Abstract
The morphology of the female Cyrtobagous salviniae Calder and Sands reproductive system is similar to other weevil species being meroistic and telotrophic. The reproductive system is composed of 2 ovaries each containing 2 ovarioles where the follicles mature. A physiological age grading system was developed where the continuum of ovarium development was divided into 2 nulliparous and 3 parous classes. This was based on the differentiation of the ovarioles, presence, and appearance of follicular relics, cuticle hardness/coloration, and fat body quantity/appearance. High correlation occurred between the parous classes and number of eggs produced where the P3 class had over 9-fold higher number of eggs in comparison with the P1 class. Mean number of eggs produced for each parous class was significantly different, however, overlap occurred. Such a system enables a determination of the past, present, and future reproductive status of field populations and mass-rearing colonies.
Keywords: Water hyacinth, ovarian development, follicular relics
Introduction
Salvinia molesta D.S. Mitchell (Salviniaceae) or giant salvinia is a floating fern that causes profound ecological, environmental, and infrastructural problems in more than 20 tropical and subtropical countries.1 It was first reported in North America in 19952 and currently negatively affects at least 90 localities in the Southern United States including Texas, North Carolina, South Carolina, Louisiana, Georgia, Florida, Alabama, Mississippi, and west to Arizona, California, and Hawaii.3 Although a variety of control techniques have been employed,4,5 the most widely successful is the use of a host-specific Curculionidae, Cyrtobagous salviniae Calder and Sands.
Morphology and biology for C salviniae have been described previously in a number of publications.6–8 Females are approximately 2.2 mm × 1.2 mm in length, and males are approximately 1.8 mm × 0.9 mm. Adults are found on or among leaves or underwater among the submersed fronds. Adults engage in multiple matings for 5 to 26 days after emergence. After mating, females typically deposit one egg every 2 to 5 days in shallow holes excavated in plant tissue. Females can continue to oviposit for at least 60 days.
Cyrtobagous salviniae is the only biocontrol agent that has been shown to have a significant impact throughout most of the introduced range of S molesta.9,10 Dramatic impacts have been documented in many locations across the world.5 However, the weevil has failed to provide a consistent level of control in giant salvinia’s more temperate range11 or under climatic extremes such as drought.12 In other cases, reasons for lack of control by the weevil are unknown.13 Several factors that have an impact on the establishment and population growth of C salviniae have been documented. These include low temperatures14,15 and plant nutrition, especially nitrogen levels.16,17 The influence of both temperature and plant nutritional composition has been documented for other biological control species that attack aquatic plant species.18–21 Methodology is needed to allow better assessments of weevil efficacy especially under diverse field conditions.
Although numerous studies have shown the impact of abiotic and biotic factors on C salviniae, no information is available on the morphology of the female reproductive system. Nor have there been any attempts to correlate changes in the reproductive system in relation to egg production. Such information would permit the development of a physiological age grading system that would enhance our understanding of past, present, and future reproductive status in both field and mass-reared colonies. In addition, such a system will provide another methodology to assess the impact of various abiotic factors (eg, temperature and nutrition) on weevil efficacy. Such an understanding may provide valuable insight into establishment failures and lack of efficacy in the more temperate regions. The main objective of this article is to describe the morphology of the female reproductive system and relate changes in morphology to egg production.
Materials and Methods
Materials
Cyrtobagous salviniae used in this study were obtained from a greenhouse colony maintained at Louisiana State University (Baton Rouge, LA, USA) founded with weevils from a field site in Gheens, LA, USA. The colony was produced under greenhouse conditions on salvinia grown in 568-L tanks (Rubbermaid Commercial Products; Rubbermaid Corporation, Winchester, VA, USA) containing reverse osmosis water (produced by model LCRO, 200 gpd; Culligan International Co., Rosemont, IL, USA). The tanks were fertilized twice weekly to maintain a concentration of 2 ppm of nitrogen, using Miracle-Gro Liquid Lawn Food (ScottsMiracle-Gro Company, Marysville, OH, USA). This product has an NPK rating of 36-6-6 with 0.325 chelated iron (0.2 g/L). Bacillus thuringiensis (15% a.i., Thuricide; Bonide Products, Oriskany, NY, USA) was sprayed twice weekly to control larval Samea multiplicalis Guené (Lepidoptera: Crambidae). To ameliorate extremes of temperature, the sides and the rim of each tank were insulated with bubble wrap/foil insulation (Reflectix, Inc., Markleville, IN, USA). Also, fresh plant material was added to culture tanks as needed to replace vegetation consumed by the weevils. High-intensity discharge lamps (models ED28 and ED18; Luna Pro-Lighting, Miami, FL, USA) were used to extend photoperiod to 14:10 (L:D) in the winter and early spring months to ensure availability of high-quality plants needed to maintain reproductive activity of females.
