Abstract
Objective
Endovascular stent-assistant angioplasty (ESAA) is a valid treatment for symptomatic vertebrobasilar artery stenosis (SVAS), but the long-term effect and the improvement of condition compared with medication treatment are unknown. This study investigated the long-term efficacy of ESAA in patients with moderate and severe SVAS, and compared the efficacy with medication treatment.
Materials and methods
We conducted a retrospective analysis of clinical data of 43 patients with moderate and severe SVAS hospitalized in our department. According to different treatment methods they were divided into 29 cases in an ESAA group and 14 cases in a medication treatment group. During the follow-up period, the degree of vascular stenosis, vascular blood flow velocity, restenosis rate, recovery of neurological function and the incidence of cerebral ischemic events in the two groups were analyzed.
Results
The average clinical follow-up period was 89.4 ± 10.2 months. Before treatment, the stenosis rate and average blood flow velocity of the two groups were not statistically significant (p > 0.05). During the follow-up period, both were significantly lower than the medication treatment group (p < 0.01). In the ESAA group, three cases of stent stenosis, and three cases in the medication treatment group were completely occluded. The total ischemic events in ESAA group were three cases, compared with nine cases in the medication treatment group; the difference was statistically significant (p < 0.05).
Conclusion
ESAA has a long-term effect in the treatment of symptomatic moderate and severe vertebrobasilar artery stenosis. It is superior to medication therapy in preventing posterior circulation ischemia (PCI), but a larger sample size is still needed to confirm the study.
Keywords: Endovascular stent, angioplasty, stenosis, vertebrobasilar artery, medication
Introduction
Ischemic stroke, the main subtype of stoke, has the characteristics of high incidence, high mortality, high disability rate, and high recurrence rate.1 Worldwide, stroke has become the most common cause of functional disability.2 More than 40% of ischemic cerebrovascular diseases are related to the vertebrobasilar circulation.3 One-fourth of patients with more than 50% atherosclerotic vertebral/basilar artery stenosis had a concurrent vertebrobasilar transient ischemic attack (TIA) or stroke. Compared with patients without stenosis, those with symptomatic vertebrobasilar artery stenosis (SVAS) had higher rates of posterior circulation stroke and recurrent stroke.4,5
When vertebrobasilar artery stenosis or occlusion occurred, the corresponding cerebral region became ischemic and hypoperfused, patients suffered from dizziness, ataxia, limb weakness, visual disturbance, speech disorder, drinking cough, and other nervous system symptoms, even coma and life-threatening states, and prognosis was poor. Early intervention with arterial stenosis can significantly reduce stroke recurrence. Clinical treatment methods include surgical treatment, medication therapy, and endovascular stent-assistant angioplasty (ESAA). ESAA has become a valid method for the treatment of vertebrobasilar artery stenosis in recent years.6 Our early short-term follow-up study of endovascular stenting for elderly patients with SVAS revealed a good clinical outcome.7 Several studies also showed that this method had a good therapeutic prospect.8–10 However, there are some complications such as cerebral hemorrhage, vasospasm, high perfusion injury, and stent restenosis in stent angioplasty. Compared with medication therapy, the safety and efficacy of stents have been questioned.11,12 Additionally, the long-term efficacy of stent angioplasty in the treatment of vertebrobasilar artery stenosis is unknown. There is also no comparative study on the efficacy of stent angioplasty versus medication therapy for vertebrobasilar artery stenosis. Therefore, this study collected the follow-up data of stent angioplasty and medication-treated patients with moderate and severe stenosis confirmed by digital subtraction angiography (DSA), retrospectively analyzed the long-term effect of endovascular stenting for symptomatic moderate and severe vertebrobasilar artery stenosis in patients, and compared outcomes of the two treating methods.
Materials and methods
This study was approved by the institutional review board with a waiver of informed consent because of its retrospective design.
We collected data from symptomatic posterior circulation ischemia (PCI) patients in our department from May 2008 to December 2010. The clinical data of patients with moderate or severe vertebrobasilar artery stenosis were confirmed by DSA. Inclusion criteria: (a) According to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) standard,13 all patients were confirmed by DSA to have moderate or severe stenosis of the vertebrobasilar artery (stenosis ≥50%); (b) there was no coagulopathy, active gastrointestinal ulcer or severe organ dysfunction; (c) National Institutes of Health Stroke Scale score (NIHSS) ≤9; (d) patients had received standardized medication; (e) follow-up data was integrity. Exclusion criteria: (a) Patients missed visit halfway; (b) patient suffered anterior circulation infarction; (c) other systemic diseases caused severe disability and death in the patient; (d) the medication treatment patients were changed to stent therapy during the follow-up period; (e) patients suffered from congenital vertebrobasilar artery stenosis.
