Abstract
Activation of innate immunity is critical for vaccine development and immunotherapy, through triggering antigen specific immune responses. Natural killer T (NKT) cells are a unique type of innate immune cells which exert potent anti-viral and anti-metastasis function, through producing interferon-γ and activating dendritic cells to present tumor antigens to CD8 T cells. alpha-Galactosylceramide, a synthetic antigen for NKT cells, is an adjuvant for protein antigens which can induce protective immunity against cancer and viral diseases, and has been proven to be safe and immune stimulatory in human cancer and hepatitis patients. Current existing problem for alpha-galactosylceramide is its induction of anergy of NKT cells, due to the non-selective presentation of alpha-galactosylceramide antigen by B cells. We hypothesized that nanoparticle formulated alpha-galactosylceramide may be selectively presented by dendritic cells and macrophages, but not B cells, thus avoiding anergy induction in NKT cells. We have prepared poly-lactic acid based nanoparticles conjugated with alpha-galactosylceramide, examined their stimulation of NKT cells in vitro and in vivo in mice, and showed that nanoparticle formulated alpha-galactosylceramide stimulates NKT cells. In contrast to soluble alpha-galactosylceramide, which caused NKT anergy after single stimulation, nanoparticle formulated alpha-galactosylceramide repeatedly stimulates NKT cells without inducing anergy. Mechanistic studies showed that nanoparticle formulated alpha-galactosylceramide is efficiently presented by mouse CD11c + population containing dendritic cells, and CD11b + population containing macrophages, but very poorly by B220 + population containing B cells. Hence, nanoparticle formulated alpha-galactosylceramide is an attractive immunomodulator for immunotherapy and vaccine development. Future studies will be focused on its application as adjuvant for protein and/or peptide antigens.
Keywords: Natural killer T cells, T cell anergy, alpha-galactosylceramide, Biodegradable nanoparticle, Dendritic cells, Phagocytosis
1. Introduction
Invariant natural killer T (NKT) cells express evolutionally conserved T cell receptors and recognize glycolipid antigens presented by the non-MHC encoded, non-polymorphic, MHC-like antigen presenting molecule CD1d. The therapeutic value of invariant NKT cells was first discovered by mechanistic studies on α-galactosylceramide (αGalCer), a glycosphingolipid which prevents lung metastasis of intravenously injected B16 melanoma cells in mice [1,2]. αGalCer, presented by CD1d in antigen presenting cells, activates the TCR signaling in NKT cells. The αGalCer activated NKT cells “jump-start” the antitumor and antiviral function of both innate and adaptive immune cells, including natural killer (NK) cells, macrophages, dendritic cells, CD4 and CD8 T cells. The functions of NKT cells are not only mediated by their secretion of cytokines (IFN-γ and IL4), but also direct cell-to-cell contact. Specifically, NKT cells enhance the “cross-priming” of tumor and viral antigen, by activating dendritic cells, up-regulating co-stimulatory molecules, as well as intracellular trafficking of phagocytosed antigens from endo-lysosome pathway to MHC class I antigen loading in endoplasmic reticulum [3,4]. Following these exciting discoveries, αGalCer has been proven to be a unique type of adjuvant for vaccine development, by multiple groups [5–12].
A major obstacle for the clinical use of αGalCer is that the soluble form of αGalCer is taken-up and presented by circulating, CD1d expressing B cells, and results in long term NKT anergy [13–15]. For treating chronic diseases such as cancer, repeated stimulation of innate cells by immunomodulators is necessary. Since soluble αGalCer induces anergy in NKT cells when injected intravenously, the rationale of repeatedly injecting soluble α-GalCer, as practiced in several previous clinical trials in cancer and hepatitis patients, has been seriously challenged. Additionally, NKT cell numbers vary greatly among healthy individuals, ranging from 0.001 to 0.1% of total peripheral blood leukocytes [16], and it is very unlikely that single treatment by a NKT cell stimulant will be effective to cause objective responses in patients. Therefore, novel therapeutic modalities that repeatedly stimulate NKT cells without inducing anergy are urgently needed.
