Abstract
Background
Spinal burst fractures are pathologies that occur in spinal injuries and cause significant mortality and morbidity as a result. Burst fractures in spinal cord injuries can result in rapid and significant oxidative stress. In addition to the primary injury in severe spinal cord injuries, subsequent secondary lesions are mainly due to inflammatory cascade activation and excessive production of free radicals. This study evaluated oxidative stress and antioxidant enzyme levels in burst fractures.
Material/Methods
Twenty patients with burst fractures were diagnosed and underwent surgery and 20 healthy control subjects were included in the study. Neurological status was evaluated using the American Spine Injury Association Impairment Scale (ASIA) before and after surgery. Neurological function was scored as ASIA A: complete deficits, ASIA B–D: incomplete deficits, and ASIA E: neurologically intact. Spectrophotometry was performed to measure malondialdehyde (MDA) and low glutathione (GSH), glutathione peroxidase (GPx) levels, which represent lipid peroxide content. Evaluations were performed within 2 days after injury in the patients.
Results
MDA levels were higher in the burst fracture group (p<0.001), whereas GSH and SOD activities were higher in the control group (both p<0.001). There was no statistically significant difference in GPx levels between the groups (p=0.482).
Conclusions
Oxidative stress appears to be related to burst fractures. Considering the importance of burst fractures in spinal cord injuries, a better understanding of these mechanisms may help in defining the role of oxidative stress after burst fractures. Prospective, randomized, controlled trials may reveal new therapeutic approaches that include antioxidants for explosive fractures focusing on oxidative stress.
MeSH Keywords: Glutathione, Glutathione Peroxidase, Malondialdehyde, Oxidative Stress, Spinal Fractures, Superoxide Dismutase
Background
Spinal column and spinal cord traumas are associated with very poor outcomes and significant neurological damage. In addition to causing physical disability and insufficiency, they cause significant psychological and socioeconomic effects. One type of such trauma is a burst fracture, which was first identified by Holdsworth in 1963 [1]. Such vertebral fractures are a common type of high-energy spinal fracture. Burst fractures constitute 15% of all spinal fractures [2,3]. The cause is mostly motor vehicle accidents and high falls. There is no consensus about treatment of these cases because of the many different conservative and surgical treatment options available [4].
Oxidative stress is defined as an increase in oxidation as a result of destruction of the balance between oxidation and antioxidant systems. Physiological adaptation is also responsible for oxidative stress, lipid peroxidation, and DNA damage [5]. Oxidative stress, which is usually the end product of lipid peroxidation malondialdehyde (MDA), can be measured with non-enzymatic markers such as 8-hydroxy-2-deoxyguanosine (8-OHdG), oxidative DNA damage indicator, protein oxidation SOD, GPx, GST, GR and antioxidant enzymes such as ascorbic acid, glutathione, ubiquinone, and cysteine [6].
Accelerated oxidative stress as a result of impaired physiological balance has been associated with many diseases, such as neurodegenerative diseases, including aging, Alzheimer’s disease, and Parkinson’s disease [7]; mental diseases, including depression, anxiety, and schizophrenia [8]; gastric cancer [9]; diabetes [10]; and heart valve diseases [11]. It has also been reported that oxidative stress may affect osteoporosis and fractures [12]. Specifically, it has recently been reported that oxidative stress may play a role in spinal cord injury and that glucocorticoid therapy may have an effect on such stress [13]. However, only limited research has been performed on the associations of oxidative stress with spinal fractures and changes in biochemical parameters.
As spinal fractures have a major impact on both patients and society in general, more research has been launched in this area, focusing on the pathogenetic mechanisms of disease and its course. We aimed to clarify the relationship between burst fractures, which constitute a significant proportion of spinal fractures, and the oxidative stress markers MDA, GSH, GPx, and SOD.
Material and Methods
Ethics Committee approval was obtained from the Ethics Committee of Scientific Research, Faculty of Medicine, Yüzüncü Yıl University (3/5/2016, approval number 12/03). Our study included 20 patients diagnosed with burst fracture who were treated at the Neurosurgery Department of Yüzüncü Yıl University Medical Faculty Hospital and 20 healthy control patients matched by sex.
This was a descriptive, prospective, controlled, and analytical. As the inclusion criteria for the study, only patients with burst fracture were selected within the last year for patients with spinal fractures. In addition, patients aged 18 years and older were included in the study. Exclusion criteria were: presence of spinal fracture other than a burst fracture, refusal to participate, and another comorbid condition that could be a source of oxidative stress.
In our study, which focused on patients diagnosed with a burst fracture and a paired control matched by sex, the patients were first informed about the study and then they provided informed consent. We collected 3-cc blood samples within 2 days after injury from the brachial veins of the patients (as well as from the controls) and centrifuged samples for 10 min at 5000 rpm in a Nude NF 800 centrifuge in EDTA tubes, after which the serum was removed and stored at −80°C. Evaluation of serum samples was carried out at a laboratory in the Department of Biochemistry, Science Faculty, Yüzüncü Yıl University. Oxidative stress markers were evaluated in the serum samples, including reduced glutathione (GSH), a low molecular weight antioxidant, superoxide dismutase (SOD), and glutathione peroxidase (GPx) in the antioxidant enzyme structure, and malondialdehyde (MDA), a product of lipid peroxidation. Measurements were performed using an ultraviolet spectrophotometer.
