Abstract
In view of the increasing prevalence of obesity in largely vegetarian Asian Indians, it is important to research a high protein, low carbohydrate vegetarian diet. The present study was designed to evaluate the effects of a “High Protein Complete (lacto) VEgetaRian Diet (Acronym; ‘PACER diet’), on weight, body composition and metabolic profiles in non-diabetic obese Asian Indians living in north India.
In this 8-week randomized control trial, 102 vegetarian subjects with body mass index (BMI) >25 kg/m2 were randomized to either a test diet (PACER diet; high protein, high fat and moderately low carbohydrate, lacto-vegetarian diet) or control diet (standard vegetarian diet formulated as the dietary guidelines for Asian Indians) after 4 weeks of diet and exercise run-in period. A standard exercise protocol was followed for both groups. Body weight, BMI, waist circumference (WC), blood pressure, fasting plasma glucose (FPG), fasting serum insulin and lipid profile were assessed before and after the intervention.
There was significant weight loss along with improvements in cardio-metabolic risk factors among both the groups post intervention. Percent reductions in the intervention group for weight (6.1± 2.9; p < 0.001), WC (3.9 ± 1.7; p < 0.001), FPG (5.9 ± 3.2; p < 0.001), total cholesterol (10.2 ± 6.3: p < 0.001), serum triacylglycerol (13.6 ± 10.6; p < 0.001) and low-density lipoprotein cholesterol (11.9 ± 7.1; p < 0.001]) were significantly greater than the control diet group. In summary, intervention with a PACER diet (high protein, high fat and moderately low carbohydrate, lacto-vegetarian diet) showed significant improvement in weight loss, body composition and cardio-metabolic profile as compared to a standard vegetarian diet among obese Asian Indians in north India.
Keyword: Nutrition
1. Introduction
India is witnessing increasing obesity due to rapid social and economic changes, increasing urbanization, rapid nutrition transition, consequent imbalanced nutrition, combined with sedentary lifestyle and genetic predisposition [1, 2]. Consequences of obesity including type 2 diabetes mellitus (T2DM) and cardiovascular diseases (CVDs) are prevalent and constantly increasing in Asian Indians [3, 4]. To curb the obesity epidemic and its associated complications, effective prevention and management approaches are needed.
Dietary modifications along with physical activity are important for management of body weight [5]. The total amount of dietary energy and the quality of nutrients are important factors responsible for the development of obesity. It is possible to modulate body composition and weight by changing dietary composition along with regular physical activity and lifestyle changes [6, 7, 8]. Diets low in carbohydrates with high fat and proteins along with a lifestyle of regular exercise have a good potential to reduce body weight and to maintain lean body tissue [9]. Based on this principle, the Atkin’s diet was developed (comprising of less than 20 gram/day of carbohydrates [naturally occurring sugars from vegetables], ∼100 grams of fat, ∼150 grams/day of protein in the induction phase and gradual increase subsequently) i.e. nearly 27% protein, 5% carbohydrates and 68% fats. This diet was successful in achieving ∼2.9% greater weight loss at 12 months as compared to control diet [10]. One potential problem with this diet was its emphasis on animal products (meat, cheese, butter etc.), which may increase the risk of dyslipidemia. Further, application of this type of diet is untenable in India since more than 50% of the Indian population is vegetarian, while the remaining population largely follow a vegetarian dietary pattern on specific days of the week for religious purposes [11]. Encouraged by the weight loss observed by Atkins diet [10], a plant based low carbohydrate diet ‘Eco-Atkins’ diet was developed by Jenkins et al. [12].
In view of the increasing prevalence of obesity in largely vegetarian Asian Indians [13, 14] and as there are no studies on vegetarian diets for weight loss in obese Asian Indians residing in India, it was important to research a high protein, low carbohydrate vegetarian (lacto-vegetarian) diet. Hence, in this study, a “High Protein (lActo) Complete VEgetaRian diet” (PACER; high protein, high fat and moderately low carbohydrate, lacto-vegetarian) diet was formulated and studied in comparison to a control diet for their effects on anthropometry and metabolic profile of obese Asian Indians living in north India.
2. Materials and methods
In this study, 104 obese vegetarian individuals (BMI >25 kg/m2 to <40 kg/m2; as per BMI classification for Asian Indians [15]) were recruited through the outpatient department of Fortis Hospital, Delhi, between April 2012 and January 2014 (Fig. 1) while the study concluded in June 2014. Exclusion criteria included those with hypothyroidism, T2DM, uncontrolled lipids (serum triacylglycerol [TAG] > 500 mg/dL and low density lipoprotein-cholesterol (LDL-c >160 mg/dL), renal disease, acute infection, known allergy for peanuts or soy/soy products, fluctuating weight (gain or loss of >3 kg) in last 6 months, or on weight loss drugs (or involved in structured weight loss programs), on lipid altering medications, mental instability or excessive alcohol consumption (more than 2 drinks/day). Women participants were excluded if they were pregnant or lactating. The study was approved by human ethics committee of Fortis hospital, New Delhi, India. Informed written consent was obtained from all the subject. This study was registered at clinicaltrials.gov as NCT02562209.
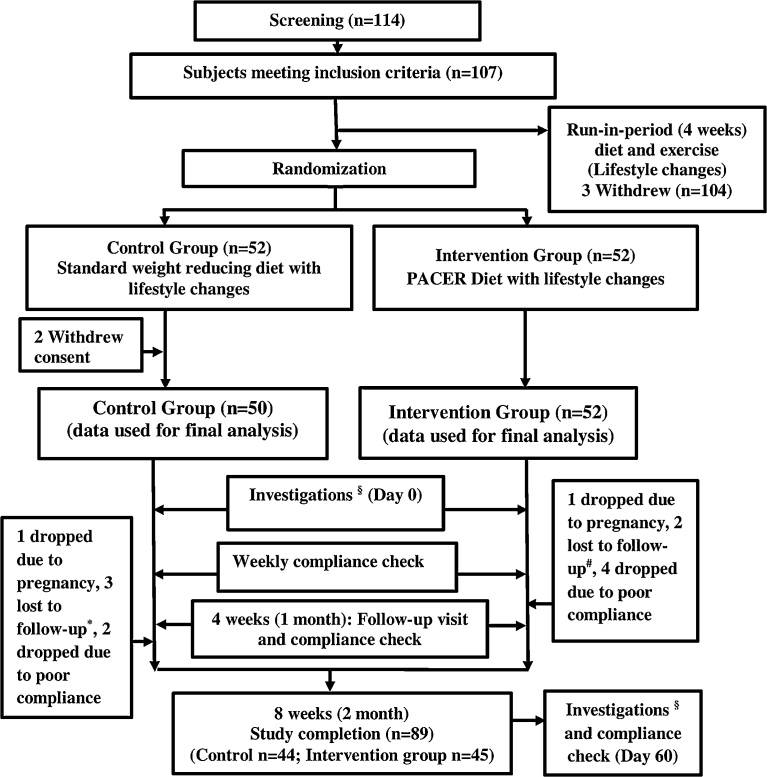
Fig. 1.
Flow chart of the study design, explaining the subject enrollment process. §Investigations: anthropometry (weight, waist circumference, hip circumference, mid upper arm circumference), biochemical investigations (fasting plasma glucose, serum insulin, lipid profile), DEXA scan, 24 hour dietary recall; *lost to follow-up in control group; shift based job (n = 2), relocation (n = 1); #lost to follow-up in intervention group; relocation (n = 1), lost interest in the study (n = 1).
