Abstract
Human MCF-7 breast cancer cells were exposed to a Random Positioning Machine (RPM). After 24 hours (h) the cells grew either adherently within a monolayer (AD) or within multicellular spheroids (MCS). AD and MCS populations were separately harvested, their cellular differences were determined performing qPCR on genes, which were differently expressed in AD and MCS cells. Gene array technology was applied to detect RPM-sensitive genes in MCF-7 cells after 24 h. Furthermore, the capability to form multicellular spheroids in vitro was compared with the intracellular distribution of NF-kappaB (NFκB) p65. NFκB was equally distributed in static control cells, but predominantly localized in the cytoplasm in AD cells and nucleus in MCS cells exposed to the RPM. Gene array analyses revealed a more than 2-fold change of only 23 genes including some whose products are affected by oxygen levels or regulate glycolysis. Significant upregulations of the mRNAs of enzymes degrading heme, of ANXA1, ANXA2, CTGF, CAV2 and ICAM1, as well as of FAS, Casp8, BAX, p53, CYC1 and PARP1 were observed in MCS cells as compared with 1g-control and AD cells. An interaction analysis of 47 investigated genes suggested that HMOX-1 and NFκB variants are activated, when multicellular spheroids are formed.
Introduction
Exposing cells to devices like the Random Positioning Machine (RPM) triggers them to change their growth behavior together with a number of cellular characteristics1,2. This phenomenon has been observed for several types of human cells including thyroid cells, chondrocytes, endothelial cells, human breast cancer MDA-MB-231 cells and others3–9. We recently demonstrated that also human Michigan Cancer Foundation-7 (MCF-7) breast cancer cells form three-dimensional (3D) aggregates. Incubated on a RPM, a part of the cells switches from a two-dimensional (2D) growth within a monolayer to a 3D growth within multicellular spheroids (MCS), the other one remains adherent and continues growing within a monolayer (AD)10. The occurrence of MCS begins within 24 h on the RPM10. After a five-day RPM-exposure, spheroids were floating in the culture supernatant. At that time, the cells of the MCS have changed their gene expression pattern. Among other mRNAs, vascular endothelial growth factor-A (VEGFA), vascular endothelial growth factor receptor 2 (FLK1), caspase-9 (Casp9), caspase-3 (Casp3), and protein kinase C alpha (PRKCA) mRNAs were downregulated in five-day MCS-samples indicating their involvement in 3D aggregation.
Isochronally with 3D aggregation, microgravity-induced apoptosis was detected in breast cancer cells6 like it has been observed in other types of cells11–13. In addition, FTC-133 thyroid cancer cells exposed to the RPM for 24 h formed spheroids and exhibited simultaneously enhanced levels of apoptosis and of NFκB proteins as compared with 1g-control cells14. NFκB incorporates a variety of transcriptional regulatory functions and is known to be of great importance in apoptosis15. It is inactivated by binding to IκB (inhibitor of NFκB). However, degradation of IκB can result in a translocation of NFκB into the nucleus, where it can activate the transcription of anti-apoptotic genes16. In a recent deep proteome analysis the translocation inhibitor IκBKB showed up in AD cells after culturing FTC-133 cells on the RPM, but could not be detected in MCS cells of the same culture flask17,18. These observations created the idea that a link between spheroid formation, initiation of apoptosis and NFκB expression may exist14. In addition, Becker-Weimann et al. postulated a link between NFκB expression and 3D organization of human breast cancer cells19.
Therefore, the principal aim of this paper was to investigate the early phases of RPM-exposure (24 h) of MCF-7 breast cancer cells and to test whether there is a link between enhancement of apoptosis, changes in NFκB expression and spheroid formation. In a first approach, we exposed MCF-7 breast cancer cells for 24 h to the RPM. Afterwards, we analyzed the intracellular distribution and expression of NFκB by means of gene array analyses as well as quantitative (q)PCR focusing on genes involved in apoptosis and cell adhesion signaling and known to play a role in spheroid formation of human thyroid cells14. Furthermore, morphological and molecular biological results were compared. These experiments should increase the knowledge about mechanisms of the self-reliant formation of tissue-comparable cell-aggregates. Finding molecules in various cell types, which mediate a microgravity-dependent cell organization in equal ways, may indicate new targets to improve tissue engineering and cancer treatment. In a further step, we investigated the impact of the poly ADP ribose polymerase (PARP) inhibitor olaparib, the effect of dexamethasone (DEX) and the phosphodiesterase-4 (PDE-4) inhibitor rolipram on spheroid formation.
Results
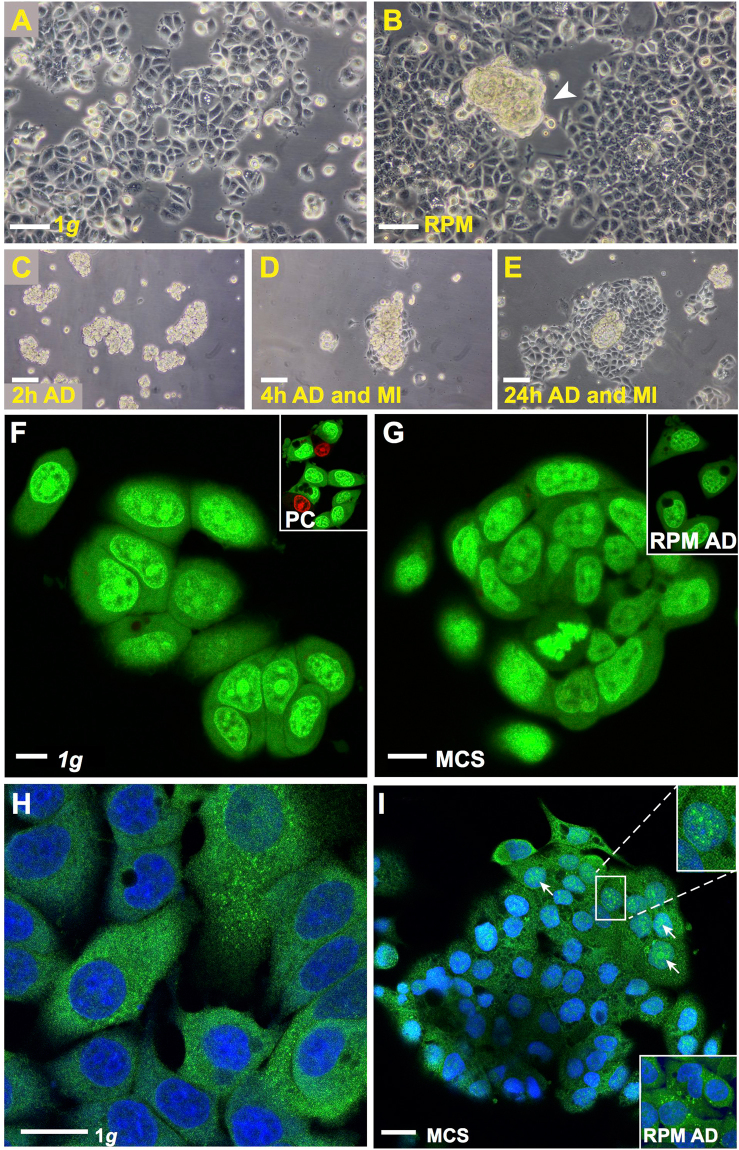
After MCF-7 human breast adenocarcinoma cells had been cultured on the RPM for 24 h, we detected two different phenotypes: Cells growing adherently within a 2D monolayer (AD) and cells growing in form of 3D aggregates exhibiting no glandular structures after this short-term exposure. The MCS had various sizes (max. 300 µm) and were floating in the supernatant (Fig. 1B). In the corresponding controls incubated under normal 1g-conditions only cells growing in 2D monolayers could be seen (Fig. 1A). The cells growing three-dimensionally in form of MCS were viable. This was demonstrated by testing the adhesion of the MCS (Fig. 1C) on slide flasks and by the migration behavior of the cells clearly seen at 4 h of incubation (Fig. 1D). After 24 h a large number of cells are migrating out of the MCS (Fig. 1E). In addition, acridine orange/ethidium bromide staining showed that MCS cells were viable and impermeable to the dye (Fig. 1G; green fluorescence) like the RPM AD cells (Fig. 1G; insert) and the 1g-control MCF-7 cells (Fig. 1F). In case of dead or necrotic cells, the cells would have taken up the dye and shown a red fluorescence. This is demonstrated in the insert of Fig. 1F, where red cells are visible when stained living cells are kept without medium under the microscope for another 5 min and photographed afterwards.
Figure 1.
(A–E) Phase contrast microscopy: (A) Native MCF-7 cells cultured for 24 h under 1g-conditions. (B) RPM-exposed sample showing two phenotypes (adherently growing MCF-7 cells and MCS). A representative example of a MCS is indicated by the white arrowhead. (C) Adhesion of MCS to the bottom of a slide flask after 2 h. (D) Migration of MCF-7 cells out of the MCS after 4 h and (E) Migration of MCF-7 cells out of the MCS after 24 h. (F–I) Confocal laser scanning microscopy: (F,G) Acridine orange/ethidium bromide staining revealed a green fluorescence in all cells after 24 h which indicates viability. (F) 1g-conditions (insert: positive control (PC) of acridine orange/ethidium bromide assay after approximately 5 min incubation). (G) viable cells in the MCS (insert: viable RPM-AD cells). (H) NFκB immunofluorescence staining of 1g-control cells: NFκB is predominantly detectable in the cytoplasm and I: RPM-exposed samples: nuclear (white arrows) and cytoplasmatic NFκB in the MCS. Upper insert: magnification of indicated area. Lower insert: NFκB detection in RPM AD cells, same magnification as in I. Nuclei are counterstained with DAPI. Scale bars in (A–E) 100 µm; scale bars in (F,G) 10 µm; scale bars in (H and I): 20 µm. 5 samples of each condition were examined separately.
