Abstract
The magnetic particles have a significant influence on the immunoassay detection and cancer therapy. Herein, the chemiluminescence immunoassay combined with the magnetic particles (MPCLIA) was presented for the clinical determination and analysis of human epididymis protein 4 (HE4) in the human serum. Under the optimized experiment conditions, the secure MPCLIA method can detect HE4 in the broader range of 0–1000 pmol/L, with a lower detection limit of 1.35 pmol/L. The satisfactory recovery rate of the method in the serum ranged from 83.62% to 105.10%, which was well within the requirement of clinical analysis. Moreover, the results showed the good correlation with enzyme-linked immunosorbent assay (ELISA), with the correlation coefficient of 0.9589. This proposed method has been successfully applied to the clinical determination of HE4 in the human serum.
Keywords: Chemiluminescence immunoassay, Magnetic particles, Human epididymis protein 4
Highlights
-
•
A high-sensitivity, relatively simple and rapid MPCLIA has been successfully developed for the clinical determination of HE4 in human serum.
-
•
The quantitative determination for HE4 in the serum samples was with satisfactory recoveries ranging from 83% to 105%.
-
•
The magnetic particle-based chemiluminescence immunoassays could be used for the quantitative detection of other tumor markers.
-
•
This assay provides apparent advantages and shows great potential in the clinical diagnosis.
1. Introduction
Epithelial ovarian cancer, which is one of the most commonly diagnosed gynecologic malignancy and the highest mortality rate, threatens the human health and life quality [1], [2], [3], [4], [5], [6]. Therefore, timely screening and detecting ovarian cancer in the earlier stage could be the significant approach to reduce the mortality. However, lack of clinical symptoms and due to the low incidence in early stage hinder the occurrence of detection sensitivity [7], [8]. Since the early and accurate prognosis analysis is the fundamental premise to improve the survival rates of patients with ovarian cancer, therefore it is highly demanded biomarkers with higher diagnostic accuracy, and setting up the sensitive and reliable analytical methods to monitor ovarian cancer in a timely and accurate way in patients.
Recently, human epididymis protein-4, also named as whey-acidic-protein four-disulfide core protein-2 (WFDC2), is one of the most promising new biomarkers [9], [10] and has been approved by the Food and Drug Administration (FDA) as the sensitive serum biomarkers for the early diagnosis and monitoring of epithelial ovarian cancer. To date, various analysis methods have been widely established for the serum HE4 detection, including enzyme-linked immunosorbent assay (ELISA), electrochemiluminescence enzyme immunoassay, time-resolved fluoroimmunoassay (TRFIA), amplified luminescent proximity homogeneous immunoassay (AlphaLISA) [11], [12], [13], [14]. Despite the considerable advancements in technology, many disadvantages still exist. ELISA and TRFIA are inferior regarding of sensitivity and accuracy. Hence, a more sensitive and convenient screening method is required in the clinical diagnoses.
Currently, chemiluminescence immunoassay (CLIA) is recognized as the most sensitive classical method in immunologic diagnosis [15], [16], with the significant merits of high sensitivity, low noise, broader linearity, reduced assay time, free of radioactive reagents, and easy to use. Meanwhile, the light intensity of chemiluminescence (CL) reaches its maximum within 1–2 min after substrate addition, thus shortening the overall analytical procedure when compared with the conventional colorimetric assays. CLIA has also been widely used in the clinical detection of tumor markers, such as alpha-fetoprotein (AFP), prostate-specific antigen (PSA), carbohydrate antigen 125 (CA125), and neuron-specific enolase (NSH) [17], [18], [19], [20]. At the same time, to further improve the detection efficiency and detection time, magnetic particles were introduced. Magnetic particles, which equipped with micron- or nano-scale iron oxide as the core component, magnetic particles can rapidly aggregate under an external magnetic field. When the external magnetic field is removed, the magnetic particles will be re-suspended in solution, which dramatically reduces the cleaning time and easy to automate. On the other hand, MPs could possess remarkable advantages, containing the large surface area, magnetic susceptibility, low toxicity, low cost of synthesis, compatibility with biomaterials and easy to separate from the matrix.
