Abstract
Background
Superselective ophthalmic artery chemotherapy (SOAC) is a proven therapy for the treatment of retinoblastomas. We describe the technique, results and complications of SOAC performed in our hospital.
Objective
The aim of this article is to demonstrate that a seemingly complex technique can be carried out with a low morbidity rate.
Methods
A retrospective analysis of patients receiving SOAC in our department from November 2014 to April 2017 was performed. Data collected were age, gender, number of procedures, arteries approached, bilaterality of treatment, and complications. The procedure was performed using a 3F sheath and a flow-dependent 1.5F microcatheter that was navigated from the femoral artery to the ostium of the ophthalmic artery (OA). When the OA was too small or a stable position could not be achieved, the microcatheter was navigated in the external carotid artery to reach an anastomotic ramus (AR) of the middle meningeal artery (MMA) to the OA. The drugs were then injected through the microcatheter in a pulsatile way.
Results
Forty-one patients underwent SOAC. A total of 248 procedures were performed in 45 eyes, and 248 arteries were approached (205 OAs and 43 MMAs). Four patients underwent tandem therapy (both eyes treated in the same procedure). Complications were: hypotension and bradycardia during the procedure (five cases), transient thrombosis of the femoral artery (two cases), retinal hemorrhage (one case), alopecia (one case), and anaphylactic shock to carboplatin (one case). No patient showed adverse effects of radiation or ischemic stroke.
Conclusion
SOAC is a safe technique with a very low complication rate.
Keywords: Superselective ophthalmic artery, retinoblastoma, ophthalmic artery, middle meningeal artery, femoral artery
Introduction
Retinoblastoma is the most frequent primary ocular tumor in pediatric patients.1 In children less than one year old, it represents 11% of the tumors.1 Worldwide, retinoblastoma is diagnosed in one of 18,000/20,000 live births.1 In Argentina, 40/45 cases are diagnosed every year.2 The tumor could be hereditary or acquired. In the former, cases are bilateral in 90% of patients and in the latter, are unilateral in 70%. In most children the unilateral cases require enucleation, while in bilateral cases conservative therapy in one eye is possible.3 Superselective ophthalmic artery chemotherapy (SOAC) allows reaching a high concentration of the chemotherapeutic drug in the eye with minimum systemic toxicity. With this technique only 10% of the drug is used in comparison to systemic chemotherapy.4,5
The procedure must be performed several times to slow the tumor growth in this low age population; therefore, great concern exists about radiation exposure because of risks of second cancers in germline mutation retinoblastomas.
For that reason, it is extremely important to perform the procedure as quickly and simply as possible to reduce procedure time and radiation exposure.6,7 At the same time, it is important to perform the procedures using small sheaths and catheters to minimize arterial injury in these very young patients.
The aim of this article is to demonstrate that a seemingly complex technique can be carried out with a low morbidity rate.
Material and methods
We retrospectively analyzed with institutional review board permission the medical charts of patients with retinoblastoma who underwent SOAC from November 2014 to April 2017 in our department. Our hospital is a tertiary pediatric center receiving 80% of children with retinoblastoma in Argentina. Some patients from Bolivia and Paraguay are sent to us for SOAC therapy.
The indication of SOAC is given by oncologists after the examination of patients having retinoblastoma performed by ophthalmologists. The inclusion criteria were the presence of unilateral or bilateral disease in children five months of age or older. Parents were informed of the risks of the procedure, namely, ischemic stroke, retinal hemorrhage, infection, anaphylaxis, loss of visual function, and death.
Events were defined as a bad result of SOAC when the treated eye must be enucleated.
The data collected were age, gender, number of procedures, arteries approached ((OA) or middle meningeal artery (MMA)), bilaterality of the treatment (tandem therapy), and complications. See Table 1.
Table 1.
