Abstract
Background:
To document antithrombotic utilization in patients with nonvalvular atrial fibrillation (NVAF), particularly, recently approved NOACs (nonvitamin K antagonist oral anticoagulants) and warfarin; and identify factors predicting the use of NOACs versus warfarin.
Methods:
A retrospective audit was conducted in an Australian hospital. Data pertaining to inpatients diagnosed with atrial fibrillation (AF) admitted between January and December 2014 were extracted. This included patient demographics, risk factors (stroke, bleeding), social history, medical conditions, medication history, medication safety issues, medication adherence, and antithrombotic prescribed at admission and discharge.
Results:
Among 199 patients reviewed, 84.0% were discharged on antithrombotics. Anticoagulants (± antiplatelets) were most frequently (52.0%) prescribed (two-thirds were prescribed warfarin, the remainder NOACs), followed by antiplatelets (33.0%). Among 41 patients receiving NOACs, 59.0% were prescribed rivaroxaban, 24.0% dabigatran, and 17.0% apixaban. Among patients aged 75 years and over, antiplatelets were most frequently used (37.0%), followed by warfarin (33.0%), then NOACs (14.0%). Compared with their younger counterparts, patients aged 75 years and over were significantly less likely to receive NOACs (14.0% versus 28.0%, p = 0.01). Among the ‘most eligible’ patients (Congestive Cardiac Failure, Hypertension (, Age ⩾ 75 years, Age= 65-74 years, Diabetes Mellitus, Stroke/ Transient Ischaemic Attack/ Thromboembolism, Vascular disease, Sex female[CHA2DS2-VASc] score ⩾2 and no bleeding risk factors), 46.0% were not anticoagulated on discharge. Patients with anaemia (68.0% versus 86.0%, p = 0.04) or a history of bleeding (65.0% versus 87.0%, p = 0.01) were less likely to receive antithrombotics compared with those without these risk factors. Warfarin therapy was less frequently prescribed among patients with cognitive impairment compared with patients with no cognitive issues (12.0% versus 23.0%, p = 0.01). Multivariate logistic regression modelling identified that patients with renal impairment were 3.6 times more likely to receive warfarin compared with NOACs (odds ratio = 3.6, 95% confidence interval = 0.08–0.90, p = 0.03, 60.0% correctly predicted; Cox and Snell R2 = 0.51, Nagelkerke R2 = 0.69).
Conclusion:
Despite the availability of NOACs, warfarin remains a preferred treatment option, particularly among patients with renal impairment. The high proportion of eligible patients still being prescribed antiplatelet therapy or ‘no therapy’ needs to be addressed.
Keywords: anticoagulants, antithrombotics, nonvitamin K antagonist oral anticoagulants, novel oral anticoagulants, stroke, warfarin
Background
Stroke prevention is critical to atrial fibrillation (AF) management, given that the risk of embolic stroke is significantly higher in patients with AF compared with persons without the arrhythmia.1 Numerous studies have confirmed the efficacy of warfarin therapy for long-term stroke prevention.2–4 However, the inherent risk of bleeding, and the complex pharmacology of warfarin, renders decisionmaking complex, such that it requires comprehensive risk–benefit assessment and careful patient management. This ultimately results in its apparent suboptimal use in ‘at-risk’ patients.4–7
The nonvitamin K antagonist oral anticoagulants (NOACs) have recently been made available for thromboprophylaxis in AF.8–11 Aside from maintaining an efficacy and safety profile that is noninferior to warfarin, NOACs have a broader therapeutic index and no dietary vitamin K restrictions, unlike warfarin. Additionally, a key advantage of the NOACs is that they do not require regular monitoring of anticoagulation parameters, unlike the need for regular International Normalized Ratio (INR) testing with warfarin. However, some studies report conflicting views in regard to the value of regular monitoring in NOAC therapy.12–15 Limited access to, or unavailability of, specific antidotes for each of the NOACs, and their relatively higher drug costs, have raised additional considerations about their use among clinicians, patients, and wider health care systems.16–21
The availability of NOACs has expanded the treatment armamentarium for stroke prevention in AF. Although other studies have reported on the utilization of antithrombotic therapies, our study considers prescribing in a particularly unique context, where the availability of NOACs has been delayed, restricted, and somewhat tainted by their initial introduction to practice. Unlike other countries (i.e. the USA and the UK) there was a considerable delay in the approval of the first NOACs (dabigatran) in Australia and GPs had their qualms about the safety and efficacy of these medications even after the Product Familiarization Program. The dearth in information regarding the antithrombotic utilization pattern immediately after the inclusion of NOACs in the Pharmaceutical Benefits Scheme (PBS) led to this study being conducted. This clinical audit might be important in the future to study the changing pattern of NOAC prescription over a period in the Australian setting. Towards the end of 2013, three NOACs (dabigatran, rivaroxaban, apixaban) were listed on the PBS for stroke prevention in patients with nonvalvular atrial fibrillation (NVAF, i.e. excluding patients with AF having valvular anomalies or those who have undergone valve replacement surgery),22–24 increasing their accessibility to prescribers and affordability for patients. Currently, there is limited information about the utilization of NOACs in the local Australian setting, following their approval for use by the Therapeutic Goods Administration.25–27
The aim of this study was to explore the utilization of antithrombotic therapy (i.e. antiplatelets and anticoagulants) in patients with AF in the local Australian setting following the PBS listing of NOACs. Specifically, the objectives of this study were to document the use of antithrombotics in hospitalized patients with AF; report the proportion of patients prescribed NOACs compared with warfarin; and identify the factors predicting the use of NOACs versus warfarin.
Methods
Study setting
A retrospective cohort study was undertaken in a tertiary hospital setting within metropolitan Sydney (New South Wales, Australia); the data were collected during the period 1–12 October 2015. Approval for this study was obtained from Western Sydney Local Health District Human Research and Ethics Committee (reference numbers: LNR/15/WMEAD/156, LNRSSA/15/WMEAD/240), and ratified by the University of Technology Sydney (protocol number: AU/6/D34E110).
Study design
Data were extracted from the medical records of previously hospitalized patients who:
were admitted to any ward between January and December 2014;
had NVAF as a primary or secondary diagnosis (diagnosed at any time from preadmission to discharge), defined per International Classification of Diseases, 10th revision codes I48.0, I48.1, I48.2, I48.91 pertaining to paroxysmal, persistent, chronic, and unspecified AF, respectively (valvular AF was excluded).
A target sample size of 196 was calculated based on the estimated (highest) proportion of patients likely to be prescribed NOACs in the local setting (15% based on previous studies),28 with 95% confidence and a 5% level of precision around the point estimate. To achieve this sample size, and allowing for an overage to account for missing or miscoded records, all admissions with AF (n = 2160; 563 as primary and 1597 as secondary diagnosis) were selected for initial review against the inclusion criteria. Patients were selected using simple randomized sampling (online random number generation).
