Abstract
Background:
Medication errors represent a significant but often preventable cause of morbidity and mortality in neonates. The objective of this systematic review was to determine the effectiveness of interventions to reduce neonatal medication errors.
Methods:
A systematic review was undertaken of all comparative and noncomparative studies published in any language, identified from searches of PubMed and EMBASE and reference-list checking. Eligible studies were those investigating the impact of any medication safety interventions aimed at reducing medication errors in neonates in the hospital setting.
Results:
A total of 102 studies were identified that met the inclusion criteria, including 86 comparative and 16 noncomparative studies. Medication safety interventions were classified into six themes: technology (n = 38; e.g. electronic prescribing), organizational (n = 16; e.g. guidelines, policies, and procedures), personnel (n = 13; e.g. staff education), pharmacy (n = 9; e.g. clinical pharmacy service), hazard and risk analysis (n = 8; e.g. error detection tools), and multifactorial (n = 18; e.g. any combination of previous interventions). Significant variability was evident across all included studies, with differences in intervention strategies, trial methods, types of medication errors evaluated, and how medication errors were identified and evaluated. Most studies demonstrated an appreciable risk of bias. The vast majority of studies (>90%) demonstrated a reduction in medication errors. A similar median reduction of 50–70% in medication errors was evident across studies included within each of the identified themes, but findings varied considerably from a 16% increase in medication errors to a 100% reduction in medication errors.
Conclusion:
While neonatal medication errors can be reduced through multiple interventions aimed at improving the medication use process, no single intervention appeared clearly superior. Further research is required to evaluate the relative cost-effectiveness of the various medication safety interventions to facilitate decisions regarding uptake and implementation into clinical practice.
Keywords: intervention, medication errors, neonatal intensive care unit, newborn infant, systematic review
Introduction
Medication errors represent a significant burden to the healthcare system.1 They can be defined as any preventable event that can cause or lead to inappropriate medication use or patient harm and can occur at any stage in the medication-use process such as prescribing, transcribing, dispensing, administering, and monitoring of medications.2 Neonates are more prone to medication errors at each stage of the medicine management process due to the increased need for calculations, dilutions, and manipulations of medications.3,4 Furthermore, many medications are used off-label in the neonatal setting, meaning that they are not specifically licensed for use in neonates and are therefore often only available in adult formulations and concentrations.5 As a result, prescribing and administration challenges often places neonates at risk of potentially fatal 10-fold or 100-fold dosing errors.6,7 There is also the associated challenge of limited dosing protocols and evidence-based information regarding the efficacy, safety, dosing, pharmacokinetic, and clinical use of medications in neonates.6 In addition, relative physiological immaturity means that neonates have less capacity in being able to buffer unintended consequences of medication errors.8 Such susceptibility towards medication errors in neonates, as previously described, is further emphasized by previous research that observed that medication errors with the potential to cause significant harm were three times more likely to occur in the neonatal intensive care unit (NICU) than in adult wards.9 Furthermore, an analysis of all medical errors occurring within the NICU identified that medication errors were the single largest contributor, accounting for 47.2% of all errors.10
Given the complexity of medication use in neonates, the high frequency in which high-risk medications are used and the potential for serious adverse events of even minor medication errors, intervention strategies to increase medication safety in neonatal care should be regularly reviewed. The identification and evaluation of such interventions are of critical importance in assisting healthcare systems and providers in understanding, implementing, and augmenting interventions to reduce neonatal medication errors.11 Despite this importance, there have been few extensive systematic reviews on interventions for preventing medication errors in the neonatal setting, with the most recent reviews only including literature up until 2013.12–14 Further, none of these reviews included both comparative and noncomparative studies. The aim of this systematic review was to identify and review different types of interventions to reduce neonatal medication errors.
Method
Search strategy
PubMed and EMBASE were searched for any studies published from 1966 until April 2016, without any language restrictions. The search included a medication errors/safety concept and a neonatal concept, with terms entered as controlled vocabulary and as keywords in all databases. MeSH search terms included: ‘medication errors’ AND ‘Infant, Newborn’, OR ‘Intensive Care Units, Neonatal’, OR ‘Intensive Care, Neonatal’, OR ‘Pediatrics’. Reference lists of all articles included in full-text review, as well as other review articles, were screened for additional studies.
Eligibility criteria
To be eligible for inclusion:
(1) An intervention specifically aimed at reducing the risk of medication errors must be carried out or reported.
(2) There must have been some measure to evaluate effectiveness in reducing risk of medication errors.
(3) The study setting must have included neonates.
Studies only published in abstract form were not eligible for inclusion.
Data abstraction
Two independent, nonblinded authors (MRN and LEG) reviewed each title and abstract for inclusion eligibility. Full-text review was also conducted by two independent, nonblinded authors (MRN and LEG) and discrepancies were resolved through author consensus discussions. For non-English language studies included in the full-text review, the primary author (MRN) translated the contents with computer translation software, which has previously been demonstrated as effective for systematic reviews.15 The study selection process was documented as per the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA).16 Data were collected in relation to study characteristics, intervention strategy, comparator treatment (if applicable), type of medication error evaluated, specific detail of medication error, and main study findings. Qualitative descriptors were utilized to describe the results of studies. We did not plan to perform a meta-analysis.
Study quality assessment
Study quality was evaluated by two independent reviewers (MRN and LEG) using the Cochrane Effective Practice and Organization of Care (EPOC) Review Group risk of bias tool, which evaluates all study types together including randomized controlled trials, nonrandomized trials, and controlled before–after studies utilizing the same eight criteria.17
Results
Search results
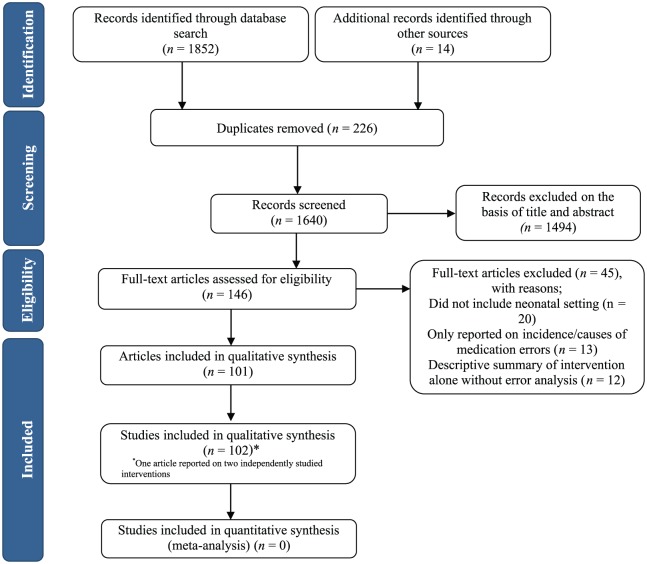
Our search identified 1852 abstracts, 226 of which were duplicates and were removed. An additional 14 articles were identified from previous systematic reviews or from checking citations of included studies and were retrieved for full-text review, leaving a total of 1640 unique records. A total of 146 articles were included in full-text review and of these, 101 were deemed eligible for inclusion in the systematic review. One article reported separately on two independently studied interventions, resulting in a total of 102 eligible studies (Figure 1).
Figure 1.
Flow diagram of included studies.
Overview of included studies
Identified studies were grouped according to their intervention type and are presented in Table 1, together with an example of an intervention within that group. The six intervention types included: technology (n = 38),18–55 organizational (n = 16),56–71 personnel (n = 13),72–84 pharmacy (n = 9),85–93 hazard and risk analysis (n = 8),10,93–99 and multifactorial (i.e. a combination of any of the previous themes; n = 18).100–117 A detailed summary of each individual study included in the review is presented in Table 2.
Table 1.
Overview of identified interventions for reducing medication errors in neonatal care.
| Intervention type | Example of intervention | References |
|---|---|---|
| Technology (n = 38) | ||
| Computerized physician order entry and clinical decision support (n = 15) | Electronic medication prescribing and atuomated dose checking | 36–50 |
| Computerized physician order entry (n = 5) | Electronic medication prescribing | 51–55 |
| Clinical decision support alone (n = 2) | Electronic tool to verify parenteral nutrition orders | 34–35 |
| Computer programmes (n = 8) | Online parenteral nutrition calculator | 23–30 |
| IV administration technology (n = 4) | Auomated infusion devices | 19–22 |
| Barcoding (n = 3) | Barcode medication administration system | 31–33 |
| Organizational (n = 16) | ||
| Guidelines, policies, and procedures (n = 9) | Development of preformatted medication order sheets | 56–64 |
| Medication distribution and supply (n = 6) | Preparation of prediluted medications for administration | 65–70 |
| Nurse prescribing (n = 1) | Transcription of paper-based orders to electronic orders by nursing staff | 71 |
| Personnel (n = 13) | ||
| Staff education (n = 13) | Personalized feedback of medication prescribing errors | 72–84 |
| Pharmacy (n = 9) | ||
| Ward based (n = 6) | Interventions identified through introduction of ward-based paediatric/neonatal clinical pharmacy service | 85–90 |
| Dispensary based (n = 3) | Interventions identified through dispensary-based pharmacy service | 91–93 |
| Hazard and risk analysis (n = 8) | ||
| Quality improvement tools (n = 4) | Use of failure modes, effects, and criticality analyses to redesign care processes | 10,95–97 |
| Error detection tools (n = 3) | Automated detection of medication errors | 93,98–99 |
| Safe learning systems (n = 1) | Critical incident reports of medication errors | 94 |
| Multifactorial (n = 18) | ||
| GPPs + education (n = 7) | Standardized IV infusion concentration list with intensive education programme | 100–106 |
| GPPs + education + technology (n = 5) | Antimicrobial stewardship programme facilitated by electronic prescribing and provision of individualized real-time feedback | 107–111 |
| GPPs + education + pharmacy (n = 4) | Preprinted medication order sheets with increase in clinical pharmacy services and provision of real-time feedback to prescribers on medication errors | 112–115 |
| Education + pharmacy (n = 1) | Pharmacist-led education programme on medication errors and daily pharmacist review of medication orders | 117 |
| Pharmacy + technology (n = 1) | Utilization of clinical pharmacists to review and intercept any adverse drug events and electronic prescribing | 116 |
GPPs, guidelines, policies and procedures; IV, intravenous.
Table 2.