The mechanisms of NKT anergy caused by αGalCer have been studied by several laboratories [13–15]. TCR signaling without co-stimulatory molecules turns on anergy-inducing transcription factors, which further up-regulate the E3 ligases (Grail, Cbl–b, and Itch) that degrade the molecular components required for T cell growth and differentiation, through ubiquitin-mediated proteasome degradation [17]. Itwas found that the co-stimulatory signals and cytokines provided by dendritic cells are critical for avoiding the NKT anergy. To overcome the anergy induction problem of soluble αGalCer, Dhodapkar and Steinmann have developed a cell therapy approach by intravenously injecting αGalCer pulsed dendritic cells generated in vitro from patients’ peripheral blood [18]. The cell therapy method avoided the NKT anergy and showed potent efficacy in human cancer patients to elicit cancer-specific CD8 responses. The efficacy of this method in curing cancer is currently being studied in larger number of patients. However, cell therapy is expensive and also impractical in virus infected patients since their tissues are excluded from GMP processing. New methods are needed to stimulate NKT cells in vivo repeatedly and effectively.
Data from several groups indicated that nanoparticles are superior carriers of vaccines due to their preferable uptake by dendritic cells [19–21]. Vaccines delivered by polyglutamic acid nanoparticles have been found to be more efficiently endocytosed by dendritic cells and macrophages than B cells [21]. Nanoparticles of 500–2000nm size were found to be more efficiently uptaken, as compared to smaller sizes (100–200 nm). Nanoparticle formulated antigens are released in the lysosome of dendritic cells, and recycled to cell surface to stimulate immune cells. Based on the above progress, we have formed the hypothesis that αGalCer packed in nanoparticles, is preferentially phagocytosed by dendritic cells, loaded to CD1d, recycled to cell surface, and activate NKT cells.
2. Materials and methods
2.1. Preparation of polylactic acid nanoparticles
Polylactic acid (PLA) nanoparticles (500–1000nm size) were prepared using a double-emulsion technique. Four hundred milligrams of PLA was dissolved in 2mL of dichloromethane in a glass tube, and 100 µL of Milli-Q water was added to the polymer solution. The polymer solution was then sonicated for 15 s to create the primary emulsion. 4mL of an aqueous 1% (w/v) solution of PEMA (poly[ethylene-alt-maleic acid]) was added to the tube, and the sonication step was repeated. After the second sonication, the emulsion was poured into 100mL of 0.3% (w/v) aqueous solution of the same stabilizer used for the second emulsion, under rapid stirring with a magnetic stirrer. The resulting nanoparticles were stirred in the solution for 3 h to evaporate the organic solvent. The nanoparticles were then washed three times with Milli-Q water, resuspended in 4mL of Milli-Q water for use.
2.2. Conjugation of streptavidin to nanoparticles
To conjugate streptavidin to nanoparticles, 12.5mg nanoparticles were resuspended in coupling buffer (50mM MES, pH 5.2) at 170 µL. 20 µL of EDAC (N-[3-Dimethylaminopropyl]-N’-ethylcarbodiimide hydrochloride) solution (200 g/L) was added to the suspension, and incubated at room temperature with continuous mixing for 15min. After, 200 µg of streptavidin was added and mixed gently for 1 h at room temperature. The strepatavidin-conjugated nanoparticles were stored in 10 mM Tris buffer, pH 0.5% with 0.05% bovine serum albumin.
2.3. Preparation of nanoparticle formulated αGalCer
The synthesis of biotinylated GalCer will be published elsewhere. For treatment of each mouse, 12.5 µg of streptavidin coated nanoparticles and 2 µg of biotinylated αGalCer were mixed and incubated for overnight at 4 °C under constant shaking. Nanoparticles were washed by PBS for 3 times by centrifugation at 2000 × g.