Data collection
The research pharmacist liaised with hospital’s Health Information Services department to retrieve patients’ medical records from the archive. The research pharmacist reviewed these records (specifically, admission form, patients’ daily progress notes, social history assessment form, discharge medication chart, laboratory reports) to extract the relevant information using a purpose-designed data collection form. The data collection form was based on a tool developed for customized stroke and bleeding risk assessment of patients diagnosed with AF,29 as well as evidence-based guidelines.26,30 The information extracted included patient demographics (age, sex, hospital length of stay), medical conditions (comorbidities, contraindications), medication management issues (cognitive impairment, visual or hearing impairment), social history (residence, family support), medication safety issue (allergies to antithrombotics, adverse drug events, polypharmacy), medication adherence (history of nonadherence), and antithrombotic medication (at admission and discharge) for AF as the primary indication, noting whether any patients were recommended recommencement of antithrombotics following admission for surgical procedures. The extracted data were utilized to calculate the patients’ stroke risk scores (per Congestive Cardiac Failure, Hypertension, Age ⩾ 75 years, Diabetes Mellitus, Stroke/ Transient Ischeamic Attack/ Thrombo-embolism [CHADS2] and CHA2DS2-VASc)31,32 and bleeding risk scores (per Hypertension (>160 mmHg systolic), Renal Disease, Liver disease, History of stroke/ TIA, History of bleeding, Labile INR, Age>65 years, Concomitant use of NSAIDs [HAS-BLED] and Hepatic/ Renal impairment, Alcohol abuse, History of Malig-nancy, Age > 75 years, Low platelet count, History of bleeding, Uncontrolled hypertension, Anaemia, Excessive risk of fall, History of stroke/ TIA [HEMORR2HAGES]),33,34 as all of these scores may be used by clinicians in real-world practice. However, for the purposes of this study, only CHA2DS2-VASc and HAS-BLED were employed for the individualized risk–benefit stratification of patients (as ‘eligible patients’ and ‘special AF populations’) owing to the better risk-predicting abilities of these scores over other scores.35–38 Patients with high stroke risk scores (CHA2DS2-VASc ⩾ 2) and low–intermediate risk of bleeding (HAS-BLED ⩽ 2) were categorized as ‘eligible candidates’ for anticoagulation therapy. Patients with high stroke risk (CHA2DS2-VASc ⩾ 2) and high bleeding risk (HAS-BLED ⩾ 3) were considered to be ‘special AF populations’ requiring specialist guidance.39 Furthermore, patients with a high stroke risk and no risk factors for bleeding (i.e. CHA2DS2-VASc ⩾ 2, and HAS-BLED and HEMMOR2HAGES = 0) were deemed to be the ‘most eligible’ candidates for anticoagulant therapy.
Prior to undertaking the main study, the data collection form was pretested on a small sample of medical records.
Data analysis
The extracted data were entered into SPSS (Statistical Package for the Social Sciences Version 21) using descriptive and inferential statistical analysis. Categorical variables were represented as frequencies and percentages. Continuous variables were summarized using means and standard deviations (SDs) for normally distributed data, or medians and interquartile ranges for nonparametric data. The Pearson χ2 test and Fisher’s exact test were used to determine the relationship between categorical variables. The univariate analysis explored the relationship between the outcome variable (use of warfarin versus NOACs) and independent variables such as individual stroke risk factors, bleeding risk factors, or medication safety and medication management issues. Further, the variables observed to have a significant association were then included in multivariate logistic regression (Forward Wald) modelling to identify factors predicting the use of therapy. A p value of less than 0.05 was considered statistically significant in all analyses.
Results
Among the 215 patients selected for review, 16 were excluded from the study (due to missing information, miscoding of patients with valvular AF as nonvalvular AF, records not being accessible for review at the time of data collection). Finally, 199 patient medical records were included in the study.
Patient characteristics
The mean age of patients was 73.8 (±15.4) years. The primary diagnosis (reason for admission) for 22.1% of patients was acute AF, followed by falls (5.5% of patients), stroke or transient ischaemic attack (TIA) (4.5%), bleeding (3%), and other illnesses or surgery in the remainder.
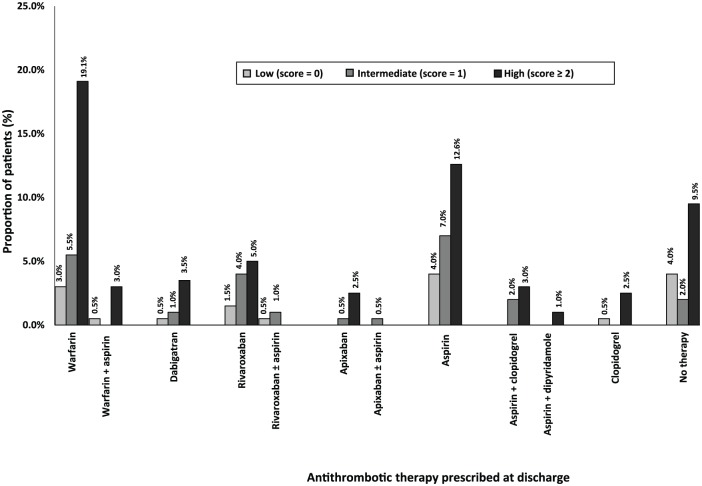
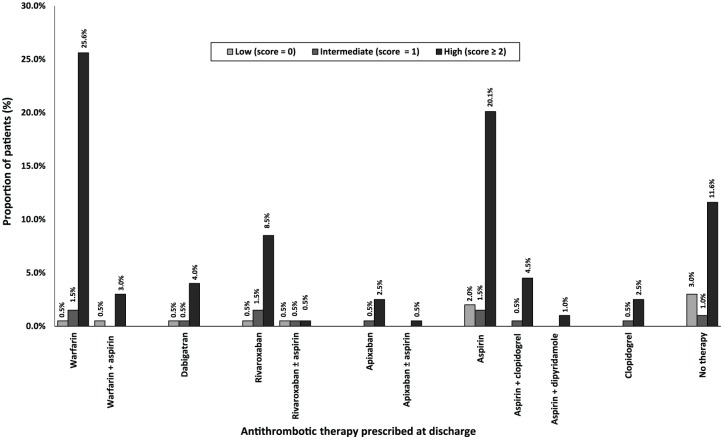
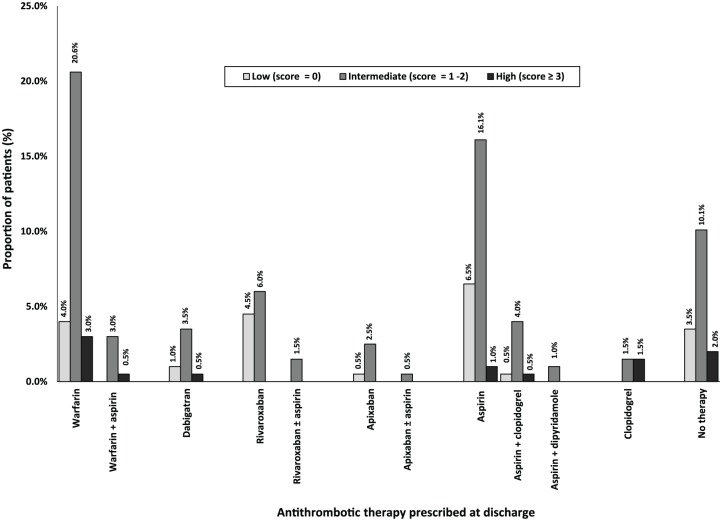
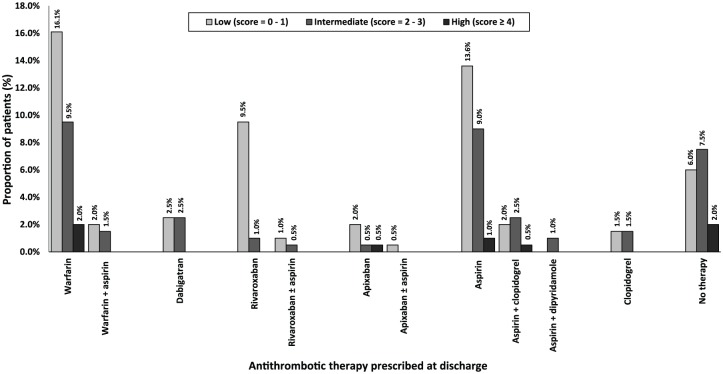
Among all 199 patients, most were assessed as having a high risk of stroke; 123 (61.8%) per CHADS2 score and 168 (84.4%) per CHA2DS2-VASC score (Table 1, Figures 1 and 2). Less than one tenth of all patients were assessed to have a high risk of bleeding; 18 (9.0%) per HAS-BLED score and 12 (6.0%) per HEMORR2HAGES score (Table 1, Figures 3 and 4). Hypertension was the most common risk factor for stroke, while falls risk was the most frequently documented risk factor for bleeding (Table 1). Regarding other medication safety considerations, one patient was reportedly allergic to warfarin, while two patients were reported to be allergic to aspirin.
