intracellular infection was first identified in a macrophage screen and then shown to also be important for infecting amoebae (21). Clearly, macrophages and amoebae provide complementary systems for the study of virulence determinants and the evolution of pathogenicity.
Prior studies have shown that the outcome of the C. neoformans-Acanthamoeba castellanii interaction is highly dependent on the conditions of the experiment. For example, confrontation experiments involving cryptococci and amoebae under nutrient-poor conditions such as phosphate-buffered saline resulted in fungal growth and the death of amoebae (4, 22). However, when cryptococci and amoebae were suspended on amoeba growth medium, the protozoa were ascendant, with a reduction of fungal cells by predation and killing (23). Those results were interpreted as suggesting that the nutritional state of amoebae was an important variable in their predatory capacity (23). However, while carrying out C. neoformans-amoeba experiments, we made the serendipitous observation that the presence of Ca2+ and Mg2+ was all that was required to potentiate amoeba predation for fungal cells. These results identify the concentration of divalent cations as an important variable in studies of microbe-amoeba interactions.
RESULTS
A serendipitous observation.
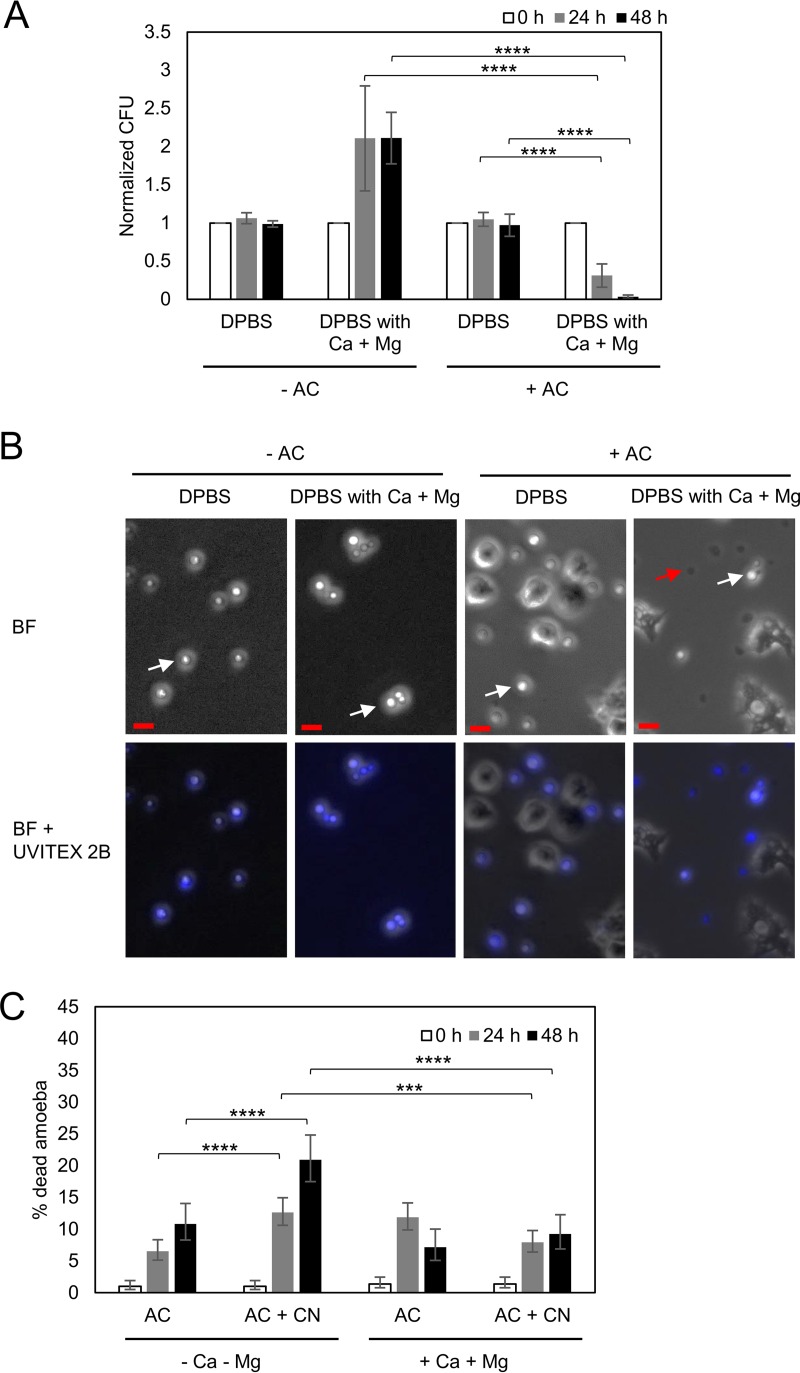
While studying C. neoformans interactions with A. castellanii in phosphate-buffered saline, we noted that the amoebae killed a substantial proportion of the fungal cells. This result was unexpected given that prior work has shown that under these conditions, C. neoformans tended to kill A. castellanii and replicate (4, 23). A review of the conditions of the experiment revealed that the solution used was not the standard laboratory formulation but instead a commercial product manufactured by Corning (Corning, NY) known as Dulbecco's phosphate-buffered saline (DPBS), which differed from the phosphate-buffered saline (PBS) used in prior experiments by supplementation with magnesium and calcium. This suggested that the difference in the findings was the presence of these two divalent cations. We then repeated the experiments with DPBS supplemented with or without Ca2+ and Mg2+, jointly and singly, and verified that the addition of the metal salts was the ingredient potentiating the activity of A. castellanii against C. neoformans. In the presence of magnesium and calcium in DPBS, C. neoformans CFU decreased when incubated with A. castellanii but increased without A. castellanii (Fig. 1A and B). A. castellanii survived better in the presence than in the absence of Ca2+ and Mg2+ during the incubation with C. neoformans (Fig. 1C). The percentage of dead A. castellanii after incubation with C. neoformans was higher than that without yeast cells in the absence of divalent metal cations (Fig. 1C). The results show that magnesium and calcium are key determinants of the outcome of the C. neoformans and amoeba interaction.
FIG 1.
