Abstract
Correction of barrier dysfunction and inflammation in acute lung injury (ALI) represents an important problem. Previous studies demonstrate barrier-protective and anti-inflammatory effects of bioactive lipid prostacyclin and its stable analog iloprost (ILO). We generated a phospholipase resistant synthetic phospholipid with iloprost attached at the sn-2 position (ILO-PC) and investigated its biological effects. In comparison to free ILO, ILO-PC caused sustained endothelial cell (EC) barrier enhancement, linked to more prolonged activation of Rap1 and Rac1 GTPases and their cytoskeletal and cell junction effectors: cortactin, PAK1, p120-catenin and VE-cadherin. ILO and ILO-PC equally efficiently suppressed acute, Rho GTPase-dependent EC hyper-permeability caused by thrombin. However, ILO-PC exhibited more sustained barrier-protective and anti-inflammatory effects in the model of chronic EC dysfunction caused by bacterial wall lipopolysacharide (LPS). ILO-PC was also more potent inhibitor of NFκB signaling and lung vascular leak in the murine model of LPS-induced ALI. Treatment with ILO-PC showed more efficient ALI recovery over 3 days after LPS challenge than free ILO. In conclusion, this study describes a novel synthetic phospholipid with barrier-enhancing and anti-inflammatory properties superior to existing prostacyclin analogs, which may be used as a prototype for future development of more efficient treatment for ALI and other vascular leak syndromes.
Introduction
Pulmonary endothelial cell (EC) barrier precisely regulates transport of fluids and macromolecules across the vessel wall, and EC barrier dysfunction developing during lung infection, injury, and other acute conditions may have devastating consequences such as pulmonary edema and acute respiratory distress syndrome (ARDS). Prostaglandin I2, or prostacyclin, is a product of cyclooxygenase-induced conversion of arachidonic acid. PGI2 exerts its biological effects via binding to G-protein coupled receptor IP, which stimulates intracellular production of cyclic AMP1. Prostacyclin is a relatively unstable prostaglandin product (in vivo half-life 40 sec), and therapeutic applications use its more stable synthetic analogs such as iloprost (ILO) with in vivo half-life 20–35 min. Prostacyclin-induced elevation of intracellular cAMP leads to smooth muscle relaxation2. This feature of prostacyclin is widely used for therapeutic correction of local and systemic circulation, as well as for relaxation of airway smooth muscle during asthma attacks.
The beneficial effects of prostacyclin extend beyond its cAMP-stimulated hemodynamic and antispastic effects. Prostacyclin and its synthetic analogs, iloprost and beraprost, exhibit direct protective action on the vascular endothelium3–6 and significantly reduce lung injury and inflammation induced by high tidal volume mechanical ventilation or bacterial pathogens7,8. Barrier-protective and anti-inflammatory properties of prostacyclin and ILO are associated with cAMP-mediated regulation of cytoskeletal dynamics, cell junctions, and inflammatory signaling. In addition to activation of cAMP-activated protein kinase (PKA), increased intracellular cAMP stimulates cAMP-activated guanine exchange factor Epac and its effector GTPase Rap1 which leads to recruitment of Rac-specific guanine nucleotide exchange factors Tiam1 and Vav2 and eventual activation of Rac1 GTPase4,5,9. Activation of Rac1 in endothelial cells stimulates peripheral actin polymerization, enforcement of peripheral F-actin rim and increases formation of VE-cadherin-based adherens junctions. These morphological and functional changes lead to enhancement of lung vascular endothelial barrier5. In turn, attenuation of agonist-induced EC permeability and inflammation by prostacyclin or ILO is mediated by Rap1/Rac1- and PKA-dependent inhibition of RhoA pathway5,10 known to trigger endothelial hyperpermeability and potentiates inflammatory response by enhancing NFkB inflammatory cascade11.
Previous studies in the models of acute lung injury and agonist-induced EC barrier dysfunction demonstrated potent and sustained protective effects of oxidized phospholipids bearing iso-prostanoid moiety12–14. In this study, we synthesized for the first time phospholipase-resistant synthetic phosphatidyl choline with ILO attached at the sn-2 position (ILO-PC). We compared barrier-protective and anti-inflammatory effects of free and phospholipid-bound ILO in human pulmonary EC treated with edemagenic agonist thrombin or bacterial wall lipopolysacharide LPS. Effects of ILO and ILO-PC were further investigated in the in vivo model of ALI caused by intratracheal instillation of LPS.
Results
Comparison of effects of ILO, ILO-PC, and OxPAPC on EC permeability
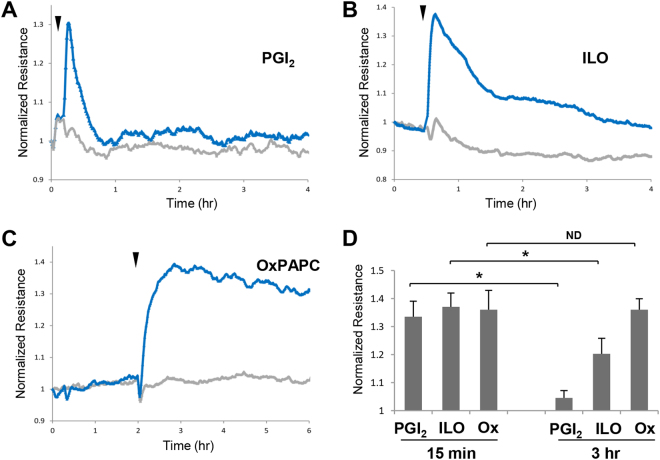
Previous studies demonstrated barrier-protective and anti-inflammatory effects of PGI2 (prostacyclin)4,10. However, relatively short (less than one minute) half-life of natural PGI2 after intravenous injection15 stimulated search for synthetic PGI2 analogs with more prolonged action, such as beraprost and iloprost (ILO) having a half-life about 30 min2,16. Stimulation of human pulmonary EC monolayers with PGI2 (Fig. 1A) caused rapid but transient increase in transendothelial electrical resistance (TER) reflecting barrier-enhancing response (Fig. 1A). In turn, ILO treatment caused more sustained TER elevation, which is in line with increased ILO stability (Fig. 1B). Effects of PGI2 and ILO were further compared with barrier enhancing effect of oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (OxPAPC) described in our previous studies12. In comparison to PGI2 and ILO, OxPAPC induced most sustained elevation of TER observed during 4 hrs after treatment (Fig. 1C,D).
Figure 1.
Effects of prostacyclin, iloprost and OxPAPC on pulmonary endothelial cell barrier properties. Pulmonary EC grown on microelectrodes were stimulated with vehicle or: (A) 0.5 µM PGI2; (B) 0.5 µM iloprost (ILO); and (C) 15 µg/ml OxPAPC. Changes in transendothelial resistance (TER) were monitored over 4-hr or 6-hr time periods. (D) Comparative measurements of TER were performed in EC monolayers stimulated with PGI2, ILO and OxPAPC after 15 min and 3 hrs of agonist treatment; n = 5; *p < 0.05. Data are expressed as mean ± SD. Biological replicates indicated by (n). Statistical significance by unpaired Student’s t-test.
Synthesis and characterization of ILO-PC effects on basal pulmonary EC barrier properties and cytoskeletal remodelling
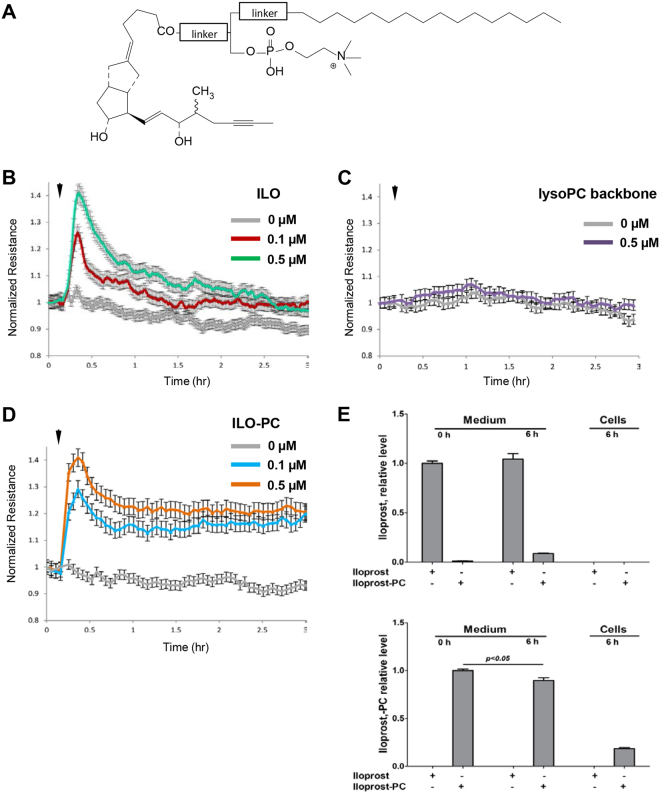
Previous study by our group demonstrated that barrier enhancing effects of OxPAPC were mediated by full length oxygenated products containing isoprostanoid-like moieties13. These moieties within natural OxPAPC in vivo are prone to cleavage by phospholipases such as lipoprotein-associated PAF-acetyl hydrolase17, which may inactivate OxPAPC barrier enhancing activity. On the other hand, presence of oxidatively fragmented molecular species in OxPAPC preparation has adverse effects on EC function13,17. In order to circumvent these limitations, we designed a novel synthetic phospholipid. The natural phospholipids contain fatty acids linked via ester bonds at both sn-1 and sn-2 positions to the glycerol backbone. The synthetic ILO-PC molecule has been designed with the ester bond at the sn-1 position and non-ester bond at the sn-2 position linking iloprost moiety rendering its resistance to phospholipase enzymatic activities. Schematic structure of ILO-PC is shown in Fig. 2A. TLC and mass spectrometry analysis confirmed purity of the compound (data not shown). ILO-PC biological activity in comparison to free ILO precursor was further tested in cell culture studies.