Table 1.
Distribution of antithrombotic therapy at hospital discharge according to patient characteristics.
| Patient characteristics (% of total, n = 199) | Warfarin ± antiplatelets | NOACs ± antiplatelets | Antiplatelet ± other antiplatelet | No therapy | Total (n = 199) |
|---|---|---|---|---|---|
| Total n (%) | 62 (31.2%) | 41 (20.6%) | 65 (32.7%) | 31 (15.6%) | 199 (100%) |
| Age group | |||||
| <65 years | 12 (6.0%) | 14 (7.0%) | 13 (6.5%) | 10 (5.0%) | 49 (24.6%) |
| 65–74 years | 16 (8.0%) | 13 (6.5%) | 15 (7.5%) | 3 (1.5%) | 46 (23.1%) |
| ⩾75 years | 34 (17.1%) | 14 (7.0%) | 38 (19.1%) | 18 (9.0%) | 104 (52.2%) |
| Sex | |||||
| Male | 33 (16.6%) | 26 (13.1%) | 34 (17.1%) | 15 (7.5%) | 108 (54.3%) |
| Female | 29 (14.6%) | 15 (7.5%) | 31 (15.6%) | 16 (8.0%) | 91 (45.7%) |
| Congestive cardiac failure | 15 (7.5%) | 4 (2.0%) | 15 (7.5%) | 1 (0.5%) | 35 (17.6%) |
| Hypertension | 41 (20.6%) | 29 (14.6%) | 41 (20.6%) | 20 (10.1%) | 131 (65.8%) |
| Diabetes mellitus | 22 (11.05%) | 11 (5.5%) | 15 (7.5%) | 6 (3.0%) | 54 (27.1%) |
| History of stroke/TIA | 13 (6.5%) | 6 (3.0%) | 15 (7.5%) | 6 (3.0%) | 37 (18.6%) |
| Thromboembolic diseases | 7 (3.5%) | 2 (1.0%) | 5 (2.5%) | 6 (3.0%) | 20 (10.1%) |
| Vascular diseases | 29 (14.57%) | 14 (7.0%) | 34 (17.08%) | 8 (3.0%) | 85 (42.7%) |
| CHADS2 | |||||
| High (score ⩾ 2) | 44 (22.1%) | 22 (11.1%) | 38 (19.1%) | 19 (9.5%) | 123 (61.8%) |
| Intermediate (score = 1) | 11 (5.5%) | 14 (7.0%) | 18 (9.0%) | 4 (2.0%) | 47 (23.5%) |
| Low (score = 0) | 7 (3.5%) | 5 (2.5%) | 9 (4.5%) | 8 (4.0%) | 29 (14.5%) |
| CHA2DS2-VASc | |||||
| High (score ⩾ 2) | 57 (28.6%) | 32 (16.1%) | 56 (28.1%) | 23 (11.5%) | 168 (84.3%) |
| Intermediate (score = 1) | 3 (1.5%) | 6 (3.0%) | 5 (2.5%) | 2 (1.0%) | 16 (8.0%) |
| Low (score = 0) | 2 (1.0%) | 3 (1.5%) | 4 (2.0%) | 6 (3.0%) | 15 (7.5%) |
| Cognitive impairment | 4 (2.0%) | 3 (1.5%) | 18 (9.0%) | 9 (4.5%) | 34 (17.0%) |
| Uncontrolled hypertension | 1 (0.5%) | 1 (0.5%) | 2 (1.0%) | 1 (0.5%) | 5 (2.5%) |
| Low platelet | 2 (1.0%) | 1 (0.5%) | 1 (0.5%) | 0 (0.0%) | 4 (2.0%) |
| Anaemia | 4 (2.0%) | 1 (0.5%) | 8 (4.0%) | 6 (3.0%) | 19 (9.5%) |
| Ethanol abuse | 2 (0.5%) | 2 (1.0%) | 3 (1.5%) | 2 (1.0%) | 9 (4.5%) |
| Rebleeding risk | 5 (2.5%) | 2 (1.0%) | 6 (3.0%) | 7 (3.5%) | 20 (10.0%) |
| Excessive falls risk | 10 (5.0%) | 5 (2.5%) | 15 (7.5%) | 8 (4.0%) | 38 (19.0%) |
| Hepatic impairment | 2 (1.0%) | 1 (0.5%) | 1 (0.5%) | 4 (2.0%) | 8 (4.0%) |
| Renal impairment | 17 (8.5%) | 4 (2.0%) | 15 (7.5%) | 9 (4.5%) | 45 (22.5%) |
| Malignancy | 5 (2.5%) | 1 (0.5%) | 3 (1.5%) | 3 (1.5%) | 12 (6.0%) |
| Concomitant use of NSAIDs | 10 (5.0%) | 4 (2.0%) | 14 (7.0%) | 0 (0.0%) | 28 (14.0%) |
| HAS-BLED | |||||
| Low (score = 0) | 8 (4.0%) | 12 (6.0%) | 14 (7.0%) | 7 (3.5%) | 41 (20.5%) |
| Intermediate (score = 1–2) | 47 (23.6%) | 28 (14.1%) | 45 (22.6%) | 20 (10.1%) | 140 (70.4%) |
| High (score ⩾ 3) | 7 (3.5%) | 1 (0.5%) | 6 (3.0%) | 4 (2.0%) | 18 (9.0%) |
| HEMORR2HAGES | |||||
| Low (score = 0–1) | 36 (18.1%) | 31 (15.5%) | 34 (17.1%) | 12 (6.0%) | 113 (56.7%) |
| Intermediate (score = 2–3) | 22 (11.1%) | 9 (4.5%) | 28 (14.1%) | 15 (7.5%) | 74 (37.2%) |
| High (score ⩾ 4) | 4 (2.0%) | 1 (0.5%) | 3 (1.5%) | 4 (2.0%) | 12 (6.0%) |
| Eligible patients (i.e. CHADS2-VASc = high and HAS-BLED = low–intermediate) | 51 (25.6%) | 31 (15.6%) | 51 (25.6%) | 20 (10.0%) | 153 (76.8%) |
| Most eligible patients (i.e. CHADS2-VASc ⩾ 2, HAS-BLED and HEMORR2HAGES = 0) | 6 (3.0%) | 6 (3.0%) | 7 (3.5%) | 2 (1.0%) | 21 (10.6%) |
| Special AF population (i.e. CHADS2-VASc = high and HAS-BLED = high) | 6 (3.0%) | 1 (0.5%) | 5 (2.5%) | 3 (1.5%) | 15 (7.5%) |
Uncontrolled hypertension defined as ‘systolic blood pressure (SBP) >160 mm Hg’. Renal impairment defined as ‘the presence of chronic dialysis or renal transplantation or serum creatinine ⩾ 200 µmol/l’. Hepatic impairment defined as ‘chronic hepatic disease (e.g. cirrhosis) or biochemical evidence of significant hepatic derangement (e.g. bilirubin >2 times ULN, in association with AST/ALT/alkaline phosphatase > 3 times ULN’.
