Abstract
A growing body of evidence has demonstrated that microRNAs (miRs) have pivotal roles in the pathophysiological development mechanisms of diabetic cardiomyopathy (DCM). Previous studies have demonstrated that miR-186-5p was significantly decreased in DCM. In addition, it has recently been reported that an imbalance of miR-186 is associated with a variety of physiological and pathological processes. Therefore, the present study was designed to investigate the role of miR-186-5p in high glucose (HG)-induced cytotoxicity and apoptosis in AC16 cardiomyocytes. Reverse transcription-polymerase chain reaction was used to demonstrate the significant decrease in the level of miR-186-5p in HG-treated AC16 cells (P<0.05). Subsequently, it was clarified that pre-transfection with miR-186-5p mimic significantly ameliorated the effects of high glucose, which induced a significant decrease in the viability of AC16 cells (P<0.05) and increases in apoptosis, as evidenced by the appearance of apoptotic nucleus and the significant upregulation of apoptosis rate in AC16 cells (P<0.05). In addition, the significantly increased expression of caspase-3 induced by HG (P<0.01) was also reversed by miR-186-5p mimic (P<0.01). Conversely, transfection with miR-186-5p inhibitor significantly reduced the viability of AC16 cells (P<0.05) and promoted apoptosis (P<0.05) as well as the expression of caspase-3 in AC16 cells (P<0.01), indicating the beneficial role of miR-186-5p in the physiological process of HG-induced damage. In conclusion, these results suggest that the distribution of miR-186-5p contributes to HG-induced cytotoxicity and apoptosis in AC16 cardiomyocytes.
Keywords: microRNA-186-5p, high glucose, cardiomyocyte injury, apoptosis, AC16 cardiomyocytes
Introduction
Diabetic cardiomyopathy (DCM), a severe and common cardiac complication of diabetes, has become the leading cause of mortality and morbidity in diabetic patients (1). Increasing evidence has suggested that hyperglycemia, increased apoptosis, insulin resistance/hyperinsulinemia and abnormal fatty acid metabolism are the most important pathophysiological mechanisms of DCM (2–4). In particular, hyperglycemia has generally been considered as a central trigger in the pathophysiology of DCM, which leads to increased oxidative stress through aggravating glucose oxidation and production of reactive oxygen species (ROS), resulting in DNA damage and accelerated apoptosis (5). Emerging evidence has also demonstrated that diabetes may be caused by an imbalance of metabolic molecules, including glucose, amino acids, lipids, and other such factors are able to promote myocardial cells damage (6). Despite this, DCM remains poorly understood and the underlying mechanisms are not completely elucidated. A number of studies have established the cellular model of DCM by using high D-glucose (HG) to simulate hyperglycemia in DCM (7,8). Therefore, it is important to investigate the molecular mechanism of HG-induced myocardial cell injury, which may provide novel insights and therapeutic strategies for the pathological process of DCM.
MicroRNAs (miRNAs or miRs) are a large class of endogenous, small, noncoding RNAs that regulate diverse biological processes including cell proliferation, cell differentiation, apoptosis, and organ development (9). Emerging evidence reveals that changes in the levels of miRNAs are associated with the occurrence and development of various diseases, including diabetes and cardiovascular diseases (10–12). In recent years, the roles of miRNAs in DCM have received more attention in research. A series of animal and cellular experiments have identified changes in several miRNAs levels and their specific mRNA targets which are associated with the pathogenetic processes of diabetic heart complication (13,14). Notably, in a previous study, the present authors determined that several miRNAs are altered in DCM, as miR-106b-5p, −144-3p, −186-5p, −22-3p and −30d-5p were downregulated, whereas miR-516a-5p, −575, and −630 were upregulated in the serum of patients with DCM (13). Of these changes, the reduction of miR-186-5p was the most marked. In addition, Bostjancic et al (15) have clarified that the dysregulation of many miRNAs, such as miR-186, is believed to be associated with a variety of physiological and pathological processes. Together, these findings provide rationale for investigating the role of miR-186-5p in the development of DCM, which will help identify the molecular mechanisms and novel therapeutic strategies for DCM.
In the present study, AC16 cardiomyocytes were used to assess the potential effect of miRNA-186-5p on HG-exerted damage in the presence or absence of miRNA-186-5p mimic and miRNA-186-5p inhibitor. To the best of our knowledge, the present results confirmed for the first time that the downregulation of miRNA-186-5p mediates HG-elicited cytotoxicity and apoptosis in AC16 cardiomyocytes.
Materials and methods
Materials
Hoechst 33258, bicinchoninic acid (BCA) protein assay kit, enhanced chemiluminescence (ECL) solution, and radioimmunoprecipitation assay (RIPA) buffer were supplied by Beyotime Institute of Biotechnology (Haimen, China). The cell counting kit-8 (CCK-8) was obtained from Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). F12/Dulbecco's modified Eagle's medium (DMEM-F12) and fetal bovine serum (FBS) were purchased from HyClone (GE Healthcare Life Sciences, Logan, UT, USA). Primary antibodies for caspase-3 (cat. no. 9662) and GAPDH (cat. no. 5174) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA), and horseradish peroxidase (HRP)-conjugated secondary antibodies (cat. no. 00001-9) were obtained from ProteinTech Group, Inc. (Danvers, MA, USA). Annexin V/propidium iodide (PI) apoptosis kit was supplied by BD Pharmingen (BD Biosciences, San Jose, CA, USA). Lipofectamine RNAiMAX and Opti-MEM medium were supplied by Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The miRcute miRNA Isolation kit, miRcute miRNA First-strand cDNA Synthesis kit and miRcute miRNA quantitative polymerase chain reaction (qPCR) detection kit were obtained from Tiangen Biotech Co., Ltd. (Beijing, China).