Figure 2.
Comparative analysis of time-dependent effects of ILO and ILO-PC on EC barrier. (A) Structure of synthetic phospholipid generated by conjugation of iloprost with lysophosphatidylcholine backbone using phospholipase-resistant linker. Agonist-induced EC barrier-enhancing response was evaluated by TER measurements. (B) Effect of 0.1 µM and 0.5 µM ILO. (C) Effect of 0.1 µM and 0.5 µM lyso-PC backbone used for generation of synthetic ILO-PC. (D) Effect of 0.1 µM and 0.5 µM ILO-PC. Shown are pooled TER data expressed as mean +/− SE of four independent measurements. (E) Mass Spectrometry analysis of ILO and ILO-PC stability in EC conditioned medium and incorporation in cells. Endothelial cells were treated with 0.4 μM ILO or ILO-PC, and the levels of both compounds were determined in the culture medium at the beginning of incubation and after six hours of treatment. Also, both compounds were determined in cell pellet collected after 6 hrs of agonist treatment. Upper panel: relative levels of ILO in cell culture medium at T = 0 hrs and culture medium and cells at T = 6 hrs. Data are expressed relative to the level of ILO in culture medium at T = 0 hrs (n = 4). Lower panel: relative levels of ILO-PC in cell culture medium at T = 0 hrs and culture medium and cells at T = 6 hrs. Data are expressed relative to the level of ILO-PC in culture medium at T = 0 hrs (n = 4). Data are expressed as mean ± SD. Biological replicates indicated by (n). Statistical significance by unpaired Student’s t-test.
Human pulmonary EC monolayers were treated in parallel studies with 0.1 μM and 0.5 μM of ILO or ILO-PC followed by measurements of TER for 3 hrs. EC stimulation with ILO caused dose-dependent, transient increase in TER reflecting EC barrier enhancement, with partial decline by 60 min after treatment (Fig. 2B). EC treatment with lysoPC backbone (0.5 μM) used for synthesis of ILO-PC did not affect EC permeability (Fig. 2C). In contrast to free ILO and lysoPC, cell treatment with synthetic ILO-PC induced sustained TER increase which was observed even after 3 hrs of stimulation (Fig. 2D). Dose-dependent responses to ILO and ILO-PC are also shown in Supplemental Figure 1. Of note, we did not observe further EC TER increase at ILO and ILO-PC concentrations greater than 0.5 µM.
The relative stability of ILO and ILO-PC in conditioned medium was analyzed using mass spectrometry approach described in Methods. Six-hour incubation with endothelial cells with ILO did not significantly decrease its level in conditioned medium (Fig. 2E). At the same time, 6-hr incubation with ILO-PC slightly decreased the levels of ILO-PC (less than 10%) in conditioned medium, but led to ILO-PC accumulation in the cell fraction. In contrast, such accumulation was not observed for free ILO. EC incubation with ILO-PC was also accompanied by release of free ILO (approximately 5%) in the culture medium.
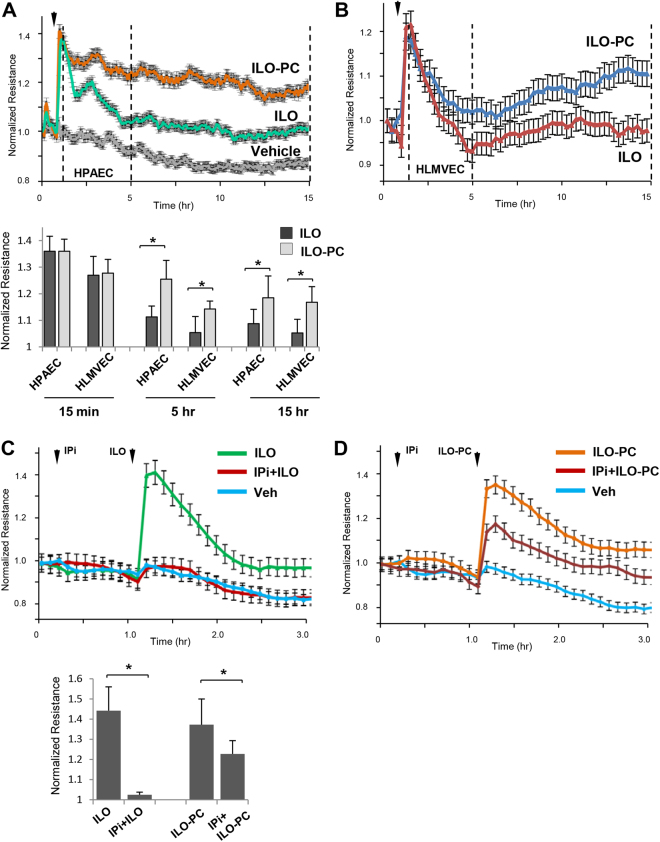
Comparative analysis of time-dependent effects of ILO and ILO-PC on pulmonary EC permeability using measurement of TER showed rapid TER increase in response to both, free ILO and ILO-PC (Fig. 3A). In contrast to sustained TER elevation caused by ILO-PC, the TER decline was observed in ILO-treated cells in the first hour. ILO-PC also caused more sustained barrier enhancing response in human lung microvascular EC (Fig. 3B). The pronounced difference in TER levels of ILO- and ILO-PC-stimulated microvascular and macrovascular EC monolayers was observed during the whole 18-hr period of observation (Fig. 3A,B). Bar graph depicts quantitative analysis of ILO- and ILO-PC-induced TER changes at the indicated time points after stimulation. EC response to prostacyclin and its stable analog, ILO, is mediated by G-protein coupled receptor IP. To evaluate IP role in ILO- ILO-PC-induced EC barrier enhancement, we pretreated human lung EC with IP pharmacological inhibitor, CAY10441 (IPi, 0.2 µM) followed by ILO or ILO-PC stimulation. IP inhibitor completely blocked ILO-induced EC barrier enhancing response (Fig. 3C), barrier response to ILO-PC was only attenuated by 50% (Fig. 3D). Bar graph depicts quantitative analysis of IPi suppressing effects on ILO- and ILO-PC-induced TER elevation at maximal point. These results, taken together with results of LC-MS-MS analysis of ILO and ILO-PC stability and differential retention in cell fraction, demonstrate novel functional effects resulting from ILO incorporation into phospholipid molecule.
Figure 3.
Barrier enhancing effects of ILO and ILO-PC on human lung macrovascular and microvascular EC and the role of prostacyclin receptor. TER measurements of: (A) Human pulmonary artery EC (HPAEC) and (B) human lung microvascular EC (HLMVEC) stimulated with ILO (0.5 µM) or ILO-PC (0.5 µM). Bar graph depicts quantitative analysis of ILO- and ILO-PC-induced TER changes at the time points after agonist indicated in panels A and B by vertical lines; n = 5; **p < 0.05. (C) HPAEC were pretreated with vehicle or prostacyclin receptor small molecule inhibitor (IPi) followed by stimulation with ILO. (D) HPAEC were pretreated with vehicle or prostacyclin receptor small molecule inhibitor CAY10441 (IPi, 0.2 µM) followed by stimulation with ILO-PC. Bar graph depicts quantitative analysis of IPi inhibitory effects on ILO- and ILO-PC-induced TER elevation at maximal point. Data are expressed as mean ± SD; n = 5; **p < 0.05. Statistical significance by two-way analysis of variance (ANOVA) and Tukey’s post hoc multiple-comparison test.
