Abstract
Background
The aim of this study was to report our single centre experience with the Medina Embolic Device (MED).
Methods
We performed a retrospective analysis of prospectively collected data to identify all patients treated with the MED. A total of 14 aneurysms (non-consecutive), in 13 patients, were treated including one ruptured and one partially thrombosed aneurysm. Fundus diameter was ≥5 mm in all cases. We evaluated the angiographic appearances, the clinical status, complications, and the need for adjunctive devices or repeat treatments.
Results
Aneurysm location was cavernous internal carotid artery (ICA; n = 1), supraclinoid ICA (n = 1), terminal ICA (n = 2), anterior communicating artery (AComA; n = 4), A2–3 (n = 1), M1–2 junction (n = 1), posterior communicating artery (PComA; n = 1), superior cerebellar artery (SCA; n = 1), and basilar tip (n = 2). The average aneurysm fundus size was 8.6 mm (range 7–10 mm) and average neck size 3.75 mm (range 1.9–6.9 mm). Immediate angiographic results were modified Raymond–Roy occlusion classification (mRRC) I n = 2, mRRC II n = 1, mRRC IIIa n = 2, mRRC IIIb n = 2, the remaining 7 aneurysms showed complete opacification. At follow-up angiography (mean 5 months) mRRC I n = 5, mRRC II n = 5, mRRC IIIa n = 3, and persistent filling was seen in 1 aneurysm. Overall, four patients had repeat treatment and one is pending further treatment. Of the aneurysms treated with more than one MED, 75% showed complete occlusion at 6-month follow up whereas only one aneurysm treated with a single device showed complete occlusion. Overall, three patients had temporary complications and there were no deaths.
Conclusions
The MED is an intra-saccular flow-diverting device with satisfactory angiographic results and an acceptable safety profile. Use of a single MED cannot be recommended and further longer term studies are needed prior to widespread clinical use.
Keywords: Aneurysm, Medina Embolic Device, flow diverter, intra-saccular
Introduction
Since the introduction of Guglielmi detachable coils in the early 1990s there have been advances in coil design with different three-dimensional (3D) designs, variable softness as well as the addition of compounds to increase the volume of the coils within the aneurysm or promote healing. The essential design of coils remains however, unchanged. Recently, after the introduction of luminal flow diversion, intra-saccular flow diversion has attracted interest. Currently two such devices, with completely different designs, are available – the Woven Endobridge (WEB, Terumo, Japan) and the Medina Embolic Device (MED) (Medtronic, Minnesota, USA).1
In this article we review our initial experience with the MED and present our early clinical and radiological data.
Materials and methods
Device description
The MED can be considered as an intra-saccular flow diverter designed to treat saccular aneurysms. It has been granted a CE mark by the European Union. It is a 3D metallic structure with a shape set radio-opaque core wire and self-expanding shape memory alloy ‘leaflets’ that account for the flow-diverting effect of the device. These lie on the long axis of the core wire and when unconstrained the device adopts a spherical shape (Figure 1). The device is pushed into an aneurysm and tumbles within the aneurysmal sac until it has been positioned suitably, similar to coils. The flow diverter mesh ‘leaflets’ distributes the forces exerted on the aneurysm wall.
Figure 1.
The Medina Embolic Device is deployed in a similar fashion to standard endovascular coils. It has a shape set core wire and flow diverter ‘leaflets’ (a). When unconstrained it adopts a spherical shape (b).
Delivery of the MED is through a 0.021-inch internal diameter microcatheter. The mechanical detachment system is identical to that of the axium coils (Medtronic). Overall, two different types of MED are currently available, framing and filling coils; the filler coil being softer and designed for deployment within the stiffer framing device.1–3
Patient selection and procedure
Patients were selected based on aneurysm size and morphology. The smallest available MED framing device was 5 mm, therefore spherical or ovoid aneurysms >5 mm were preferred. The study was approved by the local ethics committee (Regionala Etikprövningsnämnden I, Stockholm, Sweden 2017/1197-31/02).
A standard right common femoral approach and 6F system was used in all cases. The patients were not on anti-platelet medication prior to the procedure. One patient was on low dose aspirin for unrelated reasons.
An intra-operative bolus dose of heparin (5000 IU) was given at the start of the procedure with repeated intra-operative 1000 IU boluses to maintain the activated clotting time at 2–2.5 times normal.
The MED was deployed via a Prowler Select Plus microcatheter (Codman, USA). The coil sizing was based on the same measurements used to select standard coils. After placing the framing MED, additional angiographic runs were performed to assess contrast media circulation in the aneurysm, with the aim to determine whether further coils were needed. Our aim was to use only the MED and as few devices as possible initially. Additional MEDs or coils were placed in the aneurysm sac if we felt that there was insufficient stagnation within the aneurysm at treatment or follow-up angiography.