Summary of study characteristics and results by primary intervention theme.
| Study (country) | Study setting | Intervention type | Intervention detail | Comparator | Main type of error collected | Detail of medication error | Main finding |
|---|---|---|---|---|---|---|---|
| Technology | |||||||
| Abboud et al.41 (US) | N/P | CPOE + CDS | Integration of reminder for aminoglycoside monitoring into CPOE system | Pre–post | Monitoring | Failure to appropriately monitor aminoglycoside | No change in appropriate monitoring [31/177 (17.5%) versus 31/159 (19.5%)] |
| Balaguer Santamaria et al.24 (Spain) | N | Calculator | Development of Neodosis, an electronic spreadsheet to assist in calculating medication doses and standardize dilutions of commonly used drugs | Calculations performed without use of calculator | Prescribing | Errors in calculation of dose | Use of electronic calculator resulted in significant reduction in number of staff making errors [19/27 (70.3%) versus 4/27 (14.8%); p < 0.001] |
| Boling et al.40 (US) | N/P | CPOE + CDS | CPOE with dose range checking system | Pre–post | Prescribing | Dosing errors involving opioids, benzodiazepines, and potassium, requiring administration of antidote | Reduction in opioid prescribing errors [8/13,997 (0.06%) versus 1/7256 (0.01); p = 0.17], while there were no errors involving benzodiazepines or potassium in the pre- or postperiod |
| Brown et al.30 (US) | N | Computer programme | Computerized worksheet for parenteral nutrition orders | Pre–post | Prescribing | Any prescribing errors associated with TPN orders | Reduction in errors from 44/303 (14.5%) to 12/177 (6.8%); p = 0.016 |
| Cordero et al.38 (US) | N | CPOE + CDS | CPOE with NICU-specific physician order sets | Pre–post | Prescribing and administration | Caffeine loading dose administration > 3 h after being prescribed and gentamicin prescribed dose > 10% deviation from recommended dose | Significant improvement in administration of caffeine within 3 h of prescription (12% versus 63%; p < 0.05), and reduction in gentamicin dosing errors from 16/136 (11.7%) to 0/117 (0%); p < 0.05 |
| Farrar et al.37 (US) | N/P | CPOE + CDS | CPOE system with CDS implementation | Pre–post | Prescribing | Any prescribing error | Reduction in errors from 46/103 (44.7%) orders to 7/114 (6.1%) orders (p < 0.001) |
| Ferranti et al.47 (US) | N/P | CPOE + CDS | CPOE incorporating advanced dosing model | Pre–post | Any type | Any errors resulting in patient harm (e.g. transient adverse effects which required corrective therapy or increased length of stay) | Reduction in errors in NICU from 75/567 (13.2%) to 23/272 (8.5%); p = 0.006 |
| Garner et al.35 (US) | N | CDS | Interactive computerized order set with decision support for antibiotic orders | Pre–post | Prescribing | Any errors in antibiotic prescribing | Overall error rate decreased from 1.7 per medication order to 0.8 per medication order (p < 0.001) |
| Hardmeier et al.33 (US) | N | Barcoding | Implementation of barcode medication administration system | None | Administration | Any nursing-related administration errors | Total of 7/300 (2.3%) nursing-related administration errors reported during study period |
| Hennings et al.20 (US) | N/P | IV administration technology | Automated infusion devices with programmed alerts | None | Administration | Alerts requiring reprogramming events 2.5 times above or below the predefined limits for high-risk medications | Total of 36/5268 infusions (0.7%) required reprogramming; reprogramming much more common in the paediatric compared with adult ICU (RR 1.68 95% CI 1.18–2.38) |
| Hilmas et al.27 (US) | N/P | Computer programme | Computer-based order forms for parenteral nutrition ordering | Pre–post | Prescribing | Any TPN prescribing errors | Reduction in errors from 38/152 (25%) orders to 7/442 (1.6%) orders (p < 0.01) |
| Holdsworth et al.43 (US) | N/P | CPOE + CDS | CPOE system with CDS implementation | Pre–post | Any type | Any error which may result in ADE (dispensing error, overdose, underdose, wrong dose | Reduction in any errors (RR 0.34; 95% CI 0.24–0.49) and serious or life-threatening errors (RR 0.23; 95% CI 0.07–0.80) |
| Jozefczyk et al.59 (US) | N | CPOE | CPOE system implementation | Pre–post | Prescribing | Any prescribing errors | Number of orders without any prescribing errors increased from 209/500 (41.9%) to 480/500 (96%) medication orders (p = 0.001) |
| Kadmon et al.46 (Israel) | N/P | CPOE + CDS | Multistep process of introduction of CPOE, followed by introduction of CPOE + CDS | Pre–post | Prescribing | Any prescribing error | Compared with errors occurring in baseline period [103/1250 (8.2%) orders], there were significant reductions in errors occurring following the introduction of CPOE [97/1250 (7.8%) orders (p = 0.66)], CPOE and CDS [55/1250 (4.4%) orders (p < 0.001)], and further modification to CPOE and CDS system [18/1250 (1.4%) orders (<0.001)] |
| Kazemi et al.48 (Iran) | N | CPOE + CDS | Multistep process of introduction of CPOE, followed by introduction of CPOE + CDS | Pre–post | Prescribing | Dosing errors related to antibiotic and anticonvulsant orders | Compared with errors occurring in baseline period [876/1668 (52%) orders], a similar number occurred following the introduction of CPOE alone [749/1489 (50%) orders], with a reduction following introduction of CPOE and CDS [442/1331 (33%) orders (ptrend <0.001)] |
| Kelly et al.23 (US) | N/P | Computer programme | Electronic infusion calculator | Conventional calculator | Administration | Incorrect infusion rate calculation | Significant improvement in calculation accuracy from 61.9 ± 8.15% to 100 ± 0% (p < 0.001) |
| Larsen et al.19 (US) | N/P | IV administration technology | Automated infusion devices with standard infusion concentrations | Pre–post | Administration | Any administration errors involving one of the standardized medications | Absolute risk reduction of 2.3 errors per 1000 medication doses (95% CI 1.1–3.4) |
| Lehmann et al.26 (US) | N | Computer programme | Online parenteral nutrition calculator | Pre–post | Prescribing | Any TPN prescribing errors | Reduction in errors from 60/557 (10.7%) orders to 20/471 (4.2%) orders (p < 0.001) |
| Lehmann et al.28 (US) | N | Computer programme | Online parenteral nutrition calculator | Pre–post | Prescribing | Any prescribing errors associated with TPN orders | Reduction in errors from 60/557 (10.7%) orders to 8/656 (1.2%) orders (p < 0.001) |
| Lehmann et al.42 (US) | N/P | CPOE + CDS | Web-based calculator for IV continuous infusions | Pre–post | Prescribing | Any prescribing errors involving medication infusions | Reduction in errors from 35/129 (27%) orders to 8/142 (6%) orders (p < 0.001) |
| Maat et al.49 (Netherlands) | N | CPOE + CDS | Computerizing prescribing and calculating system on hypo/hyper-glycaemia | Pre–post | Prescribing | Calculation error of glucose intake | No difference in incidence of hypoglycaemia [4.0/100 hospital days (95% CI 3.2–4.8) to 3.1/100 hospital days (2.7–3.5), p = 0.88)] or hyperglycaemia [6.0/100 hospital days (95% CI 4.3–7.7) to 5.0/100 hospital days (3.7–6.3), p = 0.75] |
| MacKay et al.50 (US) | N/P | CPOE + CDS | Electronic ordering and compounding system for parenteral nutrition | Pre–post | Any type | Any errors involved in prescribing, transcribing, preparation, and administration of TPN | Reduction from 15.6/1000 orders to 2.7/1000 orders (p < 0.001) |
| Manrique-Rodriguez et al.21 (Spain) | N/P | IV administration technology | Automated infusion devices with programmed alerts | None | Administration | Compliance with drug library | After 9 months of implementation, overall compliance with the drug library was 85%, with 94% of nursing staff recommending the introduction of this technology in other units |
| Manrique-Rodriguez et al.22 (Spain) | N/P | IV administration technology | Automated infusion devices with programmed alerts | None | Administration | Compliance with drug library, and smart-pump programming errors | Overall user compliance 78%, leading to interception of 92 errors (from 486,875 programming events; 0.02%) of which 42% of intercepted errors were considered to be catastrophic |
| Menke et al.25 (US) | N/P | Computer programme | Computerized clinical documentation system | Pre–post | Administration | Medication administration delay (difference between scheduled administration times versus actual administration time) | Increase in medication administration delay from 8.5 ± 27.9 min to 16.9 ± 34.9 min (p < 0.01) |
| Morriss Jr et al.31 (US) | N | Barcoding | Barcode medication administration system | Pre–post | Administration | Any nursing-related administration errors | Reduction in likelihood of preventable ADE (HR 0.53; 95% CI 0.29–0.91) |
| Morriss Jr et al.32 (US) | N | Barcoding | Barcode medication administration system | Pre–post | Administration | Any nursing-related administration errors | Reduction in likelihood of preventable ADE (HR 0.48; 95% CI 0.23–0.98) |
| Myers et al.36 (US) | N | CPOE + CDS | CPOE system with CDS implementation | Pre–post | Any type | Any error leading to adverse drug reaction report | Reduction in errors from 3.2 to 0.6 per 1000 patient days |
| Peverini et al.34 (US) | N | CDS | Graphic user interface for parenteral nutrition decision support | Pre–post | Prescribing | Any TPN prescribing errors | Reduction in errors from 62/266 (23.3%) orders to 0/290 (0%) orders (p < 0.001) |
| Potts et al.39 (US) | N/P | CPOE + CDS | CPOE system with CDS implementation | Pre–post | Prescribing | Any prescribing errors | Reduction in errors from 2662/6803 (39.1%) orders to 110/7025 (1.6%) orders (p < 0.001) |
| Russell et al.18 (US) | N/P | CPOE + computer programmes | CPOE with bidirectional interface between pharmacy and CPOE systems for infusion-pump orders | Pre–post | Prescribing and administration | Any error related to prescribing or administration of infusions | Reduction in errors with IV fluids from 97/231 to 46/152 orders (p = 0.01), with smaller reduction in errors with medication (72/296 to 54/303 orders; p = 0.05) |
| Skouroliakou et al.29 (Greece) | N | Computer programme | Computer-assisted parenteral nutrition ordering programme | Pre–post | Prescribing | Any prescribing errors associated with TPN orders | Reduction in errors from 28/941 (3%) orders to 0/941 (0%) orders (p < 0.001) |
| Taylor et al.52 (US) | N | CPOE | CPOE system implementation | Pre–post | Administration | Any administration errors | Reduction in errors from 50/253 (20%) administration episodes to 31/268 (12%) administration episodes (RR 0.53 95% CI 0.33–0.84) |
| Trotter and Maier53 (Germany) | N/P | CPOE | CPOE system implementation | Pre–post | Prescribing | Any prescribing errors involving parenteral nutrition or IV medications | Reduction in errors from 484/4118 (12%) orders to 3/5480 (0.1%) orders (p < 0.001) |
| Upperman et al.51 (US) | N/P | CPOE | CPOE system implementation | Pre–post | Any type | Any error leading to possible or actual ADE | No reduction in total errors from 0.3 ± 0.04/1000 doses to 0.37 ± 0.04/1000 doses (p = 0.3), but reduction in harmful ADEs from 0.05 ± 0.017/1000 doses to 0.03 ± 0.003 doses (p = 0.05) |
| Vardi et al.44 (Israel) | N/P | CPOE + CDS | CPOE system with CDS implementation | Pre–post | Prescribing | Any prescribing errors related to resuscitation medication orders | Reduction in errors from 3/13,124 (0.02%) orders to 0/46,970 (0%) orders |
| Walsh et al.45 (US) | N/P | CPOE + CDS | CPOE system with CDS implementation | Pre–post | Any type | Any error leading to potential or actual patient harm | No difference in serious medication errors from 31.7/1000 patient-days to 33.0/1000 patient-days (IRR 1.04; 95% CI 0.70–1.54) with slight reduction in errors causing patient harm from 7.9/1000 patient-days to 6.5/1000 patient-days (IRR 0.83; 95% CI 0.37–1.87) |
| Warrick et al.54 (UK) | N/P | CPOE | CPOE system implementation | Pre–post | Prescribing and administration | Any prescribing and administration errors | Reduction in prescribing errors from 14/159 (9%) orders to 12/257 (5%) orders (p = 0.09) and administration errors from 43/528 (8%) to 4/278 (1%) orders (p < 0.05) |
| Organizational | |||||||
| Aguado-Lorenzo et al.70 (US) | N | Medication distribution and supply | Preparation of ready-to-use morphine infusion from pharmacy | Morphine infusions prepared on ward by nurses | Administration | Deviation (>7.5%) from labelled concentration | Number of infusions outside of acceptable concentration limits lower among those prepared by pharmacy compared with those prepared on the ward by nurses [19/99 (19.2%) versus 9/115 (7.8%); p = 0.015] |
| Allegeart et al.68 (US) | N | Medication distribution and supply | Use of paediatric amikacin vials (50 mg/ml) | Preparation of doses from adult-strength vials (250 mg/ml) | Administration | Inability to achieve target plasma concentrations/pharmacokinetic parameters | Achievement of target concentrations higher with use of paediatric vial compared with adult-strength vial [40/56 (72%) versus 44/75 (58%); p = 0.132] |
