Abstract
We report a severe adverse event occurring in the course of a cohort study (ISRCTN13784335) aimed at measuring the efficacy and safety of venous stenting in the treatment of patients with medically refractory idiopathic intracranial hypertension (IIH). The patient was a 41-year-old woman who was not overweight, who presented with severe headache, grade 1 bilateral papilledema and transient tinnitus, refractory to medical treatment. Right transverse sinus stenting was successfully performed. Following surgery, the patient’s state of consciousness decreased acutely with rapid and progressive loss of brainstem reflex. CT scan revealed acute cerebellar and intraventricular hemorrhage with obstructive hydrocephalus. Angioscan revealed normal venous sinus patency and cerebral MRI showed acute mesencephalic ischemia. Mechanical impairment of cerebellar venous drainage by the stent or venous perforation with the large guidewire used in this technique are two logical ways to explain the cerebellar hemorrhage seen in our patient. The risk of such a complication could probably be reduced using alternative tools and technique. However, given the low level of evidence around the safety of transverse sinus stenting in IIH, its formal assessment in clinical trials is required.
Keywords: Idiopathic intracranial hypertension, stenting, hemorrhage
Introduction
An increasing number of idiopathic intracranial hypertension (IIH) patients are found to have intracranial venous outflow abnormalities, including transverse sinus stenosis.1–4 Despite the fact that the exact role of venous sinus stenosis in IIH physiopathology is still debated,5–9 transverse sinus stenosis represents a potential trigger of the disease clinical manifestations and consequently, a new target of treatment for medically refractory IIH.10
While several authors reported improvement of IIH patients following the use of endovascular venous stenting,10–19 few of them reported complications with severe disabilities or death.20
We report a severe adverse event occurring in the course of a cohort study (Clinical Trial Registration URL: http://www.controlled-trials.com. Unique identifier: ISRCTN13784335) aimed at measuring the efficacy and safety of venous stenting in the treatment of patients with medically refractory IIH.
Methods
The protocol was approved by the research ethics board of the CHU de Québec and a safety committee was in charge of assessing each adverse event.
Population
We enrolled patients 18 years old or older, with diagnosis of IIH (according to Friedman),21 who failed a three-month trial or were intolerant of medical treatment (according to the referent neurologist). Patients were considered eligible if they had venous imaging showing bilateral transverse sinus stenosis or unilateral transverse sinus stenosis with contralateral transverse sinus atresia, and with pressure gradient across the stenosis of more than 8 mmHg. Informed consent was obtained for each patient. Patients with contraindication for general anesthesia, angiography, aspirin, clopidogrel or anticoagulants were excluded.
Intervention
The venous sinus stenting and peri-procedural management was defined, in the protocol, to follow a standard procedure in each patient. Catheter venography of both jugular veins was performed with catheterism of the intracranial sinuses, in order to measure venous pressure distal and proximal to the stenosis and to confirm the presence of a higher than 8 mmHg pressure gradient (under local anesthesia). Aspirin (81 mg daily) and clopidogrel (75 mg daily) were administered for at least four days before stenting. Using a femoral venous approach, retrograde catheterism of the dominant transverse sinus was performed with a 6 French Shuttle catheter, through a 75 cm long 6 French sheath, and the stent(s) were deployed within the transverse sinus, across the stenosis, with or without venous angioplasty (under general anesthesia and after heparin bolus, to obtain an activated clotting time of over 300).