Forty-three patients meeting the inclusion criteria were screened: Eighteen cases of recurrent posterior circulation TIA, and 25 cases of posterior circulation infarction. Most of the clinical symptoms were dizziness, diplopia, ataxia, dysarthria, homonymous hemianopia, hemiplegia, and sensory disturbance. All patients were treated with antiplatelet aggregation, or statin, and other risk factors were controlled for stroke. According to the different treatment methods, patients were divided into 29 cases in an ESAA group and 14 cases in a medication treatment group.
In the ESAA group, there were 12 males and 17 females, aged 65 ∼ 78 years, with an average of 71.9 ± 3.7 years. Thirteen cases had vertebrobasilar artery system TIA, and 16 cases had posterior circulation infarction. Nineteen cases had stenosis of vertebral artery opening: Three cases were V4 segment stenosis, and seven cases were basilar artery stenosis according to DSA. All ESAA procedures for treating SVAS were conducted in accordance with the ethics standards of the responsible committee on human experimentation and with the Declaration of Helsinki as revised in 1983, and received the approval of our hospital ethics committee. ESAA patients were in line with a previous study of ESAA indications, and excluded from contraindications. Patients and/or relatives signed to agree to surgery.7 The stent therapy was treated with simultaneous medication: Dual antiplatelet therapy (DAPT) (aspirin tablets (Bayer Inc), 100 mg once daily (Qd) and clopidogrel tablets (Sanofi Inc), 75 mg Qd), one to six months later changed to clopidogrel 75 mg Qd, atorvastatin (Pfizer Inc) 20–40 mg/d for lipid regulation and plaque stabilization, and control of blood pressure (Bp), blood sugar, and other risk factors for stroke. The target of treatment was blood lipid control (low-density lipoprotein cholesterol (LDL-C) <2.1 mmol/l, or LDL-C decrease >40%), Blood pressure was controlled to be Bp ≤ 140/90 mmHg which was under tolerable circumstances (diabetics, Bp ≤ 130/80 mmHg). The target of diabetes treatment was HbA1C < 7.0%.
In the medication treatment group, there were eight males and six females, aged 64 ∼ 75 years, with an average of 70.7 ± 3.9 years. Among them, five cases had vertebrobasilar artery system TIA, nine cases had posterior circulation infarction. Six cases were vertebral artery opening stenosis, one case was V2 segment stenosis, two cases were V4 segment stenosis, five cases were basilar artery stenosis. The medication treatment group had stent angioplasty indications under DSA angiography when admitted to the hospital. However, patients and/or relatives refused surgical treatment and chose medication therapy. DAPT one to four weeks later was changed to clopidogrel 75 mg Qd, atorvastatin 20–40 mg/d for lipid regulation and plaque stabilization, and control of Bp, blood sugar, and other risk factors for stroke, as in the ESAA group.
ESAA group and treatment
Preoperative management, surgical approach, and postoperative management were performed according to our past procedures,7 similar to previous studies.14,15 Antiplatelet agents (aspirin and clopidogrel) were administered at least three days before the procedure and continued for at least 1 ∼ 6 months after the ESAA. All patients were taking atorvastatin 20 mg/d, at the same time, strictly controlling the related risk factors for stroke.
All ESAA procedures were performed by two experienced interventional surgeons. Under routine electrocardiography (ECG) monitoring, using the modified Seldinger technique, a puncture of the right femoral artery was performed and an intravenous injection of heparin 70 U/kg was given for systemic heparinization. After five minutes of heparin vein injection, 2 ml blood was drawn in a myelin sheath to examine the blood coagulation function, ensuring an activated partial thromboplastin time(APTT) >120 seconds (s) or activated clotting time (ACT) >250 s. Aortic arch and cerebral angiography to determine the stenosis sites, degree, morphology, and collateral circulation situation were performed. Under the guidance of a guidewire, the head of a 6F guide tube was delivered to 1 ∼ 2 cm of the proximal end of the lesion vessel, passing the 0.014-inch microwire through the narrow segment, placing the head end 3 ∼ 4 cm distal to the stenosis section, and sending the balloon-mounted stents along the microwire, slowly pressurizing the pressure pump (according to the pressure required for stent expansion) to expand the balloon release bracket under accurate positioning of the angiography. Use an 8F catheter when the tortuous guide catheter position was unstable, we placed one guidewire (0.035-inch guidewire) at the distal end of the subclavian artery to maintain its stability. After stent implantation, use guided-catheter radiography to observe the stent shape, location, and improvement of arterial stenosis, balloon dilation (balloon system or balloon-mounted stents) again for patients whose residual stenosis were more than 30% of the diseased vessels. After the operation, the indwelling artery sheath was removed and the patient was returned to the care unit to undergo a neurologic examination to see whether there were any new positive signs in the nervous system. Heparin was neutralized naturally, and the sheath was pulled four hours later.