Results of SOAC technique.
| Case | Age | Gender | Disease | Artery | Sessions | Complications |
|---|---|---|---|---|---|---|
| 1 | 13 months | M | Bilateral | Right MMA | 8 | No |
| 2 | 28 months | M | Bilateral | Right OA | 8 | No |
| 3 | 31 months | M | Bilateral | Right MMA | 2 | Bradycardia hypotension during procedure |
| 4 | 6 months | M | Bilateral | Left OA | 9 | No |
| 5 | 16 months | M | Bilateral | Left OA | 8 | No |
| 6 | 25 months | F | Bilateral | Right OA | 6 | No |
| 7 | 48 months | F | Trilateral | Left OA | 13 | Bradycardia hypotension during procedure |
| 8 | 12 months | M | Bilateral | Right OA | 12 | Retina hemorrhage |
| 9 | 17 months | F | Bilateral | Left OA | 8 | No |
| 10 | 16 months | M | Bilateral familiar | Left MMA | 14 | Alopecia |
| 11 | 28 months | M | Bilateral | Right OA | 8 | No |
| 12 | 10 months | F | Bilateral | Right OA | 4 | Bradycardia hypotension during procedure |
| 13 | 10 months | F | Unilateral | Left OA | 8 | No |
| 14 | 21 months | F | Unilateral | Right OA | 9 | Transient FMA thrombosis |
| 15 | 15 months | F | Bilateral | Right OA | 3 | No |
| 16 | 48 months | M | Bilateral | Right OA | 1 | No |
| 17 | 13 months | M | Bilateral | Right OA | 7 | No |
| 18 | 35 months | M | Bilateral | Left OA | 1 | No |
| 19 | 11 months | M | Bilateral | Right OA | 8 | No |
| 20 | 48 months | M | Bilateral | Right OA | 5 | Transient FMA thrombosis |
| 21 | 10 months | F | Bilateral | Left and right OA | 9 | No |
| 22 | 16 months | F | Bilateral familiar | Left MMA | 4 | Bradycardia hypotension during procedure |
| 23 | 7 months | F | Bilateral | Right MMA | 5 | No |
| 24 | 12 months | F | Unilateral | Right MMA | 3 | No |
| 25 | 41 months | F | Bilateral | Left OA | 8 | No |
| 26 | 20 months | M | Bilateral | Right OA | 13 | No |
| 27 | 20 months | F | Bilateral | Left OA | 2 | No |
| 28 | 21 months | F | Bilateral | Left OA | 7 | No |
| 29 | 10 months | F | Unilateral | Left OA | 3 | No |
| 30 | 11 months | M | Trilateral | Left OA | 10 | No |
| 31 | 6 months | M | Bilateral | Right OA | 8 | No |
| 32 | 6 months | F | Bilateral | Right and left MMA | 8 | Bradycardia hypotension during procedure |
| 33 | 12 months | F | Bilateral | Right and left OA | 5 | No |
| 34 | 33 months | M | Bilateral | Right OA | 2 | No |
| 35 | 10 months | M | Unilateral | Right OA | 5 | No |
| 36 | 9 months | M | Unilateral | Left MMA | 4 | No |
| 37 | 24 months | F | Unilateral | Left OA | 2 | No |
| 38 | 12 months | F | Unilateral | Right OA | 4 | No |
| 39 | 33 months | M | Bilateral | Left OA | 3 | No |
| 40 | 11 months | F | Bilateral | Left OA | 1 | No |
| 41 | 10 months | M | Bilateral | Right and left OA | 1 | Anaphylactic shock |
M: male; F: female; MMA: middle meningeal artery; OA: ophthalmic artery; FMA: femoral artery.