Reduced glutathione (GSH)
The low molecular weight glutathione, which is synthesized in the cell as a tripeptide (glutamic acid), exists in 2 different forms: an oxide and a reductant. It is observed in all cells except erythrocytes and is important for hemostatic mechanisms [14]. It has roles in various processes, including antioxidant defense, detoxification of electrophilic xenobiotics, regulation of regulated responses via signal transduction, deoxyribonucleotide synthesis, regulation of immunological responses, and leukotriene and prostaglandin metabolism [15]. In addition to its biochemical functions, GSH is also involved in clearing ROS [16].
After mixing 0.2 ml of the blood sample with 2 ml of distilled water, 2 ml of the obtained mixture was taken and mixed with a solution containing 50.1 mg of glacial metaphosphoric acid, 6 mg of disodium EDTA, and 0.9 g of sodium chloride. This mixture was incubated for 5 min at room temperature, after which filtration with filter paper was performed.
After 0.5 ml of the filtrate had been removed and mixed with 2 ml of disodium phosphate solution, the absorbance at 412 nm was measured using a UV spectrophotometer. After 0.5 ml of filtrate was removed and mixed with 2 ml of disodium phosphate solution, the absorbance at 412 nm was measured using a UV spectrophotometer. Reductive glutathione concentrations in μmol/L were reached in the presence of both absorbances.
Superoxide dismutase (SOD)
SOD is a major factor in the defense against oxygen radicals. It prevents endothelial and mitochondrial dysfunction by inactivating nitric oxide and inhibiting peroxynitrite formation [17]. It also clears oxygen radicals produced in the respiratory chain. Dilated cardiomyopathy has been reported to be deficient or absent [18]. In SOD measurement, the xanthine–xanthine oxidase system produces superoxide radicals and reacts with radical iodonitrotetrazolium (INT) to form colored formazan. The intensity of color from this reaction was measured over a 3-min period at a wavelength of 505 nm. The reaction was inhibited in a manner dependent on the CuZn–SOD activity in the medium. The level of inhibition was measured and the SOD level in U/g was thus determined.
Glutathione peroxidase (GPx)
GPx performs the enzymatic catalysis of reactive oxygen species. GPx-1 inhibits hydrogen peroxide accumulation in the cell. It is responsible for clearing reactive oxygen radicals via glutathione together with glutathione reductase [19]. The GPx measurement method is based on the spectrophotometric demonstration of the reduction in NADPH used. Measurements were performed at a wavelength of 340 nm. GPx levels are expressed in U/g [6].
Malonaldehyde (MDA)
MDA, one of the most important products of lipid peroxidation, can affect ion exchange in the cell membrane and can lead to adverse effects, such as changes in ion permeability and enzyme activity [6]. MDA is mutagenic, genotoxic, and carcinogenic since it can react with nitrogen bases [20]. Cancer has been used to measure oxidative stress in chronic obstructive disease, asthma, and cardiovascular diseases [21]. Malondialdehyde measurement is based on the color formed with thiobarbituric acid [22]. MDA forms a pink complex as a result of incubation with thiobarbituric acid at 95°C and pH 3.4 in aerobic conditions. The level of this complex was expressed in nmol/mL of serum malondialdehyde (MDA), as determined by spectrophotometric measurement at 532 nm.
Statistical analysis
Statistical analyses were performed using the SPSS (ver. 20) statistical package. The descriptive statistics used were mean, standard deviation, median, minimum, and maximum. Preoperative and postoperative ASAI scores were statistically analyzed using the Wilcoxon signed ranks test. Normal distribution of variables was assessed visually (histogram and probability plots) and using an analytical method (Shapiro-Wilk test). The results showed that SOD, GSH, GPx, and MDA did not follow a normal distribution. The Mann-Whitney U test was thus used to compare the intra-group averages. In the analysis of GPx values, the values of the 1st and 16th patients were much higher than the group averages, and the results of these 2 patients were analyzed. Spearman correlation test was used to determine the relationships between SOD, GSH, GPx, and MDA. When Spearman correlation coefficient ranged from 0.05 to 0.30, it was considered to represent a low or negligible correlation, between 0.30 and 0.40 represented a low to medium correlation, between 0.40 and 0.60 a moderate correlation, between 0.60 and 0.70 a good correlation, and between 0.70 and 0.75 a very good correlation. Values above 0.75 were considered to reflect a perfect correlation. Statistical significance in the calculations was accepted at the 5% level (p<0.05).
Results
Our study included 20 patients with burst fracture and 20 healthy controls. The average age of the 20 fracture patients was 38.14±16.4 years, with a median age of 37. The youngest patient was 12 years old and the oldest patient was 63 years old. More traffic accidents were detected in the etiology of fracture (Figure 1). Looking at the results in terms of levels, the lumbar region was most often affected (54.5%) (Figure 2)
Figure 1.
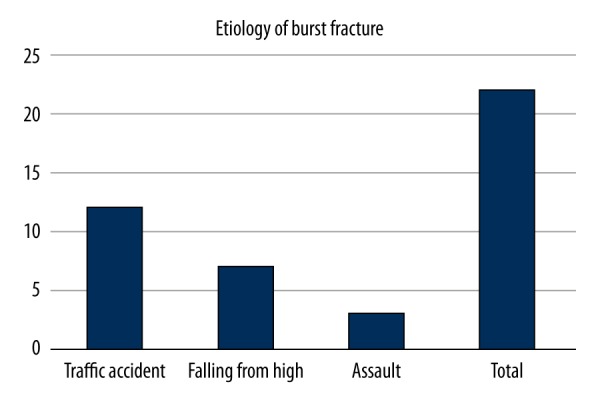
More traffic accidents were detected in the etiology of fracture.