Presence of magnesium and calcium affects the outcome of the A. castellanii-C. neoformans interaction. (A) The presence of magnesium and calcium decreases the survival of C. neoformans during the incubation with A. castellanii (AC). The survival of C. neoformans was determined by CFU after incubation with A. castellanii for 0, 24, and 48 h. The CFU counts at 24 h and 48 h were normalized to the initial CFU at time zero. Data represent the means from four biological samples. Error bars are SDs. ****, P < 0.0001 by Student's t test. (B) Phase-contrast and fluorescence images of Uvitex 2B-stained C. neoformans cells coincubated with or without A. castellanii in DPBS with or without magnesium and calcium. In the phase-contrast images, disrupted C. neoformans cells are dark (red arrow), while intact cells are refractile (white arrows). More disrupted C. neoformans cells appeared under the condition with A. castellanii incubation in DPBS containing magnesium and calcium. Scale bars are 10 μm. (C) A. castellanii cells survived better in the presence of Mg2+ and Ca2+ during the incubation with C. neoformans (CN). The viability of A. castellanii cells was determined by a trypan blue exclusion assay. The percentages of dead A. castellanii cells were determined by counting the numbers of blue stained cells per the total cell number counted. Four independent biological experiments were performed. Error bars represent 95% confidence intervals of the means. ***, P = 0.0008; ****, P < 0.0001 by Fisher's exact tests.
The commercial DPBS is supplemented with CaCl2 and MgCl2. DPBS (without Ca2+ and Mg2+) also contains KCl and NaCl, ruling out chloride ions as the component responsible for the enhanced predation of amoebae. However, to further confirm that the divalent metals were responsible for the enhanced protozoa fungicidal effects, we performed the experiment with DPBS supplemented with different forms of Ca2+ and Mg2+ (calcium nitrate and magnesium sulfate). Under these conditions, we observed an enhanced killing ability of amoebae, establishing that magnesium and calcium ions are the ones responsible for this effect (see Fig. S1 in the supplemental material).
Mg2+ is more effective than Ca2+ in potentiating A. castellanii.
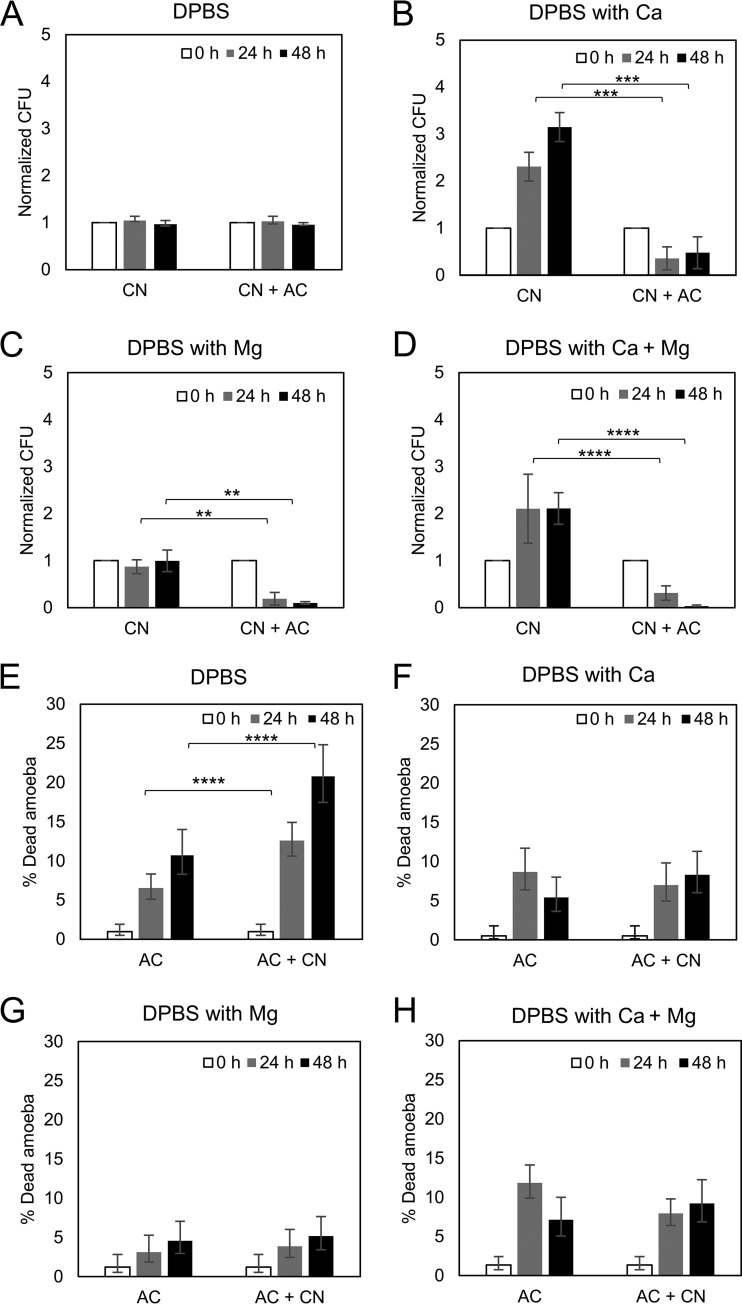
To ascertain which of the divalent cations was responsible for potentiating amoebae, we evaluated the outcome of the C. neoformans-A. castellanii interaction under conditions where DPBS was supplemented with Ca2+, Mg2+, or both. The addition of either Ca2+ or Mg2+ had a significant effect in reducing the C. neoformans CFU relative to that under conditions with no divalent cations, but the effect was much greater with Mg2+ and was maximal when both cations were present simultaneously (Fig. 2A to D). The presence of Mg2+ also increased the viability of A. castellanii in the presence and absence of C. neoformans (Fig. 2G).
FIG 2.
Magnesium had a stronger effect than calcium on reducing the survival of C. neoformans when incubated with A. castellanii. The survival of C. neoformans (CN) was determined by CFU after incubation with A. castellanii (AC) for 0, 24, and 48 h in DBPS (A), DPBS with calcium (B), DPBS with magnesium (C), and DPBS with calcium and magnesium (D). The CFU counts were normalized to the initial CFU at time zero. Data represent the means from three biological samples. Error bars are SDs. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 by Student's t tests. The viabilities of A. castellanii cells were also determined by trypan blue exclusion assays after incubation with C. neoformans in DBPS (E), DPBS with calcium (F), DPBS with magnesium (G), and DPBS with calcium and magnesium (H). The percentages of dead A. castellanii cells were determined by counting the numbers of blue stained cells per total cell number counted. Three independent biological experiments were performed. Error bars represent 95% confidence intervals of the mean. ****, P < 0.0001 by Fisher's exact tests.
Divalent cations enhanced amoeba surface area, phagocytosis, and mobility.