Ovary dissections
Dissections were performed on cohorts of 20 individuals. Teneral individuals, identified by a light brown color and soft cuticle, were initially selected. Cohorts were maintained in small containers containing 150 mL of reverse osmosis–treated water and a single salvinia ramet containing at least one apical tip. The water and ramet for each cohort were replaced weekly. Cohorts were kept at 29.5°C (±0.5°C) with a 12:12 photoperiod (L:D).
Females were removed at random from each cohort and subsequently dissected over the course of 20 weeks. This allowed for an initial description of ovarian morphology and identification of changes in morphology over time and in relation to the number of eggs produced. More than 200 weevils were examined during these initial qualitative studies. Dissection procedures consisted of placing a 5-mm drop of Krazy Glue (Elmer’s Products, Columbus, OH, USA) on a microscope slide, stirred, and allowed to partially dry. Females were placed, ventral side up in the glue and pressed lightly until secured. The glue was then allowed to dry thoroughly before dissection. Secured weevils were covered with phosphate-buffered saline (pH = 7.4). Dissections were performed under a stereo microscope (Leica MZ125; Leica, Inc., Basil, Switzerland) at varying magnifications. The edges of the elytra were carefully cut away from the abdominal tergites using a disposable scalpel. The terminalia was grasped with a forceps and pulled horizontally until the entire reproductive system was exposed. The terminal ligaments were severed using a superfine Vannas Scissors (World Precision Instruments, Sarasota, FL, USA) and the reproductive organs moved to an open area of the slide containing saline. The reproductive organs were often carefully manipulated allowing for observation of all structures. This was accomplished by gently moving the structures with a minuten pin and/or fine blade forceps or manipulating the amount of saline on the slide. The gross morphology of the ovaries was observed and structures were identified using terminology presented in the works by Grodowitz et al.21,22 In addition to identifying gross anatomy, the appearance and number of follicles were quantified and follicular relic deposition was noted and described. Somatic characters were assessed including fat body appearance which was graded as fat (almost entirely filling the hemocoel), intermediate (1/3rd-2/3rd of the hemocoel filled), or lean (<1/3rd of the hemocoel filled). In addition, cuticle color (brown or black) and cuticle hardness (soft or hard) were noted.
Relationships between ovarian morphology and egg number
To correlate the number of eggs with distinct changes in ovarian morphology, a cohort of 100 teneral females were allowed to mate and then held separately as described previously. Ramets were removed from each container weekly, carefully dissected, and number of eggs enumerated. Dissection consisted of removing the leaves and submersed fronds and cutting the stolon into 3- to 6-mm pieces, which were kept moist to prevent desiccation, and examined for eggs. The fronds for each ramet were also pulled apart at the keel and examined using a stereo microscope (Leica MZ75, Leica, Inc.) at ×5 to ×7 magnification. Females were dissected and reproductive system morphologies described as noted previously.
Statistical analysis
Homogeneity of variances was determined using Levene’s test for homogeneity of variances with significant differences noted at P ≤ .05. When variances were determined to be equal, ANOVA and Tukey post-hoc tests were used to determine differences between means (i.e., number of follicles for each physiological age) while Kruskal-Wallis analysis of variance and associated multiple comparisons of mean ranks were used to determine significant differences between number of follicles per ovariole and number of eggs produced for each physiological age.23 Mean values were considered significantly different at P ≤ .05. Relationships between numbers of eggs produced over time were determined using Pearson r or linear product moment correlation with significance determined at P ≤ .05.