Case report
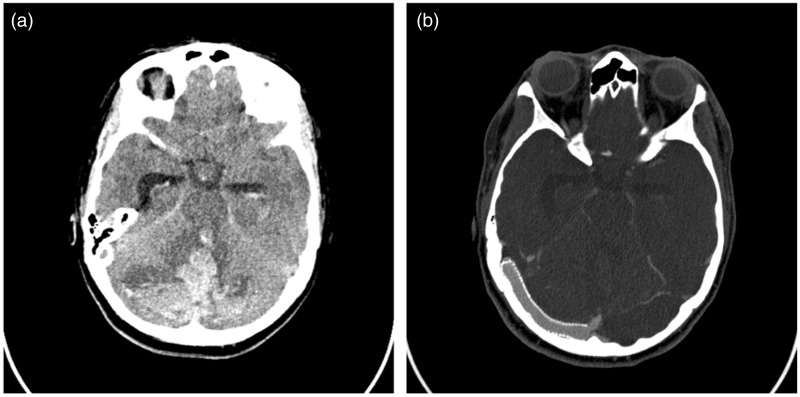
The patient was a 41-year-old woman who was not overweight, who presented with severe headache, grade 1 bilateral papilledema and transient tinnitus. Cerebrospinal (CSF) opening pressure was 36 cm H2O. Magnetic resonance imaging (MRI) revealed a small left parietal meningioma partially invading the lateral part of the superior longitudinal sinus. Venous pressure measurement did not reveal any gradient pressure across the meningioma. Cerebral angiogram and venography confirmed bilateral transverse sinus stenosis (defined by more than 50% lumen diameter narrowing). Venous pressure measurements showed a pressure gradient across the stenosis on the right and left transverse sinus of 21 and 23 mmHg, respectively. Right transverse sinus stenting with two Nitinol stents (PRECISE PRO RX® Carotid Stent System, Cordis, Milpitas, CA, USA) was successfully performed without residual stenosis after stenting (Figure 1). No angioplasty was performed. However, the 35 French guidewire used to position the guide catheter was once inadvertently positioned in a cerebellar vein during the manoeuver to position the guide catheter. Three hours following surgery, the patient’s state of consciousness decreased acutely. A computed tomography (CT) scan revealed an acute cerebellar hemorrhage in bilateral hemispheres and vermis, with intraventricular hemorrhage and obstructive hydrocephalus. Angioscan revealed normal venous sinus patency, without venous thrombosis (Figure 2). The patient received Protamine to reverse the coagulation and an external ventricular shunt was installed. Cerebral MRI was performed emergently after ventricular drainage. Cerebral MRI showed acute mesencephalic ischemia (Figure 3). Following the exams, the patient’s neurological status rapidly deteriorated, with progressive loss of brainstem reflex and neurological death.
Figure 1.
Digital angiogram (DA) and digital subtracted angiogram (DSA) before ((a), (b)) and after ((c), (d)) right transverse sinus stenting. No residual stenosis is observed.
Figure 2.
(a) Unenhanced head computed tomography (CT) showing bilateral cerebellar hemorrhage, intraventricular hemorrhage and acute obstructive hydrocephalus. (b) CT angiography showing right transverse sinus stent patency.
Figure 3.
(a) Magnetic resonance imaging diffusion weighted imaging and (b) corresponding apparent diffusion coefficient map showing acute ischemia in the left pons.
Discussion
We first planned to assess the efficacy of venous stenting to normalize CSF pressure in medically refractory IIH in a clinical trial (cohort study). We report here the occurrence of a severe adverse event leading to the death of the patient in the course of the study. Considering the increasing popularity of this technique, despite its experimental nature and the paucity of high-quality evidence on its safety, we believed that the immediate report of this event was mandatory.