Figure 2.
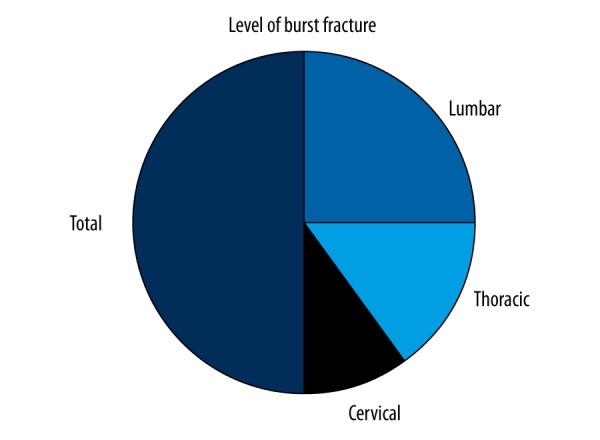
Looking at the results in terms of levels, the lumbar region is more affected by 54.5%
Figures 3 and 4 show preoperative and postoperative views of the 2 patients who underwent surgery.
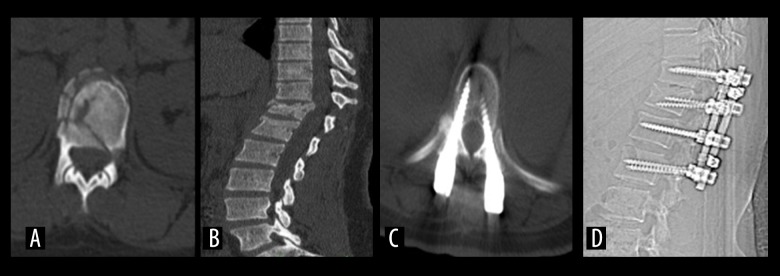
Figure 3.
A 36-year-old male (No: 13) who fell from a height. T12 burst fracture (A) Preoperative thoracolumbar CT. (B) Preoperative thoracolumbar CT Sagittal reconstruction. (C) postoperative CT. (D) posterior thoracolumbar CT (posterior instrumentation).
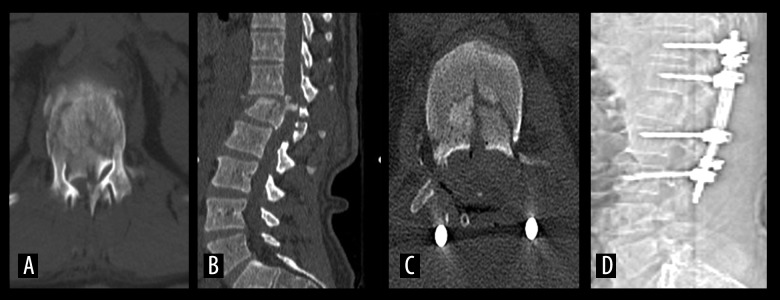
Figure 4.
A 45-year-old male who was in a traffic accident (No. 14). L1 burst fracture (A) Preoperative thoracolumbar CT. (B) Preoperative thoracolumbar CT (Sagittal reconstruction). (C) Postoperative CT(posterior instrumentation). (D) Posterior Thoracolumbar CT (Scenogram) (posterior instrumentation)
When we compared the preoperative and postoperative ASAI scores statistically, it was observed that patients’ paralysis scores improved significantly (p <0.001) (Figure 5).
Figure 5.
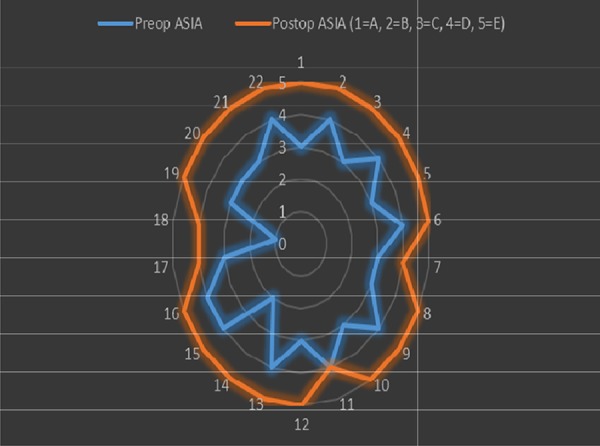
The comparison of preoperative and postoperative ASAI scores. When we compared the preoperative and postoperative ASAI scores statistically, it was observed that patients’ paralysis scores improved significantly (p<0.001).
The mean SOD value of the patient group was 2.94±1.5101 U/g, whereas that of the control group was 9.3105±0.8883 U/g, with a significant difference between them (p<0.001) (Table 1, Figure 6). The mean GPx value of the patient group was 111.2±80.0 U/g, whereas that of the control group was 111.0±3.1 U/g. There was no significant difference between them (p=0.482) (Table 2, Figure 7).
Table 1.
The SOD values of the patient and control groups.
| n | Ortalama ±SD | Median | Min. | Max. | p | |
|---|---|---|---|---|---|---|
| Patient | 20 | 2.9499±1.5100 | 2.6410 | 1.0100 | 5.6560 | <0.001 |
| Control | 20 | 9.3105±0.8882 | 9.2000 | 8.2700 | 11.0100 |
Mann-Whitney U test was used.
Figure 6.