| Broussard et al.59 (US) | N/P | GPPs | Implementation of preformatted order sheets with dosing instructions and sedation monitoring form | Pre–post | Prescribing | Any prescribing errors (e.g. wrong dose, units) relating to sedatives | Reduction in medication-ordering errors, including using units/kg (p < 0.05), ordering of the appropriate reversal agent (p = 0.02), and correct medication dosage (p < 0.001) |
| Conroy69 (US) | N/P | Medication distribution and supply | Use of medications licensed for use in paediatrics | Unlicensed/off-label medication use | Any type | All medication errors identified by clinical staff | Unlicensed/off-label medication use in neonates associated with more medication errors (OR 5.81; 2.32–14.55) |
| Hilmas et al.62 (US) | N | GPPs | Parenteral nutrition prescribing process | None | Prescribing | Any prescribing and transcribing errors related to parenteral nutrition orders | Prescribing process demonstrated 50–60% compliance with recommended standards, while pharmacist interventions were made for 5% of orders |
| Kazemi et al.71 (Iran) | N | Nurse prescribing | Transcription of order by nurse into electronic prescribing programme | Physician order directly into electronic prescribing programme | Prescribing | Any errors related to use of antibiotics or anticonvulsants | Involvement of nurses in prescribing resulted in reduction in medication errors (RR 0.50; 0.50–0.71) |
| O’Brodovich and Rappaport66 (Canada) | N/P | Medication distribution and supply | Unit dose drug distribution system | Pre–post | Administration | Any administration errors | Observed medication incident rates decreased from 10.3% to 2.9% (p < 0.05) and the nurses’ time spent on medication-related activities decreased from 23.7% to 21.6% |
| Olsen et al.65 (Denmark) | N/P | Medication distribution and supply | Implementation of a satellite pharmacy incorporating unit dose drug distribution | Pre–post | Administration | Any administration errors | Introduction of satellite pharmacy led to overall increase in errors from 389/856 (45%) to 280/540 (52%; p = 0.020), but a reduction in serious errors from 66/856 (7.7%) to 0/544 (0%; p < 0.05) |
| Palmero et al.64 (Switzerland) | N | GPPs | Implementation of preformatted order sheets | Pre–post | Prescribing | Any error related to prescribing identified by pharmacist review of all medication orders | Significant reduction in prescribing errors [146/505 (28.9%) versus 71/525 (13.5); p < 0.05] |
| Roman57 (US) | N/P | GPPs | Standardized infusion concentrations (SC) | None | Administration | Any administration-related errors | In the 2 years since the implementation of SC, only five medication errors involving medication administration were identified |
| Ross et al.56 (UK) | N/P | GPPs | Introduction of pharmacy dispensing double-check; education of nursing staff regarding IV administration; nonpunitive error reporting policy | Pre–post | Any type | Any reported medication errors | Introduction of double checking policy with pharmacy dispensing led to reduction in medication errors from 9.8 per year to 6 per year. Introduction of increased education of nursing staff regarding IV administration led to reduction in medication errors from 37 per year to 32 per year. Change in error reporting form to make it less punitive increased the error reporting rate from 33 per year to 38 per year |
| Sturgess et al.60 (UK) | N/P | GPPs | Implementation of zero-tolerance prescribing policy incorporating a dedicated prescribing area and daily feedback of prescribing errors | Pre–post | Prescribing | Any error related to prescribing (e.g. wrong drug, dose, frequency) | Reduction in prescribing errors from 969/1111 patient days (87%) to 796/1781 patient days (45%) (p < 0.001) |
| Thomas et al.61 (UK) | N | GPPs | Introduction of standardized gentamicin pathway for prescribing and monitoring | Pre–post | Administration | Errors related to gentamicin administration and monitoring (e.g. not given within 60 min of scheduled dose, inappropriate action take after level results) | Introduction led to significant improvement in number of doses given within 60 min of scheduled dosing time (82% versus 73%; p = 0.02), documentation of gentamicin level (78% versus 62%; p = 0.04), appropriate action taking according to level result (77% versus 61%; p = 0.04), and documentation of length of gentamicin therapy (61% versus 42%; p = 0.045) |
| Valizadeh et al.63 (Iran) | N | GPPs | Preparation of oral solutions using tablets | Target oral solution strength | Administration | Accuracy of prepared dose concentration for spironolactone and captopril prepared oral solutions | Significant differences and variability in prepared oral solution strength compared with the prescribed dose. The difference was statistically significant for captopril (0.35 ± 0.41 mg; p < 0.001), but not spironolactone (0.23 ± 1.58 mg; p = 0.26) |
| Watanachai et al.67 (Thailand) | N | Medication distribution and supply | Use of needle nonremovable syringes for preparation of medication dilutions | Use of needle removable syringes | Administration | Inaccuracy in preparation of insulin infusion compared with prescribed dose | Compared with target concentration of 300 µU/ml, preparation of infusion using needle nonremovable syringes was most accurate (335 ± 28 µU/ml) compared with the use of needle removable syringes [Terumo(R) 540 ± 54 µU/ml; Nipro(R) 617 ± 45 µU/ml) |
| White et al.58 (US) | N/P | GPPs | Introduction of mandatory medication request form for potassium chloride | Pre–post | Prescribing | Post-infusion elevation of serum potassium > 4.35 mmol/l | Significant reduction in errors from 103/1341 (7.7%) to 0/150 (0%); p < 0.001 |
| Personnel | |||||||
| Alemanni et al.77 (Canada) | N/P | Education | Education to nursing staff regarding medication process including drug verification, preparation, and administration | Pre–post | Administration | Any nursing errors in medication preparation and administration process | Increase in overall compliance with all steps of the medication administration process from 23/142 (16%) administration episodes to 39/140 (28%) administration episodes (p = 0.021) |
| Campino et al.74 (Spain) | N | Education | Comprehensive preventive educational strategy delivered by pharmacist on medication errors | Pre–post | Prescribing | Any prescribing errors | Reduction in errors from 868/4182 (21%) orders to 47/1512 (3%) orders (p < 0.001) |
| Chedoe et al.82 (Netherlands) | N | Education | 1 h theoretical teaching session to nurses, individual practical teaching session of commonly used medications; guided pharmacy tour | Pre–post | Administration | Any preparation and administration errors | Reduction in errors from 151/311 (49%) administration episodes to 87/284 (31%) administration episodes (p < 0.001) |
| Eisenhut et al.80 (UK) | N/P | Education | Personalized assessment and feedback for medical trainees | Pre–post | Prescribing | Any prescribing errors | Reduction in total errors from 188/421 patients to 120/588 patients (p < 0.05) and reduction in major errors from 36/421 patients to 35/588 patients (p < 0.001) |
| Gordon et al.81 (UK) | N/P | Education | E-learning resources for paediatric prescribing for trainee doctors | No access to e-learning resource | Prescribing | Total score on prescribing assessment | Intervention associated with higher mean score on prescribing assessment at 4 weeks (79 ± 12.1 versus 63 ± 13.5) and 12 weeks (79 ± 10.1 versus 69 ± 12.4; p < 0.001) postintervention |
| Leonard et al.73 (US) | N/P | Education | Educational website with competency examination; distribution of a personal digital assistant-based standardized dosing reference; zero-tolerance policy for incomplete/incorrect orders; prescriber performance feedback; publicizing of error/data | Pre–post | Prescribing | Any prescribing errors | Absolute risk reduction in errors of 38/100 orders written (p < 0.001) |
| Ligi et al.78 (France) | N | Education | Continuous incident reporting and subsequent educational interventions to combat identified errors | Pre–post | Any type | Any error leading to patient harm | Reduction in incidence of severe errors from 7.6 to 4.8 per 1000 patient-days (p = 0.005), as well as reduction in 10-fold dosing errors from 2.3/100 admissions to 0.6/100 admissions (p = 0.022) |
| Munoz Labian et al.72 (Spain) | N | Education | Education programme regarding common medication errors occurring within the neonatal unit | Pre–post | Prescribing | Any prescribing errors | Reduction in illegible orders from 22% to 8% (p = 0.005), missing route of administration from 28% to 5% (p < 0.001) and missing dose calculation from 54% to 22% (p < 0.001); no change in dosing errors from 4% to 4% |
| Niemann et al.84 (US) | N/P | Education | Multifaceted intervention including: provision of information on common medication preparation errors; training course on error prevention and drug handling; and provision of a comprehensive reference book | Pre–post | Administration | Any preparation error | Overall frequency of errors decreased from 527/581 (91%) administration episodes to 116/441 (26%) administration episodes (p < 0.001) |
| Raja Lope et al.75 (Malaysia) | N | Education | Education programme regarding medication administration process for nursing staff | Pre–post | Administration | Noncompliance to 10 standard medication administration steps | Reduction in medication administration errors from 59/188 (31%) to 26/169 (15%) administration episodes (p < 0.001) |
| Sagy76 (US) | N/P | Education | Education programme on medication prescribing for residents and nursing staff | Pre–post | Prescribing | Any prescribing error | Reduction in errors from 533/256 orders (2.1/order) to 38/140 orders (0.3/order); p < 0.05 |
| Sullivan et al.79 (US) | N/P | Education | Interactive online nursing educational module on reducing insulin administration errors | Pre–post | Administration | Administration errors involving insulin (e.g. wrong dose, incorrect documentation, inadequate monitoring following dose) | Reduction in errors from 131/882 (15%) episodes to 19/119 (2%) episodes (p < 0.001) |
| Sullivan et al.83 (US) | N | Education | Personalized performance feedback of prescribing errors | Pre–post | Prescribing | Any prescribing error involving opioids and antibiotics | Reduction in opioid related errors by 83%, with increase in number of days between opioid prescribing errors from 3.94 days to 22.63 days; no change in number of days between antibiotic prescribing errors (averaged 2.14 days) |
| Pharmacy | |||||||
| Condren et al.86 (US) | N/P | Pharmacy service | Paediatric clinical pharmacy service | None | Any type | All actual or potential medication errors requiring pharmacist intervention | 4605 interventions performed for 3978 patients with 223 adverse drug events or medications errors prevented or detected during the study period |
| Folli et al.92 (US) | N/P | Pharmacy service | Dispensary-based pharmacy service | None | Prescribing | Errors in medication order (e.g. wrong drug, dose, frequency, route, illegible order, drug–drug interaction, drug–disease interaction) | Overall error rates for the two hospitals were 1.35 and 1.77 per 100 patient-days, and 4.9 and 4.5 per 1000 medication orders, respectively |
| Gibson et al.91 (UK) | N/P | Pharmacy service | Dispensary-based pharmacy service | Pre–post | Prescribing | Errors in medication order (e.g. wrong drug, dose, frequency, route, illegible order, drug–drug interaction, drug–disease interaction) | No significant reduction in prescribing errors (53/439 (12%) versus 46/441 (10%); p = 0.577) |
| Kaushal et al.87 (US) | N/P | Pharmacy service | Ward-based paediatric clinical pharmacy service | Pre–post | Any type | Any actual or potential medication errors relating to prescribing, transcribing, dispensing, administering, or monitoring | Reduction in medication errors from 29/1000 patient-days to 6/1000 patient-days (p < 0.01) |
| Khan et al.90 (India) | N | Pharmacy service | Ward-based paediatric clinical pharmacy service | None | Any type | All actual or potential medication errors requiring pharmacist intervention | Medication errors identified in 80 of 150 patients; total of 87 interventions made, with 60 accepted by clinician |
| Krupicka et al.85 (US) | N/P | Pharmacy service | Ward-based paediatric clinical pharmacy service | None | Any type | All actual or potential medication errors requiring pharmacist intervention | A total of 172 recommendations made for 77 of 215 patients, equivalent to 35 recommendations per 100 patient-days |
| Takata et al.93 (US) | N/P | Pharmacy service | Dispensary-based pharmacy service | None | Prescribing | Errors in medication order (e.g. wrong drug, dose, frequency, route, illegible order, drug–drug interaction, drug–disease interaction) | A total of 2.67 (95% CI 2.4–3.0) interventions per 1000 patient-days and 0.82 (0.73–0.91) interventions per 1000 medication orders; 12% (8.8–15.9%) of interventions occurred in NICU |