2.4. Examining the rate of binding for biotinylated αGalCer
The biotinylated αGalCer,when present in solution, can be measured by biological assays in a NKT cell stimulation assay. We could not find detectable NKT stimulation activity of the post-binding supernatant after the biotinylated αGalCer was mixed overnight with streptavidin-nanoparticles (data not shown). Thus the biotinylated αGalCer was considered as 100% bound to nanoparticles.
2.5. Presentation of αGalCer by mouse bone marrow derived dendritic cells (BMDC)
Presentation of nanoparticle formulated αGalCer was tested in professional antigen presenting cells, dendritic cells. Mouse BMDC were generated according to our published methods [22]. 50,000 of BMDCs were mixed with soluble biotinylated αGalcer or nanoparticle formulated αGalCer for 24 h, before being incubated with 50,000 NKT cells (DN32.D2) for additional 24 h. The activation of NKT cells was represented by their cytokine (IL2) release, as measured by ELISA or bioassays [22].
2.6. Presentation of nanoparticle formulated αGalCer by mouse spleenic antigen presenting cells
Three types of antigen presenting cells (APC) were purified from mouse spleens, CD11c + population (containing dendritic cells), CD11b + (containing macrophages), and B220 + cells (containing B cells). 200,000 of each type of APCs were mixed with soluble biotinylated αGalcer or nanoparticle formulated αGalCer for 24 h, before being incubated with 100,000 NKT cells (DN32.D2) for additional 24 h. The activation of NKT cells was represented by their cytokine (IL2) release.
2.7. In vivo stimulation of NKT cells by nanoparticle formulated αGalCer
Nanoparticle containing 1 µg αGalCer was injected to C57BL6 mice, with non-conjugated nanoparticles as negative control. In parallel, we studied soluble form of αGalCer. We previously published that the biotinylation of αGalCer does not interfere or reduce its biological activity [23].
2.8. The repeated in vivo NKT cell stimulation
C57/BL6 mice were purchased from the Jackson Laboratory (Bar Arbor, ME) and housed in M.D. Anderson Cancer Center animal facilities under standard pathogen free conditions abiding institutional guidelines. 6 weeks old C57BL/6 mice were used for all experiments. 3 mice per group were used for each experiment. The treatment schedule was described in Scheme 1. For first treatment, 1 µg αGalCer, or nanoparticle formulated αGalCer in 200 µL PBS was intravenously injected (through tail vein) to each mouse. 200mL of PBS/1%DMSO was used as control.
Scheme 1.
Schedule of αGalCer (in nanoparticle form or soluble form) treatment in vivo.
2.9. Measurement of IFN-γ secretion after each stimulation
Mice were bled at 24 h after each injection. Serum IFN-γ was measured by ELISA using a kit from BD Biosciences (San Jose, CA).
2.10. Staining of NKT cells and flow cytometry analysis
24 h after in vivo drug treatment, NKT cells were purified from mouse liver, and stained by αGalCer/CD1d tetramer (provided by NIAID tetramer facility at Emory University, Atlanta, GA) at cell surface, in combination with intracellular staining (ICS) of IFN-γ.