Effects of ILO and ILO-PC on Rap1 and Rac1 GTPase signalling
Treatment of pulmonary EC with prostacyclin or ILO elevates intracellular cyclic AMP levels and stimulates cAMP-dependent signaling by protein kinase A (PKA) and cAMP-induced Epac-Rap1-Rac1 GTPase signaling cascade5. Rap1 and Rac1 GTPases play a key role in establishment of cell-cell adhesions, enforcement of cortical actin cytoskeleton, and EC barrier enhancement18 We therefore investigated the time course of Rac1 and Rap1 activation in ILO- and ILO-PC-stimulated pulmonary EC using pulldown assays.
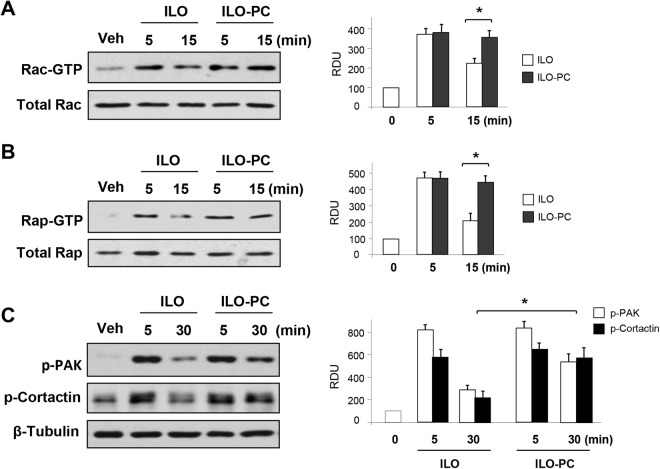
Both, ILO and ILO-PC caused prominent rapid activation of Rac1 (Fig. 4A) and Rap1 (Fig. 4B) GTPases, which was observed after 5 min of treatment. Rap1 and Rac1 activation partially declined after 15 min in ILO-treated cells, but remained significantly elevated in ILO-PC-treated ECs. These results indicate more sustained elevation of Rap1 and Rac1 activity caused by ILO-PC.
Figure 4.
ILO-PC induces more prolonged activation of Rac1 and Rap1 GTPase signaling in pulmonary EC. EC monolayers were stimulated with ILO (0.5 µM) or ILO-PC (0.5 µM) for indicated periods of time. (A) Rac-GTP pulldown assay. (A) Rap-GTP pulldown assay. (C) Phosphorylation of Rac1 targets PAK1 and cortactin was evaluated by western blot with corresponding phospho-specific antibodies. Membrane probing for β-tubulin was used as a normalization control. Bar graphs depict quantitative densitometry analysis of western blot data; n = 3; *p < 0.05. Data are expressed as mean ± SD. Biological replicates indicated by (n). Statistical significance by one-way analysis of variance (ANOVA) and Tukey’s post hoc multiple-comparison test.
Rac1-mediated PAK1 activation is manifested by PAK1 autophosphorylation at Thr428 19. In addition, tyrosine phosphorylation of another regulator of cortical actin polymerization, cortactin, is also promoted by activated Rac120. Consistent with effects on activation of Rac1, ILO and ILO-PC induced rapid phosphorylation of PAK1 and cortactin, which was transient in the ILO-stimulated EC but remained increased in ILO-PC-stimulated cells (Fig. 4C).
Effects of ILO and ILO-PC on EC adherens junctions
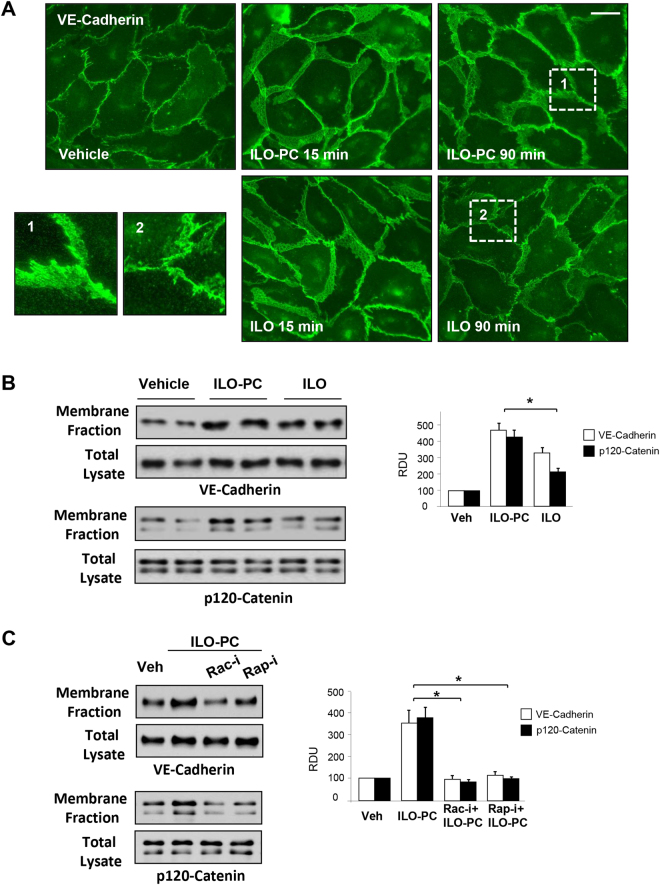
Remodeling of cell junctions and assembly of adherens junction complexes containing VE-cadherin and p120-, α-, β- γ-catenins is a key mechanism of EC barrier enhancement and barrier restoration21. We next characterized the time-dependent remodeling of adherens junctions using biochemical and imaging approaches. Immunofluorescence staining of control, ILO- and ILO-PC-stimulated EC monolayers showed that treatment with either ILO, or ILO-PC dramatically increased areas covered by adherens junctions by 15 min of stimulation, as detected by VE-cadherin-positive immunofluorescence staining (Fig. 5A). However, sustained enhancement of adherens junctions monitored after 90 min of agonist treatment was more pronounced in ILO-PC-stimulated EC monolayers. These data were supported by results of subcellular fractionation, which showed that both, ILO and ILO-PC increased accumulation of EC adherens junction proteins VE-cadherin and p120-catenin in the cell membrane fraction, which indicates increased AJ association. In contrast, this accumulation was less expressed in ILO-treated EC (Fig. 5B). ILO and ILO-PC treatment did not affect total levels of VE-cadherin and p120-catenin in cell lysates in these experiments. Inhibitory analysis using pharmacological inhibitors of Rac1 and Rap1 GTPases showed that, similarly to previously shown effects by free ILO5,6, ILO-PC-induced enhancement of cell junctions and accumulation of VE-cadherin and p120-catenin at cell membrane is mediated by Rap1 and Rac1 activities (Fig. 5C).
Figure 5.
Effects of ILO and ILO-PC on rapid and sustained enhancement of adherens junctions. (A) EC monolayers were incubated with 0.5 µM ILO or ILO-PC for 15 or 90 min. Increased areas of adherens junctions in response to agonist treatment were monitored by immunofluorescence staining of transmembrane adherens junction protein, VE-cadherin. Bar = 10 µm. Insets with higher magnification images show details of adherens junction structure. (B) Membrane accumulation of adherens junction proteins VE-cadherin and p120-catenin after 90-min stimulation with 0.5 µM ILO or ILO-PC detected by subcellular fractionation assay. (C) Membrane accumulation of VE-cadherin and p120-catenin caused by ILO or ILO-PC was analyzed in the presence or absence of Rac1 and Rap1 pharmacological inhibitors (30-min pre-treatment with 100 µM NSC23766 and 10 µM GGTI298, respectively). Probing of total lysates for VE-cadherin and p120-catenin was used as a normalization control. Bar graphs depict quantitative densitometry analysis of western blot data; n = 4; *p < 0.05. Data are expressed as mean ± SD. Biological replicates indicated by (n). Statistical significance by one-way analysis of variance (ANOVA) and Tukey’s post hoc multiple-comparison test.