| Tripathi et al.89 (US) | N/P | Pharmacy service | Ward-based paediatric clinical pharmacy service | None | Any type | All actual or potential medication errors requiring pharmacist intervention | Total of 27,773 interventions related to 10,963 admissions, with 22,765 (80%) interventions resulting in change in therapy or monitoring |
| Zhang et al.88 (China) | N/P | Pharmacy service | Ward-based paediatric clinical pharmacy service | None | Any type | All actual or potential medication errors requiring pharmacist intervention | Interventions resulted from a total of 31 medication errors identified from 683 prescriptions (4.5%) |
| Hazard and risk analysis | |||||||
| Apkon et al.95 (US) | N/P | Quality improvement tools | Redesign of medication infusion ordering; preparation; and administration process | Pre–post | Prescribing and administration | Failure mode effects analysis (FMEA) for severity (S), likelihood of error occurrence (O), likelihood that failures will escape detection (D) before causing harm RPN (risk priority number = S × O × D) assigned | According to FMEA analysis, changes in process led to significant reduction in criticality index associated with the following processes: prescribing the correct rate (136 to 26), calculating the correct amount of medication to prepare infusion (234 to 49), preparation of infusion (314–88), programming of infusion pump (269 to 99) |
| Bonnabry et al.96 (Switzerland) | N/P | Quality improvement tools | Use of failure modes; effects, and criticality analysis (FMECA) to improve TPN production process | Pre–post | Prescribing and manufacturing | Any errors related to parenteral nutrition from prescribing to manufacture | Significant 59% reduction in criticality index for TPN production process from 3415 to 1397 |
| Frey et al.94 (Switzerland) | N/P | Safe learning systems | Use of critical incident reporting to implement changes to prevent medication errors | None | Any type | Any real or potential harm resulting from errors in the medication management process | A total of 284 critical incident reports were made over a 12-month process, with suggestions to prevent such future incidents provided in 62% of reports; overall, 46 critical incident reports were followed by system changes |
| Li et al.98 (US) | N | Error detection tool | Automated detection of adverse events and medical errors | Voluntary incident reporting | Prescribing and administration | Errors related to narcotic dosing and administration | 18 errors identified through automated detection, only one of which was identified through voluntary incident reporting or use of a trigger tool with PPV of 39–100% |
| Li et al.99 (US) | N | Error detection tool | Automated detection of medication administration errors | Voluntary incident reporting | Administration | Errors related to administration of incorrect dose compared with prescription | Similar specificity (98.2% versus 100%), but much greater sensitivity (82.1% versus 5.5%) and precision (70.2% versus 50.0%) than incident reporting for error recognition |
| Suresh et al.10 (US) | N | Quality improvement tools | Numerous safety projects including education/training; use of FMEA; improving preparation and administration, etc. | None | Any type | Any real or potential harm resulting from errors in the medication management process | Multisite sharing of critical incident reports useful in identifying common medication errors occurring in the NICU setting |
| Takata et al.93 (US) | N/P | Error detection tool | Use of electronic trigger tool | Voluntary incident reporting | Any type | Any injury, large or small, caused by the use (including nonuse) of a medication | Use of trigger tool identified more ADEs than voluntary incident reports (22.3/1000 versus 1.7/1000; p < 0.001), with a positive predictive value of 4.7% (3.7–5.8%) |
| Arenas Villafranca et al.97 (Spain) | N | Quality improvement tools | Use of failure modes; effects; analysis and development of checklist to improve TPN production process | Pre–post | Prescribing and manufacturing | Any errors related to parenteral nutrition from prescribing to manufacture | Use of FMEA identified a total of 82 possible failures; the development of a checklist to address potential failures reduced mean criticality index from 137 to 48 for each item |
| Multifactorial | |||||||
| Abstoss et al.111 (US) | N/P | GPPs; education; technology | Seven overlapping interventions including: poster tracking of errors; performance metric display in staff lounge; multiple didactic curricula; unit-wide emails summarizing medication errors; CPOE; introduction of unit-based pharmacy technicians; and patient safety report form streamlining | Pre–post | Any type | Any real or potential harm resulting from errors in the medication management process | Reported error rate increased from 3.16/10,000 to 3.95/10,000 dispensed doses (p = 0.09); errors causing harm reduced from 0.56/10,000 to 0.16/10,000 doses dispensed (p < 0.001) |
| Alagha et al.115 (Egypt) | N/P | GPPs; education; pharmacy | New structured medication order chart; physician education; provision of dosing guide; and physician performance feedback | Pre–post | Prescribing | Any prescribing error | Reduction in errors from 1107/1417 to 391/1097 (78.1% versus 35.6%; p < 0.001) |
| Booth et al.104 (UK) | N/P | GPPs; education | Application of prescribing guidelines with a zero-tolerance policy; providing feedback and education | Pre–post | Prescribing | Any prescribing errors | Reduction from 892/1000 to 447/1000 errors per occupied bed days (p < 0.001) |
| Bullock et al.100 (US) | N/P | GPPs; education | Development ; dissemination and implementation of standardized IV infusion concentration list; intensive education and one-on-one coaching and mentoring | Pre–post | Administration | Any preparation or administration errors involving parenteral medications | Reduction in percentage of IV infusion orders that did not have standardized IV concentration used from 31/120 (26%) to 17/128 (13%), as well as reduction in associated medication errors related to improper dose from 26/50 (52%) to 7/28 (25%) and reduction in medication errors related to improper concentration from 6/26 (23%) to 0/7 (0%) |
| Burmester et al.102 (US) | N/P | GPPs; education | Post-cardiac surgery admission prescription forms; systematic physician education; publicizing error rates | Pre–post | Prescribing | Any prescribing error | Reduction in errors from 613/3648 to 366/8929 (16.8% versus 4.1%; p < 0.001) |
| Campino et al.106 (Spain) | N | GPPs; education | Protocol standardization and educational programme consisting of theoretical and practical teaching session | Pre–post | Administration | Calculation errors (i.e. dose drawn up versus prescribed dose), or accuracy errors (i.e. theoretical concentration versus actual concentration) in preparing IV medications | Reduction in calculation errors from 6/444 to 0/291 (1.35% versus 0%; p = 0.086) and accuracy errors from 243/444 to 61/291 (54.7% versus 23%; p < 0.001) |
| Cimino et al.112 (US) | N/P | GPPs; education; pharmacy | Various interventions delivered across different sites including: preprinted order sheets; provision of real-time feedback to prescribers on medication errors; improving availability of dosing guides; increase in pharmacist staffing; publicizing medication errors | Pre–post | Prescribing | Any prescribing error | Reduction in errors from 3259/12,026 to 217/9187 (27.9% versus 23.7%; p < 0.001) and reduction in harmful errors from 16/12,026 to 3/9187 (0.13% versus 0.03%) |
| Costello et al.113 (US) | N/P | GPPs; education; pharmacy | Multiple interventions introduced over two phases including: introduction of a clinical pharmacist; paediatrics clinical pharmacist-led medication safety team; new incident reporting form and educational forums | Pre–post | Any type | Any errors | Significant increase in number of errors reported, while errors identified as being severe reduced from 46% to 8% and then 0% over each phase |
| Davey et al.103 (UK) | N/P | GPPs; education | Junior doctor prescribing tutorial and introduction of a bedside prescribing guideline | Pre–post | Prescribing | Dose >10% deviation from guideline or good prescribing practices not followed | Prescribing tutorial associated with reduction in errors from 76/249 to 44/266 (30.5% versus 16.5%; p = 0.023) but no further reduction with subsequent implementation of bedside guideline [59/320 (18.4%) to 56/330 (17.0%) p = 0.73] |
| Di Pentima et al.109 (US) | N/P | GPPs; education; technology | Development of antimicrobial stewardship programme on vancomycin utilizing CPOE + CDS together with provision of individualized real-time feedback | Pre–post | Prescribing | Incorrect vancomycin order according to clinical indications, microbiology data or dosing guidelines | Reduction in errors from 1.8/1000 patient days to 1.4/1000 patient days (p < 0.05) |
| Hilmas et al.110 (US) | N/P | GPPs; education; technology | Development of standardized approach to deliver continuous infusions; CPOE + CDS with standardized concentrations; smart-pump infusions and intensive educational sessions | Pre–post | Prescribing and administration | Incorrect continuous infusion syringe concentration, incomplete and illegible orders; incorrect administration rate and dose | Reduction in errors from 98/200 to 0/200 (49% versus 0%) |
| Irwin et al.107 (Canada) | N/P | GPPs; education; technology | Standardizing infusion concentrations; CDS to assist with drug concentration and infusion rate; competency evaluation of staff with provision of educational programme | Pre–post | Any type | Any errors reported through incident monitoring programme involving parental medications | No change in errors from 2.4/year to 2.0/year |
| Martinez-Anton et al.105 (Spain) | N/P | GPPs; education | Standardizing of dosing guidelines; pocket tables with dosing guidelines; updated protocols; education programme on correct prescribing | Pre–post | Prescribing and administration | Any medication error related to prescribing or administration (e.g. wrong medication, frequency, route, dose) | Reduction in errors from 761/2228 to 388/1791 (34.2% versus 21.7%; p < 0.001) |
| Otero et al.114 (Argentina) | N/P | GPPs; education; pharmacy | Positive safety culture with nonpunitive management of medication errors; active interaction with pharmacists during ward rounds; provision of education regarding medication prescribing and administration | Pre–post | Prescribing and administration | Any medication error related to prescribing or administration (e.g. wrong medication, frequency, route, dose) | Reduction in errors related to prescribing, from 102/590 to 105/1144 (17% versus 9%; p < 0.05) and administration from 150/1174 to 99/1588 (13% versus 6%; p < 0.05) |
| Pallás et al.108 (Spain) | N | GPPs; education; technology | Education regarding good prescribing practice; implementation of a pocket personal computer-based automatic calculation system | Pre–post | Prescribing | Any medication error related to prescribing (e.g. wrong medication, frequency, route, dose) | Reduction in errors from 2498/6320 to 171/1435 (39.5% versus 11.9%; p < 0.001) |
| Simpson et al.117 (UK) | N | Education; pharmacy | Pharmacist-led education programme; daily pharmacist review of medication orders; competency assessment of all neonatal unit staff; and greater publicizing of medication errors | Pre–post | Prescribing and administration | Any medication error related to prescribing or administration (e.g. wrong medication, frequency, route, dose) | Reduction in errors from 24.1/1000 to 5.1/1000 patient-days (p = 0.037) |
| Wang et al.116 (US) | N/P | Pharmacy; technology | Utilization of clinical pharmacists to review and intercept any adverse drug events and CPOE | None | Any type | Any error within the medication process | Total of 865 errors identified, of which 178 considered potentially harmful; clinical pharmacists intercepted 96/178 (54%) errors, while the addition of a CPOE had the potential to intercept 130/178 (73%) errors |
| Yamanaka et al.101 (Brazil) | N/P | GPPs; education | Redevelopment of the nursing administration process; provision of nursing education regarding medication errors; provision of dosing guidelines | Pre–post | Prescribing and administration | Any medication error related to prescribing or administration (e.g. wrong medication, frequency, route, dose) | Reduction in errors from 1717/8152 to 1498/8550 (29% versus 22%; p < 0.001) |
ADE, adverse drug event; CDS, clinical decision support; CI, confidence interval; CPOE, computerized physician order entry; GPPs, guidelines, policies, and procedures; HR, hazard ratio; ICU, intensive care unit; IRR, incident rate ratio; IV, intravenous; N, neonatal only; NICU, neonatal intensive care unit; N/P, neonatal and paediatric; OR, odds ratio; PICU, paediatric intensive care unit; PPV, positive predictive value; RR, relative risk; TPN, total parenteral nutrition.