Effect of RPM-exposure on NFκB
In order to see, whether the NFκB-p65 location and content of MCF-7 cells is like that of FTC-133 follicular thyroid cancer cells14 involved in the cellular processes taking place during the first 24 h of RPM-exposure, we performed a NFκB-p65 immunofluorescence staining and Western blot analyses. The antibodies used are listed in Table 1. The Fig. 1H and I reveal MCF-7 cells stained for NFκB-p65. It can be seen that NFκB-p65 is equally distributed in the cytoplasm of cells cultured under static 1g-conditions (Fig. 1H). After a 24-hour RPM-exposure NFκB-p65 protein appeared in the nucleus of MCS cells (Fig. 1I; upper insert). Fluorescence brightness shown in the upper and lower inserts of Fig. 1I suggested more NFκB-p65 in the nucleus and cytoplasm of MCS cells than in the cytoplasm of adherent cells exposed to the RPM.
Table 1.
Antibodies applied for Western blot Analysis.
| Antibody | Dilution | Company | Molecular weight | Catalog Number |
|---|---|---|---|---|
| p-NFκB p50 | 1/1000 | Thermo Fisher | 50 kDa | #710460 |
| p-NFκB p52 | 1/1000 | Thermo Fisher | 110 kDa | #PA5-17385 |
| Anti-NFκB p105/p50 | 1/1000 | Abcam | 50, 100 kDa | #ab32360 |
| Anti-p-NFκB p65 | 1/1000 | Abcam | 70 kDa | #ab86299 |
| Anti-IκBα | 1/1000 | Cell-Signaling | 39 kDa | #9242 |
| Anti-p-IκBα | 1/1000 | Cell-Signaling | 40 kDa | #2859 |
| Anti-NFκBp65 | 1/1000 | Cell-Signaling | 65 kDa | #C22B4 |
| Anti-Cofilin | 1/1000 | Abcam | 19 kDa | #ab124979 |
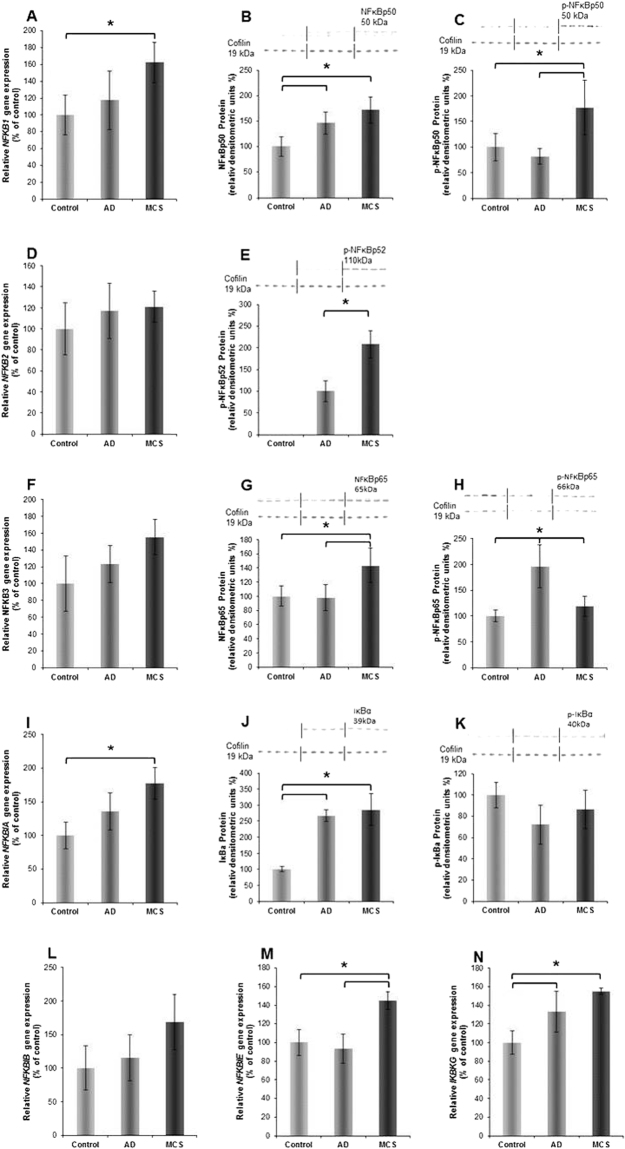
NFκB proteins comprise different variants including NFκB-p50, -p52 and -p65. They are encoded by the gene loci NFKB1, 2 and 3. The various proteins form dimeric transcription factors that regulate the expression of genes influencing a broad range of biological processes20–22. NFκB proteins are bound and inhibited by IκB proteins. Both, effectors and inhibitors may be activated by external signals, which trigger expression, phosphorylation and dimerization of various components as well as their translocation from the cytoplasm to nucleus, where it binds to specific DNA sequences (response elements). In order to investigate whether the differences in localization and amount of NFκB-p65 observed in Fig. 1H and I are only due to a re-distribution or are also due to a new synthesis of NFκB-p65 proteins, we performed qPCR of the NFKB1, NFKB2, NFKB3, NFKBIA, NFKBIB, NFKBIE and NFKBG genes (Fig. 2). Figure 2D,F,L indicate a tendency of a non-significant upregulation of NFKB2, NFKB3, NFKBIB genes under simulated microgravity (s-µg) in AD cells and especially in MCS, while Fig. 2A,I,M,N show a significant upregulation of NFKB1, NFKBIA, NFKBIE and NFKBG genes in MCS cells as compared to control cells. In AD cells only the IKBKG gene is significantly enhanced in comparison to the control cells. In general, the Western blot analyses performed on the same group as the qPCR, point in a similar direction as the corresponding gene expression pattern along with the corresponding un-phosphorylated proteins (Fig. 2B,G,J). Interestingly, the phosphorylated (p−) variants of the proteins are enhanced mainly in RPM-exposed cells (Fig. 2C,E,H). Hence, a significant phosphorylation of NFκB proteins during MCS formation on the RPM may be considered.
Figure 2.
(A) NFKB1 gene expression; (B) NFkBp50 Western blot analysis; (C) phosphorylated (p)-NFκBp50 Western blot analysis; (D) NFKB2 gene expression; (E) p-NFκBp52 Western blot analysis; (F) NFKB3 gene expression; (G) NFκBp65 Western blot analysis; (H) p-NFkBp65 Western blot analysis; (I) NFKBIA gene expression; (J) IκBα Western blot analysis; (K) p- IκBβ Western blot analysis; (L) NFKBIB gene expression; (M) NFKBIE gene expression; (N) IKBKG gene expression. The position (arrow) and molecular size (in kD) of the investigated proteins are indicated on each of the Western blot membrane images. Cofilin 1 was used as loading control. The vertical lines indicate group separation giving n = 5 per group.
Genes of factors known to be involved in spheroid formation
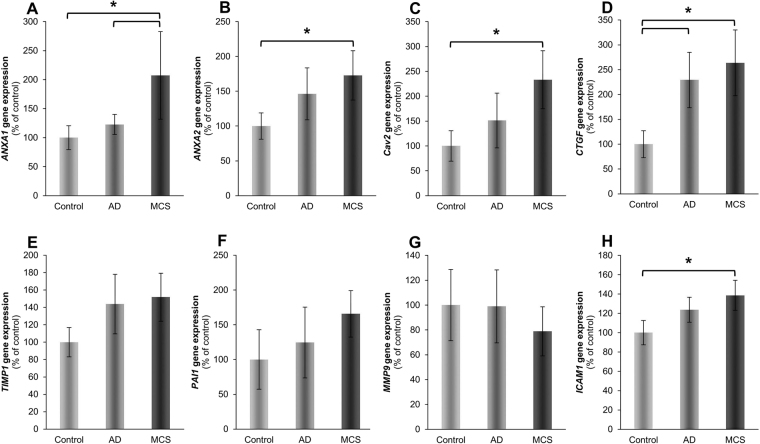
Because the growth behavior, NFKB3 gene expression and NFκB p65 protein accumulation were similar in FTC-133 and MCF-7 cells, we investigated the expression of other genes, which are assumed to play a role in the formation of 3D aggregates of human cancer cells14,23. The primers used are listed in Table 2. The gene expression status was studied after 24 h by qPCR in 1g-control cells, AD and MCS cells comparatively. These qPCR experiments revealed: The gene expression of annexin A1 (ANXA1) and annexin A2 (ANXA2) were significantly upregulated in MCS compared to 1g-control cells, while their expression was unregulated in AD samples (Fig. 3A,B). In addition, caveolin-2 (CAV2) and intercellular adhesion molecule 1 (ICAM1) mRNAs were both elevated in MCS. The expression was significantly upregulated in MCS compared to the corresponding 1g-controls (Fig. 3C,H). In contrast to CAV2, the connective tissue growth factor (CTGF) gene expression was significantly upregulated in AD as well as in MCS samples (Fig. 3D).
Table 2.