Cell culture and treatments
The AC16 human adult ventricular cardiomyocyte cell line was purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in DMEM-F12 medium with 10% FBS and 1% penicillin-streptomycin at 37°C in a humidified incubator containing 5% CO2. For HG treatment, AC16 cells grown to ~70% confluence were treated with HG (33 mM) for different durations (0, 24, 48 or 72 h). For miR-186-5p mimic treatment, cells were grown to 80–90% confluence and incubated at 37°C in fresh, serum-free and antibiotic-free medium for 3 h, and then transfected with miR-186-5p mimic (2 µg) or negative-control miRNA (2 µg) for 5 h prior to incubation at 37°C in HG for 24 h. For miR-186-5p inhibition treatment, cells were grown to 80–90% confluence and transfected with miR-186-5p inhibitor (2 µg) for 5 h and then incubated at 37°C with normal glucose (5.5 mM) for 24 h. The sequence information on miR-186-5p mimics and inhibitors are as follows: miR-186-5p mimics, 5′-CAAAGAAUUCUCCUUUUGGGCU-3′ and miR-186-5p inhibitor, 5′-AGCCCAAAAGGAGAAUUCUUUG-3′.
MTS assay
AC16 cells were seeded on 96-well plates at 5×104 cells/well overnight at 37°C. Following treatment of AC16 cells with miR-186-5p mimic or inhibitor in the presence and absence of HG, respectively, for 24 h, and 10 µl MTS reagent was added to each well for 1 h at 37°C. The absorbance values were measured at 490 nm using a microplate spectrophotometer (Thermo Fisher Scientific, Inc.). Results were expressed as a percentage of control cells (transfected with negative-control miRNA). Each assay was independently performed in triplicate. Appreciation rate (%) = (mean OD value at time point/mean OD value at 0 Day-1) ×100. Suppression rate (%)=(1-mean OD value of experimental group/control group) ×100.
Hoechst 33258 staining
Nuclear morphology of AC16 cells was assessed via Hoechst 33258 staining. Briefly, AC16 cells were seeded into a 24-well plate at a density of 1×105 cells/well overnight. Following treated as described above for 24 h, AC16 cells were washed with PBS three times and fixed with 4% formaldehyde for 10 min at 4°C. Cells were washed three times with PBS again and incubated with 10 µg/ml Hoechst 33258 at room temperature for 10 min in the dark. The morphological changes of AC16 cells were observed at ×200 magnification under a fluorescence microscope (Eclipse Ti; Nikon Corporation, Tokyo, Japan). Apoptosis rate was calculated as the number of apoptotic cells/total cells from the average of 5 random fields.
Annexin V-fluorescein isothiocyanate (FITC) and PI staining
The apoptosis of AC16 cells was evaluated using an Annexin V-PI double staining assay kit according to the manufacturer's protocol. Briefly, AC16 cells were treated for 24 h as detailed above and the culture supernatant was collected. Subsequently, AC16 cells were washed and harvested with cold PBS. AC16 cells and supernatant were co-centrifuged at 1,000 × g at room temperature for 5 min. Following washing with PBS twice, cells were resuspended in 500 µl binding buffer from the Annexin V-PI kit and then co-incubated with 5 µl Annexin V-FITC reagent and 10 µl PI reagent for 10 min at 4°C in the dark. Cellular apoptosis was detected by flow cytometry (FC500; Beckman Coulter, Inc., Brea, CA, USA).
Reverse transcription (RT)-qPCR analysis
The level of miR-186-5p in AC16 cells, following treatment as described above, was detected with RT-qPCR. Total RNA was extracted from AC16 cells using an miRcute miRNA Isolation kit according to manufacturer's protocol. Single-strand cDNA was synthesized using an miRcute miRNA First-strand cDNA Synthesis kit according to manufacturer's protocol. qPCR was performed via a one step method with an miRcute miRNA qPCR Detection kit according to the manufacturer's protocol. U6 small nuclear RNA was used as an internal reference. Bulge-loop miRNA RT-qPCR Primer Sets (one RT primer and a pair of qPCR primers for each set) specific for miR-186-5p were designed by RiboBio (Guangzhou, China). Primer sequence of miR-186-5p was as follows: forward, 5′-TCAAAGAATTCTCCTTTTGGGCT-3′ and reverse 5′-CGCTTCACGAATTTGCGTGTCAT-3′. PCR was performed for 2 min at 94°C, followed by 40–45 cycles of 94°C for 20 sec and 60°C for 34 sec. The amplified products were measured using 1% agarose gel electrophoresis and densitometry of bands was analyzed using Image J software bundled with Java 1.8.0–112 (National Institutes of Health, Bethesda, MD, USA). Relative quantitative values were calculated using the 2−ΔΔCq method (16).