3. Methods
Assessments for anthropometric parameters, blood pressure (BP), fasting plasma glucose (FPG), total cholesterol (TC), TAG, high-density lipoprotein-cholesterol (HDL-c), fasting serum insulin, serum creatinine and urine microalbuminuria were carried out as previously described [16, 17, 18, 19]. Insulin resistance was measured by two surrogate measures: fasting serum insulin and homeostasis model assessment for insulin resistance (HOMA-IR). The value of HOMA-IR was calculated by the following equation: fasting serum insulin [mU/L] × fasting plasma glucose [mg/dL]/405 [20]. Estimated glomerular filtration rate (eGFR) was calculated using MDRD formula: eGFR = 186 × Serum creatinine (mg/dL)−1.154 × Age-0.203 × [1.210 if Black] × [0.742 if Female] [21]. Body fat percent (BF%) was estimated by dual energy x-ray absorptiometry (DEXA; Hologic QDR 4500A with fan beam, Waltham, MA, USA).
Dietary intake was assessed using standardized food frequency questionnaire and 24-hour dietary recall. Participants were encouraged to maintain food diaries to check for compliance. Dietary intake of nutrients such as energy, protein, carbohydrate, and total fat was assessed using nutritive value of Indian foods [22]. Daily level of physical activity was assessed using physical activity questionnaire at each visit. Participants were required to document the type, frequency, intensity (light, moderate, or vigorous) and average duration for each activity. Assessment of total activity was done as total metabolic equivalent of tasks (MET; h/day) by summing MET values of all whole day’s activities [23]. Diet and physical activity compliance was monitored through food-frequency questionnaire, 24-hour dietary recalls, telephonic interviews (Fig. 1) and compliance check questionnaires.
3.1. Study design
The sample size for the study has been calculated from a previous study [12] with weight loss as the primary outcome. Sample size for the study was computed to detect minimum of 5% additional reduction in intervention as compared to the control arm with the following assumptions: level of significance 5%; power 90%, loss to follow up 10%. The required sample size was 40 subjects in each group. The total duration of the intervention was 8 weeks after completion of standard 4 weeks of diet and exercise run-in period (a standard time period to prepare all participants for the study and bring them at a common start point) similar to the intervention period in one of our previous studies [24]. All recruited subjects were vegetarians, thus a vegetarian diet (protein, 15%; carbohydrates, 60%; and fat, 25%) was formulated according to the standard dietary guidelines for Asian Indians. Subjects randomized to the control arm were asked to continue with the same diet as advised in the run-in period while those randomized to the experimental group were advised the PACER diet. There were no significant changes in weight during run-in period.
A randomized controlled parallel design was used to compare the effect of a test diet (PACER diet; intervention group) vs. a standard vegetarian diet (control group) over a period of 8 weeks, after a run-in period of 4 weeks as mentioned. Main outcome measure was weight loss after following PACER diet as compared to standard vegetarian diet after a period of 8 weeks. The secondary outcome measures were changes in BF%, blood pressure, lipid profile (TC, LDL-c, HDL-c, VLDL-c, TAG), FPG and serum Insulin.
After the initial enrollment, participants met the dietician to discuss the standard vegetarian diet and exercise schedule to be followed for 4 weeks of run-in period. The subjects were randomised using computer generated random number Table (nQuery Advisor, version 7) and were explained about the diet that was assigned to them. All the investigations, consultations with the dietician and investigator etc. were similar in both the groups and subjects were explained the respective diets. In case any of the subjects allocated in one arm was interested in following the other diet plan then they were suggested to follow the other diet plan after completion of the intervention period. The meal plan of the experimental and control diets have been provided in Table 1. All the subjects were satisfied with the diet regime advised to them which was evident through compliance checks. Dietary compliance in both the groups was monitored by the dietician by weekly phone calls, follow up meetings and on-site discussions. Similar exercise counselling was provided to both groups and participants were instructed to maintain the same level of physical activity throughout the study. They were advised to drink at least 8–10 glasses of water/day. After randomization two participants from the control group withdrew their consent within the first week due to hectic work schedules (Fig. 1). In total, 102 obese subjects were randomly allocated to one of the two diets; PACER diet (high protein, high fat and moderately low carbohydrate, lacto-vegetarian; n = 52) or control diet (n = 50) after a four week run-in period. During the run-in period, all participants consumed the diets according to their height, weight, and physical activity level, formulated as per the dietary guidelines for Asian Indians [25]. A 45-min brisk walk daily was recommended for all participants throughout the study. The baseline anthropometry, biochemical investigations, and DEXA was done after the 30-day run-in period and compared with post-intervention data (after completion of 8 weeks). The macronutrient targets for PACER diet were protein, 26.9%; carbohydrate, 36.6%, fat, 36.5% and of control diet was protein, 15%; carbohydrates, 60%; fat, 25% (Table 2). The baseline dietary composition is given in Table 3. The major dietary components for the PACER diet included almonds, peanuts, soy nuggets and a low carbohydrate protein supplement (Table 1, Table 4). These were provided to the participants and their family, free of cost, to ensure compliance
Table 1.
Menu plan for experimental and control diet*.
| Meals | Experimental diet (PACER diet) | Control diet |
|---|---|---|
| Early Morning | Tea (without sugar), Almonds | Tea (without sugar) |
| Breakfast | Double toned milk# | Double toned milk# |
| Spinach stuffed chapatti† (1 piece; small) | Cauliflower stuffed chapatti†(1 piece; medium) | |
| Grilled cottage cheese with capsicum and tomatoes | Sautéed cottage cheese and tomato | |
| Mid-morning | Protein supplement‡ (taken with water) | Lemonade (without sugar) |
| Cucumber salad (with lemon dressing) | Fruit Salad (without dressing) | |
| Lunch | Vegetable salad (with lemon dressing) | Vegetable salad (with lemon dressing) |
| Radish stuffed thin chapatti† (1 piece; small) | Chapatti† (2 pieces; small) | |
| Green gram whole | Kidney beans | |
| Grilled cottage cheese | Curd | |
| Sautéed mushrooms | Mixed vegetable | |
| Tea | Tea (without sugar) | Tea (without sugar) |
| Roasted groundnuts | Roasted chick pea | |
| Pre-Dinner | Protein supplement‡ (taken with water) | Spinach soup (homemade, without starch and fat/oil) |
| Dinner | Lentil soup | Lentils |
| Soybean granules with beans and tomato | Carrot and pea vegetable | |
| Chapatti† (2 pieces; small) |
Experimental diet (PACER diet); Control diet; menu resembling standard Indian dietary pattern and meal combinations.
Double toned milk is obtained by the addition of skim milk powder & water to whole milk, in India, under PFA (Prevention of food adulteration) rules, double toned milk should contain a minimum of 1.5% fat & 9.0% non-fat solids [20].
Chapatti, unleavened flatbread made from wheat flour, a staple in South Asia.
Commercial protein powder 15 g/serving (30 g/d).
Table 2.
Dietary composition of the experimental and control diet*.
| Nutrients composition | Pacer Diet | Control diet |
| Carbohydrate (%) | 36.6 | 60.0 |
| Protein (%) | 26.9 | 15.0 |
| Fat (%) | 36.5 | 25.0 |
| Distribution of protein | Protein energy percent | Protein energy percent |
| Vegetable protein (pulses, legumes, nuts) | 8.6 (31.3) | 11.1 (70.2) |
| Soy protein | 4.0 (14.9) | 0.0 (0.0) |
| Protein from dairy sources (Milk and milk products; low fat/double toned milk and milk products) [27] | 7.4 (27.9) | 4.7 (29.8) |
| Protein supplement # | 6.9 (25.9) | 0.0 (0.0) |
| Distribution of fat | Fat energy percent§ | Fat energy percent§ |
| Saturated fat | 6.4 (17.4) | 7.8 (30.5) |
| Monounsaturated fat | 10.5 (28.6) | 8.1 (31.6) |
| Polyunsaturated fat | 19.9 (54.0) | 9.6 (37.8) |
¶ values in parenthesis show percent of total protein.
Experimental diet (PACER diet); Control diet (Standard diet); menu resembling standard Indian dietary pattern and meal combinations. Values computed from Nutritive values of Indian Foods [21].
Nutritional profile (including essential amino acid content) for the protein supplement used i.
values in parenthesis show percent of total fat.
Table 3.