Of the 102 eligible studies, 86 were comparative studies and 16 were noncomparative studies (Table 3). The majority of included studies were undertaken in a combined PICU/NICU setting (n = 64; 63%). Most studies were conducted in the United States (n = 60; 59%), with 42 (41%) studies published since 2010. The majority of studies (n = 68; 66%) focused on a single medication error type, including prescribing (m = 41), administration (n = 26), or monitoring (n = 1), while the remaining 34 (33%) studies involved the investigation of multiple error types.
Table 3.
Aggregate data synthesis for included studies by comparator status.
| Characteristic | Number of studies (%) |
||
|---|---|---|---|
| Total (n = 102) | Comparative (n = 86) | Noncomparative (n = 16) | |
| Location of study | |||
| Argentina | 1 (1) | 1 (1) | 0 |
| Brazil | 1 (1) | 1 (1) | 0 |
| Canada | 3 (3) | 3 (3) | 0 |
| China | 1 (1) | 0 | 1 (6) |
| Denmark | 1 (1) | 1 (1) | 0 |
| Egypt | 1 (1) | 1 (1) | 0 |
| France | 1 (1) | 1 (1) | 0 |
| Germany | 1 (1) | 1 (1) | 0 |
| Greece | 1 (1) | 1 (1) | 0 |
| India | 1 (1) | 0 | 1 (6) |
| Iran | 3 (3) | 3 (3) | 0 |
| Israel | 2 (2) | 2 (2) | 0 |
| Malaysia | 1 (1) | 1 (1) | 0 |
| Netherlands | 2 (2) | 2 (2) | 0 |
| Spain | 9 (9) | 7 (8) | 2 (13) |
| Switzerland | 3 (3) | 2 (2) | 1 (6) |
| Thailand | 1 (1) | 1 (1) | 0 |
| UK | 9 (9) | 9 (10) | 0 |
| US | 60 (59) | 49 (57) | 11 (69) |
| Year | |||
| 1970–1979 | 1 (1) | 1 (1) | 0 |
| 1980–1989 | 2 (2) | 1 (1) | 1 (6) |
| 1990–1999 | 3 (3) | 3 (3) | 0 |
| 2000–2009 | 54 (53) | 47 (55) | 7 (44) |
| ⩾2010 | 42 (41) | 34 (40) | 8 (50) |
| Patient location | |||
| NICU only | 38 (37) | 34 (40) | 4 (25) |
| NICU/PICU | 64 (63) | 52 (60) | 12 (75) |
| Intervention theme | |||
| Technology | 38 (37) | 34 (40) | 4 (25) |
| Multifactorial | 18 (18) | 17 (20) | 1 (6) |
| Organizational | 16 (16) | 14 (16) | 2 (13) |
| Personnel | 13 (13) | 13 (15) | 0 |
| Pharmacy | 9 (9) | 2 (2) | 7 (44) |
| Hazard and risk analysis | 8 (8) | 6 (7) | 2 (13) |
| Individual type of intervention | |||
| CPOE ± CDS | 20 (20) | 20 (23) | 0 |
| Education | 13 (13) | 13 (15) | 0 |
| GPPs | 9 (9) | 7 (8) | 2 (13) |
| Pharmacy services | 9 (9) | 2 (2) | 7 (44) |
| IV administration technology | 4 (4) | 1 (1) | 3 (19) |
| Electronic computer programmes | 7 (7) | 7 (8) | 0 |
| Medication distribution and supply | 6 (6) | 6 (7) | 0 |
| Barcoding | 3 (3) | 2 (2) | 1 (6) |
| CDS | 2 (2) | 2 (2) | 0 |
| Quality improvement tools | 4 (4) | 3 (3) | 1 (6) |
| Error detection tool | 3 (3) | 3 (3) | 0 |
| Multifactorial | 18 (18) | 17 (20) | 1 (6) |
| Other | 4 (4) | 3 (3) | 1 (6) |
| Types of medication errors collected | |||
| Prescribing | 41 (40) | 38 (44) | 3 (19) |
| Administration | 26 (25) | 21 (24) | 5 (31) |
| Monitoring | 1 (1) | 1 (1) | 0 |
| Multiple types | 34 (33) | 26 (30) | 8 (50) |
Abbreviations: CDS, clinical decision support; CPOE, computerized physician order entry; GPPs, guidelines, policies, and procedures; NICU, neonatal intensive care unit; PICU, paediatric intensive care unit; IV, intravenous.
Risk of bias evaluation
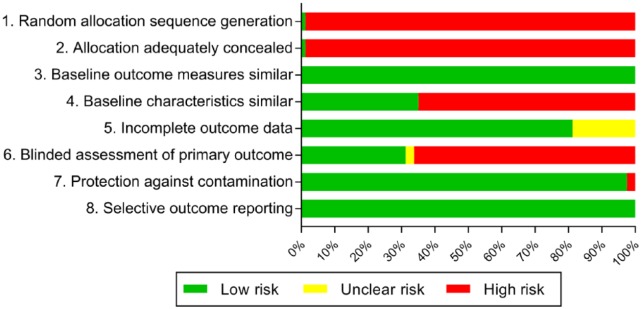
Most studies demonstrated an appreciable risk of bias (Figure 2). The lack of randomized controlled trials meant that studies were at high risk of bias across the domains related to random allocation of intervention and concealment of intervention group. Inadequate reporting or lack of accounting for differences in characteristics between the pre- and postintervention groups and nonblinding of the assessment of the primary outcome were the main areas of inconsistent bias. A number of studies had unclear risk of bias for incomplete outcome data due to the fact that the outcome was reliant on voluntary incident reports from staff rather than detailed review.
Figure 2.
Risk of bias of included studies.
Qualitative synthesis
A breakdown of medication error reduction according to intervention types and medication error types within each intervention theme is presented in Table 4. Based on a qualitative synthesis of comparative studies, the greatest median reduction in overall medication errors was seen with the use of technology-based interventions (73% reduction; n = 33 studies), but this ranged widely from an increase in medication errors of 11% to a decrease in medication errors of 100%. The remaining intervention types produced a similar reduction of medication errors of approximately 50–60%, but again, with a wide range in results.
Table 4.
Qualitative synthesis of reduction in medication errors identified in comparative studies according to intervention theme and type.
| Intervention theme | Intervention type | Medication error type |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall |
Prescribing |
Administration |
Multiple |
||||||
| Median % error reduction (range) | n * | Median % error reduction (range) | n | Median % error reduction (range) | n | Median % error reduction (range) | n | ||
| Technology (n = 34) | Any | 73 (−11–100) | 33 | 85 (25–100) | 18 | 52 (47–100) | 5 | 58 (0–83) | 9 |
| Barcoding | 50 (47–52) | 2 | – | – | 50 (47–52) | 2 | – | – | |
| CDS | 77 (53–100) | 2 | 77 (53–100) | 2 | – | – | – | – | |
| CPOE | 66 (0–99) | 5 | 96 (93–99) | 2 | 47 | 1 | 33 (0–66) | 2 | |
| CPOE + CDS | 78 (−11–100) | 15 | 83 (25–100) | 8 | −11 | 1 | 62 (0–83) | 6 | |
| Computer programmes | 96 (53–100) | 6 | 94 (53–100) | 5 | 100 | 1 | – | – | |
| IV administration technology | 73 | 1 | – | – | 73 | 1 | – | – | |
| Organizational (n = 14) | Any | 50 (−16–100) | 11 | 53 (48–100) | 5 | 37 (−16–72) | 5 | 39 | 1 |
| GPPs | 51 (37–100) | 6 | 59 (48–100) | 4 | 37 | 1 | 39 | 1 | |
| Medication distribution and supply | 46 (−16–72) | 4 | – | – | 46 (−16–72) | 4 | – | – | |
| Personnel (n = 13) | Education | 52 (14–87) | 13 | 56 (14–86) | 7 | 52 (14–87) | 5 | 37 | 1 |
| Hazard and risk analysis (n = 6) | Quality improvement tools | 65 (59–74) | 3 | – | – | – | – | 65 (59–74) | 3 |
| Pharmacy (n = 2) | Any | 48 (17–79) | 2 | 17 | 1 | – | – | 79 | 1 |
| Ward based | 79 | 1 | – | – | – | – | 79 | 1 | |
| Dispensary based | – | – | 17 | 1 | – | – | – | – | |
| Multifactorial (n = 17) | Any | 54 (15–100) | 17 | 50 (15–76) | 7 | 65 (63–67) | 2 | 61 (17–100) | 8 |
| GPPs + education | 50 (24–76) | 7 | 50 (44–76) | 3 | 65 (63–67) | 2 | 31 (24–37) | 2 | |
| GPPs + education + pharmacy | 53 (15–100) | 4 | 35 (15–54) | 2 | – | – | 75 (51–100) | 2 | |
| GPPs + education + technology | 70 (17–100) | 5 | 46 (22–70) | 2 | – | – | 71 (17–100) | 3 | |
CDS, clinical decision support; CPOE, computerized physician order entry; GPPs, guidelines, policies, and procedures; IV, intravenous.
Does not add up to total number of comparative studies within each theme as not all studies reported an error risk difference.
Eight studies [consisting of a combination of technology- (n = 4), personnel- (n = 2), organizational- (n = 1), and multifactorial-based (n = 1) interventions] reported separately on minor and major medication errors.38,43,45,51,65,78,80,112 Among these studies, the median reduction in medication errors was notably greater for major (76%; 17–100%) compared with minor (26%; −16–66%) medication errors. Notably, one of the studies identified a 16% increase in minor medication errors following the intervention, but a substantial 100% reduction in major errors.65 The corresponding median reduction in medication errors for any intervention was similar for those undertaken in a neonatal-only (56%; 25–100%) or combined neonatal/paediatric (66%; −16–100%) setting. Similarly, median reduction in medication errors for any intervention was similar regardless of whether errors were identified by incident reports (50%; 17–100%) or detailed medication order review (60%; −16–100%).