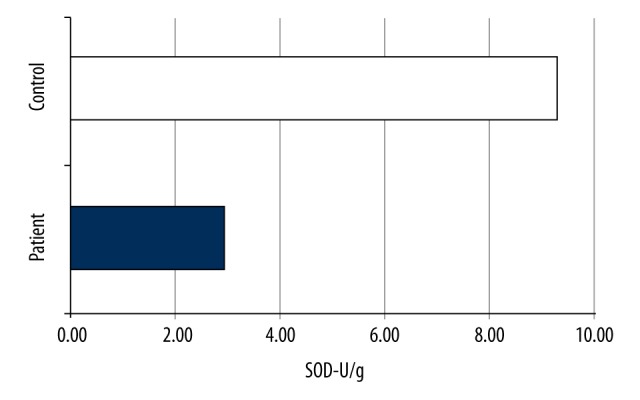
SOD values of the groups.
Table 2.
GPx values of patient and control groups.
| n | Ortalama ±SD | Median | Min. | Max. | p | |
|---|---|---|---|---|---|---|
| Patient | 18 | 111.2±80.0 | 79.9 | 16.8 | 311.2 | 0.482 |
| Control | 20 | 111.0±3.1 | 110.4 | 107.6 | 119.5 |
Mann-Whitney U test was used.
Figure 7.
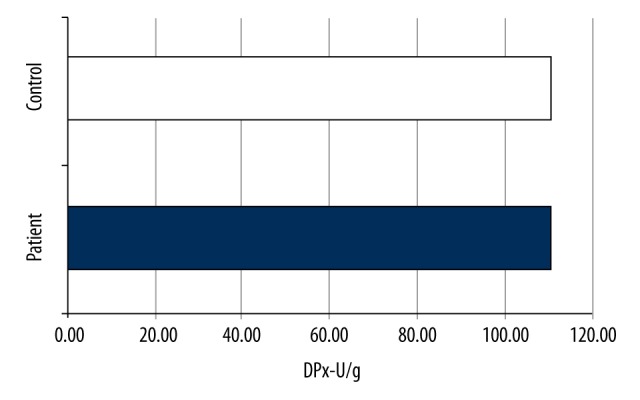
GPx values of the groups.
The mean GSH value of the patient group was 0.00485±0.00267 μmol/L, whereas that in the control group was 0.02875±00578 μmol/L, and the difference was significant (p<0.001) (Table 3, Figure 8).
Table 3.
GSH values of patient and control groups.
| n | Ortalama ±SD | Median | Min. | Max. | p | |
|---|---|---|---|---|---|---|
| Patient | 20 | 0.00485±0.00267 | 0.00474 | 0.00064 | 0.01360 | 0.001 |
| Control | 20 | 0.02875±00578 | 0.02700 | 0.01600 | 0.03700 |
Mann-Whitney U test was used.
Figure 8.
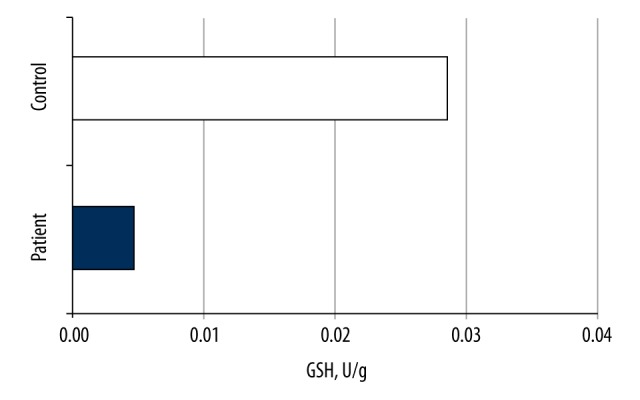
GSH values of the groups.
The mean MDA level of the patient group was 1.246±0.052 nmol/mL, whereas that of the control group was 0.598±0.091 nmol/mL, which were significantly different (p<0.001) (Table 4, Figure 9).
Table 4.
The MDA values of the patient and control groups.
| n | Ortalama ±SD | Median | Min. | Max. | p | |
|---|---|---|---|---|---|---|
| Patient | 20 | 1.246±0.052 | 1.256 | 1.083 | 1.320 | <0.001 |
| Control | 20 | 0.598±0.091 | 0.588 | 0.452 | 0.744 |
Mann-Whitney U test was used.
Figure 9.

MDA values for the groups.
When the association of oxidative stress markers was evaluated in the patient group, strong negative correlations were found between SOD and MDA (p<0.001, P=−0.723) and between GSH and MDA (p<0.001, rho=−0.759). There was also a strong positive correlation between SOD and GSH (p<0.001, rho=0.752). There was no correlation of GPx with MDA, SOD with GPx, or GPx with GSH (Table 5).
Table 5.
Relationships between oxidative stress markers.
| SOD | GPx | MDA | |
|---|---|---|---|
| SOD | |||
| GPx | p=0.514 | ||
| MDA | p<0.001, rho=−0.723 | p=0.657 | |
| GSH | p<0.001, rho=0.752 | p=0.543 | p<0.001, rho=−0.759 |
Spearman’s correlation test was used
Discussion
Spinal fractures are among the most feared type of fracture due to their associations with mortality and permanent neurological deficits [23]. Most spinal fractures from motor vehicle accidents or high-energy trauma such as falls [24,25] occur in the thoracolumbar region (65%), whereas a smaller proportion occur in the cervical region (35%) [26]. Burst fractures are the most unstable fractures of the thoracolumbar region, constituting 15% of all spinal injuries and fractures [2,3].