Results and Discussion
The reproductive system of the female salvinia weevil is similar to that of the rice weevil, Sitophilus oryzae L.24; the boll weevil, Anthonomus grandis Boheman21; the water hyacinth weevil, Neochetina eichhorniae Warner22; and the granary weevil, Sitophilus granarius L.25 All 4 have ovaries that are meroistic and telotrophic; ie, they have trophocytes (specialized nurse cells) that remain in the germarium and supply nutrients to follicles through a nutritive cord. Although no clear photos of the nutritive cord were obtained, only a few primitive orders have panoistic ovaries (ie, all oogonia, except stem-line oogonia, are eventually transformed to oocytes), and the distinctive clusters of nurse cells found in polytrophic ovaries26 were not present.
The reproductive system of the female salvinia weevil consists of a pair of ovaries, each of which is composed of 2 tubular ovarioles (Figure 1A and B). The ovarioles are joined at the proximal end, where they are connected to the lateral oviduct. The lateral oviducts unite to form the common oviduct through which eggs must pass for oviposition to take place. An ovariole is composed of 2 layers, an outer ovariole sheath (tunica externa) and an inner tunica propria, which form the walls of the ovariole. An ovariole has 2 regions: a distal germarium and a proximal vitellarium.26 Oogonia in the germarium produce the follicles, which are surrounded by epithelial tissues prior to entering the vitellarium.26 The vitellarium is an elongated chamber that houses the developing follicles. As follicles mature, they move toward the lateral oviducts, so a progression of developing follicles develops over time, with newly formed follicles adjacent to the germarium and the most mature follicles proximal to the lateral oviduct. The follicle is initially clear and then becomes translucent as it fills with yolk while developing in the vitellarium. The ovariole walls taper at the proximal end of the vitellarium. According to the work by Tyndale-Biscoe,27 the outer epithelial layer is stripped off as the oocyte exits the vitellarium and enters the lateral oviducts. The retained material is known as a follicular relic (Figure 2). Follicular relics collect at the base of the ovariole, where they eventually form a slim ring that can be seen under relatively low magnification. This is a common occurrence that has been observed in many different insect orders and is often used to identify mature reproductives.27 The ring becomes broader over time. Initially, the follicular relics are pale yellow; eventually, they turn to deeper yellow and then darken to an amber color as more ovipositions occur. After a greater number of ovipositions have taken place, dark specks or particles appear in the follicular relics due to compression from oocytes passing through the narrowed lumen of the ovariole and the accumulated mass of follicular relics at the base of the ovariole.27
Figure 1.

(A) Ovarian morphology of female Cyrtobagous salviniae and (B) details of follicle morphology. co indicates common oviducts; fe, follicular epithelia; flc, follicle; fr, follicular relics; gm, germarium; gv, germinal vesicle; if, interfollicular tissue; lo, lateral oviducts; os, ovariole sheath; ov, ovaries; ovl, ovariole; tf, terminal filament; vt, vitellarium; yk, yolk.
Figure 2.

Close-up of the follicular relics of Cyrtobagous salviniae. e indicates egg; flc, follicle; fr, follicular relics.
Because follicular relics are associated with oviposition, their characters are the main means of differentiating parous classes from each other and from the nonparous (or nulliparous) classes.27 However, other factors may contribute to follicular relic formation that is unrelated to ovulation. For example, females may reabsorb yolk and proteins from their follicles in response to environmental stress.26 In this case, yolk and proteins are removed from the oocyte, and the follicular epithelium collapses into a resorption body which persists in the proximal vitellarium27 and is indistinguishable from ordinary follicular relics.22 Because of this variation, the follicular relics may only be used to group individuals into classes on the basis of shared characteristics, such as follicular relics that encircle the base of the vitellarium, or dark specks in the follicular relics. They cannot be used to determine the exact number of ovipositions that has occurred but can be useful in ascertaining relative categories of egg production.22
The spermathecal duct (Figure 3A and B) enters the common oviduct about halfway along its length. As the egg passes along the common oviduct, sperm is released from the spermatheca and the egg is fertilized.26 The duct is connected to a light brown, sclerotized, c-shaped spermatheca that stores sperm. It terminates in a bilobed, pouch-like structure. The presence of masses of motile sperm may be confirmed through the spermathecal walls under magnification of ×100 to ×160, although sperm may be visible at lower magnifications. Muscle bands are attached to the spermatheca and a spermathecal gland is present arising from the proximal portion of the spermatheca. In other species, the spermathecal gland is known to secrete a fluid in which the sperm is conveyed to eggs in the common oviduct, aided by the contraction of the muscle bands.26 There are no apparent accessory glands.