Angiographic outcome and follow up
The angiographic appearance of the aneurysm was graded using the four-point modified Raymond–Roy occlusion classification (mRRC)4 at the end of the procedure and then again at angiographic follow up.
Routine follow-up angiography was scheduled between 4–6 months after the initial treatment. In addition to aneurysm occlusion the configuration of the MED was also assessed to determine if there had been compaction or a change in the configuration of the MED (Tables 1 and 2).
Table 1.
Aneurysm characteristics and procedural details.
| Patient no. | Aneurysm location | Aneurysm fundus size (mm) | Neck size (mm) | No. of MEDs | Devices implanted | Additional devices | Immediate angiographic result (mRRC) | Procedure time (min) | Complications | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ICA hypophyseal | 6.9 × 4.1 | 3.2 | 1 | 6 × 8 Framer | 1 × coil | IIIa | 60 | None | |
| 2 | Terminal ICA | 6.9 × 7 | 2.4 | 2 | 6 × 8 Framer, 5 × 5 Filler | N | IIIb | 30 | None | |
| 3 | AcomA | 10.5 × 6.8 | 3.8 | 2 | 9 × 13 Framer, 6 × 8 Filler | N | II | 40 | Thromboembolic | |
| 4 | M1–2 | 6.8 × 4.7 | 1.9 | 1 | 6 × 8 Framer | N | I | 30 | Thromboembolic | |
| 5 | PcomA | 9.3 × 5.8 | 2.6 | 1 | 7 × 9 Framer | N | Complete filling | 27 | None | |
| 6 | Superior cerebellar | 7.7 × 7.3 | 5.5 | 1 | 7 × 9 Framer | N | IIIb | 49 | None | |
| 7 | Terminal ICA | 9.8 × 5.8 | 2.6 | 1 | 9 × 13 Framer | N | I | 28 | None | |
| 8 | AcomA | 7.5 × 4.3 | 3.7 | 1 | 7 × 9 Framer | N | Complete filling | 74 | None | Ruptured, detachment issue |
| 9 | AcomA | 9.6 × 7.7 | 3.3 | 3 | 8 × 8 Framer, 5 × 5 Filler (2) | N | IIIa | 43 | Thromboembolic | MED loop in parent vessel |
| 10 | AcomA | 8.6 × 8.7 | 3.9 | 1 | 9 × 13 Framer | N | Complete filling | 135 (both aneurysms) | None | |
| A2–3 | 7.4 × 4.7 | 3.5 | 1 | 7 × 9 Framer | N | Complete filling | None | |||
| 11 | Cavernous ICA | 9.6 × 7.7 | 3.1 | 1 | 9 × 13 Framer | N | Complete filling | 37 | None | |
| 12 | Basilar tip | 8.8 × 4.2 | 6.9 | 1 | 7 × 9 Framer | N | Complete filling | 16 | None | |
| 13 | Basilar tip | 10.6 × 8.2 | 6.1 | 3 | 9 × 13 Framer, 8 × 10 Filler, 7 × 9 Filler | Atlas stent × 2 | Complete filling | 70 | None |
AcomA: anterior communicating artery; ICA: internal carotid artery; MED: Medina Embolic Device; mRRC: modified Raymond–Roy occlusion classification; PcomA: posterior communicating artery.
Table 2.
Follow-up results.
| Patient no. | 1st follow up (months) | Angiographic outcome (mRRC) | Coil compaction | Coil shape change | Retreatment | 2nd follow up (months) |
|---|---|---|---|---|---|---|
| 1 | 5 | I | No | No | No | N/A |
| 2 | 6 | I | No | No | No | N/A |
| 3 | 6 | I | No | No | No | N/A |
| 4 | 5 | I | No | No | No | N/A |
| 5 | 4 | IIIa – significant filling | No | Yes | Pending | N/A |
| 6 | 6 | II | No | Yes | Yes (14 months) | N/A |
| 7 | 6 | IIIa – significant filling | No | No | Yes (13 months) | N/A |
| 8 | 1 | IIIa – significant filling | No | No | Yes (3 months) coils | 3 |
| 9 | 7 | I | No | No | No | N/A |
| 10 | 6 | II | No | No | No | N/A |
| II | No | No | No | N/A | ||
| 11 | 6 | II | No | No | No | N/A |
| 12 | 6 | Unchanged | No | No | Yes (9 months) | N/A |
| 13 | 1 | II | No | No | No | 3 |
mRRC: modified Raymond–Roy occlusion classification; N/A: not applicable.
Results
A total of 13 patients with 14 aneurysms were treated with a solitary MED or in conjunction with other devices. The average patient age was 61 years (range 41–78). Aneurysm location was cavernous internal carotid artery (ICA; n = 1), supraclinoid ICA (n = 1), terminal ICA (n = 2), anterior communicating artery (AComA; n = 4), A2–3 (n = 1), M1–2 junction (n = 1), posterior communicating artery (PComA; n = 1), superior cerebellar artery (SCA; n = 1), basilar tip (n = 2). A total of 12 aneurysms were treated electively. Overall, one aneurysm was treated urgently for mass effect on the mesencephalon with acute neurological deterioration. One aneurysm had ruptured and was treated the day after the haemorrhage. The average aneurysm size was 8.6 mm in maximal diameter (range 7–10 mm). The average neck diameter was 3.75 mm (range 1.9–6.9 mm).