In this work, HE4-antibody(Ab)-alkaline phosphatase (ALP) were prepared by utilizing an improved labeling method with higher efficiency. By combining the monoclonal antibody-coated magnetic beads, a specific and sensitive determination for HE4 was developed by chemiluminescence immunoassays. The specificity and repeatability of the immunoassay were investigated by the cross-reactivity and the recycle experiments. The results obtained were in an excellent linear relationship with those from commercial ELSA kits.
2. Materials and methods
2.1. Chemicals, buffers, calibrators, and samples
1-ethyl-3-(3-dimethylaminopropyl)-carbodimide hydrochloride (EDC), N-Hydroxysuccinimide (NHS), bovine serum albumin (BSA), Dimethyl sulfoxide(DMSO), O-(carboxymethyl) hydroxylamine hemihydrochloride (CMO), N, N-dimethylformamide (DMF), 2-Aminoethanol were provided from Sigma-Aldrich, Sephadex G-25 was bought from GE Health Life Sciences. Alkaline phosphatase (ALP) was procured from BBI Enzymes. 4-(N-Maleimidomethyl) cyclohexanecarboxylic acid N-hydroxysuccinimide ester was purchased from Thermo. Lumigen APS-5 was purchased by Lumigen, Inc. tris (hydroxymethyl) aminomethane hydrochloride was from Sinopharm Chemical Reagent Co., Ltd. All chemicals were used without further treatment.
The phosphate buffer (pH 7.4) contained the 0.01 M KH2PO4 and 0.01 M Na2HPO4; the washing buffer (PBST): PBS buffer with 0.5 mL/L Tween 20.
The commercial HE4 ELISA assay kit was bought from Cusabio Biotech. Co., LTD. CEA, AFP, carbohydrate antigen 125 (CA125), carbohydrate antigen 153 (CA153), carbohydrate antigen199 (CA199) were bought from HyTest biotech Co. LTD. Elafin, Secretory leukocyte protease inhibitor(SLPI) were purchased from US Biological Life Sciences (America), Bilirubin, Hemoglobins, Triglycerides were bought from Solarbio Science &Technology Co., LTD. Rheumatoid factor was provided from Beijing Labo Biotech. Co., LTD.
2.2. Instrumentation
The immunomagnetic particles were provided by Merck (Beijing, China), which the diameter was 1.0 µm. The magnetic separator was provided by the Tianjin Baseline Chromtech Research Centre (Tianjin, China). The chemiluminescence analyzer (BHP9507, Beijing Hamamatsu Photon Techniques Inc, China) were utilized to estimate the chemiluminescence (CL) signal.
2.3. Methods
2.3.1. Preparation of monoclonal antibody-coated magnetic beads
The conjugates of the magnetic beads and antibody were prepared as follows. To activate carboxyl groups on the beads, 230 μL fresh EDC (10 mg/mL) and 260 μL NHS (10 mg/mL) were added to 2 mg magnetic beads in 1 mL coating buffer, reacting for 30 min at room temperature. The activation solution was then discarded, and the magnetic beads were washed with the solution for several times. Activated magnetic beads have the ability to couple with biological ligands via primary amines.
Afterward, 100 μg monoclonal antibody was added in 1 mL binding buffer and both were mixed with the above activated magnetic beads by gentle rotation (60 rpm) overnight at room temperature. Then the supernatant was removed by the magnetic separator and the beads suspended was blocked. The washing process was repeated three times followed by incubation with 1 mL blocking buffer for 3 h at room temperature. After the final washing step, the antibody-magnetic bead conjugates were resuspended in 5% MCHE-020 buffer and stored at 4 ℃ until required.
2.3.2. Preparation of HE4-Antibody(Ab)-Alkaline phosphatase (ALP)
Before coupling with ALP, HE4-antibody was activated. Briefly, 1 mg antibody in 0.05 M PBS (pH 8.0, 5 mg/mL) was incubated with the 2IT solution at room temperature for 30 min and purified by a Sephadex G-25 column primed by 0.05 M PBS (pH 7.3).
2 mg ALP was activated by 10 μL of 10 mg/mL SMCC for 30 min at room temperature. The activated detection antibody was mixed with the pretreated ALP. Excess functional groups in SMCC were blocked by maleimide (50 μL, 10 mg/mL). The reaction of antibody and ALP was performed in the dark for 12 h at room temperature. The labeled antibody mixture was dialyzed in PBS (pH 6.5) overnight and purified by Sephadex G-25. Finally, the purified labeled antibody was stored at 4 ℃, denoted as HE4-Ab-ALP.