To date, hemorrhagic complications reported after stent placement for venous hypertension treatment are few and essentially related to intracranial stent thrombosis.10,11,20,22 In a series by Higgins et al., two patients also complained of transient partial hearing loss on the stented side, one of unsteadiness, and two patients presented with intraluminal venous thrombus formation in immediate post-op venogram, requiring thrombolysis therapy.11 On a retrospective series of 52 IIH patients treated with sinus stenting, Ahmed et al. reported transient hearing loss in two patients, a subdural hematoma due to cortical vein perforation, and a contralateral subdural, subarachnoidal and intracerebral hemorrhage.10 However, the authors did not attempt to explain this latter complication. We believe that the contralateral occurrence of a subdural hematoma is most probably related to guidewire injury. Indeed, to allow positioning of the guide catheter in the transverse sinus, a 35-inch guidewire was required for support. This guidewire has been designed for use in large extracranial arteries. Its use in intracranial sinus could be considered relatively safe considering the stiffness of the sinus wall. However, its manipulation in the sinus also implies the risk of inadvertence placement in a cerebral vein, which carries a very high risk of venous perforation when using this large guidewire. Despite the absence of contrast extravasation seen during the procedure, cerebellar vein perforation with the large guidewire coupled with dual antiplatelet therapy and intra-procedural anticoagulotherapy, remains a potential mechanism to explain the large and rapidly increasing hematoma seen in our patient. Using an alternative technique with a smaller microcatheter wire system, and potentially with an intermediate catheter if more support was required, as in this case, could have reduced the chance of wire perforation. Moreover, applying an anticoagulation/antiplatelet regimen at the more aggressive end of the reported spectrum may have contributed to the severity of the hemorrhage. It is generally considered that stents in large venous structures are less liable to thrombosis than in arterial structures, and a single agent may probably have sufficed.
In our patient, angioscan showed complete patency of the sinus, ruling out the potential for in-stent thrombosis. Angio-architectural analysis of dural arteriovenous shunting in transverse sinus has shown the presence of dural compartments.23,24 Assuming that these compartments may also be seen without dural arteriovenous shunts, we can hypothesize that the stent may have been placed in one of two sinus lumens, with occlusion of a cerebellar vein drainage through the other lumen, collapsed by the stented one. However, in our particular case, we cannot find any evidence that could have suggested the presence of a venous compartment on the venous phase of the arterial angiography performed before stent placement. Venous MRI performed before stenting did not show any septation suggestive of a double lumen within the right transverse sinus.
Raper et al. recently reported a 19% and 3% decrease in filling and occlusion of the vein of Labbé (VOL) respectively in 56 cases having transverse sinus stenting for IIH, but without any neurological sequelae.25 The authors concluded that stent coverage of the VOL should not deter the therapeutic use of venous sinus stenting in IIH. In our opinion, this unique report cannot accurately estimate the risk of a symptomatic occlusion of any cortical vein with this technique. In our case, careful comparison of digital subtraction angiography and venous MRI performed before stenting showed a cerebellar vein that was no longer visible on the angioscan performed after stenting. Even if the absence of this vein on angioscan after stenting may be due to a delay in contrast enhancing due to the compression by the cerebellar hematoma, rather than its cause, venous ischemia related to mechanical obstruction of a cerebellar vein drainage remains a potential way to explain the complication seen in this patient. Stents used in venous sinus to treat IIH were first designed for use in extracranial arterial conduit. Since their comportment and safety have been specifically studied for arterial disease, veins reaction to these devices is essentially unknown. Venous elastic properties and variable anatomy make morphological changes to stenting less predictable in venous system. In our case, venous expansion with stenting may have displaced a cerebellar vein outflow and have impaired its drainage within the sinus. Venous obstruction may also have directly resulted from stent apposition on the wall of the sinus, with mechanical impairment of a cerebellar vein to drain into the sinus. In this case, we placed two stents in order to completely open the transverse sinus. The increased amount of metal at the overlapping section of the stents may have decreased the lumen of a cerebellar vein outflow. Since venous anatomy is highly variable and complex, individual characterization of venous anatomy could help identify higher-risk patients with critical venous drainage into the targeted sinus. Being aware of angio-architecture variants of transverse venous sinus and its tributaries, interventionists should better examine the venous phase of the arterial angiography and use superposition with venous angiography before placing the stent. Imaging protocols could include in-depth angiogram study with particular incidences showing veins and their outflow into the sinus, coupled with image fusion with venous CT or venous MR to better understand the anatomical relation of these veins with the sinus. However, even the most comprehensive analysis of patient venous anatomy can hardly guarantee prevention of such an event in future patients treated with transverse sinus stenting.