Baseline characteristics at randomization.
| Variables | Control group (n = 50) | Intervention group (n = 52) | p value# |
|---|---|---|---|
| Age (years) | 38.7 ± 9.9 | 38.9 ± 8.7 | 0.879 |
| Male (%) | 17 (34.0) | 21 (40.4) | 0.505† |
| Female (%) | 33 (66.0) | 31 (59.6) | |
| Medical history* | |||
| Hypertension (%) | 14 (28.0) | 17 (32.7) | 0.265 |
| Lifestyle profile | |||
| Smoking (%) | 14 (28.0) | 12 (23.1) | 0.325 |
| Alcohol (%) | 11 (22.0) | 17 (32.7) | 1.463 |
| Nutritional profile | |||
| Energy (Kcal) | 1868.7 ± 204.1 | 1824.7 ± 236.7 | 0.318 |
| Protein (%) | 12.1 ± 1.03 | 11.8 ± 08 | 0.085 |
| Carbohydrate (%) | 57.6 ± 3.2 | 57.3 ± 2.8 | 0.237 |
| Fat (%) | 31.0 ± 3.0 | 30.6 ± 2.8 | 0.411 |
| Physical activity profile | |||
| Metabolic Equivalent of Task (MET) score | 32.9 ± 2.8 | 34.02 ± 3.8 | 0.115 |
Values are presented as Means ± SD, p < 0.05: statistically significant.
none of the participants had any history of heart disease or chronic liver disease.
calculated using Students two-sample t test.
calculated using Pearson chi squared test.
Table 4.
Nutritional information for the low carbohydrate protein supplement used for intervention (PACER) diet#.
| Nutrients | Approximate composition/10 g (1 scoop serving size) |
|---|---|
| Macro-nutrients | |
| Energy | 36 kcal |
| Protein | 8.0 g |
| Carbohydrate | 0.5 g |
| Fat | 0.3 g |
| Minerals | |
| Calcium | 70 mg |
| Iron | 1 mg |
| Essential Amino Acids | |
| Isoleucine | 408 mg |
| Leucine | 696 mg |
| Lysine | 544 mg |
| Methionine(& Cysteine) | 224 mg |
| Phenylalanine (& Tyrosine) | 752 mg |
| Threonine | 320 mg |
| Tryptophan | 104 mg |
| Valine | 432 mg |
| Histidine | 216 mg |
As mentioned on nutrient label of the product (AMWAY Nutrilite protein powder).
On an average, each subject was counseled for 20–25 min during each visit. It was ensured that all participants received standardized advice regarding exercise and other lifestyle factors. All participants were encouraged to come along with their spouse for the dietary counseling to ensure better understanding and compliance. Three methods of dietary assessment were adopted. Firstly, the macronutrient calculation has been done on the basis of 3-day 24-hour dietary recall (including 2 weekdays and 1 weekend) and the information from food frequency was used to assess the overall pattern of food intake. The food frequency questionnaire listed all the commonly consumed food items from different foods groups and also various commonly consumed junk foods. This questionnaire is validated for Asian Indians. The subjects were instructed to maintain food diaries to ensure compliance. The data from 24-hour dietary recall for three days and food frequency questionnaire were collected during subjects’ visits to the study site every month. Subjects were asked to record data of food intake in food diaries every day and to share information with the investigator during their visits. Hence the 24-hour dietary recall, food frequency questionnaire and food diaries were used to capture data on dietary intake.
In total there were 5 visits of subjects: first at screening; second on enrollment (beginning of run-in period); third at visit 1 or randomization; fourth visit was on the 4th week, fifth visit on the 8 week of intervention respectively. The compliance checks were done on weekly basis. Subjects adhered to diet and exercise regime, on at least 6 out of the 7 days in a week i.e. 80%. Compliance to the protocol was good, as indicated by compliance checks: weekly compliance questionnaires, weekly telephone calls, messages, discussion, and cross checking with the spouse or any close relative. If any subject was observed to be deviating from compliance, they were repeatedly counselled and followed up. Subsequently greater degree of compliance was observed. Further, to rule out sharing of study product with non-participants such as friends and family members, the subjects were also asked to bring empty packets of dietary components (almonds, peanuts, soy nuggets, low carbohydrate protein supplement) while meeting the study investigator. However, 2 subjects in the control group and 4 subjects in intervention group were excluded from the study due to poor compliance. Further, dietary and physical activity recall in the presence of the spouse of the subject was performed to check compliance and concordance. The Global Physical Activity Questionnaire (GPAQ) was used to assess level of physical activity as this has been validated for Asian Indians [26].
3.2. Analysis
The intervention and the control groups were balanced as no statistical difference was observed for age and gender. The effect of Pacer diet was found to be consistent in the stratified analysis by gender, i.e. there was no effect modification due to gender. The intention-to-treat (ITT) analysis was the primary analysis. Baseline observation was carried forward for subjects with missing values. Unless otherwise stated, ITT data are presented throughout. Per-protocol analysis (all compliant participants) has also been conducted. Student’s two-sample t-test was used to compare difference in mean values at baseline and post intervention between the two groups. Paired t-test was used to compare the pre and post intervention mean values within the group. For every outcome variable, results are presented as mean ± SD. The difference between the two groups post intervention is also presented. P value < 0.05 at (95% CI) was considered statistically significant. Statistical analysis was done using STATA 12.0 (STATA Corp, Houston, TX, USA) software
4. Results
Of the 102 participants (64 females and 38 males, all included in ITT analysis), 89 (44 control and 45 intervention group) completed the study. The mean (± SD) age of the subjects was 38.8 ± 9.2 years. There were no significant differences in the socio-demographic characteristics (Table 5). The actual dietary intake and physical activity details of the study subjects have been provided in Table 6. Importantly, there were no significant differences in the baseline measurements of obesity (weight, BMI, waist circumference, hip circumference and BF%) and metabolic parameters between the intervention and the control groups (Table 7). The overall compliance for participants completing the trial was 81%.
Table 5.
Sociodemographic profile of the study participants.
| Variables | Control Group (n = 50) | Intervention Group (n = 52) | p value |
|---|---|---|---|
| Education | |||
| Postgraduate (%) | 54.0 | 38.5 | 0.278* |
| Graduate (%) | 44.0 | 57.7 | |
| Intermediate (%) | 2.0 | 3.9 | |
| Occupation | |||
| Homemaker (%) | 16.0 | 17.0 | 0.867* |
| Service (%) | 52.0 | 53.9 | |
| Business (%) | 14.0 | 9.6 | |
| Student (%) | 2.0 | 3.9 | |
| Monthly household income (INR) | 142500 ± 53664.2 | 135769.2 ± 64141.27 | 0.568# |
| Marital status | |||
| Married (%) | 86.0 | 90.4 | 0.472* |
| Unmarried (%) | 14.0 | 9.6 | |
INR; Indian National Rupee.
Calculated using two-sample t-test.
Calculated using chi squared test.
Table 6.
Nutrition and physical activity profile during the study.
| Control Group (n = 44) | Intervention Group (n = 45) | p value* | |
|---|---|---|---|
| Nutritional profile | |||
| Energy (Kcal) | 1443.2 ± 130.4 | 1405.9 ± 127.6 | 0.176 |
| Protein (%) | 14.7 ± 1.6 | 24.9 ± 2.2 | < 0.001 |
| Carbohydrate (%) | 59.3 ± 2.7 | 38.5 ± 3.1 | < 0.001 |
| Fat (%) | 26.1 ± 1.7 | 36.6 ± 2.4 | < 0.001 |
| Physical Activity profile | |||
| Metabolic Equivalent of Task (MET) | 36.5 ± 2.8 | 37.5 ± 4.1 | 0.202 |
Calculated using Student’s two sample t-test.
Table 7.