At initial treatment, a single MED was used in 10 aneurysms with 2 MEDs used in 2 aneurysms and 3 MEDs used in 2 aneurysms. Additional devices were used in two cases with single devices at the time of initial treatment. In one basilar tip aneurysm two stents were used in a Y-configuration to prevent encroachment of the MEDs into the parent vessel. In another case a single coil was placed in the aneurysm after the MED had been deployed. The median procedure time (defined as the time taken from acquisition of the working projection to the final angiographic run in this projection) was 40 min.
At the end of the procedure two aneurysms (14%) showed no persisting contrast media circulation whereas seven aneurysms (50%) showed circulation in most of the aneurysm. Of the remaining five aneurysms, two (14%) exhibited contrast media filling the fundus of the aneurysm while the other two (14%) showed contrast media permeating between the MED and the wall of the aneurysm. Overall, one had (7%) filling only of the neck of the aneurysm (Table 1).
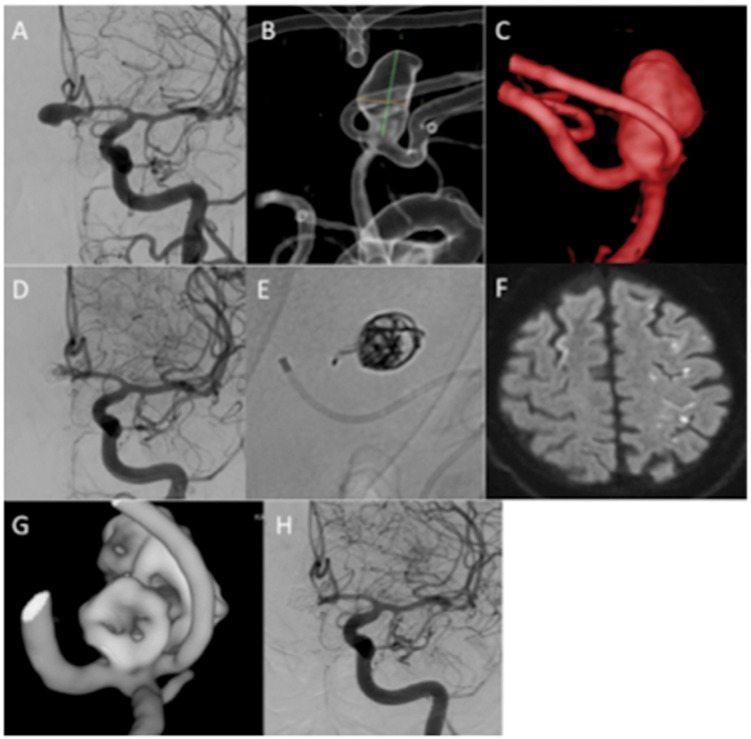
Clinical and angiographic follow up was available for all patients at mean 5 months after treatment (range 1–7 months). The patient with a symptomatic partially thrombosed aneurysm (patient 13) underwent early angiography at 1 month post-procedure to ensure the MED had not compacted or displaced within the thrombus. This patient underwent repeat follow up and required retreatment of the aneurysm at 10 months post-procedure, as the aneurysm had not become occluded at the 6-month follow-up angiography. The patient with a ruptured aneurysm (patient 8) underwent early angiography at 1 month with persistence of contrast media circulation in the aneurysm. This patient was retreated with standard endovascular coils at three months post-procedure (Figure 2).
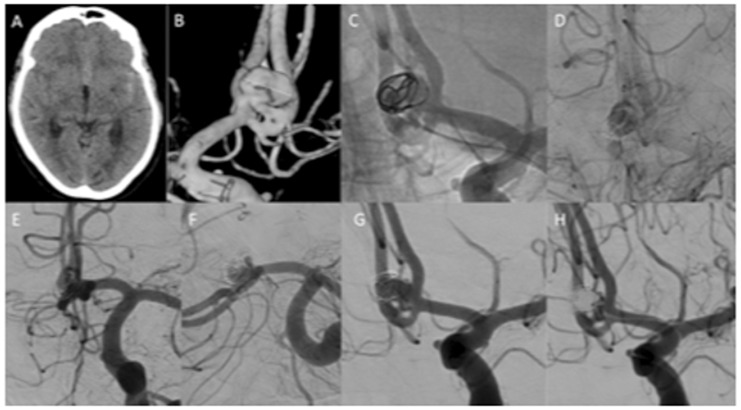
Figure 2.
