Abstract
Revascularization for chronic limb-threatening ischemia (CLTI) is necessary to alleviate symptoms and wound healing. When it fails or is not possible, there are few alternatives to avoid limb amputation in these patients. Although experimental studies with stem cells and growth factors have shown promise, clinical trials have demonstrated inconsistent results because CLTI patients generally need arteriogenesis rather than angiogenesis. Moreover, in addition to the perfusion of the limb, there is the need to improve the neuropathic response for wound healing, especially in diabetic patients. Growth hormone (GH) is a pleiotropic hormone capable of boosting the aforementioned processes and adds special benefits for the redox balance. This hormone has the potential to mitigate symptoms in ischemic patients with no other options and improves the cardiovascular complications associated with the disease. Here, we discuss the pros and cons of using GH in such patients, focus on its effects on peripheral arteries, and analyze the possible benefits of treating CLTI with this hormone.
Keywords: angiogenesis, arteriogenesis, chronic limb-threatening ischemia, growth hormone, neuropathic response, redox balance, wound healing
Introduction
Peripheral arterial disease (PAD), otherwise known as the narrowing of the arteries of the lower limb and subsequent restriction of blood flow, is increasing in prevalence due to an aging population. It affects 4–12% of people aged 55–70 years, and up to 20% of those aged over 70 years, and it is more common among men.1,2 This prevalence is affected by the existence of cardiovascular risk factors, such as smoking, diabetes mellitus (DM), arterial hypertension, or hyperlipidemia.
Chronic limb-threatening ischemia (CLTI) is the most severe manifestation of PAD, and it is seen in patients with ischemic rest pain or skin lesions (ulcers or gangrene). In this kind of ischemia, treatments to improve arterial perfusion become necessary to mitigate the symptoms and prevent amputation of the limb. The quality of life is low in patients with CLTI, as they need continuous care, lose their independence, have reduced social contact, and, finally, become depressed.3 The rate of limb loss increases in patients with diabetes or chronic end stage renal failure. If treatment does not work properly in the first few months, it is doubtful whether initial success will be sustained after 12 months.4 Moreover, CLTI represents an independent risk factor for mortality,5 as almost 50% of patients do not survive >5 years after diagnosis.6
Although bypass graft surgery and endovascular techniques have been developed to increase perfusion in this pathology, there may be cases in which the patient is not suitable for these approaches, the invasive treatment fails, or a high surgical risk discourages the patient from both kind of management. Figure 1 depicts a CLTI diabetic patient, with multilevel arterial occlusive disease, illustrating the challenge of revascularization.
Figure 1.

Computed tomography angiogram (CTA) showing the leg flow of a patient with chronic limb-threatening ischemia. Note the diffuse disease and heavy calcification (green arrows) of the arteries with several and long occlusions (red arrow) affecting the femoropopliteal and distal vessels.
For this reason, several alternative vascular therapies (e.g. vasodilator drugs, lumbar sympathectomy and growth factors) have emerged in recent years with the aim of relieving pain at rest, healing wounds and avoiding limb loss in the aforementioned situations. Here, we focus on therapy with growth factors, whose objective is to induce vascular regeneration that follows an arterial occlusion by stimulating angiogenesis and arteriogenesis.7–9
It should be kept in mind that critical ischemia of the leg is a proinflammatory state, in which the redox imbalance, attributable to an overproduction of reactive oxygen species (ROS) that overwhelm the protective defense mechanism of cells, plays a primary role. Most of the cardiovascular risk factors eventually converge and result in this critical situation, making the patient vulnerable. As we will discuss later, a drug that repair this general imbalance, can help improve the symptoms of vascular disease, and can also provide other benefits related to the morbidity and mortality associated with this condition.
First, we will try to clearly define how the formation of new blood vessels occurs. Currently, it is accepted that angiogenesis is the formation of new capillaries, by sprouting and intussusception, from preexisting capillaries, and is dependent on hypoxia signals. In turn, arteriogenesis is the mechanism by which preexisting and normoxic collateral arterioles enlarge as a consequence of shear stress forces following a major artery occlusion. Both, angiogenesis and arteriogenesis, are encompassed in the term neovascularization. However, the formation of a primitive vascular plexus during embryonic development from angioblast is known as vasculogenesis.10–12 It should be highlighted that vasculogenesis appears in the embryo, but may also be present in adulthood; conversely, angiogenesis has also been demonstrated during embryogenesis.13,14
Therefore, angiogenesis and arteriogenesis are different mechanisms for the recovery of decreased blood flow that is secondary to arterial occlusion, and involves a series of events intended to compensate for the lack of flow (Figure 2). Arteriogenesis leads to the vasodilation of collateral vessels, and decreases the resistance to blood flow, which results in a 30% increase in blood flow distally to the occlusion.13,15 In the typical form of CLTI, oxygen tension drops in the foot, while a collateral network of blood vessels is generated, mainly in the proximal thigh, where they are surrounded by healthy and adequately oxygenated tissues.13 Consequently, although therapeutic interventions aimed at salvaging a limb affected by CLTI may include attempts to stimulate both angiogenesis and arteriogenesis, the latter should be the objective if attempting to pursue a real clinical benefit in these patients.13
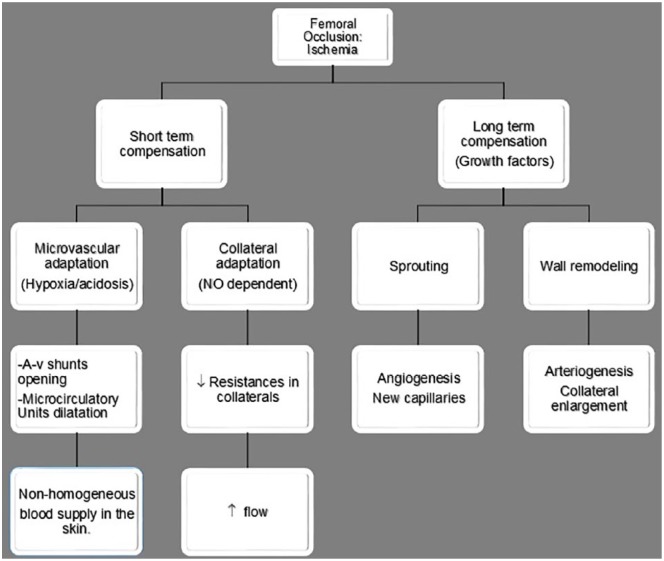
Figure 2.
Vascular adaptation following an ischemic insult. It has been divided into two different periods: short and long term. Observe both kinds of compensation process: microcirculatory and collateral.
A-v, arteriovenous; NO, nitric oxide.
Growth hormone and the vascular system
Currently, the vascular endothelium is considered an important internal secretion gland due to its many functions, including the paracrine/autocrine production of growth factors, such as growth hormone (GH),16 for which the endothelium has specific receptors.17 This is consistent with the widely accepted fact that GH is a pleiotropic hormone produced in, practically, all tissues and organs.18
Human GH is a mixture of two main proteins (22 and 20 kDa), and a number of a large (dimers, trimmers, etc.) and short isoforms produced inside the pituitary gland, whose role in the organism remains unestablished. The main form of GH (GH-N or GH 22 kDa) shares 25% homologous sequence with prolactine (PRL), and 96% with the placental GH variant (GH-V).17,19 All these originate from a common ancestral gene.17–20 Although the relationship between these three hormones has not yet been properly clarified, they are all thought to act as proangiogenic factors.17,19
In the vascular bed, GH helps regulate the production of vascular growth factors and their function.17 Interestingly, the first information available on the effects of GH on the vascular bed did not come from experimental studies, but rather from clinical evidence observed in GH-deficient patients. Many studies have shown that these patients suffer an endothelial dysfunction reflected as a lesser endothelium-dependent vasodilatation,21 which is probably due to a depletion of endothelial nitric oxide (NO) production.22 GH treatment stimulates the production of NO, while it restores endothelial function,21,23 and decreases the associated oxidative stress.21 Similarly, GH treatment is capable of reversing structural vascular lesions, such as the intima-media thickness.21,24 Therefore, it is likely that the hormone can play an important role as a modulator or inductor of the physiological mechanisms involved in recovery after a cardiovascular injury. The skin of GH-deficient patients has reduced capillary density and permeability, which improves after they receive GH treatment.25 Furthermore, adults and children with this illness have reduced retinal vascularization.26,27 In fact, the retina has been considered a major target for the proangiogenic actions of the GH–insulin-like growth factor 1 (IGF-1) axis.28,29
GH and peripheral blood vessels
Angiogenesis and GH
In addition to its effects on the heart,19,30–37 GH also acts on the peripheral vascular system. Its role in angiogenesis has been comprehensively reviewed.17 Briefly, GH receptors have been detected in blood vessels from different adult and fetal vascular beds,38–40 and in cultured endothelial cells,40,41 where GH stimulates endothelial cell proliferation42–44 and tube formation.17,45 In vitro studies with endothelial cells have shown that GH produces a mitogenic effect, affects cell morphology (modulates the cell cytoskeleton), increases extracellular matrix (ECM) and boosts the formation of capillary-like structures in Matrigel®-coated plates.46 Moreover, it promotes the mobilization of endothelial progenitor cells (EPCs) into the bloodstream and improves the process of vascularization.47,48 This effect of GH appears to be mediated by vascular endothelial growth factor (VEGF), stromal cell-derived factor 1 (SDF1) or erythropoietin (EPO).47
IGF-1 may also mediate the proangiogenic action of GH, as its receptors are expressed in endothelial cells, and it stimulates angiogenesis both in vivo and in vitro.49 Although IGF-1 is the main mediating factor of GH’s effects, some of them are independent.23 For instance, it promotes the expression and activity of endothelial nitric oxide synthase (eNOS).41 NO stimulates vasorelaxation, vasopermeability, and angiogenesis.50 Moreover, systemic40 or local infusions of GH23 acutely increase forearm blood flow and NO delivery in healthy humans, without causing significant changes in plasma IGF-1 levels or in muscle IGF-1 mRNA expression.
