Abstract
Basilar trunk perforator artery aneurysms are rare. Their diagnosis and treatment are difficult, controversial, and challenging. Analysis of 52 cases (49 documented in the literature and three personal cases) clearly shows a re-bleeding rate of 15% in patients whose aneurysm has not been occluded and 0% in treated patients (p < 0.05). The most effective treatment, and the one that presents the least complication, is double-stenting across the basilar trunk.
Keywords: Perforator aneurysm, basilar trunk, stent
Introduction
Basilar trunk perforator artery aneurysms are rare. Moreover their definition is controversial. According to Aboukais et al.,1 these are aneurysms whose neck is entirely located on a perforator artery without direct involvement of the basilar trunk. Satti and colleagues2 describe three types: aneurysms arising from the basilar trunk adjacent to a perforating arterial branch but not involving a perforating artery (Type I); aneurysms originating from the perforating artery base (Type II), with IIa aneurysms incorporating the origin of the perforating arteries and IIb aneurysms having the perforating artery arising from the dome of the aneurysm. Type III aneurysms are fusiform and arise beyond the parent vessel. Because of their rarity, the natural history and ideal approach to treatment has not been established. Following are three personal case reports, a statistical analysis of the published cases is presented and, lastly, a conduct is proposed.
Case 1
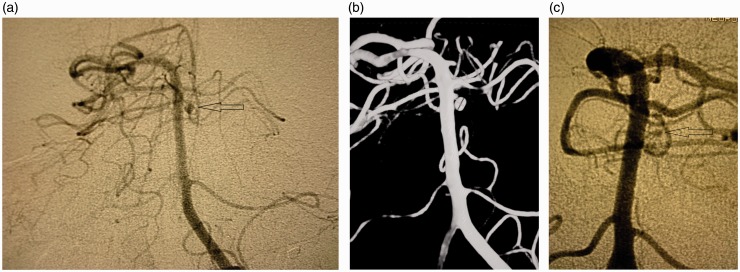
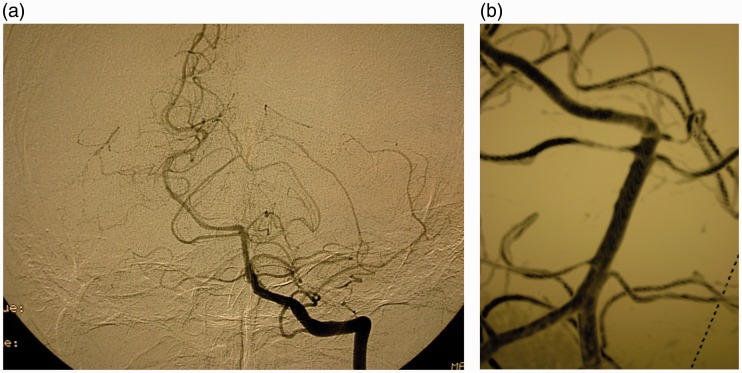
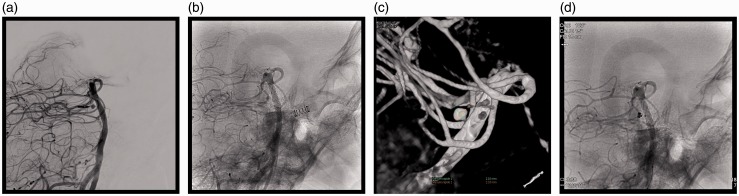
A 69-year-old man presented with vomiting after sudden headaches. The Glasgow Score (GS) was 11 without neurological deficit (Hunt and Hess score and World Federation of Neurosurgical Societies (WFNS) score 4). Cerebral computed tomography (CT) showed posterior subarachnoid haemorrhage (SAH), intraventricular haemorrhage (Fisher grade 4) and hydrocephalus. After the placement of an external ventricular derivation, an initial four-vessel cerebral angiogram was carried out which did not reveal the source of the haemorrhage. During his hospitalisation, clinical conditions slowly improved despite several pulmonary infections, and the patient was discharged one month later without any neurological symptom. Two months afterwards, a computed tomographic angiography (CTA) and a new cerebral angiogram were performed and showed a 2.5 mm aneurysm behind the basilar artery (BA), originating from a rostral perforating artery (Figure 1(a) and (b)). An endovascular treatment was decided, and planned one week later. The pre-embolisation angiogram showed a significant decrease of the lesion and a conservative management was decided (Figure 1(c)). One year later, the patient was free of symptoms (modified Rankin Score (mRS) 0) and a new cerebral angiogram showed no sign of the lesion (Figure 2(a) and (b)).