Figure 3.
(A, B) The spermatheca of Cyrtobagous salviniae is a sclerotized pouch-like structure which houses the sperm until fertilization occurs. The cloudy material within the spermatheca is sperm. mb indicates muscle bands; spt, spermatheca; sptd, spermathecal duct; sptg, spermathecal gland.
Fat body typically fills the hemocoel and obscures the organs in the nulliparous classes of reproductive development. Fat body in teneral females appears creamy white or ivory and is present in a smooth semisolid form. As the individual ages and produces follicles, the fat body decreases, occupies a smaller portion of the hemocoel, and becomes distinctly granular or “clumped.” It may also take on a greenish cast in many individuals.
Changes in ovarian morphology were observed with age and higher number of ovipositions. This continuum can be divided into a series of physiological classes and their diagnostic characters are listed in Table 1. Females in the first nulliparous or N1 class can be identified by the absence of visible follicles within the ovariole, which is a slim, clear tube with no clear differentiation between germarium and vitellarium (Figure 4A). Females in the N1 class have fat body that obscures the internal organs and appears creamy white. A secondary characteristic is a soft brown cuticle, indicating recent (ie, less than 1 week) emergence. Sclerotization of the cuticle is a gradual process that takes 5 to 6 days, so some individuals had both black and brown areas of a partially sclerotized cuticle.
Table 1.
Characteristics associated with the nulliparous (N1, N2) and parous (P1, P2, P3) age classes for female Cyrtobagous salviniae.
| Characteristics | N1 | N2 | P1 | P2 | P3 |
|---|---|---|---|---|---|
| Reproductive system characteristics | |||||
| No. of follicles per ovariolea (mean ± SE) | 0 | 3.9 ± 2.1 (5; a) | 8.0 ± 1.2 (10; b) | 7.7 ± 1.6 (15; b) | 6.1 ± 2.7 (29; b) |
| Eggs present in oviducts | No | No | Yes | Yes | Yes |
| Follicular relics present | No | No | Yes | Yes | Yes |
| Follicular relics encircling ovariole base | No | No | No | Yes | Yes |
| Inclusions present in follicular relics | No | No | No | No | Yes |
| Sperm present in spermatheca | No | Yes/No | Yes | Yes | Yes |
| Somatic characteristics | |||||
| Fat body | Fat | Fat | Intermediate | Lean | Lean |
| Cuticle hardness | Soft | Soft | Hard | Hard | Hard |
| Cuticle color | Brown | Brown | Black | Black | Black |
Mean values followed by the same letter are significantly different at P ≤ .05 (based on Tukey analysis).
Number of follicles for each physiological age are significantly different at P < .00018 (df = 3, 58; F = 5.67) (based on ANOVA).
Figure 4.

The nulliparous classes of Cyrtobagous salviniae: (A) N1—note the reduced length and lack of ovariole differentiation, ie, no developing follicles present and (B) N2—ovarioles are differentiated with distinct follicles present. flc indicates follicle.
Follicles begin to appear in the vitellarium during the N2 class and enlarge as they accumulate yolk (Figure 4B). There is a clear differentiation between germarium and vitellarium. A continuum develops over time, with less developed (ie, immature) follicles located toward the distal portion of the ovariole, and more developed follicles closer to the proximal end of the ovariole, near the oviducts. The mean number of follicles in N2 ovarioles is 3.9 ± 2.1 (mean ± SE; n = 5; Table 1). Fat body remains smooth and creamy and still partially obscures the organs. As ovulation has not taken place, no follicular relics are present.
In the P1 class, follicular relics are pale yellow and do not completely encircle the ovariole base (Figure 5A). Initially, follicular relics loosely occupy a region at the base of the ovariole. The ovarioles contain a progression of developing follicles with more mature ones located at the proximal portion of the ovariole. A mature P1 female averages 8.0 ± 1.2 (mean ± SE; n = 10) follicles per ovariole (Table 1). Eggs may be present in the lateral or common oviducts. The fat body has a granular or clumped appearance and is intermediate in quantity (only occupies half the abdominal hemocoel). The cuticle is fully sclerotized (hardened and black).