In addition to the stimulation of the aforementioned growth factors and its direct effect, during the process of vascularization GH can induce the expression of other factors contributing to the effects of the hormone, among them: fibroblast growth factor (FGF), epidermal growth factor (EGF), brain-derived neurotrophic factor (BDNF), EPO, and cytokines as interleukin (IL)-1β, IL-2 or tumor necrosis factor (TNF)-α.24,51,52 Even more, GH is capable of interacting with receptors for PRL, which can also trigger proangiogenic signals17 (Figure 3).
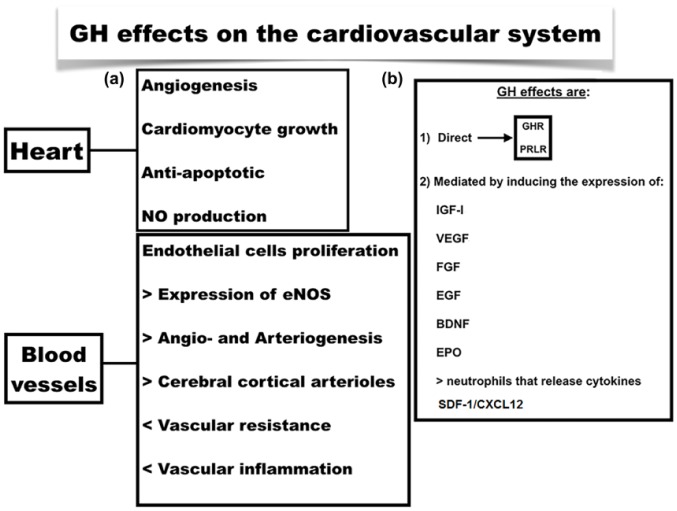
Figure 3.
(a) GH effects on the cardiovascular system at the heart and peripheral level. (b) (1) GH effects can be mediated by the activation of its own receptor (GHR) or that of PRL (PRLR). (2) Factors involved in the response to GH.
BDNF, brain-derived neurotrophic factor; EGF, epidermal growth factor; eNOS, endothelial NO synthase; EPO, erythropoietin; FGF, fibroblast growth factor; GH, growth hormone; GHR, growth hormone receptor; IGF-1, insulin-like growth factor 1; NO, nitric oxide; PRL, prolactin; PRLR, prolactin receptor; SDF-1/CXCL12, stromal cell-derived factor 1/C-X-C motif chemokine 12; VEGF, vascular endothelial growth factor.
Vasculogenesis and GH
During embryogenesis, the onset of vessel formation in the limbs takes place during the seventh week of pregnancy, when the release of GH and PRL, that slowly began at week 5, seems to reach more significant levels.17 This timing suggests that both, GH and PRL, might play pivotal roles in organizing the sequential actions of a number of growth factors that can then act in this fetal proangiogenic environment.
Another fact supporting the possible role of GH in the embryo’s vasculogenesis is the relationship of GH and SDF1 or C-X-C motif chemokine ligand 12 (CXXL12). This molecule has been implied in the migration of hematopoietic cells from the fetal liver to the bone marrow and the formation of large blood vessels.53 During adulthood, SDF1 accomplishes an important role in angiogenesis by recruiting EPCs from the bone marrow through the CXCR4 receptor.54 GH and SDF1 are related through this receptor, which is present in somatotropic cells.55 SDF1 boosts the GH gene and the release of the hormone from the anterior pituitary gland, either in normal or tumoral cells.55,56 GH also seems to stimulate SDF1 production, because the chemokine is strongly elevated in GH-transgenic mice57,58 and in GH-treated animals compared with that in controls.58 Furthermore, both molecules activate the JAK/STAT pathway, which has been implicated in endothelial migration and differentiation during angiogenesis.55
Nevertheless, countering the assumption that GH plays a determining role in embryonic vasculogenesis, is the fact that, in anencephalic fetuses, which usually lack a pituitary gland, no alterations in limb vessels have been reported, despite the fact that they tend to have significant abnormalities in major cerebral arteries and the retinal vascular development process.59 A possible explanation for this unexplained finding might be that the locally produced GH in endothelial cells compensates for the lack of endocrine GH secretion.
Arteriogenesis and GH
Although several signaling pathways converge to facilitate the growth of collateral arteries, the NO pathway, which partially controls endothelial function and leukocytes adhesion, appears to be the most important, at least, as a first step after an arterial occlusion60 (Figure 4).
Figure 4.
Arteriogenesis. The increased shear stress forces produced by an arterial occlusion trigger the NO pathway activation, which in last term is the factor responsible for the onset of new functional collaterals. NO production inhibits VE-cadherin expression, which physiologically plays a pivotal role for maintaining the integrity of the vascular membrane, therefore allowing increased vascular permeability and monocytes and macrophages entry into the vascular wall. Blue arrows indicate stimulation and red arrows inhibition.
NO, nitric oxide; VE, vascular-endothelial.
As described previously, the GH/IGF-1 axis activates the eNOS enzyme and regulates other nonendothelial-dependent actions.23,25,26,47,48,61 NO production relaxes arterial smooth muscle cells, and thereby reduces vascular tone, and inhibits its proliferation, migration, and platelet adhesion.13,62–64 Some models of PAD have shown that NO depletion is not a major factor involved in the onset of arterial disease. In these models, there is a marked insensitivity to NO resulting from a redox imbalance.15 This finding is consistent with recent reports of elevated H2O2 and NO insensitivity in humans and animals with arterial disease.65–71 Beyond the role of NO on vascular tone, the GH/IGF-1 axis regulates gene expression of the vascular smooth muscle ATP-sensitive potassium (KATP) channel,72 and therefore, lowers blood pressure.
ECM is essential in both angiogenesis and arteriogenesis. It plays a conspicuous role in cell proliferation and migration and metabolism of growth factors. GH is one of the hormones that regulates the actions of ECM.46,73 It produces a dose-dependent and significant increase of some proteins in cultures of human aortic smooth muscle cells such as hyaluronic acid and chondroitin. This action is not mediated by IGF-1, as this molecule did not exert any effect on ECM in the same study.73
Additionally, GH has vasoactive effects by acting on the autonomic nervous system. For instance, a marked increase in sympathetic nerve activity can be seen in GH-deficient patients,74 which tends to be reverted after GH replacement therapy.75 This increase suggests that GH may regulate central sympathetic activity, affecting vascular peripheral resistance. The removal of sympathetic constrictor tone from the arterial walls, along with increased blood flow in the denervated area may stimulate collateral enlargement, and consequently facilitate arteriogenesis, particularly in diabetic patients with neuropathy, whom show an increased vasomotor tone in the skeletal muscle arterioles secondary to an elevated noradrenaline-induced contraction.12 In experimental studies, α-adrenergic tone of the iliac artery was higher in diabetic animals compared with nondiabetic controls.76 However, the effect of diabetic neuropathy on vasoreactivity in the lower extremity have not been clearly understood, as arteriovenous shunt flow is raised due to the release of the tonic vasoconstrictor tone, particularly in rich zones of shunts, as toe pulp.77 Those areas of skin that are deficient in arteriovenous anastomosis, as the foot dorsum, are less affected. This supports the idea that the sympathetic damage in diabetic neuropathy is partial and patched, and that we have to difference between central and peripheral autonomic nervous system damage. This damage will depend on the type of DM, the presence of comorbidities, the experimental model used and the vascular bed, but, in general, collateral arteries in DM are prone to present an increased constriction and decreased dilatation.12
Then, GH seems to be an important arteriogenic factor, particularly if we consider that it strongly induces the expression of VEGF and other growth factors that upregulate adhesion molecules, which are essential for the development of collateral arteries.13 In addition, GH may also play a role in arteriogenesis as a result of its action on the immune system. It has been shown that monocytes, monocyte chemoattractant protein-1 (MCP-1) and T-lymphocytes play a key role in the control of vascular remodeling during arteriogenesis,13 as their deficiency decreases and delays this process. GH is a strong inductor of these cells,19,78,79 and activates human monocyte chemotaxis and migration.79 Exogenous GH administration induces MCP-1 mRNA up to eight-fold at concentrations as low as 0.5 ng/ml.78 However, the stimulation of immune cells by GH, not only benefits arteriogenesis, but also the neurogenic inflammatory response, and eventually the wound healing process.80 Figure 5 summarizes the effects of GH at different levels.
Figure 5.