A patient with a ruptured AComA aneurysm (a and b) was treated with a single MED (c). The MED had to be repositioned several times before stagnation of contrast was achieved (d). At control angiography there was still contrast media filling of the aneurysm (e) although this appeared incomplete (f and g). Retreatment was performed at 3 months with conventional coiling within the MED frame. There was still a neck remnant (h).
AComA: anterior communicating artery; MED: Medina Embolic Device.
At follow up none of the MEDs had compacted. The configuration of the MED had changed slightly in two aneurysms. A total of five aneurysms (36%) were completely occluded (mRRC I) (Figure 3), five aneurysms (36%) had small neck remnants (mRRC II), 3 aneurysms (21%) showed continued contrast media circulation within the aneurysm although this did not completely fill the aneurysm (mRRC IIIa) and in one aneurysm (7%) there was no appreciable change at the follow-up angiogram.
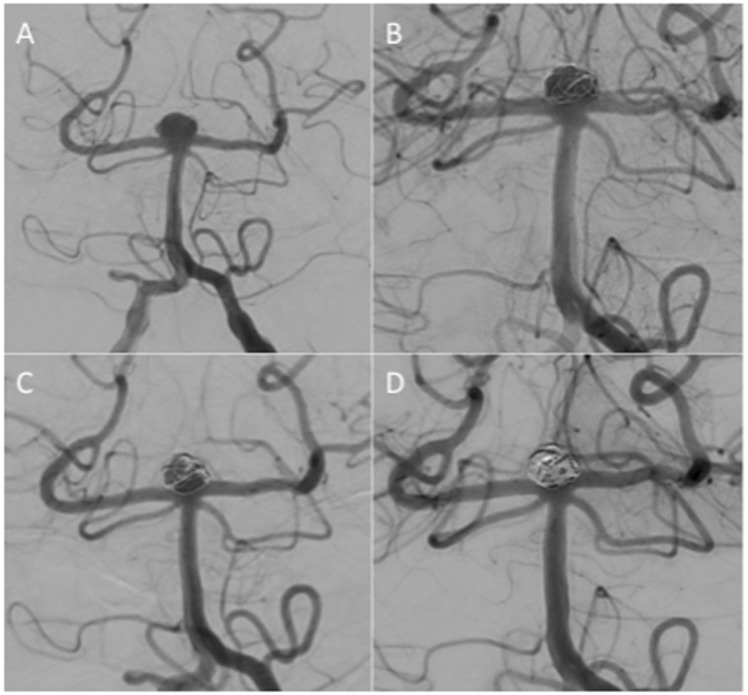
Figure 3.
An unruptured terminal carotid aneurysm (a and b) was treated with 2 MEDs at the end of the procedure there was still filling in the aneurysm (c, mRRC IIIb) however, at follow up at 6 months the aneurysm was completely excluded from the circulation (d).
MED: Medina Embolic Device; mRRC: modified Raymond–Roy occlusion classification.
Of the four aneurysms treated with multiple MEDs, three had complete aneurysm occlusion at follow up and the last patient had a neck remnant. Of the remaining nine aneurysms one had complete aneurysm occlusion while four had neck remnants, three showed partial occlusion and in one there was still contrast opacification of the aneurysm. Furthermore, of the seven aneurysms that showed persistent and complete contrast media filling of the aneurysm at the end of the procedure, none went on to complete occlusion (mRRC I) at the follow-up angiography (Table 2).
A total of four patients have undergone retreatment because of persistent aneurysmal opacification (Figure 4 and 5) and one patient is awaiting retreatment.
Figure 4.
An unruptured basilar tip aneurysm (patient 12) (a) was treated with a single MED framer with persistent contrast media circulation in the aneurysm at the end of the procedure (b). Follow up and 6 months showed persistent filling of the aneurysm (c) and therefore repeat treatment at 9 months was performed with balloon assistance in a Y-configuration. Delayed follow-up angiography is awaited.
MED: Medina Embolic Device.
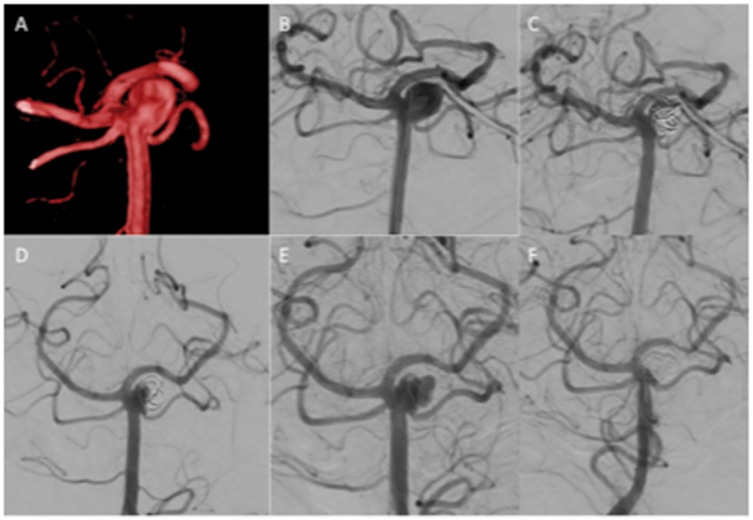
Figure 5.
