Abstract
Importance
Current assessment of visual field loss in diseases such as glaucoma suffers from subjectivity of patient responses and lack of portability of standard perimeters.
Objective
To describe the development and initial validation of the nGoggle (nGoggle, Inc., San Diego, CA), a portable brain-computer interface (BCI) for objective assessment of visual field loss.
Design, Setting and Participants
This case-control study involved 62 eyes of 33 glaucomatous patients and 30 eyes of 17 healthy subjects. Glaucoma was diagnosed based on masked grading of optic disc stereophotographs. All subjects underwent testing with the nGoggle and standard automated perimetry (SAP) within 3 months. The nGoggle integrates wearable, wireless, dry electroencephalogram and electrooculogram systems and a cellphone-based head-mounted display, allowing detection of multifocal steady-state visual-evoked potentials (mfSSVEP) associated with visual field stimulation. The performance of global and sectoral nGoggle mfSSVEP metrics to discriminate glaucomatous from healthy eyes were compared to global and sectoral SAP parameters. Repeatability of the nGoggle measurements was assessed by collecting repeated testing in 20 eyes of 10 glaucomatous subjects for 3 sessions of measurements separated by weekly intervals.
Main Outcomes and Measures
Receiver Operating Characteristic (ROC) curves summarizing diagnostic accuracy. Intraclass correlation coefficient (ICC) and coefficient of variation (CV) for assessing repeatability.
Results
The ROC curve area for the global nGoggle mfSSVEP parameter was 0.924 (95% CI: 0.863 – 0.964), which was larger than for SAP MD (AUC=0.813; 95% CI: 0.716 – 0.896), SAP MS (AUC = 0.797; 95% CI: 0.687 – 0.880; P=0.030) and SAP PSD (AUC = 0.768; 95% CI: 0.657 – 0.858; P = 0.012). No statistically significant differences were seen for sectoral measurements between nGoggle and SAP. ICCs for global and sectoral parameters ranged from 0.74 to 0.92 and mean CVs ranged from 3.03% to 7.45%.
Conclusions and Relevance
The nGoggle represents a portable BCI platform for assessing electrical brain responses associated with visual field stimulation. The device was able to discriminate eyes with glaucomatous neuropathy from healthy eyes in a clinic-based setting. Further studies should investigate the feasibility of the nGoggle for home-based testing as well as for detecting visual function loss over time.
INTRODUCTION
Glaucoma is a group of optic neuropathies that have in common a progressive degeneration of retinal ganglion cells (RGCs) and their axons, resulting in a characteristic appearance of the optic disc and visual field loss. Assessment of functional loss in glaucoma has been traditionally made using standard automated perimetry (SAP). However, SAP requires considerable subjective input from the patient and is limited by large test-retest variability.1 As SAP testing is generally performed in clinic-based settings, limited resources frequently result in patients not undergoing the necessary number of tests over time, which may result in late diagnosis or delayed detection of progression.
Objective assessment of visual field damage has been attempted with the use of visual evoked potential (VEP) techniques, especially multifocal VEP (mfVEP). The multifocal technique allows many areas of the retina to be stimulated simultaneously and separate responses from each part of the visual field to be obtained. Results published using mfVEP have demonstrated a good correspondence between visual field sensitivity and local mfVEP responses.2 However, current mfVEP recording techniques can only be performed with non-portable devices in clinic- or laboratory-based settings, requiring cumbersome setup for placement of electrodes, skin preparation and gel application, which are time consuming and may be uncomfortable for the patient.
Progress has been recently achieved in the development of Brain-Computer Interfaces (BCIs) that can successfully process electrical brain signals such as VEPs. BCIs commonly rely on steady-state visual evoked potentials (SSVEPs), which, in contrast to the transient event-related potentials, are elicited by rapid flickering stimulation producing a brain response characterized by a “quasi-sinusoidal” waveform whose frequency components are constant in amplitude and phase.3 SSVEPs have desirable properties for use in the assessment of the integrity of the visual system. The technique is faster than mfVEP, less susceptible to artifacts produced by blink and eye movements,4 to electromyographic noise contamination5 and may present better signal to noise (SNR) ratio.3,6–11 The feasibility of wireless SSVEP data acquisition has also been demonstrated for monitoring high temporal resolution brain dynamics without requiring conductive gels applied to the scalp.12–16 In addition, advanced analytical techniques, such as independent component analysis, have been successfully employed to improve detectability of SSVEP signals.17–26 These advances in the use of SSVEP technique make it an ideal candidate technique for development of a portable objective method of assessment of visual field loss in glaucoma.
In the current study we present the development and initial validation of the nGoggle (nGoggle, Inc., San Diego, CA), a portable brain-computer interface (BCI) for objective assessment of visual field deficits using multifocal steady-state visual-evoked potentials (mfSSVEP). The portable platform integrates a wearable, wireless, dry Electroencephalogram (EEG) system and a head-mounted display allowing monitoring of the electrical brain activity associated with visual field stimulation. We investigated the ability of nGoggle measurements to discriminate glaucomatous from healthy eyes as well as their repeatability.