Western blot analysis
AC16 cells were treated as detailed above and lysed in RIPA buffer containing 1 mM phenylmethane sulfonyl fluoride (Beyotime Institute of Biotechnology). Cellular protein was centrifuged at 12,000 × g for 10 min at 4°C and quantified using a BCA protein assay kit according to the manufacturer's protocol. Equal amounts of proteins (50 µl) were separated on 12% SDS-PAGE and electrotransferred onto a polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA). The membrane was then blocked with 5% no-fat milk in 0.05% Tween 20/TBS (TBST) for 2 h at room temperature. Following washing three times with TBST, the membrane was incubated with primary antibodies against cleaved caspase-3 (1:2,000) and GAPDH (1:2,000) overnight at 4°C. The membrane was subsequently incubated with a HRP-conjugated secondary antibody (1:5,000) for 2 h at room temperature and developed using an ECL solution. GAPDH was used as the internal loading control. The band densities were determined with Quantity One 4.6 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Each assay was independently performed in triplicate.
Statistical analysis
Data are presented as the mean ± standard error of the mean. The difference between groups was determined by one-way analysis of variance followed by Fisher's least significance difference test. P<0.05 was considered to indicate a statistically significant difference.
Results
The level of miR-186-5p is downregulated in HG-treated AC16 cardiomyocytes
RT-qPCR was performed to measure the change of miR-186-5p level in HG-treated AC16 cardiomyocytes. As presented in Fig. 1, HG treatment for 12, 24 and 48 h significantly decreased the level of miR-186-5p in AC16 cells compared with control cells. The greatest decrease in miR-186-5p was observed following 24 h treatment. Therefore, 24 h was identified as the optimal treatment time for subsequent experiments.
Figure 1.
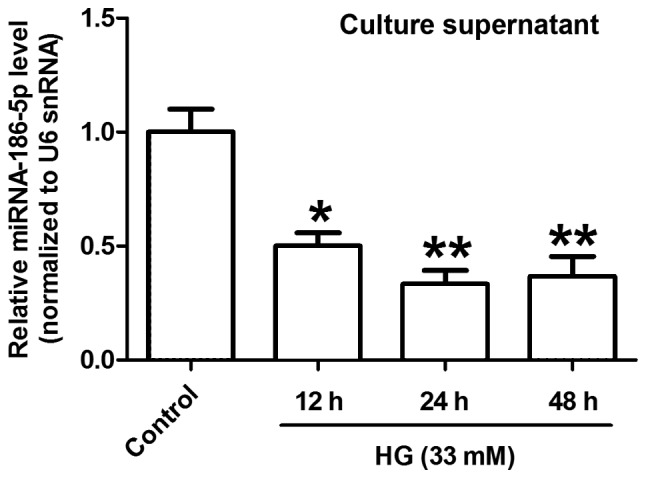
The level of miR-186-5p was downregulated in HG-treated AC16 cells. AC16 cells were treated with HG (33 mM) for 48 h. The level of miR-186-5p in AC16 cells was measured via reverse transcription-quantitative polymerase chain reaction. Data are presented as the mean ± standard error of the mean (n=3). *P<0.05, **P<0.01 vs. control group. miR, microRNA; HG, high glucose; snRNA, small nuclear RNA.
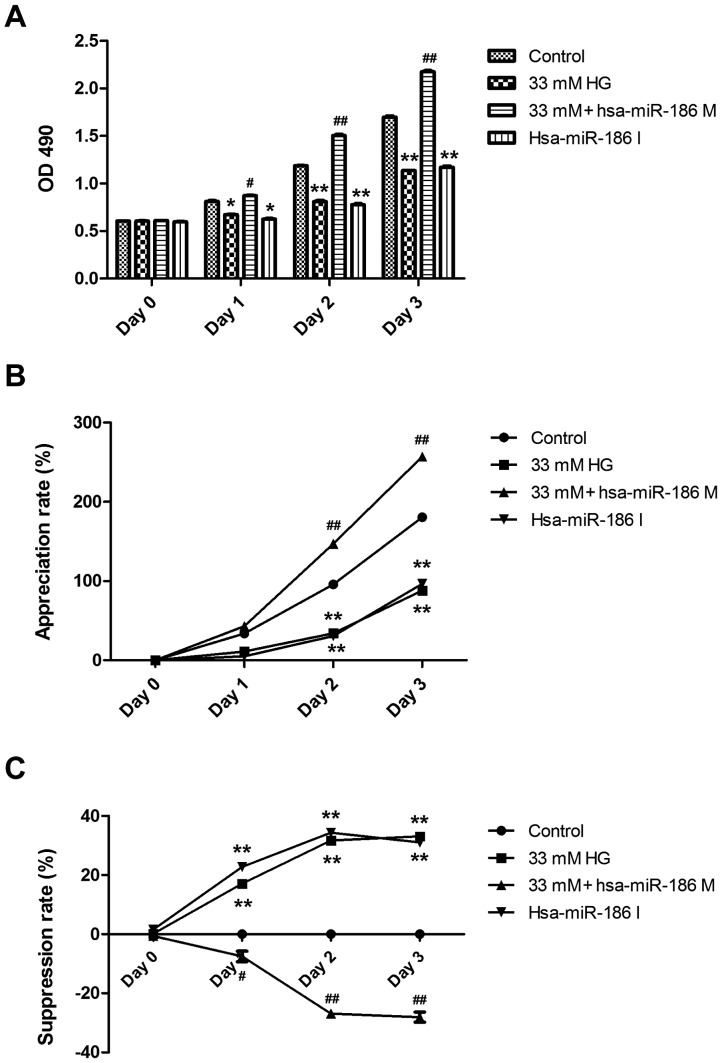
miR-186-5p mimic transfection reverses HG-induced downregulation of cell viability in AC16 cardiomyocytes