NSAID, nonsteroidal anti-inflammatory drug; TIA, transient ischaemic attack. CHA2DS2VASc score, Congestive Cardiac Failure, Hypertension (,Age ⩾ 75 years, Age= 65-74 years, Diabetes Mellitus, Stroke/ Transient Ischaemic Attack/ Thromboembolism, Vascular disease, Sex female; CHADS2, Congestive Cardiac Failure, Hypertension, Age ⩾ 75 years, Diabetes Mellitus, Stroke/ Transient Ischeamic Attack/ Thromboembolism 2) HAS-BLED: Hypertension (>160 mmHg systolic), Renal Disease, Liver disease, History of stroke/ TIA, History of bleeding, Labile INR, Age>65 years, Concomitant use of NSAIDs; HEMMOR2HAGES, Hepatic/ Renal impairment, Alcohol abuse, History of Malignancy, Age > 75 years, Low platelet count, History of bleeding, Uncontrolled hypertension, Anaemia, Excessive risk of fall, History of stroke/ TIA.
Figure 1.
Discharge antithrombotic therapy distribution based on CHADS2 scores (n = 199). Congestive Cardiac Failure, Hypertension, Age ⩾ 75 years, Diabetes Mellitus, Stroke/ Transient Ischeamic Attack/ Thromboembolism.
Figure 2.
Antithrombotics utilization at discharge according to CHA2DS2-VASc scores (n = 199). CHA2DS2VASc score, Congestive Cardiac Failure, Hypertension (, Age ⩾ 75 years, Age= 65-74 years, Diabetes Mellitus, Stroke/ Transient Ischaemic Attack/ Thromboembolism, Vascular disease, Sex female.
Figure 3.
Discharge antithrombotic utilization based on HAS-BLED scores (n = 199). HAS-BLED, Hypertension (>160 mmHg systolic), Renal Disease, Liver disease, History of stroke/ TIA, History of bleeding, Labile INR, Age > 65 years, Concomitant use of NSAIDs.
Figure 4.
Utilization of antithrombotic therapy based on HEMORR2HAGES scores (n =199). HEMMOR2HAGES, Hepatic/ Renal impairment, Alcohol abuse, History of Malignancy, Age > 75 years, Low platelet count, History of bleeding, Uncontrolled hypertension, Anaemia, Excessive risk of fall, History of stroke/ TIA.
Antithrombotic therapy on admission
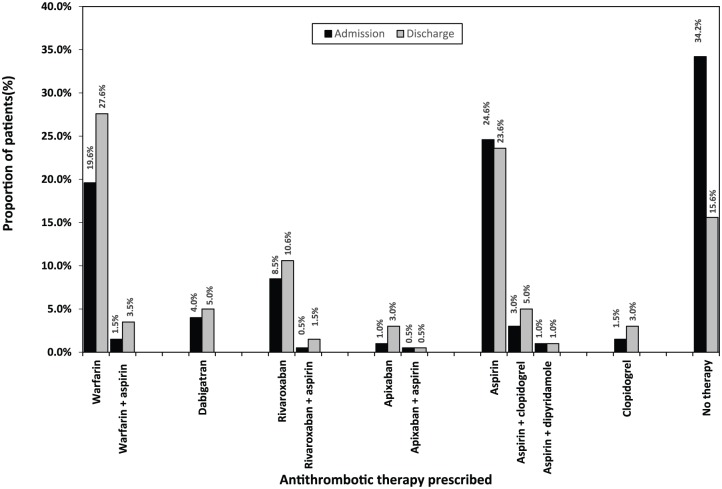
From the total of 199 patients, 131 (65.8%) were using some form of antithrombotic therapy on admission to hospital, leaving one third 68 (34.2%) on ‘no therapy’ (Figure 5). Overall, anticoagulants were the most frequently prescribed therapy (35.7%), followed by antiplatelets (30.2%). Among 71 patients prescribed an anticoagulant, 42 (59.2%) were on warfarin (± antiplatelet): 39 (54.9%) received warfarin alone and 3 (4.2%) warfarin + aspirin. The remaining 29 (40.8%) patients were using NOACs (± antiplatelet): 27 (38.0%) were on an NOAC alone and 2 (2.8%) were on an NOAC + aspirin.
Figure 5.
Distribution of antithrombotic therapy: admission versus discharge (n = 199).
Antithrombotic therapy utilization at discharge
Among the 199 patients discharged from hospital, 168 (84.4%) were prescribed some form of antithrombotic therapy (Table 1, Figure 5). Patients were most frequently discharged on anticoagulants 103 (51.8%), followed by antiplatelets 65 (32.7%). Among 103 patients prescribed anticoagulants, 62 (60.2%) were prescribed warfarin (± antiplatelet): 55 (53.4%) were on warfarin alone and 7 (6.8%) were on warfarin + antiplatelets. The remaining 41 (39.8%) patients were discharged on NOACs (± antiplatelet): 37 (35.9%) were on an NOAC alone and 4 (3.9%) were on an NOAC + antiplatelets. Among 41 patients prescribed NOACs, rivaroxaban 24 (58.5%) was the most frequently prescribed agent, followed by dabigatran 10 (24.4%), then apixaban 7 (17.1%).
Changes in antithrombotic therapy use from hospital admission to discharge
Overall, the utilization of antithrombotic therapy increased at discharge compared with that observed at admission (84.4% versus 65.8%, p < 0.01). Specifically, the proportion of patients prescribed warfarin (± antiplatelet therapy) significantly increased at discharge from that observed at admission (31.2% versus 21.1%, p < 0.01) (Figure 5). Among 68 patients using ‘no therapy’ on admission, 40 (58.8%) were prescribed an antithrombotic prior to discharge; 18 (26.5%) received aspirin (± other antiplatelet), 9 (13.2%) received warfarin (± antiplatelet), 5 (7.4%) received (rivaroxaban), 3 (4.4%) received apixaban, 3 (4.4%) received clopidogrel, and 2 (2.9%) received dabigatran (Figure 5). Among 57 patients on aspirin during admission, 16 (28.1%) were switched to an anticoagulant prior to discharge; 11 (19.3%) changed to warfarin (± antiplatelet), and 5 (8.8%) to rivaroxaban (± antiplatelet).