METHODS
This was a prospective study conducted at the Visual Performance Laboratory of the University of California San Diego (UCSD). Written informed consent was obtained from all participants. The institutional review board and human subjects committee approved all methods, which adhered to the tenets of the Declaration of Helsinki for research involving human subjects and were conducted in accordance with the regulations of the Health Insurance Portability and Accountability Act. Study design and implementation started in April 2015, with data collection performed from October 2015 to July 2016. The study was completed on October 2016.
All participants underwent a comprehensive ophthalmologic examination, including medical history, best-corrected visual acuity, slit-lamp biomicroscopy, intraocular pressure measurement with Goldman tonometry, gonioscopy, dilated stereoscopic fundus examination, stereoscopic optic disc photography and SAP. Only subjects with open angles on gonioscopy were included. Subjects were excluded if they presented with a best-corrected visual acuity less than 20/40, spherical refraction outside ± 5.0 diopters and/or cylinder correction outside 3.0 diopters, or any ocular/systemic disease besides glaucoma that could affect the optic nerve or the visual field, such as coexisting retinal disease.
Diagnosis of glaucoma was based on the presence of glaucomatous optic neuropathy as determined by masked grading of optic disc stereophotographs by two graders. If these graders disagreed, a third observer served as an adjudicator. Simultaneous stereoscopic optic disc photographs (TRC-SS; Topcon Instrument Corporation of America, Paramus, NJ) were reviewed using a stereoscopic viewer (Asahi Pentax Stereo Viewer II; Asahi Optical Co., Tokyo, Japan). Signs of glaucomatous optic neuropathy were considered rim thinning, excavation, presence of retinal nerve fiber layer defects and cup/disc ratio asymmetry greater than 0.2. Normal subjects were recruited from the general population and were required to have normal optic disc appearance in both eyes as well as no history of elevated intraocular pressure.
The nGoggle
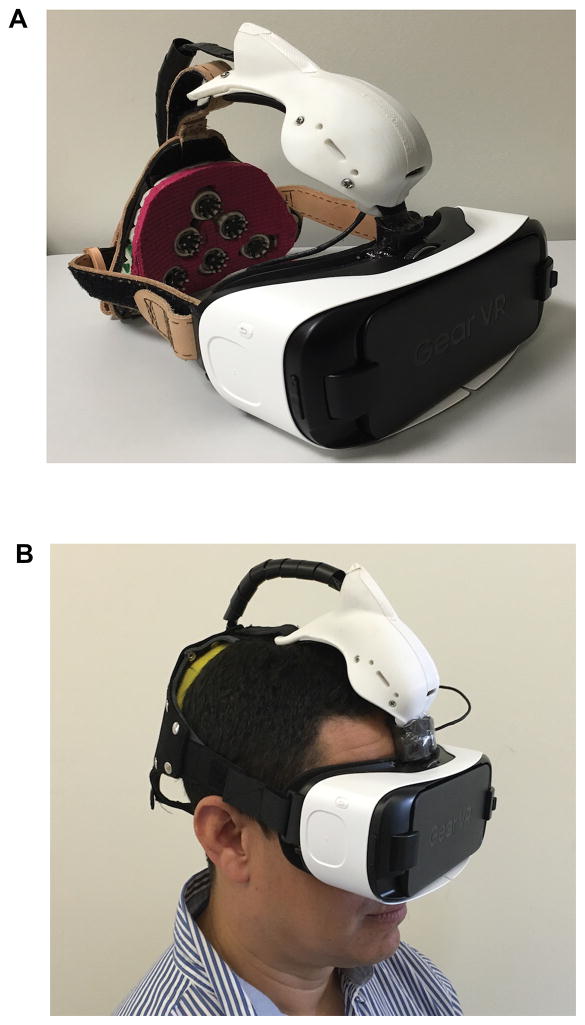
The nGoggle (nGoggle, Inc., San Diego, CA) (Figure 1) consists of a portable, objective BCI integrating wireless, easy-to-wear dry electroencephalogram (EEG) and electrooculogram (EOG) systems and a head-mounted display. The device contains a wireless neuromonitoring system-on-module, equipped with a dual-core embedded processor, assorted I/O interfaces and a dual-band 602.11a/b/g/n Wi-Fi + Bluetooth 4 radio. The device also detects 3D linear acceleration and 3D angular velocity simultaneously at 200sps.
Figure 1. The nGoggle, a portable brain-computer interface for assessment of visual function.
A. The nGoggle consists of a portable multifocal steady-state visual evoked potential-based visual function assessment platform, integrating wearable, wireless, dry electroencephalogram (EEG) and electrooculogram (EOG) systems and a head-mounted display. B. Subject photograph while undergoing testing with the nGoggle.
The nGoggle is capable of capturing electrophysiological signals from six EEG and four EOG channels. It uses customized flexible polymer-based dry EEG electrodes and foam-based dry EOG electrodes for no-preparation wearing. Integrated low-noise pre-amplifiers and 24-bit sigma-delta analog-digital converters can perform synchronous data sampling up to 1000 samples per second (sps). Each channel is equipped with lead-off detection capability to check whether the electrode makes good contact with the scalp. The mfSSVEP Visual Stimuli Rendering Mobile App is based on OpenGL ES 2.0 to render multi-frequency multifocal visual stimuli on an Android phone with a 60fps display. The Signal Processing and Data Analysis Tool is equipped with a proprietary software that has been used in our high-speed BCI speller,27–29 which holds successive world records, and adapted to estimate the relative amplitudes, SNRs, phases, and correlations of mfSSVEP with multi-focal visual stimuli.