An unruptured aneurysm of the superior cerebellar artery (a and b) was treated initially with a single MED with mRRC IIIb occlusion at the end of the procedure (c). Follow-up angiography at 6 months showed a slight change in the configuration of the MED and a residual neck remnant (d). Delayed angiography demonstrated a large recurrence (e). The patient was retreated with conventional coils (f).
MED: Medina Embolic Device; mRRC: modified Raymond–Roy occlusion classification.
Complications
In total three patients had thromboembolic complications. In patient 9 (Figure 6), after successful deployment of the MED, a coil loop was displaced into the parent vessel (A1) upon removal of the coiling catheter. We believe that this occurred because the detachment zone of the MED coil re-entered the deployment catheter after deployment, and was pulled out of the aneurysm together with the microcatheter at the end of the procedure. The patient was given an intravenous (IV) bolus dose of aspirin (500 mg) at the end of the procedure and commenced on dual anti-platelet agents however, magnetic resonance imaging (MRI) performed 72 h post-operatively showed multiple embolic infarcts, the majority of which were in the territory of the anterior cerebral artery (ACA) and thought to be secondary to the displaced MED leaflet and our efforts to push it back into the aneurysm. The patient will remain on dual anti-platelet agents for 12 months and aspirin for life. At clinical follow up at 6 months post-procedure, the patient had returned to baseline neurology. In another case a similar detachment issue occurred where, after detachment, the coil loop repeatedly entered the microcatheter. As we recognised the issue on this occasion we repeatedly pushed the pusher wire out of the microcatheter until the MED leaflet stopped re-entering the microcatheter.
Figure 6.
A patient with an unruptured AComA aneurysm (a–c) was treated with 2 MEDs. There was good stasis of contrast in the aneurysm at the end of the procedure (d) however some persistent filling was seen. Upon removal of the microcatheter a single MED loop was displaced and could be seen in the parent artery (e). The patient was started on dual anti-platelet agents. He developed symptoms consistent with an ACA territory infarction and MRI demonstrated multiple small embolic infarctions within the ACA territory (f). He recovered completely and follow-up angiography showed complete occlusion of the aneurysm (g and h).
ACA: anterior cerebral artery; AComA: anterior communicating artery; MED: Medina Embolic Device; MRI: magnetic resonance imaging.
Small thromboembolic lesions were seen on post-operative Diffusion Weighted Imaging (DWI) MRI in two other patients, however, neither of these could be definitively attributed to the MED. At clinical follow up both of these patients were at baseline neurology.
Discussion
The introduction of flow diversion technology represented a milestone in endovascular therapeutics. While this technology began with intra-luminal flow diverters the potential benefits of an intra-saccular flow-diverting device were quickly seized upon. The principle advantage of intra-saccular devices is that they avoid coverage of side branches and anti-platelet medication is believed not to be necessary.
The MED represents a major shift in the design of intra-saccular flow diverters. A total of three groups have published their early experiences with the device.2,5 The original publication by Turk et al.2 included nine patients, seven of whom were treated solely with the MED. A Framer coil was placed in all the aneurysms and the average number of devices used was 2.8 (range 1–7) and the aneurysms treated varied in size from ≈4.5–17 mm. Although in this initial paper only three of the aneurysms had early follow up at 1 month, all three showed >95% occlusion and the authors state that this limited follow up provided an early indication that the device caused progressive aneurysm occlusion over time. This is similar to that seen with traditional coils and with endoluminal flow diverters. The authors also noted that relatively few MEDs were placed, compared with the number of coils that would have been required to achieve adequate occlusion, therefore the overall procedure time was short, in some cases less than 10 minutes, which may translate into improved safety. The device appeared to have a good safety profile.