Effects of ILO and ILO-PC in cell models of acute and chronic EC barrier dysfunction
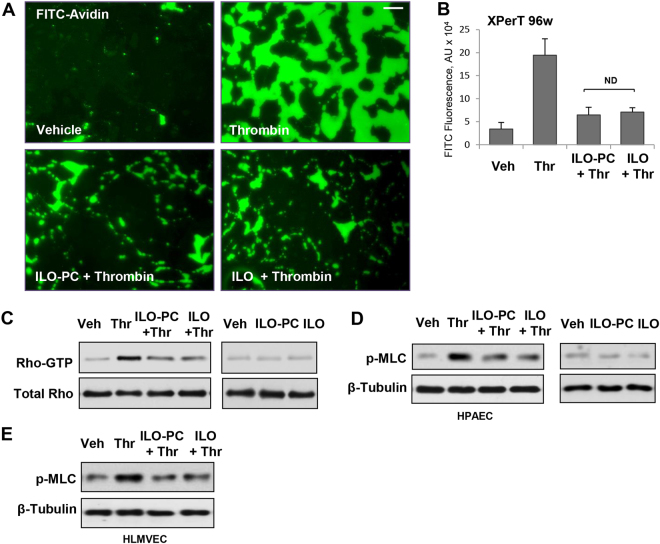
Thrombin-induced permeability in pulmonary vascular EC is a well-known rapid and transient response developing within 5–10 min and followed by complete recovery of EC barrier 40–50 min after thrombin addition22,23. Rapid permeability increase caused by thrombin is mediated by RhoA GTPase activation and MLC phosphorylation24. Pretreatment with both, ILO and ILO-PC effectively blocked the development of thrombin-induced EC permeability, which was evaluated in FITC-avidin permeability visualization assays (Fig. 6A) and fluorimetry-based quantitative assays in 96-well plates (Fig. 6B). Thrombin-induced increase in EC permeability developed within 5–15 min and was associated with rapid increase in RhoA activity (Fig. 6C) and MLC phosphorylation (Fig. 6D). Both, ILO and ILO-PC blunted thrombin-induced RhoA activation and MLC phosphorylation with comparable efficiency. Alone, ILO or ILO-PC did not activate RhoA signaling (Fig. 6C,D, right panels). Similar suppression of thrombin-induced MLC phospohorylation by free ILO and ILO-PC was observed in human lung microvascular EC (Fig. 6E).
Figure 6.
Effects of ILO and ILO-PC on thrombin-induced EC permeability and RhoA pathway activation. EC monolayers were incubated with 0.5 µM ILO or ILO-PC for 15 min prior to thrombin treatment (0.5 U/ml, 5 min). Visualization of FITC-avidin accumulation underneath EC monolayers was performed which reflects an extent of EC barrier dysfunction; bar = 20 µm (A). EC permeability for FITC-labeled avidin tracer was assessed by quantitative fluorimetry analysis described in Methods; n = 3; *p < 0.05; ND - no difference (B). Pulmonary EC were incubated with 0.5 µM ILO or ILO-PC for 15 min followed by treatment with thrombin or vehicle (C and D left panels). In separate control experiments, the cells were treated with 0.5 µM ILO or ILO-PC alone (C and D right panels). Activation of RhoA GTPase measured by pulldown assays after 5-min thrombin stimulation (C). Rho kinase mediated phosphorylation of myosin light chains (MLC) 15 min after thrombin addition was detected by western blot in human pulmonary artery EC (D) and human lung microvascular EC (E). Probing with antibody to β-tubulin was used as a normalization control. Results are representative of three independent experiments. Data are expressed as mean ± SD. Biological replicates indicated by (n). Statistical significance by one-way analysis of variance (ANOVA) and Tukey’s post hoc multiple-comparison test.
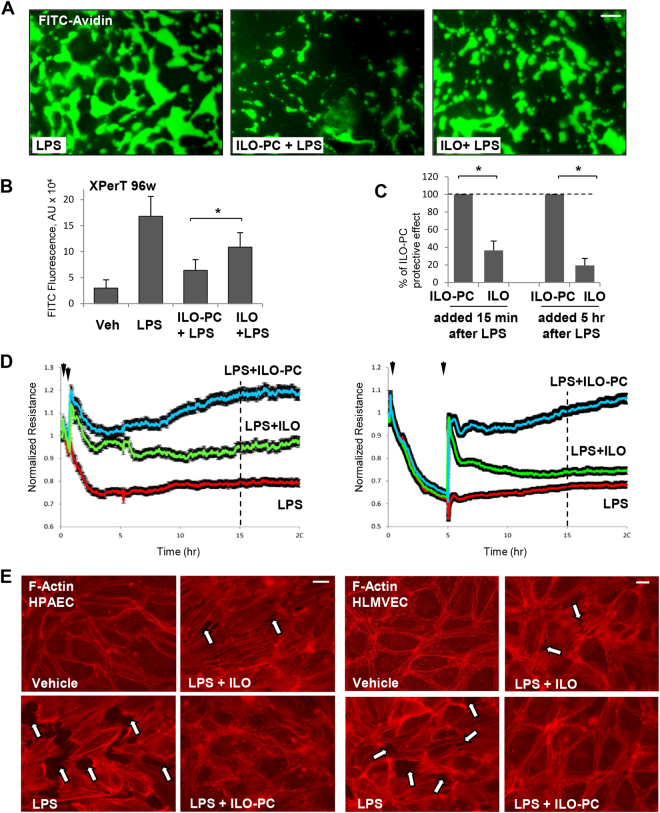
EC exposure to LPS causes gradual barrier disruption, which develops within 4–8 hrs and is accompanied by increased expression of inflammatory markers and cytokines25. This model was used in the following studies to evaluate effects of ILO and ILO-PC on EC barrier dysfunction in clinically relevant model of LPS-induced inflammation. In contrast to thrombin model of EC permeability, ILO-PC exhibited more pronounced inhibitory effect than free ILO in the model of chronic EC barrier dysfunction caused by LPS. Visualization of FITC-avidin penetration through EC monolayer challenged with LPS for 6 hrs shows better barrier-protective effects of ILO-PC co-treatment, as compared to treatment with free ILO (Fig. 7A). These results were further confirmed by quantitative analysis using 96-well format of this assay (Fig. 7B).
Figure 7.
Effects of ILO and ILO-PC on LPS-induced EC barrier dysfunction. Cells were treated with LPS (200 ng/ml) alone or in combination with 0.5 µM ILO or ILO-PC for 6 hrs. (A) Visulaization of LPS-induced FITC-avidin binding to substrate underlying EC monolayers reflecting extent of EC barrier dysfunction; bar = 20 µm. (B) Analysis of EC monolayer permeability for FITC-avidin was assessed by fluorimetry approach described in Methods; n = 4; *p < 0.05. (C) Comparative analysis of effects of ILO- and ILO-PC post-treatment on EC barrier after LPS challenge monitored by TER changes. Agonists were added 15 min or 5 hrs after HPAEC challenge with LPS, and analysis was performed at time points marked by vertical lines in panel D. Protective effect of ILO-PC post-treatment against LPS-induced EC barrier dysfunction at each time point was taken as 100%. Data are expressed as mean ± SD; n = 5; **p < 0.05. Statistical significance by two-way analysis of variance (ANOVA) and Tukey’s post hoc multiple-comparison test. (D) Effect of ILO and ILO-PC post-treatment on LPS-induced EC permeability. Pulmonary EC plated on microelectrodes were stimulated with LPS. Vehicle, ILO or ILO-PC was added 15 min (left panel) or 5 hrs after LPS addition (right panel). TER reflecting EC monolayer barrier properties was monitored over 20 hours. (E) Attenuation of LPS-induced formation of actin stress fibers and paracellular gaps (marked by arrows) by ILO and ILO-PC in human lung macrovascular (left panels, bar = 10 µm) and microvascular (right panels, bar = 5 µm) EC. F-actin was visualized by cell staining with Texas Red phalloidin. Results are representative of three independent experiments.
Protective effects of ILO and ILO-PC were also evaluated using TER measurements in the more clinically relevant setting of post-treatment, when agonists were added to EC monolayers 15 min or 5 hrs after LPS challenge. In both experimental settings, ILO-PC exhibited more potent protective effects than free ILO, which were registered after 15 hrs of agonist addition (Fig. 7C). Time-resolved analysis of ILO or ILO-PC effects on LPS-induced EC permeability using TER measurements (Fig. 7D) showed similar levels of rapid EC barrier enhancement by both ILO and ILO-PC in the first 15 mins of treatment. However, ILO-PC exhibited more sustained protection of EC barrier in LPS-stimulated EC than free ILO did. Sustained protective effects of ILO-PC were observed not only after 15 min-post-treatment, but also when ILO-PC was added 5 hrs after LPS challenge (Fig. 7C,D). Morphological analysis of ILO and ILO-PC effects on LPS-induced EC actin cytoskeleton remodeling and barrier disruption was further performed by visualization of F-actin using staining with Texas Red phalloidin. Treatment with ILO-PC for 2 hrs after 5-hr EC exposure to LPS caused complete restoration of EC monolayer integrity and disappearance of intercellular gaps, while protective effect of free ILO post-treatment was weaker, as evidenced by the presence of gaps in treated human lung macrovascular (left panels) and macrovascular (right panels) monolayers (Fig. 7E).