2.3.3. Immunoassay procedure
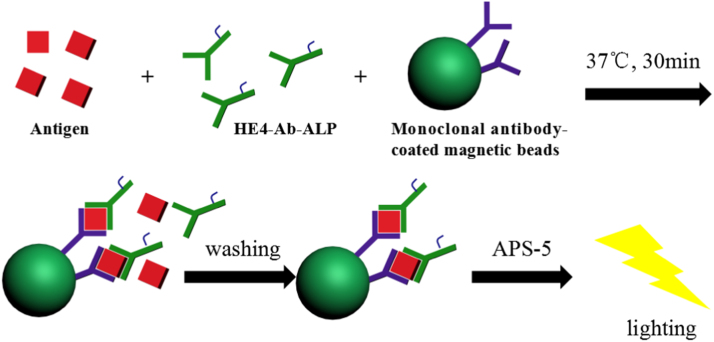
The schematic diagram of the MPCLIA analysis was shown in Fig. 1, first of all, 300 μL of standard solutions or samples, 60 μL monoclonal antibody-coated magnetic beads, 60 μL HE4-Ab-ALP were added into the assigned test tube stepwise, shaking by the oscillator for 30 s, then the mixture was incubated at 37 ℃ for 30 min. In the washing process, the materials were dragged by the magnets in the test tubes rack for the full separation (2 min). The beads were washed five times with 300 μL of washing buffer. After repeating 5 times of washing, 300 μL Lumigen APS-5 solution (substrate solution) was added to each tube, and the chemiluminescence signs were measured immediately.
Fig. 1.
The schematic principle of the magnetic particle based-chemiluminescence immunoassay.
2.3.4. Clinical application and analysis
Clinical samples used in this study were obtained from the Second Affiliated Hospital of Nanchang University. HE4 levels of 60 clinical serum samples were determined by the proposed method and the commercial traditional method (ELISA). Linear correlation analysis was performed. The regression equation and correlation coefficients were used to judge the correlation between two methods.
3. Results and discussion
3.1. Sensitivity, linear range, and hook effect
A sandwich immunoassay was designed. Some experimental conditions, including the concentration of monoclonal antibody-coated magnetic beads and HE4-Ab-ALP, were optimized with the objectives: (1) to study immunoassay performance under the optimized conditions, (2) to improve the immunoassay sensitivity (Table 1).
Table 1.
Optimization of the concentration of magnetic beads and HE4-Ab-ALP.
| Magnetic beads concentration (mg/mL) | HE4 level (pmol/L) | HE4-Ab-ALP (ug/mL) |
||
|---|---|---|---|---|
| 0.05 | 0.1 | 0.2 | ||
| 0.05 | 0 | 784 | 1670 | 2852 |
| 50 | 19,631 | 32,239 | 50,163 | |
| 1000 | 726,893 | 1,561,379 | 1,778,456 | |
| 0.1 | 0 | 1236 | 2365 | 4174 |
| 50 | 38,926 | 53219 | 68,362 | |
| 10,000 | 1,182,536 | 1,957,056 | 2,170,327 | |
| 0.2 | 0 | 3125 | 4721 | 7893 |
| 50 | 55,224 | 83219 | 101120 | |
| 1000 | 2,380,375 | 2,531,973 | 3,039,672 | |
| 0.3 | 0 | 5184 | 7420 | 9405 |
| 50 | 71,027 | 87,932 | 133,032 | |
| 1000 | 2,642,198 | 3,238,745 | 3,125,371 | |
The concentration of monoclonal antibody-coated magnetic beads and HE4-Ab-ALP was optimized to obtain higher CL response for positive group (50 and 1000 pmol/L HE4) and lower CL signal for the blank group. For each HE4 level, as the amount of monoclonal antibody-coated magnetic beads and HE4-Ab-ALP increased, the CL signal for positive groups and blank group also increased. A higher CL ratio was achieved for 0.1 μg/mL HE4-Ab-ALP and 0.1 mg/mL magnetic beads concentration.