To investigate whether miR-186-5p is associated with HG-induced myocardial cytotoxicity, we detected the effect of miR-186-6p mimic, which is able to simulate the high level of mature miR-186-6p in cells, or miR-186-5p inhibitor on the viability of AC16 cells. As presented in Fig. 2, pre-transfection of AC16 cells with miR-186-5p mimic significantly reversed the downregulation of cell viability induced by HG (Fig. 2A). In addition, HG induced a significant decrease in the appreciation rate of AC16 cells, compared with controls (Fig. 2B) and a significant increase in the suppression rate of AC16 cells (Fig. 2C), which were both significantly ameliorated by miR-186-5p mimic, which suggests that the AC16 cells transfected with miR-186-5p mimic had the ability to protect against long-term HG-induced injury. Furthermore, it was demonstrated that miR-186-5p inhibitor significantly downregulated the viability and appreciation rate (Fig. 2A and B) and significantly upregulated the suppression rate of AC16 cells (Fig. 2C) compared with controls, similar to treatment with HG, which suggests that miR-186-5p may have an important role in maintaining cell survival. These results showed that HG was able to induce cytotoxicity through downregulating the level of miR-186-5p in AC16 cells.
Figure 2.
HG induced a decrease in the viability of AC16 cells through downregulating the miR-186-5p level. AC16 cells were transfected with miR-186-5p M prior to HG treatment for 1–4 days, or AC16 cells were transfected with miR-186-5p I in normal glucose conditions. (A) The viability of AC16 cells was measured by MTS assay. (B) Appreciation rate (%) = (mean OD value at time point/mean OD value at 0 Day-1) ×100. (C) Suppression rate (%) = (1-mean OD value of experimental group/control group) ×100. Data are presented as the mean ± standard error of the mean (n=3). *P<0.05, **P<0.01 vs. control group; #P<0.05, ##P<0.01 vs. HG group. HG, high glucose; miR, microRNA; M, mimic; I, inhibitor; hsa, human.
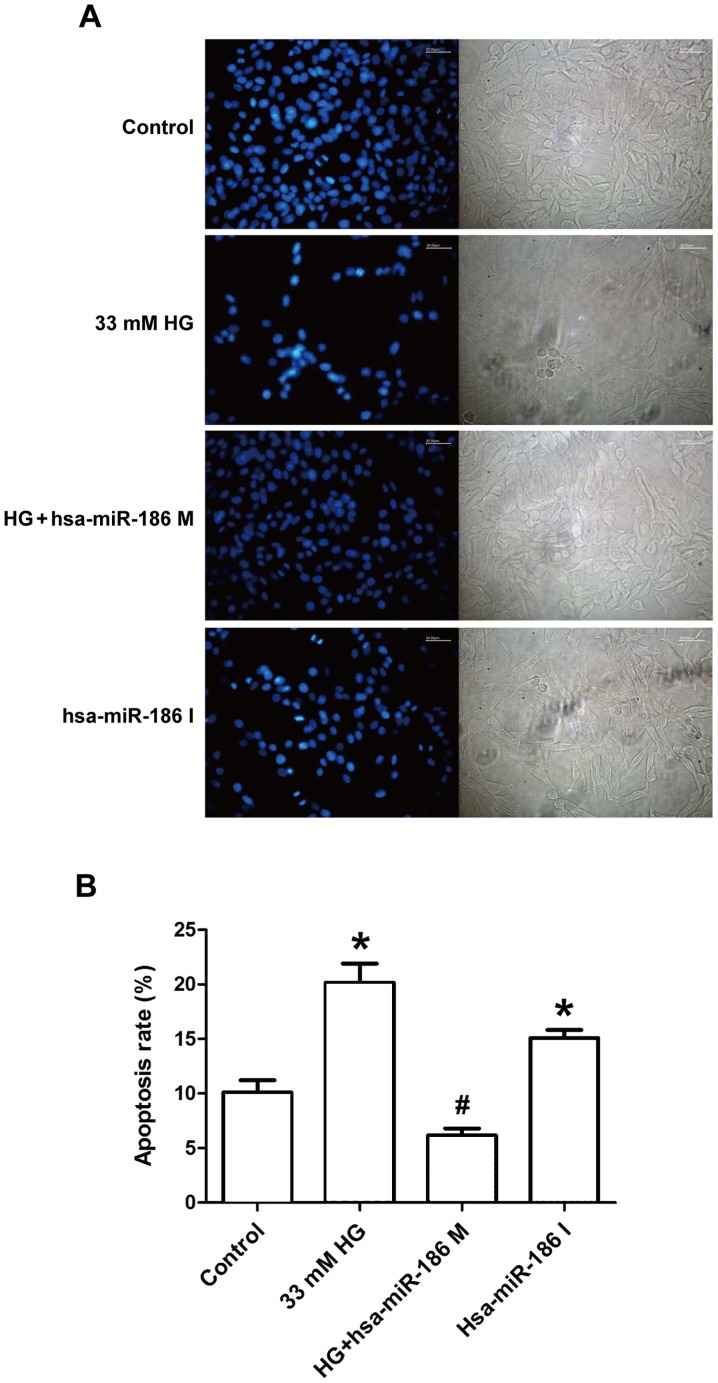
miR-186-5p mimic ameliorates HG-induced morphological changes of AC16 apoptotic cells
To determine whether an association exists between HG-induced cardiomyocyte apoptosis and miR-186-5p, the effect of miR-186-5p mimic and miR-186-5p inhibitor was observed on apoptosis in AC16 cells via Hoechst 33258 staining. As shown in Fig. 3, in the control group, AC16 cells exhibited regular-shaped nuclei and low intensity blue uniform fluorescence, whereas the numbers of AC16 cells with fragmented or condensed nuclei and bright blue fluorescence, which were characteristics of apoptotic cells. were markedly increased in HG-treated cells (Fig. 3A). However, compared with HG group, the number of AC16 cells with bright blue fluorescence was markedly decreased in the miR-186-5p mimic group. Furthermore, the rate of apoptosis was significantly upregulated by HG, in comparison with control cells; however, this was significantly ameliorated in miR-186-5p mimic cells (Fig. 3B). In addition, transfection with miR-186-5p inhibitor led to fragmented or condensed nuclei in AC16 cells. These results suggest that HG downregulated the level of miR-186-5p in AC16 cells, and therefore induced apoptosis.