Primers used for quantitative real-time PCR
| Factor | Primer name | Sequence 5′ - 3′ |
|---|---|---|
| 18 S | 18S-F | GGAGCCTGCGGCTTAATTT |
| 18S-R | CAACTAAGAACGGCCATGCA | |
| Annexin A1; ANXA1 | ANXA1-F | GCCAAAGACATAACCTCAGACACAT |
| ANXA1-R | GAATCAGCCAAGTCTTCATTCACA | |
| Annexin A2; ANXA2 | ANXA2-F | GGTACAAGAGTTACAGCCCTTATGACA |
| ANXA2-R | CATGGAGTCATACAGCCGATCA | |
| Apoptosis Regulator BAX; BAX | BAX-F | GTCAGCTGCCACTCGGAAA |
| BAX-R | AGTAACATGGAGCTGCAGAGGAT | |
| Apoptosis Regulator BCL-2; BCL2 | BCL2-F | TCAGAGACAGCCAGGAGAAATCA |
| BCL2-R | CCTGTGGATGACTGAGTACCTGAA | |
| Caspase 3; CASP3 | Casp3-F | CTCCAACATCGACTGTGAGAAGTT |
| Casp3-R | GCGCCAGCTCCAGCAA | |
| Caspase 8; CASP8 | Casp8-F | TGCAAAAGCACGGGAGAAAG |
| Casp8-R | CTCTTCAAAGGTCGTGGTCAAAG | |
| Caspase 9; CASP9 | Casp9-F | CTCCAACATCGACTGTGAGAAGTT |
| Casp9-R | GCGCCAGCTCCAGCAA | |
| Caveolin 2; CAV2 | Cav2-F | GATCCCCACCGGCTCAAC |
| Cav2-R | CACCGGCTCTGCGATCA | |
| Connective Tissue Growth Factor; CTGF | CTGF-F | ACAAGGGCCTCTTCTGTGACTT |
| CTGF-R | GGTACACCGTACCACCGAAGAT | |
| Cytochrome C; CYC | Cyc-F | CACTGCGGGAAGGTCTCTAC |
| Cyc-R | GGGGTGCCATCGTCAAACTC | |
| NF-kappa-B transcription complex P105/P50; NFKB1 | NFkB1-F | CTTAGGAGGGAGAGCCCAC |
| NFkB1-R | TGAAACATTTGTTCAGGCCTTC | |
| NF-kappa-B transcription complex P100/P52; NFKB2 | NFkB2-F | GTACAAAGATACGCGGACCC |
| NFkB2-R | CCAGACCTGGGTTGTAGCA | |
| NF-kappa-B transcription complex P65 | NFkB-F | CGCTTCTTCACACACTGGATTC |
| NFkB-R | ACTGCCGGGATGGCTTCT | |
| NF-kappa-B essential modulator (NEMO); IKBKG | IkBKG-F | AACTGGGACTTTCTCGGAGC |
| IkBKG-R | GGCAAGGGCTGTCAGCAG | |
| NF-kappa-B inhibitor alpha; NFKBIA | NFkBIa-F | AATGCTCAGGAGCCCTGTAAT |
| NFkBIa-R | CTGTTGACATCAGCCCCACA | |
| NF-kappa-B inhibitor beta; NFKBIB | NFkBIb-F | CCCGGAGGACCTGGGTT |
| NFkBIb-R | GCAGTGCCGTGTCCCC | |
| NF-kappa-B inhibitor epsilon; NFKBIE | NFkBIe-F | TGGGCATCTCATCCACTCTG |
| NFkBIe-R | ACAAGGGATTCCTCAGTCAGGT | |
| Tumor necrosis factor receptor superfamily member 6 (Fas); FAS | CD95-F | AGTCTGGTTCATCCCCATTGAC |
| CD95-R | AGGGATTGGAATTGAGGAAGACT | |
| Intercellular adhesion molecule 1; ICAM1 | ICAM1-F | CGGCTGACGTGTGCAGTAAT |
| ICAM1-R | CTTCTGAGACCTCTGGCTTCGT | |
| Matrix metalloproteinase-9; MMP9 | MMP9-F | CCTGGAGACCTGAGAACCAATC |
| MMP9-R | TTCGACTCTCCACGCATCTCT | |
| Cellular tumor antigen p53; p53 | p53-F | CCTGGATTGGCCAGACTGC |
| p53-R | TTTTCAGGAAGTAGTTTCCATAGGT | |
| Plasminogen activator inhibitor 1; PAI1 | PAI1-F | AGGCTGACTTCACGAGTCTTTCA |
| PAI1-R | CACTCTCGTTCACCTCGATCTTC | |
| Poly [ADP-ribose] polymerase 1; PARP1 | PARP1-F | CGAGTCGAGTACGCCAAGAG |
| PARP1-R | CATCAAACATGGGCGACTGC | |
| Metalloproteinase inhibitor 1; TIMP1 | TIMP1-F | GCCATCGCCGCAGATC |
| TIMP1-R | GCTATCAGCCACAGCAACAACA |
All sequences are given in the 5′–3′ direction.
Figure 3.
Expression of genes putatively involved in MCS-formation. (A) Annexin A1 (ANXA1), (B) Annexin A2 (ANXA2), (C) Caveolin-2 (Cav2), (D) Connective tissue growth factor (CTGF), (E) Metalloproteinase inhibitor 1 (TIMP1), (F) Plasminogen activator inhibitor 1 (PAI1), (G) Matrix metalloproteinase 9 (MMP9), (H) Intercellular adhesion molecule 1 (ICAM1), were analysed after 24 h of RPM-exposure. All values are given as mean ± standard deviation. *p < 0.05 vs. corresponding 1g-control. AD: adherent cells, MCS: multicellular spheroids, after RPM-exposure. Number of replicates is 4.
Both, tissue inhibitor of metalloproteinases 1 (TIMP1) and plasminogen activator inhibitor 1 (PAI1) mRNAs were not significantly regulated, however, a tendency of an upregulation in AD and MCS was visible (Fig. 3E,F). Both factors inhibit metalloproteinases including metalloproteinase 9 (MMP9), whose mRNA was not significantly regulated (Fig. 3G).
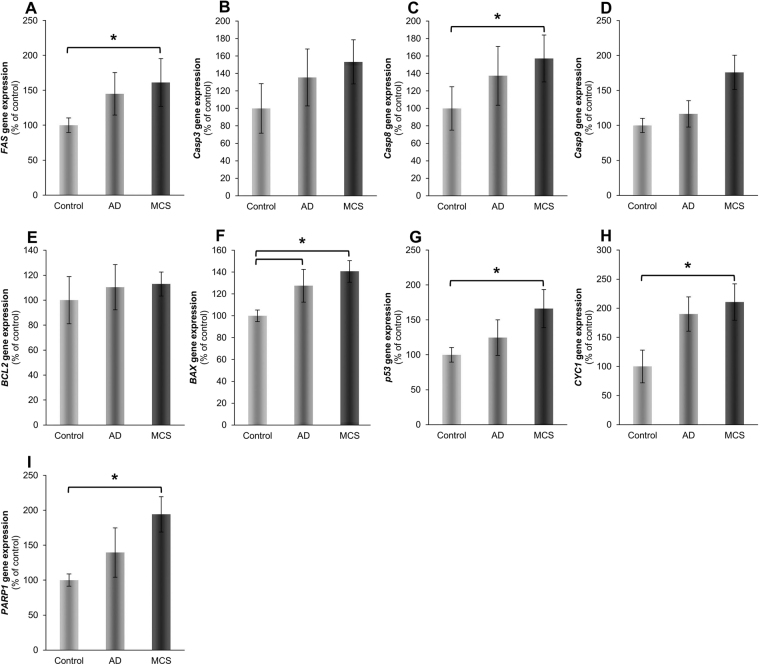
Additional genes involved in intrinsic and extrinsic pathways of programmed cell death, were selected, because apoptosis was found repeatedly to accompany cell response to removing gravity1,5,11,14. Casp3 and Casp9 mRNAs were not significantly changed in AD and MCS compared to their corresponding controls (Fig. 4B,D). However, only MCS versus control showed a significant upregulation of caspase-8 (Casp8) (Fig. 4C). Furthermore, the cellular tumor antigen p53 (p53) gene expression was significantly enhanced in MCS versus control cells (Fig. 4G). The cytochrome c1 (CYC1), poly(ADP-ribose)-polymerase 1 (PARP1) (Fig. 4H,I) and tumor necrosis factor receptor superfamily member (FAS) mRNAs (Fig. 4A) were upregulated in MCS samples compared to the control group. In addition, the apoptosis regulator Bcl-2 (BCL2) mRNA remained unregulated in AD and MCS cells (Fig. 4E). In contrast, the apoptosis regulator BAX (BAX) gene expression was significantly upregulated in AD and MCS samples compared to the control cells (Fig. 4F). However, many of them exhibited significant regulations in at least one of the three possible permutations of gene expression comparisons between our experimental groups.
Figure 4.
Gene expression of genes whose products are associated with apoptosis pathway. (A) Fas, (B) Caspase-3 (Casp3), (C) Caspase-8 (Casp8), (D) Caspase-9 (Casp9), (E) Apoptosis regulator Bcl-2 (BCL2), (F) Apoptosis regulator BAX (BAX), (G) Cellular tumor antigen p53 (p53), (H) Cytochrome C 1 (Cyc1), (I) Poly ADP-ribose polymerase (PARP1) were analyzed after 24 h of RPM-exposure. All values are given as mean ± standard deviation. *p < 0.05 vs. corresponding 1g-control. AD: adherent cells, MCS: multicellular spheroids, after RPM-exposure. Number of replicates is 4.