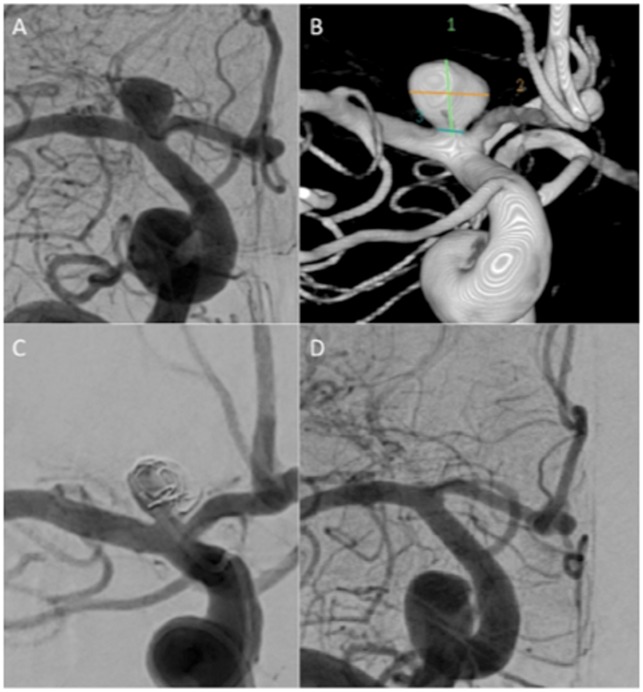
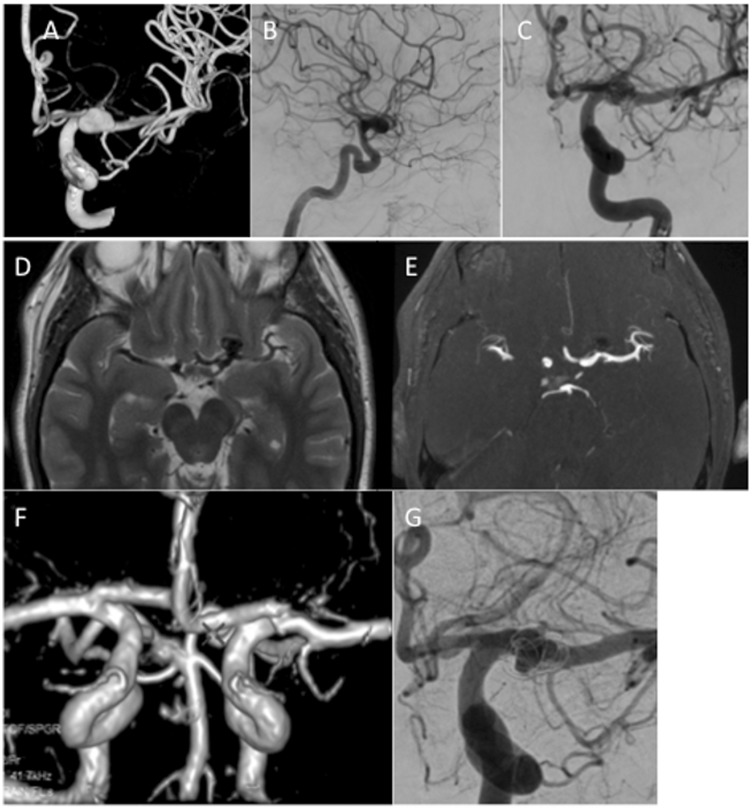
In the more recent publication of Aguilar-Pérez et al.3 the results of 15 consecutive patients with 16 aneurysms were reported. In this series 3.4 MEDs per aneurysm were used (range 1–9). Follow-up imaging was available in 11 patients with mRRC II or better seen in 8 patients. The authors discuss in detail the potential advantages and disadvantages of the device including the problems regarding aneurysmal shape. As mentioned earlier the MED forms a spherical shape however, many aneurysms are not spherical.6 This then creates a problem regarding the applicability of the device to many aneurysms. In the publications of Turk et al. and Aguilar-Pérez the device was used in non-spherical aneurysms. While the device can be implanted and cause flow disruption its exact shape in the aneurysm is difficult to assess. The shapes of the aneurysms are not clearly indicated in the paper by Sourour et al.7 In the paper of Aguilar-Pérez et al.3 this difficulty was well described and in one case the authors detailed how the leaflets of the device actually redirected the remaining flow towards a bleb on the aneurysm which then required coils to be placed. This difficulty in assessing the position of the leaflets of the MED within the aneurysm comes from the fact that these leaflets are almost radiolucent, even when using magnified exposures, which leads us to another issue – assessment of coverage at the aneurysmal neck by the MED. An option that we initially believed could negate this problem was the use of on table high-resolution intra-arterial rotational angiography or flat panel compu5ted tomography (CT) with multi-planar reformat (XPerCT, Philips Healthcare, the Netherlands). However, it could not be used to reliably assess coverage at the neck due to the significant metallic artefact caused by the core wire. The combination of radiolucent flow-diverting leaflets and radio-opaque core wire make the accurate assessment of neck coverage extremely challenging and this has been one of the major difficulties we have experienced in using this device. In addition to the difficulties with assessing coverage at the neck we found that follow-up assessment with CT angiography was limited by the metallic artefact. Follow-up imaging with MRI was also impaired since the MED appears to act in a similar manner to a Faraday cage with no signal return from within the leaflets of the device and therefore, an inability to determine if there is persistent filling between the leaflets of the MED. Despite this, one is able to detect the presence of a neck remnant/recurrence by using standard MRI sequences (Figure 7).
Figure 7.