Comparison of anthropometric and biochemical parameters pre- and post- intervention between the two diet groups.
| Intention to treat analysis† |
Per Protocol Analysis‡ |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Control group§ (n = 50)¶ | Intervention groupǁ (n = 52)¶ | Difference# (95% CI) | p-value* | Control group§ (n = 44)¶ | Intervention group║ (n = 45)¶ | Difference# (95% CI) | p-value* |
| Weight (kg) | ||||||||
| Pre-intervention | 85.2 ± 10.4 | 83.8 ± 10.4 | 0.49 | 85.8 ± 10.8 | 83.5 ± 10.9 | 0.325 | ||
| (Week 0) | ||||||||
| Post-intervention | 82.7 ± 9.9 | 78.9 ± 10.2 | 0.06 | 82.9 ± 10.2 | 77.9 ± 10.4 | 0.027 | ||
| (Week 8) | ||||||||
| % Change ǂ | 2.9 ± 1.7 | 6.1 ± 2.9 | −3.2 (−4.1 to −2.3) | <0.001 | 3.4 ± 1.3 | 7.1 ± 1.9 | −3.7 (−4.4 to −2.9) | <0.001 |
| BMI (kg/m2) | ||||||||
| Pre-intervention (Week 0) | 31.2 ± 2.9 | 31.5 ± 3.1 | 0.6 | 31.2 ± 3.1 | 31.1 ± 3.1 | 0.887 | ||
| Post-intervention | 30.3 ± 2.9 | 29.7 ± 3.2 | 0.35 | 30.2 ± 2.9 | 29.0 ± 2.9 | 0.074 | ||
| (Week 8) | ||||||||
| % Change ǂ | 2.9 ± 1.6 | 5.7 ± 2.6 | −2.9 (−3.7 to −2.0) | <0.001 | 3.3 ± 1.3 | 6.6 ± 1.7 | −3.3 (−3.9 to −2.9) | < 0.001 |
| Waist circumference (cm) | ||||||||
| Pre-intervention | 104.1 ± 5.7 | 102.7 ± 5.9 | 0.23 | 103.8 ± 5.7 | 103.2 ± 5.9 | 0.645 | ||
| (Week 0) | ||||||||
| Post-intervention (Week 8) | 101.9 ± 5.9 | 98.7 ± 5.8 | 0.01 | 101.4 ± 5.7 | 98.7 ± 5.9 | 0.034 | ||
| % Change ǂ | 2.1 ± 1.1 | 3.9 ± 1.7 | −1.8 (−2.3 to −1.2) | <0.001 | 2.4 ± 0.9 | 4.4 ± 0.9 | −2.1 (−2.4 to −1.7) | < 0.001 |
| Hip circumference (cm) | ||||||||
| Pre-intervention | 110.0 ± 4.6 | 108.8 ± 6.3 | 0.26 | 110.3 ± 4.7 | 109.5 ± 6.5 | 0.507 | ||
| (Week 0) | ||||||||
| Post-intervention (Week 8) | 108.1 ± 4.6 | 105.5 ± 6.1 | 0.01 | 108.1 ± 4.8 | 105.6 ± 6.4 | 0.041 | ||
| % Change ǂ | 1.7 ± 0.9 | 3.0 ±1.4 | −1.3 (−1.8 to −0.8) | <0.001 | 1.9 ± 0.7 | 3.5 ± 0.8 | −1.5 (−1.9 to −1.2) | <0.001 |
| Mid upper arm circumference (cm) | ||||||||
| Pre-intervention (Week 0) | 37.3 ±3.01 | 36.4 ± 3.64 | 0.18 | 37.3 ± 3.1 | 36.8 ± 3.7 | 0.47 | ||
| Post-intervention (Week 8) | 36.4 ± 2.8 | 34.8 ± 3.4 | 0.01 | 36.4 ± 2.9 | 34.9 ± 3.5 | 0.046 | ||
| % Change ǂ | 2.2 ± 1.4 | 4.3 ± 2.2 | −2.1 (−2.8 to −1.3) | <0.001 | 2.5 ± 1.1 | 4.9 ± 1.6 | −2.4 (−2.9 to −1.8) | <0.001 |
| Body fat percent (%) | ||||||||
| Pre-intervention (Week 0) | 45.4 ± 3.1 | 46.5 ± 2.4 | 0.06 | 45.3 ± 3.3 | 46.3 ± 2.5 | 0.101 | ||
| Post-intervention (Week 8) | 44.9 ± 3.2 | 45.7 ± 2.3 | 0.18 | 44.7 ± 3.3 | 45.4 ± 2.3 | 0.258 | ||
| % Change ǂ | 1.0 ± 0.9 | 1.6 ± 0.9 | −0.6 (−0.9 to −0.2) | 0 | 1.2 ± 0.8 | 1.9 ± 0.7 | −0.7 (-0.9 to −0.4) | < 0.001 |
| Systolic blood pressure (mmHg) | ||||||||
| Pre-intervention (Week 0) | 136.1 ± 11.4 | 133.4 ± 12.1 | 0.25 | 136.8 ± 11.7 | 134 ± 12.4 | 0.276 | ||
| Post-intervention (Week 8) | 134.3 ± 10.7 | 129.7 ± 11.2 | 0.03 | 134.8 ± 10.9 | 129.8 ± 11.3 | 0.035 | ||
| % Change ǂ | 1.2 ± 1.9 | 2.8 ± 1.8 | −1.53 (−2.28 to −0.78) | <0.001 | 1.4 ± 2.07 | 3.1 ± 1.6 | −1.7 (-2.5 to −0.9) | < 0.001 |
| Diastolic blood pressure (mmHg) | ||||||||
| Pre-intervention (Week 0) | 83.9 ± 5.8 | 85.9 ± 5.1 | 0.07 | 83.8 ± 5.7 | 86.2 ± 5.0 | 0.036 | ||
| Post-intervention (Week 8) | 83.6 ± 5.4 | 85.2 ± 4.5 | 0.11 | 83.4 ± 5.2 | 85.3 ± 4.6 | 0.065 | ||
| % Change ǂ | 0.3 ± 1.8 | 0.8 ± 1.9 | −0.42 (−1.16 to 0.32) | 0.26 | 0.5 ± 1.8 | 1.0 ± 1.9 | −0.6 (−1.3 to 0.2) | 0.165 |
| Fasting plasma glucose (mg/dL) | ||||||||
| Pre-intervention (Week 0) | 99.9 ± 10.6 | 97.5 ± 9.5 | 0.23 | 100.3 ± 10.8 | 97.2 ± 9.7 | 0.16 | ||
| Post-intervention (Week 8) | 96.6 ± 9.8 | 91.7 ± 9.6 | 0.01 | 96.6 ± 10.2 | 90.8 ± 9.6 | 0.007 | ||
| % Change ǂ | 3.2 ± 2.7 | 5.9 ± 3.2 | −2.7 (−3.9 to −1.6) | <0.001 | 3.7 ± 2.4 | 6.7 ± 2.6 | −2.9 (−4.1 to −1.9) | <0.001 |
| Fasting serum insulin (mU/L) | ||||||||
| Pre-intervention (Week 0) | 16.9 ± 6.1 | 17.0 ± 6.3 | 0.89 | 16.2 ± 5.9 | 17.1 ± 6.6 | 0.514 | ||
| Post-intervention (Week 8) | 15.4 ± 5.9 | 15.4 ± 6.0 | 0.98 | 14.6 ± 5.4 | 15.2 ± 6.2 | 0.605 | ||
| % Change ǂ | 8.0 ± 10.5 | 10.5 ± 7.3 | −2.5 (−6.0 to 1.0) | 0.16 | 8.9 ± 10.8 | 11.7 ± 6.9 | −2.8 (−6.7 to 0.9) | 0.145 |
| HOMA-IR! | ||||||||
| Pre-intervention (Week 0) | 4.2 ± 1.6 | 4.1 ± 1.5 | 0.85 | 4.0 ± 1.5 | 4.1 ± 1.6 | 0.823 | ||
| Post-intervention (Week 8) | 3.7 ± 1.4 | 3.5 ± 1.4 | 0.51 | 3.5 ±1.3 | 3.42 ± 1.4 | 0.833 | ||
| % Change ǂ | 10.9 ± 10.3 | 15.8 ± 8.0 | −4.9 (−8.5 to −1.2) | 0.009 | 12.4 ± 10.2 | 17.7 ± 6.7 | −5.4 (−8.9 to −1.7) | 0.004 |
| Total cholesterol (mg/dL) | ||||||||