Successful standard of operation: After stent placement, no stent-related cerebrovascular hemorrhage or cerebral ischemic events occurred during the perioperative period, and the residual stenosis rate of diseased vessels was less than 30%.
Follow-up
Forty-three patients were followed by outpatient visits, and all assessments were completed by the same neurologist. All episodes of stroke, death, and TIAs were observed within 30 days. The NIHSS16 was scored before admission and discharge. Neurological assessments were performed at one, three, six, and 12 months after discharge, and clinical follow-up was followed annually. During the clinical follow-up, head computed tomography (CT) or magnetic resonance imaging (MRI) examinations were performed in patients suspected of recurrence of stroke; stented patients were reexamined by head and neck CT angiography (CTA) and magnetic resonance angiography (MRA) after discharge at six months. Two groups of patients had an annual review of head and neck CTA/MRA, and DSA was reexamined according to patient symptoms or angiography. The incidence of ischemic events associated with stenosis vessels, such as TIA, new onset cerebral infarction, and the NIHSS and Malek17 scores were recorded in the two groups. Stenosis of the vertebrobasilar artery was examined by DSA or head and neck CTA/MRA.
Measurement of blood flow velocity in stenotic vessels
Blood flow velocity measurement was performed by trained and qualified professional technicians with more than five years’ experience and completed by the same technical staff at the same location. They were assessed at admission, and at one, three, and six months after treatment by transcranial Doppler (TCD). Maximum median velocity (Vm) in the stenosis area of the diseased vessels was used as the evaluation index. The blood velocity was also measured at 12 months at the follow-up period and each year thereafter.
Evaluating indicator
We recorded the success rate of the ESAA, the incidence of perioperative complications of the stented patients and the degree of restenosis, the rate of restenosis, the rate of occlusion, the blood flow velocity in stenotic vessels, the incidence of ischemic events, and the NIHSS and Malek scores of the two groups at the end of the follow-up period. Restenosis was defined when the diameter of the artery after stent placement was less than 50% of the diameter of the artery immediately after implantation.18
Statistical analysis
Applying the SPSS 16.0 version statistical package, the measurement data were expressed by mean ± standard deviation (x ± S), two samples were compared by t test, and multiple mean was compared by one-way analysis of variance (ANOVA). The chi square test was used to compare the enumeration data. P < 0.05 was statistically significant.
Results
Baseline characteristics of patients in the two groups
According to the inclusion and exclusion criteria, 43 cases were included in the study, including 29 cases in the ESAA group and 14 cases in the medication treatment group. There was no significant difference between the two groups with respect to gender, age, hypertension, abnormal lipid metabolism, smoking, diabetes mellitus, body mass index (BMI), etc. (p > 0.05). See Table 1.
Table 1.
Baseline characteristics of two groups.
| ESAA group n = 29 | Medication treatment group n = 14 | p value | |
|---|---|---|---|
| Gender (male/female) | 12/17 | 8/6 | 0.3315 |
| Age (average ± standard deviation) | 71.9 ± 3.7 | 70.7 ± 3.9 | 0.3313 |
| Degree of vertebrobasilar artery stenosis | 81.7 ± 8.5 | 78.8 ± 6.6 | 0.2687 |
| Hypertension (cases %) | 24 (82.8) | 12 (85.7) | 0.8057 |
| Abnormal lipid metabolism (cases %) | 10 (34.5) | 5 (35.7) | 0.9367 |
| Smoking (cases %) | 9 (31.0) | 4 (28.6) | 0.8691 |
| Diabetes mellitus (cases %) | 12 (41.4) | 6 (42.9) | 0.9267 |
| BMI (kg/m2 (average ± standard deviation)) | 26.2 ± 2.5 | 25.8 ± 3.1 | 0.6519 |
ESAA: endovascular stent-assistant angioplasty; BMI: body mass index.