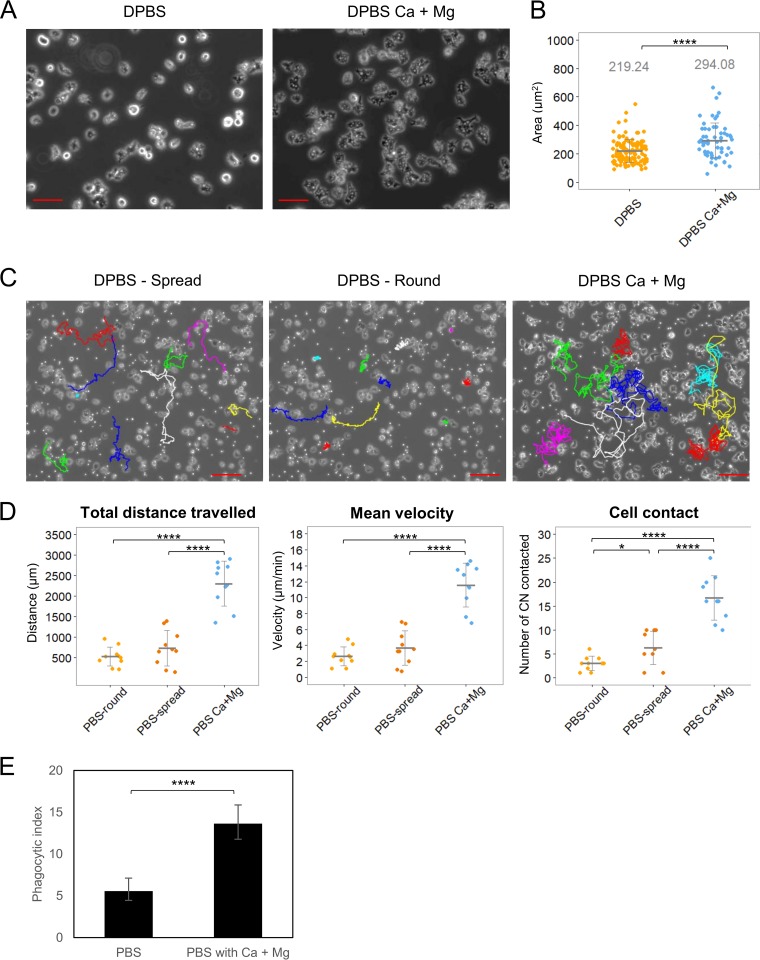
To investigate the mechanism by which divalent cations potentiated A. castellanii against C. neoformans, we evaluated three parameters with a high likelihood to impact the fungal-protozoal interaction: amoeba adhesion, phagocytosis, and mobility. Both Ca2+ and Mg2+ are known to be important for cellular adhesion, with Mg2+ having a stronger effect than Ca2+ (24, 25). We measured cellular areas as a measure of adhesion and noted that amoebae in the presence of Ca2+ and Mg2+ manifested significantly greater areas when placed on a glass surface (Fig. 3A and B). Amoebae moved more and made more contacts with C. neoformans in the presence of Ca2+ and Mg2+ (Fig. 3C and D), which translated into a significantly greater phagocytosis of yeast cells (Fig. 3E).
FIG 3.
Presence of calcium and magnesium enhances the adherence of A. castellanii to the surface and its motility. (A) Phase-contrast images of A. castellanii cells on glass surfaces in DPBS with or without Ca2+ and Mg2+. Scale bars are 50 μm. (B) To quantify cell spreading, the areas of A. castellanii cells in DPBS with or without Ca2+ and Mg2+ were measured. At least 100 cells were analyzed for each condition. Error bars indicate SDs. *, P = 0.01572; ****, P < 0.0001 by Student's t test. (C) Images of A. castellanii cell trajectories on glass surfaces in DPBS with or without Ca2+ and Mg2+. Ten A. castellanii cells were randomly selected for manually centroid tracked for a total duration of 2 h under each condition. The interval of each track is 30 s. Color lines indicate manually generated tracks. Two distinct morphologies of amoeboid cells, round and spread, in DPBS were tracked separately. Scale bars are 100 μm. (D) The total distance, the mean velocity, and the frequency of contact with C. neoformans cells were quantified. The total distance was defined as the sum of the distances the amoeboid cells travelled from the starting point to the endpoint of the cell trajectory. The mean velocity was calculated as the mean from all velocity measurements from amoeboid cells moving in each 30-s interval. Error bars indicate SDs. ****, P < 0.0001 by Student's t tests. (E) The presence of magnesium and calcium induces the phagocytosis of C. neoformans by A. castellanii. A. castellanii and Uvitex 2B-labeled C. neoformans were incubated at a 1:1 ratio in DPBS with or without magnesium and calcium for 2 h to allow phagocytosis. The phagocytic index was determined by the number of internalized C. neoformans cells per 100 A. castellanii cells. Data were obtained from four biologically independent experiments. Error bars represent 95% confidence intervals of the means. ****, P < 0.0001 by Fisher's exact test.
Amoeba fungicidal activity requires protozoa-fungus cell contact.