The six dry EEG sensors on the nGoggle were located at positions Pz, PO4, PO3, O1, Oz, and O2 according to the 10–20 international system. Visual stimuli (eFigure 1, online only) consisted of two patterns of 20 sectors involving the central 35° field of view, flickering at different frequencies (8 – 11.8 Hz with an interval of 0.2 Hz). The platform employs a frequency approximation approach in order to approximate flexible frequencies with variable number of frames in a stimulating period. This allows successful presentation of a large number of visual stimuli with different frequencies, overcoming limitations arising from the fixed display frequency rate. We have previously presented details of this technique elsewhere.30,31 Two patterns of visual stimuli were presented separately to enhance the signal-to-noise ratio in eliciting SSVEPs. The experiment consisted of three A-pattern sessions and three B-pattern sessions for each eye. Subjects were instructed to sit in a comfortable chair and to gaze at a red dot located in the center of visual stimuli. Each session per eye contained 30 trials of 6s duration, including 5s of visual stimulation followed by a 1s short break, totaling 3 minutes. Ninety-second data epochs comprising 6-channel mfSSVEPs were extracted from the recorded EEG data after band-pass filtering from 6 Hz to 25 Hz. Epochs with artifacts due to fixation losses were detected by the analysis of EOG channels and removed automatically using a customized algorithm. In this study, epochs whose EOG amplitudes exceeded a pre-defined threshold of ± 150 μV were removed.22
Spatial filters based on canonical correlation analysis (CCA) were applied to data epochs, according to a previously described technique.32,33 In brief, CCA is an extension of the ordinary correlation analysis to measure the underlying correlation between two sets of multidimensional variables. In the analysis of SSVEPs, the coefficients obtained by the CCA between EEG signals and synthesized computational models of SSVEPs were used as a spatial filter to remove noisy channels, leading to a measure robust to artifacts and spontaneous EEG activities.33 As CCA represents a correlation measure, it varies from 0 to 1 with higher values indicating higher correlation between evoked EEG signals and ideal waveforms of SSVEPs. Therefore, normal eyes would be expected to show higher CCA values than eyes with glaucomatous neuropathy.
A global metric representing the overall mfSSVEP CCA metric for each eye was calculated as the average of values for each sector and used in the study. In addition, we also calculated sectoral measurements to correspond to superior, inferior, temporal and central areas of the field of view, approximating a previously published structure-function map by Garway-Heath et al (eFigure 2, online only).34
Standard automated perimetry
SAP testing was conducted using program 24-2 and the SITA Standard testing algorithm (Humphrey Visual Field Analyzer II, Carl Zeiss Meditec, Dublin, CA). Visual fields with more than 25% fixation losses or more than 15% false-positive errors were excluded. SAP threshold sensitivities were obtained for each target location and averaged to calculate a global mean sensitivity (MS) value. The two locations just above and below the blind spot were not included in the analysis. SAP mean deviation (MD) and pattern standard deviation (PSD) were also evaluated as global parameters in this study. In addition, threshold sensitivities were averaged to correspond to nGoggle sectors as shown in eFigure 2 (online only).
Subjects underwent testing with SAP and the nGoggle within 3-months.
Statistical Analyses
Receiver operating characteristic (ROC) curves were constructed to assess the diagnostic ability of the nGoggle and SAP in discriminating glaucomatous eyes from normal eyes. The ROC curve provides the tradeoff between the sensitivity and 1 – specificity. The area under the ROC curve (AUC) was used to summarize the diagnostic accuracy of each parameter. An AUC of 1.0 represents perfect discrimination, whereas an area of 0.5 represents chance discrimination.
To account for the use of both eyes of the same subject in the analyses, a bootstrap resampling procedure (n = 1000 resamples) was used to derive confidence intervals. To account for the correlation between eyes, the cluster of data for the study subject was considered as the unit of resampling in order to adjust standard errors. This procedure has been previously used to adjust for the presence of multiple correlated measurements from the same unit.35
Sample Size and Power Calculation
For this investigation of diagnostic accuracy, sample size was calculated to detect a minimally significant difference of 0.1 between the areas under the ROC curves of diagnostic parameters, with a correlation of 0.6 between measurements and a 2:1 ratio of glaucoma to healthy eyes. For a power of 80%, the required sample size was calculated as 54 glaucomatous and 27 healthy eyes.
Repeatability Assessment
Initial assessment of the repeatability of nGoggle measurements was obtained by collecting repeated testing in 20 eyes of 10 glaucomatous subjects. Subjects had 3 sessions of measurements separated by weekly intervals between sessions. For each eye, a coefficient of variation (CV) was calculated as the ratio of the standard deviation of the three measurements and the corresponding mean. Intraclass correlation coefficients (ICCs) were also used to evaluate test-retest variability. ICCs above 0.75 are usually considered to indicate good reproducibility.
Statistical analyses were performed with commercially available software (Stata version 12; StataCorp, College Station, TX). The alpha level (type I error) was set at 0.05.