Summary of GH actions for neovascularization and wound healing at different levels. At the BM level the hormone enhances the production and release of EPCs which peripherally mature to ECs. On ECs the hormone activates eNOS leading to NO production; this contributes to improve redox balance. GH decreases peripheral resistances by decreasing SNS. The hormone also acts on the IS leading to an increase in T and B-lymphocytes and the production of antibodies. GH exerts actions on the neurogenic response that results in an increase in SP production. These effects may lead to: (A) angiogenesis, as a result of hypoxia there is a sprout of new capillaries; (B) arteriogenesis, the occlusion of an artery (a) increases shear stress forces that act on preexisting collateral arterioles (b, c) enlarging them and allowing blood flow to bypass the occlusion (b1, c1). Wound healing is one of the consequences of the recovery of blood flow.
BM, bone marrow; EC, endothelial cell; eNOS, endothelial nitric oxide synthase; EPC, endothelial progenitor cell; GH, growth hormone; IS, immune system; NO, nitric oxide; ROS, reactive oxygen species; SNS, sympathetic nervous system; NS, peripheral nervous system;SP, Substance P. SP, Substance P.
Wound healing and GH
Wound healing may not be achieved despite revascularization in CTLI patients. That is the case, for example, of diabetic patients, in whom an accurate function of the neurogenic inflammatory response is also needed in addition to good flow around the wound to achieve the final closure. Figure 6 summarizes the characteristics of a neuropathic wound in a diabetic patient from the clinical point of view. The same occurs in patients with neuropathy secondary to tretraplegia, alcohol or trauma, in whom, although the flow is conserved, can equally suffer from skin ulcers, which are normally difficult to heal. Figure 7 depicts a neuropathic wound evolution in a tetraplegic patient, treated with a topical administration of GH. In fact, there is some debate regarding whether ischemic diabetic patients always require revascularization, as a reasonable healing rate of 46% has been observed when revascularization is not achievable.81 For this reason, these kinds of patients should not be driven directly for major amputation without first trying to heal their wounds by other methods.
Figure 6.

Typical neuropathic wound with torpid evolution in a diabetic patient that previously underwent endovascular revascularization. Note the hyperkeratotic edge of the wound and the lack of healing.
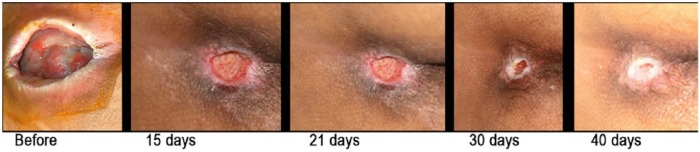
Figure 7.
Evolution of a neuropathic wound in a tetraplegic patient treated with topic GH administration (0.5 mg/day).
GH, growth hormone.
Neuropathy typically appears in almost 50-70% of diabetic patients, and may affect this neurogenic response.
The neurogenic response is mediated by nociceptive afferent fibers which release the neuropeptide Substance P (SP). SP is essential for the activation of macrophages, chemoattraction, and the action of cytokines, natural killer (NK) cells and T-lymphocytes,82–86 all of them participating in the arteriogenic and angiogenic processes, and in wound healing. SP increases the production of nerve growth factor (NGF),87 which is stimulated by GH, along with its receptor.88 SP mediates interactions between neurons and immune cells, and modulates immune cell proliferation rates and cytokine production89 (Figure 8).
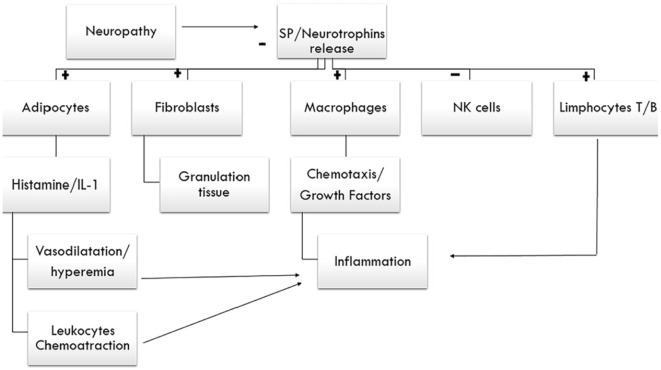
Figure 8.
Neurogenic response. Schematic design of the mechanisms involved in the inflammation process triggered by neuropathy. It represents the connection between nervous and immune system that contributes to the wound healing. SP and neurotrophins are the mediator molecules.
+, stimulation; -, inhibition; IL-1, interleukin-1; NK-cells, natural killer-cells; SP, Substance P.
Although the relationship between SP and GH at the peripheral level has not been properly studied, their relationship at the level of the central nervous system has been well established,90–93 and it is tempting to speculate that they must participate in a common reparative response aimed at wound healing.
On the other hand, GH is a strong inducer of the immune system. It mainly acts on macrophages and T-lymphocytes, that play a key role in wound healing.13,78,79 A bidirectional relationship between the neuroendocrine system and immune function has been shown.94 The presence of GH receptors in lymphocytes membranes95 supports the immunomodulatory properties of this hormone.
During the inflammatory phase of healing, macrophages deliver growth factors that attract fibroblasts and facilitate the next phase or proliferative phase. GH promotes the release of some of these factors, such as EGF, which is responsible for stimulating fibroblasts and their actions; VEGF, which promotes angiogenesis in the wound; and FGF, which stimulates macrophages, mast cells and T-lymphocytes, and facilitates granulation and epithelization.96,97 Effectively, GH stimulates wound healing in experimental studies with diabetic rats and mice.80,98 These statements are valid, both for skin lesions and for injuries of internal organs, as the general healing concepts are the same. Surprisingly, it is not the concentration of GH, but the previous alteration of the immune system that really influences the final effect of the hormone on this system. That is, a different response is observed in healthy people than in those with allergies,94 which supports the idea that GH may play an important role when the immune system is altered, as is the case in patients with CLTI, particularly in the elderly or diabetic population.
The wound healing timetable may be variable. Wounds become stuck in the inflammatory phase because of poor perfusion or deficit or dysfunction of the growth factors. The latter typically appears in patients with neuropathy, that usually affects DM patients with CLTI, and GH could help improve the wound flow, and the growth factors needed for the wound to reach the final cure. Although out of the scope of this review, wound infection and tissue necrosis are also another factors that clearly influence on healing.
Special considerations for angio/arteriogenesis in atherosclerotic CLTI patients
Cardiovascular risk factors
Age
Both cardiovascular structure and function are under a continuous remodeling process as we age. Aging may attenuate angiogenesis and arteriogenesis, and either produces less proangiogenic cytokines or increases the expression of antiangiogenic factors.99 Vascular aging is characterized by increased mitochondrial ROS production in endothelial cells, which, in turn, decreases the bioavailability of the vasodilator and anti-apoptotic NO, increases cardiac oxygen demand, and promotes vascular inflammation by inducing nuclear factor kappa-B (NF-kB). These adverse effects are reverted by GH treatment.61 For example, it has been reported that GH treatment increases the number of cerebral cortical arterioles in aging rats.100 Among these age-related changes in the cardiovascular system some aspects increase, such as fibrosis, and other aspects decrease, such as myocyte number, stress-induced cardiovascular response, exercise capacity, vessel rarefaction, arterial compliance and endothelial function.33
By examining a sample obtained from an ischemic muscle of an elderly patient (Figure 9), it is easy to understand this process. The lack of blood in a particular territory leads to an inflammatory response aimed at releasing growth factors and cells that trigger neovascularization [Figure 9(b)]. Scarce capillaries and CD 31 cells are usually seen in elderly patients with CLTI [Figure 9(c)], which indicates that the physiological response on its own might not be enough.
Figure 9.
Hematoxylin-eosin stained histological section of an ischemic muscle. (a) Note the fat infiltration with numerous vacuoles. (b) Extensive inflammatory infiltration, centralization of nuclei and interfiber edema. (c) Immunohistochemistry showing CD31 cells; note the small number of stained capillaries. Magnification 40×.
Nonetheless, elderly people still remain responsive to physical (e.g. exercise training) or biochemical stimuli (e.g. exogenous angiogenic growth factors), which improve the angiogenic and arteriogenic responses. NO-donors (nitrates, sodium nitroprusside, NONOates, S-Nitrosothiols, NO hybrid drugs), as well as single or combined angiogenic growth factors, were found to be effective in promoting collateral function in the ischemic tissues of animals of old age.99
Many patients suffering from an arterial occlusion are elderly. Aging also affects the ECM, integrins, the eNOS system, inflammatory responses and neurohormonal factors,99 and it also has an impact on GH production. Throughout life, the pulsatile secretion of the hormone decreases, both in number and amplitude, until it is imperceptible.33 This fact can contribute to an imbalance between pro and antiangiogenic factors, favoring the latter. This is one of the reasons why the administration of GH to elderly people has been proposed,101 though it must be balanced with its possible side effects.33
DM
DM raises the risk of suffering from arterial occlusion, complicates its treatment and impairs arteriogenesis. Diabetic patients show a lower sensitivity for shear stress, also due to multilevel arterial occlusive disease, and an elevated vasomotor tone, which impairs the activation of the endothelium, elevates the threshold of response to vasodilator stimuli, and reduces the enlargement of collateral arteries in the end.12,102,103 In addition, EPCs function and signaling of growth factors are also impaired in diabetes.12 It is important to highlight the dysfunction of eNOS in this disease, which explains the attenuated arterial remodeling. Either endothelial or platelet-derived NO are affected.12,102 Maintained hyperglycemia activates protein C and NF-kB, which increase ROS and subsequently interfere with the redox balance, promoting atherosclerosis.102
GH could aid in the recovery of vasomotor tone and eNOS dysfunction in patients with DM. Knowing that the expression of growth factors is altered in diabetic patients with neuropathy, and that their administration induces neuronal regeneration in both, in vitro and in vivo models of diabetic nerve damage,104 treatment with a combination of proangiogenic and antineurogenic factors could be an attractive option for such patients in a clinical scenario.