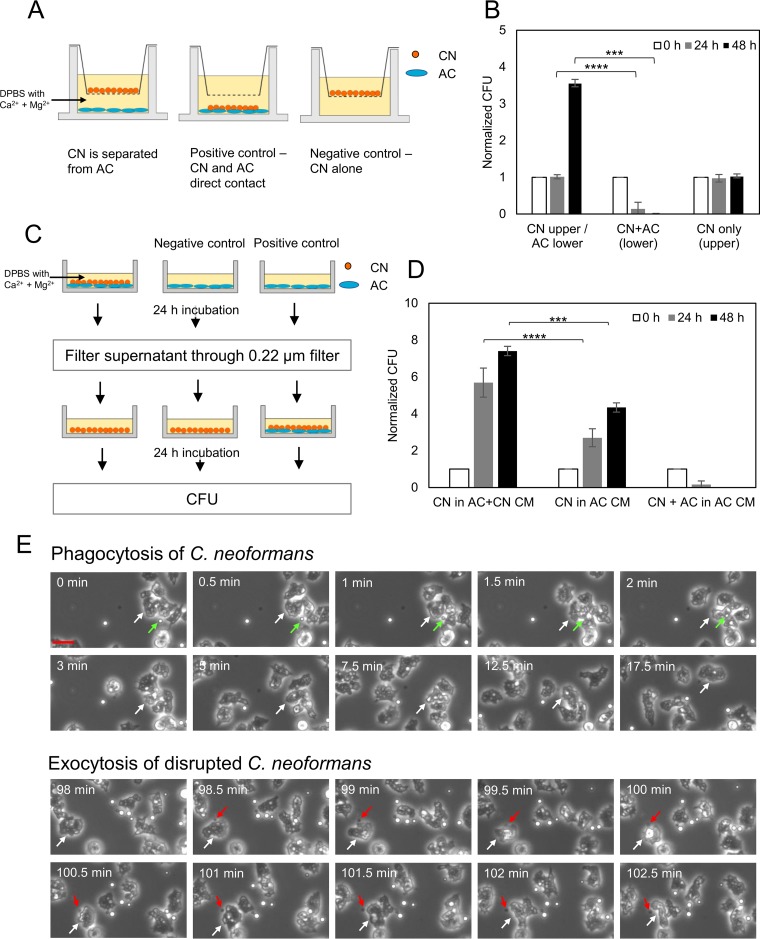
Next, we considered whether contact was necessary for an amoeba to kill C. neoformans by separating fungal and amoeboid cells in wells where the fluid was connected through a semipermeable membrane. We observed no reduction in fungal CFU under conditions where the fungal and protozoal cells were separated. However, when C. neoformans and amoeba were placed in the same chamber, there was a reduction in fungal colonies (Fig. 4A and B). The incubation of C. neoformans in amoeba-conditioned medium, which was collected after the coincubation of A. castellanii and C. neoformans, had no effect on fungal colonies, arguing against the release of fungicidal products from amoebae (Fig. 4C and D). These experiments indicated a necessity for contact between the amoeba and C. neoformans for protozoal fungicidal activity. Cinematographic analysis of the C. neoformans-A. castellanii interaction revealed the phagocytosis followed by the regurgitation of fungal carcasses, with the latter being easily distinguishable from live fungal cells, as these were shriveled structures that had lost their characteristic translucent appearance by light microscopy (Fig. 4E; see also Movie S1). The average time from phagocytosis to regurgitation of cryptococcal cellular remnants was 2.04 ± 0.53 h (n = 5).
FIG 4.
Phagocytosis is the major mechanism for A. castellanii to kill C. neoformans. (A) Scheme of the Transwell assay. A. castellanii and C. neoformans were separated in DPBS with Ca2+ and Mg2+ by porous membrane (pore size, 0.4 μm). The upper compartment contained C. neoformans while the lower compartment contained A. castellanii. As a positive control, C. neoformans was incubated with A. castellanii in the lower compartment. As a negative control, C. neoformans was incubated without A. castellanii in the upper compartment. (B) The survival of C. neoformans was determined by CFU after the Transwell assay for 0, 24, and 48 h. The CFU counts were normalized to the initial CFU at time zero. Data represent the means from four biological samples. Error bars are SDs. ***, P = 0.0004; ****, P < 0.0001 by Student's t tests. (C) Schematic for the production of conditioned media from coincubation of C. neoformans (CN) and A. castellanii (AC) and exposure of the fungal cells. After exposure of A. castellanii to C. neoformans in DPBS with Ca2+ and Mg2+, conditioned media were collected, filtered, and added to fresh cultures of C. neoformans. As a negative control, A. castellanii-conditioned medium was collected and added to C. neoformans. As a positive control, A. castellanii-conditioned medium was collected and added to the coincubation of C. neoformans and A. castellanii. (D) The survival of C. neoformans (CN) was determined by CFU after exposure to conditioned medium (CM) for 0, 24, and 48 h. The CFU counts were normalized to the initial CFU at time zero. Data represent the means from three biological samples. Error bars are SDs. ***, P = 0.00223; ****, P < 0.0001 by Student's t tests. (E) Time-lapse phase-contrast images of coincubation of C. neoformans and A. castellanii in DPBS with Ca2+ and Mg2+. Top images show the phagocytosis of C. neoformans (green arrows) by A. castellanii (white arrows). Bottom images show the exocytosis of disrupted C. neoformans (appears dark; red arrows) from A. castellanii (white arrows). Scale bar is 25 μm.
Interaction of C. neoformans and amoeba.
To better understand how divalent cations potentiated an amoeba against C. neoformans, we carried out several experiments to probe variables that might contribute to their interaction. Given that the capsule of C. neoformans has negatively charged glucuronic acid residues that can interact with cations and that capsule enlargement can be triggered by Ca2+ (26), we first incubated yeast cells with Mg2+ and Ca2+, and then washed and added the fungal cells to the amoebae (see Fig. S2A and B). We measured no effect, suggesting that the increased susceptibility of C. neoformans to an amoeba in the presence of cations was not due to effects on the yeast cell and/or capsule. The pretreatment of the amoeba or both the amoeba and C. neoformans with Mg2+ and Ca2+ (Fig. S2C to F) followed by washing and incubation with C. neoformans also had no effect on fungicidal activity. These results indicate that the divalent cations have to be in the solution containing amoeboid and fungal cells for their potentiating effect on amoeba fungicidal activity.
DISCUSSION
Amoebae are an increasingly popular alternative host system for studying interactions between microbes and environmental phagocytic cells, but relatively little work has been done on the effect of experimental conditions on the outcome of their interaction with fungi. In earlier studies, we had noted that carrying out confrontation experiments between A. castellanii and C. neoformans in ATCC medium 712 with peptone-yeast extract-glucose (PYG) potentiated the fungicidal capacity of the protozoa and inferred that it reflected an improved nutritional status for the amoeboid cells (23). Here, we show that the addition of the divalent cations Ca2+ and Mg2+ is sufficient to potentiate A. castellanii activity against C. neoformans, making it very likely that they are the active ingredients in ATCC medium 712, which has 0.05 M CaCl2 and 0.4 M MgSO4. Our findings establish that the ionic composition of the solution can have an important effect on the outcome of the experiment with C. neoformans and suggest that the same may apply in other amoeba-microbe confrontation systems.
The ability of amoebae to prey on C. neoformans was first described in the 1970s (27, 28). The evidence for the importance of this interaction in the environment comes from the observation that the prevalence of C. neoformans in soils is reduced proportionally to the presence of amoebae (29). Consequently, amoebae probably play a major role in reducing the fungal burden in contaminated soils and, as such, may reduce the amount of human and animal exposure. Our results suggest that the efficacy of amoebae in soils may depend on environmental divalent cation concentrations. In this regard, soils can vary greatly on free Ca and Mg depending on the presence of vegetable matter detritus and the pH, which in turn can affect worm concentrations (30). In the United States, soil Ca and Mg concentrations average 0.375 M (range, 0.02 to >4 M) and 0.6 M (range, 0.0025 to 8 M), respectively (31). Such ranges in concentrations imply that the availability of these cations to soil amoebae would vary greatly depending on the soils, given that that these concentrations do not necessarily imply that the element would be available in a soluble cationic form.