Among the 16 patients switched to NOACs at discharge, 10 (62.5%) were originally on ‘no therapy’, 5 (31.3%) were on aspirin (± other antiplatelet), and 1 (6.3%) was on warfarin. Conversely, of the 5 patients switched from NOACs to other therapies at discharge, 1 (20.0%) was changed from rivaroxaban to aspirin (due to haematuria and gastrointestinal bleeding), 1 was changed (20.0%) from apixaban to warfarin (due to declining renal function; estimated glomerular filtration rate = 12 ml/min/1.73 m2), and the reasons for three changes were not documented [2 (40.0%) patients changed from rivaroxaban to aspirin, 1 (20.0%) patient changed from rivaroxaban to ‘no therapy’].
Age and antithrombotic therapy utilization at discharge
Among 104 patients aged 75 years and over (Table 1), most patients (46.2%) were discharged on anticoagulants [34 (32.7%) on warfarin and 14 (13.5%) on NOACs] and the remainder on antiplatelets 38 (36.5%) and ‘no therapy’ 18 (17.3%). There was no significant difference in the proportion of patients aged 75 years and over receiving anticoagulants (46.2%) compared with their younger counterparts (57.9%; p = 0.10). Compared with their younger counterparts, a significantly lower proportion of patients aged 75 years and over received NOACs (13.5% versus 28.4%, p = 0.01).
Discharge antithrombotic therapy according to stroke risk
Among patients with a high stroke risk (n = 168, CHA2DS2-VASc ⩾ 2), most patients (53.0%) were discharged on anticoagulants [57 (33.9%) on warfarin and 32 (19.1%) on NOACs]; the remaining 56 (33.3%) patients were on antiplatelets, and 31 (18.5%) on ‘no therapy’ (Table 2 and Figure 2). Among 16 patients with an intermediate stroke risk, most (56.3%) were anticoagulated: 6 (37.5%) were on NOACs and 3 (18.8%) were on warfarin. Within the low stroke risk group (n = 15), two thirds (66.7%) were either on antiplatelet therapy or ‘no-therapy’, while 5 (33.3%) were on anticoagulants (Table 2).
Table 2.
Antithrombotic utilization according to patient stroke risk (CHA2DS2-VASc) versus bleeding risk (HAS-BLED).
| CHA2DS2-VASc | HAS-BLED | No therapy | Warfarin ± antiplatelets | NOACs ± antiplatelets | Antiplatelets ± other antiplatelets | Total (n = 199) |
|---|---|---|---|---|---|---|
| Low Total |
Low | 4 (2.0%) | 1 (0.5%) | 2 (1.0%) | 4 (2.0%) | 11 (5.5%) |
| Intermediate | 2 (1.0%) | 1 (0.5%) | 1 (0.5%) | 0 (0.0%) | 4 (2.0%) | |
| High | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| 15 (7.5%) | ||||||
| Intermediate Total |
Low | 1 (0.5%) | 1 (0.5%) | 4 (2.0%) | 3 (1.5%) | 9 (4.5%) |
| Intermediate | 0 (0.0%) | 1 (0.5%) | 2 (1.0%) | 1 (0.5%) | 4 (2.0%) | |
| High | 1 (0.5%) | 1 (0.5%) | 0 (0.0%) | 1 (0.5%) | 3 (1.5%) | |
| 16 (8.0%) | ||||||
| High Total |
Low | 2 (1.0%) | 6 (3.0%) | 6 (3.0%) | 7 (3.5%) | 21 (10.6%) |
| Intermediate | 18 (9.0%) | 45 (22.6%) | 25 (12.6%) | 44 (22.1%) | 132 (6.6%) | |
| High | 3 (1.5%) | 6 (3.0%) | 1 (0.5%) | 5 (2.5%) | 15 (7.5%) | |
| 168 (84.4%) | ||||||
| Total | 31 (15.6%) | 62 (31.2%) | 41 (20.6%) | 65 (32.7%) | 199 (100%) | |
HAS-BLED: low (score = 0); intermediate (score = 1–2); high (score ⩾ 3).
CHA2DS2-VASc: low (score = 0); intermediate (score = 1); high (score ⩾ 2).
NOAC, nonvitamin K antagonist oral anticoagulant.
CHA2DS2VASc score, Congestive Cardiac Failure, Hypertension, Age ⩾ 75 years, Age= 65-74 years, Diabetes Mellitus, Stroke/ Transient Ischaemic Attack/ Thromboembolism, Vascular disease, Sex female; HAS-BLED, Hypertension (>160 mmHg systolic), Renal Disease, Liver disease, History of stroke/ TIA, History of bleeding, Labile INR, Age>65 years, Concomitant use of NSAIDs.
A higher (albeit not statistically significant) proportion of patients with a high stroke risk received warfarin therapy (33.9%) compared with patients with low–intermediate stroke risk (16.1%, p = 0.06). Although not significant, a higher proportion of patients with low–intermediate stroke risk were discharged on ‘no therapy’ (25.8%) compared with patients with high stroke risk (13.7%, p = 0.09). A lower proportion of patients with high stroke risk received NOACs (19.0%) compared with those with low–intermediate stroke risk (29.0%, p = 0.21)
Taking into account specific stroke risk factors, a higher proportion of patients with congestive cardiac failure (CCF) received antithrombotic therapy (97.1%) compared with patients without CCF (81.7%, p = 0.02). Similarly, a significantly higher proportion of patients with vascular disease received antithrombotic therapy (90.6%) compared with those without vascular disease (79.8%, p = 0.03).
Bleeding risk and antithrombotic utilization
The mean HEMORR2HAGES and HAS-BLED scores of the patients were 1.3 (±1.2) and 1.2 (±0.9) respectively, representing an intermediate bleeding risk for the overall sample. Among 18 patients with a high risk of bleeding (HAS-BLED ⩾ 3), most (44.4%) were prescribed anticoagulants (7 (38.9%) were on warfarin and 1 (5.6%) was on an NOAC), and the remainder were discharged on antiplatelets (n = 6, 33.3%) and ‘no therapy’ (n = 4, 22.2%).
Taking into account specific bleeding risk factors, patients with anaemia were less frequently prescribed antithrombotic therapy (64.8%) compared with those without anaemia (86.1%, p = 0.04). Patients with history of bleeding were less frequently prescribed antithrombotic therapy (65.0%) compared with those without a history of bleeding (86.6%, p = 0.01). Patients with renal impairment were less frequently prescribed NOACs (8.9%) compared with those without renal impairment (24.0%, p = 0.02). Patients with cognitive impairment were less frequently discharged on warfarin (11.8%) compared with those without cognitive impairment (23.0%, p = 0.01).
Antithrombotic therapy based on stroke risk versus bleeding risk
Overall, 168 (84.4%) patients had a high stroke risk (as per CHA2DS2-VASc score), and were subsequently categorized as eligible, most eligible and special AF population, based on their corresponding bleeding risk (Tables 1 and 2). Among ‘eligible’ patients (n = 153), most (53.5%) were discharged on anticoagulants: 51 (33.3%) were on warfarin and 31 (20.3%) were on NOACs. The remaining 71 (46.4%) patients in the ‘eligible’ group did not receive an anticoagulant, consisting of 51 (33.3%) on antiplatelets and 20 (13.1%) prescribed ‘no therapy’.