Figure 3.
HG treatment induced morphological changes of apoptotic AC16 cells through downregulating the miR-186-5p level. AC16 cells were transfected with miR-186-5p M prior to HG treatment or the AC16 cells were transfected with miR-186-5p I in normal glucose. (A) The morphological indicators of apoptosis were detected by Hoechst 33258 staining with fluorescence microscopy (magnification, ×200). Images are representative of at least three independent determinations. Scale bar, 30.00 µm. (B) Statistical analysis of Hoechst 33258 staining. Data are presented as the mean ± standard error of the mean (n=3). *P<0.05 vs. control group; #P<0.05 vs. HG group. HG, high glucose; miR, microRNA; M, mimic; I, inhibitor; hsa, human.
miR-186-5p mimic alleviates HG-induced increase in the expression of cleaved caspase-3 protein in AC16 cardiomyocytes
Caspase-3 is considered as a key effector protease in the cellular apoptotic response, which is activated in the process of apoptosis followed by substrate binding, resulting in cell apoptosis through amplifying the cascade reaction (17). Therefore, the effect of miR-186-5p on cleaved caspase-3 level was measured in AC16 cells. As presented in Fig. 4, AC16 cells treated with HG or transfected with miR-186-5p inhibitor exhibited a significantly increased expression of cleaved caspase-3 protein compared with that of the control group. However, compared with the HG group, transfection with miR-186-5p mimic significantly decreased the expression of cleaved caspase-3 protein in AC16 cells. This suggests that HG induced an upregulation of cleaved caspase-3 protein expression via reducing the miR-186-5p level.
Figure 4.
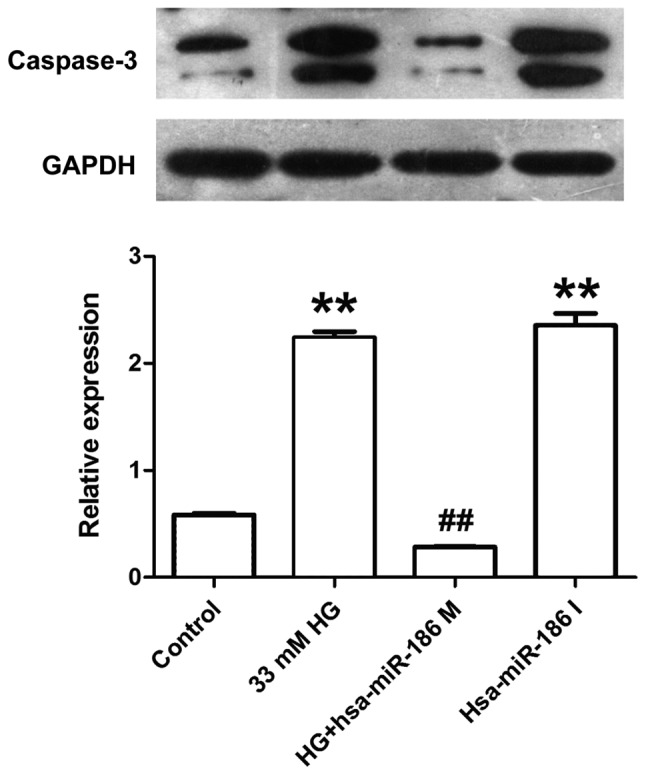
HG induced an increase in the expression of cleaved caspase-3 in AC16 cells through downregulating miR-186-5p level. The AC16 cells were transfected with miR-186-5p M prior to treatment with HG (33 mM, 24 h), or AC16 cells were transfected with miR-186-6p I in normal glucose conditions. The expression of cleaved caspase-3 was detected by western blotting. GAPDH was used as a reference gene. Data are presented as the mean ± standard error of the mean (n=3). **P<0.01 vs. control group; ##P<0.01 vs. HG group. HG, high glucose; miR, microRNA; M, mimic; I, inhibitor; hsa, human.