The potentiation of amoeba fungicidal activity by divalent cation ions was associated with increases in surface area, mobility, contact with fungal cells, and phagocytosis. Hence, divalent cations appear to have global effects on amoeboid cell biological function that may work synergistically to enhance their predatory capacity. An increased surface area together with greater mobility and enhanced contacts with C. neoformans cells are likely to increase the probability of phagocytosis, which was indeed measured. Amoebae were visualized ingesting cryptococcal cells that were later regurgitated as opaque and shriveled forms that were easily distinguishable from live translucent cells. The requirement for contact and phagocytosis in fungal killing was shown by the absence of fungicidal activity through diffusible substances. In contrast, facilitating the contact between amoebae and yeast cells reduced the fungal CFU. The treatment of fungal cells with divalent cations had no discernible impact on the interaction, suggesting that the effect of Ca2+ and Mg2+ in augmenting amoeba fungicidal activity was primarily, if not exclusively, due to the effects on the protozoan cells.
Although the mechanism by which Ca2+ and Mg2+ affect A. castellanii has not been investigated, there is evidence from related systems that these cations can have powerful effects on amoebae. Pinocytosis and locomotion of amoebae can be influenced by monovalent and divalent cations (32). A. castellanii is known to accumulate Ca2+ in the presence of excess cations, with cells forming electron-dense deposits (33). The displacement of cell surface-associated Ca2+ with drugs was shown to inhibit phagocytosis (34). Both Ca2+ and Mg2+ trigger signaling pathways that promote chemotaxis in the social amoeba Dictyostelium discoideum (35). For Entamoeba invadens, the signaling program that triggers encystation is dependent on Ca2+ (36). Ca2+ has been shown to increase the adhesion of Acanthamoeba polyphaga to extracellular matrix proteins (37) in a manner that recapitulates the increased adhesion effects we describe in this study. However, not all interactions of amoebae with other cells require divalent cations. In this regard, Entamoeba histolytica adhered and killed Chinese hamster ovary cells in the presence of EDTA, suggesting that the divalent cations were not needed for the interaction (38). These observations illustrate the protean effects of divalent cations on protozoa and, in aggregate, support the view for major differences in the physiological states of amoeboid cells under conditions with and without Ca2+ and Mg2+, which translate into large differences in their ability to kill C. neoformans. However, it is noteworthy that C. neoformans also responds to divalent cations with capsular enlargement (26), which protects against amoebae (39). Hence, the fungal-protozoal balance of power could reflect not only cation concentrations but also the time each type of organism had to adapt to the prevailing conditions.
Several publications describing amoeba interactions with various pathogens report the use of different experimental conditions that could vary in divalent cations. For example, A. castellanii interactions with Pseudomonas aeruginosa (41), Corynebacterium spp. (41), and Campylobacter jejuni (43) were studied in peptone-yeast extract-glucose (PYG) medium, while interactions with Vibrio cholerae (40) and Aspergillus fumigatus (11) were done separately in defined artificial seawater and Dulbecco's modified Eagle medium that included Ca2+ and Mg2+ cations and interactions with Bacillus anthracis (44) was studied in autoclaved creek water supplemented with divalent cations. Given the powerful effects of divalent cations on amoeba function, comparisons across studies need to take into account the possibility that differences in Ca2+ and Mg2+ could affect experimental outcomes.
C. neoformans is commonly found in soils enriched in bird feces (reviewed in reference 1). In urban centers, C. neoformans is often found in high densities in soils near sites where pigeons roost, where guano provides a rich source of nutrients (45). Human and animal infection is believed to originate from the inhalation of desiccated yeast cells or basidiospores that become aerosolized from C. neoformans-contaminated soils (1, 46). Consistent with an environmental source of infection, isolates recovered from environmental sites were indistinguishable from those recovered from patients by the use of several genetic markers (47, 48). The contaminated soils near a hospital have been suggested as sites for hospital-acquired infection of C. neoformans (49). The remediation of C. neoformans-contaminated soils is a formidable challenge, and current methods involve the application of noxious chemicals such as formalin (50) and quaternary ammonium salts (51). Given that amoeba predation can reduce fungal burden in contaminated soils (29), our results suggest that increasing the soil concentration of divalent cations with calcium and magnesium salts could potentiate the fungicidal capacity of amoebae and provide a more environmentally friendly biological alternative to chemical decontamination.
In summary, we establish the divalent cation concentration as a major variable in the outcome of A. castellanii interactions with C. neoformans. Given the dramatic effects that divalent cations have on amoeba physiology, we suspect that this effect will apply to other microbe-amoeba interactions and is likely to be an important experimental variable in other systems. This effect may also have applications for the environmental control of C. neoformans, since increasing Ca and Mg concentrations in soils could potentiate amoeba predation and reduce the fungal burden in contaminated sites.
MATERIALS AND METHODS
Cell culture.
Acanthamoeba castellanii strain 30234 was obtained from the American Type Culture Collection (ATCC). Cultures were maintained in PYG broth (ATCC medium 712) at 25°C according to instructions from ATCC. C. neoformans var. grubii serotype A strain H99 was used in all experiments, and this strain was originally obtained from John Perfect (Durham, NC). Cryptococcal cells were cultivated in Sabouraud dextrose broth with shaking (120 rpm) at 30°C overnight (16 h) prior to use in amoeba assays.
Assay of A. castellanii and C. neoformans interaction.
The survival of C. neoformans in amoeba culture was performed as described previously (22). Briefly, A. castellanii cells were washed twice with Dulbecco's phosphate-buffered saline (DPBS; Corning, Corning, NY) and diluted in DPBS to an appropriate density. This buffer is available with and without calcium and magnesium supplementation. A. castellanii cells (1 × 104 cells/well) were added to 96-well plates and allowed to adhere for 1 h at 25°C. C. neoformans cells were washed twice with DPBS and diluted in DPBS to an appropriate density. Fungal cells (1 × 104) were added to wells containing amoebae or control wells containing DPBS alone, and the plates were incubated at 25°C. At 0, 24, and 48 h, the amoebae were lysed by pulling the culture through 27-gauge syringe needles five to seven times. The extent of cell lysis was examined using a light microscope, and approximately 98% of amoeboid cells were lysed. The lysates were serially diluted, plated on Sabouraud agar, and incubated at 30°C for 48 h for CFU determination. To study the effect of calcium and/or magnesium on the survival of C. neoformans during the interaction with amoebae, DPBS was replaced with DPBS containing 0.9 mM calcium and/or 0.5 mM magnesium. To study if the cations affect the adhesion between C. neoformans and A. castellanii, either or both C. neoformans and A. castellanii cells were pretreated with DPBS containing 0.9 mM calcium and 0.5 mM magnesium for 1 h. Cells were then washed with DPBS. The viability of A. castellanii was also determined under the same conditions and time intervals by adding a 1:80 dilution of trypan blue stain. The percentage of dead amoebae was determined by counting the number of trypan blue-stained cells per total cell number counted. A minimum of 100 cells were counted. Control wells contained A. castellanii without C. neoformans.