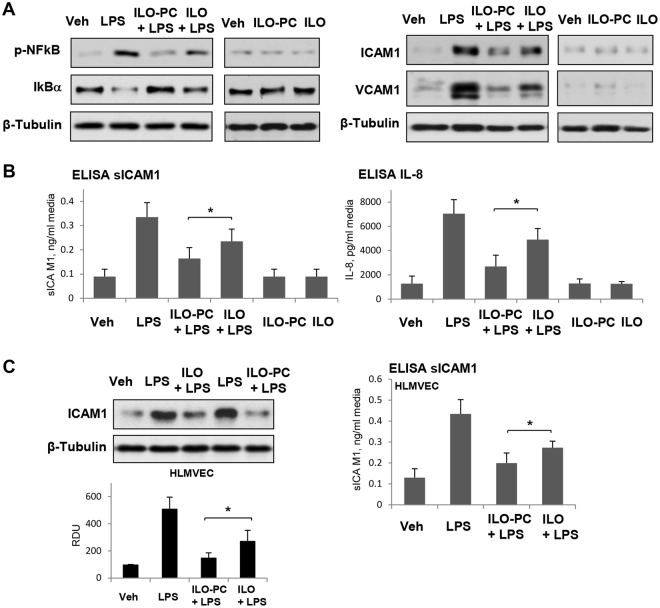
Activation of NFκB-mediated inflammatory signaling cascade is induced by various inflammatory stimuli including LPS and manifested by phosphorylation of NFκB subunit and degradation of inhibitory IκBα subunit. LPS-induced induced phosphorylation of NFκB and degradation of IκBα was attenuated by both, ILO and ILO-PC, although anti-inflammatory effect of ILO-PC was more pronounced (Fig. 8A, left panels).
Figure 8.
Effects of ILO and ILO-PC on LPS-induced inflammatory activation. Cells were treated with LPS (200 ng/ml) alone or in combination with 0.5 µM ILO or ILO-PC. In separate control experiments, the cells were treated with 0.5 µM ILO or ILO-PC alone. (A) IkBα degradation, NFkB phosphorylation (2 hrs after treatment), ICAM1 and VCAM1 expression (6 hrs after treatment) in EC stimulated with LPS, ILO, ILO-PC, or theirs combination was analyzed by western blot. Probing for β-tubulin was used as a normalization control. (B) The levels of soluble ICAM1 (sICAM1) and IL-8 in conditioned medium after 6 hrs of HPAEC co-treatment with LPS and ILO or ILO-PC was measured using ELISA assay. (C) Expression of cell-bound ICAM1 (left panel) and soluble ICAM1 in conditioned medium after 6 hrs of HLMVEC co-treatment with LPS and ILO or ILO-PC was measured by western blot and ELISA assays; n = 5, *P < 0.05. Data are expressed as mean ± SD. Biological replicates indicated by (n). Statistical significance by one-way analysis of variance (ANOVA) and Tukey’s post hoc multiple-comparison test.
Activation of vascular endothelium by LPS is known to stimulate NFκB-dependent expression of adhesion molecules ICAM1 and VCAM1 promoting increased neutrophil adhesion and neutrophil transmigration through EC monolayer thus leading to neutrophil recruitment to the inflamed lung tissue. EC treatment with both, ILO and ILO-PC inhibited LPS-induced expression of ICAM1 and VCAM1. However, in agreement with effects on NFκB suppression, ILO-PC was a more potent inhibitor of LPS-induced ICAM1 and VCAM1 expression than free ILO (Fig. 8A, right panels). ILO-PC was also a more potent inhibitor of LPS-activated secretion of soluble ICAM1 and IL-8 by EC exposed to LPS (Fig. 8B). More potent than ILO, anti-inflammatory effects of ILO-PC were also observed in lung microvascular EC (Fig. 8C).
These results clearly demonstrate more sustained and potent protective effects of ILO-PC, as compared to free ILO, against the endothelial barrier dysfunction and inflammatory activation induced by LPS in vitro. In the next experiments, we compared protective effects of free ILO and ILO-PC in the murine model of LPS-induced lung injury.
Effects of ILO and ILO-PC on LPS-induced lung injury
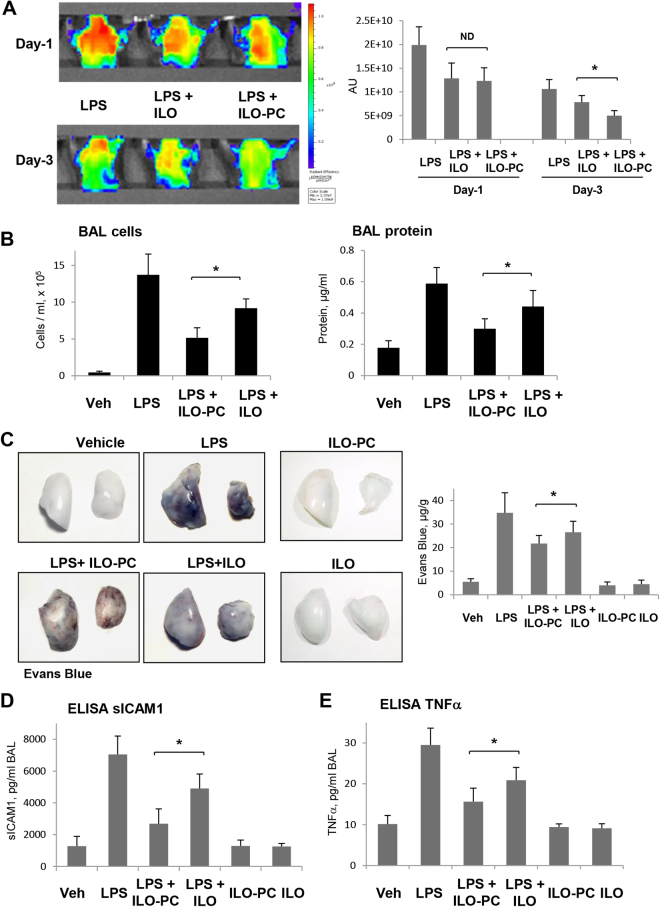
Mice were treated with LPS followed by concurrent ILO or ILO-PC intravenous injection followed by second injection 5 hrs after LPS challenge. We used non-invasive fluorescence optical imaging approach to evaluate effects of ILO and ILO-PC on LPS-induced lung vascular leak in same animals prospectively, 1 and 3 days after treatment. Angiosense 680 EX tracer was injected intravenously, and tracer accumulation analysis in the lungs reflecting ALI-associated lung vascular leak was performed in anesthetized animals as described in Methods. Angiosense 680 EX accumulation in the lungs was maximal at day-1 after LPS injection and subsided at day-3 (Fig. 9A). Tracer clearance from the injured lungs reflecting lung vascular barrier repair was more evident in the LPS-challenged lungs with ILO-PC treatment as compared to lungs treated with free ILO.
Figure 9.
Effects of ILO and ILO-PC on LPS-induced lung injury and barrier dysfunction. (A) Live imaging analysis of lung vascular barrier dysfunction after LPS intratracheal injection with and without intravenous administration of ILO or ILO-PC. LPS-induced accumulation of fluorescent Angiosense 680 EX imaging agent in the lungs of same animals was detected by Xenogen IVIS 200 Spectrum imaging system 1 day and 3 days after LPS challenge and presented in arbitrary colors. Bar graph represents quantitative analysis of imaging; n = 4; *p < 0.05. (B) Analysis of protein concentration and total cell count was performed in BAL samples obtained from control and experimental groups after LPS challenge; n = 4; *p < 0.05. (C) Evans blue dye (30 ml/kg, i/v) was injected 2 hr before termination of the experiment. Photographs depict Evans blue accumulation in the lung tissue illustrating increased lung vascular permeability. Bar graph depicts quantitative analysis of Evans blue labeled albumin extravasation performed by spectrophotometric analysis of Evans blue extracted from the lung tissue samples; n = 4 per condition; *p < 0.05. The levels of soluble ICAM1 (D) and TNFα (E) were measured in BAL samples using ELISA assay; n = 4; *p < 0.05. Data are expressed as mean ± SD. Biological replicates indicated by (n). Statistical significance by one-way analysis of variance (ANOVA) and Tukey’s post hoc multiple-comparison test.