RESULTS
The investigation of the ability of the nGoggle to detect visual field loss included 62 eyes of 33 glaucomatous patients and 30 eyes of 17 healthy subjects. Table 1 summarizes the clinical and demographic variables for the subjects included in the study. There was no statistically significant difference in mean age between glaucomatous and healthy subjects (68.2 ± 11.0 vs. 66.1 ± 9.9; P = 0.57). There were also no statistically significant differences in race or gender between glaucoma and healthy subjects.
Table 1.
Demographic and clinical variables of the glaucomatous and healthy participants/eyes included in the study. Values represent mean ± standard deviation unless otherwise noted.
| Glaucoma (n = 62 eyes of 33 subjects) | Healthy (n = 30 eyes of 17 subjects) | P | |
|---|---|---|---|
| Age, years | 68.2 ± 11.0 | 66.1 ± 9.9 | 0.57 |
| Gender, female, n (%) | 8 (47) | 16 (48) | 0.92 |
| Race, n(%) | |||
| White | 19 (58) | 9 (53) | 0.498 |
| Black | 12 (36) | 8 (47) | |
| Asian | 2 (6) | 0 | |
| SAP MS, dB* | 24.8 (17.5 – 27.7) | 29.1 (26.8 – 30.7) | <0.001 |
| SAP MD, dB* | −4.0 (−12.7 – −1.8) | −0.6 (−2.4 – 1.0) | <0.001 |
| SAP PSD, dB* | 4.7 (2.2 – 9.9) | 1.9 (1.4 – 3.0) | <0.001 |
| nGoggle global mfSSVEP | 0.289 ± 0.020 | 0.334 ± 0.024 | <0.001 |
median (interquartile range).
Abbreviations: MS – mean sensitivity, MD – mean deviation; PSD – pattern standard deviation; mfSSVEP – multifocal steady-state visual evoked potential; CCA – canonical correlation analysis
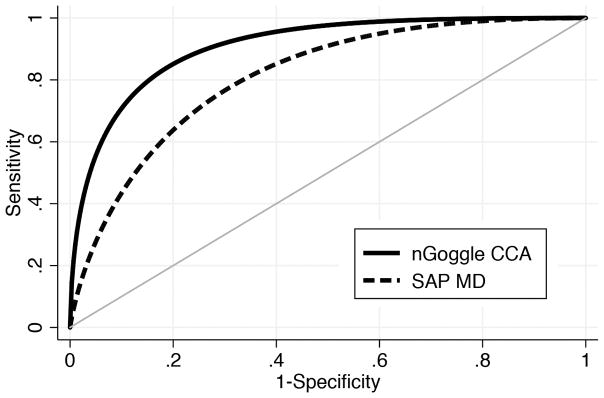
The mean nGoggle global mfSSVEP parameter was lower for glaucoma eyes (0.289 ± 0.020) compared to normal eyes (0.334 ± 0.024; P <0.001) (Table 1). Table 2 shows AUCs and sensitivities at fixed specificities for the different nGoggle and SAP parameters. The AUC for the global nGoggle mfSSVEP parameter was 0.924 (95% CI: 0.863 – 0.964), which was larger than that for SAP MD (AUC = 0.813; 95% CI: 0.716 – 0.896; P=0.046), SAP MS (AUC = 0.797; 95% CI: 0.687 – 0.880; P=0.030) and SAP PSD (AUC = 0.768; 95% CI: 0.657 – 0.858; P = 0.012). Figure 2 shows ROC curves for the mfSSVEP CCA parameter and for MD. For specificity at 80%, the mfSSVEP CCA parameter had sensitivity of 85%, compared to 64% for SAP MD. For specificity at 90%, the mfSSVEP CCA parameter had sensitivity of 71% versus 43% for SAP MD.
Table 2.
Areas under the receiver operating characteristic (AUC) curves and sensitivities at fixed specificities to discriminate glaucoma from healthy eyes for the nGoggle global multifocal steady-state visual evoked parameter and standard automated perimetry (SAP) parameters.
| AUC (95% CI) | Sensitivity for Specificity at 80% | Sensitivity for Specificity at 90% | |
|---|---|---|---|
| nGoggle global mfSSVEP | 0.924 (0.863 – 0.964). | 85% (71% – 95%) | 71% (53% – 87%) |
| SAP MD | 0.813 (0.716 – 0.896) | 64% (45% – 82%) | 43% (23% – 68%) |
| SAP MS | 0.797 (0.687 – 0.880) | 60% (39% – 77%) | 39% (18% – 59%) |
| SAP PSD | 0.768 (0.657 – 0.858) | 61% (40% – 75%) | 47% (24% – 66%) |
Abbreviations: MS – mean sensitivity, MD – mean deviation; PSD – pattern standard deviation; mfSSVEP – multifocal steady-state visual evoked potential
Figure 2. Receiver operating characteristic curves for the global nGoggle parameter and SAP mean deviation.
Receiver operating characteristic curves for the nGoggle global multifocal steady-state visual evoked potential (mfSSVEP) parameter and standard automated perimetry mean deviation (MD).
eTable 1 shows ROC curve areas for sectoral measurements obtained by the nGoggle and SAP. The ROC curve areas were generally larger for nGoggle than SAP, notably for the central area, although without statistically significant difference between corresponding sectors (P>0.10 for all comparisons).