Microarray analysis
In order to detect further genes, which change their expression activity, during the first 24 h of culturing MCF-7 cells on the RPM, we performed microarray analyses on 1g-control, AD and MCS cells (Fig. 5A). The microarray analysis (Table 3 and Supplemental Table 1) did not show a significant microgravity-dependent change in NFκB-p65 expression. Moreover, it revealed a rather stable mRNA expression pattern. In total 319 genes (331 probes, 330 probes annotated to 319 genes) transcripts were significantly differentially expressed (5% false discovery rate (FDR) in Analysis of variance (ANOVA)). In the pairwise comparison of control cells, AD and MCS, the expression of 140 significantly differentially expressed genes and open reading frames was changed 1.4-fold (Supplemental Table 1). However, a two- or more-fold change of the mRNA was merely found in 23 genes (Table 3). As a two- or more-fold change is usually considered to indicate biological relevance, we studied these genes in more detail.
Figure 5.
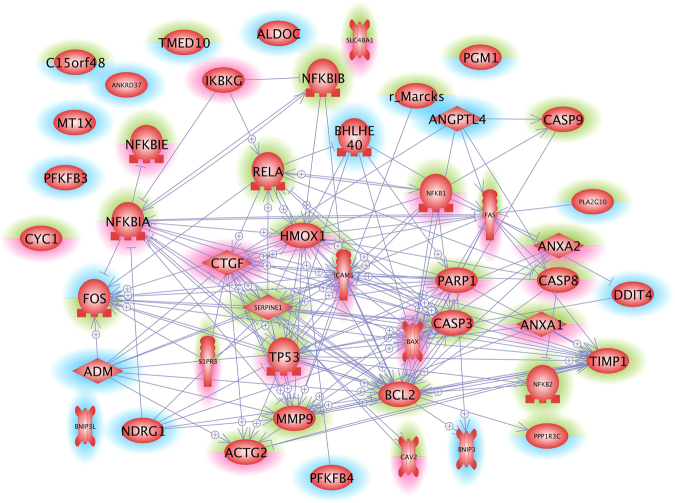
(A) K-mean clustering of significant expression differences in the microarray experiment (5% FDR, N = 331 probes). Using k = 4 the first cluster comprises 103 probes downregulated in MCS and AD. The second and forth cluster comprise 66 and 63 probes upregulated in MCS. Cluster 2 genes have an intermediate expression in AD. The 99 probes in cluster three are downregulated in MCS. (B) Pathway studio analysis of genes detected by the gene array analysis: The arrows indicate interaction. HMOX1 is most net-worked.
Table 3.
Genes changed two- or more-fold after a 24 h-RPM-exposure.
| Gene symbol | Fold change AD vs. controls | Fold change MCS vs. controls | Oxygen |
|---|---|---|---|
| ADM | −3.97341 | −3.71790 | sensitive |
| ALDOC | −2.72112 | −2.93859 | sensitive |
| ANGPTL4 | −2.82266 | −2.52424 | sensitive |
| ANKRD37 | −3.30216 | −3.47036 | sensitive |
| BHLHE40 | −2.63050 | −2.12209 | |
| BNIP3 | −2.06507 | −1.99325 | sensitive |
| BNIP3L | −2.10573 | −2.65328 | sensitive |
| C15ORF48 | −1.28973 | −2.14925 | |
| DDIT4 | −2.36264 | −2.80513 | sensitive |
| FOS | −2.23903 | −1.62010 | |
| MARCKS | −1.23023 | −2.21407 | |
| MT1X | −2.30906 | −2.49814 | |
| NDRG1 | −2.95903 | −2.78486 | sensitive |
| PFKFB3 | −2.10202 | −3.13231 | sensitive |
| PFKFB4 | −3.20572 | −2.84829 | sensitive |
| PGM1 | −1.77618 | −2.05219 | |
| PLA2G10 | −1.17541 | −2.21319 | |
| PPP1R3C | −1.72085 | −2.07604 | |
| TMED10 | −1.13444 | −2.60338 | |
| SLC48A1 | 1.31299 | 2.07465 | |
| S1PR3 | 1.46902 | 2.07908 | |
| ACTG2 | 1.67742 | 2.17152 | |
| HMOX1 | 1.23716 | 2.78409 |
19 of the genes were downregulated more than two-fold and 4 genes were upregulated at least two-fold (Table 3). 10 of the down-regulated genes code for proteins linked to oxygen levels or hypoxia24–30. The degree of downregulation of the expression of these genes was very similar in AD and MCS cells. But myristoylated alanine-rich C-kinase substrate (MARCKS), which codes for an actin interacting protein, is more significantly (5% FDR ANOVA) downregulated in MCS than in AD cells31. A more profound difference between AD and MCS cells was seen, when the upregulated genes were analyzed, which code for the cytoskeletal protein gamma-enteric smooth muscle actin (ACTG) and additional three proteins suppressing apoptosis and regulating the concentration of heme, which influences apoptosis32–34. All 4 genes indicated were significantly upregulated (>2 fold) only in MCS cells (Table 3).
Because 10 of the 19 genes found significantly downregulated are related to oxygen homeostasis, we applied the Pathway Studio analysis to see whether there is an interaction between them (Figs 6 and 7). Interestingly, we did not see significant interactions of these factors, neither at protein nor at gene level. Interaction at gene and protein level was, however, detected, when all 23 factors shown in Table 3 were analyzed. A cross-linking goes through 7 of the 23 genes (Fig. 5B) which code for 3 extracellular, 1 membrane, 5 nuclear, 2 mitochondrial and 12 cytosolic proteins (see also Fig. 7, Table 3). Hemoxygenase (HMOX1) is the most networked gene.
Figure 6.
Pathway studio analysis of genes analyzed in the study by the gene array analysis and qPCR. Arrows indicate influence. Rims around the icon indicate up- (red) and down-regulation (blue). Green means un-regulated. The lower half of a rim indicates regulation in MCS, the upper half indicates regulation in the AD cells.
Figure 7.
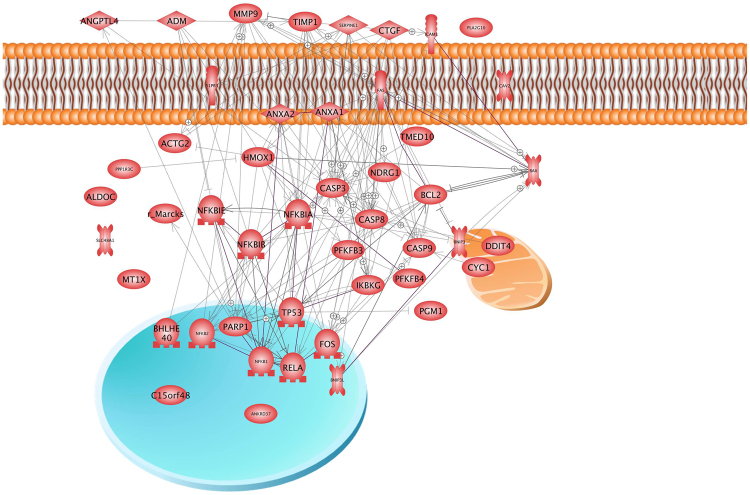
Pathway studio analysis of proteins whose genes were analysed in the study by the gene array analysis and qPCR. Arrows indicate interaction. Mitochondria (yellow), cell membrane (red) and nucleus (blue) are indicated to support localization of the various proteins.
In order to examine, whether the genes determined by qPCR as upregulated in MCS cells interact with the differentially expressed genes detected in the gene array analysis, we subjected the experimental results to the Pathway studio analysis. The candidates comprised 41 items including RELA (NFKB3) and Hemox-1. They completed a complicated network consisting of 31 of the 41 factors at gene (Fig. 6) and protein levels (Fig. 7). Figures 5 and 6 indicate that the genes recognized before to be important in spheroid formation and the genes noticed in gene array analysis very well fit into the networks (see also Table 3). HMOX1 and RELA show strong interaction at a genetic level (Fig. 6), but no mutual influence has so far been detected on the protein level (Fig. 7). In addition, the ICAM1 gene, which codes for a cell adhesion protein is under the positive influence of upregulated genes such as FAS, PARP1, P53, CTGF, and NFKB1, but may be suppressed by caspase-3. Besides of CTGF, these genes are significantly upregulated only in MCS cells.
Impact of targeting PARP and NFκB on spheroid formation
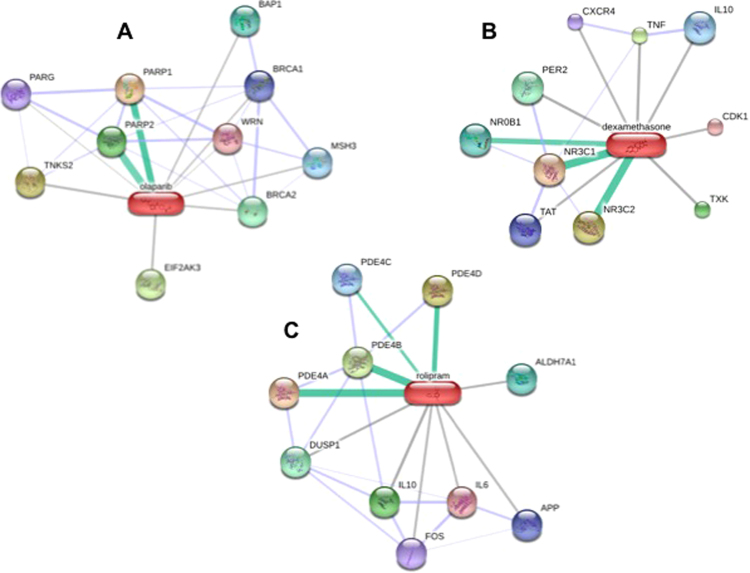
In order to evaluate the functional effects of PARP and NFκB, we performed inhibition assessments using the drugs olaparib, dexamethasone (DEX) and rolipram. Figure 8 shows the target proteins of the three drugs. According to a STITCH 4 database search (chemical-protein interaction networks; http://stitch.embl.de/), it can be assumed that DEX preferentially inhibits NROB1, NR3C1 and NR3C2 (Fig. 8, green bars), but has side effects cross-reacting with a couple of other factors including IL10 and CDK1 (Fig. 8). Similarly, olaparib inhibits PARP1 and PARP2 (Fig. 8, green bars) and has additional effects on other eight proteins (Fig. 8). Rolipram mainly blocks the action of PDE4A, PDE4B and PDE4D (Fig. 8, green bars). To some degree it also interacts with factors like IL6, IL10, APP, and FOS.