| Pre-intervention (Week 0) | 177.1 ± 44.6 | 174.7 ± 38.6 | 0.77 | 181.7 ± 42.8 | 174 ± 39.1 | 0.373 | ||
| Post-intervention (Week 8) | 167.3 ± 42.6 | 156.9 ± 36.8 | 0.19 | 170.6 ± 41.8 | 154.1 ± 36.1 | 0.049 | ||
| % Change ǂ | 5.4 ± 4.8 | 10.2 ± 6.3 | −4.8 (−7.0 to −2.6) | <0.001 | 6.2 ± 4.6 | 11.5 ± 5.6 | −5.3 (−7.5 to −3.2) | <0.001 |
| Serum triacylglycerol (mg/dL) | ||||||||
| Pre-intervention (Week 0) | 139.9 ± 36.3 | 140.9 ± 34.8 | 0.89 | 140.5 ± 37.3 | 138.4 ± 33.1 | 0.779 | ||
| Post-intervention (Week 8) | 129.9 ± 31.4 | 121.1 ± 32.0 | 0.16 | 130.3 ± 32.47 | 115.8 ± 27.1 | 0.025 | ||
| % Change ǂ | 6.4 ± 6.2 | 13.6 ± 10.6 | −7.2 (−10.6 to −3.8) | 6.6 ± 5.8 | 15.6 ± 10.0 | −8.9 (−12.4 to −5.5) | <0.001 | |
| <0.001 | ||||||||
| HDL-c (mg/dL) | ||||||||
| Pre-intervention (Week 0) | 44.3 ± 9.5 | 42.4 ± 9.1 | 0.32 | 44.9 ± 9.8 | 42.7 ± 9.4 | 0.262 | ||
| Post-intervention (Week 8) | 43.2 ± 8.6 | 41.9 ± 8.1 | 0.42 | 43.8 ± 8.9 | 42.2 ± 7.9 | 0.369 | ||
| % change ǂ | 1.9 ± 5.9 | 0.6 ± 8.2 | 1.4 (−1.5 to 4.2) | 0.34 | 2.1 ± 6.3 | 0.2 ± 8.5 | 1.8 (−1.3 to 5.0) | 0.251 |
| LDL-c (mg/dL) | ||||||||
| Pre-intervention (Week 0) | 106.1 ± 31.2 | 100.9 ± 26.01 | 0.36 | 110.1 ± 28.8 | 100.4 ± 23.6 | 0.085 | ||
| Post-intervention (Week 8) | 100.5 ± 29.1 | 89.4 ± 26.4 | 0.05 | 103.6 ± 27.67 | 87.7 ± 22.8 | 0.004 | ||
| % Change ǂ | 4.9 ± 5.3 | 11.9 ± 7.1 | −7.0 (−9.5 to −4.6) | <0.001 | 5.9 ± 3.7 | 13.1 ± 6.4 | −7.1 (−9.3 to −4.9) | <0.001 |
| VLDL-c (mg/dL) | ||||||||
| Pre-intervention (Week 0) | 27.9 ± 7.3 | 28.2 ± 6.9 | 0.89 | 28.1 ± 7.5 | 27.7 ± 6.6 | 0.779 | ||
| Post-intervention (Week 8) | 25.9 ± 6.3 | 24.2 ± 6.4 | 0.16 | 26.1 ± 6.5 | 23.2 ± 5.4 | 0.025 | ||
| % Change ǂ | 6.4 ± 6.2 | 13.7 ± 10.6 | −7.2 (−10.6 to −3.8) | <0.001 | 6.6 ± 5.8 | 15.6 ± 10.0 | −8.9 (−12.4 to −5.5) | <0.001 |
BMI, Body mass index; HDL-c, high density lipoprotein cholesterol; HOMA-IR, Homeostasis model assessment for insulin resistance; LDL-c, low density lipoprotein cholesterol; VLDL-c, Very low density lipoprotein cholesterol.
Intention to treat analysis was the primary analysis, baseline observations were carried forward for subjects with missing values.
Per protocol analysis, included all compliant participants.
Control group; following standard diet.
Intervention group; following “High Protein Complete (lacto) VEgetaRian” (PACER) diet.
Values are means ± SD.
Difference calculated using Student’s two-sample t-test between the post intervention percent changes of the two groups; 95% CI in parentheses.
Student’s two sample t-test was used to compare difference in mean values between the two groups.
Percent change was calculated between baseline and post intervention values for each group (values presented as %).
The value of HOMA-IR was calculated using the following equation: fasting serum insulin [mU/L] × fasting plasma glucose [mg/dL]/405 [20] The effect size and its 95% CI (mean percentage reduction in the intervention group-control group) was statistically significant for the following parameters: weight: −3.2 (−4.1 to −2.3); BMI: −2.9 (−3.7 to −2.0); WC: −1.8 (−2.3 to −1.2); HC: −1.3 (−1.8 to −0.8) and MUAC: −2.1 (−2.8 to −1.3).
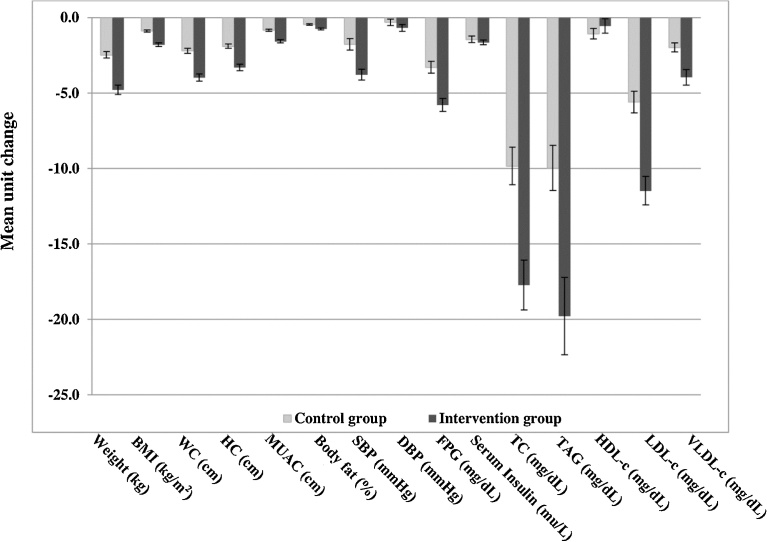
The post intervention effect size and its 95% CI for FPG levels was significantly higher −2.7 (−3.9 to −1.6) in the intervention group as compared to the control group (Table 4), the percent reduction in serum insulin levels despite being more in the intervention group −2.5 (−6.0 to 1.0) were not statistically significant. The percent reduction for systolic blood pressure (SBP) in the intervention group was also significantly higher −1.53 (−2.28 to −0.78). The percentage reductions (difference, 95% CI) in the intervention group were higher for TC −4.8 (−7.0 to −2.6), triacylglycerol −7.2 (−10.6 to −3.8), LDL-c 1.4 (−1.5 to 4.2) while no significant effect was seen on HDL-c levels (Fig. 2).
Fig. 2.
Changes in outcome variables between intervention and control group after 8 week intervention. Intervention group, participants following high Protein Complete (lacto) VEgetaRian diet (PACER diet); Control group, participants following standard diet. Values represent mean unit change (reduction) ± SD. BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HC, hip circumference; HDL-c, high density lipoprotein-cholesterol; LDL-c, low density lipoprotein-cholesterol; MUAC, mid upper arm circumference; SBP, systolic blood pressure; TC, total cholesterol; TAG, serum triacylglycerol; VLDL-c, very low density lipoprotein-cholesterol; WC: waist circumference.