Figure 1.
(a) Arteriography (anteroposterior view) two months after subarachnoid haemorrhage (SAH), a 2.5 mm distal one-third perforator aneurysm (arrow). (b) Three-dimensional angiography two months after SAH. (c) Pre-embolisation angiogram: significant decrease of the aneurysm.
Figure 2.
(a) Arteriography (anteroposterior view) one year later: total disappearance of the aneurysm (arrow). (b) Three-dimensional angiography one year later.
Case 2
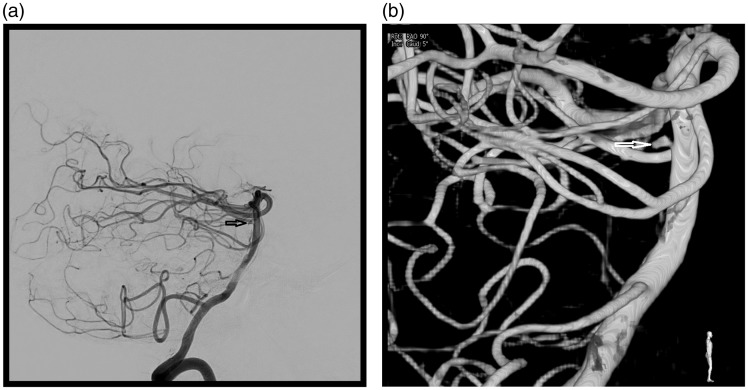
A 53-year-old patient with no previous medical history was admitted to the emergency department with sudden-onset headache. On admission, the GS, WFNS, and Hunt and Hess scores were 15, 1, and 1, respectively. The cerebral CT scan revealed an SAH grade 4 on the Fisher scale (intraventricular haemorrhage with no hydrocephalus). CTA did not reveal any intracranial aneurysms. The cerebral angiography performed just after showed a left perforator artery aneurysm in the upper third of the BA trunk measuring 1.8 mm at its widest point (Figure 3(a) and (b)). The treatment was performed the following day (D1). Under general anaesthesia, an 8F sheath was placed on each femoral artery. An intra-arterial dose of 20 mg eptifibatide was administered. Three-dimensional rotational acquisition by bilateral injection on the two vertebral arteries showed a decrease in the lesion’s size, now measuring 0.5 mm at its widest point (Figure 4(a)). In view of the extremely small size of the lesion and its highly probable fragility, it was decided not to risk selective catheterisation and coiling, but to treat the patient by stenting. In order to minimise the risk of occlusion of BA perforators, it was decided to deploy two LEO stents, one within the other, instead of using a flow diverter. Two LEO Baby stents (2.5–18 and 2.5–12) were deployed one within the other adjacent to the perforator artery with the aneurysm, resulting in the complete disappearance of the lesion from view (Figure 4(b)). Upon awakening, the patient’s clinical status was unchanged. Sixty milligrams of prasugrel was administered orally and 250 mg of acetylsalicylic acid was injected intravenously, followed by a daily dose of 10 mg of prasugrel combined with 75 mg of acetylsalicylic acid orally.
Figure 3.
(a) Lateral view of the initial angiography showed a 1.8 mm distal perforator aneurysm (arrow). (b) Three-dimensional angiography (arrow).
Figure 4.
(a) Three-dimensional angiography at day one. (b) Anteroposterior view after stent-in-stent technique: no opacification of the aneurysm.