3. Results and discussion
3.1. Nanoparticle formulated αGalCer can be presented by mouse bone marrow derived dendritic cells
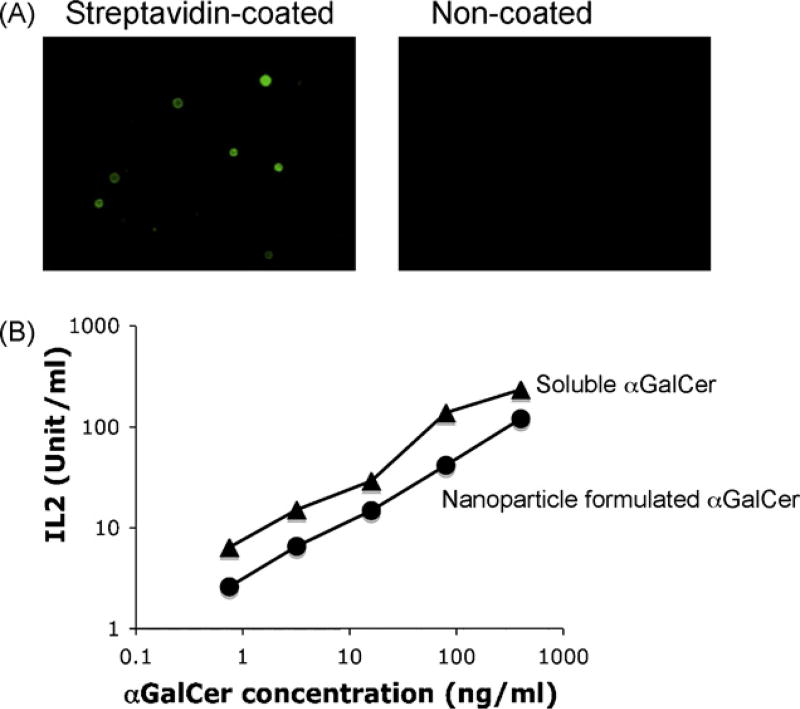
As shown in Fig. 1, nanoparticle formulated αGalCer was processed and presented by bone marrow derived dendritic cells, triggering activation of NKT cells. At similar concentrations, nanoparticle formulated αGalCer showed 30% stimulatory activity as compared to free αGalCer (Fig. 1B). This could be due to incomplete release of αGalCer in the lysosome to CD1d antigen presenting pathway.
Fig. 1.
Presentation of nanoparticle formulated αGalCer by dendritic cells. (A) Strepatavidin coated nanoparticles were stained by biotin-FITC, and visualized by fluorescence microscope. Nanoparticles without streptavidin coating (non-coated) were stained as negative control. (B) Strepatvidin coated nanoparticles were conjugatedwith biotinylated αGalCer, and presented by mouse dendritic cells to stimulate NKT cells. Soluble form of biotinylated αGalCer were used as positive control. NKT cell activation was represented by their secretion of cytokine (IL2). Data were representative of three independent experiments: (▲) soluble biotinylated αGalCer and (●) nanoparticle conjugated αGalCer.
3.2. Nanoparticle overcomes the anergy of NKT cells
Similarly to αGalCer [13–15], the first injection of nanoparticle formulated αGalCer elicited cytokine release, peaked at 24–48 h post injection, as measured by serum IFN-γ concentration. As shown in Table 1, nanoparticle formulated αGalCer induced IFN-γ secretion after each stimulation. In contrast, soluble αGalCer caused IFN-γ secretion only at the first treatment and failed to induce upon subsequent stimulations. Thus our new formulation of αGalCer can repeatedly stimulate NKT cells and induce IFN-γ production without leading to anergy. However, the nanoparticle formulated αGalCer induced 10-fold lower cytokine secretion in mouse serum at a same drug dose (1 µg of αGalCer). This could be due to lower retention of these nanoparticles in immune organs (liver and spleen) and the efficacy of αGalCer releasing from nanoparticles (Fig. 1).
Table 1.
αGalCer-nanoparticles repeatedly activate NKT cells to produce IFN-γ (pg/mL).
| αGalCer-nanoparticles | Nanoparticles w/o αGalCer |
αGalCer | |
|---|---|---|---|
| ×1 injection | 286 ± 39a | 48 ± 1 | 2601 ± 257 |
| ×2 injection | 252 ± 62 | 42 ± 5 | 65 ± 2 |
| ×3 injection | 261 ± 102 | 45 ± 3 | 40 ± 11 |
Data were average of three mice per group along with standard deviation.