Figure 8.
Pharmacological studies. STITCH 4 search for targets of olaparib (A), dexamethasone (B) and rolipram (C). Primary targets are indicated by a green bar between the drug and the protein. Factors affected to a minor degree are shown by grey lines86.
To determine the formation of spheroids when molecules of interest are inhibited, we treated the MCF-7 cells with various concentrations of olaparib (0 M, 2.5 µM, 5 µM and 10 µM), DEX (0 M, 0.01 µM, 0,1 µM, 1 µM) and rolipram (0 M, 1 µM, 10 µM), while exposed to the RPM for 24 h. To exclude toxic effects of the used solvent and/or the drugs on the MCF-7 cells, we prepared 24 h static 1g-experiments with solvent and the mentioned drug concentrations (Supplemental Fig. 1). After a 24 hour-exposure, the cells were stained with acridine orange/ethidium bromide to examine the cell viability (Supplemental Fig. 1). None of the used concentrations of solvent and drugs had a cytotoxic effect on the cells, as presented by the green staining, while no red staining of the nuclei was detectable (Supplemental Fig. 1). In addition, no increased cell detachment or formation of cell aggregates was noticed due to drug or solvent supplementation.
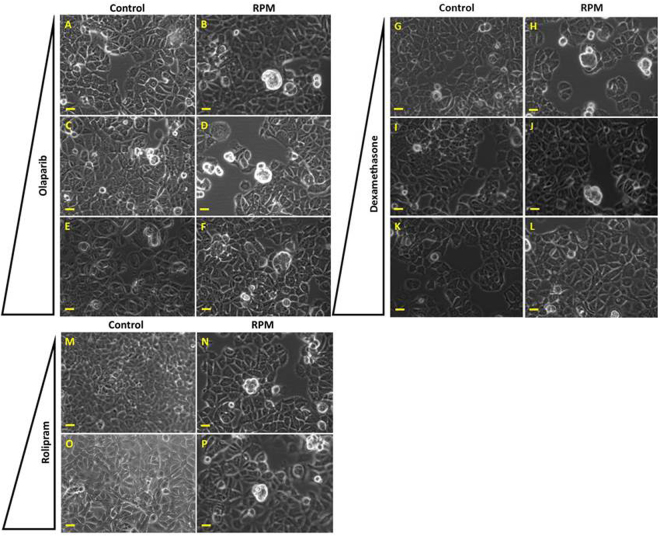
We exposed the MCF-7 cells to the RPM for 24 h including the drug concentrations mentioned. While treatment with olaparib and rolipram did not show visible effects on the formation of early spheroids (Fig. 9A–F,M–P), the number of visible MCSs seemed to decrease with increasing concentration of DEX (Fig. 9G–L). Acridine orange/ethidium bromide staining revealed viable cells in RPM-AD cells as well as in MCS (Supplemental Fig. 1N, arrow indicates a MCS).
Figure 9.
Phase contrast microscopy of drug-treated cells exposed to the RPM. Olaparib-treated cells static control: (A) 2.5 µM, (C) 5 µM, (E) 10 µM. Olaparib-treated cells exposed to the RPM for 24 h: (B) 2.5 µM, (D) 5 µM, (F) 10 µM. DEX-treated cells static control: (G) 0.01 µM, (I) 0.1 µM, (K) 1 µM. DEX-treated cells exposed to the RPM for 24 h: (H) 0.01 µM, (J) 0.1 µM, (L) 1 µM. Rolipram-treated cells static control: M) 1 µM, (O) 10 µM. Rolipram-treated cells exposed to the RPM for 24 h: (N) 1 µM, (P) 10 µM. The pyramid indicates increasing drug concentration. Scale bar: 100 µm.
Discussion
Organisms live on Earth under the permanent influence of gravity. Removing this influence results in remodeling of various tissues in humans. For example bone loss and muscle atrophy can be observed in astronauts and cosmonauts after long-term spaceflights35. In addition, various changes in different types of human cells were detected. Examples are macrophages producing less reactive oxygen in microgravity compared to 1 g36 and human thyroid cancer cells which form 3D cell aggregates, when cultured for a longer time in microgravity2. This makes microgravity a valuable environment for studies on a number of cellular characteristics not understood so far37. However, long-term removal of gravity achieved by spaceflights is very expensive and seldom performed. With the help of ground-based facilities, which were constructed, to simulate microgravity on Earth, some aspects of annulling gravity can be studied. Such devices, including the RPM, trigger at least a part of the incubated cells to detach from the bottom of a culture flask and to form 3D aggregates like they are observed after spaceflights1,2. Even though the RPM produces sheer forces, which are nearly completely absent in real microgravity38,39, it is generally accepted that the cause of spheroid formation of cells cultured either on a RPM or in space is the absence of cell sedimentation in both conditions1,2,40, as no other cause could be identified until today.
Also MCF-7 cells form spheroids when exposed to the RPM10. In contrast to thyroid cancer cells, which only form spheres, the MCF-7 cells form 3D structures that have central holes after a five-day RPM-exposure, which remind of duct structures of normal epithelial breast cells10. After a 24 h RPM-exposure dense MCS of MCF-7 cells similar to FTC-133 thyroid cancer spheroids or MCS from normal thyroid cells were visible14,41.
The MCF-7 breast cancer cell line is characterized as estrogen-receptor (ER)-positive, and progesterone receptor (PR)-positive. It belongs to the luminal A molecular subtype42. MCF-7 cells show characteristics of differentiated breast epithelium and are positive for epithelial markers and negative for mesenchymal markers like vimentin42. MCF-7 cells have demonstrated the ability to form MCS under static 1g-conditions using non-adherent Petri dishes43. These MCS can mature after long-term culture to lumen-containing spheroids43.
Normal cells such as endothelial blood vessel cells for example form tubular structures when cultured on a RPM5. Human chondrocytes form cartilage pieces when cultured in space and on the Rotating Wall Vessel44 or when they were exposed to the RPM45.
These observations point to the possibility that the technique of microgravity-dependent spheroid formation could be exploited for tissue engineering purposes in the future46, perhaps also in breast reconstruction after surgery. In this study, we investigated the early phase of spheroid formation of MCF-7 cells exposed to simulated microgravity created by a RPM.
MCF-7 cells of a parent population, which grow either as MCS cells or as a monolayer showed similar and different features after a 24 hour-exposure to the RPM. Both states of cellular appearance exhibited an increased expression of CTGF and BAX and a rather similarly decreased expression of adrenomedullin (ADM), aldolase (ALDOC), angiopoietin-like 4 (ANGPTL4), ankyrin repeat domain 37 (ANKRD37), BCL2 interacting protein 3 (BNIP3), N-myc downstream regulated 1 (NDRG1), whose products are affected by oxygen levels24–30. The decrease of ADM, ALDOC, ANGPTL4, ANKRD37, BNIP3, NDRG1 may be explained by an increase in oxygen concentration within vented culture flasks observed after 24 h of incubation on the RPM47. Such an increase may be the reason for the down-regulation of various genes whose products were changed in response to oxygen levels and regulate glycolysis, as the regulation was very similar in adherent and in MCS cells (Supplemental Table 1). In addition, spheroid formation is observed in closed and not-vented incubation chambers during a spaceflight and during clinorotation (clinostat device), which is another method of preventing cell sedimentation on ground2,40. This indicates that different oxygen concentrations are not accountable for spheroid formation. Therefore, an enhanced oxygen concentration together with the down-regulation of hypoxia-inducible factor (HIF)-related genes may be an epiphenomenon, when cells are incubated on the RPM in vented culture flasks. As oxygen enhancement cannot be considered to trigger the switch from a 2D to a 3D growth behavior, we focused on genes which have been differently regulated in AD and MCS cells during the early 24 h of incubation.
Differences in gene expression changes were observed in genes of factors playing a role in apoptosis. These genes were clearly elevated in MCS than in AD samples. They comprise p53, CYC1, PARP1, FAS, Casp8 as well as ANXA148–50. These elevated apoptotic factors in MCS are accompanied by factors promoting cell survival. One of these genes is sphingosine-1-phosphate receptor 3 (S1PR3), the other one is heme oxygenase-1. They are both up-regulated in MCS as detected by microarray and code for proteins favoring cell survival33,34.