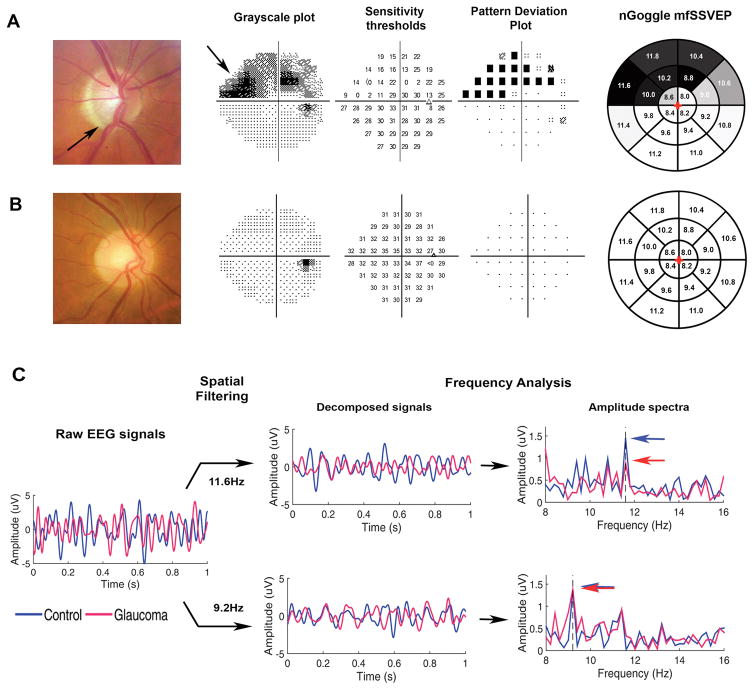
Figure 3 illustrates an example of test results obtained by the nGoggle in a glaucomatous and a healthy eye, as well as SAP results in the same eyes.
Figure 3. Results from nGoggle and standard perimetry of glaucomatous and healthy eyes included in the study.
A. Eye with glaucoma showing inferior loss of neuroretinal rim (arrow) and superior nasal visual field defect (arrow). B. Optic disc photograph and perimetric results of a healthy eye. C. Multifocal steady-state visual evoked potentials obtained by the nGoggle for the eyes in A (red) and B (blue). The figure illustrates processing for sectors 11.6Hz, located in the superior nasal region of defect; and 9.2Hz, located in the inferior temporal normal region. For the 11.6Hz frequency, the amplitude for the glaucoma eye (red arrow) is much lower than that for the healthy eye (blue arrow). For the 9.2Hz frequency, the amplitudes are almost identical. The final pattern of nGoggle sectoral results are show in greyscale in the rightmost column.
Assessment of Repeatability
Repeatability of measurements obtained by the nGoggle was investigated in 20 eyes of 10 glaucomatous subjects. These eyes had median SAP MD of −3.7 dB, ranging from −21.7dB to 0.1dB. Mean global mfSSVEP value on all tests of 0.289, ranging from 0.248 to 0.347. The average intraclass correlation coefficient (ICC) of the global mfSSVEP parameter was 0.92 (95% CI: 0.82 – 0.97), which was greater than 0.75 (P<0.001). ICCs for sectors ranged from 0.74 to 0.90. Mean coefficient of variation (CV) of the mfSSVEP global parameter was 3.03% (95% CI: 2.19% – 3.87%), whereas CVs for sectors ranged from 4.14% to 7.45%.
DISCUSSION
In the present study, we described and provided initial validation of the nGoggle, a portable BCI device for objective assessment of visual field loss. The proposed device integrates EEG and a head-mounted display for assessment of mfSSVEP potentials in response to visual stimulation. Results from our study showed that the nGoggle was able to discriminate eyes with glaucomatous neuropathy from healthy eyes. In addition, measurements from the nGoggle showed adequate test-retest repeatability, suggesting that they may be useful for longitudinal monitoring of neural losses.
SSVEP signals obtained by the nGoggle were significantly lower in glaucomatous compared to healthy eyes, with an area under the ROC curve of 0.924. The ROC curve area for the global mfSSVEP parameter was superior to those obtained for SAP global parameters MS, MD and PSD. In order to allow an unbiased comparison between the diagnostic accuracies of SAP and the nGoggle, glaucoma diagnosis was based on masked assessment of optic disc photographs. Such approach has been used by several authors when investigating and comparing the diagnostic accuracies of multiple visual function tests for glaucoma. Sample et al36 reported ROC curve areas ranging from 0.60 to 0.80 for different SAP parameters when detecting glaucoma diagnosed based on assessment of optic disc photographs. Importantly, damage to the optic disc and retinal nerve fiber layer as seen on photographs has been shown to precede and predict the development of visual field defects in many eyes with glaucoma.37–39 In our study, 11 (18%) of the 62 eyes with glaucomatous optic neuropathy had SAP MD and PSD with P>5%, as well as Glaucoma Hemifield Test (GHT) result within normal limits, as compared to the Humphrey normative database, and would be classified as having normal fields in clinical practice. These eyes had average global mfSSVEP of 0.280 ± 0.010, which was significantly lower than that of the healthy eyes included in the study (0.334 ± 0.024; P<0.001). This finding suggests that the mfSSVEP signals obtained by the nGoggle may be able to detect glaucoma before the appearance of visual field defects on standard perimetry, a finding that has been previously shown in studies with conventional (non steady-state) and wet-electrode-based multifocal VEP devices in glaucoma.2,40–42
Although ROC curve areas tended to be larger for sectoral measurements from the nGoggle compared to SAP, no statistically significant differences were noted. Of note, the largest difference was seen for the central sector, with ROC curve areas of 0.805 vs. 0.680, respectively. This might reflect the relative insensitivity of the SAP 24-2 strategy for detection of glaucomatous damage in the central area, as shown by previous authors.43
The assessment of the repeatability of the nGoggle showed its measurements to exhibit low test-retest variability. The ICC was 0.92 and the coefficient of variation was 3.03% for the global mfSSVEP CCA parameter. These numbers are comparable to those previously described for traditional wired and wet-electrode multifocal VEP assessment.44 The low test-retest variability of the nGoggle seems to reflect the stability of SSVEP potentials and their known relatively high SNR and less susceptibility to artifacts, supporting its application for assessment of longitudinal change over time. However, future longitudinal studies are necessary to investigate the ability of the device in detecting progressive glaucomatous damage over time.