Although mechanical impairment of cerebellar venous drainage or venous perforation are two logical way to explain the cerebellar hemorrhage seen in our patient, the brainstem ischemia cannot be fully explained by the same mechanism. Indeed, the venous drainage of the infarcted territory is anatomically unrelated to vermian or cerebellar venous drainage26 and its radiological appearance (very well-defined border) corresponds to an arterial ischemia more likely due to arterial compression by the enlarging hematoma.
Conclusion
Transverse sinus venous stenting for treatment of resistant IIH should not be prematurely abandoned based on technical complications. Indeed, the risk of such complications could probably be reduced by using alternative tools and improved technique, including the use of a triaxial system with a smaller guidewire/microcatheter and intermediate catheter, as well as a less aggressive antiplatelet/anticoagulotherapy regimen. On the other hand, given the low level of evidence around the safety of this technique, including the risk of a symptomatic occlusion of a cortical vein, physicians should acknowledge the incertitude related with its risk when obtaining consent from a patient, while the scientific community should encourage its formal assessment in clinical trials.
Acknowledgments
The authors would like to thank Frédéric Morin for data collection as well as Drs Jacques Brochu and François Pouliot for their work within the security committee.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: MEA, JLG, PL and GM received a research grant from the Canadian Heads of Academic Radiology/GE Healthcare development program and the Department of Medical Imaging of Laval University.
References
- 1.King JO, Mitchell PJ, Thomson KR, et al. Cerebral venography and manometry in idiopathic intracranial hypertension. Neurology 1995; 45: 2224–2248. [DOI] [PubMed] [Google Scholar]
- 2.Johnston I, Kollar C, Dunkley S, et al. Cranial venous outflow obstruction in the pseudotumour syndrome: Incidence, nature and relevance. J Clin Neurosci 2002; 9: 273–278. [DOI] [PubMed] [Google Scholar]
- 3.Karahalios DG, Rekate HL, Khayata MH, et al. Elevated intracranial venous pressure as a universal mechanism in pseudotumor cerebri of varying etiologies. Neurology 1996; 46: 198–202. [DOI] [PubMed] [Google Scholar]
- 4.Farb RI, Vanek I, Scott JN, et al. Idiopathic intracranial hypertension: The prevalence and morphology of sinovenous stenosis. Neurology 2003; 60: 1418–1424. [DOI] [PubMed] [Google Scholar]
- 5.Baryshnik DB, Farb RI. Changes in the appearance of venous sinuses after treatment of disordered intracranial pressure. Neurology 2004; 62: 1445–1446. [DOI] [PubMed] [Google Scholar]
- 6.Higgins JN, Gillard JH, Owler BK, et al. MR venography in idiopathic intracranial hypertension: Unappreciated and misunderstood. J Neurol Neurosurg Psychiatry 2004; 75: 621–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins JN, Pickard JD. Lateral sinus stenoses in idiopathic intracranial hypertension resolving after CSF diversion. Neurology 2004; 62: 1907–1908. [DOI] [PubMed] [Google Scholar]
- 8.King JO, Mitchell PJ, Thomson KR, et al. Manometry combined with cervical puncture in idiopathic intracranial hypertension. Neurology 2002; 58: 26–30. [DOI] [PubMed] [Google Scholar]