HMOX-1 codes for an enzyme, degrading heme and exhibits together with its degradation products, cytoprotective properties33. This enzyme may be localized in various cellular compartments. In caveolae HMOX-1 is inhibited by caveolin-151. In thyroid cells an increased concentration of caveolin-1 prevents spheroid formation52,53. Therefore, it may not be excluded that HMOX-1 plays a role in spheroid formation.
According to the literature, both HMOX-1 and NFκB p65 were mutually downregulating their gene expressions54,55. In our experimental setting on breast cancer cells grown on the RPM, HMOX-1 was upregulated, while NFKB3 remained unregulated. The HMOX-1 upregulation could be due to a positive influence of ADM and PARP-156,57. Despite the NFKB3 gene expression remained unchanged (Fig. 2F), NFκB p65 protein was accumulated in MCS (Fig. 2G) and enriched within the nucleus. This fact points to an enhanced NFκB p65 translocation activity regulated by NFκBIA and NFκBIB (Figs 6 and 7)18,58. Becker-Weimann et al.19 found that NFκB is a key regulator in the formation of organized spheres in breast cancer cells cultured in 3D matrigels. While organized spheres had a low expression of NFκB p65, unorganized spheres presented an upregulation19. This is in concert with our findings that NFκB p65 is enhanced in 24 h adherent and MCS cells as these early spheroids showed a random accumulation of cells (Fig. 1). The random accumulation of MCF7 cells was organized in glandular structures with polarized cells after 5 days of cultivation on the RPM10. A deeper investigation of the correlation between cell polarization and NFκB in RPM exposed cells will surely shed further light on the process of spheroid formation under microgravity. Furthermore, NFκB p65 translocation triggers the expression of various proteins including ICAM1, which in our experiments was upregulated in MCS (Fig. 3H). Hence, NFκB seems to play a central role in spheroid formation, which suggests that manipulation of NFκB activity by biological or pharmacological agents could influence spheroid formation or related processes59. The effect of NFκB p65 is directed towards ICAM1 also by PARP160. CTGF, via mitogen-activated protein kinase and NFκB activation, can induce proinflammatory genes in murine tubuloepithelial cells61. Interestingly, the S1PR3 gene was upregulated in the MCS samples (Table 3). A recent paper demonstrated that sphingosine-1-phosphate increases the expansion of cancer stem cells via S1PR3 by a ligand-independent Notch activation in breast cancer62.
It is known that high levels of PARP-1 were associated with a poor prognosis in early breast cancer. PARP1 overexpression was detectable in various cancer cell lines and was associated with malignant progression63. We found a high PARP1 expression in the spheroids and targeted it by PARP inhibition. Thus, we investigated the impact of olaparib (a competitive PARP-1/2 inhibitor) on the growth of MCF-7 breast cancer cells exposed to the RPM and to 1g-conditions (Fig. 9). MCF-7 cells had been already treated with olaparib in earlier studies and three different doses were tested (2.5, 5 and 10 µM)64. Recent data suggested that PARP inhibitors might be useful to treat estrogen receptor-positive and estrogen-dependent tumors65. Here we could show that targeting PARP1/2 with olaparib did not alter the 3D aggregation of the MCF-7 cells cultured on the RPM, which indicated that PARP seems not to be the main key factor responsible for 3D spheroids formation in simulated microgravity.
In a second step, we used DEX to modulate the NFκB activity. It has been shown that the application of DEX promoted the NFκB transcriptional activity in MCF-7 cells66. Khan et al. identified that NFκB was also regulated by glucocorticoids and their receptor in MCF-7 breast cancer cells66. Here, we applied DEX to evaluate its impact on spheroid formation. After a 24-hour RPM-exposure we detected that 1 µM might inhibit the spheroid formation. This finding supports the hypothesis that NFκB might be involved in spheroid formation on the RPM. It is known that DEX suppresses the IL8 gene expression in airway epithelial cells67. We had shown earlier that the application of IL-8 protein facilitated the formation of MCS in thyroid cancer cell lines using the liquid-overlay technique68. Therefore, DEX might interact with IL8 to reduce MCS formation in this study, which has to be confirmed in the future.
In addition, we tested a second agent known to target NF-κB. Rolipram is a cyclic adenosine monophosphate (cAMP)-specific phosphodiesterase (PDE-4) inhibitor and earlier used to influence MCF-7 cancer cells69. The agent has shown to prevent the NFκB binding activity in human chorionic cells70. In this experimental setting, we can report that the drug is not influencing 3D spheroid formation in the first 24 h of RPM-exposure (Fig. 9).
Future studies are necessary to clarify the exact mechanisms involved in the process of 3D aggregation and spheroid formation, such as genetic knockouts or knockins in cell lines71.
In addition, the genes CTGF, FAS and P53, which were upregulated in MCS and their products, have positive influence on the gene expression of ICAM172,73. ICAM1 is a surface protein, mainly detectable in endothelial cells, but also expressed in human breast cancer cells74. It may contribute to the cell-cell interaction required for spheroid formation either by direct binding to integrin beta 275 or by changing the structure of the cell adhesion complex as it was observed recently on normal thyroid cells17. Interestingly, during earlier studies on MCF-7 cells, we detected a downregulation of ICAM1 in AD and MCS cells after a 24-hour-exposure to the RPM10. The reason might be either due to the changed serum supplementation, as growth factor concentration is unpredictable76, or due to the random walk of the RPM which incorporates different variances of stress39. In both cases the onset of apoptosis may vary, which reduces ICAM1 expression via caspase-311,77.
Taken together, our experiments suggest that NFκB family members and HMOX-1 interact on a gene level, when breast cancer cells transit from a 2D to a 3D growth on the RPM. They are changed in the same direction, when adherent and MCS-cells are compared (Fig. 6). Whether these alterations are accidental parallel events or mutually caused remains to be determined. Whether the up-regulated ANXA2 or the down-regulated MARCKS genes which both code for cytoskeleton interacting proteins or the genes of CAV2, TIMP1, PAI1 and MMP-9, which either code for membrane proteins or for enzymes regulating the extracellular matrix constitution, contribute to this process remains to be determined in future studies. Interestingly, CAV2, TIMP1, and PAI1 show an up-regulation in these experiments, but have exhibited together with MMP9 a downregulation in earlier experiments on thyroid cells9.
In addition, our studies deliver new knowledge about how these cells might behave in real microgravity. This data can be used to prepare future spaceflight missions. Hence, using these and earlier results as a basis10,78,79, we plan to conduct a future NASA and DLR space experiment like the successfully flown Cellbox-1 (NanoRacks-CellBox-Thyroid Cancer: http://www.nasa.gov/mission_pages/station/research/experiments/1648.html 52,53) onboard the International Space Station in order to increase the current knowledge of the behavior of human breast cancer cells under real microgravity in space with a special focus on early cytoskeletal changes and 3D growth.
Methods
Cell culture
MCF-7 human breast adenocarcinoma cells (Fig. 1A) were purchased from the American type culture collection (ATCC) (Manassas, VA, USA). Cells were cultivated in RPMI 1640 medium (Life Technologies, Naerum, Denmark) supplemented with 10% fetal calf serum (FCS) (Biochrom, Berlin, Germany) and 1% penicillin/streptomycin (Biochrom) at 37 °C and 5% CO2. One day prior to the experimental run on the RPM, cells were seeded either in slide flasks (Thermo Fisher Scientific, Roskilde, Denmark) (3 × 105 cells/cm²) for fluorescence staining or in T25 (1 × 106 cells) vented cell culture flasks (Sarstedt, Nümbrecht, Germany) for RNA and protein extraction. Before starting the run, flasks were filled up with medium, taking care that no air bubbles remain. A detailed procedure has been published previously9,23. To test the viability of the cells in the multicellular spheroids (MCS), the MCS were collected after 24 h (Fig. 1B) and seeded in slide flasks. The adhesion and migration behavior of the cells of the MCS was examined by phase contrast microscopy after 2 h, 4 h and 24 h (Fig. 1C–E).
Drug treatment
For targeting molecules of interest, we seeded 106 MCF-7 cells in slideflasks. After 24 h the cells were synchronized for 4 h and afterwards treated with the respective chemical agents for 24 h during RPM-exposure or without RPM-exposure. To target PARP we used the PARP 1/2 inhibitor olaparib (Selleckchem, Absource Diagnostics, Munich, Germany). We prepared a stock solution in DMSO. The aliquots were stored at −80 °C until use. Concentrations of 2.5, 5, or 10 µM olaparib in medium were applied64. Negative controls were incubated with DMSO only.
For targeting NFκB we applied dexamethasone (DEX) (Sigma-Aldrich, Taufkirchen, Germany). According to Bruxant et al.80 and Khan et al.66 we treated the MCF-7 cells with DEX (0 M, 0,001 µM, 0,1 µM, 1 µM). The MCF-7 cells were treated with DEX dissolved in water for 24 h.
Moreover, we applied the PDE4 inhibitor rolipram. We used the following doses 0 M, 1 µM, and 10 µM69. Rolipram was first prepared as a stock solution in ethanol. Control MCF-7 cells were treated with an equivalent volume of the solvent.
Random Positioning Machine
The desktop RPM (Airbus Defense and Space (ADS), Leiden, The Netherlands) was located in a standard incubator with 37 °C and 5% CO2. The RPM was operated in real random mode with random direction and interval and a maximum speed of 12.5 revolutions per minute. Sample flasks to be tested were placed onto the middle frame with a maximal distance of 7 cm to the center of rotation allowing a µg quality between 10−4 and 10−2 g, which is reached over time40,81. Corresponding static 1g-controls, which were completely filled with medium, were placed next to the RPM in the same incubator (n = 15 samples each group/run).