Prominent lung inflammatory response caused by LPS was accompanied by increase in cell count and protein content in bronchoalveolar lavage (BAL) samples (Fig. 9B). These BAL changes still observed at day-3 after LPS treatment were significantly attenuated by ILO and ILO-PC, although ILO-PC displayed more potent protective effect. In consistence with the effects on BAL parameters, treatment with ILO or ILO-PC suppressed Evans Blue dye extravasation in the lung resulting from lung vascular leak. ILO-PC again was more potent in inhibiting LPS-induced vascular leak in comparison to ILO (Fig. 9C).
LPS challenge also significantly increased soluble ICAM1 and TNFα levels detected in BAL samples. In turn, intravenous injection of ILO or ILO-PC significantly attenuated production of TNFα and soluble ICAM1, and ILO-PC demonstrated more pronounced inhibitory effects on these parameters of inflammation (Fig. 9D). Altogether, these data demonstrate more potent protective effects of ILO-PC over free ILO against lung inflammation and barrier dysfunction induced by vasoactive mediators and bacterial pathogens.
Discussion
This study describes for the first time the biological properties of a phospholipase resistant synthetic phospholipid with incorporated iloprost moiety. Generation of such compound was inspired by previous findings by our group, which demonstrated pronounced and sustained barrier-enhancing and anti-inflammatory effects of isoprostanee-containing products of membrane phospholipid oxidation13. These effects of OxPAPC exceeded protective effects of free prostaglandin I2 or iloprost (Fig. 1). However, uncertain stability and variable composition of oxidized phospholipid preparations represent a serious obstacle in potential therapeutic application of this group of bioactive lipids. These limitations motivated our efforts in design of an individual phospholipid molecule bearing iloprost, a modified prostacyclin characterized by prolonged action. The results of this study show that incorporation of ILO into synthetic phospholipid did not compromise ILO beneficial effects on vascular endothelial barrier function and its anti-inflammatory properties, but similarly to OxPAPC, led to prolonged EC barrier enhancement and more pronounced anti-inflammatory effect. The lysophospholipid backbone used for ILO-PC synthesis did not enhance EC barrier when added alone at equivalent concentration (Fig. 1), but instead, at higher concentrations it caused modest EC permeability increase (data not shown). These results suggest that ILO conjugation with phospholipid scaffold adds new quantitative and qualitative characteristics to the ILO-PC molecule.
Similarly to free ILO, ILO-PC activated Rac1 and Rap1 GTPases and inhibited thrombin-induced RhoA pathway of EC permeability. In consistence with rapid nature of thrombin-induced EC permeability response associated with acute and transient RhoA activation, ILO-PC did not exhibit superior barrier protective effects over free ILO.
Both ILO and ILO-PC attenuated more sustained LPS-induced barrier dysfunction triggered by inflammatory mechanisms manifested by increased IκBα degradation and NFκB phosphorylation. Such attenuation of inflammatory signaling suppressed LPS-induced ICAM1 and VCAM1 expression in cultured human pulmonary EC and LPS-challenged lungs. These findings strongly suggest that ILO and ILO-PC exhibit their beneficial effects via similar vascular protective and anti-inflammatory mechanisms. However, synthetic ILO-PC revealed several advantages over free ILO or OxPAPC including higher potency and longer effect. First, ILO-PC causes sustained EC barrier enhancement at 50–200 ng/ml concentration range compared to 5–20 µg/ml typical concentration range for OxPAPC. In comparison to free ILO, ILO-PC at the same molar concentration caused more sustained barrier enhancing effect. Second, ILO-PC exhibited more potent barrier-protective and anti-inflammatory effects in the cell and animal models of LPS-induced injury. In addition to analysis of BAL parameters of lung injury, we monitored the time course of lung vascular leak in ILO- and ILO-PC-treated mice with LPS-induced ALI using a non-invasive live imaging approach. The results demonstrated a significant acceleration of lung recovery in mice treated with ILO-PC in comparison to ILO-treated counterparts. These results were consistent with stronger inhibition of LPS-induced Evans blue extravasation reflecting lung vascular leak, accumulation of inflammatory cells, cytokines, and protein levels in BAL samples of ILO-PC-treated mice (Fig. 7).
More potent ILO-PC effects were associated with more sustained activation of Rap1/Rac1 signaling and their cytoskeletal targets resulting in preservation of actin cytoskeletal arrangement and pulmonary EC barrier integrity. This pathway drives barrier-protective and anti-inflammatory effects of prostacyclin and its stable analogs4,6,8. Prolonged Rap1/Rac1 activation may also explain better efficiency of ILO-PC in clinically relevant scenario of ILO-PC post-treatment after LPS challenge (Fig. 6).
Sustained EC barrier enhancement and more prolonged activation of Rac1 and Rap1 signaling by ILO-PC is an important finding which warrants further investigation. Interestingly, in contrast to HPAEC, HLMVEC developed induced a bi-phasic barrier enhancement response to ILO-PC, whereby the increase in TER decreased to baseline before increasing again. At present we cannot unequivocally explain the differences in dynamics of barrier enhancement response to ILO-PC by microvascular (HLMVEC) and microvascular (HPAEC) cells, and this aspect deserves further investigation. One may speculate that these differences may reflect: a) donor-to-donor HLMVEC variability in the magnitude of initial spike and following sustained phase; b) phenotypic and functional differences between micro- and microvascular EC reflecting regional heterogeneity of vascular endothelium; or c) differences in gene expression activation between micro-and macrovascular EC developing at a later time point after ILO-PC stimulation.
MS analysis of ILO and ILO-PC in conditioned medium shows similar stability of both compounds. However, we observed retention of ILO-PC, but not free ILO, in EC samples. These findings suggest the increased ILO-PC interaction with cell (in extracellular or intracellular compartment) which may be a factor defining strong long-lasting physiological effect exhibited by ILO-PC. Alternatively, our data showing more sustained barrier enhancing response to ILO-PC and only partial attenuation of this response by IP inhibitor suggest the possibility of less efficient desensitization of IP receptor by ILO-PC, as compared to free ILO, which would lead to more sustained activation of IP receptor signaling. Finally, partial attenuation of ILO-PC response by IP inhibitor, taken together with selective cell uptake of ILO-PC, but not free ILO, indicate a possibility of additional intracellular ILO-PC signaling contributing to EC barrier enhancement and inhibition of LPS-induced EC inflammation. Among factors contributing to more sustained effects of ILO-PC as compared to free ILO may be also activation of additional mechanisms leading to Rap1 and Rac1 activation. As illustration of latter point, oxPAPC, which contains esterified isoprostanes and thus structurally resembles ILO-PC, engages cell membrane-associated pool of GRP78, a chaperone which is increasingly recognized as cell surface signaling receptor26 triggering, inter alia, Rac1-dependent mechanism of EC barrier enhancement27. Thus, precise characterization of unique pharmacodynamics properties and molecular mechanisms underlying long lasting ILO-PC effects is an important follow up of this study and a subject of future research.
In conclusion, we generated and characterized biological properties of a prototype molecule representing a new class of synthetic phospholipids with anti-inflammatory and barrier-protective properties. Our results demonstrate a new way to improve in vitro and in vivo efficiency of barrier-protective and anti-inflammatory prostaglandin analogs. This novel class of molecules may accelerate recovery in ALI and other inflammatory conditions by improvement of lung vascular barrier function and modulation of innate immune mechanisms and inflammatory signaling cascades.
Materials and Methods
Reagents and cell culture
1-Palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (PAPC) was obtained from Avanti Polar Lipids (Alabaster, AL) and oxidized by exposure of dry lipid to air as previously described28–30. The extent of oxidation was monitored by positive ion electrospray mass spectrometry (ESI-MS)30. PGI2, iloprost, and IP receptor inhibitor CAY10441 were obtained from Cayman Chemical (Ann Arbor, MI). Rac1 inhibitor NSC23766 and Rap1 inhibitor GGTI298 were obtained from EMD Millipore. Antibodies to Rac1, Rap1, and p120-catenin were obtained from BD Transduction Laboratories (San Diego, CA); RhoA, VE-cadherin, ICAM1 and VCAM1 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA); phospho-MLC, phospho-PAK1, phospho-cortactin, phospho-NFκB, and IκBα antibodies were obtained from Cell Signaling (Beverly, MA); antibody to β-tubulin was obtained from Sigma (St. Louis, MO). Texas Red phalloidin and Alexa Flour 488 conjugated secondary antibodies were purchased form Molecular Probes (Eugene, OR). Unless specified, biochemical reagents were obtained from Sigma (St. Louis, MO). Solvents used were of analytical grade. Human pulmonary artery endothelial cells (HPAEC) were obtained from Lonza (East Rutherford, NJ), cultured according to manufacturer’s protocol, and used at passages 5–9.