- 9.McGonigal A, Bone I, Teasdale E. Resolution of transverse sinus stenosis in idiopathic intracranial hypertension after L-P shunt. Neurology 2004; 62: 514–515. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed R, Friedman DI, Halmagyi GM. Stenting of the transverse sinuses in idiopathic intracranial hypertension. J Neuroophthalmol 2011; 31: 374–380. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JN, Cousins C, Owler BK, et al. Idiopathic intracranial hypertension: 12 cases treated by venous sinus stenting. J Neurol Neurosurg Psychiatry 2003; 74: 1662–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Métellus P, Levrier O, Fuentes S, et al. Endovascular treatment of benign intracranial hypertension by stent placement in the transverse sinus. Therapeutic and pathophysiological considerations illustrated by a case report [article in French]. Neurochirurgie 2005; 51: 113–120. [DOI] [PubMed] [Google Scholar]
- 13.Rajpal S, Niemann DB, Turk AS. Transverse venous sinus stent placement as treatment for benign intracranial hypertension in a young male: Case report and review of the literature. J Neurosurg 2005; 102(3 Suppl): 342–346. [DOI] [PubMed] [Google Scholar]
- 14.Fields JD, Javedani PP, Falardeau J, et al. Dural venous sinus angioplasty and stenting for the treatment of idiopathic intracranial hypertension. J Neurointerv Surg 2013; 5: 62–68. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JN, Owler BK, Cousins C, et al. Venous sinus stenting for refractory benign intracranial hypertension. Lancet 2002; 359: 228–230. [DOI] [PubMed] [Google Scholar]
- 16.Ogungbo B, Roy D, Gholkar A, et al. Endovascular stenting of the transverse sinus in a patient presenting with benign intracranial hypertension. Br J Neurosurg 2003; 17: 565–568. [DOI] [PubMed] [Google Scholar]
- 17.Owler BK, Parker G, Halmagyi GM, et al. Pseudotumor cerebri syndrome: Venous sinus obstruction and its treatment with stent placement. J Neurosurg 2003; 98: 1045–1055. [DOI] [PubMed] [Google Scholar]
- 18.Elder BD, Rory Goodwin C, Kosztowski TA, et al. Venous sinus stenting is a valuable treatment for fulminant idiopathic intracranial hypertension. J Clin Neurosci 2015; 22: 685–689. [DOI] [PubMed] [Google Scholar]
- 19.Dinkin MJ, Patsalides A. Venous sinus stenting in idiopathic intracranial hypertension: Results of a prospective trial. J Neuroophthalmol 2017; 37: 113–121. [DOI] [PubMed] [Google Scholar]
- 20.Kalyvas AV, Hughes M, Koutsarnakis C, et al. Efficacy, complications and cost of surgical interventions for idiopathic intracranial hypertension: A systematic review of the literature. Acta Neurochir (Wien) 2017; 159: 33–49. [DOI] [PubMed] [Google Scholar]
- 21.Friedman DI. Idiopathic intracranial hypertension. Curr Pain Headache Rep 2007; 11: 62–68. [DOI] [PubMed] [Google Scholar]
- 22.Albuquerque FC, Dashti SR, Hu YC, et al. Intracranial venous sinus stenting for benign intracranial hypertension: Clinical indications, technique, and preliminary results. World Neurosurg 2011; 75: 648–652. discussion 592–595. [DOI] [PubMed] [Google Scholar]
- 23.Piske RL, Campos CM, Chaves JB, et al. Dural sinus compartment in dural arteriovenous shunts: A new angioarchitectural feature allowing superselective transvenous dural sinus occlusion treatment. AJNR Am J Neuroradiol 2005; 26: 1715–1722. [PMC free article] [PubMed] [Google Scholar]
- 24.de Paula Lucas C, Prandini MN, Spelle L, et al. Parallel transverse-sigmoid sinus harboring dural arteriovenous malformation. How to differentiate the pathological and normal sinus in order to treat and preserve patency and function. Acta Neurochir (Wien) 2010; 152: 523–527. [DOI] [PubMed] [Google Scholar]
- 25.Raper DMS, Ding D, Chen CJ, et al. Patency of the vein of Labbé after venous stenting of the transverse and sigmoid sinuses. J Neurointerv Surg 2017; 9: 587–590. [DOI] [PubMed] [Google Scholar]
- 26.Rhoton AL., Jr The cerebellar arteries. Neurosurgery 2000; 47(3 Suppl): S29–S68. [DOI] [PubMed] [Google Scholar]