The efficacy and complications of the ESAA group
In the ESAA group, a total of 32 stents were released in place in 29 cases, using Genesis 11, Cypher 2, Intec 9 and Apollo 10. The operation success rate was 100%. The average preoperative arterial stenosis rate was 65% ∼ 95%, with an average of 81.7 ± 8.5%. The immediate rate of arterial stenosis after operation was 0 ∼ 10%, with an average of 3.6 ± 3.7%, and the stenosis rate was significantly improved. The difference was statistically significant (p < 0.01). All patients had no new ischemic symptoms, with no TIA attack. No serious complications occurred during the perioperative period in all cases.
Stenosis degree, restenosis rate and occlusion rate before and after treatment in the two groups
The average clinical follow-up was 89.4 ± 10.2 months (73 ∼ 104 months). Before treatment, the stenosis rate had no significant difference (p > 0.05); immediately after stent implantation, the vascular stenosis rate compared with the preoperative rate between two groups, the differences were statistically significant (p < 0.01). During the follow-up period, 11 patients in the ESAA group underwent DSA, of whom three had stent stenosis, all of which were stenosis of the vertebral artery opening (stenosis rates were 20%, 35%, and 70%). The other patients underwent CTA/MRA examination, without stent displacement, rupture and restenosis, and the anterior blood flow was good. In the medication treatment group, no DSA examination was performed. CTA/MRA examination was performed and three cases of stenosis vascular occlusion were found.
The arterial blood flow velocity before and after treatment in the two groups
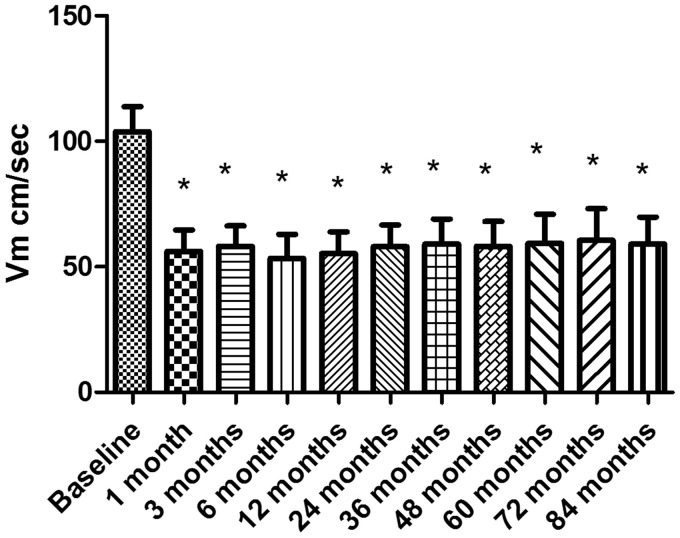
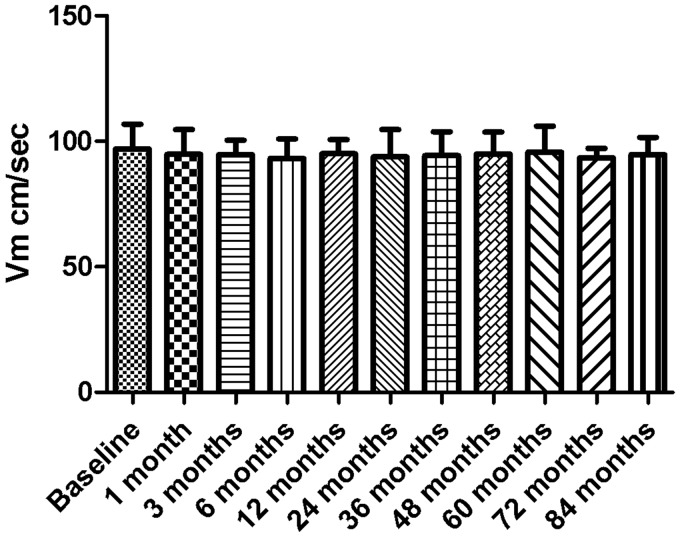
All patients acquired satisfactory vertebrobasilar blood flow signals. Vm was within the follow-up period of the two groups (see Figures 1 and 2). The results showed that the Vm of all the stenosis vessels in the ESAA group was very high, and dropped down to the normal range after one month. The difference between preoperative Vm and postoperative Vm in each period was significant (p < 0.01). After the operation, Vm was similar in each follow-up period (p > 0.05). There was no significant difference in the blood velocity in the medication treatment group between each time point during the follow-up period (p > 0.05). The blood flow velocity in the ESAA group was significantly lower than that in the medication treatment group at the same period of follow-up (p < 0.01).
Figure 1.
The blood velocity (Vm) of stenotic arteries in the endovascular stent-assistant angioplasty group at each follow-up time (cm/sec) means ± SD. *p < 0.01 vs baseline.