Microscopy and time-lapse imaging.
For measuring the spread area of A. castellanii in the presence or absence of calcium and magnesium, A. castellanii cells (5 × 105 cells) were allowed to adhere for 2 h on a glass surface of poly-d-lysine-coated coverslip-bottom MatTek petri dishes with 14-mm microwells (MatTek Corporation, Ashland, MA) in DPBS with or without magnesium and calcium. Images were then taken using a Zeiss Axiovert 200M inverted microscope with a 20× phase objective. The areas of amoeboid cells (minimum 100 cells) were measured using ImageJ software.
For fluorescence imaging, A. castellanii cells were washed twice with DPBS, and 2 × 104 amoeboid cells were seeded on an 8-well-chambered cover glass (Nunc, Roskilde, Denmark) in DPBS with or without magnesium and calcium at 25°C for 2 h. C. neoformans cells were stained with 0.01% Uvitex 2B (Polysciences, Warminster, PA) for 10 min and washed twice with DPBS. Uvitex 2B-stained C. neoformans cells (2 × 104 cells) were added to an A. castellanii culture and incubated at 25°C. After 2 h and 24 h of incubation, images were taken using a DAPI (4′,6-diamidino-2-phenylindole) filter-equipped Zeiss Axiovert 200M inverted microscope with 20× phase objective. Images were also used for measuring the phagocytosis index, which was determined by the number of C. neoformans cells per 100 amoeboid cells.
For time-lapse imaging, A. castellanii cells were seeded (5 × 105 cells) on MatTek petri dishes in DPBS with or without magnesium and calcium. Cells were then incubated at 25°C for 2 h. Cryptococcal cells (5 × 105 cells/well) were added to an amoeba culture. After a 15-min incubation to allow fungal cells to settle down, images were taken every 30 s for 24 h using a Zeiss Axiovert 200M inverted microscope with a 10× phase objective in an enclosed chamber under conditions of 25°C.
The movements of A. castellanii cells were tracked manually using ImageJ manual tracking. Ten amoeboid cells were randomly selected and tracked after the first 2 h of incubation with C. neoformans under each tested condition. The total distance, mean velocity, and the number of contacts between A. castellanii and C. neoformans were measured. The total distance was defined as the sum of the distances the amoeboid cells travelled from the starting point to the endpoint of the cell trajectory. The mean velocity was calculated as the mean from all velocity measurements from amoeboid cells moving in each 30-s interval.
Supernatant toxicity assay.
To examine the effect of the secretion of A. castellanii on C. neoformans, Transwell plates (6-well, polycarbonate membrane, 0.4-μm-pore size; Corning) were used to separate C. neoformans and A. castellanii into two compartments with a continuity of saline. A. castellanii cells were washed twice and suspended with DPBS containing magnesium and calcium, and 2 × 105 cells were added to the lower compartments. The plates were then incubated at 25°C for 2 h. C. neoformans cells were washed and suspended with DPBS containing magnesium and calcium, and 2 × 105 cryptococcal cells were added to the inserts. After 0 h and 24 h of incubation, C. neoformans cells in the inserts were serially diluted, plated on Sabouraud agar, and incubated at 30°C for 48 h for CFU determination.
To examine whether the secretion of A. castellanii products required direct cell contact between amoebae and fungi, C. neoformans cells were treated with amoeba-conditioned medium collected after the incubation of A. castellanii and C. neoformans. A. castellanii cells (1 × 104 cells) in DPBS containing magnesium and calcium were added to 96-well plates and allowed to adhere for 2 h at 25°C. C. neoformans cells were washed twice with DPBS containing magnesium and calcium, and then 1 × 104 cells were added to wells containing amoebae and the plates were incubated at 25°C for 24 h. The conditioned medium was collected and filtered through 0.22-μm syringe filters. Fresh cultures of C. neoformans cells were washed twice with DPBS containing magnesium and calcium and suspended under the conditioned medium. Fungal cells (1 × 104) were added to wells and incubated at 25°C. Control wells contained a coincubation of A. castellanii and C. neoformans as well as C. neoformans alone with A. castellanii-conditioned medium. After 0 h and 24 h of incubation, the amoebae were lysed by pulling the cultures through 27-gauge syringe needles five to seven times. The lysates were serially diluted, plated on Sabouraud agar, and incubated at 30°C for 48 h for CFU determination.
Statistical analysis.
All comparisons were analyzed by either unpaired two-tailed Student's t tests or two-tailed Fisher's exact tests.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by grants 5R01HL059842, 5R01AI033774, 5R37AI033142, and 5R01AI052733.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01717-17.