Technique
The technique used in this series of patients is a slight variation of the one described by Gobin et al.4,8
We do not use any decongestant in the nose or skin. The procedure is performed under general anesthesia with endotracheal intubation. The femoral artery (FMA) is punctured and 50 IU/kg of heparin is administered. Alternatively, the right or left FMA is punctured with a 21 G needle and a 3F sheath is inserted. A microcatheter flow-dependent Magic 1.5 (Balt Extrusion, Montmorency, France) supported by a 0.008-inch microwire Hybrid (Balt Extrusion, Montmorency, France) is placed through the sheath and navigated from the FMA to the distal part of the supraclinoid internal carotid artery (ICA). The microwire is then removed and the microcatheter is pulled while injecting contrast media using a 1 cc syringe with fluoroscopy until it remains anchored in the ostium of the OA. Care must be taken during the injection to avoid moving the microcatheter away from the ostium of the OA. When the microcatheter has reached a stable position, the force and rhythm applied to the syringe must be such to cause the chemotherapeutic drugs injected to be directed to the OA. The injection is given in a pulsatile way at a rate of 5 cc/min. It takes no more than 10 minutes for every drug (Figure 1). Fluoroscopy is used as minimally as possible during drug injection. One or two times the stability of the microcatheter is checked taking into account the position of the tip in relation to the anterior clinoid process.
Figure 1.
Digital subtraction angiogram (DSA) of the ophthalmic artery (OA). The tip of the microcatheter is in the ostium of the OA. Both the choroidal and ciliary body blush are clearly seen.
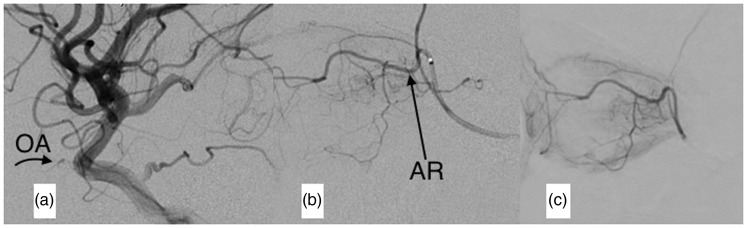
If the OA is too small for an appropriate selective infusion or it cannot be approached, the anastomic ramus (AR) coming from the MMA directly to the OA or its supraorbital branch is catheterized with the Magic 1.5 microcatheter and the Hybrid microguidewire. Both the choroidal and ciliary body blush prove that the AR is useful to deliver the chemotherapeutic agents. Drugs are then injected through the microcatheter (Figures 2 and 3).
Figure 2.
Digital subtraction angiogram (DSA) of the middle meningeal artery (MMA) in a patient in whom the microcatheter does not reach a stable position in the ostium of the ophthalmic artery (OA). (a) The microcatheter was navigated through the MMA in order to reach the anastomotic ramus (AR) with the supraorbital branch of the OA. (b) The microcatheter is in the AR and after contrast media injection, the OA, its ostium and the internal carotid artery (ICA) fill retrogradely. (c) Late-phase DSA shows both the choroidal and ciliary body blush.
Figure 3.
Digital subtraction angiogram (DSA) of the internal carotid artery (ICA) and the middle meningeal artery (MMA) in a patient with a small ophthalmic artery (OA). (a) DSA of the ICA. The OA (arrow) and its ostium are too small to be reached by the microcatheter. (b) An anastomotic ramus (AR) (arrow) joins the MMA to the intraorbital portion of the OA that is bigger than the intracranial portion. (c) Late-phase DSA of the MMA. Both the choroidal and ciliary body bush are seen.
Only two radiological projections are used: the anteroposterior view to navigate the microcatheter and microguidewire from the FMA to the common carotid artery and the lateral view to reach the ICA and the ostium of the OA or external carotid artery (ECA) and the MMA.
To decrease the radiation exposure, we did not perform a diagnostic cerebral angiogram before or after the intervention. SOAC is performed in cycles at three- or four-week intervals using a scheme of two (melphalan hydrochloride and topotecan hydrochloride) or three (carboplatin) drugs. The drugs are diluted with saline to obtain a volume of 30 cm3 of solution.8
FMA hemostasis is performed by 10 minutes of manual compression.