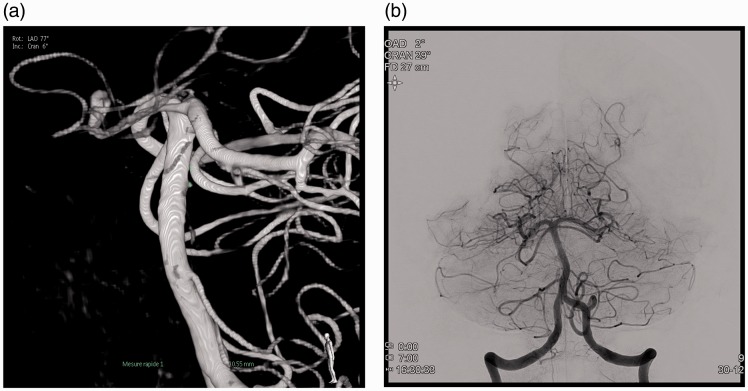
On the eighth day, the patient presented a diminished consciousness level (GS 11), leading to the diagnosis of a diffuse arterial vasospasm. Angioplasty was performed on segments M1, A1 and at the carotid terminations, bilaterally. There was no vasospasm of the BA, but there was evidence of a 2 mm repermeabilisation of the aneurysmal lesion. On the ninth day, the patient was taken back to the angio suite and the angiography found an increased size in the aneurysm, now measuring 2.5 mm (Figure 5(a)–(c)). It was possible to deliver two coils through the mesh of the two stents to occlude this repermeabilisation (Figure 2(b)). Upon awakening, the patient presented with right-side hemiplegia and dysarthria. An emergency magnetic resonance imaging scan was performed, revealing a left-sided medial-pontine ischaemic lesion. Six hours later, the patient had completely recovered from the neurological deficit. Six months after the SAH, the patient had no neurological symptoms (mRS 0) and the follow-up angiography showed a completely occluded aneurysm.
Figure 5.
(a) Lateral view (subtracted) at ninth day showed a 2.5 mm aneurysmal repermeabilisation. (b) A lateral view (non-subtracted). (c) Three-dimensional reconstruction. (d) Two coils were delivered through the mesh of the two stents.
Case 3
A 59-year-old patient with no previous medical history was admitted to the emergency department with sudden-onset headache. On admission, the GS, WFNS, and Hunt and Hess scores were 15, 1, and 1, respectively. The cerebral CT scan showed an SAH Fisher grade 3. A CTA and a cerebral angiogram performed on the same day did not find any aneurysms. The angiogram performed on the fifth day revealed a 1.5 mm aneurysm on the right rostral perforating branch of the BA. An injection of 20 mg of eptifibatide was administered and, as with the previous patient, two LEO Baby stents (2.5–12 mm) were placed in the BA adjacent to the perforator artery. At the end of the procedure, a marked reduction in the filling of that artery was observed. The same antiplatelet protocol as described in clinical Case 2 was instituted. At six months, the patient presented with an mRS score 0 and the control angiogram revealed complete occlusion of the aneurysm, with no intra-stent stenosis.
Literature analysis
A comprehensive literature search using PubMed was performed. The keywords ‘cerebral aneurysm’, ‘perforator aneurysm’ and ‘basilar artery’ were used for this search. Twenty-three articles with case reports or case series were found, relating to 57 patients (including those described here).1–23 Five patients were excluded: One had a post-traumatic aneurysm,6 one a high-flow aneurysm related to an arteriovenous malformation,10 and three patients were described in two different articles.14,23 Their mean age was 58 years, the median age was 59 years, with a maximum of 82 years and a minimum of 27 years. There were twice as many men as women. The mean aneurysm size was 2.5 mm, with a median of 2 mm, a minimum of 0.5 mm and a maximum of 7 mm. The median Fisher score was 3, with a minimum of 3 and a maximum of 4. All patients presented with SAH. For the analysis of the literature data, patients were divided into three groups: patients submitted to surgical treatment (surgery group), patients submitted to endovascular treatment (endovascular group), and patients receiving neither surgical nor endovascular treatment (conservative group).
There were nine patients in the surgery group.3–6,8,12,13,19 There was one (11%) failure: The aneurysm could not be clipped, and the patient underwent endovascular treatment.5 Two (22%) aneurysms were selectively excluded with preservation of the perforator artery.3,19 Six (67%) perforator arteries were sacrificed.4,6,8,12,13 There were five (56%) postoperative complications,3,4,8,12,13 one (11%) was permanent following a stroke in the region of the sacrificed perforator artery.13 There were no cases of re-bleeding after surgical treatment (Table 1).
Table 1.