A patient with an unruptured left ICA bifurcation aneurysm (a–c) was treated with a single MED. At initial follow up performed at 6 months axial T2-weighted MRI (d) showed a signal void in the aneurysm suggestive of continued filling however, on TOF MRA (e) there was no evidence flow which was confirmed on 3D reconstruction of the MRA data (f). On angiography, there was persistent filling of the aneurysm (mRRC IIIa). The discrepancy between the MRI and the angiographic data was thought to be due to a ‘Faraday cage’ like effect of the MED.
ICA: internal carotid artery; MED: Medina Embolic Device; MRA: Magnetic Resonance Angiography; MRI: magnetic resonance imaging; mRRC: modified Raymond–Roy occlusion classification; TOF: Time of Flight.
Naturally, several devices could be placed in the aneurysm just as with coils however, this then adds to the procedure length and the cost of the treatment and one is still faced with the difficulty of knowing when to stop. In our series, there was excellent occlusion of the aneurysms in which several MEDs or MED and coil or MED and stents were placed with the majority of these aneurysm being occluded at follow up. Similarly, assessment of flow stasis within the aneurysm could possibly be used to determine the number of MEDs required to obtain occlusion. However, at the current time insufficient data is available to determine how much stasis in the aneurysm is needed to ensure long-term occlusion with no clear relationship between post-procedure contrast stasis and occlusion stasis seen in our cohort. In our experience, we believe that aneurysms demonstrating complete contrast opacification at the end of the procedure are likely to remain patent at follow up.
The most recent publication by Sourour et al.7 evaluated 12 patients with 13 aneurysms. The anterior communicating artery was the most common location for the aneurysms followed by the Middle Cerebral Artery (MCA) bifurcation and carotid-ophthalmic. In this series, the authors used a single MED framer to obtain a basket in 12/13 aneurysms. They then proceeded to implant further MED fillers, standard coils or both. The authors state that, as this was their first experience with the device, even if total exclusion of the aneurysm was seen coils were used to fill the basket in order to prevent any retraction of the MED. In this regard the authors opted to use the MED in a similar fashion to a framing coil rather than an intra-saccular flow diverter. Turk et al.2 suggest that the potential improved coverage at the neck may prevent herniation of future coil loops if they are placed in the aneurysm and this seems to have been successfully achieved by Sourour et al.7 as they did not report a single coil protrusion despite coils being placed in 11 of the aneurysms. While a combination of MED and coils may certainly be a useful strategy to achieve occlusion of the aneurysm it does not allow assessment of the flow-diverting properties of the MED nor the risk of recurrence. Aguilar-Pérez et al.5 treated several of the aneurysms with both the MED and endoluminal flow diverters. This strategy may prove useful for large aneurysms since the dual layer of flow diversion at the neck and significant flow disruption that is likely to occur with both devices together may promote extremely rapid thrombosis. However, this would then require the patients to be on dual anti-platelet medication. Therefore, in both the papers by Aguilar-Pérez et al. and Sourour et al., while appropriate strategies to prevent delayed complications and encourage aneurysmal healing were adopted, they do not allow an accurate assessment of the ability of the MED alone to occlude aneurysms.
In our series one single ruptured aneurysm was treated in the acute phase. In the series of Sourour et al.7 three ruptured aneurysms were treated in the acute phase (<24 h). The MED theoretically could offer advantages over standard coils in the acute setting. The larger surface area of the device will cover a greater internal surface area compared with a single or even several coils and the larger surface over which force is distributed may mean that the risk of re-rupture during treatment is reduced. Nevertheless, as discussed earlier, the main issue is to ensure coverage across the neck and therefore implanting several MEDs may be considered. This would not only have the advantage of increasing coverage across the neck and the rupture site but also increasing stasis within the aneurysm which would hopefully promote more rapid thrombosis. However, the MED is stiffer than standard coils, which may increase wall stress and thus the risk for rupture. It should be noted that this far there has been no reports of procedural aneurysmal rupture.
Although the mean average procedure time in our series was not particularly short at 49 min this is skewed given the long procedure time of the patient with two aneurysms. Similarly, given that these are our early cases it is likely that the procedure time will shorten significantly once familiarity with the device improves. Our shortest procedure time was 16 min, similar to that achieved in several of the cases in the Turk et al.2 series. In the series by Sourour et al.7 two procedures were performed in less than 15 min and 50% were performed in ≈30 min or less. In the cases of extremely rapid treatment (one case in 12 min and another in 13 min) only a single MED was deployed. Given these results and our own experience we believe that procedure times below 30 min for aneurysms treated solely with MED are likely with improved operator experience.