Two participants in the intervention group reported flatulence and heaviness of abdomen; they were advised to keep adequate gaps (minimum two hours) between two consecutive meals and eat food at a slower pace. The problem resolved after three days in both the cases. Apart from this, no clinical adverse events were reported during the course of the study.
5. Discussion
This is the first higher protein, higher fat and moderately lower carbohydrate dietary intervention as compared to standard vegetarian control diet in Asian Indians, which demonstrates several benefits on adiposity and cardio-metabolic risk factors among obese subjects.
Several studies and meta-analyses have shown greater loss of weight, body fat mass, and waist circumference following high protein energy restricted diets as compared to standard protein diets [27, 28, 29, 30] for upto 6 months, however certain studies have found no evident difference in weight loss when high protein diets and standard protein diets were compared [31]. In a three month randomized controlled dietary intervention among 60 overweight/obese Iranian women following either a high protein (HP) diet (25% protein, 45% carbohydrate, 30% fat; total protein divided between animal and plant sources in a 1:1 ratio) or energy-restricted control diet (15% protein, 55% carbohydrate, 30% fat), Azadbakht et al. [32], demonstrated significantly higher weight loss (−6.10 ± 0.34% vs. −3.90 ± 0.26; p < 0.0001) and reduction in waist circumference (−5.06 ± 0.28% vs. −3.03 ± 0.21; p <0.0001) in the HP group. In a four week randomized dietary intervention trial Baba et al. [33], higher weight loss in the high protein group as compared to the high carbohydrate group was reported. These studies included animal proteins as a significant part of the total protein content.
Vegetarian and plant based diets have been less researched in context of weight loss. Jenkins et al. [12], researched a plant based low-carbohydrate diet (“eco-Atkins diet”; 26% carbohydrates, 31% protein, and 43% fat) compared to a high carbohydrate lacto-ovo vegetarian diet (58% carbohydrates, 16% protein, and 25% fat). Application of this diet lead to reduction in the levels of TC (−19.8% vs. −12.7%), serum triacylglycerol (TAG; −29.2% vs. −17.8%), and LDL-c (−20.4% vs. −12.3%) as compared to the high-carbohydrate diet, respectively. Both the diets showed similar weight loss of 3.9 ± 0.4 kg vs. 4.2 ± 0.3 kg (p = 0.94), respectively, with no significant percentage changes in values of fasting plasma glucose, serum insulin and insulin resistance. In a two week randomized cross-over study between iso-caloric (30% protein, 30% fat, 40% carbohydrate) soy based vegetarian high protein and meat based high protein diet, Neacsu et al. [34], showed similar weight loss of 2.41 ± 0.22 kg vs. 2.27 ± 0.19 kg; p = 0.352; (±Standard Error of the difference between sample means, SED: 0.1), subjectively rated hunger (p = 0.569; SED between sample means: 3.8), fullness (p = 0.404; ±SD between the sample means: 4.1), desire to eat (p = 0.356; SD between the sample means: 3.7), preservation of lean body mass (p = 0.334; SD: 0.2), and loss of percent fat mass (p = 0.179; SD between the sample means: 0.2) suggesting that vegetarian diets can be as effective as meat-based diets for appetite control during weight loss. In the present study, the intervention group following PACER diet showed greater percent improvements for weight loss, TC, TAG, LDL-c as compared to control diets, similar to previous trials. Importantly, this intervention also lead to decrease in fasting plasma glucose and insulin resistance as compared to those in the control group, results not shown in previous studies with vegetarian diets.
Increasing dietary protein intake promotes weight loss through several mechanisms. On an average, dietary protein requires 20–30% of its usable energy to be expended for metabolism and/or storage as compared to 5–10% and 0–3% required by carbohydrates and dietary fats respectively [35]. Diets high in protein promote energy expenditure through increased postprandial thermogenesis [36], and increase total daily expenditure while preventing decline in resting energy expenditure during weight loss [37, 38]. Interventional studies with dietary protein have shown to promote weight loss by increasing satiety as compared to carbohydrates [39, 40]. Weigle et al. [38], demonstrated an increase in satiety among participants following a high protein diet (30% protein, 20% fat, 50% carbohydrate) as compared to a standard vegetarian diet (15% protein, 35% fat, 50% carbohydrate). The effect of high-protein diets on satiety involves multiple metabolic pathways; including enhancement of satiating effects of circulating gut related hormones [38]. Similar to the findings of previous studies [38, 39] participants in the present study following intervention diet also reported higher satiety as compared to those following control diets.
Concerning palatability of the diet, we observed that the prescribed diets in both the arms were liked by the study subjects. Slight variations in recipes were permitted to avoid monotony in diet and to increase palatability. Every effort was made to design the recipes in accordance to the preferences of the study subjects. Subjects enthusiastically followed the prescribed diets during the study period. Long-term adherence to any single diet is always difficult, however a longer period of study is required to definitely answer this question.
Although the exact mechanism of blood pressure decrement and high protein diets is not clear, it is stipulated that dairy proteins contain natural angiotensin-converting-enzyme inhibitors (ACEI), one of the major types of blood pressure lowering medications [41]. Previously, studies have demonstrated the beneficial effects of vegetable protein on blood pressure [34, 41] and are suggestive of an inverse association between protein intake particularly from plant sources and blood pressure [42]. The result for SBP in the present study was in line with previous studies showing significant reduction in the intervention group.
In the present study, there was significant reduction of glycemia (FPG) and surrogate marker of insulin resistance (HOMA-IR) in the intervention group. Consumption of a high protein diet stabilizes blood glucose and reduces the postprandial insulin response [43, 44]. Several studies including systematic review and meta-analysis have demonstrated that high-protein diet as compared to other diets leads to a significant decrease in glycosylated hemoglobin levels [45]. Piatti et al. [46], highlighted that high protein intake during weight loss may enhance insulin sensitivity. These benefits may be due to the effect of protein on satiety and/or due to a lower glycemic load because of a lower carbohydrate intake. However, some studies indicate a reduction in fasting plasma glucose and serum insulin concentrations following weight loss irrespective of the type of diet (high protein or standard protein diet). The results of the present study were in line with previous studies showing significant reduction for TC, TAG and LDL-c levels in the intervention group. High protein weight loss diets have beneficial effects on TC and TAG in overweight and obese subjects and achieved better lipid results in subjects at increased risk of cardiovascular disease [27, 28]. Studies have also shown convincing evidence for an inverse relationship between soya protein intake and LDL-c [46]. Appel et al. [47], in OmniHeart study demonstrated that high protein diet lowered LDL-c by 3.3 mg/dL (p = 0.01) and TAG by 15.7 mg/dL (p < 0.001) as compared to high carbohydrate diet.
Studies done in Western populations have shown that vegetarians have lower BMI and lower cholesterol than non-vegetarians [48, 49]. However, a direct comparison between vegetarian and non-vegetarian has not been done. A prospective study on elderly women has shown that long term adherence to vegetarian diet was associated with improved nutrient intake and reductions in blood glucose and lipid levels. Specifically, vegetarians had significantly lower blood glucose, LDL-c, and TC levels when compared to non-vegetarians [48]. It may be noted that vegetarian diets in the Indian context may not show similar impact on metabolic parameters because of multiple unique characteristics of Indian diet like, the choice of oils, high carbohydrate saturated fat and trans fat contents etc. In cohort studies, it has been shown that adherence to vegetarian diets result in a moderate reduction in mortality from coronary heart disease as vegetarian diets are rich n-6 polyunsaturated fatty acids, fibre, carotenoids which may positively impact cardiovascular risk factors [50].
Macronutrient composition of vegetarian diets, as shown by our study, could be manipulated to achieve reasonable weight loss in obese individuals. Research examining vegetarian diets and weight loss is much needed across diverse ethnic groups including older adults [51].