After awakening, patients are transported to the recovery room and discharged home the day after the procedure.
Results
Results are summarized in Table 1.
We treated 41 patients, performing 248 procedures in 45 eyes. A total of 248 arteries were cannulated (205 OAs and 43 MMAs). There were two patients with trilateral retinoblastoma, both of them with an enucleated eye previous to the beginning of the SOAC. Thirty-one patients had bilateral retinoblastoma. Four of them received SOAC in both eyes, so-called tandem therapy. The other children with bilateral disease had an eye enucleated previously. Only one patient had to be enucleated because the tumor did not respond to the SOAC.
The youngest patients were six months old (cases 31 and 32) and the oldest patients were four years old (cases 7, 16, and 20). Case 10 had the highest number of procedures, 14. Bradycardia and hypotension during the navigation of the microcatheter through the cavernous ICA was present in five cases. They responded very well to intravenous epinephrine bitartrate infusion. FMA transient thrombosis was found after the procedure in two patients (cases 14 and 20). They were successfully managed with antiplatelet therapy. Alopecia (case 10) was observed in a patient after administration of topotecan and melphalan in the MMA, probably due to reflux of drugs in the temporal artery.
The post-procedure examination in patient eight revealed retinal hemorrhage. It resolved completely in subsequent explorations. One patient suffered an anaphylactic shock to carboplatin. He fully recovered after receiving epinephrine and corticoids. All patients have been followed up with clinical and ophthalmologic examinations as well as magnetic resonance imaging; none of them showed adverse effect attributable to radiation or ischemic stroke.
Discussion
The story of intra-arterial chemotherapy for the treatment of retinoblastomas began in 1954 when Reese injected into the ICA a nitrogen mustard analog.9 In 1988 Yamane et al. described a technique consisting of positioning a microballoon in the supraclinoid ICA distal to the orifice of the OA to redirect the drug into the OA.10 In 2006, David Abramson of the Department of Ophthalmic Oncology at Memorial Sloan-Kettering Cancer Center and the interventional radiologist Y. Pierre Gobin of the Weill Cornell/New York-Presbyterian Hospital devised a technique to reach the OA with a microcatheter. Doing so, the chemotherapeutic drugs could be injected through the microcatheter.4,8
SOAC is today an established tool in the armamentarium of retinoblastoma therapy.11–17 It is used worldwide for unilateral and bilateral (tandem-therapy) retinoblastomas.18 The technique has enabled the salvage of eyes that would otherwise need to be enucleated.13,18 The “dark side” of SOAC is the multiple sessions needed to stop the tumor growth. There is concern about the radiation received by children. Angiography equipment used in neurointerventional procedures has high radiation exposure.19,20 Most patients who undergo SOAC are younger than two years old and have a high susceptibility to irradiation,7 being at risk of developing secondary tumors.6,7,20
In order to decrease the radiation exposure to a minimum, no diagnostic cerebral angiography was performed prior to the SOAC; we focused only on reaching the OA or MMA. Fluoroscopy is used at a minimum using the relation of the anterior clinoid process and tip of the microcatheter to control the position during drug injection.
Another important issue in these very young patients receiving multiple interventions in a short period of time is the repeated punctures to which the FMA must be subjected. The use of low-caliber sheaths (3F) reduces the damage and probabilities of thrombosis of the FMA. That is why the microcatheter is navigated directly to the OA or MMA without guide-catheter assistance. Once it reaches the supraclinoid portion of the ICA, the flow-dependent 1.5 F microcatheter takes advantage of the hemodynamic changes between this vessel and the OA, reaching the ostium of the OA when it has a normal diameter (Figure 1). We do not navigate the microcatheter beyond the ostium of the OA. It is faster, easier and safer than trying to cannulate the OA with a microwire and a microcatheter. In patients with small OA most of the blood flow to the eyeball comes from the AR of the MMA to the OA or its branches. The MMA and the AR branches are approached with a 1.5 F microcatheter navigating through the ECA21 (Figures 2 and 3).