Figure 2.
The blood velocity (Vm) of stenotic arteries in the medication treatment group at each follow-up time (cm/sec) means ± SD.
NIHSS scores of the two groups before and after treatment
Before treatment, the NIHSS score of the ESAA group was 3.4 ± 2.0. When discharged from the hospital, patient NIHSS scores were 1.3 ± 0.6; the difference was statistically significant (p < 0.01). The NIHSS scores of patients in the medication treatment group before treatment were 3.2 ± 1.3. When discharged from the hospital, patients’ NIHSS scores were 1.1 ± 0.9; the difference was statistically significant (p < 0.01). There was no significant difference in NIHSS scores between the two groups before treatment, at discharge time, or during follow-up (p > 0.05).
Ischemic events occurred in the two groups during follow-up
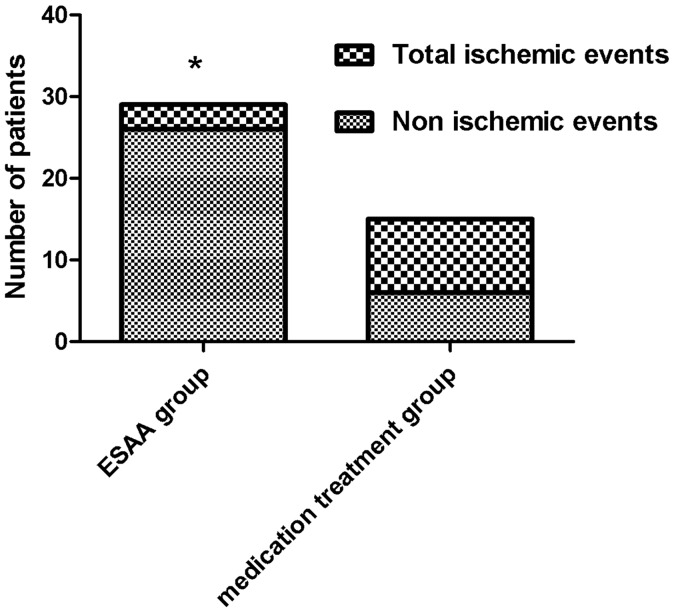
ESAA patients during the follow-up period experienced new-onset cerebral infarction in two cases and TIA in one case. In the medication group patients with new-onset cerebral infarction included seven cases (including one death) and TIA in two cases. The occurrence of cerebral infarction and TIA were related to the vertebrobasilar artery stenosis. The total ischemic events between the two groups were statistically significant (p < 0.05). See Figure 3.
Figure 3.
Ischemic events occurring in the two groups during the follow-up period. Stent implantation significantly reduced postoperative ischemic events. Chi-square analysis was used, *p < 0.05. ESAA: endovascular stent-assistant angioplasty.
The average follow-up time of the ESAA group was 88.2 ± 10.4 months (73 ∼ 104 months). The results showed that the Malek score was 1 point in 26 cases, 2 points in two cases, and 3 points in one case. All patients underwent brain MRI examination in follow-up, which found that one patient had a TIA and one patient with new symptomatic/asymptomatic infarction, respectively, consistent with the site of the vertebrobasilar artery. One patient seven months later relapsed with a TIA; one patient 16 months later relapsed with a new symptomatic cerebral infarction (the NIHSS score was 6 at admission). No significant arterial stenosis was observed in both cases (residual stenosis was 10%). When admitted, they received aspirin tablets 100 mg and clopidogrel 75 mg oral DAPT, atorvastatin 40 mg, at the same time with cerebral circulation improving treatment, the symptoms improved and they were discharged with no recurrence of TIA symptoms. The NIHSS score of the patient with cerebral infarction was 3. Other patients had no new infarction at follow-up, no episodes of TIA, or deaths.
The average follow-up time of the medication treatment group was 91.9 ± 9.5 months (76∼104 months). The results showed that the Malek score was 1 point in five cases, 2 points in two cases, 3 points in three cases, 4 points in three cases, and 5 points in one case. Two vertebral artery stenosis patients presented, respectively, in the follow-up period of three months, 15 months, 62 months (one patient in March, 15 months after the first attack) with one TIA. Upon MRA review, the degree of arterial stenosis was the same as before. According to the etiology, patients with TIA relapse were considered the low perfusion type. After fluid infusion increases blood-volume, acute phase suspension of antihypertensive drugs, improvement of cerebral perfusion, and other comprehensive treatment, no similar episodes occurred after intensive medical treatment. Four patients presented with cerebral infarction during the follow-up period, of whom two patients had vertebral artery occlusion, and one patient was followed up for 71 months with a sudden disturbance of consciousness. Cranial MRA showed basilar artery occlusion, and the patient died after treatment. At the same time, during the follow-up period, three patients developed new asymptomatic minor infarction, and the infarct site was consistent with the site of the vertebrobasilar artery. Four cases of patients with cerebral infarction were admitted to the hospital, treated with DAPT, statins (atorvastatin 20–40 mg), and risk factors control and cerebral circulation improving therapy, and three cases of asymptomatic cerebral infarction received no special treatment.