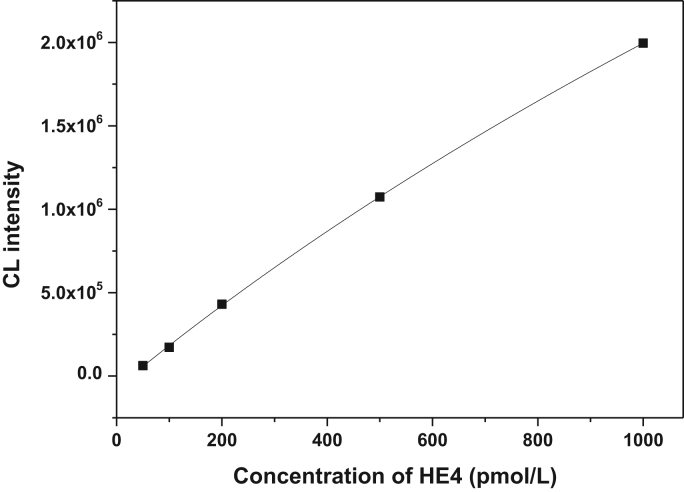
To establish the detection system, the number of magnetic particles, HE4-Ab-ALP was optimized successively according to the above discussion. The standard calibration curve was successfully obtained for RLU values against HE4 concentrations of 0–1000 pmol/L. As shown in Fig. 2, there is a good linearity and the square of the correlation coefficient was 0.9997.
Fig. 2.
Calibration curve of CL intensity versus the concentration of HE4.
The sensitivity of the immunoassay was measured according to the function equation as given below:
| (1) |
where SD was the standard deviation of chemiluminescence intensities obtained with 20 replicate blanks (zero concentration), K is the absolute value of the slope of the calibration curve obtained using the average of the various concentrations of antigen (standards) and blanks. According to the function equation, the calculated analytical sensitivity was 1.35 pmol/L.
3.2. Specificity
The specificity of the immunoassay was studied by cross-reactivity (CR) experiments. The CR was estimated in terms of the percentage of the IC50 of HE4 over the IC50 of several associated with ovarian cancer, including CEA, AFP, CA125, CA153, CA199, Elafin, SLPI. These results were illustrated in Table 2. Since the cross-reactivity for each of these tumor markers in the samples was negligible, we supposed that those tumor markers would not affect the accuracy of results in the clinical diagnosis determination.
Table 2.
Cross-reactivity of variously related tumor markers as determination for HE4 by CLIA.
| Antigen | Tested level | Cross-reactivity |
|---|---|---|
| CEA | 500 μg/L | 0 |
| AFP | 500 μg/L | 0 |
| CA125 | 3000 u/mL | 0 |
| CA153 | 3000 u/mL | 0 |
| CA199 | 3000 u/mL | 0 |
| Elafin | 10,000 pmol/L | ≦ 0.1% |
| SLPI | 10,000 pmol/L | ≦ 0.1% |
3.3. Recovery
HE4 antigen was added (spiked in) to normal human serum at theoretical concentrations of 0, 50 and 200 pmol/L, and the increase in the measured concentration of HE4 for each spiked serum was determined by comparison to normal serum. Recovery rates were calculated by the following formula.
| (2) |
These experiments were analyzed and repeated five times by the MPCLIA method, the final results listed in Table 3 showed that the acceptable and favorable recoveries were ranging from 83.62% to 105.10%. Moreover, each concentration of antigen was diluted two-, three-, and five-fold in normal human serum to assess the linearity of the assay, mean recovery rates in these diluted samples ranged from 82.78% to 103.87% (Table 4). These data were within the requirement of analysis and in good agreement with the previously reported literature [20], revealing the developed method could be reliable and useful as a quantitative tool for the clinical analysis.
Table 3.
Recovery rate of HE4 tested in normal human serum.
| Sample | Added | Detection | Recoveries (%) |
|---|---|---|---|
| Sample 1 | 0 | 15.57 | |
| 50 | 57.55 | 83.96 | |
| 50 | 61.445 | 91.75 | |
| 50 | 66.535 | 101.93 | |
| 50 | 58.92 | 86.70 | |
| 50 | 62.32 | 93.50 | |
| 200 | 181.57 | 83.00 | |
| 200 | 205.17 | 94.80 | |
| 200 | 195.57 | 90.00 | |
| 200 | 190.69 | 87.56 | |
| 200 | 186.21 | 85.32 | |
| Sample 2 | 0 | 208.19 | |
| 50 | 250.87 | 85.36 | |
| 50 | 253.46 | 90.54 | |
| 50 | 250 | 83.62 | |
| 50 | 252.44 | 88.50 | |
| 50 | 251.74 | 87.10 | |
| 200 | 415.19 | 103.50 | |
| 200 | 380.99 | 86.40 | |
| 200 | 403.09 | 97.45 | |
| 200 | 418.39 | 105.10 | |
| 200 | 391.71 | 91.76 | |
| 200 | 388.29 | 90.05 |
Table 4.