Our study was intended to perform an initial proof-of-concept of the feasibility of the nGoggle as a portable objective device for assessment of visual function. The portability and objectivity make the device promising for home-based assessment of visual function. With home-based testing, a much higher number of tests could be acquired over time, potentially making it easier to separate true change from test-retest variability. Although our study only investigated the use of the nGoggle in a controlled office-based setting, in a previous study we have shown the feasibility of acquiring reliable wireless SSVEP signals in subjects performing ordinary activities, such as walking on a treadmill.45 It is important to recognize, however, that home-based testing is likely to introduce unforeseen challenges and, therefore, carefully conducted studies will be necessary to validate the device for this application.
In conclusion, we presented the development and application of the nGoggle, a portable platform for objective assessment of visual function. The device was able to identify eyes with glaucomatous optic neuropathy and its measurements showed adequate repeatability. Future longitudinal investigations should assess whether the nGoggle is able to detect progressive glaucomatous damage over time.
Supplementary Material
KEY POINTS.
Question
Is a portable brain-computer interface (BCI) for objective assessment of visual function able to discriminate glaucomatous from healthy eyes?
Findings
The nGoggle is a BCI that assesses multifocal steady-state visual evoked potentials in response to visual field stimulation. In this case-control study, nGoggle parameters were able to discriminate eyes with glaucomatous optic neuropathy from healthy eyes, as compared to standard automated perimetry.
Meaning
The BCI was able to detect glaucomatous damage in a clinic-based study and shows promise as a portable device for objectively assessing visual function loss.
Acknowledgments
Funding: Supported in part by National Institutes of Health/National Eye Institute grants EY021818 (FAM) and R21 EY025056 (FAM), UCSD Calit2 Strategic Research Opportunity (FAM), Army Research Lab CTA W911NF-10-2-0022 (TPJ, YTW, YPL, YW). The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Dr. Medeiros had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial disclosures: M.N.: none; Y.T.W.: none; T.P.J.: Co-Founder, nGoggle, Inc.; J.K.Z.: Co-Founder, nGoggle, Inc.; Y.Y.C.: none; A.D.F: none; F.B.D.: none; Y.P.L:none; Y.W: none; F.A.M.: research support from Alcon Laboratories, Bausch & Lomb, Carl Zeiss Meditec, Heidelberg Engineering, Merck, Allergan, Sensimed, Topcon, Reichert; consultant for Allergan, Carl Zeiss Meditec, and Novartis. Co-Founder, nGoggle, Inc.