Several complications of SOAC are described in the literature.8,16
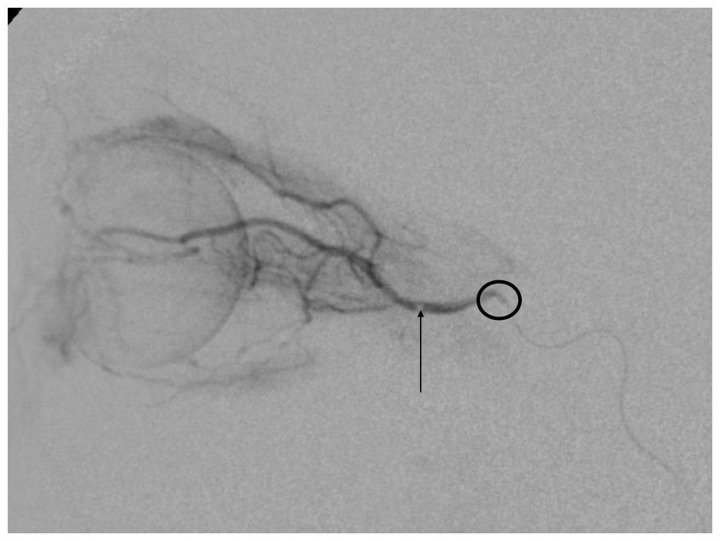
The most serious complication we had was a retinal hemorrhage that resolved after several weeks. It was the only case in which the microcatheter was navigated distal in the OA. The force exerted on the syringe for drug injection probably caused damage to the retina (Figure 4). An anaphylactic shock was caused by administration of carboplatin during the procedure; the patient responded well to medication without consequences. Some patients presented bradycardia and hypotension during the procedure; it is probably produced by a reflex triggered by navigation of the microcatheter in the cavernous segment of the ICA.8
Figure 4.
Digital subtraction angiogram (DSA). The arrow shows the tip of the microcatheter in the ophthalmic artery (OA), too distal to be safe for superselective ophthalmic artery chemotherapy. The circle surrounds the ostium of the OA.
They responded very well to the intravenous infusion of epinephrine bitartrate. Although stroke is a potential complication of the procedure, no patient in our series presented with this event.
Conclusions
We described our experience with the SOAC technique. Taking the necessary precautions, this endovascular procedure is fast and effective with a very low rate of complications. Lowering the time of radiation exposure and using a small sheath is an important issue in these young children undergoing multiple procedures in a short period of time. Longer follow-up is needed to evaluate more accurately our results.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Dimaras H, Corson TW, Cobrinik D, et al. Retinoblastoma. Nat Rev Dis Primers 2015; 1: 15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreno F, Sinaki B, Fandiño A, et al. A population-based study of retinoblastoma incidence and survival in Argentine children. Pediatr Blood Cancer 2014; 61: 1610–1615. [DOI] [PubMed] [Google Scholar]
- 3.Chantada GL, Fandiño AC, Schvartzman E, et al. Impact of chemoreduction for conservative therapy for retinoblastoma in Argentina. J Pediatr Blood Cancer 2014; 61: 821–826. [DOI] [PubMed] [Google Scholar]
- 4.Gobin YP, Cloughesy TF, Chow KL, et al. Intraarterial chemotherapy for brain tumors by using a spatial dose fractionation algorithm and pulsatile delivery. Radiology 2001; 218: 724–732. [DOI] [PubMed] [Google Scholar]
- 5.Abramson DH, Dunkel IJ, Brodie SE, et al. A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology 2008; 115: 1398–1404, 1404.e1. [DOI] [PubMed] [Google Scholar]
- 6.Miller DL, Balter S, Cole PE, et al. Radiation doses in interventional radiology procedures: The RAD-IR study: Part I: Overall measures of dose. J Vasc Interv Radiol 2003; 14: 711–727. [DOI] [PubMed] [Google Scholar]