However, GH is a counter-regulatory hormone that opposes the hypoglycemic effects of insulin, but is not a clear contraindication for the possible use of the hormone in cases of PAD in diabetic patients. Moreover, a recent study in rats reported that GH and its receptor are critically involved in pancreatic β-cell growth, survival, differentiation and insulin secretion.105 Perhaps these are the mechanisms by which GH improves long-term glycemic control,106–109 without causing an increase in glycated hemoglobin (HbA1C) levels (a reference parameter in DM control).110
Redox balance
The main mechanism of damage of the cardiovascular factors remains unknown; however, their deleterious mechanism may be due to the fact that they modify the normal redox balance.13,15 Antioxidant molecules such as TEMPOL (a superoxide dismutase agonist) and apocynin (a nicotinamide adenine dinucleotide phosphate oxidase inhibitor), improve arteriogenic compensation when administered before and after the occlusion of an artery, whereas their benefit was interrupted when L-NAME (nitroarginine methyl ester), an inhibitor of nitric oxide synthase, was administered.15 Supporting these data, anti-hypertensive drugs, such as captopril or ramipril, increase the tissue flow mediated by arteriogenesis in animal models of ischemia and humans with PAD. The underlying mechanism originates from its antioxidant action, because of their sulfhydryl group, which corrects the characteristic superoxide-ion overload.15 The accumulation of the latter is characteristic of patients with arterial hypertension, and is responsible for collateral flow deterioration. Therefore, cardiovascular risk factors worsen oxidative stress, and contribute to the redox imbalance that begins with aging.
Oxidative stress seems to have a great influence on arterial function and collateral remodeling. The correction of oxidative stress together with the prevention and treatment of vascular risk factors play important roles in the chronic setting of vascular disease. It must be highlighted that GH, not only activates eNOS, but also reduces the redox imbalance,21 decreasing the respiratory chain activity on the mitochondria111 (Figure 5).
Embryonic origin of the vessels
There are several differences among vessels pertaining to different territories according to their embryonic origin with respect to their response to growth factors. That is, not all factors function equally for all vessels.112 There are several embryonic differences between coronary and peripheral arteries. For example, VEGF yielded positive angiogenic results in myocardial infarction but failed in PAD.113,114 Some explanations have been proposed with regard to this matter. First, different expression patterns of growth factor receptors may be determined by different locations; second, endothelial cells from different arteries may have different abilities to recruit monocytes/macrophages; and finally, there are different tissues surrounding both kinds of arteries (cardiomyocytes and skeletal muscle cells) which might imply different needs as a result of their different metabolisms.112 All these facts favor arteriogenesis or angiogenesis in one territory or another, or favor the action of a specific factor in one place and not in the other.
In addition, the genetic make-up of the embryonic development will determine the number of preexisting collateral vessels, and is a key point in the repercussion of an arterial occlusion. In fact, cardiovascular risk factors have a negative impact on the load of preexisting collateral vessels, and, hence, also on the final compensation in the case of occlusion. The collateral load is affected in patients with diabetes, or those with old age,115 which could be another fact explaining the lesser benefit of the use of proangiogenic growth factors in these groups of patients.
Type of vascular disease
The type of vascular disease may determine the final choice of therapy for the neovascularization process, as not all diseases that cause CLTI affect major arteries. There are some conditions affecting smaller vessels in which the main approach should be to promote angiogenesis. That is the case of nonatherosclerotic inflammatory disorders observed in cases of vasculitis, such as thromboangiitis obliterans (TAO). Clinical trials with growth factors, including stem cells, such as the TACT trial, yielded better results in patients with TAO than with PAD,116 showing that atherosclerotic PAD is more aggressive. Stem cells seem to promote mainly angiogenesis, as was demonstrated in an animal model studied by the same authors of the TACT trial, in which they observed that neovessels were located at the capillary level rather than at the arteriolar level.117
Microangiopathy with or without the occlusion of distal vessels of the leg, that typically affects diabetic patients and chronic renal failure, is another situation in which the main therapeutic approach could be angiogenesis, as major arteries are unaffected in this condition. As we will discuss later, neuropathy also has to be addressed in such patients, as it alters wound healing.
Antiangiogenesis and GH
In spite of this extensive analysis of the vascular effects induced by GH, high levels of this hormone are not always associated with angiogenesis. In fact, GH does not stimulate the proliferation of some harvested endothelial cells,17,43 which implies that, some of the vascular effects induced by the hormone depend on the local environment in which it is acting, for example, the presence of other angiogenic agents, such as IGF-1, NO, VEGF and even the autocrine GH. As described above, the secretion of GH by endothelial cells promotes the proliferation, migration, survival and formation of capillary vessels.17 It is possible that GHRs might be either occupied or desensitized by the autocrine hormone, and, therefore, prevent the action of the endocrine GH or that of the exogenously administered hormone.
Another more feasible possibility is that GH could suffer a cellular proteolytic cleavage, which would generate vasoinhibins. Vasoinhibins are a family of peptides resulting from the proteolytic cleavage of PRL and GH, which block the vasodilatation, permeability, growth and survival of blood vessels.118,119 Therefore, they control the balance between the growth and regression of blood vessels under physiological conditions, especially in the female reproductive system.17
The inhibition of GH signaling by proteins from the suppressor of cytokine signaling (SOCS) family has also been reported. GH induces the expression of several SOCS family members (CIS, SOCS1-3), which suggests that these proteins may also regulate GH signaling. It is thought that SOCS2 acts as a negative regulator of GH activity.120 In this sense, proinflammatory cytokines such as IL-1B or TNF-α, and endotoxins, which are frequently increased in PAD patients, may induce SOCS proteins and could lead to GH insensitivity. Paradoxically, it has been found that high concentrations of SOCS2 may upregulate GH signaling in mice120 (Figure 10).
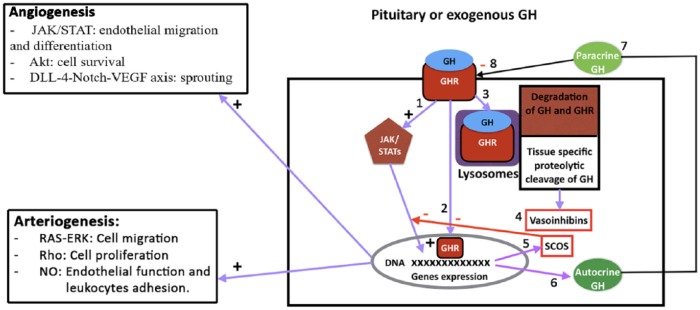
Figure 10.
Intracellular mechanisms by which GH produces angiogenesis or arteriogenesis or may be inactivated. (1) After the interaction between GH and its receptor (GHR) a cascade of signaling pathways is initiated by JAK2 activation leading to the expression of a number of genes. (2) The GHR may be internalized together with GH and translocated to the nucleus where it may also activate gene expression. (3) GH and GHR may suffer a lysosomal degradation after being internalized, but also, depending on the tissue, the hormone may suffer a specific proteolytic cleavage giving origin to vasoinhibins (4) which may block both angiogenesis and arteriogenesis. This represents a mechanism of control for both processes. (5) Among the genes expressed SOCS acts by inhibiting GH signaling, directly or affecting the translocation of the GHR to the nucleus of the cell. (6, 7) Cells may express GH that acts in an autocrine (6) or paracrine (7) manner. This cellular production of the hormone may lead to an interaction with the membrane GHR (8) impeding the effects of endocrine or exogenously administered hormone, or even may produce the desensitization of GHR.
On the left of the figure, signals responsible for angiogenesis (upper) and arteriogenesis (lower) can be seen. Blue arrows, stimulation; red arrows and squares, inhibition.
+, activation; -, inhibition; Akt, serin threonine kinase; DLL4-Notch-VEGF axis, Delta like 4-Notch-vascular endothelial growth factor axis; GH, growth hormone; GHR, growth hormone receptor; JAK/STATs, Janus kinase/signal transducer and activator of transcription; NO, nitric oxide; RAS-ERK, rats sarcoma-extracellular signal-regulated kinases; Rho, hexameric protein found in prokaryotes, necessary for the process of terminating the transcription of some genes; SOCs, suppressor of cytokine signaling.