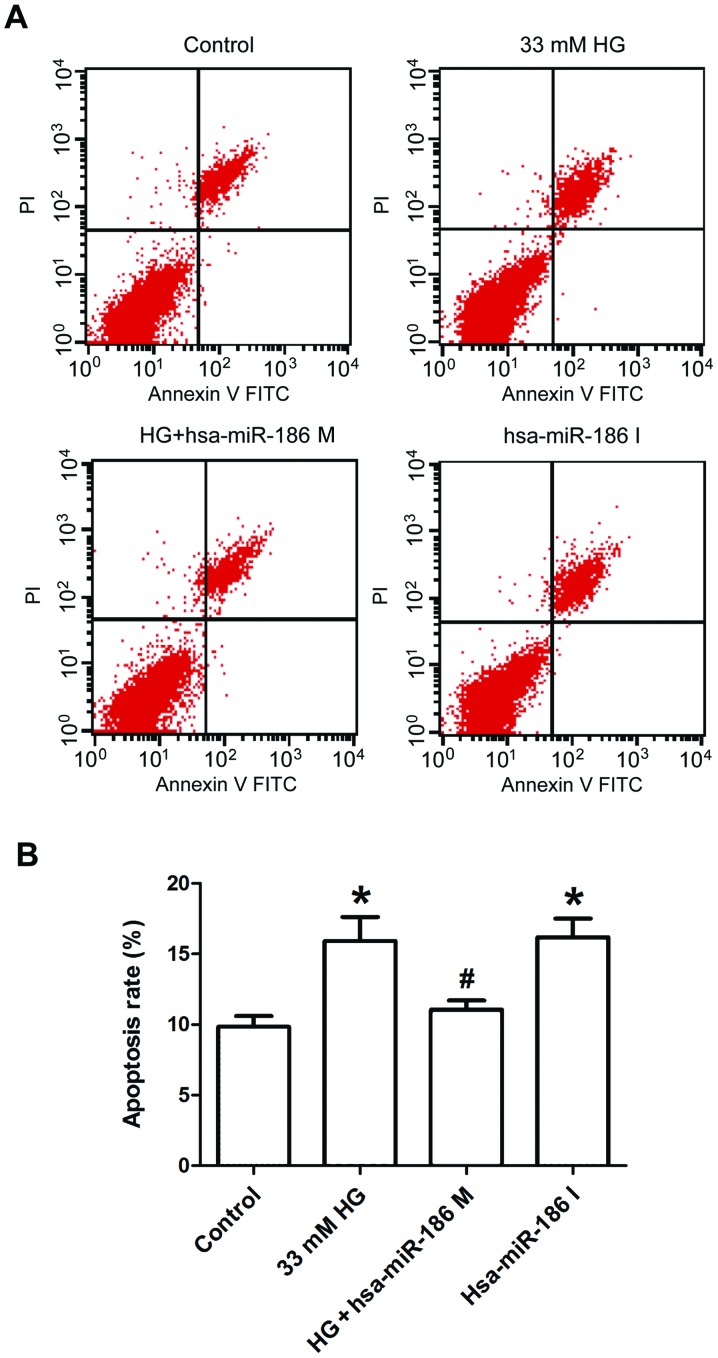
miR-186-5p mimic ameliorates HG-induced apoptosis in AC16 cardiomyocytes
Finally, Annexin V/PI staining and flow cytometry were used to further test the effect of miR-186-5p on apoptosis in AC16 cells. As presented in Fig. 5, AC16 cells treated with HG or transfected with miR-186-6p inhibitor significantly increased the percentage of apoptotic cells in comparison with the control group, whereas transfection with miR-186-5p mimic significantly ameliorated the HG-induced increase in the apoptotic ratio. This result suggested that HG induced apoptosis through downregulating the miR-186-5p level in AC16 cells.
Figure 5.
HG induced apoptosis in AC16 cells through downregulating miR-186-5p level. AC16 cells were transfected with miR-186-5p M prior to treatment with HG (33 mM, 24 h) or AC16 cells were transfected with miR-186-5p I in normal glucose conditions. (A) Apoptosis was detected by Annexin V/PI double staining and (B) quantitative analysis of percentage of early-stage apoptotic cells (Annexin V+/PI−) and late-stage apoptotic (Annexin V+/PI+) cells. Data are presented as the mean ± standard error of the mean (n=3). *P<0.05 vs. control group; #P<0.05 vs. HG group. HG, high glucose; miR, microRNA; M, mimic; I, inhibitor; FITC, fluorescein isothiocyanate; PI, propidium iodide; hsa, human.