References
- 1.Chauhan BC, Garway-Heath DF, Goni FJ, Rossetti L, Bengtsson B, Viswanathan AC, Heijl A. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92(4):569–573. doi: 10.1136/bjo.2007.135012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hood DC, Thienprasiddhi P, Greenstein VC, Winn BJ, Ohri N, Liebmann JM, Ritch R. Detecting early to mild glaucomatous damage: a comparison of the multifocal VEP and automated perimetry. Invest Ophthalmol Vis Sci. 2004;45(2):492–498. doi: 10.1167/iovs.03-0602. [DOI] [PubMed] [Google Scholar]
- 3.Vialatte FB, Maurice M, Dauwels J, Cichocki A. Steady-state visually evoked potentials: focus on essential paradigms and future perspectives. Prog Neurobiol. 2010;90(4):418–438. doi: 10.1016/j.pneurobio.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Perlstein WM, Cole MA, Larson M, Kelly K, Seignourel P, Keil A. Steady-state visual evoked potentials reveal frontally-mediated working memory activity in humans. Neurosci Lett. 2003;342(3):191–195. doi: 10.1016/s0304-3940(03)00226-x. [DOI] [PubMed] [Google Scholar]
- 5.Gray M, Kemp AH, Silberstein RB, Nathan PJ. Cortical neurophysiology of anticipatory anxiety: an investigation utilizing steady state probe topography (SSPT) Neuroimage. 2003;20(2):975–986. doi: 10.1016/S1053-8119(03)00401-4. [DOI] [PubMed] [Google Scholar]
- 6.Abdullah SN, Aldahlawi N, Rosli Y, Vaegan, Boon MY, Maddess T. Effect of contrast, stimulus density, and viewing distance on multifocal steady-state visual evoked potentials (MSVs) Invest Ophthalmol Vis Sci. 2012;53(9):5527–5535. doi: 10.1167/iovs.11-9325. [DOI] [PubMed] [Google Scholar]
- 7.Abdullah SN, Vaegan, Boon MY, Maddess T. Contrast-response functions of the multifocal steady-state VEP (MSV) Clin Neurophysiol. 2012;123(9):1865–1871. doi: 10.1016/j.clinph.2012.02.067. [DOI] [PubMed] [Google Scholar]
- 8.Vaegan, Rahman AM, Sanderson GF. Glaucoma affects steady state VEP contrast thresholds before psychophysics. Optom Vis Sci. 2008;85(7):547–558. doi: 10.1097/OPX.0b013e31817dba51. [DOI] [PubMed] [Google Scholar]
- 9.Vaegan, Anderton PJ, Millar TJ. Transient and steady state focal and pattern electroretinogram nerve section losses in cats with unilateral optic. Doc Ophthalmol. 2002;105(2):105–127. doi: 10.1023/a:1020592701609. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Wang R, Gao X, Hong B, Gao S. A practical VEP-based brain-computer interface. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2006;14(2):234–240. doi: 10.1109/TNSRE.2006.875576. [DOI] [PubMed] [Google Scholar]
- 11.Bin GY, Gao XR, Yan Z, Hong B, Gao SK. An online multi-channel SSVEP-based brain-computer interface using a canonical correlation analysis method. Journal of Neural Engineering. 2009;6(4):046002. doi: 10.1088/1741-2560/6/4/046002. (046006pp) [DOI] [PubMed] [Google Scholar]
- 12.Chi YM, Jung TP, Cauwenberghs G. Dry-contact and noncontact biopotential electrodes: methodological review. IEEE Rev Biomed Eng. 2010;3:106–119. doi: 10.1109/RBME.2010.2084078. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Jung TP, Makeig S, Rao BD. Compressed sensing for energy-efficient wireless telemonitoring of noninvasive fetal ECG via block sparse Bayesian learning. IEEE Trans Biomed Eng. 2013;60(2):300–309. doi: 10.1109/TBME.2012.2226175. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, Jung TP, Makeig S, Rao BD. Compressed sensing of EEG for wireless telemonitoring with low energy consumption and inexpensive hardware. IEEE Trans Biomed Eng. 2013;60(1):221–224. doi: 10.1109/TBME.2012.2217959. [DOI] [PubMed] [Google Scholar]
- 15.Lin CT, Ko LW, Chang MH, Duann JR, Chen JY, Su TP, Jung TP. Review of wireless and wearable electroencephalogram systems and brain-computer interfaces--a mini-review. Gerontology. 2010;56(1):112–119. doi: 10.1159/000230807. [DOI] [PubMed] [Google Scholar]
- 16.Mullen T, Kothe C, Chi YM, Ojeda A, Kerth T, Makeig S, Cauwenberghs G, Jung TP. Real-time modeling and 3D visualization of source dynamics and connectivity using wearable EEG. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:2184–2187. doi: 10.1109/EMBC.2013.6609968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gramann K, Gwin JT, Ferris DP, Oie K, Jung TP, Lin CT, Liao LD, Makeig S. Cognition in action: imaging brain/body dynamics in mobile humans. Rev Neurosci. 2011;22(6):593–608. doi: 10.1515/RNS.2011.047. [DOI] [PubMed] [Google Scholar]
- 18.Gramann K, Gwin JT, Bigdely-Shamlo N, Ferris DP, Makeig S. Visual evoked responses during standing and walking. Front Hum Neurosci. 2010;4:202. doi: 10.3389/fnhum.2010.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gwin JT, Gramann K, Makeig S, Ferris DP. Removal of movement artifact from high-density EEG recorded during walking and running. J Neurophysiol. 2010;103(6):3526–3534. doi: 10.1152/jn.00105.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delorme A, Sejnowski T, Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage. 2007;34(4):1443–1449. doi: 10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onton J, Westerfield M, Townsend J, Makeig S. Imaging human EEG dynamics using independent component analysis. Neurosci Biobehav Rev. 2006;30(6):808–822. doi: 10.1016/j.neubiorev.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ. Dynamic brain sources of visual evoked responses. Science. 2002;295(5555):690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- 24.Jung TP, Makeig S, McKeown MJ, Bell AJ, Lee TW, Sejnowski TJ. Imaging Brain Dynamics Using Independent Component Analysis. Proc IEEE Inst Electr Electron Eng. 2001;89(7):1107–1122. doi: 10.1109/5.939827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin Neurophysiol. 2000;111(10):1745–1758. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- 26.Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, Sejnowski TJ. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37(2):163–178. [PubMed] [Google Scholar]