Surgery group.
| First author/Year | Patient number | Localisation | Preservation of perforator artery | Definitive ischaemic complication | Re-SAH |
|---|---|---|---|---|---|
| Ghogawala3/1996 | 1 | Distal one-third | Yes | No | No |
| Hamel4/2005a | 2 | Middle one-third | No | No | No |
| Sanchez-Mejia6/2007 | 3 | Distal one-third | No | No | No |
| 4 | Middle one-third | No | No | No | |
| Mathieson8/2010a | 5 | Distal one-third | No | No | No |
| Gross12/2013 | 6 | Distal one-third | No | No | No |
| Apok13/2013a | 7 | Distal one-third | No | Yes | No |
| Sivakanthan19/2015 | 8 | Distal one-third | Yes | No | No |
Failed endovascular treatment. SAH: subarachnoid haemorrhage.
The endovascular group consisted of 31 patients including the three cases presented here.2,4,5,8,9–11,13–18,20,22,23 There were 10 (32%) failures of the endovascular treatment due to the impossibility of catheterising the perforator artery, three (10%) patients underwent surgery,4,8,13 and the seven (23%) remaining patients were kept under observation.14,18,20 Twenty (65%) were treated as follows: 10 (32%) stent-within-stent (including the two patients described here),2,5,9,11,15,23 five (16%) flow diverters,16,17,22 one (3%) single stent,5 for two (6%) patients, the perforator artery and the aneurysm were occluded with Onyx14,20 and two (6%) aneurysms were coiled.10,20 There was one (3%) death due to a rupture of the perforator artery during catheterisation.23 There were nine (29%) complications: Five (17%) patients were made worse by the treatment14,16,20,23 (four ischaemias,14,16,20 one patient died due to perforator rupture23); four (including one presented here) presented a transient ischaemic complication.2,16 All ischaemic complications were secondary to occlusion of the perforating artery carrying the aneurysm. There was no recurrence of bleeding in this group (Table 2). The conservative group comprised 40 patients (including one described here).1–3,6–9,11,13,14,16,18–23 There were 12 (30%) complications. Six (15%) patients presented re-bleeding.6,7,13,20,22 (Table 3). The mean time to re-bleeding was 21.6 days and the median was 13 days, with a minimum of five and a maximum of 60 days. Two (5%) patients were secondarily treated by surgery,6,13 two by the endovascular approach20,22 and two conservatively.7,20 Six (15%) patients7,20,22 presented an ischaemic complication, two (5%) of which led to a worsening of the patients’ initial clinical status (mRS 5 at the end of follow-up).20,22 Regarding the 15 (37%) patients for whom data were available, the aneurysm thrombosed spontaneously on average on the 137th day (median 90 days, standard deviation 159 days, minimum 4 days, maximum 480 days).2,7,11,14,18,20,22
Table 2.
Endovascular treatment.
| First author/Year | Patient number | Localisation | Treatment modalities | Preservation of perforator artery | Definitive ischaemic complication |
|---|---|---|---|---|---|
| Fiorella5/2006 | 1a | Distal one-third | One stent | Unknown | No |
| 2 | Basilar trunk | Stent in stent | Unknown | No | |
| Deshaies9/2011 | 3 | Distal one-third | Stent in stent | Yes | No |
| Chen10/2012 | 4 | Right pontine circumferential artery | Coils | Yes | No |
| Nyberg11/2013 | 5 | Middle one-third | Stent in stent | Yes | No |
| 6 | Middle one-third | Stent in stent | Yes | No | |
| Ding14/2013 | 7 | Distal one-third | Onyx | No | Yes |
| Kim15/2014 | 8 | Distal one-third | Stent in stent | Yes | No |
| Chalouhi17/2014 | 9 | Middle one-third | Flow diverter | Yes | No |
| Satti2/2017 | 10 | Middle one-third | Stent in stent then one more stent | No | No |
| Peschillo16/2016 | 11 | Distal one-third | Flow diverter | No | Yes |
| 12 | Distal one-third | Stent plus flow diverter | Yes | No | |
| 13 | Distal one-third | Flow diverter | Unknown | Unknown | |
| Forbrig20/2016 | 14 | Distal one-third | Coil | No | Yes |
| 15 | Distal one-third | Onyx | No | Yes | |
| Finitsis22/2017 | 16 | Middle one-third | Flow diverter | Yes | No |
| Buell23/2017 | 17 | Middle one-third | Stent in stent | Yes | No |
| 18 | Middle one-third | Stent in stent | Yes | No | |
| Present series | 19 | Distal one-third | Stent in stent | No | No |
| 20 | Distal one-third | Stent in stent | Yes | No |
In this group there is no re-bleeding; one patient died because of perforator rupture.23
Failed surgery.