Coil compaction is believed to be one of the most important factors that can lead to aneurysm recurrence and it is thought to occur secondary to the water-hammer effect of the pulsatile flow as well as the dissolution of intra-aneurysmal thrombus.8 Coil unravelling has also been reported and this occurs when the coil loops loosen from the tight packing obtained at the time of operation that can then alter the flow within the aneurysm and account for aneurysmal regrowth.9,10 Similar changes in shape have also been seen with the WEB device with Cognard and Januel11 reporting a radiological deterioration in the appearance of 71% of treated aneurysms however, other groups have shown markedly lower rates of shape change with the WEB device.12 In our study, we did not see compaction of the MED in any of the cases however, we did see a change in the configuration of the device in at least two aneurysms. This change in configuration did not occur in either the ruptured aneurysm or the partially thrombosed aneurysm and we are unable to determine any specific features that could explain this change. Similarly, compaction was not reported in the study of Sourour et al.;7 however, in this paper the authors inserted coils within a MED frame in order to prevent compaction. An additional factor to consider is the potential for the device to alter the sheer stress on the inner wall of the aneurysm sac. It is possible that good apposition of the MED leaflets against the inner wall of the aneurysm may alter the effects of circulating blood within the aneurysm and stabilise the aneurysm wall. Although this is yet to proved it certainly represents an interesting further avenue of research for intra-saccular flow-diverting devices such as the MED.
It is yet to be determined if stability of the MED construct alone persists in the long-term or exactly what factors determine stability of the device and further studies without adjunctive coiling or endoluminal flow diversion are required.
Our study suffers from the inherent limitations of a retrospective study and heterogeneity in the patients as well as the relatively small patient number and relatively short follow-up period.
Conclusion
The MED is an intra-saccular flow-diverting device with an acceptable angiographic outcome in this early series. The treatment safety profile is acceptable. The majority of aneurysms treated with multiple MEDs went on to complete occlusion. However, only about 50% of aneurysms treated with one MED alone were occluded at the 6-month follow up. The long-term stability of the aneurysm occlusion is yet to be determined. Currently we cannot recommend the use of MED as a single device in the endovascular treatment of intracranial arterial aneurysms. The MED should be evaluated in a study prior to widespread clinical use.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PB serves as proctor and consultant for Phenox and Neurvana. PB is a consultant for Medtronic and principal investigator for Medina PMCF study. MiSö is a consultant for Phenox (Bochum, Germany), Medtronic (Minnesota, USA), Neuravi (Galway, Ireland).
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Henkes H, Weber W. The past, present and future of endovascular aneurysm treatment. Clin Neuroradiol 2015; 25(Suppl. 2): 317–324. [DOI] [PubMed] [Google Scholar]
- 2.Turk AS, Maia O, Ferreira CC, et al. Periprocedural safety of aneurysm embolization with the Medina Coil System: the early human experience. J Neurointerventional Surg 2016; 8: 168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguilar Perez M, Bhogal P, Martinez Moreno R, et al. The Medina Embolic Device: early clinical experience from a single center. J Neurointerventional Surg 2017; 9: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mascitelli JR, Moyle H, Oermann EK, et al. An update to the Raymond–Roy Occlusion Classification of intracranial aneurysms treated with coil embolization. J Neurointerventional Surg 2015; 7: 496–502. [DOI] [PubMed] [Google Scholar]
- 5.Aguilar Perez M, Bhogal P, Martinez Moreno R, et al. The Medina Embolic Device: early clinical experience from a single center. J Neurointerventional Surg 2017; 9: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.You S-H, Kong D-S, Kim J-S, et al. Characteristic features of unruptured intracranial aneurysms: predictive risk factors for aneurysm rupture. J Neurol Neurosurg Psych 2010; 81: 479–484. [DOI] [PubMed] [Google Scholar]
- 7.Sourour N-A, Vande Perre S, Maria FD, et al. Medina® Embolization Device for the treatment of intracranial aneurysms: safety and angiographic effectiveness at 6 months. Neurosurgery. Epub ahead of print 10 April 2017. DOI: 10.1093/neuros/nyx161. [DOI] [PubMed] [Google Scholar]
- 8.Sluzewski M, van Rooij WJ, Slob MJ, et al. Relation between aneurysm volume, packing, and compaction in 145 cerebral aneurysms treated with coils. Radiology 2004; 231: 653–658. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Xie X. Treatment of an unraveled intracerebral coil. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv 2010; 76: 746–750. [DOI] [PubMed] [Google Scholar]
- 10.Mori K, Nakao Y, Horinaka N, et al. Cerebral aneurysm regrowth and coil unraveling after incomplete Guglielmi detachable coil embolization: serial angiographical and histological findings. Neurol Med Chir (Tokyo) 2003; 43: 293–297. [DOI] [PubMed] [Google Scholar]
- 11.Cognard C, Januel AC. Remnants and recurrences after the use of the WEB intrasaccular device in large-neck bifurcation aneurysms. Neurosurgery 2015; 76: 522–530, discussion 530. [DOI] [PubMed] [Google Scholar]
- 12.Sivan-Hoffmann R, Gory B, Riva R, et al. One-year angiographic follow-up after WEB-SL endovascular treatment of wide-neck bifurcation intracranial aneurysms. Am J Neuroradiol 2015; 36: 2320–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]