Discussion
Based on the findings of our review, no single intervention appeared clearly superior in reducing the risk of medication errors, with significant variability evident among studies within and across themes with respect to methods, definitions, and outcomes. Identified interventions often targeted different aspects of the medication management process, highlighting that a combination of interventions is most likely required to achieve a significant reduction in medication errors.
Santesteban and colleagues have published the most recent systematic review on interventions for preventing medication errors in neonatal care.14 They restricted their search to studies undertaken in the neonatal unit setting only, identifying a total of 16 intervention studies published up until 2013. Our search was much more extensive, identifying 34 comparative studies undertaken in the neonatal setting, out of our total of 86 comparative studies included in this review. Despite this discrepancy in number of studies, the findings remain similar in that while many interventions demonstrated significant potential for reducing medication errors, no firm conclusions could be drawn as to which interventions were most effective. Our findings are also similar to that of an earlier systematic review by Rinke and colleagues that included 63 studies across both neonatal and paediatric settings,12 as well as a recent Cochrane review that included findings from just seven studies.13 Both of these reviews observed that the inability to draw firm conclusions was partly due to limited studies in some areas, while also due to significant methodological heterogeneity evident across studies. Further, a consistent issue raised across reviews is whether decreases in medication errors truly relate to benefits for patients in terms of reducing actual harm.
Despite challenges in linking medication errors directly to patient harm, there is evidence of additional benefits from various interventions beyond a reduction in medication errors. For example, Myers and colleagues observed that the introduction of a computerized physician order entry (CPOE) system with clinical decision support (CDS) was also associated with a reduction in phone calls to pharmacy.36 Similarly, Vardi and colleagues also demonstrated that this technology was associated with a reduction in the time taken to order resuscitation medications from 14.4 min to 2.1 min (p < 0.001).44 Notably, Maat and colleagues identified no reduction in medication errors associated with their implementation of CDS to assist in managing hypo/hyperglycaemia, but they did observe reductions in time taken to perform simple (1.3; 0.3–2.3 min) or complex orders (8.6; 5.1–12.1 min).49
The potential for altered intervention effectiveness due to local variable factors is raised by Abboud and colleagues, who identified no reduction in gentamicin monitoring errors following the introduction of CDS as part of the prescribing process.41 In this case, the authors suggested that the intervention had minimal benefit because they already had a clinical pharmacist responsible for ensuring monitoring was performed correctly, but results could differ in settings where clinical pharmacists are not present.
A common observation across studies that utilized a staged design to implement CPOE and then CDS was that maximal benefits were not gained until CDS was added.46,48 Intuitively, this makes sense as CDS or computer programmes are usually developed to address activities that have already been predetermined to be high risk, and so have greater potential for reduction in medication errors. Notably, such improvements were not necessarily restricted to technology-based interventions. Less costly interventions involving paper-based prescribing and CDS, such as use of preprinted order forms, were identified as achieving similar reductions in medication errors to more expensive, computer-based approaches.59,64 Therefore, in settings where CPOE systems are not readily available, lower-cost alternative approaches towards CDS are ideal.
An issue common across a number of studies was the need to adequately support staff in the implementation of any interventions, especially those that significantly change current practices. For example, with the introduction of automated infusion devices in drug libraries, there is a reliance on staff using the technology to its full extent in order to obtain maximal benefit. For various reasons, whether it be staff who consider the new process more complicated or too time consuming, work arounds may be created which can lead to medication errors. In introducing automated infusion devices, Manrique and colleagues monitored their use and identified an overall compliance rate of 78–85%.21,22 While the automated infusion devices appeared extremely effective in preventing potentially catastrophic medication errors, compliance was still not ideal. Hennings and colleagues identified that neonatal ICU staff were almost twice as likely (RR 1.68; 1.18–2.38) to reprogramme pumps than adult ICU staff.20 Whether this was just due to staff ignoring or overriding the alerts or because the medication library and associated functions were not sufficiently programmed for use in the neonatal unit is unclear. However, these examples highlight the importance around thorough implementation strategies and the requirement to constantly monitor and evaluate the use of new technologies as they are implemented within the neonatal unit. There is also the constant requisite to review and update such technologies as time goes on, as further advancements are made.
Strengths of our review include the comprehensive literature search strategy and inclusion of a broad range of comparative and noncomparative studies to explore the breadth of research previously undertaken on interventions for reducing medication errors in neonates. This is of particular usefulness in exploring the evaluation of different interventions to support implementation into clinical practice, as well as guide future research priorities.
Notwithstanding the comprehensive nature of our systematic review, several limitations bear consideration. First, significant variability was evident across all included studies, with differences in intervention strategies, trial methods, types of medication errors evaluated, and how medication errors were identified and evaluated. Such heterogeneity has been observed in previous systematic reviews of interventions to reduce paediatric medication errors.12 A key aspect for overcoming limitations in the existing evidence base identified in this review lies in standardization of definitions and research methodologies for medication error studies. In particular, consistent grading of medication errors using universal reporting standards, such as the one endorsed by the National Coordinating Council for Medication Error Reporting and Prevention, would facilitate a greater understanding of the impact of interventions on harmful medication errors.118 This is of importance as only eight of the identified studies reported separately on minor and major medication errors, demonstrating a significant difference in error reduction depending on the definition utilized. Notably, one of the studies identified a 16% increase in minor medication errors following the introduction of the intervention, largely thought due to increased awareness and reporting of errors, but a substantial 100% reduction in major errors.65 Others have also called for more consistent use of denominators that better reflect the total opportunities for error (e.g. prescribing errors per 1000 medication orders), rather than the use of other denominators such as medication errors per patient or per patient day;12 the latter being considered more susceptible to bias from factors such as the criticality of the patient and number of medications being ordered, and limiting ability to accurately compare results across studies.
Conclusion
While neonatal medication errors can be reduced through multiple interventions aimed at improving the medication use process, no single intervention appeared superior. Despite the significant increase in the number of published studies focused on reducing neonatal medication errors, our knowledge of interventions to prevent neonatal medication errors remains hampered through a lack of uniformity in study design, data collection methodology, and outcome reporting. This heterogeneity leads to difficulties in developing clear guidance as to which interventions are best to adopt. Further research is required to evaluate the relative cost-effectiveness of the various medication safety interventions to facilitate decisions regarding uptake and implementation into clinical practice. Ultimately, the choice of the ideal interventions for improving medication safety will likely be an individual one, taking into consideration local resources, together with an understanding of the types and severity of errors that occur within the organization.
Acknowledgments
Luke E Grzeskowiak acknowledges salary support provided through an Australian National Health and Medical Research Council (NHMRC) Early Career Fellowship (ID 1070421).
Footnotes
Authors’ Note: Luke E Grzeskowiak is also affiliated to SA Pharmacy, Flinders Medical Centre, Bedford Park, SA, Australia.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Luke E. Grzeskowiak  http://orcid.org/0000-0001-8554-4696
http://orcid.org/0000-0001-8554-4696
Contributor Information
Minh-Nha Rhylie Nguyen, SA Pharmacy, Flinders Medical Centre, Bedford Park, SA, Australia.
Cassandra Mosel, SA Pharmacy, Flinders Medical Centre, Bedford Park, SA, Australia.
Luke E. Grzeskowiak, Adelaide Medical School, Robinson Research Institute, University of Adelaide, Level 6, AHMS, Adelaide, SA 5000, Australia.
References
- 1. Roughead EE, Semple SJ, Rosenfeld E. The extent of medication errors and adverse drug reactions throughout the patient journey in acute care in Australia. Int J Evid Based Healthc 2016; 14: 113–122. [DOI] [PubMed] [Google Scholar]
- 2. Aronson JK. Medication errors: definitions and classification. Br J Clin Pharmacol 2009; 67: 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kunac DL, Reith DM. Identification of priorities for medication safety in neonatal intensive care. Drug Saf 2005; 28: 251–261. [DOI] [PubMed] [Google Scholar]
- 4. Chedoe I, Molendijk HA, Dittrich ST, et al. Incidence and nature of medication errors in neonatal intensive care with strategies to improve safety. Drug Saf 2007; 30: 503–513. [DOI] [PubMed] [Google Scholar]
- 5. Conroy S, McIntyre J, Choonara I. Unlicensed and off label drug use in neonates. Arch Dis Child Fetal Neonatal Ed 1999; 80: F142–F145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krzyzaniak N, Bajorek B. Medication safety in neonatal care: a review of medication errors among neonates. Ther Adv Drug Saf 2016; 7: 102–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chappell K, Newman C. Potential tenfold drug overdoses on a neonatal unit. Arch Dis Child Fetal Neonatal Ed 2004; 89: F483–F484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Samra HA, McGrath JM, Rollins W. Patient safety in the NICU: a comprehensive review. J Perinat Neonatal Nurs 2011; 25: 123–132. [DOI] [PubMed] [Google Scholar]
- 9. Kaushal R, Bates DW, Landrigan C, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA 2001; 285: 2114–2120. [DOI] [PubMed] [Google Scholar]
- 10. Suresh G, Horbar JD, Plsek P, et al. Voluntary anonymous reporting of medical errors for neonatal intensive care. Pediatrics 2004; 113: 1609–1618. [DOI] [PubMed] [Google Scholar]