Atherosclerosis and GH
Patients with acromegaly are at increased cardiovascular risk compared with that of healthy individuals.121 A proatherogenic state has been described in these patients. Cardiovascular abnormalities, such as hypertension and cardiomyopathy, are frequent in this condition and lead to heart failure.122,123 In this process, characterized by an increased and continuous pituitary GH release, an endothelial dysfunction seems to exist that can be measured by several markers. For example, some atherogenic factors, such as oxidized low-density lipoproteins or lipoprotein (a),124,125 and hemostatic parameters like fibrinogen and tissue plasminogen activator, are increased, whereas lower concentrations of protein S have been found,126 which may represent an atherogenic and hypercoagulable environment. At the clinical level, intima-media thickness is elevated, although this finding is not consistent,127 and other markers of atherogenesis, such as lipoprotein-associated phospholipase A (2) or homocysteine seem to be unchanged.124,128
Although a premature atherosclerotic state in acromegaly has been suggested as a consequence of the insulin resistance and direct vascular effects of GH and IGF-1,129 there are controversial data in the studies and a methodological bias.130 In any case, the concentration of the hormone in acromegaly is considerably high and sustained over a long period of time compared with that occurring physiologically. Another question to be elucidated is if the atherosclerotic findings observed in acromegaly are due to GH or due to the cardiovascular risk factors that appear in such patients.121
Potential clinical use and drawbacks
With regard to the potential use of GH as an angiogenic/arteriogenic therapy, there might be some concern about its theoretical oncogenic potential. However, long-term studies in children with a deficit of the hormone secondary to the administration of prophylactic brain radiotherapy to treat leukemia, reported only a slightly increased incidence of the occurrence of a second neoplasm compared with that in the equivalent age population treated with GH due to GH-deficiency. Furthermore, it has recently been published that this second neoplasm appears as a consequence of radiotherapy and not of GH treatment. Acromegaly has been proven to increase thyroid cancer risk;131 however, this disease evolves over many years, and the responsible pituitary tumor releases high and sustained amounts of GH. It is yet to be established whether the hormone is the factor responsible for this increased risk or whether it depends on the metabolic and hormonal alterations that occur as a result of the consistently elevated plasma IGF-1 levels. Both, in clinical practice conditions and in our own experience, short-term treatment with the hormone at the appropriate dosage is safe. In any case, the presence of a malignancy has to be ruled out whenever a treatment with GH is scheduled.
Assuming the positive effects of GH on PAD, a possible question is in regard to the best method of administering the hormone. Theoretically, nanocapsules containing slowly released GH, placed just before the arterial occlusion, would be an effective method of administration. In fact, these GH-nanocapsules are being developed and tested in animal and cell studies for other purposes (unpublished data). However, we do not consider that this way of administration may represent a clear advantage over conventional subcutaneous administration. In our experience, the topical administration has demonstrated to be effective for treating neuropathic wounds. Nevertheless, more studies are needed in this direction. It has to be highlighted that those cases with severe foot infection and sepsis should be a contraindication for GH treatment, because it has been reported that the administration of high doses of the hormone in critical ill patients is associated with increased morbidity and mortality.132,133
The dose of GH needed for treating these usually elderly patients is not yet clear; however, monitoring IGF-1 levels should be enough. As stated above, high doses of GH do not achieve more of a benefit and could induce the collateral effects.
According to the analysis of the published literature, and our unpublished experience, we believe that translational data will eventually need to make a leap from the bench to bedside, and this hormone should be tested as a bail out tool for the treatment of patients with CLTI when no other option is available, either alone or in combination with others growth factors, including stem cells.
We are currently conducting a phase III randomized controlled trial called: Growth Hormone Angiogenic Study (GHAS), Eudract 2012-002228-34, approved by the Spanish Agency of Drugs and Health Products (AEMPs) and the Autonomic Committee on Research Ethics in Galicia (CAEIG), Spain, in patients suffering from CLTI. We are also conducting an experimental study, which was approved by the Bioethics Committee of Galicia (CBG), Spain, with the objective of testing different animal models of ischemia and GH actions. Interestingly, early data obtained among several genes analyzed in human ischemic muscle samples from 16 patients with CLTI, suggest that there is a significant increase in eNOS and VEGF-A expression in the treatment group. Regarding the diabetic patients enrolled thus far in this trial, none of them have experienced a significant modification of their glycemic control, or a change in the dose of their antidiabetic drugs.
Conclusion
Based on the current knowledge of the arteriogenic and angiogenic mechanisms, and on the data in this paper, the treatment with growth factors in patients suffering from CLTI may be of high utility. A key point for lower limbs is to search for molecules that enhance arteriogenesis. Antioxidants might play an important role in the future. Knowledge of the wound healing process and the neurogenic response could improve the results by using specific growth factors, and hence, decreasing the failure to reach the primary endpoints of the clinical trials.
In summary, GH is a pleiotropic hormone whose actions depend on the environment. Thus, after an arterial occlusion, the hormone may be able to boost angiogenic and arteriogenic responses. The more severe the ischemia is, the more number of signals there will be for the hormone, and the more effective it will be. However, these mechanisms are still poorly understood.
Other possible benefits of this hormone for wound healing and cardiovascular morbidity in critical limb ischemia have been highlighted, especially in the elderly and diabetic populations. Nevertheless, the pros and cons of using this hormone must be weighed, and the best dosage and route of administration needs to be investigated.
It is unlikely that only one factor will be enough to improve the physiological mechanisms needed for recovery. The combination of growth factors and stem cells will result in the greatest benefit in these kinds of patients. Clinical trials will determine whether GH is useful for treating patients with severe PAD, mitigating symptoms and avoiding amputations.
Acknowledgments
We thank Dr Santiago Pérez Cachafeiro, for providing an important stimulus to develop this review. Without his help and knowledge this article would not have been possible.
We equally thank the Center of Research in Molecular Medicine of the University of Santiago of Compostela (CIMUS), Spain for the genetic analysis in ischemic muscle samples from the GHAS study, and Dr Álvaro Gómez Castro, for the histological images
All authors have contributed to: (1) the conception and design of the work, acquisition or analysis or interpretation of data, (2) drafting the article or revising it critically for important intellectual content, and (3) final approval of the version to be published.
Footnotes
Funding: The authors disclose the receipt of financial support for the publication of this article. This review was funded by the Carlos III Health Institute and the European Regional Development Fund (ISCIII-FEDER), Madrid, Spain, (grant number PI 13-00790), and was funded by the Spanish Society of Angiology and Vascular Surgery (SEACV).
ORCID iD: Diego Caicedo  https://orcid.org/0000-0002-9599-7047
https://orcid.org/0000-0002-9599-7047
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Diego Caicedo, Department of Angiology and Vascular Surgery, Complexo Hospitalario Universitario de Pontevedra, Avda. de Mourente, s/n; 36071-Pontevedra, Spain.
Pablo Devesa, Research and Development Medical Center Foltra, Teo, Spain.
Víctor M. Arce, Department of Physiology, Santiago University School of Medicine, Spain
Julia Requena, Department of Angiology and Vascular Surgery, Complexo Hospitalario Universitario de Pontevedra, Spain.
References