- 7.Vijayakrishnan R, Shields C, Ramasubramanian A, et al. Irradiation toxic effects during intra-arterial chemotherapy for retinoblastoma. Should we be concerned? Arch Ophthalmol 2010; 128: 1427–1431. [DOI] [PubMed] [Google Scholar]
- 8.Gobin YP, Dunkel IJ, Marr BP, et al. Intra-arterial chemotherapy for the management of retinoblastoma: Four-year experience. Arch Ophthalmol 2011; 129: 732–737. [DOI] [PubMed] [Google Scholar]
- 9.Reese AB, Hyman GA, Merriam GR, Jr, et al. Treatment of retinoblastoma by radiation and triethylenemelamine. AMA Arch Ophthalmol 1954; 53: 505–513. [DOI] [PubMed] [Google Scholar]
- 10.Yamane T, Kaneko A, Mohri M. The technique of ophthalmic arterial infusion therapy for patients with intraocular retinoblastoma. Int J Clin Oncol 2004; 9: 69–73. [DOI] [PubMed] [Google Scholar]
- 11.Chantada GL, Fandiño AC, Carcaboso AM, et al. A phase I study of periocular topotecan in children with intraocular retinoblastoma. Invest Ophthalmol Vis Sci 2009; 50: 1492–1496. [DOI] [PubMed] [Google Scholar]
- 12.Abramson DH, Dunkel IJ, Brodie SE, et al. Superselective ophthalmic artery chemotherapy as primary treatment for retinoblastoma (chemosurgery). Ophthalmology 2010; 117: 1623–1629. [DOI] [PubMed] [Google Scholar]
- 13.Abramson DH. Retinoblastoma: Saving life with vision. Annu Rev Med 2014; 65: 171–184. [DOI] [PubMed] [Google Scholar]
- 14.Abramson DH, Shields CL, Munier FL, et al. Treatment of retinoblastoma in 2015: Agreement and disagreement. JAMA Ophthalmol 2015; 133: 1341–1347. [DOI] [PubMed] [Google Scholar]
- 15.Chantada G, Schaiquevich P. Management of retinoblastoma in children: Current status. Paediatr Drugs 2015; 17: 185–198. [DOI] [PubMed] [Google Scholar]
- 16.Shields CL, Bianciotto CG, Jabbour P, et al. Intra-arterial chemotherapy for retinoblastoma. Report no. 2, treatment complications. Arch Ophthalmol 2011; 129: 1407–1415. [DOI] [PubMed] [Google Scholar]
- 17.Chantada GL, Qaddoumi I, Canturk S, et al. Strategies to manage retinoblastoma in developing countries. Pediatr Blood Cancer 2011; 56: 341–348. [DOI] [PubMed] [Google Scholar]
- 18.Abramson DH, Marr BP, Francis JH, et al. Simultaneous bilateral ophthalmic artery chemosurgery for bilateral retinoblastoma (tandem therapy). Plos One 2016; 11: e0156806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schueler BA, Kallmes DF, Cloft HJ. 3D cerebral angiography: Radiation dose comparison with digital subtraction angiography. AJNR Am J Neuroradiol 2005; 26: 1898–1901. [PMC free article] [PubMed] [Google Scholar]
- 20.Orbach DB, Stamoulis C, Strauss JK, et al. Neurointerventions in children: Radiation exposure and its import. AJNR Am J Neuroradiol 2014; 35: 650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bracco S, Venturi C, Leonini S, et al. Transorbital anastomotic pathways between the external and internal carotid systems in children affected by intraocular retinoblastoma. Surg Radiol Anat 2016; 38: 79–87. [DOI] [PubMed] [Google Scholar]