Discussion
Diabetes is associated with hyperglycemia, insulin resistance and dyslipidemia, which are risk factors for cardiovascular diseases. The prevalence of cardiac diabetic diseases such as DCM has been increasing worldwide, and is the leading cause of morbidity and mortality among diabetic patients (18). However, the underlying mechanisms of DCM remain to be elucidated. In recent years, the role of miRNAs in the development of DCM has been an area of interest for research (13,19). Therefore, the aim of the present study was to investigate whether changes in miR-186-5p expression are associated with HG-induced AC16 cardiomyocyte injury, which is an in vitro cellular model of hyperglycemia-induced myocardial injury (20).
miRNAs have been demonstrated to have important roles in various forms of cardiovascular disease. Bostjancic et al (15) used miRNA microarrays to screen the differential expression of miRNA in human myocardial infarction and found that miRNA-186 was dysregulated under myocardial infarction. Consistent with this finding, the present study also demonstrated that miR-186-5p was downregulated in HG-treated AC16 cells, indicating the potential mechanism for expanding the therapeutic strategies. There is also evidence that miR-186 participates in modulating glucose uptake as well as activating cell cycle checkpoint under disease conditions (21). In the present study, HG-induced injury resulted in the decreased viability and appreciation rate as well as increased suppression ratio of AC16 cardiomyocytes, which is consistent with previous findings. These findings suggest that the (22) decreased level of miR-186-5p contributes to HG-induced cell damage.
Accumulating evidence demonstrates that myocardial cell death is considered as a major event in the progression of cardiovascular diseases, and suppression of myocardial cell death for apoptosis-specific signaling pathways results in a significant prevention of DCM (23,24). The activity of caspase-3 and apoptosis were markedly increased in a mouse model of streptozotocin-induced DCM (25) and in hyperglycemia-induced H9c2 cardiac myoblasts (26). In addition, there is also evidence that miR-186 transfection induced apoptosis, whereas anti-miR-186 transfection reduced apoptosis under disease conditions (27,28), indicating that miR-186 promotes apoptosis. Notably, Zhang et al (29) investigated the effects of miRNA-186 overexpression or inhibition on apoptosis in A549 cells, and demonstrated that the significant downregulation of miRNA-186 expression was associated with curcumin-induced apoptosis. Based on these research findings, it can be determined that miR-186 has a complex association with apoptosis and the varying roles of miR-186 in apoptosis may be associated with the regulation of different downstream signaling pathways or different subtypes. In the present study, whether changes in miR-186 level were associated with HG-induced apoptosis was also investigated. it was demonstrated that miR-186-5p mimic downregulated the expression of caspase-3 protein, whereas miR-186-5p inhibitor significantly upregulated the expression of caspase-3 protein in AC16 cardiomyocytes, which was consistent with the observation delineated by Sha et al (30). In addition, morphological changes of apoptotic cells induced by HG were ameliorated by miR-186-5p mimic transfection. The HG-induced upregulation of apoptosis rate was also ameliorated by miR-186-5p mimic, which suggests that the downregulation of miR-186-5p is associated with HG-induced apoptosis, likely through activation of caspase-3. However, a limitation of the present study is that the downstream target(s) of miRNA-186 were not further explored. In addition, performing in vivo experiments is necessary to elucidate the underlying molecular mechanism of this phenomenon.
In conclusion, the present study demonstrated that miR-186-5p was downregulated in HG-treated AC16 cells, miR-186-5p mimic reversed HG-exhibited cytotoxicity and apoptosis and miR-186-5p inhibitor increased apoptosis, which was the same effect as HG in AC16 cells. These findings suggest that miR-186-5p deletion ameliorates HG-induced injury, likely by modestly promoting apoptosis, which may be a potential mechanism for expanding the therapeutic strategies of DCM.
Acknowledgements
The present study was supported by Guangdong Natural Science Foundation (grant nos. 2015A030310359 and S2011010002620) and Science and Technology Planning Project of Guangdong in China (grant no. 2012A080202020).