Recovery of HE4 tested in normal human serum after two-, three-, and five-fold dilution.
| Dilution | Serum 1 |
Serum 1 |
||||
|---|---|---|---|---|---|---|
| factor | Detection value | Mean value | Recovery(%) | Detection value | Mean value | Recovery(%) |
| 1 | 496.3 | 469.33 | 286.5 | 291.70 | ||
| 473.2 | 312.7 | |||||
| 438.5 | 275.9 | |||||
| 2 | 234.1 | 216.43 | 92.23 | 115.6 | 133.60 | 91.60 |
| 216.7 | 152.7 | |||||
| 198.5 | 132.5 | |||||
| 3 | 147.5 | 140.33 | 89.70 | 87.6 | 85.67 | 88.10 |
| 129.3 | 85.9 | |||||
| 144.2 | 83.5 | |||||
| 5 | 81.3 | 77.70 | 82.78 | 61.2 | 60.60 | 103.87 |
| 78.6 | 57.5 | |||||
| 73.2 | 63.1 | |||||
3.4. Accuracy
To determine the accuracy of the MPCLIA method, the two samples were repeating detected for 10 times and calculated on a single day; the results were depicted in Table 5. The coefficient of variation (CV) for measuring the concentration of 90 and 600 pmol/L in the sample, was 3.46% and 7.57%, respectively. Both the CV values were less than 10.0%. Such reproducibility was highly acceptable and in favor of the MPCLIA assay.
Table 5.
Coefficient of variation of HE4 from spiked samples.
| HE4 added (pmol/L) | HE4 detection (pmol/L) | CV% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 90 | 92.39 | 86.52 | 85.77 | 84.28 | 90.03 | 92.36 | 92.90 | 89.73 | 89.19 | 92.33 | 3.46 |
| 600 | 602.41 | 560.33 | 555.35 | 559.95 | 577.67 | 686.21 | 639.02 | 636.64 | 661.42 | 622.42 | 7.57 |
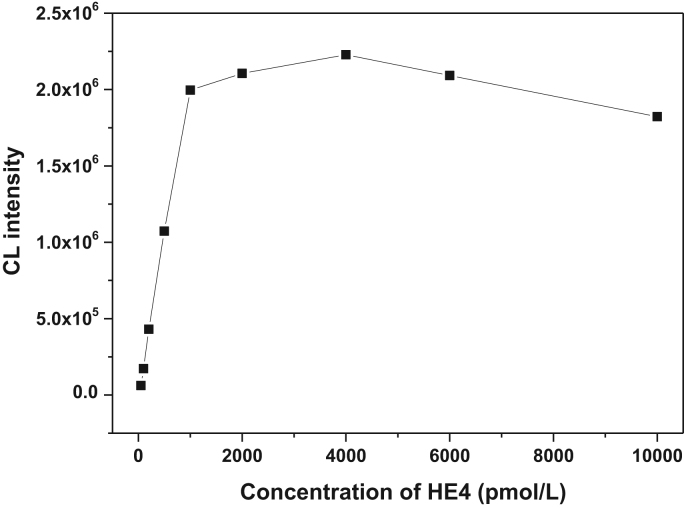
3.5. Hook effect
Hook effect is an issue that plagues many sandwich immunoassays measuring analytes at high concentrations, which resulted in false negatives or inaccurately low results. Hence, we need to seek the hook effect point by increasing the dose of the target examined sample to improve the detection accuracy. As shown in Fig. 3. Nonlinearity of the signal became pronounced at 1000 pmol/L and the signal decreased at 6000 pmol/L.
Fig. 3.
Evaluation of the hook effect.
3.6. Interference
To determine factors of serum interference in the assay, the known antigen concentrations were prepared in normal serum with the addition of common serum constituents known to cause interference in immunoassays: bilirubin (200 mg/mL), hemoglobins (500 mg/mL), triglycerides (1000 mg/mL) and rheumatoid factor (1000 IU/mL), respectively. The mean value and detection deviation ware displayed in Table 6. Thus, the proposed method was determined to exhibit minimal interference caused by these substances.