The neurological status of the patients was assessed according to the ASIA scale (Table 6, Figure 5). Preoperative neurologic dysfunction was present in all patients and AISA A (n=1, 4.5%); AISA (n=1, 4.5%); AISA C (n=11, 50%); and AISA D (n=9, 40.9%). Normal preoperative function AISA E was not observed in any patient.
Table 6.
Demographic data, level of burst fracture, level of stabilization, pre-postop ASIA score in patients.
| No | Age | Sex | Etiology | Level of burst fracture | Level of stabilization | Preop ASIA | Postop ASIA |
|---|---|---|---|---|---|---|---|
| 1 | 59 | M | Traffic accident | L1 | T11–L3 | C | E |
| 2 | 51 | M | Falling from high | L1 | T11–L3 | D | E |
| 3 | 38 | F | Traffic accident | L2 | T12–L4 | C | E |
| 4 | 12 | M | Traffic accident | T11–T12 | T10–L2 | D | E |
| 5 | 29 | M | Falling from high | L5 | L4–L5 | C | E |
| 6 | 20 | M | Assault | T12 | T10–L1 | D | E |
| 7 | 17 | M | Traffic accident | L4 | L3–L5 | C | D |
| 8 | 38 | F | Traffic accident | L2 | L1–L4 | C | E |
| 9 | 19 | M | Falling from high | L2 | T12–L3 | D | E |
| 10 | 63 | F | Traffic accident | L2 | L1–L3 | C | E |
| 11 | 59 | F | Traffic accident | T12 | T10–L1 | D | D |
| 12 | 34 | M | Assault | L5 | L5 | C | E |
| 13 | 36 | M | Falling from high | T12 | T11–L2 | D | E |
| 14 | 45 | M | Traffic accident | L1 | T11–L3 | B | E |
| 15 | 23 | M | Falling from high | T12 | T10–L2 | D | E |
| 16 | 24 | M | Assault | T12 | T10–L1 | D | E |
| 17 | 62 | M | Falling from high | L3 | L1–L4 | C | D |
| 18 | 32 | F | Traffic accident | C5, 6, and 7 | C5, 6, and 7 | A | D |
| 19 | 46 | F | Traffic accident | T2 | T1–T3 | C | E |
| 20 | 58 | M | Traffic accident | L2 | L1–L4 | C | E |
| 21 | 56 | M | Falling from high | T11 | T9–L1 | C | E |
| 22 | 21 | M | Traffic accident | T12 | T11–L1 | D | E |
M – Male; F – Female T – thoracic; L – lumbar; C – cervical; ASIA – American Spinal Injury Association.
All patients except one patient had improved ASIA score (No: 11). The postoperative ASIA score increased to E level in 18 patients. In a similar study, Louis et al. reported 66% complete recovery in patients with incomplete injury. None of the patients in our series had a neurological decline, during the course of the treatment [27].
Physical, chemical, and inflammatory damage as well as aging increase the production of free oxygen radicals [28]. Free oxygen radicals are also used in physiological processes such as phagocyte activation, activities of the immune system, lipid peroxidation, and the electron transport chain in mitochondria [29,30]. However, such radicals must be controlled by the antioxidant system. To achieve this, the antioxidant system is constantly involved in a battle against oxidative stress. Oxidative stress occurs when free radicals increase or the antioxidant system becomes insufficient, leading to a prooxidant–antioxidant imbalance and an excess amount of prooxidants. Oxidative stress has been shown to be involved in post-traumatic stress disorder [31], diabetes, and cardiovascular diseases [32], and neurodegenerative diseases [33,34]. In addition, it has been reported that the oxidative stress level increases after trauma. However, the data on this issue in cases of burst fractures from major trauma are rather limited [28].
The present study sheds light on this issue by showing that SOD enzyme activity, which constitutes the major defense system against oxygen radicals causing oxidative stress, was low in patients with burst fracture compared with that in the control group (p<0.001). Physical trauma-activated polymorphonuclear leukocytes (PMNL) have also been shown to trigger the production of free oxygen radicals [35].
With the production of these radicals, cell integrity deteriorates as various systems in the cell are damaged. Similarly, Rana et al. reported that patients with abdominal trauma had reduced SOD levels compared with those in the control group [28]. Moreover, there have been a range of reports about reductions of SOD levels, such as by Wang et al. in acute traumatic brain injury [36], Alzoghaibi et al. in Crohn’s disease [37], Esposito et al. [38] in Alzheimer’s disease, Regan et al. in osteoarthritis [39], and Vachier et al. in asthmatic patients [40]. However, there have been few studies examining the association of SOD with the skeletal system compared with those on other diseases. Smietana et al. reported that bone strength and stiffness decreased upon SOD deficiency [41], which was confirmed by Muller et al. [42]. In addition, an animal study by Halıcı et al. showed that SOD levels were reduced in cases of femur fracture [43]. Moreover, Göktürk et al. reported that free oxygen radicals delayed the healing of fractures [44]. These findings were supported by our study. However, although SOD activity is believed to play physiologically essential roles, it is not known exactly whether a decrease in its level is caused by burst fractures, which increases the level of oxidative stress as a primer, or because the role of the deficiency in the process of healing is reduced. This issue cannot be resolved here because of the particular design of our study. However, in a study by Grabitz et al., this issue was researched and an increase in SOD levels was shown to be protective against spinal cord injury by inhibiting spinal neuron damage [45], which was confirmed by Taoka et al. [46].