Figure 5.
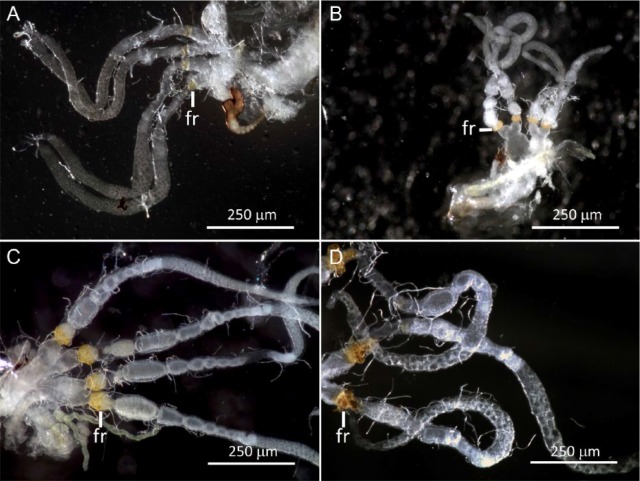
The parous classes of Cyrtobagous salviniae: (A) P1—follicular relics do not completely encircle the base of the ovariole, (B) P2—follicular relics encircle the base of the ovariole, (C) P3—dark particles or inclusions present in follicular relics apparently due to their compression from a high number of ovulations, and (D) degenerate ovaries; follicular relics dark, dark particles present and limited number of healthy follicles present. fr indicates follicular relics.
Females in the P2 class have follicular relics that fully encircle the base of the ovariole (Figure 5B). They appear typically deeper yellow and broader in width than P1 follicular relics. No dark inclusions (particles) are present. The continuum of developing follicles and the overall appearance of the ovarioles are similar to that of a P1 individual. The ovarioles can contain from 8 to 10 follicles with a mean of 7.7 ± 1.6 (mean ± SE; n = 15) follicles per ovariole (Table 1). Fat body is intermediate to lean, and the cuticle tends to be black and somewhat harder than in the P1 class.
Ovaries that are in the P3 class (Figure 5C) resemble P2 ovaries, except that inclusions (ie, dark particles) are present in the follicular relics. These are caused by continual compression of the follicular relics from the large number of follicles that have shed their epithelial tissue as they passed out of the ovariole. There may be ovulated eggs in the lateral oviducts and the common oviduct. Fat body is lean (little or no visible fat body), and the cuticle is hard and black. There may be as many as 8 to 11 follicles within the ovariole with a mean of 6.1 ± 2.7 (mean ± SE; n = 29; Table 1).
However, as weevils approached 20 weeks of age, the number of follicles declined sharply as individuals began to exhibit signs of senescence (Figure 5D). The degenerate ovaries of aged individuals (in the late stages of the P3 class) contain few, if any, healthy follicles. Instead, these individuals showed signs of declining reproductive capacity, such as grossly enlarged germaria, fewer healthy follicles, unyoked or misshapen follicles, follicles that were absent from or not maturing in sequence, and narrowed vitellaria with very dense follicular relics. Senescent follicular relics cannot be reliably distinguished from follicles that have been reabsorbed, which usually takes place due to inadequate nutrition.
Differences in mean number of ovipositions were noted for each parous age class with highest number of ovipositions associated with the P3 class (Figure 6). Mean oviposition values for the 3 parous classes were significantly different (F2,92 = 90.4, P < .0001) with over 9-fold higher ovipositions for the P3 class in comparison with the P1 class. However, overlap occurred for each class as indicated by examination of the range. The mean number of eggs produced for P1 and P2 age class was significantly different from the P3 age class at P < .05. A strong positive correlation (P < .05, r = .881, n = 20) was observed between number of ovipositions and chronological age (Figure 7). This is not unexpected. It has been documented that physiological age and chronological age are not directly related unless temperature and nutrition are controlled which they were in these experiments.24
Figure 6.