We acknowledge that the present study has a higher number of female participants (62.8%) as compared to males (37.2%) but there was no statistical difference that would influence the outcomes of the study. Moreover, our previous studies have reported that obesity is more prevalent among Asian Indian women as compared to men, specifically after child birth and in the postmenopausal phase [52, 53]. Further, the trial duration of 8 weeks in the present study could be a possible limitation, but studies of 1 and 4 weeks duration have been published previously [12, 54]. Studies of longer duration should be conducted to evaluate the long-term effect on kidney function, reproductive health, bone matrix and life span. Finally, research into finding methods to improve long-term compliance of such diets should be carried out.
In conclusion, this 8-week randomized control trial demonstrates that a higher protein, higher fat and moderately lower carbohydrate from lacto-vegetarian source as compared to standard vegetarian control diets leads to significant reductions in weight, waist circumference, and improves the cardio-metabolic profile among obese Asian Indians.
Declarations
Author contribution statement
Swati Bhardwaj: Performed the experiments; Wrote the paper.
Anoop Misra, Seema Gulati, Shajith Anoop: Conceived and designed the experiments; Wrote the paper.
Vinit Kumar Kamal, Ravindra Mohan Pandey: Analyzed and interpreted the data.
Competing interest statement
The authors declare no conflict of interest.
Funding statement
This work was supported by the Indian Council of Medical Research (ICMR; Grant number SSD/WS/076/2008).
Additional information
The clinical trial described in this paper was registered at https://clinicaltrials.gov/ under the registration number NCT02562209.
Acknowledgements
We are thankful to all the participants of the study for their co-operation.
References
- 1.Misra A., Bhardwaj S. Obesity and the metabolic syndrome in developing countries: focus on South Asians. Nestle Nutr. Inst. Workshop Ser. 2014;78:133–140. doi: 10.1159/000354952. [DOI] [PubMed] [Google Scholar]
- 2.Bhardwaj S., Misra A. Obesity, diabetes and the Asian phenotype. World Rev. Nutr. Diet. 2015;111:116–122. doi: 10.1159/000362308. [DOI] [PubMed] [Google Scholar]
- 3.Misra A., Ramchandran A., Jayawardena R. Diabetes in South Asians. Diabet. Med. 2014;31:1153–1162. doi: 10.1111/dme.12540. [DOI] [PubMed] [Google Scholar]
- 4.Skolnik N.S., Ryan D.H. Pathophysiology, epidemiology, and assessment of obesity in adults. J. Fam. Pract. 2014;63:S3–S10. PMID; 25198218. [PubMed] [Google Scholar]
- 5.Shukla A.P., Buniak W.I., Aronne L.J. Treatment of obesity in 2015. J. Cardiopulm Rehabil. Prev. 2015;35:81–92. doi: 10.1097/HCR.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 6.Gardner C.D., Kiazand A., Alhassan S. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A to Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969–977. doi: 10.1001/jama.297.9.969. www.http://jamanetwork.com/journals/jama/fullarticle/205916 [DOI] [PubMed] [Google Scholar]
- 7.Shai I., Schwarzfuchs D., Henkin Y. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 2008;359:229–241. doi: 10.1056/NEJMoa0708681. http://www.nejm.org/doi/full/10.1056/NEJMoa0708681#t=article [DOI] [PubMed] [Google Scholar]
- 8.Malik V.S., Hu F.B. Popular weight-loss diets: from evidence to practice. Nat. Clin. Pract. Cardiovasc. Med. 2007;4:34–41. doi: 10.1038/ncpcardio0726. [DOI] [PubMed] [Google Scholar]
- 9.Gudzune K.A., Doshi R.S., Mehta A.K. Efficacy of commercial weight-loss programs: an updated systematic review. Ann. Intern. Med. 2015;162:501–512. doi: 10.7326/M14-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atkins R.C. New York; Harper Collins: 2002. Dr Atkins' New Diet Revolution.www.http://biblioteca.usv.ro/Carti/Nutrition/Dr%20Atkins'%20New%20Diet%20Revolution.pdf [Google Scholar]
- 11.Agrawal S., Millett C.J., Dhillon P.K. Type of vegetarian diet, obesity and diabetes in adult Indian population. Nutr. J. 2014;13:89. doi: 10.1186/1475-2891-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkins D.J., Wong J.M., Kendall C.W. The effect of a -based low-carbohydrate (eco-Atkins) diet on body weight and blood lipid concentrations in hyperlipidemic subjects. Arch. Intern. Med. 2009;169:1046–1054. doi: 10.1001/archinternmed.2009.115. [DOI] [PubMed] [Google Scholar]
- 13.Singh P.N., Arthur K.N., Orlich M.J. Global epidemiology of obesity, vegetarian dietary patterns, and noncommunicable disease in Asian Indians. Am. J. Clin. Nutr. 2014;100(Suppl. 1):359S–364S. doi: 10.3945/ajcn.113.071571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gammon C.S., von Hurst P.R., Coad J. Vegetarianism, vitamin B12 status, and insulin resistance in a group of predominantly overweight/obese South Asian women. Nutrition. 2012;28:20–24. doi: 10.1016/j.nut.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Misra A., Chowbey P., Makkar B.M. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J. Assoc. Phys. India. 2009;57:163–170. http://www.japi.org/february_2009/R-1.html [PubMed] [Google Scholar]
- 16.Vikram N.K., Misra A., Dwivedi M. Correlations of C-reactive protein levels with anthropometric profile, percentage of body fat and lipids in healthy adolescents and young adults in urban North India. Atherosclerosis. 2003;168:305–313. doi: 10.1016/s0021-9150(03)00096-0. [DOI] [PubMed] [Google Scholar]
- 17.Lenfant C., Chobanian A.V., Jones D.W. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension. 2003;41:1178–1179. doi: 10.1161/01.HYP.0000075790.33892.AE. [DOI] [PubMed] [Google Scholar]
- 18.Vikram N.K., Misra A., Pandey R.M. Association between subclinical inflammation & fasting insulin in urban young adult north Indian males. Indian J. Med. Res. 2006;124:677–682. http://medind.nic.in/iby/t06/i12/ibyt06i12p677.pdf [PubMed] [Google Scholar]
- 19.Misra A., Arora N., Mondal S. Relation between plasma leptin and anthropometric and metabolic covariates in lean and obese diabetic and hyperlipidaemic Asian Northern Indian subjects. Diabetes Nutr. Metab. 2001;14:18–26. [PubMed] [Google Scholar]
- 20.Matthews D.R., Hosker J.P., Rudenski A.S. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am. J. Kidney Dis. 2002;39:S1–S266. https://www.kidney.org/sites/default/files/docs/ckd_evaluation_classification_stratification.pdf [PubMed] [Google Scholar]
- 22.Gopalan C., Rama Sastri B.V., Balasubramanian S.C. 2nd ed. National Institute of Nutrition, Indian Council of Medical research; Hyderabad: 2007. Nutritive Value of Indian Foods. [Google Scholar]
- 23.Ainsworth B.E., Haskell W.L., Whitt M.C. Compendium of physical activities: an update of activity codes and MET intensities. Med. Sci. Sports. Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. https://insights.ovid.com/pubmed?pmid=10993420 [DOI] [PubMed] [Google Scholar]
- 24.Nigam P., Bhatt S., Misra A. Effect of a 6-month intervention with cooking oils containing a high concentration of monounsaturated fatty acids (olive and canola oils) compared with control oil in male Asian Indians with nonalcoholic fatty liver disease. Diabetes Technol. Ther. 2014;16:255–261. doi: 10.1089/dia.2013.0178. [DOI] [PubMed] [Google Scholar]
- 25.Misra A., Sharma R., Gulati S. Consensus dietary guidelines for healthy living and prevention of obesity, the metabolic syndrome, diabetes, and related disorders in Asian Indians. Diabetes Technol. Ther. 2011;13:683–694. doi: 10.1089/dia.2010.0198. [DOI] [PubMed] [Google Scholar]