Taking these findings together, it can be considered that oxidative stress plays an important role in spinal fracture or spinal cord injury, and that the removal of oxidative stress by antioxidants can lead to better clinical results. However, it has also been reported that SOD levels after trauma are first reduced and then decreased. Unfortunately, there have been few long-term studies on this issue. For this reason, there is a need for further studies on how SOD activities decrease after trauma. In this context, the results of our work go some way to bridging this gap by reflecting the levels of oxidative stress in the acute phase.
The vast majority of glutathione, an endogenous antioxidant, is in reduced form (GSH). GSH, GPx, and GR together bring about an important antioxidant defense system [47]. In our study, the GSH values of the patient group were lower than in the control group (p<0.001). Luo et al. reported a reduction in GSH levels after abdominal surgery, supporting the findings of our work [48]. In addition, Kamencic et al. [49] and Lucas et al. [50] reported that an improvement in functional outcome was induced by promoting GSH synthesis in patients with spinal cord trauma. Studies have also reported that GSH levels are elevated after trauma. Furthermore, Wang et al. reported that GSH levels in acute traumatic brain injury patients were higher than those in controls. These findings contradict the results of our study, but in the study of Wang et al., GSH levels were studied within the first week after trauma, showing that GSH and SOD levels were associated with prognosis [36]. In our work, evaluations were made within the first 2 days. The difference in the obtained findings may thus be due to the fact that the underlying GSH levels decrease early in response to trauma, and then tend to rise in the long-term period.
GPx is an important enzyme in the antioxidant system that works with GSH and GR to prevent the formation of hydrogen peroxide. In our study, GPx levels were similar in the control group with burst fractures (p=0.482). In the literature, contradictory results of post-traumatic GPx levels have been reported. The causes of cellular and molecular events such as spinal fractures or spinal cord trauma persist for a long time after injury, causing lesion enlargement and tissue damage. For this reason, the future functional status in spinal cord injury depends on the initial injury and secondary events and complications. Vaziri et al. found that GPx levels did not change on the 1st day of the experiment and that the experimental spinal cord injury showed a significant increase on the 14th day [51]. However, irrespective of the inconsistent results regarding an increase or a decrease in GPx level after spinal injury reported previously, an increase in GPx levels caused significant improvement in clinical outcomes.
Lipid peroxidation is an oxidative stress indicator resulting from MDA. In our study, the MDA levels of patients with burst fractures were found to be significantly higher than those in the control group (p<0.001). This study supports the findings of increased oxidative stress levels in burst fractures using rats obtained by Göktürk et al. In this study, after the tibia fracture of the MDA levels, there was an increase at 2–3 weeks [52].
An experimental fracture model was established using rats in a study by Halıcı et al., in which MDA levels were found to be increased after fracture [42]. It was also shown that MDA levels, which are high when using the antioxidant melatonin, are reduced upon fracture. Prasad et al. similarly demonstrated that MDA levels increased in single or multiple fractures in humans [53]. MDA levels were also found to be higher in multiple fractures and were thought to be proportional to the severity of trauma. The oxidative stress cascade triggered after trauma is thought to lead to secondary problems independently of the trauma itself. For this purpose, it is estimated that the results obtained when oxidative stress is neglected will be well developed. In this context, Porter and colleagues performed antioxidant treatment for 18 trauma patients and compared them with 18 control trauma patients who did not receive such treatment. The results showed that there were fewer complications such as organ dysfunction and infection in the patients given antioxidants [54].
As a result, it can be considered that oxidative stress increases upon spinal trauma, which negatively affects healing, and oxidative stress is decreased.
Our work has some limitations. First, parameters that affect oxidative stress, such as sociodemographic characteristics, fracture time, used treatments, and antioxidant use, were not evaluated. However, this issue was partially overcome by matching of the control group according to sex and excluding comorbid conditions that can be sources of oxidative stress. Another limitation is that the oxidative stress markers were only evaluated once. Therefore, it was not possible for us to draw any conclusions about the temporal changes in oxidative stress markers in this study. Moreover, because the patients were often evaluated in the acute phase, our results reflect the level of oxidative stress in this phase alone.
Results and Recommendations
Our study is the first to show increased levels of oxidative stress in burst-type spinal fractures from high-energy trauma. Our findings show that the antioxidant effect was slowed in major traumas, especially in the late period, such as via increased MDA, and decreased SOD and GSH. In the case of burst fractures, which are frequently accompanied by spinal cord injury, antioxidant treatment, especially from an early stage, can improve the functional status of patients by preventing the formation of free radicals or at least by reducing their levels. Prospective, randomized, controlled trials may reveal new therapeutic approaches that include antioxidants for explosive fractures focusing on the oxidative stress.
Acknowledgements
This work is Fetullah Kuyumcu’s expertise thesis. We thank to Prof. Dr. Halit Demir for his biochemical analysis, Associate Professor Mehmer Arslan, Assistant MD. M Edip Akyol for their contribution in the process of treating patients, and Sadi Elasan for his statistical analysis.
Footnotes
This study was presented as oral presentation at the 31st Scientific Congress of the Turkish Neurosurgical Society 16th place
Source of support: This work was carried out by YYU Scientific Research Projects Coordination in ID-1494 Supported as Project No 2015-TF-U215
Conflicts of interest
None.