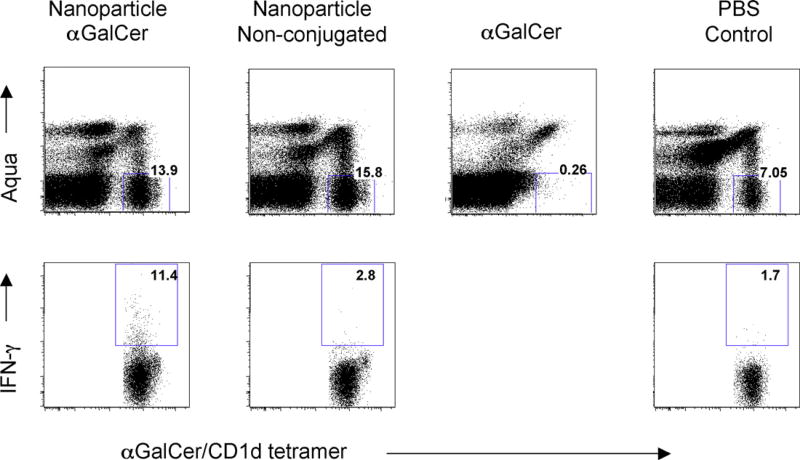
It is well known that soluble form of αGalCer, when administered intravenously to mice, will cause down regulation of TCR on NKT cells within 24 h. T cell receptors will come back to cell surface after 48 h, and NKT cells start massive expansion between day 4 and day 6 after drug treatment, followed by long term anergy [13–15]. In a clear contrast, we found nanoparticle formulated αGalCer did not cause the down regulation of cell surface T cell receptor (Fig. 2), although the NKT cells were activated and produced INF-γ. We also found the nanoparticle formulated α-GalCer only caused 2–3-fold of increase of NKT cell number in peripheral blood peaked between day 5 and day 8 after drug treatment (data not shown), which is in clear contrast to the massive expansion of NKT cells (10–20-fold increase) when the mice were treated by soluble form of α-GalCer.
Fig. 2.
In vivo stimulation of NKT cells by nanoparticle formulated αGalCer. C57BL6 mice were intravenously treated with 2 µg of nanoparticle formulated αGalCer. 24 h after treatment, mice were sacrificed, and liver lymphocytes were prepared for cell surface staining of NKT cells by αGalCer/CD1d tetramer, and intracellular staining of IFN-γ. Mice treated with non-conjugated nanoparticles, as well as mice treated by soluble form of αGalCer, were studied in parallel. (Up panel) Liver NKT cells were stained by αGalCer/CD1d tetramer. Numbers indicate percentage of NKT cells. Aqua (Invitrogen, Carlsberg, CA) positive cells were excluded to ensure only living cells were stained. (Lower panel) Intracellular production of IFN-γ was stained by intracellular staining technology. Numbers indicate the percentage of NKT cells producing IFN-γ. Data were representative of three mice in each treated group. Note that soluble form of αGalCer, when administered intravenously to mice, will cause down regulation of TCR on NKT cells within 24 h, thus the NKT cells could not be detected by αGalCer/CD1d tetramer staining.
3.3. Nanoparticle formulated αGalCer is preferentially presented by dendritic cells
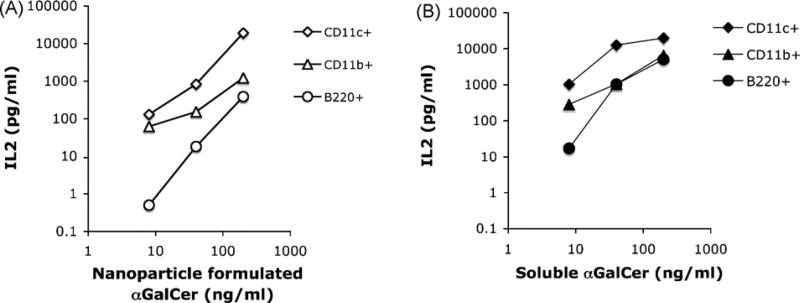
Parekh et al. demonstrated that intravenous injection of αGalCer loaded B cells caused the anergy of NKT cells [15]. Thus we have examined the efficacy of B cells in presenting nanoparticle formulated αGalCer. Fig. 3A showed that nanoparticle formulated αGalCer was presented at a 100-fold lower efficiency as compared to CD11c + cells, at different concentrations between 1 ng/mL and 1 µg/mL. In contrast, soluble form of αGalCer (Fig. 3B) was very efficiently presented by B cells, a finding similar to previous published results by several other groups [13–15]. Thus nanoparticle formulated αGalCer avoided the presentation by B cell population due to their poor capacity of phagocytosis.