Mean number and range of ovulations/ovipositions for each parous age class for Cyrtobagous salviniae. Numbers above the min/max lines are the actual min/max values.
Figure 7.

Correlation between mean weekly counts of number of eggs oviposited by Cyrtobagous salviniae (P < .05, r = .868, n = 20).
Over 97% of the total ovipositions had taken place by the 18th week, and many of those left alive had degenerate ovaries when they were dissected. At 2 months (8 weeks), most of the weevils were entering the P3 class. The number of ovipositions per weevil per week varied widely. Some weevils did not oviposit during a weekly period, in contrast to the findings of Sands et al28 who reported that oviposition was continuous in all individuals. The highest weekly number of ovipositions by a female was 63. This was unexpected, as there are relatively few studies that address oviposition, and there are no reports of a weevil ovipositing 9 eggs a day, except those maintained on salvinia that was “very rich in nitrogen” for short periods.28 Weevils in this study oviposited up to 15 times as many eggs as reported by Forno et al19 in their 60-day study. Female weevils oviposited 32.1% of their eggs in the keel of the leaf. There is only a passing mention of this location in the literature, except for Jayanth,29 who reported finding 59.3% of eggs in the keel of the leaf in his fecundity study. The most common site for oviposition was in the rhizomes of mature ramets. Very few eggs were located in buds or young rhizomes, which are often consumed by larvae and adults. We found no eggs on the outside of leaves or on roots.
Physiological age grading techniques can be used to obtain data on population age structure27 or gain insight into the reproductive health of a population. Some of this information, for instance, assessments of reproductive health, may be difficult or impossible to obtain by other methods.30 For example, Grodowitz et al22 described the reproductive morphology of N eichhorniae and devised an age grading system that allowed the assessment of the reproductive age structure of field populations. With this information, it was possible to discern that parous females were at their highest levels in March and decreased to low levels in June before rising again in September. There was no apparent resorption of follicles, an indication that host plant nutrition was adequate. Tyndale-Biscoe et al,27 working with Onthophagus granulatus Boheman, identified a number of important factors related to population dynamics by quantifying the distribution of individuals in various reproductive classes. These included threshold temperatures for development, optimal brood temperature, and drought-related stress, by quantifying the distribution of individuals in various reproductive classes. Guillot et al31 reported that parous females of a population of horn flies, Haematobia irritans L., were less likely to disperse than newly mated flies, and that immigrant populations of flies had different age structures. Spurgeon et al32 related female reproductive development, fat body characters, and environmental conditions to the onset of diapause in the boll weevil, A grandis.
A baseline study that links the physiological development and oviposition of C salviniae provides a powerful tool for research and understanding the potential impact of a biocontrol agent. In the case of C salviniae, whose efficacy regarding S molesta is a function of its population growth, information on fecundity, life span, and reproductive development can provide valuable insight about present and potential efficacy against the target organism. Such techniques can be applied to mass-reared colonies to maximize the release of individuals in prime (ie, early parous) reproductive condition. Assessing the physiological age of field populations will allow a more refined investigation of reproductive age structure that could provide information on past and present reproductive health as well as potential future impact. Information from physiological age grading studies could be used to anticipate potential problems related to establishment and growth of field populations. This may be especially important in understanding populations that fail to establish in the more temperate range of giant salvinia.
Acknowledgments
The authors thank Dr Wendell Lorio, our cooperator at the Gheens, LA, field site, who provided samples and fresh salvinia to maintain the colony. Technical assistance and discussions on the conclusions are attributed to Katheryn Parys. They also thank industrious student workers, Ming Wang, Katie (Clarke) Kogler, Alan Mouton, Kiah Williams, and Kelli Jo Richard. Without their dedication, it would have been impossible to conduct a study of this size. Thanks are also given to Eric Riddick and Ted Center for critical review of the manuscript. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported by Louisiana Department of Wildlife and Fisheries, US Army Engineers Research and Development Center, and Aquatic Plant Control Research Program.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: LE, SJ, and MJG conceived and designed experiments; contributed to writing the manuscript; agree with manuscript results; made critical revisions; and approved the final version. LE and MJG analyzed the data; jointly developed the structure and arguments for the paper. LE wrote the first draft. All authors reviewed and approved the final manuscript.