References
- 1.Holdsworth F. Fractures, dislocations, and fracture-dislocations of the spine. J Bone Joint Surg Am. 1963;45:6–20. [PubMed] [Google Scholar]
- 2.Wilcox RK, Boerger TO, Allen DJ, et al. A dynamic study of thoracolumbar burst fractures. J Bone Joint Surg Am. 2003;85-A(11):2184–89. doi: 10.2106/00004623-200311000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Bohlman HH. Current concepts review: Treatment of fractures and dislocations of the thoracic and lumbar spine. J Bone Joint Surg Am. 1985;67-A(1):165–69. [PubMed] [Google Scholar]
- 4.Benzel EC, Larson SJ. Functional recovery after decompressive operation for thoracic and lumbar spine fractures. Neurosurgery. 1986;19:772–78. doi: 10.1227/00006123-198611000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Yoshikawa T, Naito Y. What is oxidative stress? JMAJ. 2002;45(7):271–76. [Google Scholar]
- 6.Eken A. rat kan ve doku örneklerinde oksidatif stres parametreleri. Journal of Clinical and Analytical Medicine. 2017 Online. ET: 03/01/2017 [in Turkish] [Google Scholar]
- 7.Uttara B, Singh AV, Zamboni P, Mahajan R. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salim S. Oxidative stress and psychological disorders. Curr Neuropharmacol. 2014;12(2):140–47. doi: 10.2174/1570159X11666131120230309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasavidevi VB, Kishor HD, Adinath NS, et al. Depleted nitrite and enhanced oxidative stress in urolithiasis. Indian J Clin Biochem. 2006;21:177–80. doi: 10.1007/BF02912938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haydent MR, Tyagi SC. Neural redox stress and remodeling in metabolic syndrome, type 2 diabetes. JOP. 2002;3:126–38. [PubMed] [Google Scholar]
- 11.Peña-Silva RA, Miller JD, Chu Y, Heistad DD. Serotonin produces monoamine oxidase-dependent oxidative stress in human heart valves. Am J Physiol Heart Circ Physiol. 2009;297:1354–60. doi: 10.1152/ajpheart.00570.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheweita SA, Khoshhal KI. Calcium metabolism and oxidative stress in bone fractures: Role of antioxidants. Curr Drug Metab. 2007;8:519–25. doi: 10.2174/138920007780866852. [DOI] [PubMed] [Google Scholar]
- 13.Jia Z, Zhu H, Li J, et al. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord. 2012;50(4):264–74. doi: 10.1038/sc.2011.111. [DOI] [PubMed] [Google Scholar]
- 14.Aksoy Y. Antioksidan mekanizmada glutatyonun rolü. T Klin Tıp Bilimleri. 2002;22:442–48. [in Turkish] [Google Scholar]
- 15.Kerksick C, Willoughby D. The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J Int Soc Sports Nutr. 2005;2(2):38–44. doi: 10.1186/1550-2783-2-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohen R, Nyska A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30(6):620–50. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- 17.Fukai T, Ushio-Fukai M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15(6):1583–606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Huang TT, Carlson EJ, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–81. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 19.Antunes F, Han D, Cadenas E. Relative contributions of heart mitochondria glutathione peroxidase and catalase to H(2)O(2) detoxification in in vivo conditions. Free Radic Biol Med. 2002;33:1260–67. doi: 10.1016/s0891-5849(02)01016-x. [DOI] [PubMed] [Google Scholar]
- 20.Mercan U. Toksikolojide serbest radikallerin önemi. YYU Vet Fak Derg. 2004;15(1–2):91–96. [in Turkish] [Google Scholar]
- 21.Khoubnasabjafari M, Ansarin K, Jouyban A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. Bioimpacts. 2015;(3):123–27. doi: 10.15171/bi.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain SK. Membrane lipid peroxidation in erythrocytes of the newborn. Clin Chim Acta. 1986;161(3):301–6. doi: 10.1016/0009-8981(86)90014-8. [DOI] [PubMed] [Google Scholar]
- 23.Cassar-Pullicino VN. Spinal injury: Optimising the imaging options. Eur J Radiol. 2002;42:85–91. doi: 10.1016/s0720-048x(02)00049-9. [DOI] [PubMed] [Google Scholar]
- 24.Bensch FV, Kiuru MJ, Koivikko MP, Koskinen SK. Spine fractures in falling accidents: analysis of multidetector CT findings. Eur Radiol. 2004;14(4):618–24. doi: 10.1007/s00330-003-2090-6. [DOI] [PubMed] [Google Scholar]
- 25.Trivedi JM, Jones R, Hunt A. Spinal trauma: Therapy – options and outcomes. Eur J Radiol. 2002;42:127–34. doi: 10.1016/s0720-048x(02)00045-1. [DOI] [PubMed] [Google Scholar]
- 26.Hu R, Mustard CA, Burns C. Epidemiology of incident spinal fracture in a complete population. Spine. 1996;21(4):492–99. doi: 10.1097/00007632-199602150-00016. [DOI] [PubMed] [Google Scholar]
- 27.Louis CV, Gauthier VY, Louis RP. Posterior approach with Louis plates for fractures of the thoracolumbar and lumbar spine with and without neurological deficits. Spine. 1998;23(18):2030–39. doi: 10.1097/00007632-199809150-00022. [DOI] [PubMed] [Google Scholar]
- 28.Rana SV, Kashinath D, Singh G, et al. Study on oxidative stress in patients with abdominal trauma. Mol Cell Biochem. 2006;291(1–2):161–66. doi: 10.1007/s11010-006-9210-y. [DOI] [PubMed] [Google Scholar]