Table 3.
Conservative group: treatment after re-subarachnoid haemorrhage (SAH (6/40, 15%).
In total, of the 52 patients investigated, 28 (54%) patients had their aneurysm occluded (eight (15%) of the patients were treated surgically, 20 (38%) by the endovascular approach), one (2%) patient died due to rupture of the perforator artery during catheterisation, and 23 (44%) patients were managed conservatively.
There is a significant difference in the re-bleeding rate (15%) between the patients in the conservative group and those (0%) treated by open or endovascular surgery (Fisher’s exact test, p = 0.04). There is no significant difference between these two groups in terms of ischaemic complications.
Discussion
BA perforator aneurysms are a rare cause of SAH.22 Their diagnosis is difficult because it consists of tiny and often partially thrombosed lesions8 fed by a small-calibre artery.21 Cerebral angiography should be performed following a strict technique, and repeated if the SAH is strongly suggestive of aneurismal rupture, the exact timing of this repeat imaging still being a matter of debate.1,6,8,14,18,23 The natural history is not completely understood, which makes the therapeutic choice difficult.2,18,22,23 The latter should be discussed on a multidisciplinary basis.
Conservative management is favoured by several authors; thus Park et al.7 suggest that this is a subtype of spontaneous SAH that has a unique haemorrhage localisation (pre-mesencephalic cistern), specific cause (tiny aneurysms at the origin of the mesencephalic perforator artery), and common benign clinical course. Forbrig20 and colleagues estimate that a conservative treatment might be an eligible first-line treatment option particularly in tiny BA perforator aneurysms with a small parent vessel taking into account the perioperative risks of endovascular and microsurgical treatment. Buell et al.23 propose surveillance with, maybe, in the absence of any contraindication, the introduction of antifibrinolytic therapy, and recommends endovascular treatment if the lesion grows. Finitsis et al.22 also propose endovascular treatment in the event of increasing size, re-bleeding, or lack of regression. However, considering the potentially serious ischaemic complications due to spontaneous occlusion of the perforator artery14,20,22 and the significant difference in the re-bleeding rate between patients who had undergone aneurismal exclusion and surveyed patients (0% vs 15%, p < 0.05), a treatment should be proposed for these patients. This treatment may consist of the selective occlusion of the aneurysm, with sacrifice of the perforator artery, or in stenting the BA. Selective occlusion could be performed only twice (2/8, 25%) surgically3,19 as, according to Sanchez-Mejia and Lawton,6 these types of aneurysms are not amenable to conventional clip occlusion because they have no necks. According to Mathieson et al.,8 it is difficult to obtain proximal control prior to securing the lesion, and it is clearly not possible to perform subpial dissection around the dome of the aneurysm to completely assess the anatomy of the lesion, with the necessity to preserve all the other perforator vessels, and avoid damage to the surrounding cranial nerves. It is equally difficult to selectively exclude this type of aneurysm by the endovascular approach, because the perforator artery that has the aneurysm is rarely suitable for catheterisation. That was achieved with only one (1/20, 5%) Type 3 aneurysm.10 Moreover, catheterisation manoeuvres can be risky and result in aneurysmal rupture.23,24 The second possibility is to sacrifice the parent artery, either surgically or by the endovascular approach. Although it has been stressed by several surgeons that all basilar perforator arteries must be preserved, because it is not possible to judge the functional significance of these perforator arteries based on size or location,3,19 due to the existence of anastomoses between the three groups of perforator arteries,25 it is possible to sacrifice one perforator artery without causing cerebral trunk