References
- 1. Storrs MJ, Julien MH. Salvinia: A Handbook for the Integrated Control of Salvinia molesta in Kakadu National Park. (Northern Landscapes Occasional Papers No. 1). Darwin, NT, Australia: Australian Nature Conservation Agency; 1996. [Google Scholar]
- 2. Johnson D. Giant salvinia found in South Carolina. Aquatics. 1995;17:22. [Google Scholar]
- 3. Jacono CC, Davern TR, Center TD. The adventive status of Salvinia minima and Salvinia molesta in the Southern U.S. and the related distribution of the weevil Cyrtobagous salviniae. Castanea. 2001;66:214–226. [Google Scholar]
- 4. Tasker AV. USDA demonstration project: giant Salvinia (Toledo Bend Reservoir and Surrounding Areas in Louisiana and Eastern Texas. Environmental Assessment, March 2001). http://www.aphis.usda.gov/plant_health/ea/downloads/salvinia.pdf. Accessed May 31, 2017.
- 5. Mcfarland DG, Nelson LS, Grodowitz MJ, Smart RM, Owens CS. Salvinia molesta DS Mitchell (Giant Salvinia) in the United States: A Review of Species Ecology and Approaches to Management. (ERDC/EL SR-04-2). Vicksburg, MS: U.S. Army Engineer Research and Development Center; 2001. [Google Scholar]
- 6. Calder AA, Sands DPA. A new Brazilian Cyrtobagous hustache (Coleoptera: Curculionidae) introduced into Australia to control salvinia. J Aust Entomol Soc. 1985;24:57–64. [Google Scholar]
- 7. Forno IW, Sands DPA, Sexton W. Distribution, biology and host specificity of Cyrtobagous singularis Hustache (Coleoptera: Curculionidae) for the biological control of Salvinia molesta. Bull Entomol Res. 1983;73:85–95. [Google Scholar]
- 8. Sands DPA, Schotz M, Bourne AS. The feeding characteristics and development of larvae of a salvinia weevil Cyrtobagous sp. Entomol. Exp Appl. 1983;34:291–296. [Google Scholar]
- 9. Julien MH, Center TD, Tipping PW. Floating fern (Salvinia). In: Van Driesche R, Blossey B, Hoddle M, Lyon S, Reardon R, eds. Biological Control of Invasive Plants in the Eastern United States (Forest Health Technology Enterprise Team). Morgantown, WV: USDA Forest Service Publication; 2002:17–32. [Google Scholar]
- 10. Thomas PA, Room PM. Successful control of the floating weed Salvinia molesta in Papua New Guinea: a useful biological invasion neutralizes a disastrous one. Environ Conserv. 1986;13:242–248. [Google Scholar]
- 11. Hennecke B, Postle L. The key to success: an investigation into oviposition of the salvinia weevil in cool climate regions. Paper presented at: 15th Australian Weeds Conference Papers & Proceedings; September 24-28, 2006; Adelaide, SA, Australia. [Google Scholar]
- 12. Tipping PW, Martin MR, Center TD, Davern TM. Suppression of Salvinia molesta Mitchell in Texas and Louisiana by Cyrtobagous salviniae Calder and Sands. Aquat Bot. 2008;88:196–202. [Google Scholar]
- 13. Skeat A. Biological control of Salvinia molesta in Kakadu National Park, Northern Territory. Paper presented at: Proceedings of the Ninth Australian Weeds Conference; August 6-10, 1990; Adelaide, SA, Australia. Council of Australian Weed Science Societies: 130–133. [Google Scholar]
- 14. Forno IW, Bourne AS. Feeding by adult Cyrtobagous salviniae on Salvinia molesta under different regimes of temperature and nitrogen content and the effects on plant growth. BioControl. 1985;30:279–286. [Google Scholar]
- 15. Forno IW, Bourne AS. Temperature-related effects of three insects on growth of Salvinia molesta in Brazil. Entomophaga. 1986;31:19–26. [Google Scholar]
- 16. Room PM, Gunatilaka GA, Shivanathan P, Fernando IVS. Control of Salvinia molesta in Sri Lanka by Cyrtobagous salviniae. Paper presented at: Proceedings of the VII International Symposium on Biological Control of Weeds; September 11-16, 1989; Rome, Italy. [Google Scholar]