- 1. Blanes JI, Cairols MA, Marrugat J; ESTIME. Prevalence of peripheral artery disease and its associated risk factors in Spain: the ESTIME Study. Int Angiol 2009; 28: 20–25. [PubMed] [Google Scholar]
- 2. Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001; 286: 1317–1324. [DOI] [PubMed] [Google Scholar]
- 3. Belch JJF, Topol EJ, Agnelli G, et al. ; Prevention of Atherothrombotic Disease Network. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med 2003; 163: 884–892. [DOI] [PubMed] [Google Scholar]
- 4. Fowkes G, Gillespie IN. Angioplasty (versus non surgical management) for intermittent claudication. Cochrane Database Syst Rev 2000;(2):CD000017. [DOI] [PubMed] [Google Scholar]
- 5. Ix JH, Biggs ML, Kizer JR, et al. Association of body mass index with peripheral arterial disease in older adults: the Cardiovascular Health Study. Am J Epidemiol 2011; 174: 1036–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis 1991; 87: 119–128. [DOI] [PubMed] [Google Scholar]
- 7. Sneider EB, Nowicki PT, Messina LM. Regenerative medicine in the treatment of peripheral arterial disease. J Cell Biochem 2009; 108: 753–761. [DOI] [PubMed] [Google Scholar]
- 8. Powell RJ. Update on clinical trials evaluating the effect of biologic therapy in patients with critical limb ischemia. J Vasc Surg 2012; 56: 264–266. [DOI] [PubMed] [Google Scholar]
- 9. Clair D, Shah S, Weber J. Current state of diagnosis and management of critical limb ischemia. Curr Cardiol Rep 2012; 14: 160–170. [DOI] [PubMed] [Google Scholar]
- 10. Cai W, Schaper W. Mechanisms of arteriogenesis. Acta Biochim Biophys Sin (Shanghai) 2008; 40: 681–692. [PubMed] [Google Scholar]
- 11. Schierling W, Troidl K, Troidl C, et al. The role of angiogenic growth factors in arteriogenesis. J Vasc Res 2009; 46: 365–374. [DOI] [PubMed] [Google Scholar]
- 12. Ruiter MS, van Golde JM, Schaper NC, et al. Diabetes impairs arteriogenesis in the peripheral circulation: review of molecular mechanisms. Clin Sci (Lond) 2010; 119: 225–238. [DOI] [PubMed] [Google Scholar]
- 13. Heil M, Schaper W. Insights into pathways of arteriogenesis. Curr Pharm Biotechnol 2007; 8: 35–42. [DOI] [PubMed] [Google Scholar]
- 14. Drake CJ. Embryonic and adult vasculogenesis. Birth Defects Res C Embryo Today 2003; 69: 73–82. [DOI] [PubMed] [Google Scholar]
- 15. Unthank J, Haas TL, Miller S. Impact of shear level and cardiovascular risk factors on bioavailable nitric oxide and outward vascular remodeling in mesenteric arteries. In: Schaper W, Deindl E. (eds) Arteriogenesis-molecular regulation, patophysiology and therapeutics I. Herzogenrath: Shaker Verlag, 2011, pp.89–119. [Google Scholar]
- 16. Corbacho AM, Martínez de la Escalera G, Clapp C. Roles of prolactin and related members of the prolactin/growth hormone/placental lactogen family in angiogenesis. J Endocrinol 2002; 173: 219–238. [DOI] [PubMed] [Google Scholar]
- 17. Clapp C, Thebault S, Jeziorski MC, et al. Peptide hormone regulation of angiogenesis. Physiol Rev 2009; 89: 1177–1215. [DOI] [PubMed] [Google Scholar]
- 18. Walker W, Fitzpatrick S, Barrera-Saldana H, et al. The human placental lactogen genes: structure, function, evolution and transcriptional regulation. Endocr Rev 1991; 12: 316–328. [DOI] [PubMed] [Google Scholar]
- 19. Devesa J, Almengló C, Devesa P. Multiple effects of growth hormone in the body: is it really the hormone for growth? Clin Med Insights Endocrinol Diabetes 2016; 9: 47–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller WL, Eberhardt NL. Structure and evolution of the growth hormone gene family. Endocr Rev 1983; 4: 97–130. [DOI] [PubMed] [Google Scholar]
- 21. Evans LM, Davies JS, Anderson RA, et al. The effect of GH replacement therapy on endothelial function and oxidative stress in adult growth hormone deficiency. Eur J Endocrinol 2000; 142: 254–262. [DOI] [PubMed] [Google Scholar]
- 22. Böger RH. Nitric oxide and the mediation of the hemodynamic effects of growth hormone in humans. J Endocrinol Invest 1999; 22: 75–81. [PubMed] [Google Scholar]
- 23. Napoli R, Guardasole V, Angelini V, et al. Acute effects of growth hormone on vascular function in human subjects. J Clin Endocrinol Metab 2003; 88: 2817–2820. [DOI] [PubMed] [Google Scholar]
- 24. Devesa J, Devesa P, Reimunde P. Growth hormone revisited. Med Clin 2010; 135: 665–670. [DOI] [PubMed] [Google Scholar]
- 25. Oomen PHN, Beentjes JAM, Bosma E, et al. Reduced capillary permeability and capillary density in the skin of GH-deficient adults: improvement after 12 months GH replacement. Clin Endocrinol (Oxf) 2002; 56: 519–524. [DOI] [PubMed] [Google Scholar]
- 26. Hellström A, Svensson E, Carlsson B, et al. Reduced retinal vascularization in children with growth hormone deficiency. J Clin Endocrinol Metab 1999; 84: 795–798. [DOI] [PubMed] [Google Scholar]
- 27. Hellström A, Carlsson B, Niklasson A, et al. IGF-I is critical for normal vascularization of the human retina. J Clin Endocrinol Metab 2002; 87: 3413–3416. [DOI] [PubMed] [Google Scholar]
- 28. Frystyk J. The growth hormone hypothesis - 2005 revision. Horm Metab Res 2005; 37(Suppl. 1): 44–48. [DOI] [PubMed] [Google Scholar]
- 29. Chen J, Smith LEH. Retinopathy of prematurity. Angiogenesis 2007; 10: 133–140. [DOI] [PubMed] [Google Scholar]
- 30. Kusano K, Tsutsumi Y, Dean J, et al. Long-term stable expression of human growth hormone by rAAV promotes myocardial protection post-myocardial infarction. J Mol Cell Cardiol 2007; 42: 390–399. [DOI] [PubMed] [Google Scholar]
- 31. Rong SL, Lu YX, Liao YH, et al. Effects of transplanted myoblasts transfected with human growth hormone gene on improvement of ventricular function of rats. Chin Med J (Engl) 2008; 121: 347–354. [PubMed] [Google Scholar]
- 32. Maison P, Chanson P. Cardiac effects of growth hormone in adults with growth hormone deficiency: a meta-analysis. Circulation 2003; 108: 2648–2652. [DOI] [PubMed] [Google Scholar]
- 33. Khan AS, Sane DC, Wannenburg T, et al. Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc Res 2002; 54: 25–35. [DOI] [PubMed] [Google Scholar]
- 34. Osterziel K. Role of nitric oxide in the vasodilator effect of recombinant human growth hormone in patients with dilated cardiomyopathy. Cardiovasc Res 2000; 45: 447–453. [DOI] [PubMed] [Google Scholar]
- 35. Marleau S, Mulumba M, Lamontagne D, et al. Cardiac and peripheral actions of growth hormone and its releasing peptides: relevance for the treatment of cardiomyopathies. Cardiovasc Res 2006; 69: 26–35. [DOI] [PubMed] [Google Scholar]
- 36. Granata R, Trovato L, Gallo MP, et al. Growth hormone-releasing hormone promotes survival of cardiac myocytes in vitro and protects against ischaemia-reperfusion injury in rat heart. Cardiovasc Res 2009; 83: 303–312. [DOI] [PubMed] [Google Scholar]
- 37. Lucchesi P. Growth hormone-releasing peptides and the heart: secretagogues or cardioprotectors? Cardiovasc Res 2004; 61: 7–8. [DOI] [PubMed] [Google Scholar]
- 38. Temmim L, Kolle S, Baker H, et al. Expression of growth hormone receptor in human liposarcomas and lipomas 1. Oncol Rep 2000; 7: 757–760. [DOI] [PubMed] [Google Scholar]
- 39. Werther GA, Haynes K, Waters MJ. Growth hormone (GH) receptors are expressed on human fetal mesenchymal tissues: identification of messenger ribonucleic acid and GH-binding protein. J Clin Endocrinol Metab 1993; 76: 1638–1646. [DOI] [PubMed] [Google Scholar]
- 40. Li G, Del Rincon JP, Jahn LA, et al. Growth hormone exerts acute vascular effects independent of systemic or muscle insulin-like growth factor I. J Clin Endocrinol Metab 2008; 93: 1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thum T, Tsikas D, Frölich JC, et al. Growth hormone induces eNOS expression and nitric oxide release in a cultured human endothelial cell line. FEBS Lett 2003; 555: 567–571. [DOI] [PubMed] [Google Scholar]
- 42. Lobie PE, García-Aragón J, Wang BS, et al. Cellular localization of the growth hormone binding protein in the rat. Endocrinology 1992; 130: 3057–3065. [DOI] [PubMed] [Google Scholar]
- 43. Rymaszewski Z, Cohen RM, Chomczynski P. Human growth hormone stimulates proliferation of human retinal microvascular endothelial cells in vitro. Proc Natl Acad Sci USA 1991; 88: 617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Struman I, Bentzien F, Lee H, et al. Opposing actions of intact and N-terminal fragments of the human prolactin/growth hormone family members on angiogenesis: an efficient mechanism for the regulation of angiogenesis. Proc Natl Acad Sci USA 1999; 96: 1246–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Frerich B, Kurtz-Hoffmann J, Lindemann N. Influence of growth hormone on maintenance of capillary-like structures in an in vitro model of stromal vascular tissue: results from morphometric analysis. Artif Organs 2005; 29: 338–341. [DOI] [PubMed] [Google Scholar]