ILO-PC synthesis
Amino group of 1-O-hexadecyl-2-amino-2-deoxy-sn-glycerol (Bachem, Switzerland) was protected by the Boc group. The protected amino-glycerol was phosphitylated and used for introduction of the choline polar head group as described previously31. After the cleavage of the Boc group, the free amino-group was acylated by iloprost succinimide ester. The latter was produced from iloprost (Cayman Chemicals, Ann Arbor, MI) and N-hydroxysuccinimide (Sigma-Aldrich), using EDC (Pierce) as a coupling agent31. Purification of the synthesized ILO-PC was performed on an SPE cartridge (SupelClean LC18, Sigma-Aldrich, St. Louis, MO) using a gradient of methanol in water. The purity of the synthesized product was checked by TLC on Kieselgel 60 plates in chloroform-methanol-water (100:50:10, v/v/v) by staining with 10% CuSO4/8.5% H3PO4 and followed by heating to 120 °C as well as by mass-spectrometry using Sciex 6500 QTRAP triple quadrupole ion trap hybrid mass spectrometer interfaced with Agilent 1290 UHPLC system. Synthetic ILO-PC was dissolved in chloroform and stored at −70 °C. Phospholipid concentration was determined by measurement of organic phosphate as described previously32.
Mass Spectrometry analysis of ILO and ILO-PC in conditioned medium and cell samples
Standards and reagents
Methanol, water and chloroform (LC/MS or HPLC grade) were purchased from Thermo Fisher Scientific (Waltham, MA). Lipoxin A4-d5 (LXA4-d5) was purchased from Cayman Chemicals (Ann Arbor, MI), 21:0/22:6-phosphatidylcholine (21:0/22:6-PC) was purchased from Avanti Polar Lipids (Alabaster, AL). The standards were dissolved in methanol and stored at −80 °C.
Lipid extraction and sample preparation for LC-MS/MS
Medium and cellular lipids were extracted by modified Bligh and Dyer procedure33 with the use of 2% formic acid for phase separation. Internal standards (LXA4-d5 and 21:0/22:6-PC) were added at the beginning of the extraction process.
UHPLC-ESI-MS/MS analysis of iloprost and iloprost-PC
The content of iloprost and iloprost-PC were determined by liquid chromatography electrospray ionization tandem mass spectrometry using Sciex 6500QTRAP mass spectrometer coupled with Shimadzu Nexera X2 UHPLC system. Iloprost was detected in negative ions mode as a transition from the m/z 359.1 to the m/z 231.2. Lipoxin A4-d5 was used as the internal standard and detected as a transition from the m/z 356.2 to the m/z 115.0. Chromatography was performed using Ascentis Express RP-Amide 2.7 µm 2.1 × 50 mm column and gradient elution from methanol:water:formic acid (35:65:0.5) to methanol:chloroform:water:formic acid (90:10:0.5:0.5). Iloprost-PC was detected in positive ions mode as a transition from the m/z 823.8 to the m/z 184.1; 21:0/22:6-PC (detected as a transition from the m/z 876.6 to the m/z 184.1) was used as the internal standard for the quantitation of iloprost-PC. Chromatography was performed on the same column using a gradient elution from methanol:water:formic acid (65:35:0.5, 5 mM ammonium formate) to methanol:chloroform:water:formic acid (90:10:0.5:0.5, 5 mM ammonium formate). Standard curves of variable amounts of analytes versus fixed amount of corresponding internal standard were created for the absolute quantitation of iloprost and iloprost-PC.
Measurements of endothelial monolayer permeability
The cellular barrier properties were analyzed by measurements of transendothelial electrical resistance (TER) across confluent human pulmonary artery endothelial monolayers using an electrical cell-substrate impedance sensing system (Applied Biophysics, Troy, NY) as previously described34,35. Endothelial permeability to macromolecules was monitored by permeability visualization assay (XperT)36 available from Millipore (Vascular Permeability Imaging Assay, cat. #17–10398). The method is based on high affinity binding of avidin-conjugated FITC-labeled tracer added in cell culture medium during agonist stimulation to the biotinylated ligand in the substrate coating underlying EC monolayers. In permeability visualization experiments, cells grown on biotinylated gelatin-coated glass coverslips were fixed with 3.7% formaldehyde in PBS (10 min, room temperature), and imaging of areas with substrate-bound FITC-avidin was performed.
GTPase activation assays
Rac, Rap and Rho activation was evaluated in pulldown assays using agarose beads with immobilized PAK1-PBD, Ral GDS-RBD and rhotekin, respectively, as described elsewhere37. The levels of activated small GTPases bound to beads and evaluated by Western blot analysis were normalized to total Rac, Rap and Rho levels.
Immunofluorescence
Endothelial monolayers plated on glass cover slips were subjected to immunofluorescence staining with VE-cadherin antibody as described previously38. Texas Red phalloidin was used to visualize F-actin. After immunostaining, slides were analyzed using a Nikon video imaging system (Nikon Instech Co., Tokyo, Japan). Images were processed with Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA) software.
Differential protein fractionation and immunoblotting
Confluent HPAEC were stimulated with thrombin; cytosolic and membrane fractions were isolated using subcellular protein fractionation kit (Thermo Fisher Scientific, Rockford, IL) according to manufacturer protocol. For analysis of protein phosphorylation profile, cells were stimulated, then lysed, and protein extracts were separated by SDS-PAGE, transferred to polyvinylidene fluoride (PVDF) membrane, and probed with specific antibodies. Equal protein loading was verified by reprobing membranes with antibody to β-tubulin or specific protein of interest. The relative intensities of immunoreactive protein bands (RDU, relative density units) were analyzed and quantified by scanning densitometry using Image Quant software (Molecular Dynamics, Sunnyvale, CA).
Measurement of cytokines and chemokines
For cytokine measurements in preconditioned medium of human pulmonary EC cultures, supernatants from treated EC were collected and centrifuged to remove debris. IL-8 and soluble ICAM1 (sICAM1) levels were determined by ELISA (R&D Systems, Minneapolis, MN) following manufacturer’s protocol. The concentrations of sICAM1 and TNFα in mouse bronchoalveolar lavage (BAL) fluid samples were measured using mouse-specific ELISA kits (R&D Systems, Minneapolis, MN). Absorbance was read at 450 nm within 30 min in Victor X5 Multilabel Plate Reader (Perkin Elmer, Waltham, MA).
Animal studies
All animal care and treatment procedures were approved by the University of Chicago Institutional Animal Care and Use Committee. Eight-week old C57Bl/6j mice were purchased from Jackson Laboratories (Bar Harbor, ME). Animals were handled according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Bacterial lipopolysaccharide (LPS, 0.63 mg/kg body wt; Escherichia coli O55:B5) or sterile water was injected intratracheally in a small volume (20–30 µl) using a 20-gauge catheter (Exelint International, Los Angeles, CA). Iloprost (10 μg/kg), ILO-PC (10 μg/kg) or sterile saline solution was administrated concurrently and 5 hrs after LPS instillation by intravenous injection in the external jugular vein. Animals were sacrificed at day-1 or day-3 by exsanguination under anesthesia. BAL was performed using 1 ml of sterile Hanks balanced salt buffer and measurements of cell count and protein concentration were conducted as previously described39. For analysis of LPS-induced lung vascular leak, Evans blue dye (30 ml/kg) was injected into the external jugular vein 2 hrs before termination of the experiment. In vivo optical imaging: Mice were injected with 100 µl of 2 nmol Angiosense 680 EX (a vascular fluorescent blood pool imaging agent purchased from PerkinElmer, Boston, MA), intravenously via tail vein. Fluorescence optical imaging was performed in the Integrated Small Animal Imaging Research Resource (iSAIRR) at the University of Chicago using Xenogen IVIS 200 Spectrum (Caliper Life Sciences. Alameda, CA). Mice were exposed to isoflurane anesthesia with O2 through the gas anesthesia manifold and placed on the imaging stage. Acquisition and image analysis were performed with Living Image 4.3.1 Software.
Statistical analysis
Results are expressed as means ± SD of three to five independent experiments. Stimulated samples were compared with controls by unpaired Student’s t-test. For multiple-group comparisons, one-way analysis of variance (ANOVA) and Tukey’s post hoc multiple-comparison test were used. P < 0.05 was considered statistically significant.