- 17. Sands DPA, Schotz EM, Bourne FAS. A comparative study on the intrinsic rates of increase of Cyrtobagous singularis and C. salviniae on the water weed Salvinia molesta. Entomol Experiment et Applic. 1986;42:231–237. [Google Scholar]
- 18. Center TD, Dray FA. Associations between Waterhyacinth Weevils (Neochetina Eichhorniae and N. Bruchi) and phenological stages of Eichhornia Crassipes in Southern Florida. Florida Entomol. 1992;75:196–211. [Google Scholar]
- 19. Center TD, Dray FA, Jubinsky GP, Grodowitz MJ. Biological control of water hyacinth under conditions of maintenance management: can herbicides and insects be integrated? Environ Manage. 1999;23:241–256. [DOI] [PubMed] [Google Scholar]
- 20. Center TD, Dray FA. Bottom-up control of water hyacinth weevil populations: do the plants regulate the insects? J Appl Ecol. 2010;47:329–337. [Google Scholar]
- 21. Grodowitz MJ, Smart M, Doyle RD, et al. Hydrellia pakistanae and H. balciunasi, insect biological control agents of hydrilla: boon or bust? Paper presented at: XI International Symposium on Biological Control of Weeds; April 28-May 2, 2004; Canberra, ACT, Australia. [Google Scholar]
- 22. Grodowitz MJ, Brewer FD. Ovarian anatomy and physiological age-grading of the female boll weevil, Anthonomus grandis grandis Boheman (Coleoptera: Curculionidae). Ann Entomol Soc Am. 1987;80:642–651. [Google Scholar]
- 23. Grodowitz MJ, Center TD, Freedman JE. A physiological age-grading system for Neochetina eichhorniae (Warner) (Coleoptera: Curculionidae), a biological control agent of water hyacinth, Eichhornia crassipes (Mart.) Solms. Biol Contr. 1997;9:89–105. [Google Scholar]
- 24. Dell Inc. Dell Statistica (Data Analysis Software System), version 13. software.dell.com. Austin, TX: Dell Inc; 2015. http://www.onthehub.com/statistica/. Palo Alto, CA: TIBCO Software Inc; 2017. [Google Scholar]
- 25. Perez-Mendoza J, Throne JE, Baker JE. Ovarian physiology and age-grading in the rice weevil, Sitophilus oryzae (Coleoptera: Curculionidae). J Stored Prod Res. 2004;40:179–196. [Google Scholar]
- 26. Richards OW. Observations on grain-weevils, Calandra (Col. Curculionidae), general biology and oviposition. J Zoology. 1947;117:1–43. [Google Scholar]
- 27. Chapman RF. The Insects: Structure and Function. 4th ed. Cambridge, UK: Cambridge University Press; 1998. [Google Scholar]
- 28. Tyndale-Biscoe M. Age-grading methods in adult insects: a review. Bull Entomol Res. 1984;74:341–377. [Google Scholar]
- 29. Sands DPA, Schotz M, Bourne AS. A comparative study on the intrinsic rates of increase of Cyrtobagous singularis and C. salviniae on the water weed Salvinia molesta. Entomol Exp Appl. 1986;42:231–237. [Google Scholar]
- 30. Jayanth KP. Studies on the fecundity and longevity of Cyrtobagous salviniae (Coleoptera: Curculionidae), an effective biocontrol agent of Salvinia molesta in India. J Biol Control. 1989;3:62-64. [Google Scholar]
- 31. Hayes EJ, Wall R. Age-grading adult insects: a review of techniques. Physiol Entomol. 1999;24:1–10. [Google Scholar]
- 32. Guillot FS, Miller JA, Kunz SE. The physiological age of female horn flies (Diptera: Muscidae) emigrating from a natural population. J Econ Entomol. 1988;81:555–561. [DOI] [PubMed] [Google Scholar]
- 33. Spurgeon DW, Sappington TW, Suh CP. A system for characterizing reproductive and diapause morphology in the boll weevil (Coleoptera: Curculionidae). J Entomol Sci. 2003;42:320–328. [Google Scholar]