References
- 1.Acar E, Ural D, Bildirici U, Sahin T, Yilmaz I. Diabetic cardiomyopathy. Anadolu Kardiyol Derg. 2011;11:732–737. doi: 10.5152/akd.2011.196. [DOI] [PubMed] [Google Scholar]
- 2.Bertoni AG, Tsai A, Kasper EK, Brancati FL. Diabetes and idiopathic cardiomyopathy: A nationwide case-control study. Diabetes Care. 2003;26:2791–2795. doi: 10.2337/diacare.26.10.2791. [DOI] [PubMed] [Google Scholar]
- 3.Kain V, Halade GV. Metabolic and biochemical stressors in diabetic cardiomyopathy. Front Cardiovasc Med. 2017;4:31. doi: 10.3389/fcvm.2017.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–671. doi: 10.1007/s00125-014-3171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isfort M, Stevens SC, Schaffer S, Jong CJ, Wold LE. Metabolic dysfunction in diabetic cardiomyopathy. Heart Fail Rev. 2014;19:35–48. doi: 10.1007/s10741-013-9377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramasarma T, Rafi M. A glucose-centric perspective of hyperglycemia. Indian J Exp Biol. 2016;54:83–99. [PubMed] [Google Scholar]
- 8.Feuvray D. Diabetic cardiomyopathy. Arch Mal Coeur Vaiss. 2004;97:261–265. [PubMed] [Google Scholar]
- 9.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 10.Vickers KC, Rye KA, Tabet F. MicroRNAs in the onset and development of cardiovascular disease. Clin Sci (Lond) 2014;126:183–194. doi: 10.1042/CS20130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClelland AD, Kantharidis P. microRNA in the development of diabetic complications. Clin Sci (Lond) 2014;126:95–110. doi: 10.1042/CS20130079. [DOI] [PubMed] [Google Scholar]
- 12.Tyagi AC, Sen U, Mishra PK. Synergy of microRNA and stem cell: A novel therapeutic approach for diabetes mellitus and cardiovascular diseases. Curr Diabetes Rev. 2011;7:367–376. doi: 10.2174/157339911797579179. [DOI] [PubMed] [Google Scholar]
- 13.León LE, Rani S, Fernandez M, Larico M, Calligaris SD. Subclinical detection of diabetic cardiomyopathy with MicroRNAs: Challenges and perspectives. J Diabetes Res. 2016;2016:6143129. doi: 10.1155/2016/6143129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asrih M, Steffens S. Emerging role of epigenetics and miRNA in diabetic cardiomyopathy. Cardiovasc Pathol. 2013;22:117–125. doi: 10.1016/j.carpath.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Bostjancic E, Zidar N, Glavac D. MicroRNA microarray expression profiling in human myocardial infarction. Dis Markers. 2009;27:255–268. doi: 10.1155/2009/641082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Juraver-Geslin HA, Durand BC. Early development of the neural plate: New roles for apoptosis and for one of its main effectors caspase-3. Genesis. 2015;53:203–224. doi: 10.1002/dvg.22844. [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz S, Canpolat U, Aydogdu S, Abboud HE. Diabetic cardiomyopathy; summary of 41 years. Korean Circ J. 2015;45:266–272. doi: 10.4070/kcj.2015.45.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Liu S. Role of microRNAs in the pathogenesis of diabetic cardiomyopathy. Biomed Rep. 2017;6:140–145. doi: 10.3892/br.2017.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.You Q, Wu Z, Wu B, Liu C, Huang R, Yang L, Guo R, Wu K, Chen J. Naringin protects cardiomyocytes against hyperglycemia-induced injuries in vitro and in vivo. J Endocrinol. 2016;230:197–214. doi: 10.1530/JOE-16-0004. [DOI] [PubMed] [Google Scholar]
- 21.Sun P, Hu JW, Xiong WJ, Mi J. miR-186 regulates glycolysis through Glut1 during the formation of cancer-associated fibroblasts. Asian Pac J Cancer Prev. 2014;15:4245–4250. doi: 10.7314/APJCP.2014.15.10.4245. [DOI] [PubMed] [Google Scholar]
- 22.Liang JL, Xiao DZ, Liu XY, Lin QX, Shan ZX, Zhu JN, Lin SG, Yu XY. High glucose induces apoptosis in AC16 human cardiomyocytes via macrophage migration inhibitory factor and c-Jun N-terminal kinase. Clin Exp Pharmacol Physiol. 2010;37:969–973. doi: 10.1111/j.1440-1681.2010.05420.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee Y, Gustafsson AB. Role of apoptosis in cardiovascular disease. Apoptosis. 2009;14:536–548. doi: 10.1007/s10495-008-0302-x. [DOI] [PubMed] [Google Scholar]
- 24.Cai L, Kang YJ. Cell death and diabetic cardiomyopathy. Cardiovasc Toxicol. 2003;3:219–228. doi: 10.1385/CT:3:3:219. [DOI] [PubMed] [Google Scholar]
- 25.Zheng D, Ma J, Yu Y, Li M, Ni R, Wang G, Chen R, Li J, Fan GC, Lacefield JC, Peng T. Silencing of miR-195 reduces diabetic cardiomyopathy in C57BL/6 mice. Diabetologia. 2015;58:1949–1958. doi: 10.1007/s00125-015-3622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun X, Chen RC, Yang ZH, Sun GB, Wang M, Ma XJ, Yang LJ, Sun XB. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food Chem Toxicol. 2014;63:221–232. doi: 10.1016/j.fct.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Sun KX, Jiao JW, Chen S, Liu BL, Zhao Y. MicroRNA-186 induces sensitivity of ovarian cancer cells to paclitaxel and cisplatin by targeting ABCB1. J Ovarian Res. 2015;8:80. doi: 10.1186/s13048-015-0207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He W, Feng J, Zhang Y, Wang Y, Zang W, Zhao G. microRNA-186 inhibits cell proliferation and induces apoptosis in human esophageal squamous cell carcinoma by targeting SKP2. Lab Invest. 2016;96:317–324. doi: 10.1038/labinvest.2015.134. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Du Y, Wu C, Ren X, Ti X, Shi J, Zhao F, Yin H. Curcumin promotes apoptosis in human lung adenocarcinoma cells through miR-186* signaling pathway. Oncol Rep. 2010;24:1217–1223. doi: 10.3892/or_00000975. [DOI] [PubMed] [Google Scholar]
- 30.Sha WG, Shen L, Zhou L, Xu DY, Lu GY. Down-regulation of miR-186 contributes to podocytes apoptosis in membranous nephropathy. Biomed Pharmacother. 2015;75:179–184. doi: 10.1016/j.biopha.2015.07.021. [DOI] [PubMed] [Google Scholar]