- 29.Taoka Y, Okajima K, Uchiba M, et al. Gabexate mesilate, a synthetic protease inhibitor, prevents compression-induced spinal cord injury by inhibiting activation of leukocytes in rats. Crit Care Med. 1997;25:874–79. doi: 10.1097/00003246-199705000-00026. [DOI] [PubMed] [Google Scholar]
- 30.Gutteridge JMC. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–28. [PubMed] [Google Scholar]
- 31.Miller MW, Sadeh N. Traumatic stress, oxidative stress and posttraumatic stress disorder: Neurodegeneration and the accelerated-aging hypothesis. Mol Psychiatry. 2014;19(11):1156–62. doi: 10.1038/mp.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–23. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 33.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 34.Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–14. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 35.Henrich D, Zimmer S, Seebach C, et al. Trauma-activated polymorphonucleated leukocytes damage endothelial progenitor cells: Probable role of CD11b/CD18-CD54 interaction and release of reactive oxygen species. Shock. 2011;36(3):216–22. doi: 10.1097/SHK.0b013e3182236eba. [DOI] [PubMed] [Google Scholar]
- 36.Wang H-C, Lin Y-J, Shih F-Y, et al. The role of serial oxidative stress levels in acute traumatic brain injury and as predictors of outcome. World Neurosurgery. 2016;87:463–70. doi: 10.1016/j.wneu.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Alzoghaibi MA, Al-Mofleh IA, Al-Jebreen AM. Antioxidant activities for superoxide dismutase in patients with Crohn’s disease. J Basic Clin Physiol Pharmacol. 2014;25(1):59–62. doi: 10.1515/jbcpp-2013-0042. [DOI] [PubMed] [Google Scholar]
- 38.Esposito L, Raber J, Kekonius L, et al. Reduction in mitochondrial superoxide dismutase modulates Alzheimer’s disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J Neurosci. 2006;26(19):5167–79. doi: 10.1523/JNEUROSCI.0482-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Regan E, Flannelly J, Bowler R, et al. Extracellular superoxide dismutase and oxidant damage in osteoarthritis. Arthritis Rheum. 2005;52(11):3479–91. doi: 10.1002/art.21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vachier I, Damon M, Le Doucen C, et al. Increased oxygen species generation in blood monocytes of asthmatic patients. Am Rev Respir Dis. 1992;146:1161–66. doi: 10.1164/ajrccm/146.5_Pt_1.1161. [DOI] [PubMed] [Google Scholar]
- 41.Smietana MJ, Arruda EM, Faulkner JA, et al. Reactive oxygen species on bone mineral density and mechanics in Cu, Zn superoxide sismutase (Sod1) knockout mice. Biochem Biophys Res Commun. 2010;403:149–53. doi: 10.1016/j.bbrc.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller FL, Song W, Liu Y, et al. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 43.Halıcı M, Öner M, Güney A, et al. Melatonin promotes fracture healing in the rat model. Eklem Hastalik Cerrahisi. 2010;21(3):172–77. [PubMed] [Google Scholar]
- 44.Göktürk E, Turgut A, Bayçu C, et al. Oxygen-free radicals impair fracture healing in rats. Acta Orthop Scand. 1995;66:473–75. doi: 10.3109/17453679508995590. [DOI] [PubMed] [Google Scholar]
- 45.Grabitz K, Freye E, Prior R, et al. The role of superoxide dismutase (SOD) in preventing postischemic spinal cord injury. Adv Exp Med Biol. 1990;264:13–16. doi: 10.1007/978-1-4684-5730-8_3. [DOI] [PubMed] [Google Scholar]
- 46.Taoka Y, Naruo M, Koyanagi E, et al. Superoxide radicals play important roles in the pathogenesis of spinal cord injury. Paraplegia. 1995;33(8):450–53. doi: 10.1038/sc.1995.98. [DOI] [PubMed] [Google Scholar]
- 47.Lu SC. Glutathione synthesis. Biochimica et Biophysica Acta. 2013;1830:3143–53. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo JL, Hammarqvist F, Andersson K, Wernerman J. Skeletal muscle glutathione after surgical trauma. Annals Surg. 1996;223(4):420–27. doi: 10.1097/00000658-199604000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamencic H, Griebel RW, Lyon AW, et al. Promoting glutathione synthesis after spinal cord trauma decreases secondary damage and promotes retention of function. FASEB J. 2001;15(1):243–25. doi: 10.1096/fj.00-0228com. [DOI] [PubMed] [Google Scholar]
- 50.Lucas JH, Wheeler DG, Guan Z, et al. Effect of glutathione augmentation on lipid peroxidation after spinal cord injury. J Neurotrauma. 2002;19(6):763–75. doi: 10.1089/08977150260139138. [DOI] [PubMed] [Google Scholar]
- 51.Vaziri ND, Lee YS, Lin CY, et al. NAD(P)H oxidase, superoxide dismutase, catalase, glutathione peroxidase and nitric oxide synthase expression in subacute spinal cord injury. Brain Res. 2004;995(1):76–83. doi: 10.1016/j.brainres.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 52.Göktürk E, Turgut A, Bayçu C, et al. Oxygen-free radicals impair fracture healing in rats. Acta Orthop Scand. 1995;66:473–75. doi: 10.3109/17453679508995590. [DOI] [PubMed] [Google Scholar]
- 53.Prasad G, Dhillon MS, Khullar M, Nagi ON. Evaluation of oxidative stress after fractures. A preliminary study. Acta Orthop Belg. 2003;69(6):546–51. [PubMed] [Google Scholar]
- 54.Porter JM, Rao A, Azimuddin L, Rajswami Antioxidant therapy in the prevention of organ dysfunction syndrome and infectious complications after trauma. Am Surg. 1999;65:478–83. [PubMed] [Google Scholar]