Phase contrast microscopy
Cells were observed and photographed using an Axiovert 25 Microscope (Carl Zeiss Microscopy, LLC, USA) and a Canon EOS 550D camera (Canon GmbH, Krefeld, Germany)23.
Sample collection
After 24 h the cells were investigated and photographed. The MCS were collected by mild centrifugation at 3000 g for 5 min and 4 °C and stored in liquid nitrogen. Harvesting of the adherent cells was performed, by using a cell scraper after carefully adding ice-cold phosphate buffered saline (PBS, Life Technologies). The suspension was collected and centrifuged at 3000 g for 5 min and 4 °C followed by discarding the PBS and storage of the pellet in liquid nitrogen.
Acridine orange/ethidium bromide staining
Control and RPM-exposed MCF-7 cells of both phenotypes MCS and adherently growing cells were stained with acridine orange/ethidium bromide (Molecular Probes, Darmstadt, Germany) as performed in previous studies82. The stained MCF-7 cells were immediately investigated by using a Zeiss LSM 710 confocal laser scanning microscope (Zeiss, Jena, Germany) as previously described9.
Indirect immunofluorescence staining of NFκB
Immunofluorescence analysis of NFκB p65 was performed on 80% confluent MCF-7 cells after a 24 hour exposure to the RPM. The cells were fixed with 4% paraformaldehyde for 25 minutes at room temperature (RT), permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 h at RT. Afterwards, the cells were labelled with NFκB [p65] rabbit polyclonal antibody (Thermo Fisher Scientific) at 2 µg/mL in 1% BSA and incubated overnight at 4 °C, then labelled with Alexa Fluor 488 goat anti-rabbit IgG secondary antibody (Thermo Fisher Scientific) at a dilution of 1:400 for 1 h at RT and washed 3 times. For nuclear staining, we used DAPI (4′,6-diamidin-2-phenylindol) (Sigma-Aldrich, Taufkirchen, Germany) for 5 min and washed the cells twice with DPBS. For evaluation, the slides were mounted with Fluoroshield (Sigma-Aldrich, Taufkirchen, Germany) and analysed with a Zeiss LSM 710 confocal laser scanning microscope9.
Western Blot Analysis
Western blot analysis was performed as recently published17. The RPM experiment for the Western blot analyses was performed three times. In each of these experiments five different culture flasks were mounted on the RPM. In parallel five 1g-control flasks were cultured next to the RPM. At the end of the experiment cells were harvested and an aliquot from each flask was subjected to Western blot analysis. In RPM-samples we detected two phenotypes (RPM-AD cells and RPM-MCS). Hence, the Western blot contains ten lanes loaded with RPM samples (AD and MCS), and five lanes loaded with 1g-control samples. The Western blot experiment was repeated thrice. The concentration was adjusted to a total protein load of 30 µg per well in Laemmli buffer. Criterion XT 4–12% precast gels (Biorad, Hercules, USA) were loaded and run for 1 h at 150 volts. Transfer to a PVDF membrane was performed at 100 volts and 30 minutes. Membranes were blocked in 0.3% I-Block (Applied Biosystems, Foster City, USA) in TBS-T overnight. The antibodies listed in Table 1 were applied for 2 h at room temperature followed by washing steps. The applied secondary antibody, a Horseradish peroxidase (HRP)-linked antibody was utilized at a dilution of 1:4000 (Cell Signaling Technology, Inc., Danvers, MA, USA) for additional 2 h at room temperature. Membranes were developed using Biorad Clarity Western ECL and imaged with an Image Quant LAS 4000 mini (GE Healthcare Life Science, Freiburg, Germany). Cofilin (CFL1) [EPR6375] (ABCAM, Cambridge, UK) was used as a loading control. The membranes were analyzed using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA; http://rsb.info.nih.gov/ij/) for densitometric quantification of the bands83.
RNA and protein extraction
The RNA and protein extraction were performed using the AllPrep RNA/Protein kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s protocol. The concentrations were determined with the spectrophotometer Ultrospec 2100 pro (Amersham Biosciences, Amersham, Great Britain).
Quantitative real-time PCR
Complementary DNA was produced using the First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) following manufacturer’s instructions. qrtPCR was performed using the SYBR® Select Master Mix (Applied Biosystems, Darmstadt, Germany) and the 7500 Real-Time PCR System (Applied Biosystems) to determine the expression levels of target genes, shown in Table 2. Selective primers were designed to span exon-exon boundaries and to have a Tm of 60 °C using Primer Express software (Applied Biosystems), and were synthesized by TIB Molbiol (Berlin, Germany). Samples were measured in triplicate and were normalized to the housekeeper 18 S rRNA. Comparative threshold cycle (ΔΔCT) methods were used for relative quantification of transcription levels, with 1 g set as 100%84.
Microarray technique
The 25 Illumina HumanWG-6_V2_0_R3 arrays have been normalized using the BeadStudio Gene Expression Module v3.3.7, and quantile normalization without background correction. After quantile normalization and exclusion of low or not expressed genes (minimum Illumina detection p-value > 0.05) a parametric ANOVA comparing the conditions control, AD and MCS was performed. Probes which undergo 5% FDR85 were selected as differential expressed. Differentiation of the expression profiles was performed using hierarchical and k-mean clustering. The cluster analysis was done using Partek Genomic Suite 6.3 applying hierarchical average linkage clustering and k-mean clustering with Euclidean distance function on standardized log2 signal values. K was selected by analyses of the hierarchical clustering dendrograms.
Pathway analyses
To investigate mutual regulation of genes and to visualize localization and interactions between proteins, we entered relevant UniProtKB entry numbers in the Pathway Studio v.11 software (Elsevier Research Solutions, Amsterdam, The Netherlands). Graphs were generated for gene expression and protein regulation and binding. The method was described previously9,52. STITCH 4 (Chemical-Protein Interaction Networks, European Molecular Biology Laboratory (EMBL), Heidelberg, Germany) was applied to investigate the interaction of DEX, olaparib and rolipram with their targets. The data is given in Fig. 8.
Statistical evaluation
Statistical evaluation was performed using SPSS 15.0 (SPSS, Inc., Chicago, IL, USA). The Mann-Whitney-U-Test was used to compare 1 g and s-μg conditions, as well as AD cells and MCS cells. All data is presented as mean ± standard deviation (SD) with a significance level of *p < 0.05.
Electronic supplementary material
Supplemental Table 1 and Supplemental Fig. 1
Acknowledgements
This study was supported by the German Space Agency DLR (D.G.) (BMWi grant 50WB1524), and Aarhus University, Denmark (D.G., T.J.C.).
Author Contributions
D.G., T.J.C. and S.K. designed the experiment. S.K. and T.I. executed the experiments and collected the material. A.G.P., L.S., J.S. and S.R. performed western blot analyses. S.K. and M.W. performed qrtPCR analyses. J.B. performed the pathway analyses. K.S., N.H. and H.S. performed and analysed the microarray. S.K. performed the olaparib-, DEX- and rolipram-drug tests. D.G., J.B., T.J.C. and S.K. wrote the manuscript. M.I. and R.L. contributed reagents, materials and analysis tools. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-18556-8.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grimm D, et al. Simulated microgravity alters differentiation and increases apoptosis in human follicular thyroid carcinoma cells. FASEB J. 2002;16:604–606. doi: 10.1096/fj.01-0673fje. [DOI] [PubMed] [Google Scholar]
- 2.Pietsch J, et al. Spheroid formation of human thyroid cancer cells in an automated culturing system during the Shenzhou-8 Space mission. Biomaterials. 2013;34:7694–7705. doi: 10.1016/j.biomaterials.2013.06.054. [DOI] [PubMed] [Google Scholar]
- 3.Pietsch J, et al. A proteomic approach to analysing spheroid formation of two human thyroid cell lines cultured on a random positioning machine. Proteomics. 2011;11:2095–2104. doi: 10.1002/pmic.201000817. [DOI] [PubMed] [Google Scholar]
- 4.Ulbrich C, et al. Characterization of human chondrocytes exposed to simulated microgravity. Cell. Physiol. Biochem. 2010;25:551–560. doi: 10.1159/000303059. [DOI] [PubMed] [Google Scholar]
- 5.Grimm D, et al. Different responsiveness of endothelial cells to vascular endothelial growth factor and basic fibroblast growth factor added to culture media under gravity and simulated microgravity. Tissue Eng. Part A. 2010;16:1559–1573. doi: 10.1089/ten.tea.2009.0524. [DOI] [PubMed] [Google Scholar]
- 6.Masiello MG, et al. Phenotypic switch induced by simulated microgravity on MDA-MB-231 breast cancer cells. Biomed. Res. Int. 2014;2014:652434. doi: 10.1155/2014/652434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingram M, et al. Three-dimensional growth patterns of various human tumor cell lines in simulated microgravity of a NASA bioreactor. In Vitro Cell. Dev. Biol. Anim. 1997;33:459–466. doi: 10.1007/s11626-997-0064-8. [DOI] [PubMed] [Google Scholar]