Table 6.
Interference of added bilirubin, hemoglobins, triglyceride, and rheumatoid factor in serum samples.
| Interference | Serum 1 |
Serum 2 |
||||
|---|---|---|---|---|---|---|
| Detection value | Mean value | Detection deviation (%) | Detection value | Mean value | Detection deviation (%) | |
| No Interference | 136.82 | 128.70 | 362.89 | 390.76 | ||
| 118.29 | 378.60 | |||||
| 130.98 | 430.80 | |||||
| Bilirubin (concentration: 200 mg/mL) | 140.08 | 127.27 | − 1.11 | 362.23 | 404.78 | 3.59 |
| 123.10 | 415.95 | |||||
| 118.64 | 436.16 | |||||
| Hemoglobins (concentration: 500 mg/mL) | 123.30 | 123.56 | − 3.99 | 439.01 | 442.35 | 13.20 |
| 130.15 | 404.04 | |||||
| 117.24 | 484.00 | |||||
| Triglyceride (concentration: 1000 mg/mL) | 130.94 | 131.94 | 2.52 | 380.72 | 367.14 | −6.04 |
| 122.35 | 383.18 | |||||
| 142.54 | 337.52 | |||||
| Rheumatoid factor (concentration: 1000 IU/mL) | 129.57 | 137.81 | 7.08 | 364.16 | 413.87 | 5.92 |
| 138.88 | 435.23 | |||||
| 144.99 | 442.24 | |||||
3.7. Correlation of clinical samples tested
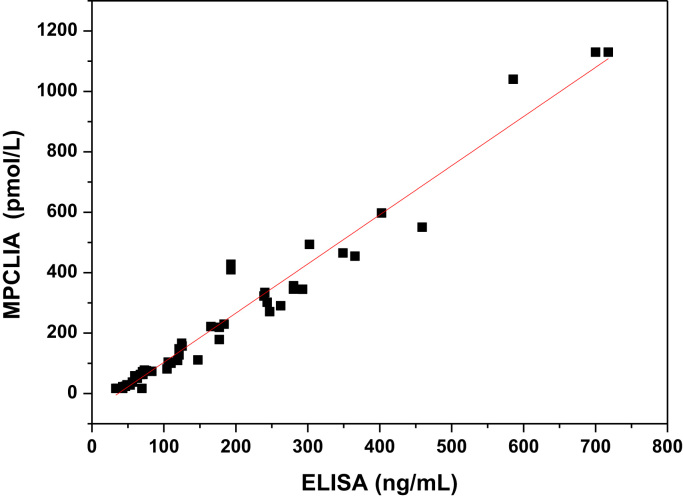
To evaluate this novel assay for clinical applications, 60 serum samples were evaluated by two methods (Fig. 4). The x-axis represented the concentrations of HE4 detected by ELISA, and the y-axis was the concentrations of HE4 detected by MPCLIA. The correlation equation was y = 1.6270x −59.31 and the correlation coefficient was 0.9589. Obviously, the two methods are well correlated. Thus, the MPCLIA method we have established appears to be well suited for use as a clinical diagnostic to detect HE4 in human serum.
Fig. 4.
Correlation between the results measured by the proposed MPCLIA and ELISA.
Acknowledgments
The work is supported by the National Natural Science Foundation of China, China (31370002, 3171101088 and 81301561) and the Provincial Youth Fund of Jiangxi (20142BAB215072).
Acknowledgments
Ethical approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Consent for publication
has been approved by all individual participants.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.01.002.