ischaemia. This was performed in six (6/8, 75%) cases surgically4,6,8,12,13 with one permanent ischaemic complication13 and in three (3/20, 15%) cases by the endovascular route with two permanent ischaemic complications.14,20 An alternative to selective aneurysmal exclusion and sacrifice of the perforator artery is stenting of the BA adjacent to the perforator arterial branch, with the purpose of reducing the flow in the aneurysm to bring its thrombosis, while preserving the patency of the perforator artery. Digital particle velocimetry flow studies in sidewall aneurysm models have demonstrated a significant decrease in intra-aneurysmal flow velocity and vorticity and wall shear stress when one or more stents are placed across the orifice of the aneurysm.26,27 The choice of stent depends on its porosity: The less porous the stent, the greater the intra-saccular haemodynamic effect28 but also the greater the risk of occlusion of a perforator artery.9,17,29 Thus, Phillips et al.30 report 14% perforator territory infarctions when placing a flow diverter stent in the treatment of posterior circulation aneurysms. For these reasons, 50% of BA perforator aneurysms treated by the endovascular approach have relied on the stent-within-stent technique2,5,9,11,15,23 without any definitive ischaemic complications being observed around the covered perforator arteries, whereas of the five (25%) patients treated with flow diverter stents,16,17,22 one presented a cerebral trunk infarction by occlusion of a perforator artery covered by the device.16 The disadvantage of stenting is the need to start a double anti-aggregant therapy during the bleeding period.16,20
In conclusion, BA perforator aneurysms responsible for SAH should be treated, and the stent-within-stent technique should be given preference because it represents a relatively low-risk treatment option.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Aboukais R, Zairi F, Estrade L, et al. A dissecting aneurysm of a basilar perforating artery. Neurochirurgie 2016; 62: 263–265. [DOI] [PubMed] [Google Scholar]
- 2.Satti SR, Vance AZ, Fowler D, et al. Basilar artery perforator aneurysms (BAPAs): Review of the literature and classification. J Neurointerv Surg 2017; 9: 669–673. [DOI] [PubMed] [Google Scholar]
- 3.Ghogawala Z, Shumacher JM, Ogilvy CS. Distal basilar perforator artery aneurysm: Case report. Neurosurgery 1996; 39: 393–396. [DOI] [PubMed] [Google Scholar]
- 4.Hamel W, Grzyska U, Westpha, et al. Surgical treatment of a basilar perforator aneurysm not accessible to endovascular treatment. Acta Neurochir (Wien) 2005; 147: 1283–1286. [DOI] [PubMed] [Google Scholar]
- 5.Fiorella D, Albuquerque FC, Deshmukh VR, et al. Endovascular reconstruction with the Neuroform stent as monotherapy for the treatment of uncoilable intradural pseudoaneurysms. Neurosurgery 2006; 59: 291–300. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Mejia RO, Lawton MT. Distal aneurysms of basilar perforating and circumferential arteries. Report of three cases. J Neurosurg 2007; 107: 654–659. [DOI] [PubMed] [Google Scholar]
- 7.Park SQ, Kwon OK, Kim SH, et al. Pre-mesencephalic subarachnoid hemorrhage: Rupture of tiny aneurysms of the basilar artery perforator. Acta Neurochir (Wien) 2009; 151: 1639–1646. [DOI] [PubMed] [Google Scholar]
- 8.Mathieson CS, Barlow P, Jenkins S, et al. An unusual case of spontaneous subarachnoid haemorrhage – a ruptured aneurysm of a basilar perforator artery. Br J Neurosurg 2010; 24: 291–293. [DOI] [PubMed] [Google Scholar]
- 9.Deshaies E, Jacobsen W, Krishnamurthy S. Enterprise stent-within-stent embolization of a basilar artery perforator aneurysm. World J Neurosci 2011; 1: 45–48. [Google Scholar]
- 10.Chen L, Chen E, Chotai S, et al. An endovascular approach to ruptured aneurysms of the circumferential branch of the basilar artery. J Clin Neurosci 2012; 19: 527–531. [DOI] [PubMed] [Google Scholar]
- 11.Nyberg EM, Chaudry MI, Turk AS, et al. Report of two cases of a rare cause of subarachnoid hemorrhage including unusual presentation and an emerging and effective treatment option. J Neurointerv Surg 2013; 5: e30. [DOI] [PubMed] [Google Scholar]