REFERENCES
- 1.Casadevall A, Perfect JR. 1998. Cryptococcus neoformans. American Society for Microbiology, Washington, DC. [Google Scholar]
- 2.Casadevall A, Pirofski LA. 2007. Accidental virulence, cryptic pathogenesis, martians, lost hosts, and the pathogenicity of environmental microbes. Eukaryot Cell 6:2169–2174. doi: 10.1128/EC.00308-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guimaraes AJ, Gomes KX, Cortines JR, Peralta JM, Peralta RH. 2016. Acanthamoeba spp. as a universal host for pathogenic microorganisms: one bridge from environment to host virulence. Microbiol Res 193:30–38. doi: 10.1016/j.micres.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Steenbergen JN, Shuman HA, Casadevall A. 2001. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc Natl Acad Sci U S A 18:15245–15250. doi: 10.1073/pnas.261418798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadevall A, Nosanchuk JD, Steenbergen JN. 2003. “Ready-made” virulence and “dual-use” virulence factors in pathogenic environmental fungi–the Cryptococcus neoformans paradigm. Curr Opin Microbiol 112:1164–1175. [DOI] [PubMed] [Google Scholar]
- 6.Adiba S, Nizak C, van Baalen M, Denamur E, Depaulis F. 2010. From grazing resistance to pathogenesis: the coincidental evolution of virulence factors. PLoS One 5:e11882. doi: 10.1371/journal.pone.0011882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steenbergen JN, Nosanchuk JD, Malliaris SD, Casadevall A. 2003. Cryptococcus neoformans virulence is enhanced after intracellular growth in the genetically malleable host Dictyostelium discoideum. Infect Immun 71:4862–4872. doi: 10.1128/IAI.71.9.4862-4872.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzo J, Albuquerque PC, Wolf JM, Nascimento R, Pereira MD, Nosanchuk JD, Rodrigues ML. 2017. Analysis of multiple components involved in the interaction between Cryptococcus neoformans and Acanthamoeba castellanii. Fungal Biol 121:602–614. doi: 10.1016/j.funbio.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Steenbergen JN, Nosanchuk JD, Malliaris SD, Casadevall A. 2004. Interaction of Blastomyces dermatitidis, Sporothrix schenckii, and Histoplasma capsulatum with Acanthamoeba castellanii. Infect Immun 72:3478–3488. doi: 10.1128/IAI.72.6.3478-3488.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hillmann F, Novohradska S, Mattern DJ, Forberger T, Heinekamp T, Westermann M, Winckler T, Brakhage AA. 2015. Virulence determinants of the human pathogenic fungus Aspergillus fumigatus protect against soil amoeba predation. Environ Microbiol 17:2858–2869. doi: 10.1111/1462-2920.12808. [DOI] [PubMed] [Google Scholar]
- 11.Van Waeyenberghe L, Bare J, Pasmans F, Claeys M, Bert W, Haesebrouck F, Houf K, Martel A. 2013. Interaction of Aspergillus fumigatus conidia with Acanthamoeba castellanii parallels macrophage-fungus interactions. Environ Microbiol Rep 5:819–824. doi: 10.1111/1758-2229.12082. [DOI] [PubMed] [Google Scholar]
- 12.Bidochka MJ, Clark DC, Lewis MW, Keyhani NO. 2010. Could insect phagocytic avoidance by entomogenous fungi have evolved via selection against soil amoeboid predators? Microbiology 156:2164–2171. doi: 10.1099/mic.0.038216-0. [DOI] [PubMed] [Google Scholar]
- 13.Mylonakis E, Casadevall A, Ausubel FM. 2007. Exploiting amoeboid and non-vertebrate animal model systems to study the virulence of human pathogenic fungi. PLoS Pathog 3:e101. doi: 10.1371/journal.ppat.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilbi H, Weber SS, Ragaz C, Nyfeler Y, Urwyler S. 2007. Environmental predators as models for bacterial pathogenesis. Environ Microbiol 9:563–575. doi: 10.1111/j.1462-2920.2007.01238.x. [DOI] [PubMed] [Google Scholar]
- 15.Lin J, Idnurm A, Lin X. 2015. Morphology and its underlying genetic regulation impact the interaction between Cryptococcus neoformans and its hosts. Med Mycol 53:493–504. doi: 10.1093/mmy/myv012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madu UL, Ogundeji AO, Mochochoko BM, Pohl CH, Albertyn J, Swart CW, Allwood JW, Southam AD, Dunn WB, May RC, Sebolai OM. 2015. Cryptococcal 3-hydroxy fatty acids protect cells against amoebal phagocytosis. Front Microbiol 6:1351. doi: 10.3389/fmicb.2015.01351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magditch DA, Liu TB, Xue C, Idnurm A. 2012. DNA mutations mediate microevolution between host-adapted forms of the pathogenic fungus Cryptococcus neoformans. PLoS Pathog 8:e1002936. doi: 10.1371/journal.ppat.1002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derengowski LS, Paes HC, Albuquerque P, Tavares AH, Fernandes L, Silva-Pereira I, Casadevall A. 2013. The transcriptional response of Cryptococcus neoformans to ingestion by Acanthamoeba castellanii and macrophages provides insights into the evolutionary adaptation to the mammalian host. Eukaryot Cell 12:761–774. doi: 10.1128/EC.00073-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Araujo Gde S, Fonseca FL, Pontes B, Torres A, Cordero RJ, Zancope-Oliveira RM, Casadevall A, Viana NB, Nimrichter L, Rodrigues ML, Garcia ES, Souza W, Frases S. 2012. Capsules from pathogenic and non-pathogenic Cryptococcus spp. manifest significant differences in structure and ability to protect against phagocytic cells. PLoS One 7:e29561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chrisman CJ, Albuquerque P, Guimaraes AJ, Nieves E, Casadevall A. 2011. Phospholipids trigger Cryptococcus neoformans capsule enlargement during interactions with amoebae and macrophages. PLoS Pathog 7:e1002047. doi: 10.1371/journal.ppat.1002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danelishvili L, Wu M, Stang B, Harriff M, Cirillo SL, Cirillo JD, Bildfell R, Arbogast B, Bermudez LE. 2007. Identification of Mycobacterium avium pathogenicity island important for macrophage and amoeba infection. Proc Natl Acad Sci U S A 104:11038–11043. doi: 10.1073/pnas.0610746104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malliaris SD, Steenbergen JN, Casadevall A. 2004. Cryptococcus neoformans var. gattii can exploit Acanthamoeba castellanii for growth. Med Mycol 42:149–158. doi: 10.1080/13693786310001616500. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Solache MA, Izquierdo-Garcia D, Smith C, Bergman A, Casadevall A. 2013. Fungal virulence in a lepidopteran model is an emergent property with deterministic features. mBio 4:e00100-13. doi: 10.1128/mBio.00100-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeichi M, Okada TS. 1972. Roles of magnesium and calcium ions in cell-to-substrate adhesion. Exp Cell Res 74:51–60. doi: 10.1016/0014-4827(72)90480-6. [DOI] [PubMed] [Google Scholar]