Discussion
The vertebral basilar artery system is also called posterior circulation, and is composed of vertebral artery extracranial segment, intracranial segment, basilar artery, posterior cerebral artery (PCA) and its branches, which supply blood for one-third of the posterior cerebral hemisphere and part of the diencephalon, brainstem, and cerebellar region. Atherosclerotic vascular stenosis can lead to chronic occlusion of the vessel and plaque abscission can result in the embolism of the distal vessels; they are important causes of PCI and can have severe consequences with poor prognosis.19 Compared with the anterior circulation of ischemia, both in clinical manifestation, evaluation methods, and management measures, the difference between them often causes great challenges to clinicians. For first-time PCI suffering patients, when combined with SVAS (confirmed by brain CTA/MRA vertebral artery and basilar artery stenosis ≥50%), the risk of recurrent stroke or TIA within 90 days is three times higher than in patients without vascular stenosis, and the risk of recurrence is highest within two weeks after stroke. The risk of early recurrence with intracranial arterial stenosis is higher in patients with PCI compared with extracranial arterial stenosis (16.2% vs 33.0%).20 Therefore, it is important to strengthen the management of SVAS. Early intervention in SVAS can effectively reduce the risk of stroke recurrence.
Although surgical treatment, medication therapy, and endovascular intervention have been applied in the clinic, these therapies have not been confirmed by large sample clinical randomized trials. The difficulties of exposing the target vessels during the operation process and postoperative complications, such as acute thrombosis, vagus nerve/recurrent laryngeal nerve paralysis, and chylothorax, have greatly limited the implementation of open surgery.21–23
In terms of medication, for symptomatic patients with severe vertebrobasilar artery stenosis, the risk of stroke recurrence remains high even with formal medical treatment.4,5 The Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS)24 study showed that 12.2% of patients had a stroke during the one-year follow-up period, even with aggressive medical treatment for patients with severe intracranial basilar artery stenosis. In our study, 43 patients were treated with drugs before admission, but the results were poor, which also showed that medication treatment could not fundamentally relieve the stenosis problem.
Recent studies have suggested that stent therapy has the following advantages over medication therapy: (a) to restore the normal diameter of stenotic vessels, improve cerebral perfusion and relieve ischemic symptoms; (b) the stent presses the sclerosis plaque outside the stent, preventing the embolus from falling and causing cerebral infarction; (c) stent implantation is minimally invasive and safe. In this study, 43 patients with SVAS were followed for seven years. For the ESAA group, both short-term and long-term follow-up were effective. Only three ischemic events had a recurrence during the seven-year follow-up, and the hemodynamics returned to normal and remained stable after operation. The efficacy of the ESAA group was better than that of the medication treatment group. The treatment of SVAS with ESAA can reduce the recurrence rate of stroke, and the prevention of posterior circulation ischemia events is better than that of medication therapy. Although medication therapy can improve brain cell function, increase the oxygen tolerance of brain tissue, and establish collateral circulation, the curative effect is limited, and optimal therapeutic effect cannot be achieved.25 Medication therapy alone cannot improve the role of vascular stenosis, and the efficacy of cerebral embolism due to plaque loss caused by soft plaque or ulcer plaque is poor. These reasons account for a lower incidence of posterior ischemic events in the ESAA group than in the medication therapy group.
With the development of ultrasound technology, TCD has been widely used in the clinical screening of stenosis or occlusion of cerebral arteries.26 It’s believed that TCD diagnosis of vertebrobasilar artery lesions has high accuracy.27,28 Previous studies29,30 have confirmed that TCD technology is an important method for economical, simple, and repeatable evaluation of long-term effect of comprehensive evaluation of the vascular structure and hemodynamic parameters before and after carotid artery stenting and vertebral artery stenting.