- 12.Gross BA, Puri AS, Du R. Basilar trunk perforator artery aneurysms. Case report and literature review. Neurosurg Rev 2013; 36: 163–168. [DOI] [PubMed] [Google Scholar]
- 13.Apok V, Tarnaris A, Brydon HL. An unusual aneurysm of a basilar perforating artery presenting with a subarachnoid haemorrhage. Br J Neurosurg 2013; 27: 105–107. [DOI] [PubMed] [Google Scholar]
- 14.Ding D, Starke RM, Jensen ME, et al. Perforator aneurysms of the posterior circulation: Case series and review of the literature. J Neurointerv Surg 2013; 5: 546–551. [DOI] [PubMed] [Google Scholar]
- 15.Kim YJ, Ko JH. Sole stenting with large cell stents for very small ruptured intracranial aneurysms. Interv Neuroradiol 2014; 20: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peschillo S, Caporlingua A, Cannizzaro D, et al. Flow diverter stent treatment for ruptured basilar trunk perforator aneurysms. Neurointerv Surg 2016; 8: 190–196. [DOI] [PubMed] [Google Scholar]
- 17.Chalouhi N, Jabbour P, Starke RM, et al. Treatment of a basilar trunk perforator aneurysm with the pipeline embolization device: Case report. Neurosurgery 2014; 74: E697–E701. [DOI] [PubMed] [Google Scholar]
- 18.Chavent A, Lefevre PH, Thouant P, et al. Spontaneous resolution of perforator aneurysms of the posterior circulation. J Neurosurg 2014; 121: 1107–1111. [DOI] [PubMed] [Google Scholar]
- 19.Sivakanthan S, Carlson AP, van Loveren H, et al. Surgical clipping of a basilar perforator artery aneurysm: A case of avoiding perforator sacrifice. J Neurol Surg A Cent Eur Neurosurg 2015; 76: 79–82. [DOI] [PubMed] [Google Scholar]
- 20.Forbrig R, Eckert B, Ertl L, et al. Ruptured basilar artery perforator aneurysms – treatment regimen and long-term follow-up in eight cases. Neuroradiology 2016; 58: 285–291. [DOI] [PubMed] [Google Scholar]
- 21.Daruwalla VJ, Syed FH, Elmokadem AH, et al. Large basilar perforator pseudoaneurysm: A case report. Interv Neuroradiol 2016; 22: 662–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finitsis S, Derelle AL, Tonnelet R, et al. A dissecting aneurysm of a basilar perforating artery. Basilar perforator aneurysms: Presentation of 4 cases and review of the literature. World Neurosurg 2017; 97: 366–373. [DOI] [PubMed] [Google Scholar]
- 23.Buell TJ, Ding D, Raper DM, et al. Posterior circulation perforator aneurysms: A proposed management algorithm. Neurointerv Surg. Epub ahead of print 6 January 2017. DOI: 10.1136/neurintsurg-2016-012891. [DOI] [PubMed]
- 24.Darsaut TE, Costalat V, Salazkin I, et al. Fatal avulsion of choroidal or perforating arteries by guidewires. Case reports, ex vivo experiments, potential mechanisms and prevention. Interv Neuroradiol 2014; 20: 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marinković SV, Gibo H. The surgical anatomy of the perforating branches of the basilar artery. Neurosurgery 1993; 33: 80–87. [DOI] [PubMed] [Google Scholar]
- 26.Cantón G, Levy DI, Lasheras JC, et al. Flow changes caused by the sequential placement of stents across the neck of sidewall cerebral aneurysms. J Neurosurg 2005; 103: 891–902. [DOI] [PubMed] [Google Scholar]
- 27.Lieber BB, Livescu V, Hopkins LN, et al. Particle image velocimetry assessment of stent design influence on intra-aneurysmal flow. Ann Biomed Eng 2002; 30: 768–777. [DOI] [PubMed] [Google Scholar]
- 28.Bouillot P, Brina O, Ouared R, et al. Particle imaging velocimetry evaluation of intracranial stents in sidewall aneurysm: Hemodynamic transition related to the stent design. PLoS One 2014; 9: e113762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roszelle BN, Babiker MH, Hafner W, et al. In vitro and in silico study of intracranial stent treatments for cerebral aneurysms: Effects on perforating vessel flows. J Neurointerv Surg 2013; 5: 354–360. [DOI] [PubMed] [Google Scholar]
- 30.Phillips TJ, Wenderoth JD, Phatouros CC, et al. Safety of the pipeline embolization device in treatment of posterior circulation aneurysms. AJNR Am J Neuroradiol 2012; 33: 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]