- 11. Levine SR, Cohen M, Blanchard N, et al. Guidelines for preventing medication errors in pediatrics. J Pediatr Pharmacol Ther 2001; 6: 426–442. [Google Scholar]
- 12. Rinke ML, Bundy DG, Velasquez CA, et al. Interventions to reduce pediatric medication errors: a systematic review. Pediatrics 2014; 134: 338–360. [DOI] [PubMed] [Google Scholar]
- 13. Maaskant JM, Vermeulen H, Apampa B, et al. Interventions for reducing medication errors in children in hospital. Cochrane Database Syst Rev 2015; 3: CD006208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Santesteban E, Arenas S, Campino A. Medication errors in neonatal care: a systematic review of types of errors and effectiveness of preventive strategies. J Neonatal Nurs 2015; 21: 200–208. [Google Scholar]
- 15. Balk EM, Chung M, Hadar N, et al. Accuracy of data extraction of non-English language trials with Google Translate (Prepared by the Tufts Evidence-based Practice Center under Contract No. 290–2007–10055 I). AHRQ Publication No. 12-EHC056-EF, April 2012. Rockville, MD: Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269. [DOI] [PubMed] [Google Scholar]
- 17. Effective Practice and Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services, http://epoc.cochrane.org/epoc-specific-resources-review-authors (2016, accessed 12 December 2016). [Google Scholar]
- 18. Russell RA, Triscari D, Murkowski K, et al. Impact of computerized order entry to pharmacy interface on order-infusion pump discrepancies. J Drug Deliv 2015; 2015: 686598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Larsen GY, Parker HB, Cash J, et al. Standard drug concentrations and smart-pump technology reduce continuous-medication-infusion errors in pediatric patients. Pediatrics 2005; 116: e21–e25. [DOI] [PubMed] [Google Scholar]
- 20. Hennings S, Romero A, Erstad BL, et al. A comparison of automated infusion device technology to prevent medication errors in pediatric and adult intensive care unit patients. Hosp Pharm 2010; 45: 464–471. [Google Scholar]
- 21. Manrique-Rodriguez S, Sanchez-Galindo A, Fernandez-Llamazares CM, et al. Developing a drug library for smart pumps in a pediatric intensive care unit. Artif Intell Med 2012; 54: 155–161. [DOI] [PubMed] [Google Scholar]
- 22. Manrique-Rodriguez S, Sanchez-Galindo AC, Lopez-Herce J, et al. Impact of implementing smart infusion pumps in a pediatric intensive care unit. Am J Health Syst Pharm 2013; 70: 1897–1906. [DOI] [PubMed] [Google Scholar]
- 23. Kelly KJ, Neu J, Rice TB, et al. Efficacy of a programmed calculator for constant-infusion medication calculations. Pediatrics 1984; 73: 68–70. [PubMed] [Google Scholar]
- 24. Balaguer Santamaría JA, Fernández Ballart JD, Escribano Subias J. Usefulness of a software package to reduce medication errors in neonatal care. An Esp Pediatr 2001; 55: 541–545. [PubMed] [Google Scholar]
- 25. Menke JA, Broner CW, Campbell DY, et al. Computerized clinical documentation system in the pediatric intensive care unit. BMC Med Inform Decis Mak 2001; 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lehmann CU, Conner KG, Cox JM. Provider error prevention: online total parenteral nutrition calculator. Proc AMIA Symp 2002: 435–439. [PMC free article] [PubMed] [Google Scholar]
- 27. Hilmas E, Partyka CM. Implementation of computerized parenteral nutrition orders in a community pediatric hospital. Am J Health Syst Pharm 2004; 61: 273–277. [DOI] [PubMed] [Google Scholar]
- 28. Lehmann CU, Conner KG, Cox JM. Preventing provider errors: online total parenteral nutrition calculator. Pediatrics 2004; 113: 748–753. [DOI] [PubMed] [Google Scholar]
- 29. Skouroliakou M, Konstantinou D, Papasarantopoulos P, et al. Computer assisted total parenteral nutrition for pre-term and sick term neonates. Pharm World Sci 2005; 27: 305–310. [DOI] [PubMed] [Google Scholar]
- 30. Brown CL, Garrison NA, Hutchison AA. Error reduction when prescribing neonatal parenteral nutrition. Am J Perinatol 2007; 24: 417–427. [DOI] [PubMed] [Google Scholar]
- 31. Morriss FH, Jr, Abramowitz PW, Nelson SP, et al. Effectiveness of a barcode medication administration system in reducing preventable adverse drug events in a neonatal intensive care unit: a prospective cohort study. J Pediatr 2009; 154: 363–368, 368.e1. [DOI] [PubMed] [Google Scholar]
- 32. Morriss FH, Jr, Abramowitz PW, Nelson SP, et al. Risk of adverse drug events in neonates treated with opioids and the effect of a bar-code-assisted medication administration system. Am J Health Syst Pharm 2011; 68: 57–62. [DOI] [PubMed] [Google Scholar]
- 33. Hardmeier A, Tsourounis C, Moore M, et al. Pediatric medication administration errors and workflow following implementation of a bar code medication administration system. J Healthc Qual 2014; 36: 54–63. [DOI] [PubMed] [Google Scholar]
- 34. Peverini RL, Beach DS, Wan KW, et al. Graphical user interface for a neonatal parenteral nutrition decision support system. Proc AMIA Symp 2000: 650–654. [PMC free article] [PubMed] [Google Scholar]
- 35. Garner SS, Cox TH, Hill EG, et al. Prospective, controlled study of an intervention to reduce errors in neonatal antibiotic orders. J Perinatol 2015; 35: 631–635. [DOI] [PubMed] [Google Scholar]
- 36. Myers TF, Venable HH, Hansen JA. Computer-enhanced neonatology practice evolution in an academic medical center. NICU clinical effectiveness task force. J Perinatol 1998; 18: S38–S44. [PubMed] [Google Scholar]
- 37. Farrar K, Caldwell NA, Robertson J, et al. Use of structured paediatric prescribing screens to reduce the risk of medication errors in the care of children. Br J Healthcare Comput Inform Manag 2003; 20: 25–27. [Google Scholar]
- 38. Cordero L, Kuehn L, Kumar RR, et al. Impact of computerized physician order entry on clinical practice in a newborn intensive care unit. J Perinatol 2004; 24: 88–93. [DOI] [PubMed] [Google Scholar]
- 39. Potts AL, Barr FE, Gregory DF, et al. Computerized physician order entry and medication errors in a pediatric critical care unit. Pediatrics 2004; 113: 59–63. [DOI] [PubMed] [Google Scholar]
- 40. Boling B, McKibben M, Hingl J, et al. ; Clinical Informatics Outcomes Research Group. Effectiveness of computerized provider order entry with dose range checking on prescribing errors. J Patient Saf 2005; 1: 190–194. [Google Scholar]
- 41. Abboud PA, Ancheta R, McKibben M, et al. ; Clinical Informatics Outcomes Research Group. Impact of workflow-integrated corollary orders on aminoglycoside monitoring in children. Health Informatics J 2006; 12: 187–198. [DOI] [PubMed] [Google Scholar]
- 42. Lehmann CU, Kim GR, Gujral R, et al. Decreasing errors in pediatric continuous intravenous infusions. Pediatr Crit Care Med 2006; 7: 225–230. [DOI] [PubMed] [Google Scholar]
- 43. Holdsworth MT, Fichtl RE, Raisch DW, et al. Impact of computerized prescriber order entry on the incidence of adverse drug events in pediatric inpatients. Pediatrics 2007; 120: 1058–1066. [DOI] [PubMed] [Google Scholar]
- 44. Vardi A, Efrati O, Levin I, et al. Prevention of potential errors in resuscitation medications orders by means of a computerised physician order entry in paediatric critical care. Resuscitation 2007; 73: 400–406. [DOI] [PubMed] [Google Scholar]
- 45. Walsh KE, Landrigan CP, Adams WG, et al. Effect of computer order entry on prevention of serious medication errors in hospitalized children. Pediatrics 2008; 121: e421–e427. [DOI] [PubMed] [Google Scholar]
- 46. Kadmon G, Bron-Harlev E, Nahum E, et al. Computerized order entry with limited decision support to prevent prescription errors in a PICU. Pediatrics 2009; 124: 935–940. [DOI] [PubMed] [Google Scholar]
- 47. Ferranti JM, Horvath MM, Jansen J, et al. Using a computerized provider order entry system to meet the unique prescribing needs of children: description of an advanced dosing model. BMC Med Inform Decis Mak 2011; 11: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kazemi A, Ellenius J, Pourasghar F, et al. The effect of computerized physician order entry and decision support system on medication errors in the neonatal ward: experiences from an Iranian teaching hospital. J Med Syst 2011; 35: 25–37. [DOI] [PubMed] [Google Scholar]
- 49. Maat B, Rademaker CM, Oostveen MI, et al. The effect of a computerized prescribing and calculating system on hypo- and hyperglycemias and on prescribing time efficiency in neonatal intensive care patients. JPEN J Parenter Enteral Nutr 2013; 37: 85–91. [DOI] [PubMed] [Google Scholar]
- 50. MacKay M, Anderson C, Boehme S, et al. Frequency and severity of parenteral nutrition medication errors at a large children’s hospital after implementation of electronic ordering and compounding. Nutr Clin Pract 2016; 31: 195–206. [DOI] [PubMed] [Google Scholar]
- 51. Upperman JS, Staley P, Friend K, et al. The impact of hospitalwide computerized physician order entry on medical errors in a pediatric hospital. J Pediatr Surg 2005; 40: 57–59. [DOI] [PubMed] [Google Scholar]
- 52. Taylor JA, Loan LA, Kamara J, et al. Medication administration variances before and after implementation of computerized physician order entry in a neonatal intensive care unit. Pediatrics 2008; 121: 123–128. [DOI] [PubMed] [Google Scholar]
- 53. Trotter A, Maier L. Computerized physician order entry system in pediatric inpatients: prevention of medication errors and adverse drug events. Monatsschrift fur Kinderheilkunde 2009; 157: 160–165. [Google Scholar]
- 54. Warrick C, Naik H, Avis S, et al. A clinical information system reduces medication errors in paediatric intensive care. Intensive Care Med 2011; 37: 691–694. [DOI] [PubMed] [Google Scholar]
- 55. Jozefczyk KG, Kennedy WK, Lin MJ, et al. Computerized prescriber order entry and opportunities for medication errors: comparison to tradition paper-based order entry. J Pharm Pract 2013; 26: 434–437. [DOI] [PubMed] [Google Scholar]
- 56. Ross LM, Wallace J, Paton JY. Medication errors in a paediatric teaching hospital in the UK: five years operational experience. Arch Dis Child 2000; 83: 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roman N. Innovative solutions: standardized concentrations facilitate the use of continuous infusions for pediatric intensive care unit nurses at a community hospital. Dimens Crit Care Nurs 2005; 24: 275–278. [DOI] [PubMed] [Google Scholar]
- 58. White JR, Veltri MA, Fackler JC. Preventing adverse events in the pediatric intensive care unit: prospectively targeting factors that lead to intravenous potassium chloride order errors. Pediatr Crit Care Med 2005; 6: 25–32. [DOI] [PubMed] [Google Scholar]
- 59. Broussard M, Bass PF, III, Arnold CL, et al. Preprinted order sets as a safety intervention in pediatric sedation. J Pediatr 2009; 154: 865–868. [DOI] [PubMed] [Google Scholar]
- 60. Sturgess E, Booth R, Taberner-Stokes A, et al. Reduction in prescription errors on paediatrics intensive care with ‘zero tolerance prescription’. Pediatr Crit Care Med 2011; 12: A147. [Google Scholar]
- 61. Thomas C, Kamalanathan AN, Subhedar NV. The impact of the introduction of a gentamicin pathway. Arch Dis Child Fetal Neonatal Ed 2011; 96: Fa50–Fa51. [Google Scholar]
- 62. Hilmas E, Peoples JD. Parenteral nutrition prescribing processes using computerized prescriber order entry: opportunities to improve safety. JPEN J Parenter Enteral Nutr 2012; 36: 32S–35S. [DOI] [PubMed] [Google Scholar]
- 63. Valizadeh S, Rasekhi M, Hamishehkar H, et al. Medication errors in oral dosage form preparation for neonates: the importance of preparation technique. J Res Pharm Pract 2015; 4: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Palmero D, Di Paolo ER, Beauport L, et al. A bundle with a preformatted medical order sheet and an introductory course to reduce prescription errors in neonates. Eur J Pediatr 2016; 175: 113–119. [DOI] [PubMed] [Google Scholar]