Among the ‘most eligible’ patients (n = 21, Table 1), 9 (42.9%) did not receive and anticoagulant. The remaining 12 (57.1%) ‘most eligible’ patients were discharged on anticoagulants: 6 (28.6%) patients were on warfarin and 6 (28.6%) were on NOACs. Within the ‘special AF’ group (n = 15, Table 1), 8 (53.3%) patients were discharged on ‘no therapy’ and the remaining 7 (46.7%) were prescribed anticoagulants: 6 (40.0%) patients were on warfarin and 1 (6.7%) was on an NOAC.
No patients were categorized as having a low risk of stroke and a high risk of bleeding (Table 2).
Factors influencing NOAC utilization at discharge
All the stroke risk factors, bleeding risk factors and individual patients’ scores for both bleeding (HEMMOR2HAGES and HAS-BLED) and stroke (CHA2DS2-VASc and CHADS2) were included in the univariate analysis. Patients aged 75 years and over were 2.4 times less likely [odds ratio (OR) = 0.41, 95% confidence interval (CI) = 0.181–0.935, p = 0.03] and patients with renal impairment were 3.6 times less likely (OR = 0.28, 95% CI = 0.08–0.90, p = 0.03) to be prescribed NOACs (i.e. they were more likely to receive warfarin). Subsequently, both these factors were included in multivariate logistic regression (Forward–Wald) modelling. Finally, renal impairment was retained as the only predictor of NOAC use over warfarin; patients with renal impairment were 3.6 times less likely to receive NOACs; that is, they were more likely to receive warfarin (OR = 0.28, 95% CI = 0.08–0.90, p = 0.03, 59.8% correctly predicted; Cox and Snell R2 = 0.51, Nagelkerke R2 = 0.69).
Reasons for not prescribing anticoagulant therapy
The reasons for clinicians’ treatment choices when an anticoagulant was not prescribed in patients with high stroke risk were documented for 12 (6.0%, n = 199) patients. In 5 cases the patient or their family preferred to continue using their preadmission aspirin or ‘no therapy’. In 4 cases, falls risk was cited by the clinician as the reason for not prescribing an anticoagulant. In 2 cases, medication nonadherence by the patient was cited as the reason for not prescribing anticoagulants (although it was not stated whether there was an actual history of nonadherence or simply concern about the potential for nonadherence). One patient did not receive an anticoagulant due to a history of epistaxis and haematuria (related to previous warfarin use).
What is new and conclusion
Overall in this study, 84.4% of patients with AF were discharged on some type of antithrombotic therapy for stroke prevention. This is slightly less than the 85% antithrombotic utilization rate among patients with AF reported in a previous Australian study, prior to the PBS listing of NOACs.40 The GLORIA AF registry reported that for the years 2008–2014, the utilization of NOACs in North and South America, Asia, and Europe (23 countries) ranged from as low as 25.5% to a high of 52.4%,41 while for other studies conducted in the USA, Canada, and Europe (7 countries) between 2011 and 2013 it ranged from 6.6% to 13%.28,42,43 Our study observed that 20.6% of patients were prescribed NOACs in a local Australian hospital setting.
This research observed that anticoagulants were the most frequently used therapy among eligible patients, prescribed in 50.3% of patients. Warfarin was preferred over NOACs in patients who were at risk. This might be due to the recommendation in the then contemporary local clinical guideline that patients should be prescribed NOACs only if they cannot be managed well on warfarin.26 However, the current local guideline now considers NOACs as the first line of therapy44 and this might improve prescription of NOACs in the future. A systematic review on studies conducted prior to NOAC approval demonstrated that the suboptimal use of anticoagulants in eligible patients ranges from 39% to 70%.6 Our study showed that a considerable proportion, that is, 46.4% of eligible patients, did not receive an anticoagulant despite the availability of NOACs. These high-risk patients were either prescribed antiplatelets or ‘no therapy’. A recent randomized controlled trial, BAFTA (the Birmingham Atrial Fibrillation Treatment of the Aged Group), found that antiplatelet therapy does not offer a thromboprophylactic benefit and carries a high risk of bleeding compared with warfarin.45 Additionally, evidence shows that risk–benefit ratios of NOACs are far superior to antiplatelets, especially in at-risk patients.46,47 Hence current guidelines no longer recommend aspirin for stroke prevention in the high-risk AF population.44,48–51 Moreover, this study also observed that some of the patients were prescribed anticoagulants in combination with antiplatelet therapy, and this may be for coexisting ischaemic heart disease and related interventions (e.g. previous myocardial infarction, coronary artery disease, percutaneous coronary intervention).52,53 However, a recent meta-analysis has reported that combination therapy does not provide any additional stroke prevention benefit compared with anticoagulant therapy alone and increases the risk of bleeding. Hence, it is recommended that patients with AF on an anticoagulant therapy should be cautiously assessed for the indications for antiplatelet therapy.54 Previous studies28,55 have demonstrated that older patients with AF are less likely to be prescribed NOACs. This audit reported that renal impairment was observed as a predictor negatively influencing clinicians’ choice for NOACs over warfarin therapy, and this factor might be a surrogate to old age. This is in keeping with a similar study conducted to report antithrombotic utilization when NOACs were newly approved in the USA.56 This might be due to limited or no information regarding safety and efficacy of NOACs versus warfarin in patients with declining renal function in ‘real-world’ clinical practice. Two subgroup analyses, ROCKET AF and ARISTOTLE, observed that both rivaroxaban and apixaban have a noninferior safety and efficacy profile compared with warfarin in patients with mild–moderate renal impairment.57,58 It is suggested that any anticoagulants, especially the NOACs, offer benefits in patients with high stroke and bleeding risk compared with aspirin or ‘no therapy’. Nonetheless, more information is required regarding the use of anticoagulants in patients with prior intracranial bleeding, severe renal or hepatic impairment and active malignancy.39 It is likely that NOAC prescription might improve over time with the availability of more evidence regarding their risk–benefit ratios in patients with impaired renal function and the procedures to manage acute bleeding episodes in such patients.
Although the inclusion of NOACs has expanded the scope for prescribing prophylactic therapy in patients with AF, the complexity of decisionmaking when selecting available treatment options persists. Studies have demonstrated that decision-support tools may help facilitate comprehensive and individualized risk–benefit assessment for selecting antithrombotic therapy in patients with AF to optimize the prescription of anticoagulants in those who are at risk.59–61 However, other studies have reported differences in physicians’ and patients’ perspectives regarding stroke risk and bleeding risk, and their individual preferences for therapy,62,63 which might not be readily addressed in such tools. Indeed, this study reports that some patients were unwilling to use warfarin, reinforcing recommendations that patients’ perspectives must also be incorporated into decisionmaking to obtain maximum adherence and benefit from the selected therapy.11,30,64
In interpreting the findings from this study, some potential limitations need to be acknowledged. First, the data represent practice in an Australian hospital and may not be generalizable to any other settings. Second, given the retrospective nature of data collection in this study, it is possible that reasons for the treatment decisions made for these patients were not fully documented in the medical records, and therefore appropriate avoidance of certain therapies was not ascertained. Our approach may underestimate the number of people eligible for anticoagulation because the risk categories across the scores may not always be directly equivalent. For example, CHA2DS2-VASc=4 and HAS-BLED=4 is associated with a stroke risk of around 5% without anticoagulation, and around 10% major bleeding risk with anticoagulation. In this regard, our approach potentially emphasizes safety (as opposed to benefit) in the decisionmaking equation. Furthermore, this study reflects prescribing habits in 2014, and these habits may change as prescribers become more familiar with the newer anticoagulants; for this reason, this audit should be repeated over time to identify any temporal changes in prescribing and to reflect a more contemporary utilization of the NOACs. Finally, it was not possible to determine the level of patient engagement in the decisionmaking process for stroke prevention treatment selection.