Fig. 3.
Poor presentation of nanoparticle formulated αGalCer by B cells. B220 + B cells were purified from mouse spleen by cell sorting. 200,000 B cells were mixed with different concentration of nanoparticle formulated αGalCer, and incubated with 100,000 NKT cells (DN32.D3) for 24 h.NKT cell activation was represented by their secretion of cytokine (IL2). CD11c + cells and CD11b + cells were studied in parallel. Data were representative of two independent experiments: (A) presentation of nanoparticle formulated αGalCer and (B) presentation of free αGalCer.
In summary, our data strongly support our hypothesis that nanoparticle formulated αGalCer repeatedly activates NKT cells, avoiding anergy induction. Further improvement may be achieved by testing more lysosome degradable materials, to enhance the efficacy of releasing αGalCer. Surface charges may be modified to enhance the uptake of nanoparticles by dendritic cells in immune organs. Dendritic cell specific ligands, such as carbohydrate ligands for DC lectins, may be utilized for targeting to dendritic cells.
Nanoparticle formulated αGalCer may be used as novel immunomodulators to treat cancer and viral diseases. Furthermore, a same nanoparticle may be packed with both αGalCer and protein/peptide antigens, as unique vaccine adjuvant. Our preliminary studies have shown that both OVA protein and gp100 melanoma peptide antigens [24], when conjugated to αGalCer containing nanoparticles, induced potent antigen specific CD8 T cell responses (data not shown).
Acknowledgments
We thank Bhanu Prakash Pappu, Yeonseok Chung and Chen Dong for advice and discussion. This project is supported by M.D. Anderson Cancer Center (D.Z. and C.L.), Ohio State University (P.G.W.) and NIH grant AI42694 and 46969 (K.J.S.), andCA123195-01 (P.G.W.). C.L. is supported by John S. Dunn Foundation. D.Z. is recipient of a Developmental Award from Baylor-UTHouston Center for AIDS Research AIDS Research Core Support Grant (AI36211).
References
- 1.Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res. 1995;7(10–11):529–34. [PubMed] [Google Scholar]
- 2.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997 Nov;278(5343):1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 3.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003 Jul;198(2):267–79. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou D. OX40 signaling directly triggers the antitumor effects of NKT cells. J Clin Invest. 2007 Nov;11(117):3169–72. doi: 10.1172/JCI33976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi YS, Hoory T, Monie A, Wu A, Connolly D, Hung CF. alpha-Galactosylceramide enhances the protective and therapeutic effects of tumor cell based vaccines for ovarian tumors. Vaccine. 2008 Oct;26(46):5855–63. doi: 10.1016/j.vaccine.2008.08.027. Epub 2008 Sep 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y, Chen A, Li X, Chen Z, Zhang W, Song Y, et al. Enhancement of HIV DNA vaccine immunogenicity by the NKT cell ligand, alpha-galactosylceramide. Vaccine. 2008 Mar;26(15):1807–16. doi: 10.1016/j.vaccine.2008.02.002. Epub 2008 Feb 20. [DOI] [PubMed] [Google Scholar]
- 7.Ko SY, Lee KA, Youn HJ, Kim YJ, Ko HJ, Heo TH, et al. Mediastinal lymph node CD8alpha-DC initiate antigen presentation following intranasal coadministration of alpha-GalCer. Eur J Immunol. 2007 Aug;37(8):2127–37. doi: 10.1002/eji.200636909. [DOI] [PubMed] [Google Scholar]
- 8.Youn HJ, Ko SY, Lee KA, Ko HJ, Lee YS, Fujihashi K, et al. A single intranasal immunization with inactivated influenza virus and alpha-galactosylceramide induces long-term protective immunity without redirecting antigen to the central nervous system. Vaccine. 2007 Jul;25(28):5189–98. doi: 10.1016/j.vaccine.2007.04.081. Epub 2007 May 21. [DOI] [PubMed] [Google Scholar]