- 26.Anjana R.M., Pradeepa R., Das A.K., Deepa M., Bhansali A., Joshi S.R. Physical activity and inactivity patterns in India – results from the ICMR-INDIAB study (Phase-1) [ICMR-INDIAB-5] Int. J. Behav. Nutr. Phys. Act. 2014;11(1):26. doi: 10.1186/1479-5868-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasiakos S.M., Lieberman H.R., Fulgoni V.L., 3rd. Higher-protein diets are associated with higher HDL cholesterol and lower BMI and waist circumference in US adults. J. Nutr. 2015;145:605–614. doi: 10.3945/jn.114.205203. [DOI] [PubMed] [Google Scholar]
- 28.Clifton P.M., Bastiaans K., Keogh J.B. High protein diets decrease total and abdominal fat and improve CVD risk profile in overweight and obese men and women with elevated triacylglycerol. Nutr. Metab. Cardiovasc. Dis. 2009;19:548–554. doi: 10.1016/j.numecd.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Mirmiran P., Hajifaraji M., Bahadoran Z. Dietary protein intake is associated with favorable cardiometabolic risk factors in adults: Tehran Lipid and Glucose Study. Nutr. Res. 2012;32:169–176. doi: 10.1016/j.nutres.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 30.St Jeor S.T., Howard B.V., Prewitt T.E. Dietary protein and weight reduction: a statement for healthcare professionals from the Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism of the American Heart Association. Circulation. 2001;104:1869–1874. doi: 10.1161/hc4001.096152. [DOI] [PubMed] [Google Scholar]
- 31.Yang M.U., Van Itallie T.B. Composition of weight lost during short-term weight reduction metabolic responses of obese subjects to starvation and low-calorie ketogenic and nonketogenic diets. J. Clin. Invest. 1976;58:722–730. doi: 10.1172/JCI108519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azadbakht L., Izadi V., Surkan P.J., Esmaillzadeh A. Effect of a High Protein Weight Loss Diet on Weight, High-Sensitivity C-Reactive Protein, and Cardiovascular Risk among Overweight and Obese Women: A Parallel Clinical Trial. Int. J. Endocrinol. 2013;2013:971724. doi: 10.1155/2013/971724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baba N.H., Sawaya S., Torbay N. High protein vs high carbohydrate hypoenergetic diet for the treatment of obese hyperinsulinemic subjects. Int. J. Obes. Relat. Metab. Disord. 1999;23:1202–1206. doi: 10.1038/sj.ijo.0801064. http://www.stockton-press.co.uk/ijo [DOI] [PubMed] [Google Scholar]
- 34.Neacsu M., Fyfe C., Horgan G. Appetite control and biomarkers of satiety with vegetarian (soy) and meat-based high-protein diets for weight loss in obese men: a randomized crossover trial. Am. J. Clin. Nutr. 2014;100:548–558. doi: 10.3945/ajcn.113.077503. [DOI] [PubMed] [Google Scholar]
- 35.Westerterp-Plantenga M.S., Nieuwenhuizen A., Tome D. Dietary protein, weight loss, and weight maintenance. Annu. Rev. Nutr. 2009;29:21–41. doi: 10.1146/annurev-nutr-080508-141056. [DOI] [PubMed] [Google Scholar]
- 36.Halton T.L., Hu F.B. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J. Am. Coll. Nutr. 2004;23:373–385. doi: 10.1080/07315724.2004.10719381. http://www.colorado.edu/intphys/Class/IPHY3700_Greene/pdfs/discussionEssay/thermogenesisSatiety/HaltonProtein2004.pdf [DOI] [PubMed] [Google Scholar]
- 37.Leidy H.J., Clifton P.M., Astrup A. The role of protein in weight loss and maintenance. Am. J. Clin. Nutr. 2015 doi: 10.3945/ajcn.114.084038. [DOI] [PubMed] [Google Scholar]
- 38.Weigle D.S., Breen P.A., Matthys C.C. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am. J. Clin. Nutr. 2005;82:41–48. doi: 10.1093/ajcn.82.1.41. www.http://ajcn.nutrition.org/content/82/1/41.long [DOI] [PubMed] [Google Scholar]
- 39.Ortinau L.C., Hoertel H.A., Douglas S.M. Effects of high-protein vs high- fat snacks on appetite control, satiety, and eating initiation in healthy women. Nutr. J. 2014;13:97 doi: 10.1186/1475-2891-13-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wycherley T.P., Moran L.J., Clifton P.M. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012;96:1281–1298. doi: 10.3945/ajcn.112.044321. [DOI] [PubMed] [Google Scholar]
- 41.Fekete A.A., Givens D.I., Lovegrove J.A. The impact of milk proteins and peptides on blood pressure and vascular function: a review of evidence from human intervention studies. Nutr. Res. Rev. 2013;26:177–190. doi: 10.1017/S0954422413000139. [DOI] [PubMed] [Google Scholar]
- 42.Alonso A., Beunza J.J., Bes-Rastrollo M. Vegetable protein and fiber from cereal are inversely associated with the risk of hypertension in a Spanish cohort. Arch. Med. Res. 2006;37:778–786. doi: 10.1016/j.arcmed.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Layman D.K., Shiue H., Sather C. Increased dietary protein modifies glucose and insulin homeostasis in adult women during weight loss. J. Nutr. 2003;133:405–410. doi: 10.1093/jn/133.2.405. http://jn.nutrition.org/content/133/2/405.full.pdf+html [DOI] [PubMed] [Google Scholar]
- 44.Boden G., Sargrad K., Homko C. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann. Intern. Med. 2005;142:403–411. doi: 10.7326/0003-4819-142-6-200503150-00006. [DOI] [PubMed] [Google Scholar]
- 45.Ajala O., English P., Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am. J. Clin. Nutr. 2013;97:505–516. doi: 10.3945/ajcn.112.042457. [DOI] [PubMed] [Google Scholar]
- 46.Piatti P.M., Monti F., Fermo I. Hypocaloric high-protein diet improves glucose oxidation and spares lean body mass: comparison to hypocaloric high-carbohydrate diet. Metabolism. 1994;43:1481–1487. doi: 10.1016/0026-0495(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 47.Appel L.J., Sacks F.M., Carey V.J. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 48.Nieman D.C., Underwood B.C., Sherman K.M., Arabatzis K., Barbosa J.C., Johnson M., Shultz T.D. Dietary status of seventh-day adventist vegetarian and non-vegetarian elderly women. J. Am. Diet Assoc. 1989;89(12):1763–1769. [PubMed] [Google Scholar]
- 49.Pedersen A.N., Kondrup J., Borsheim E. Health effects of protein intake in healthy adults: a systematic literature review. Food Nutr. Res. 2013;57 doi: 10.3402/fnr.v57i0.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Key T.J., Appleby P.N., Rosell M.S. Health effects of vegetarian and vegan diets. Proc. Nutr. Soc. 2006;65(1):35–41. doi: 10.1079/pns2005481. [DOI] [PubMed] [Google Scholar]
- 51.Turner-McGrievy G., Mandes T., Crimarco A. A plant-based diet for overweight and obesity prevention and treatment. J. Geriatr. Cardiol. 2017;14(5):369–374. doi: 10.11909/j.issn.1671-5411.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chopra S.M., Misra A., Gulati S. Overweight, obesity and related non-communicable diseases in Asian Indian girls and women. Eur. J. Clin. Nutr. 2013;67:688–696. doi: 10.1038/ejcn.2013.70. [DOI] [PubMed] [Google Scholar]
- 53.Bhardwaj S., Misra A., Misra R. High prevalence of abdominal, intra-abdominal and subcutaneous adiposity and clustering of risk factors among urban Asian Indians in North India. PLoS One. 2011;6:e24362. doi: 10.1371/journal.pone.0024362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Triffoni-Melo Ade T., Dick-de-Paula I., Portari G.V. Short-term carbohydrate-restricted diet for weight loss in severely obese women. Obes. Surg. 2011;21:1194–1202. doi: 10.1007/s11695-010-0110-6. [DOI] [PubMed] [Google Scholar]