- 46. Messias de, Lima CF, Dos Santos Reis MD, da Silva Ramos FW, et al. Growth hormone modulates in vitro endothelial cell migration and formation of capillary-like structures. Cell Biol Int 2017; 41: 577–584. [DOI] [PubMed] [Google Scholar]
- 47. Devin JK, Vaughan DE, Blevins LS, Jr, et al. Low-dose growth hormone administration mobilizes endothelial progenitor cells in healthy adults. Growth Horm IGF Res 2008; 18: 253–263. [DOI] [PubMed] [Google Scholar]
- 48. Thum T, Hoeber S, Froese S, et al. Age-dependent impairment of endothelial progenitor cells is corrected by growth hormone mediated increase of insulin-like growth factor-1. Circ Res 2007; 100: 434–443. [DOI] [PubMed] [Google Scholar]
- 49. Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol 2004; 24: 435–444. [DOI] [PubMed] [Google Scholar]
- 50. Walford G, Loscalzo J. Nitric oxide in vascular biology. J Thromb Haemostasis 2003; 1: 2112–2118. [DOI] [PubMed] [Google Scholar]
- 51. Clapp C, Thebault S, Jeziorski MC, et al. Peptide hormone regulation of angiogenesis. Physiol Rev 2009; 89: 1177–1215. [DOI] [PubMed] [Google Scholar]
- 52. Bozzola M, De Benedetti F, De Amici M, et al. Stimulating effect of growth hormone on cytokine release in children. Eur J Endocrinol 2003; 149: 397–401. [DOI] [PubMed] [Google Scholar]
- 53. Mazo IB, Massberg S, von Andrian UH. Hematopoietic stem and progenitor cell trafficking. Trends Immunol 2011; 32: 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zheng H, Fu G, Dai T, et al. Migration of endothelial progenitor cells mediated by stromal cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal transduction pathway. J Cardiovasc Pharmacol 2007; 50: 274–280. [DOI] [PubMed] [Google Scholar]
- 55. Lee Y, Kim JM, Lee EJ. Functional expression of CXCR4 in somatotrophs: CXCL12 activates GH gene, GH production and secretion, and cellular proliferation. J Endocrinol 2008; 199: 191–199. [DOI] [PubMed] [Google Scholar]
- 56. Barbieri F, Bajetto A, Porcile C, et al. Role of stromal cell-derived factor 1 (SDF1/CXCL12) in regulating anterior pituitary function. J Mol Endocrinol 2007; 38: 383–389. [DOI] [PubMed] [Google Scholar]
- 57. Smaniotto S, Martins-Neto AA, Dardenne M, et al. Growth hormone is a modulator of lymphocyte migration. Neuroimmunomodulation 2011; 18: 309–313. [DOI] [PubMed] [Google Scholar]
- 58. Smaniotto S, De Mello-Coelho V, Villa-Verde DMS, et al. Growth hormone modulates thymocyte development in vivo through a combined action of laminin and CXC chemokine ligand 12. Endocrinology 2005; 146: 3005–3017. [DOI] [PubMed] [Google Scholar]
- 59. Kim JH, Yu YS, Kim K-W, et al. Impaired retinal vascular development in anencephalic human fetus. Histochem Cell Biol 2010; 134: 277–284. [DOI] [PubMed] [Google Scholar]
- 60. Eitenmüller I, Volger O, Kluge A, et al. The range of adaptation by collateral vessels after femoral artery occlusion. Circ Res 2006; 99: 656–662. [DOI] [PubMed] [Google Scholar]
- 61. Ungvari Z, Orosz Z, Labinskyy N, et al. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 2007; 293: H37–H47. [DOI] [PubMed] [Google Scholar]
- 62. Walsh MF, Barazi M, Pete G, et al. Insulin-like growth factor I diminishes in vivo and in vitro vascular contractility: role of vascular nitric oxide. Endocrinology 1996; 137: 1798–1803. [DOI] [PubMed] [Google Scholar]
- 63. Tsukahara H, Gordienko DV, Tonshoff B, et al. Direct demonstration of insulin-like growth factor-I-induced nitric oxide production by endothelial cells. Kidney Int 1994; 45: 598–604. [DOI] [PubMed] [Google Scholar]
- 64. Capaldo B, Guardasole V, Pardo F, et al. Abnormal vascular reactivity in growth hormone deficiency. Circulation 2001; 103: 520–524. [DOI] [PubMed] [Google Scholar]
- 65. Stasch JP, Schmidt PM, Nedvetsky PI, et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest 2006; 116: 2552–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gladwin MT. Deconstructing endothelial dysfunction: soluble guanylyl cyclase oxidation and the NO resistance syndrome. J Clin Invest 2006; 116: 2330–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bauersachs J, Bouloumié A, Mülsch A, et al. Vasodilator dysfunction in aged spontaneously hypertensive rats: changes in NO synthase III and soluble guanylyl cyclase expression, and in superoxide anion production. Cardiovasc Res 1998; 37: 772–779. [DOI] [PubMed] [Google Scholar]
- 68. Klöss S, Bouloumié A, Mülsch A. Aging and chronic hypertension decrease expression of rat aortic soluble guanylyl cyclase. Hypertension 2000; 35: 43–47. [PubMed] [Google Scholar]
- 69. Ruetten H, Zabel U, Linz W, et al. Downregulation of soluble guanylyl cyclase in young and aging spontaneously hypertensive rats. Circ Res 1999; 85: 534–541. [DOI] [PubMed] [Google Scholar]
- 70. Melichar VO, Behr-Roussel D, Zabel U, et al. Reduced cGMP signaling associated with neointimal proliferation and vascular dysfunction in late-stage atherosclerosis. Proc Natl Acad Sci 2004; 101: 16671–16676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lacy F, O’Connor DT, Schmid-Schönbein GW. Plasma hydrogen peroxide production in hypertensives and normotensive subjects at genetic risk of hypertension. J Hypertens 1998; 16: 291–303. [DOI] [PubMed] [Google Scholar]
- 72. Tivesten Å, Barlind A, Caidahl K, et al. Growth hormone-induced blood pressure decrease is associated with increased mRNA levels of the vascular smooth muscle KATP channel. J Endocrinol 2004; 183: 195–202. [DOI] [PubMed] [Google Scholar]
- 73. Erikstrup C, Pedersen LM, Heickendorff L, et al. Production of hyaluronan and chondroitin sulphate proteoglycans from human arterial smooth muscle: the effect of glucose, insulin, IGF-I or growth hormone. Eur J Endocrinol 2001; 145: 193–198. [DOI] [PubMed] [Google Scholar]
- 74. Sverrisdóttir YB, Elam M, Herlitz H, et al. Intense sympathetic nerve activity in adults with hypopituitarism and untreated growth hormone deficiency. J Clin Endocrinol Metab 1998; 83: 1881–1885. [DOI] [PubMed] [Google Scholar]
- 75. Sverrisdóttir YB, Elam M, Caidahl K, et al. The effect of growth hormone (GH) replacement therapy on sympathetic nerve hyperactivity in hypopituitary adults: a double-blind, placebo-controlled, crossover, short-term trial followed by long-term open GH replacement in hypopituitary adults. J Hypertens 2003; 21: 1905–1914. [DOI] [PubMed] [Google Scholar]
- 76. Martínez-Nieves B, Dunbar JC. Vascular dilatatory responses to sodium nitroprusside (SNP) and alpha-adrenergic antagonism in female and male normal and diabetic rats. Proc Soc Exp Biol Med 1999; 222: 90–98. [DOI] [PubMed] [Google Scholar]
- 77. Stevens MJ, Edmonds ME, Douglas SL, et al. Influence of neuropathy on the microvascular response to local heating in the human diabetic foot. Clin Sci 1991; 80: 249–256. [DOI] [PubMed] [Google Scholar]
- 78. Fasshauer M, Klein J, Kralisch S, et al. Monocyte chemoattractant protein 1 expression is stimulated by growth hormone and interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun 2004; 317: 598–604. [DOI] [PubMed] [Google Scholar]
- 79. Meazza C, Pagani S, Travaglino P, et al. Effect of growth hormone (GH) on the immune system. Pediatr Endocrinol Rev 2004; 1: 490–495. [PubMed] [Google Scholar]
- 80. Thorey IS, Hinz B, Hoeflich A, et al. Transgenic mice reveal novel activities of growth hormone in wound repair, angiogenesis, and myofibroblast differentiation. J Biol Chem 2004; 279: 26674–26684. [DOI] [PubMed] [Google Scholar]
- 81. Spanos K, Saleptsis V, Athanasoulas A, et al. Factors associated with ulcer healing and quality of life in patients with diabetic foot ulcer. Angiology 2017; 68: 242–250. [DOI] [PubMed] [Google Scholar]
- 82. Payan DG. Neuropeptides and inflammation: the role of Substance P. Annu Rev Med 1989; 40: 341–352. [DOI] [PubMed] [Google Scholar]
- 83. O’Connor TM, O’Connell J, O’Brien DI, et al. The role of Substance P in inflammatory disease. J Cell Physiol 2004; 201: 167–180. [DOI] [PubMed] [Google Scholar]
- 84. Harrison S, Geppetti P. Substance P. Int J Biochem Cell Biol 2001; 33: 555–576. [DOI] [PubMed] [Google Scholar]
- 85. Matsuda H, Kawakita K, Kiso Y, et al. Substance P induces granulocyte infiltration through degranulation of mast cells. J Immunol 1989; 142: 927–931. [PubMed] [Google Scholar]
- 86. Ni T, Liu Y, Peng Y, et al. Substance P induces inflammatory responses involving NF-κB in genetically diabetic mice skin fibroblasts co-cultured with macrophages. Am J Transl Res 2016; 8: 2017–2188. [PMC free article] [PubMed] [Google Scholar]
- 87. Amann R, Egger T, Schuligoi R. The tachykinin NK(1) receptor antagonist SR140333 prevents the increase of nerve growth factor in rat paw skin induced by Substance P or neurogenic inflammation. Neuroscience 2000; 100: 611–615. [DOI] [PubMed] [Google Scholar]
- 88. Scharfmann R, Atouf F, Tazi A, et al. Growth hormone and prolactin regulate the expression of nerve growth factor receptors in INS-1 cells. Endocrinology 1994; 134: 2321–2328. [DOI] [PubMed] [Google Scholar]