The study also found that in the ESAA group of patients, Vm was significantly lower (p < 0.01), and maintained a good hemodynamic in the follow-up period, while in the medication treatment group during the follow-up period, Vm had no significant change (p > 0.05), despite the fact that the rate of vascular stenosis in the follow-up group was higher than before treatment (83.1 ± 10.8 vs 78.8 ± 6.6), but there was no significant difference (p = 0.207). Therefore, Vm may be one parameter for evaluating the vascular structures and hemodynamics, which can be used for vascular stenosis screening. But the TCD operation was a “Blind Detective”; the accuracy of the results are closely related to the technique and clinical experience of the operator, so there may be errors simply on the basis of Vm to determine the degree of stenosis, and therefore it should be combined with CTA, MRA, or even DSA examination for comprehensive evaluation.
The NIHSS score was the most commonly used scale for assessing neurological deficits in patients with acute stroke. The results of our study showed no statistically significant difference in NIHSS scores between the two groups during the follow-up period. The reason is speculated to be that the NIHSS score carries less weight in posterior circulation strokes.31 Also, the clinical manifestations of posterior circulation stroke, such as vertigo, swallowing, choking, ataxia, and other subjective feelings, are not easily quantified. Therefore, no significant difference in NIHSS scores between the two groups might not reflect the actual differences in neurological deficits after treatment.
Therefore, we believe that it is suitable to make the best possible choice and comprehensive use of existing methods including medication therapy and stent therapy to prevent stroke and improve the clinical symptoms in patients with SVAS. ESAA may be a promising approach for patients with SVAS who are resistant to the treatment of internal medicine. It’s also worth noting that there are some shortcomings in this study. First, it is a retrospective study; second, this small sample size may cause a deviation in the result. The sample size is small because the patients were strictly screened (strictly according to inclusion and excluded criteria), and followed for a long time; meanwhile, there was a gap between the CTA/MRA and the DSA for vascular evaluation. In addition, cerebral blood flow was evaluated with Doppler ultrasound, which has lower sensitivity than positron emission computed tomography. Therefore, larger-sample, multicenter, randomized controlled studies are needed for comparing standardized medication therapy with endovascular stenting in SVAS.
Conclusions
Taken together, this study suggests that ESAA has long-term efficacy in symptomatic moderate and severe vertebrobasilar artery stenosis, can improve the severity of arterial stenosis and is superior to medication therapy in the prevention of PCI.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Wang Y, Cui L, Ji X, et al. The China National Stroke Registry for patients with acute cerebrovascular events: Design, rationale, and baseline patient characteristics. Int J Stroke 2011; 6: 355–361. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics—2017 Update: A report from the American Heart Association. Circulation 2017; 135: e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrera E, Maeder-Ingvar M, Rossetti AO, et al. Trends in risk factors, patterns and causes in hospitalized strokes over 25 years: The Lausanne Stroke Registry. Cerebrovasc Dis 2007; 24: 97–103. [DOI] [PubMed] [Google Scholar]
- 4.Marquardt L, Kuker W, Chandratheva A, et al. Incidence and prognosis of > or = 50% symptomatic vertebral or basilar artery stenosis: Prospective population-based study. Brain 2009; 132(Pt 4): 982–988. [DOI] [PubMed] [Google Scholar]
- 5.Gulli G, Khan S, Markus HS. Vertebrobasilar stenosis predicts high early recurrent stroke risk in posterior circulation stroke and TIA. Stroke 2009; 40: 2732–2737. [DOI] [PubMed] [Google Scholar]
- 6.Weber R, Kraywinkel K, Diener HC, et al. Symptomatic intracranial atherosclerotic stenoses: Prevalence and prognosis in patients with acute cerebral ischemia. Cerebrovasc Dis 2010; 30: 188–193. [DOI] [PubMed] [Google Scholar]
- 7.Yan Y, Liang L, Chen T, et al. Treatment of symptomatic vertebrobasilar artery stenosis with stent-assistant angioplasty in the elderly. Rev Assoc Med Bras (1992) 2012; 58: 422–426. [PubMed] [Google Scholar]
- 8.Edgell RC, Zaidat OO, Gupta R, et al. Multicenter study of safety in stenting for symptomatic vertebral artery origin stenosis: Results from the Society of Vascular and Interventional Neurology Research Consortium. J Neuroimaging 2013; 23: 170–174. [DOI] [PubMed] [Google Scholar]