Although most patients with AF are using antithrombotic therapy for stroke prevention, only half are receiving an anticoagulant. Despite the availability of NOACs as alternatives to warfarin, not all eligible patients are receiving an anticoagulant, leading to the potential underutilization of guideline-recommended therapy. Further work is needed to map the temporal trends in the utilization of antithrombotics in AF and identify the factors underpinning treatment selection in this context.
Acknowledgments
We sincerely thank the hospital’s Health Information Services unit for their cooperation and assistance throughout this study.
Footnotes
Authors’ Note: Beata Bajorek is also affiliated to Royal North Shore Hospital, Department of Pharmacy, St. Leonards, Sydney, Australia.
Clara Chow is also affiliated to The George Institute for Global Health, Sydney, Australia.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Ekta Yogeshkumar Pandya, University of Technology Sydney Faculty of Health, Broadway, Ultimo, Sydney, NSW 2007, Australia.
Elizabeth Anderson, Westmead Hospital, Westmead, NSW, Australia.
Clara Chow, Westmead Hospital, Westmead, NSW, Australia.
Yishen Wang, University of Technology Sydney Faculty of Health, Broadway, Sydney, NSW, Australia.
Beata Bajorek, University of Technology Sydney Faculty of Health, Broadway, Sydney, NSW, Australia.
References
- 1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991; 22: 983–988. [DOI] [PubMed] [Google Scholar]
- 2. Petersen P, Godtfredsen J, Boysen G, et al. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation: the Copenhagen AFASAK study. Lancet 1989; 333: 175–179. [DOI] [PubMed] [Google Scholar]
- 3. Investigators SPiAF. Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet 1996; 348: 633–638. [PubMed] [Google Scholar]
- 4. Hart RG, Benavente O, McBride R, et al. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med 1999; 131: 492–501. [DOI] [PubMed] [Google Scholar]
- 5. Adhiyaman V, Kamalakannan D, Oke A, et al. Underutilization of antithrombotic therapy in atrial fibrillation. J R Soc Med 2000; 93: 138–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ogilvie IM, Newton N, Welner SA, et al. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med 2010; 123: 638–645. e4. [DOI] [PubMed] [Google Scholar]
- 7. Munschauer F, Priore R, Hens M, et al. Thromboembolism prophylaxis in chronic atrial fibrillation practice patterns in community and tertiary-care hospitals. Stroke 1997; 28: 72–76. [DOI] [PubMed] [Google Scholar]
- 8. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 9. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–891. [DOI] [PubMed] [Google Scholar]
- 10. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–992. [DOI] [PubMed] [Google Scholar]
- 11. Heidbuchel H, Verhamme P, Alings M, et al. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J 2013; 34: 2094–2106. [DOI] [PubMed] [Google Scholar]
- 12. Bounameaux H, Reber G. New oral antithrombotics: a need for laboratory monitoring. Against. J Thromb Haemost 2010; 8: 627–630. [DOI] [PubMed] [Google Scholar]
- 13. Mismetti P, Laporte S. New oral antithrombotics: a need for laboratory monitoring. J Thromb Haemost 2010; 8: 621–626. [DOI] [PubMed] [Google Scholar]
- 14. Ramos-Esquivel A. Monitoring anticoagulant therapy with new oral agents. World J Methodol 2015; 5: 212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gosselin RC, Adcock DM. Assessing nonvitamin K antagonist oral anticoagulants (NOACs) in the laboratory. Int J Lab Hematol 2015; 37(Suppl. 1): 46–51. [DOI] [PubMed] [Google Scholar]
- 16. Cate HT. New oral anticoagulants: discussion on monitoring and adherence should start now! Thromb J 2013; 11: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bauer KA. Pros and cons of new oral anticoagulants. Hematology Am Soc Hematol Educ Program 2013; 2013: 464–470. [DOI] [PubMed] [Google Scholar]
- 18. Strunets A, Mirza M, Sra J, et al. Novel anticoagulants for stroke prevention in atrial fibrillation: safety issues in the elderly. Expert Rev Clin Pharmacol 2013; 6: 677–689. [DOI] [PubMed] [Google Scholar]
- 19. Yang E. A clinician’s perspective: novel oral anticoagulants to reduce the risk of stroke in nonvalvular atrial fibrillation – full speed ahead or proceed with caution? Vasc Health Risk Manag 2014; 10: 507–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Choi J, DiBonaventura M, Kopenhafer L, et al. Survey of patients with atrial fibrillation on the use of warfarin and the newer anticoagulant, dabigatran. Circ Cardiovasc Qual Outcomes 2012; 1 http://circoutcomes.ahajournals.org/content/5/Suppl_1/A240 (accessed 28 November 2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elewa HF, DeRemer CE, Keller K, et al. Patients satisfaction with warfarin and willingness to switch to dabigatran: a patient survey. J Thromb Thrombolysis 2014; 38: 115–120. [DOI] [PubMed] [Google Scholar]
- 22. The Pharmaceutical Benefits Scheme-Dabigatran. http://www.pbs.gov.au/medicine/item/9318k-9319l-9320m-9321n-9322p-9323q (accessed 28 November 2017).
- 23. The Pharmaceutical Benefits Scheme-Rivaroxaban. http://www.pbs.gov.au/pbs/search?term=rivaroxaban (accessed 28 November 2017).
- 24. Community Affairs References Committee. Out-of-pocket costs in Australian healthcare, August 2014, http://www.aph.gov.au/Parliamentary_Business/Committees/Senate/Community_Affairs/Australian_healthcare/Report (2014, accessed 28 November 2017).
- 25. NPS MedicineWise. Dabigatran (Pradaxa) for stroke prevention in patients with non-valvular atrial fibrillation, August 2011, https://www.nps.org.au/radar/articles/dabigatran-pradaxa-for-stroke-prevention-in-patients-with-non-valvular-atrial-fibrillation.( accessed 28 November 2017).
- 26. Western Australian Therapeutic Advisory Group. Prescribing guidelines for dabigatran, rivaroxaban and apixaban, November 2013, http://scghed.com/wp-content/uploads/2014/01/WATAG-NOAC-Guidelines.pdf. (accessed 28 November 2017).