- 65. Olsen P, Lorentzen H, Thomsen K, et al. [Medication errors in a pediatric department]. Ugeskr Laeger 1997; 159: 2392–2395. [PubMed] [Google Scholar]
- 66. O’Brodovich M, Rappaport PA. A study pre and post unit dose conversion in a pediatric hospital. Can J Hosp Pharm 1991; 44: 5–15. [PubMed] [Google Scholar]
- 67. Watanachai A, Suprasongsin C. Deadspace: a potential error in concentration of medication during dilutional process in neonates. J Med Assoc Thai 2003; 86: 1128–1132. [PubMed] [Google Scholar]
- 68. Allegaert K, Anderson BJ, Vrancken M, et al. Impact of a paediatric vial on the magnitude of systematic medication errors in neonates. Paediatr Perinat Drug Ther 2006; 7: 59–63. [Google Scholar]
- 69. Conroy S. Association between licence status and medication errors. Arch Dis Child 2011; 96: 305–306. [DOI] [PubMed] [Google Scholar]
- 70. Aguado-Lorenzo V, Weeks K, Tunstell P, et al. Accuracy of the concentration of morphine infusions prepared for patients in a neonatal intensive care unit. Arch Dis Child 2013; 98: 975–979. [DOI] [PubMed] [Google Scholar]
- 71. Kazemi A, Fors UG, Tofighi S, et al. Physician order entry or nurse order entry? Comparison of two implementation strategies for a computerized order entry system aimed at reducing dosing medication errors. J Med Internet Res 2010; 12: e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Munoz Labian M, Pallas Alonso C, de La Cruz Bertolo J, et al. Medication errors in a neonatal unit. An Esp Pediatr 2001; 55: 535–540. [PubMed] [Google Scholar]
- 73. Leonard MS, Cimino M, Shaha S, et al. Risk reduction for adverse drug events through sequential implementation of patient safety initiatives in a children’s hospital. Pediatrics 2006; 118: e1124–e1129. [DOI] [PubMed] [Google Scholar]
- 74. Campino A, Lopez-Herrera MC, Lopez-de-Heredia I, et al. Educational strategy to reduce medication errors in a neonatal intensive care unit. Acta Paediatr 2009; 98: 782–785. [DOI] [PubMed] [Google Scholar]
- 75. Raja Lope RJ, Boo NY, Rohana J, et al. A quality assurance study on the administration of medication by nurses in a neonatal intensive care unit. Singapore Med J 2009; 50: 68–72. [PubMed] [Google Scholar]
- 76. Sagy M. Optimizing patient care processes in a children’s hospital using Six Sigma. J Clin Outcomes Manag 2009; 16: 411–414. [Google Scholar]
- 77. Alemanni J, Touzin K, Bussières JF, et al. An assessment of drug administration compliance in a university hospital centre. J Eval Clin Pract 2010; 16: 920–926. [DOI] [PubMed] [Google Scholar]
- 78. Ligi I, Millet V, Sartor C, et al. Iatrogenic events in neonates: beneficial effects of prevention strategies and continuous monitoring. Pediatrics 2010; 126: e1461–e1468. [DOI] [PubMed] [Google Scholar]
- 79. Sullivan MM, O’Brien CR, Gitelman SE, et al. Impact of an interactive online nursing educational module on insulin errors in hospitalized pediatric patients. Diabetes Care 2010; 33: 1744–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Eisenhut M, Sun B, Skinner S. Reducing prescribing errors in paediatric patients by assessment and feedback targeted at prescribers. ISRN Pediatr 2011; 2011: 545681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gordon M, Chandratilake M, Baker P. Improved junior paediatric prescribing skills after a short e-learning intervention: a randomised controlled trial. Arch Dis Child 2011; 96: 1191–1194. [DOI] [PubMed] [Google Scholar]
- 82. Chedoe I, Molendijk H, Hospes W, et al. The effect of a multifaceted educational intervention on medication preparation and administration errors in neonatal intensive care. Arch Dis Child Fetal Neonatal Ed 2012; 97: F449–F455. [DOI] [PubMed] [Google Scholar]
- 83. Sullivan KM, Suh S, Monk H, et al. Personalised performance feedback reduces narcotic prescription errors in a NICU. BMJ Qual Saf 2013; 22: 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Niemann D, Bertsche A, Meyrath D, et al. Drug handling in a paediatric intensive care unit: can errors be prevented by a three-step intervention? Klin Padiatr 2014; 226: 62–67. [DOI] [PubMed] [Google Scholar]
- 85. Krupicka MI, Bratton SL, Sonnenthal K, et al. Impact of a pediatric clinical pharmacist in the pediatric intensive care unit. Crit Care Med 2002; 30: 919–921. [DOI] [PubMed] [Google Scholar]
- 86. Condren ME, Haase MR, Luedtke SA, et al. Clinical activities of an academic pediatric pharmacy team. Ann Pharmacother 2004; 38: 574–578. [DOI] [PubMed] [Google Scholar]
- 87. Kaushal R, Bates DW, Abramson EL, et al. Unit-based clinical pharmacists’ prevention of serious medication errors in pediatric inpatients. Am J Health Syst Pharm 2008; 65: 1254–1260. [DOI] [PubMed] [Google Scholar]
- 88. Zhang C, Zhang L, Huang L, et al. Clinical pharmacists on medical care of pediatric inpatients: a single-center randomized controlled trial. PLoS One 2012; 7: e30856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tripathi S, Crabtree HM, Fryer KR, et al. Impact of clinical pharmacist on the pediatric intensive care practice: an 11-year tertiary center experience. J Pediatr Pharmacol Ther 2015; 20: 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Khan SN, Joseph S, Sasidharan P. A study of clinical pharmacist initiated intervention for the optimal use of medications in a neonatal intensive care unit (NICU) of a tertiary care hospital, South India. Int J Pharm Pharm Sci 2016; 8: 23–26. [Google Scholar]
- 91. Gibson J, Alexander V, Newton D. Influence on medication therapy of increased patient services by pharmacists in a pediatric hospital. Am J Health Syst Pharm 1975; 32: 495–500. [PubMed] [Google Scholar]
- 92. Folli HL, Poole RL, Benitz WE, et al. Medication error prevention by clinical pharmacists in two children’s hospitals. Pediatrics 1987; 79: 718–722. [PubMed] [Google Scholar]
- 93. Takata GS, Taketomo CK, Waite S; California Pediatric Patient Safety Initiative. Characteristics of medication errors and adverse drug events in hospitals participating in the California Pediatric Patient Safety Initiative. Am J Health Syst Pharm 2008; 65: 2036–2044. [DOI] [PubMed] [Google Scholar]
- 94. Frey B, Buettiker V, Hug MI, et al. Does critical incident reporting contribute to medication error prevention? Eur J Pediatr 2002; 161: 594–599. [DOI] [PubMed] [Google Scholar]
- 95. Apkon M, Leonard J, Probst L, et al. Design of a safer approach to intravenous drug infusions: failure mode effects analysis. Qual Saf Health Care 2004; 13: 265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bonnabry P, Cingria L, Sadeghipour F, et al. Use of a systematic risk analysis method to improve safety in the production of paediatric parenteral nutrition solutions. Qual Saf Health Care 2005; 14: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Arenas Villafranca JJ, Gomez Sanchez A, Nieto Guindo M, et al. Using failure mode and effects analysis to improve the safety of neonatal parenteral nutrition. Am J Health Syst Pharm 2014; 71: 1210–1218. [DOI] [PubMed] [Google Scholar]
- 98. Li Q, Melton K, Lingren T, et al. Phenotyping for patient safety: algorithm development for electronic health record based automated adverse event and medical error detection in neonatal intensive care. J Am Med Inform Assoc 2014; 21: 776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Li Q, Kirkendall ES, Hall ES, et al. Automated detection of medication administration errors in neonatal intensive care. J Biomed Inform 2015; 57: 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bullock J, Jordan D, Gawlinski A, et al. Standardizing IV infusion medication concentrations to reduce variability in medication errors. Crit Care Nurs Clin North Am 2006; 18: 515–521. [DOI] [PubMed] [Google Scholar]
- 101. Yamanaka TI, Pereira DG, Pedreira ML, et al. Redesigning nursing activities to reduce medication errors in pediatrics. Rev Bras Enferm 2007; 60: 190–196. [DOI] [PubMed] [Google Scholar]
- 102. Burmester MK, Dionne R, Thiagarajan RR, et al. Interventions to reduce medication prescribing errors in a paediatric cardiac intensive care unit. Intensive Care Med 2008; 34: 1083–1090. [DOI] [PubMed] [Google Scholar]
- 103. Davey AL, Britland A, Naylor RJ. Decreasing paediatric prescribing errors in a district general hospital. Qual Saf Health Care 2008; 17: 146–149. [DOI] [PubMed] [Google Scholar]
- 104. Booth R, Sturgess E, Taberner-Stokes A, et al. Zero tolerance prescribing: a strategy to reduce prescribing errors on the paediatric intensive care unit. Intensive Care Med 2012; 38: 1858–1867. [DOI] [PubMed] [Google Scholar]
- 105. Martinez-Anton A, Sanchez JI, Casanueva L. Impact of an intervention to reduce prescribing errors in a pediatric intensive care unit. Intensive Care Med 2012; 38: 1532–1538. [DOI] [PubMed] [Google Scholar]
- 106. Campino A, Santesteban E, Pascual P, et al. Strategies implementation to reduce medicine preparation error rate in neonatal intensive care units. Eur J Pediatr 2016; 175: 755–765. [DOI] [PubMed] [Google Scholar]
- 107. Irwin D, Vaillancourt R, Dalgleish D, et al. Standard concentrations of high-alert drug infusions across paediatric acute care. Paediatr Child Health 2008; 13: 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pallás CR, De-la-Cruz J, Del-Moral MT, et al. Improving the quality of medical prescriptions in neonatal units. Neonatology 2008; 93: 251–256. [DOI] [PubMed] [Google Scholar]
- 109. Di Pentima MC, Chan S. Impact of antimicrobial stewardship program on vancomycin use in a pediatric teaching hospital. Pediatr Infect Dis J 2010; 29: 707–711. [DOI] [PubMed] [Google Scholar]
- 110. Hilmas E, Sowan A, Gaffoor M, et al. Implementation and evaluation of a comprehensive system to deliver pediatric continuous infusion medications with standardized concentrations. Am J Health Syst Pharm 2010; 67: 58–69. [DOI] [PubMed] [Google Scholar]
- 111. Abstoss KM, Shaw BE, Owens TA, et al. Increasing medication error reporting rates while reducing harm through simultaneous cultural and system-level interventions in an intensive care unit. BMJ Qual Saf 2011; 20: 914–922. [DOI] [PubMed] [Google Scholar]
- 112. Cimino MA, Kirschbaum MS, Brodsky L, et al. ; Child Health Accountability Initiative. Assessing medication prescribing errors in pediatric intensive care units. Pediatr Crit Care Med 2004; 5: 124–132. [DOI] [PubMed] [Google Scholar]
- 113. Costello JL, Torowicz DL, Yeh TS. Effects of a pharmacist-led pediatrics medication safety team on medication-error reporting. Am J Health Syst Pharm 2007; 64: 1422–1426. [DOI] [PubMed] [Google Scholar]
- 114. Otero P, Leyton A, Mariani G, et al. ; Patient Safety Committe. Medication errors in pediatric inpatients: prevalence and results of a prevention program. Pediatrics 2008; 122: e737–e743. [DOI] [PubMed] [Google Scholar]
- 115. Alagha HZ, Badary OA, Ibrahim HM, et al. Reducing prescribing errors in the paediatric intensive care unit: an experience from Egypt. Acta Paediatr 2011; 100: e169–e174. [DOI] [PubMed] [Google Scholar]
- 116. Wang JK, Herzog NS, Kaushal R, et al. Prevention of pediatric medication errors by hospital pharmacists and the potential benefit of computerized physician order entry. Pediatrics 2007; 119: e77–e85. [DOI] [PubMed] [Google Scholar]
- 117. Simpson JH, Lynch R, Grant J, et al. Reducing medication errors in the neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed 2004; 89: F480–F482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hartwig S, Denger S, Schneider P. Severity-indexed, incident report-based medication error-reporting program. Am J Health Syst Pharm 1991; 48: 2611. [PubMed] [Google Scholar]