- 27.Nakanishi M, Wang Y, Wang YT, Mitsukura Y, Jung TP. A high-speed brain speller using steady-state visual evoked potentials. Int J Neural Syst. 2014;24(6):1450019. doi: 10.1142/S0129065714500191. [DOI] [PubMed] [Google Scholar]
- 28.Nakanishi M, Wang Y, Wang YT, Jung TP. A Comparison Study of Canonical Correlation Analysis Based Methods for Detecting Steady-State Visual Evoked Potentials. PLoS One. 2015;10(10):e0140703. doi: 10.1371/journal.pone.0140703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Wang Y, Nakanishi M, Gao X, Jung TP, Gao S. High-speed spelling with a noninvasive brain-computer interface. Proc Natl Acad Sci U S A. 2015;112(44):E6058–6067. doi: 10.1073/pnas.1508080112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakanishi M, Wang Y, Wang YT, Mitsukura Y, Jung TP. Generating visual flickers for eliciting robust steady-state visual evoked potentials at flexible frequencies using monitor refresh rate. PLoS One. 2014;9(6):e99235. doi: 10.1371/journal.pone.0099235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Wang YT, Jung TP. Visual stimulus design for high-rate SSVEP BCI. Electron Lett. 2010;46(15):1057–1058. [Google Scholar]
- 32.Wang Y, Gao X, Gao S. Computational modeling and application of steady-state visual evoked potentials in brain-computer interfaces. Sci Suppl. 2015;350(6256):43–46. [Google Scholar]
- 33.Lin Z, Zhang C, Wu W, Gao X. Frequency recognition based on canonical correlation analysis for SSVEP-based BCIs. IEEE Trans Biomed Eng. 2007;54(6 Pt 2):1172–1176. doi: 10.1109/tbme.2006.889197. [DOI] [PubMed] [Google Scholar]
- 34.Garway-Heath DF, Poinoosawmy D, Fitzke FW, Hitchings RA. Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology. 2000;107(10):1809–1815. doi: 10.1016/s0161-6420(00)00284-0. [DOI] [PubMed] [Google Scholar]
- 35.Medeiros FA, Sample PA, Zangwill LM, Liebmann JM, Girkin CA, Weinreb RN. A statistical approach to the evaluation of covariate effects on the receiver operating characteristic curves of diagnostic tests in glaucoma. Invest Ophthalmol Vis Sci. 2006;47(6):2520–2527. doi: 10.1167/iovs.05-1441. [DOI] [PubMed] [Google Scholar]
- 36.Sample PA, Medeiros FA, Racette L, Pascual JP, Boden C, Zangwill LM, Bowd C, Weinreb RN. Identifying glaucomatous vision loss with visual-function-specific perimetry in the diagnostic innovations in glaucoma study. Invest Ophthalmol Vis Sci. 2006;47(8):3381–3389. doi: 10.1167/iovs.05-1546. [DOI] [PubMed] [Google Scholar]
- 37.Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR, Kass MA. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714–720. doi: 10.1001/archopht.120.6.714. discussion 829–730. [DOI] [PubMed] [Google Scholar]
- 38.Medeiros FA, Alencar LM, Zangwill LM, Bowd C, Sample PA, Weinreb RN. Prediction of functional loss in glaucoma from progressive optic disc damage. Arch Ophthalmol. 2009;127(10):1250–1256. doi: 10.1001/archophthalmol.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quigley HA, Katz J, Derick RJ, Gilbert D, Sommer A. AN EVALUATION OF OPTIC DISK AND NERVE-FIBER LAYER EXAMINATIONS IN MONITORING PROGRESSION OF EARLY GLAUCOMA DAMAGE. Ophthalmology. 1992;99(1):19–28. doi: 10.1016/s0161-6420(92)32018-4. [DOI] [PubMed] [Google Scholar]
- 40.Hood DC, Greenstein VC. Multifocal VEP and ganglion cell damage: applications and limitations for the study of glaucoma. Prog Retin Eye Res. 2003;22(2):201–251. doi: 10.1016/s1350-9462(02)00061-7. [DOI] [PubMed] [Google Scholar]
- 41.Goldberg I, Graham SL, Klistorner AI. Multifocal objective perimetry in the detection of glaucomatous field loss. Am J Ophthalmol. 2002;133(1):29–39. doi: 10.1016/s0002-9394(01)01294-6. [DOI] [PubMed] [Google Scholar]
- 42.Graham SL, Klistorner AI, Goldberg I. Clinical application of objective perimetry using multifocal visual evoked potentials in glaucoma practice. Arch Ophthalmol. 2005;123(6):729–739. doi: 10.1001/archopht.123.6.729. [DOI] [PubMed] [Google Scholar]
- 43.Hood DC, Raza AS, de Moraes CG, Liebmann JM, Ritch R. Glaucomatous damage of the macula. Prog Retin Eye Res. 2013;32:1–21. doi: 10.1016/j.preteyeres.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Punjabi OS, Stamper RL, Bostrom AG, Lin SC. Repeatability of the multifocal visual evoked potentials in a clinical glaucoma setting. Can J Ophthalmol. 2008;43(4):435–440. doi: 10.3129/i08-078. [DOI] [PubMed] [Google Scholar]
- 45.Lin YP, Wang Y, Jung TP. Assessing the feasibility of online SSVEP decoding in human walking using a consumer EEG headset. J Neuroeng Rehabil. 2014;11:119. doi: 10.1186/1743-0003-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.