- 9.Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, Tortoli M, et al. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci USA. 2007 Mar;104(10):3984–9. doi: 10.1073/pnas.0700191104. Epub 2007 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko SY, Ko HJ, Chang WS, Park SH, Kweon MN, Kang CY. alpha-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol. 2005 Sep;175(5):3309–17. doi: 10.4049/jimmunol.175.5.3309. [DOI] [PubMed] [Google Scholar]
- 11.Silk JD, Hermans IF, Gileadi U, Chong TW, Shepherd D, Salio M, et al. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J Clin Invest. 2004 Dec;114(12):1800–11. doi: 10.1172/JCI22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, Wilson JM, Schmieg J, Kronenberg M, et al. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med. 2002 Mar;195(5):617–24. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002 Sep;3(9):867–74. doi: 10.1038/ni827. Epub 2002 Aug 5. [DOI] [PubMed] [Google Scholar]
- 14.Uldrich AP, Crowe NY, Kyparissoudis K, Pellicci DG, Zhan Y, Lew AM, et al. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005 Sep;5(175):3092–101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parekh VV, Wilson MT, Olivares-Villagómez D, Singh AK, Wu L, Wang CR, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005 Sep;115(9):2572–83. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee PT, Putnam A, Benlagha K, Teyton L, Gottlieb PA, Bendelac A. Testing the NKT cell hypothesis of human IDDM pathogenesis. J Clin Invest. 2002 Sep;110(6):793–800. doi: 10.1172/JCI15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heissmeyer V, Macian F, Im SH, Varma R, Feske S, Venuprasad K, et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004 Mar;5(3):255–65. doi: 10.1038/ni1047. Epub 2004 Feb 15. [DOI] [PubMed] [Google Scholar]
- 18.Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005 May;201(9):1503–17. doi: 10.1084/jem.20042592. [Erratum in: J Exp Med 2007;204(October (10)):2487] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zwiorek K, Bourquin C, Battiany J, Winter G, Endres S, Hartmann G, et al. Delivery by cationic gelatin nanoparticles strongly increases the immunostimulatory effects of CpG oligonucleotides. Pharm Res. 2008 Mar;25(3):551–62. doi: 10.1007/s11095-007-9410-5. Epub 2007 Oct 3. [DOI] [PubMed] [Google Scholar]
- 20.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007 Oct;25(10):1159–64. doi: 10.1038/nbt1332. Epub 2007 Sep 16. [DOI] [PubMed] [Google Scholar]
- 21.Uto T, Wang X, Sato K, Haraguchi M, Akagi T, Akashi M, et al. Targeting of antigen to dendritic cells with poly (gamma-glutamic acid) nanoparticles induces antigen-specific humoral and cellular immunity. J Immunol. 2007 Mar;178(5):2979–86. doi: 10.4049/jimmunol.178.5.2979. [DOI] [PubMed] [Google Scholar]
- 22.Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004 Dec;306(5702):1786–99. doi: 10.1126/science.1103440. Epub 2004 Nov 11. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Goff RD, Zhou D, Mattner J, Sullivan BA, Khurana A, et al. A modified alpha-galactosyl ceramide for staining and stimulating natural killer T cells. J Immunol Methods. 2006 Dec;312(1–2):34–9. doi: 10.1016/j.jim.2006.02.009. Epub 2006 Mar 6. [DOI] [PubMed] [Google Scholar]
- 24.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8 + T cells. J Exp Med. 2003 Aug;198(4):569–80. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]