Appendix A. Transparency document
Supplementary material
References
- 1.Anderson Garnet L. Assessing lead time of selected ovarian cancer biomarkers: a nested case–control study. J. Natl. Cancer Inst. 2010;102(1):26–38. doi: 10.1093/jnci/djp438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drapkin Ronny. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005;65(6):2162–2169. doi: 10.1158/0008-5472.CAN-04-3924. [DOI] [PubMed] [Google Scholar]
- 3.Junhong Guo. Serum CA125, CA199 and CEA combined detection for epithelial ovarian cancer diagnosis&58; a meta-analysis. Open Med. 2017;12(1):131–137. doi: 10.1515/med-2017-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel Rebecca L., Miller Kimberly D., Jemal Ahmedin. Cancer statistics, 2016. CA: Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 5.Speeckaert Marijn M., Speeckaert Reinhart, Delanghe Joris R. Human epididymis protein 4 in cancer diagnostics: a promising and reliable tumor marker. Adv. Clin. Chem. 2013;59(1):21. doi: 10.1016/b978-0-12-405211-6.00001-2. [DOI] [PubMed] [Google Scholar]
- 6.Scaletta Giuseppe. The role of novel biomarker HE4 in the diagnosis, prognosis and follow-up of ovarian cancer: a systematic review. Expert Rev. Anticancer Ther. 2017;17(9):827–839. doi: 10.1080/14737140.2017.1360138. [DOI] [PubMed] [Google Scholar]
- 7.PRESL J.I.R.I. Importance of preoperative knowledge of the biomarker HE4 in early-stage endometrial cancer regarding surgical management. Anticancer Res. 2017;37(5):2697–2702. doi: 10.21873/anticanres.11619. [DOI] [PubMed] [Google Scholar]
- 8.Chang Xiaohong. Human epididymis protein 4 (HE4) as a serum tumor biomarker in patients with ovarian carcinoma. Int. J. Gynecol. Cancer. 2011;21(5):852–858. doi: 10.1097/IGC.0b013e31821a3726. [DOI] [PubMed] [Google Scholar]
- 9.Han Xiao, Zou Chen-chen, Fang Xiang-zhong. Early screening of ovarian cancer. Acta Math. Appl. Sin. English Ser. 2017;33(2):463–474. [Google Scholar]
- 10.Ferraro Simona. Serum human epididymis protein 4 vs carbohydrate antigen 125 for ovarian cancer diagnosis: a systematic review. J. Clin. Pathol. 2013:2012. doi: 10.1136/jclinpath-2012-201031. [DOI] [PubMed] [Google Scholar]
- 11.CHEN Shao-lang. Development of time-resolved fluoroimmunoassay kit for detection of human epididymis protein 4 (HE4) in serum. Asian Pac. J. Trop. Med. 2014;4:012. [Google Scholar]
- 12.Zhou Lijun. Detection of human epididymis protein 4 (HE4) in human serum samples using a specific monoclonal antibody‐based sandwich enzyme‐linked immunosorbent assay (ELISA) J. Clin. Lab Anal. 2016;30(5):581–589. doi: 10.1002/jcla.21906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Hui. Rapid quantitation of human epididymis protein 4 in human serum by amplified luminescent proximity homogeneous immunoassay (AlphaLISA) J. Immunol. Methods. 2016;437:64–69. doi: 10.1016/j.jim.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Fu Xiaoling. Chemiluminescence enzyme immunoassay using magnetic nanoparticles for detection of neuron specific enolase in human serum. Anal. Chim. Acta. 2012;722:114–118. doi: 10.1016/j.aca.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Qiong. The establishment of an HE4‐CLIA method and the combined analysis of HE4 and CA125 in ovarian cancer. J. Clin. Lab Anal. 2016;30(5):709–718. doi: 10.1002/jcla.21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamd-Ghadareh Somayeh. An amplified comparative fluorescence resonance energy transfer immunosensing of CA125 tumor marker and ovarian cancer cells using green and economic carbon dots for bio-applications in labeling, imaging and sensing. Biosens. Bioelectron. 2017;96:308–316. doi: 10.1016/j.bios.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Toit Du, Andrew Stephen. Comparison of 2 human chorionic gonadotropin assays as tumor markers assays. Clin. Chem. 2010;56(9):1502–1503. doi: 10.1373/clinchem.2010.149351. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Xin. A sandwich electrochemiluminescence immunosensor for highly sensitive detection of alpha fetal protein based on MoS2-PEI-Au nanocomposites and Au@ BSA core/shell nanoparticles. Sens. Actuators B Chem. 2017;253:470–477. [Google Scholar]
- 19.Ma Ting. Preparation of an acridinium ester-labeled antibody and its application in goldmag nanoparticle-based, ultrasensitive chemiluminescence immunoassay for the detection of human epididymis protein 4. Micromachines. 2017;8(5):149. [Google Scholar]
- 20.Liu Zhenshi. Magnetic microbead-based enzyme-linked immunoassay for detection of Schistosoma japonicum antibody in human serum. Anal. Biochem. 2010;404(2):127–134. doi: 10.1016/j.ab.2010.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material