Electronic supplementary material
Author Contributions
Study conception and design: O.O., A.B., K.B., V.B.; Acquisition of data: O.O., N.S., Y.T., G.G., F.M., E.B.; Analysis and interpretation of data: O.O., F.M., E.B., A.B., K.B, V.B.; Drafting of manuscript: O.O., A.B., K.B.; Critical revision: O.O., A.B., K.B, V.B. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-19197-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol. 2004;36:1187–1205. doi: 10.1016/j.biocel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Melian EB, Goa KL. Beraprost: a review of its pharmacology and therapeutic efficacy in the treatment of peripheral arterial disease and pulmonary arterial hypertension. Drugs. 2002;62:107–133. doi: 10.2165/00003495-200262010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Baumer Y, Drenckhahn D, Waschke J. cAMP induced Rac 1-mediated cytoskeletal reorganization in microvascular endothelium. Histochem Cell Biol. 2008;129:765–778. doi: 10.1007/s00418-008-0422-y. [DOI] [PubMed] [Google Scholar]
- 4.Fukuhara S, et al. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birukova AA, et al. Prostaglandins PGE2 and PGI2 promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res. 2007;313:2504–2520. doi: 10.1016/j.yexcr.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birukova AA, Fu P, Xing J, Birukov KG. Rap1 mediates protective effects of iloprost against ventilator induced lung injury. J Appl Physiol. 2009;107:1900–1910. doi: 10.1152/japplphysiol.00462.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birukova AA, Fu P, Xing J, Cokic I, Birukov KG. Lung endothelial barrier protection by iloprost in the 2-hit models of ventilator-induced lung injury (VILI) involves inhibition of Rho signaling. Transl Res. 2010;155:44–54. doi: 10.1016/j.trsl.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birukova AA, et al. Iloprost improves endothelial barrier function in lipopolysaccharide-induced lung injury. Eur Respir J. 2013;41:165–176. doi: 10.1183/09031936.00148311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579:4966–4972. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- 10.Birukova AA, et al. Prostacyclin post-treatment improves LPS-induced acute lung injury and endothelial barrier recovery via Rap1. Biochim Biophys Acta. 2015;1852:778–791. doi: 10.1016/j.bbadis.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Essler M, Staddon JM, Weber PC, Aepfelbacher M. Cyclic AMP blocks bacterial lipopolysaccharide-induced myosin light chain phosphorylation in endothelial cells through inhibition of Rho/Rho kinase signaling [In Process Citation] J Immunol. 2000;164:6543–6549. doi: 10.4049/jimmunol.164.12.6543. [DOI] [PubMed] [Google Scholar]
- 12.Birukov KG, et al. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res. 2004;95:892–901. doi: 10.1161/01.RES.0000147310.18962.06. [DOI] [PubMed] [Google Scholar]
- 13.Birukova AA, et al. Fragmented oxidation products define barrier disruptive endothelial cell response to OxPAPC. Transl Res. 2013;161:495–504. doi: 10.1016/j.trsl.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meliton A, et al. Oxidized phospholipids protect against lung injury and endothelial barrier dysfunction caused by heat-inactivated Staphylococcus aureus. Am J Physiol Lung Cell Mol Physiol, ajplung. 2015;00248:02014. doi: 10.1152/ajplung.00248.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cawello W, Schweer H, Muller R, Bonn R, Seyberth HW. Metabolism and pharmacokinetics of prostaglandin E1 administered by intravenous infusion in human subjects. European journal of clinical pharmacology. 1994;46:275–277. doi: 10.1007/BF00192562. [DOI] [PubMed] [Google Scholar]
- 16.Hildebrand M. Pharmacokinetics and tolerability of oral iloprost in thromboangiitis obliterans patients. European journal of clinical pharmacology. 1997;53:51–56. doi: 10.1007/s002280050336. [DOI] [PubMed] [Google Scholar]
- 17.McIntyre TM. Bioactive oxidatively truncated phospholipids in inflammation and apoptosis: formation, targets, and inactivation. Biochim Biophys Acta. 2012;1818:2456–2464. doi: 10.1016/j.bbamem.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pannekoek WJ, Kooistra MR, Zwartkruis FJ, Bos JL. Cell-cell junction formation: The role of Rap1 and Rap1 guanine nucleotide exchange factors. Biochim Biophys Acta. 2009;1788:790–796. doi: 10.1016/j.bbamem.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Bokoch GM. Biology of the p21-Activated Kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 20.Head JA, et al. Cortactin tyrosine phosphorylation requires Rac1 activity and association with the cortical actin cytoskeleton. Mol Biol Cell. 2003;14:3216–3229. doi: 10.1091/mbc.E02-11-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dejana E, Orsenigo F, Molendini C, Baluk P, McDonald DM. Organization and signaling of endothelial cell-to-cell junctions in various regions of the blood and lymphatic vascular trees. Cell Tissue Res. 2009;335:17–25. doi: 10.1007/s00441-008-0694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birukova AA, Tian X, Tian Y, Higginbotham K, Birukov KG. Rap-afadin axis in control of Rho signaling and endothelial barrier recovery. Mol Biol Cell. 2013;24:2678–2688. doi: 10.1091/mbc.E13-02-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holinstat M, et al. Suppression of RhoA activity by focal adhesion kinase-induced activation of p190RhoGAP: role in regulation of endothelial permeability. J Biol Chem. 2006;281:2296–2305. doi: 10.1074/jbc.M511248200. [DOI] [PubMed] [Google Scholar]
- 24.van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res. 2000;87:335–340. doi: 10.1161/01.RES.87.4.335. [DOI] [PubMed] [Google Scholar]
- 25.Togbe D, et al. Toll-like receptor and tumour necrosis factor dependent endotoxin-induced acute lung injury. Int J Exp Pathol. 2007;88:387–391. doi: 10.1111/j.1365-2613.2007.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misra UK, Deedwania R, Pizzo SV. Binding of activated alpha2-macroglobulin to its cell surface receptor GRP78 in 1-LN prostate cancer cells regulates PAK-2-dependent activation of LIMK. J Biol Chem. 2005;280:26278–26286. doi: 10.1074/jbc.M414467200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birukova AA, et al. GRP78 is a novel receptor initiating a vascular barrier protective response to oxidized phospholipids. Mol Biol Cell. 2014;25:2006–2016. doi: 10.1091/mbc.E13-12-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bochkov VN, et al. Oxidized phospholipids stimulate tissue factor expression in human endothelial cells via activation of ERK/EGR-1 and Ca(++)/NFAT. Blood. 2002;99:199–206. doi: 10.1182/blood.V99.1.199. [DOI] [PubMed] [Google Scholar]
- 29.Leitinger N, et al. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc Natl Acad Sci USA. 1999;96:12010–12015. doi: 10.1073/pnas.96.21.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson AD, et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;272:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 31.Oskolkova OV, Watanabe Y, Nakatomi M. Synthesis of PI(3,4,5)P-3 with unsaturated and saturated fatty acid chains. Tetrahedron. 1999;55:9743–9754. doi: 10.1016/S0040-4020(99)00551-7. [DOI] [Google Scholar]
- 32.Broekhuyse RM. Phospholipids in tissues of the eye. I. Isolation, characterization and quantitative analysis by two-dimensional thin-layer chromatography of diacyl and vinyl-ether phospholipids. Biochim Biophys Acta. 1968;152:307–315. doi: 10.1016/0005-2760(68)90038-6. [DOI] [PubMed] [Google Scholar]
- 33.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian journal of biochemistry and physiology. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- 34.Birukova AA, et al. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2006;290:L540–548. doi: 10.1152/ajplung.00259.2005. [DOI] [PubMed] [Google Scholar]
- 35.Birukova AA, et al. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J. 2004;18:1879–1890. doi: 10.1096/fj.04-2328com. [DOI] [PubMed] [Google Scholar]
- 36.Dubrovskyi O, Birukova AA, Birukov KG. Measurement of local permeability at subcellular level in cell models of agonist- and ventilator-induced lung injury. Lab Invest. 2013;93:254–263. doi: 10.1038/labinvest.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian Y, et al. Hepatocyte growth factor-induced Asef-IQGAP1 complex controls cytoskeletal remodeling and endothelial barrier. J Biol Chem. 2015;290:4097–4109. doi: 10.1074/jbc.M114.620377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birukova AA, Malyukova I, Poroyko V, Birukov KG. Paxillin - {beta}-catenin interactions are involved in Rac/Cdc42-mediated endothelial barrier-protective response to oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol. 2007;293:L199–211. doi: 10.1152/ajplung.00020.2007. [DOI] [PubMed] [Google Scholar]
- 39.Fu P, et al. Amifostine reduces lung vascular permeability via suppression of inflammatory signalling. Eur Respir J. 2009;33:612–624. doi: 10.1183/09031936.00014808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.