- 25.Hornby JE. 1973. Measurements of cell adhesion. II. Quantitative study of the effect of divalent ions on cell adhesion. J Embryol Exp Morphol 30:511–518. [PubMed] [Google Scholar]
- 26.Nimrichter L, Frases S, Cinelli LP, Viana NB, Nakouzi A, Travassos LR, Casadevall A, Rodrigues ML. 2007. Self-aggregation of Cryptococcus neoformans capsular glucuronoxylomannan is dependent on divalent cations. Eukaryot Cell 6:1400–1410. doi: 10.1128/EC.00122-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bunting LA, Neilson JB, Bulmer GS. 1979. Cryptococcus neoformans: gastronomic delight of a soil ameba. Sabouraudia 17:225–232. doi: 10.1080/00362177985380341. [DOI] [PubMed] [Google Scholar]
- 28.Neilson JB, Ivey MH, Bulmer GS. 1978. Cryptococcus neoformans: pseudohyphal forms surviving culture with Acanthamoeba polyphaga. Infect Immun 20:262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz A, Neilson JB, Bulmer GS. 1982. Control of Cryptococcus neoformans in nature by biotic factors. Sabouraudia 20:21–29. doi: 10.1080/00362178285380051. [DOI] [PubMed] [Google Scholar]
- 30.Reich PB, Oleksyn J, Modrzynski J, Mrozinski P, Hobbie SE, Eissenstat DM, Chorover J, Chadwick OA, Hale CM, Tjoelker MG. 2005. Linking liter calcium, earthworms and soil properties: a common garden test with 14 tree species. Ecol Lett 8:811–818. doi: 10.1111/j.1461-0248.2005.00779.x. [DOI] [Google Scholar]
- 31.Shacklette HB, JG. 1984. Element concentration in soils and superficial materials of the conterminous United States, p 1–10. U.S. Geological Survey professional paper 1270. United States Government Printing Office, Washington, DC. [Google Scholar]
- 32.Stockem W, Klein HP. 1988. Pinocytosis and locomotion of amoebae: XVII. Influence of different cations on induced pinocytosis in Amoeba proteus. Eur J Protistol 23:317–326. doi: 10.1016/S0932-4739(88)80021-X. [DOI] [PubMed] [Google Scholar]
- 33.Sobota A, Przelecka A, Janossy AG. 1978. X-ray microanalysis of calcium-dependent deposits at the plasma membrane of Acanthamoeba castellanii. Cytobiologie 17:464–469. [PubMed] [Google Scholar]
- 34.Głowacka SK, Sobota A, Przelecka A. 1985. Displacement of cell-surface associated calcium inhibits phagocytosis and Ca-ATPase activity in amoeba. Cell Biol Int Rep 9:183–191. doi: 10.1016/0309-1651(85)90093-1. [DOI] [PubMed] [Google Scholar]
- 35.Korohoda W, Madeja Z, Sroka J. 2002. Diverse chemotactic responses of Dictyostelium discoideum amoebae in the developing (temporal) and stationary (spatial) concentration gradients of folic acid, cAMP, Ca(2+) and Mg(2+). Cell Motil Cytoskeleton 53:1–25. doi: 10.1002/cm.10052. [DOI] [PubMed] [Google Scholar]
- 36.Makioka A, Kumagai M, Kobayashi S, Takeuchi T. 2002. Possible role of calcium ions, calcium channels and calmodulin in excystation and metacystic development of Entamoeba invadens. Parasitol Res 88:837–843. doi: 10.1007/s00436-002-0676-6. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Asem EK, McLaughlin GL. 1994. Calcium enhances Acanthamoeba polyphaga binding to extracellular matrix proteins. Invest Ophthalmol Vis Sci 35:2421–2426. [PubMed] [Google Scholar]
- 38.Ravdin JI, Guerrant RL. 1981. Role of adherence in cytopathogenic mechanisms of Entamoeba histolytica. Study with mammalian tissue culture cells and human erythrocytes. J Clin Invest 68:1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaragoza O, Chrisman CJ, Castelli MV, Frases S, Cuenca-Estrella M, Rodriguez-Tudela JL, Casadevall A. 2008. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell Microbiol 10:2043–2057. doi: 10.1111/j.1462-5822.2008.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van der Henst C, Scrignari T, Maclachlan C, Blokesch M. 2016. An intracellular replication niche for Vibrio cholerae in the amoeba Acanthamoeba castellanii. ISME J 10:897–910. doi: 10.1038/ismej.2015.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siddiqui R, Lakhundi S, Khan NA. 2015. Interactions of Pseudomonas aeruginosa and Corynebacterium spp. with non-phagocytic brain microvascular endothelial cells and phagocytic Acanthamoeba castellanii. Parasitol Res 114:2349–2356. doi: 10.1007/s00436-015-4432-0. [DOI] [PubMed] [Google Scholar]
- 42. Reference deleted.
- 43.Bui XT, Winding A, Qvortrup K, Wolff A, Bang DD, Creuzenet C. 2012. Survival of Campylobacter jejuni in co-culture with Acanthamoeba castellanii: role of amoeba-mediated depletion of dissolved oxygen. Environ Microbiol 14:2034–2047. doi: 10.1111/j.1462-2920.2011.02655.x. [DOI] [PubMed] [Google Scholar]
- 44.Dey R, Hoffman PS, Glomski IJ. 2012. Germination and amplification of anthrax spores by soil-dwelling amoebas. Appl Environ Microbiol 78:8075–8081. doi: 10.1128/AEM.02034-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larsen RA, Pappas PG, Perfect JR, Aberg JA, Casadevall A, Cloud GA, James R, Filler S, Dismukes WE. 2005. A phase I evaluation of the safety and pharmacodynamic activity of a murine-derived monoclonal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob Agents Chemother 49:952–958. doi: 10.1128/AAC.49.3.952-958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Botts MR, Hull CM. 2010. Dueling in the lung: how Cryptococcus spores race the host for survival. Curr Opin Microbiol 13:437–442. doi: 10.1016/j.mib.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Currie BP, Freundlich LF, Casadevall A. 1994. Restriction fragment length polymorphism analysis of Cryptococcus neoformans isolates from environmental (pigeon excreta) and clinical isolates in New York City. J Clin Microbiol 32:1188–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nosanchuk JD, Shoham S, Fries BC, Shapiro DS, Levitz SM, Casadevall A. 2000. Evidence for zoonotic transmission of Cryptococcus neoformans from a pet cockatoo to an immunocompromised patient. Ann Intern Med 132:205–208. doi: 10.7326/0003-4819-132-3-200002010-00032. [DOI] [PubMed] [Google Scholar]
- 49.Rustan ME, Rubinstein HR, Siciliano C, Masih DT. 1992. Possibility of in-hospital infection by Cryptococcus neoformans in patients with AIDS. Rev Inst Med Trop Sao Paulo 34:383–387. doi: 10.1590/S0036-46651992000500002. [DOI] [PubMed] [Google Scholar]
- 50.Pal M, Dave P. 2016. Cryptococcosis: an emerging airborne mycosis of global concern. Air Water Borne Dis 5:127. doi: 10.4172/2167-7719.1000127. [DOI] [Google Scholar]
- 51.Krangvichain P, Niyomtham W, Prapasarakul N. 2016. Occurrence and susceptibilities to disinfectants of Cryptococcus neoformans in fecal droppings from pigeons in Bangkok, Thailand. J Vet Med Sci 78:391–396. doi: 10.1292/jvms.15-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.