- 27. Curnow J. Practical issues with using novel oral anticoagulants. Cardiology Today 2014; 4: 23–26. [Google Scholar]
- 28. Kirchhof P, Ammentorp B, Darius H, et al. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC guidelines on atrial fibrillation: primary results of the prevention of thromboemolic events – European Registry in Atrial Fibrillation (PREFER in AF). Europace 2014; 16: 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bajorek BV, Masood N, Krass I. Development of a Computerised Antithrombotic Risk Assessment Tool (CARAT) to optimise therapy in older persons with atrial fibrillation. Australas J Ageing 2012; 31: 102–109. [DOI] [PubMed] [Google Scholar]
- 30. Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace 2006; 8: 651–745. [DOI] [PubMed] [Google Scholar]
- 31. Karthikeyan G, Eikelboom JW. The CHADS2 score for stroke risk stratification in atrial fibrillation – friend or foe? Thromb Haemost 2010; 104: 45–48. [DOI] [PubMed] [Google Scholar]
- 32. Olesen JB, Torp-Pedersen C, Hansen ML, et al. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0–1: a nationwide cohort study. Thromb Haemost 2012; 107: 1172–1179. [DOI] [PubMed] [Google Scholar]
- 33. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro heart survey. Chest 2010; 138: 1093–1100. [DOI] [PubMed] [Google Scholar]
- 34. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the national registry of atrial fibrillation (NRAF). Am Heart J 2006; 151: 713–719. [DOI] [PubMed] [Google Scholar]
- 35. Fauchier L, Chaize G, Gaudin AF, et al. Predictive ability of HAS-BLED, HEMORR2HAGES, and ATRIA bleeding risk scores in patients with atrial fibrillation. A French nationwide cross-sectional study. Int J Cardiol 2016; 217: 85–91. [DOI] [PubMed] [Google Scholar]
- 36. Zhu W, He W, Guo L, et al. The HAS-BLED score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol 2015; 38: 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu W-G, Xiong Q-M, Hong K. Meta-analysis of CHADS2 versus CHA2DS2-VASc for predicting stroke and thromboembolism in atrial fibrillation patients independent of anticoagulation. Tex Heart Inst J 2015; 42: 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen J-Y, Zhang A-D, Lu H-Y, et al. CHADS2 versus CHA2DS2-VASc score in assessing the stroke and thromboembolism risk stratification in patients with atrial fibrillation: a systematic review and meta-analysis. J Geriatr Cardiol 2013; 10: 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Potpara TS, Lip GYH. Oral anticoagulant therapy in atrial fibrillation patients at high stroke and bleeding risk. Prog Cardiovasc Dis 2015; 58: 177–194. [DOI] [PubMed] [Google Scholar]
- 40. Bajorek BV, Ren S. Utilisation of antithrombotic therapy for stroke prevention in atrial fibrillation in a Sydney hospital: then and now. Int J Clin Pharm 2012; 34: 88–97. [DOI] [PubMed] [Google Scholar]
- 41. Huisman MV, Rothman KJ, Paquette M, et al. Antithrombotic treatment patterns in patients with newly diagnosed nonvalvular atrial fibrillation: the GLORIA-AF registry, phase II. Am J Med 2015; 128: 1306–1313. e1. [DOI] [PubMed] [Google Scholar]
- 42. Lip GY, Laroche C, Dan G-A, et al. ‘Real-world’ antithrombotic treatment in atrial fibrillation: the EORP-AF pilot survey. Am J Med 2014; 127: 519–529. e1. [DOI] [PubMed] [Google Scholar]
- 43. Brais C, Larochelle J, Turgeon M, et al. Patterns of oral anticoagulants use in atrial fibrillation. J Popul Ther Clin Pharmacol 2014; 22: e90–e95. [PubMed] [Google Scholar]
- 44. Government of Western Australia Department of Health. Quick Reference guide: Atrial Fibrillation Information for the Health Practitioner, http://rockyed.com.au/upload/data/d_pdf/pdf_8_24_1.pdf. (accessed 28 November 2017).
- 45. Mant J, Hobbs FR, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007; 370: 493–503. [DOI] [PubMed] [Google Scholar]
- 46. Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364: 806–817. [DOI] [PubMed] [Google Scholar]
- 47. Roskell NS, Lip GY, Noack H, et al. Treatments for stroke prevention in atrial fibrillation: a network meta-analysis and indirect comparisons versus dabigatran etexilate. Thromb Haemost 2010; 104: 1106–1115. [DOI] [PubMed] [Google Scholar]
- 48. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64: e1–e76. [DOI] [PubMed] [Google Scholar]
- 49. Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 2015; 17: 1467–1507. [DOI] [PubMed] [Google Scholar]
- 50. NICE Guidelines. Preventing stroke in people with atrial fibrillation, September 2015, http://pathways.nice.org.uk/pathways/atrial-fibrillation#path=view%3A/pathways/atrial-fibrillation/preventing-stroke-in-people-with-atrial-fibrillation.xml&content=view-index (2015, accessed May 2016).
- 51. Hobbs FR, Taylor CJ, Geersing GJ, et al. European Primary Care Cardiovascular Society (EPCCS) consensus guidance on stroke prevention in atrial fibrillation (SPAF) in primary care. Eur J Prev Cardiol 2016; 23: 460–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. So CH, Eckman MH. Combined aspirin and anticoagulant therapy in patients with atrial fibrillation. J Thromb Thrombolysis 2017; 43: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pandya E, Masood N, Wang Y, et al. Impact of a computerized antithrombotic risk assessment tool on the prescription of thromboprophylaxis in atrial fibrillation: hospital setting. Clin Appl Thromb Hemost 2018; 24: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kumar S, Danik SB, Altman RK, et al. Non-vitamin K antagonist oral anticoagulants and antiplatelet therapy for stroke prevention in patients with atrial fibrillation: a meta-analysis of randomized controlled trials. Cardiol Rev 2016; 24: 218–223. [DOI] [PubMed] [Google Scholar]
- 55. Desai NR, Krumme AA, Schneeweiss S, et al. Patterns of initiation of oral anticoagulants in patients with atrial fibrillation- quality and cost implications. Am J Med 2014; 127: 1075–1082. e1. [DOI] [PubMed] [Google Scholar]
- 56. Baik SH, Hernandez I, Zhang Y. Evaluating the initiation of novel oral anticoagulants in Medicare beneficiaries. J Manag Care Spec Pharm 2016; 22: 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fox KAA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J 2011; 32: 2387–2394. [DOI] [PubMed] [Google Scholar]
- 58. Hohnloser SH, Hijazi Z, Thomas L, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J 2012; 33: 2821–2830. [DOI] [PubMed] [Google Scholar]
- 59. Oliveira R, Grilo S, Moreira C, et al. A quality study to improve prophylactic antithrombotic therapy prescribed to patients with atrial fibrillation. Rev Port Cardiol 2014; 33: 89–94. [DOI] [PubMed] [Google Scholar]
- 60. Simona Bo, Valpreda S, Scaglione L, et al. Implementing hospital guidelines improves warfarin use in non-valvular atrial fibrillation: a before-after study. BMC Public Health 2007; 7: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bajorek BV, Krass I, Ogle SJ, et al. Optimizing the use of antithrombotic therapy for atrial fibrillation in older people: a pharmacist-led multidisciplinary intervention. J Am Geriatr Soc 2005; 53: 1912–1920. [DOI] [PubMed] [Google Scholar]
- 62. Protheroe J, Fahey T, Montgomery AA, et al. Effects of patients’ preferences on the treatment of atrial fibrillation: observational study of patient-based decision analysis. West J Med 2001; 174: 311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thomson RG, Eccles MP, Steen IN, et al. A patient decision aid to support shared decision-making on anti-thrombotic treatment of patients with atrial fibrillation: randomised controlled trial. Qual Saf Health Care 2007; 16: 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pandya EY, Bajorek B. Factors affecting patients’ perception on, and adherence to, anticoagulant therapy: anticipating the role of direct oral anticoagulants. Patient 2017; 10: 163–185. [DOI] [PubMed] [Google Scholar]