- 89. Mashaghi A, Marmalidou A, Tehrani M, et al. Neuropeptide Substance P and the immune response. Cell Mol Life Sci 2016; 73: 4249–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Arita J, Kojima Y, Yamamoto I, et al. Somatotropes and thyrotropes in the rat anterior pituitary gland cosecrete Substance P: analysis by the sandwich cell immunoblot assay. Neuroendocrinology 1994; 60: 567–574. [DOI] [PubMed] [Google Scholar]
- 91. Lemamy GJ, Guillaume V, Ndéboko B, et al. Substance P stimulates Growth Hormone (GH) and GH-Releasing Hormone (GHRH) secretions through tachykinin NK2 receptors in sheep. Peptides 2012; 35: 60–64. [DOI] [PubMed] [Google Scholar]
- 92. Coiro V, Volpi R, Capretti L, et al. Intravenously infused Substance P enhances basal and growth hormone (GH) releasing hormone-stimulated GH secretion in normal men. Peptides 1992; 13: 843–846. [DOI] [PubMed] [Google Scholar]
- 93. Cheng K, Wei L, Chaung LY, et al. Inhibition of L-692,429-stimulated rat growth hormone release by a weak Substance P antagonist: L-756,867. J Endocrinol 1997; 152: 155–158. [DOI] [PubMed] [Google Scholar]
- 94. Borrione P, Grasso L, Pautasso M, et al. Impact of different concentrations of human recombinant growth hormone on T lymphocytes. Int J Immunopathol Pharmacol 2012; 25: 87–97. [DOI] [PubMed] [Google Scholar]
- 95. Argetsinger LS, Campbell GS, Yang X, et al. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 1993; 74: 237–244. [DOI] [PubMed] [Google Scholar]
- 96. Broughton G, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg 2006; 117: 1e-S–32e-S. [DOI] [PubMed] [Google Scholar]
- 97. Barrientos S, Stojadinovic O, Golinko MS, et al. Growth factors and cytokines in wound healing. Wound Repair Regen 2008; 16: 585–601. [DOI] [PubMed] [Google Scholar]
- 98. García-Esteo F, Pascual G, Gallardo A, et al. A biodegradable copolymer for the slow release of growth hormone expedites scarring in diabetic rats. J Biomed Mater Res B Appl Biomater 2007; 81: 291–304. [DOI] [PubMed] [Google Scholar]
- 99. Yang H. Effect of aging on angiogenesis and arteriogenesis. Curr Cardiol Rev 2007; 3: 65–74. [Google Scholar]
- 100. Sonntag WE, Lynch CD, Cooney PT, et al. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology 1997; 138: 3515–3520. [DOI] [PubMed] [Google Scholar]
- 101. Rudman D, Feller AG, Nagraj HS, et al. Effects of human growth hormone in men over 60 years old. N Engl J Med 1990; 323: 1–6. [DOI] [PubMed] [Google Scholar]
- 102. Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol 2006; 47: 921–929. [DOI] [PubMed] [Google Scholar]
- 103. Eton D, Zhou G, He TC, et al. SS18. Enhancing neovascularization in chronic limb-threatening ischemia. J Vasc Surg 2015; 61: 106S. [Google Scholar]
- 104. Leinninger GM, Vincent AM, Feldman EL. The role of growth factors in diabetic peripheral neuropathy. J Peripher Nerv Syst 2004; 9: 26–53. [DOI] [PubMed] [Google Scholar]
- 105. Huang Y, Chang Y. Regulation of pancreatic islet beta-cell mass by growth factor and hormone signaling. Prog Mol Biol Transl Sci 2014; 121: 321–349. [DOI] [PubMed] [Google Scholar]
- 106. Johannsson G, Mårin P, Lönn L, et al. Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab 1997; 82: 727–734. [DOI] [PubMed] [Google Scholar]
- 107. Weaver JU, Monson JP, Noonan K, et al. The effect of low dose recombinant human growth hormone replacement on regional fat distribution, insulin sensitivity, and cardiovascular risk factors in hypopituitary adults. J Clin Endocrinol Metab 1995; 80: 153–159. [DOI] [PubMed] [Google Scholar]
- 108. Hwu CM, Kwok CF, Lai TY, et al. Growth hormone (GH) replacement reduces total body fat and normalizes insulin sensitivity in GH-deficient adults: a report of one-year clinical experience. J Clin Endocrinol Metab 1997; 82: 3285–3292. [DOI] [PubMed] [Google Scholar]
- 109. Jeffcoate W. Can growth hormone therapy cause diabetes? Lancet 2000; 355(9204): 589–590. [DOI] [PubMed] [Google Scholar]
- 110. Mathioudakis N, Salvatori R. Adult-onset growth hormone deficiency: causes, complications and treatment options. Curr Opin Endocrinol Diabetes Obes 2008; 15: 352–358. [DOI] [PubMed] [Google Scholar]
- 111. Ardail D, Debon A, Perret-Vivancos C, et al. Growth hormone internalization in mitochondria decreases respiratory chain activity. Neuroendocrinology 2010; 91: 16–26. [DOI] [PubMed] [Google Scholar]
- 112. Pelisek J, Shimizu M, Nikol S. Differential developmental origin of arteries: impact on angiogenesis and arteriogenesis. Med Chem Rev 2004; 1: 317–326. [Google Scholar]
- 113. Mohler ER, Rajagopalan S, Olin JW, et al. Adenoviral-mediated gene transfer of vascular endothelial growth factor in critical limb ischemia: safety results from a phase I trial. Vasc Med 2003; 8: 9–13. [DOI] [PubMed] [Google Scholar]
- 114. Rasmussen HS, Rasmussen CS, Macko J. VEGF gene therapy for coronary artery disease and peripheral vascular disease. Cardiovasc Radiat Med 2002; 3: 114–117. [DOI] [PubMed] [Google Scholar]
- 115. Collinson DJ, Donnelly R. Therapeutic angiogenesis in peripheral arterial disease: can biotechnology produce an effective collateral circulation? Eur J Vasc Endovasc Surg 2004; 28: 9–23. [DOI] [PubMed] [Google Scholar]
- 116. Matoba S, Tatsumi T, Murohara T, et al. ; TACT Follow-up Study Investigators. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J 2008; 156: 1010–1018. [DOI] [PubMed] [Google Scholar]
- 117. Iba O, Matsubara H, Nozawa Y, et al. Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation 2002; 106: 2019–2025. [DOI] [PubMed] [Google Scholar]
- 118. Clapp C, Aranda J, González C, et al. Vasoinhibins: endogenous regulators of angiogenesis and vascular function. Trends Endocrinol Metab 2006; 17: 301–307. [DOI] [PubMed] [Google Scholar]
- 119. Clapp C, Thebault S, Martínez de la Escalera G. Role of prolactin and vasoinhibins in the regulation of vascular function in mammary gland. J Mammary Gland Biol Neoplasia 2008; 13: 55–67. [DOI] [PubMed] [Google Scholar]
- 120. Croker BA, Kiu H, Nicholson SE. SOCS regulation of the JAK/STAT signalling pathway. Semin Cell Dev Biol 2008; 19: 414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Rizzo M, Montalto G, Rizvi AA, et al. The role of elevated growth hormone on the increased atherosclerosis in patients with acromegaly. Angiology 2012; 63: 492–494. [DOI] [PubMed] [Google Scholar]
- 122. Damjanovic SS, Neskovic AN, Petakov MS, et al. High output heart failure in patients with newly diagnosed acromegaly. Am J Med 2002; 112: 610–616. [DOI] [PubMed] [Google Scholar]
- 123. Rajasoorya C, Holdaway IM, Wrightson P, et al. Determinants of clinical outcome and survival in acromegaly. Clin Endocrinol (Oxf) 1994; 41: 95–102. [DOI] [PubMed] [Google Scholar]
- 124. Vilar L, Naves LA, Costa SS, et al. Increase of classic and nonclassic cardiovascular risk factors in patients with acromegaly. Endocr Pract 2007; 13: 363–372. [DOI] [PubMed] [Google Scholar]
- 125. Boero L, Cuniberti L, Magnani N, et al. Increased oxidized low density lipoprotein associated with high ceruloplasmin activity in patients with active acromegaly. Clin Endocrinol (Oxf) 2010; 72: 654–660. [DOI] [PubMed] [Google Scholar]
- 126. Erem C, Nuhoglu I, Kocak M, et al. Blood coagulation and fibrinolysis in patients with acromegaly: increased plasminogen activator inhibitor-1 (PAI-1), decreased tissue factor pathway inhibitor (TFPI), and an inverse correlation between growth hormone and TFPI. Endocrine 2008; 33: 270–276. [DOI] [PubMed] [Google Scholar]
- 127. Paisley AN, Banerjee M, Rezai M, et al. Changes in arterial stiffness but not carotid intimal thickness in acromegaly. J Clin Endocrinol Metab 2011; 96: 1486–1492. [DOI] [PubMed] [Google Scholar]
- 128. Boero L, Manavela M, Gómez Rosso L, et al. Alterations in biomarkers of cardiovascular disease (CVD) in active acromegaly. Clin Endocrinol (Oxf) 2009; 70: 88–95. [DOI] [PubMed] [Google Scholar]
- 129. Boysan SN, Kantarci F, Celik O, et al. Atherosclerotic risk factors and premature atherosclerosis in acromegaly before and after 48 months of octreotide-LAR treatment. Angiology 2012; 63: 522–527. [DOI] [PubMed] [Google Scholar]
- 130. Kartal I, Oflaz H, Pamukçu B, et al. Investigation of early atherosclerotic changes in acromegalic patients. Int J Clin Pract 2010; 64: 39–44. [DOI] [PubMed] [Google Scholar]
- 131. Dagdelen S, Cinar N, Erbas T. Increased thyroid cancer risk in acromegaly. Pituitary 2014; 17: 299–306. [DOI] [PubMed] [Google Scholar]
- 132. Takala J, Ruokonen E, Webster NR, et al. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med 1999; 341: 785–792. [DOI] [PubMed] [Google Scholar]
- 133. Uronen-Hansson H, Allen ML, Lichtarowicz-Krynska E, et al. Growth hormone enhances proinflammatory cytokine production by monocytes in whole blood. Growth Horm IGF Res 2003; 13: 282–286. [DOI] [PubMed] [Google Scholar]