- 9.Mohammadian R, Sharifipour E, Mansourizadeh R, et al. Angioplasty and stenting of symptomatic vertebral artery stenosis clinical and angiographic follow-up of 206 cases from Northwest Iran. Neuroradiol J 2013; 26: 454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YJ, Lee JH, Choi JW, et al. Long-term outcome of vertebral artery origin stenosis in patients with acute ischemic stroke. BMC Neurol 2013; 13: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SSALVIA Study Investigators. Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA): Study results. Stroke 2004; 35: 1388–1392. [DOI] [PubMed] [Google Scholar]
- 12.Chimowitz MI, Lynn MJ, Turan TN, et al. Design of the stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis trial. J Stroke Cerebrovasc Dis 2011; 20: 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991; 325: 445–453. [DOI] [PubMed] [Google Scholar]
- 14.Kocak B, Korkmazer B, Islak C, et al. Endovascular treatment of extracranial vertebral artery stenosis. World J Radiol 2012; 4: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuura M, Terada T, Masuo O, et al. Clinical results of percutaneous transluminal angioplasty and stenting for intracranial vertebrobasilar atherosclerotic stenoses and occlusions. Interv Neuroradiol 2004; 10(Suppl 2): 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spilker J, Kongable G, Barch C, et al. Using the NIH Stroke Scale to assess stroke patients. The NINDS rt-PA Stroke Study Group. J Neurosci Nurs 1997; 29: 384–392. [DOI] [PubMed] [Google Scholar]
- 17.Malek AM, Higashida RT, Phatouros CC, et al. Treatment of posterior circulation ischemia with extracranial percutaneous balloon angioplasty and stent placement. Stroke 1999; 30: 2073–2085. [DOI] [PubMed] [Google Scholar]
- 18.Taylor RA, Siddiq F, Suri MF, et al. Risk factors for in-stent restenosis after vertebral ostium stenting. J Endovasc Ther 2008; 15: 203–212. [DOI] [PubMed] [Google Scholar]
- 19.Nouh A, Remke J, Ruland S. Ischemic posterior circulation stroke: A review of anatomy, clinical presentations, diagnosis, and current management. Front Neurol 2014; 5: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gulli G, Marquardt L, Rothwell PM, et al. Stroke risk after posterior circulation stroke/transient ischemic attack and its relationship to site of vertebrobasilar stenosis pooled data analysis from prospective studies. Stroke 2013; 44: 598–604. [DOI] [PubMed] [Google Scholar]
- 21.Ota T, Usami K, Iijima A, et al. Staged surgical treatment for symptomatic vertebrobasilar artery stenosis: Combined treatment with endovascular angioplasty and bypass surgery. World Neurosurg 2012; 78: 90–94. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez CA, Febrer G, Gaudric J, et al. Open repair of vertebral artery: A 7-year single-center report. Ann Vas Surg 2012; 26: 79–85. [DOI] [PubMed] [Google Scholar]
- 23.Lee CJ, Morasch MD. Treatment of vertebral disease: Appropriate use of open and endovascular techniques. Semin Vasc Surg 2011; 24: 24–30. [DOI] [PubMed] [Google Scholar]
- 24.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011; 365: 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang CW, Chang FC, Chern CM, et al. Stenting versus medical treatment for severe symptomatic intracranial stenosis. Am J Neuroradiol 2011; 32: 911–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao L, Barlinn K, Sharma VK, et al. Velocity criteria for intracranial stenosis revisited: An international multicenter study of transcranial Doppler and digital subtraction angiography. Stroke 2011; 42: 3429–3434. [DOI] [PubMed] [Google Scholar]
- 27.Felberg RA, Christou I, Demchuk AM, et al. Screening for intracranial stenosis with transcranial Doppler: The accuracy of mean flow velocity thresholds. J Neuroimaging 2002; 12: 9–14. [DOI] [PubMed] [Google Scholar]
- 28.Ghorbani A, Ashtari F, Fatehi F. The assessment value of transcranial Doppler sonography versus magnetic resonance angiography in vertebrobasilar stroke. J Res Med Sci 2010; 15: 133–139. [PMC free article] [PubMed] [Google Scholar]
- 29.Younis GA, Gupta K, Mortazavi A, et al. Predictors of carotid stent restenosis. Catheter Cardiovasc Interv 2007; 69: 673–682. [DOI] [PubMed] [Google Scholar]
- 30.Fiorella D, Chow MM, Anderson M, et al. A 7-year experience with balloon-mounted coronary stents for the treatment of symptomatic vertebrobasilar intracranial atheromatous disease. Neurosurgery 2007; 61: 236–243. [DOI] [PubMed] [Google Scholar]
- 31.Martin-Schild S, Albright KC, Tanksley J, et al. Zero on the NIHSS does not equal the absence of stroke. Ann Emerg Med 2011; 57: 42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]