- 8.Chang TT, Hughes-Fulford M. Monolayer and spheroid culture of human liver hepatocellular carcinoma cell line cells demonstrate distinct global gene expression patterns and functional phenotypes. Tissue Eng. Part A. 2009;15:559–567. doi: 10.1089/ten.tea.2007.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corydon TJ, et al. Reduced Expression of Cytoskeletal and Extracellular Matrix Genes in Human Adult Retinal Pigment Epithelium Cells Exposed to Simulated Microgravity. Cell. Physiol. Biochem. 2016;40:1–17. doi: 10.1159/000452520. [DOI] [PubMed] [Google Scholar]
- 10.Kopp S, et al. Identifications of novel mechanisms in breast cancer cells involving duct-like multicellular spheroid formation after exposure to the Random Positioning Machine. Sci. Rep. 2016;6:26887. doi: 10.1038/srep26887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kossmehl P, et al. Weightlessness induced apoptosis in normal thyroid cells and papillary thyroid carcinoma cells via extrinsic and intrinsic pathways. Endocrinology. 2003;144:4172–4179. doi: 10.1210/en.2002-0171. [DOI] [PubMed] [Google Scholar]
- 12.Uva BM, et al. Microgravity-induced apoptosis in cultured glial cells. Eur. J. Histochem. 2002;46:209–214. doi: 10.4081/1681. [DOI] [PubMed] [Google Scholar]
- 13.Schatten H, Lewis ML, Chakrabarti A. Spaceflight and clinorotation cause cytoskeleton and mitochondria changes and increases in apoptosis in cultured cells. Acta. Astronaut. 2001;49:399–418. doi: 10.1016/S0094-5765(01)00116-3. [DOI] [PubMed] [Google Scholar]
- 14.Grosse J, et al. Gravity-sensitive signaling drives 3-dimensional formation of multicellular thyroid cancer spheroids. FASEB J. 2012;26:5124–5140. doi: 10.1096/fj.12-215749. [DOI] [PubMed] [Google Scholar]
- 15.Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J. Clin. 2005;55:178–194. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- 16.Song JK, et al. Cell growth inhibition and induction of apoptosis by snake venom toxin in ovarian cancer cell via inactivation of nuclear factor kappaB and signal transducer and activator of transcription 3. Arch. Pharm. Res. 2012;35:867–876. doi: 10.1007/s12272-012-0512-1. [DOI] [PubMed] [Google Scholar]
- 17.Bauer, J. et al. Proteome Analysis of Human Follicular Thyroid Cancer Cells Exposed to the Random Positioning Machine. Int. J. Mol. Sci. 18, 10.3390/ijms18030546 (2017). [DOI] [PMC free article] [PubMed]
- 18.Wu H, et al. A negative feedback loop between miR-200b and the nuclear factor-kappaB pathway via IKBKB/IKK-beta in breast cancer cells. FEBS J. 2016;283:2259–2271. doi: 10.1111/febs.13543. [DOI] [PubMed] [Google Scholar]
- 19.Becker-Weimann S, et al. NFkB disrupts tissue polarity in 3D by preventing integration of microenvironmental signals. Oncotarget. 2013;4:2010–2020. doi: 10.18632/oncotarget.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Perkins ND. Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene. 2006;25:6717–6730. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Chen ZJ. Regulation of NF-kappaB by ubiquitination. Curr. Opin. Immunol. 2013;25:4–12. doi: 10.1016/j.coi.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kopp S, et al. Mechanisms of three-dimensional growth of thyroid cells during long-term simulated microgravity. Sci. Rep. 2015;5:16691. doi: 10.1038/srep16691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benita Y, et al. An integrative genomics approach identifies Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res. 2009;37:4587–4602. doi: 10.1093/nar/gkp425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu P, Goh YY, Chin HF, Kersten S, Tan NS. Angiopoietin-like 4: a decade of research. Biosci. Rep. 2012;32:211–219. doi: 10.1042/BSR20110102. [DOI] [PubMed] [Google Scholar]
- 26.Bellot G, et al. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol. Cell Biol. 2009;29:2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Said HM, et al. Oxygen-dependent regulation of NDRG1 in human glioblastoma cells in vitro and in vivo. Oncol. Rep. 2009;21:237–246. [PubMed] [Google Scholar]
- 28.Lv Y, et al. PFKFB3-mediated glycolysis is involved in reactive astrocyte proliferation after oxygen-glucose deprivation/reperfusion and is regulated by Cdh1. Neurochem. Int. 2015;91:26–33. doi: 10.1016/j.neuint.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, et al. HIF-1alpha activates hypoxia-induced PFKFB4 expression in human bladder cancer cells. Biochem. Biophys. Res. Commun. 2016;476:146–152. doi: 10.1016/j.bbrc.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 30.Kim SM, Kim JY, Lee S, Park JH. Adrenomedullin protects against hypoxia/reoxygenation-induced cell death by suppression of reactive oxygen species via thiol redox systems. FEBS Lett. 2010;584:213–218. doi: 10.1016/j.febslet.2009.11.063. [DOI] [PubMed] [Google Scholar]
- 31.Aderem A. Signal transduction and the actin cytoskeleton: the roles of MARCKS and profilin. Trends Biochem. Sci. 1992;17:438–443. doi: 10.1016/0968-0004(92)90016-3. [DOI] [PubMed] [Google Scholar]
- 32.Dunn LL, et al. New insights into intracellular locations and functions of heme oxygenase-1. Antioxid. Redox Signal. 2014;20:1723–1742. doi: 10.1089/ars.2013.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.An S, Zheng Y, Bleu T. Sphingosine 1-phosphate-induced cell proliferation, survival, and related signaling events mediated by G protein-coupled receptors Edg3 and Edg5. J. Biol. Chem. 2000;275:288–296. doi: 10.1074/jbc.275.1.288. [DOI] [PubMed] [Google Scholar]
- 34.Khan AA, Quigley JG. Heme and FLVCR-related transporter families SLC48 and SLC49. Mol. Aspects Med. 2013;34:669–682. doi: 10.1016/j.mam.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White RJ, Averner M. Humans in space. Nature. 2001;409:1115–1118. doi: 10.1038/35059243. [DOI] [PubMed] [Google Scholar]
- 36.Adrian A, et al. The oxidative burst reaction in mammalian cells depends on gravity. Cell Commun. Signal. 2013;11:98. doi: 10.1186/1478-811X-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becker JL, Souza GR. Using space-based investigations to inform cancer research on Earth. Nat. Rev. Cancer. 2013;13:315–327. doi: 10.1038/nrc3507. [DOI] [PubMed] [Google Scholar]
- 38.Hauslage J, Cevik V, Hemmersbach R. Pyrocystis noctiluca represents an excellent bioassay for shear forces induced in ground-based microgravity simulators (clinostat and random positioning machine) NPJ microgravity. 2017;3:12. doi: 10.1038/s41526-017-0016-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wuest SL, Stern P, Casartelli E, Egli M. Fluid Dynamics Appearing during Simulated Microgravity Using Random Positioning Machines. PloS one. 2017;12:e0170826. doi: 10.1371/journal.pone.0170826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warnke E, et al. Spheroid formation of human thyroid cancer cells under simulated microgravity: a possible role of CTGF and CAV1. Cell Commun. Signal. 2014;12:32. doi: 10.1186/1478-811X-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warnke E, et al. Cytokine Release and Focal Adhesion Proteins in Normal Thyroid Cells Cultured on the Random Positioning Machine. Cell. Physiol. Biochem. 2017;43:257–270. doi: 10.1159/000480368. [DOI] [PubMed] [Google Scholar]
- 42.Comsa S, Cimpean AM, Raica M. The Story of MCF-7 Breast Cancer Cell Line: 40 years of Experience in Research. Anticancer Res. 2015;35:3147–3154. [PubMed] [Google Scholar]
- 43.do Amaral JB, Urabayashi MS, Machado-Santelli GM. Cell death and lumen formation in spheroids of MCF-7 cells. Cell Biol Int. 2010;34:267–274. doi: 10.1042/CBI20090024. [DOI] [PubMed] [Google Scholar]
- 44.Freed LE, Langer R, Martin I, Pellis NR, Vunjak-Novakovic G. Tissue engineering of cartilage in space. Proc. Natl. Acad. Sci. USA. 1997;94:13885–13890. doi: 10.1073/pnas.94.25.13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aleshcheva G, et al. Scaffold-free Tissue Formation Under Real and Simulated Microgravity Conditions. Basic Clin. Pharmacol. Toxicol. 2016;119(Suppl 3):26–33. doi: 10.1111/bcpt.12561. [DOI] [PubMed] [Google Scholar]
- 46.Grimm D, et al. Growing tissues in real and simulated microgravity: new methods for tissue engineering. Tissue Eng. Part B Rev. 2014;20:555–566. doi: 10.1089/ten.teb.2013.0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pietsch J, et al. Metabolic enzyme diversity in different human thyroid cell lines and their sensitivity to gravitational forces. Proteomics. 2012;12:2539–2546. doi: 10.1002/pmic.201200070. [DOI] [PubMed] [Google Scholar]
- 48.Zadeh MM, Motamed N, Ranji N, Majidi M. & Falahi, F. Silibinin-Induced Apoptosis and Downregulation of MicroRNA-21 and MicroRNA-155 in MCF-7 Human Breast Cancer Cells. J. Breast Cancer. 2016;19:45–52. doi: 10.4048/jbc.2016.19.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parente L, Solito E. Annexin 1: more than an anti-phospholipase protein. Inflamm. Res. 2004;53:125–132. doi: 10.1007/s00011-003-1235-z. [DOI] [PubMed] [Google Scholar]
- 50.Cregan SP, Dawson VL, Slack RS. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene. 2004;23:2785–2796. doi: 10.1038/sj.onc.1207517. [DOI] [PubMed] [Google Scholar]
- 51.Kim HP, Wang X, Galbiati F, Ryter SW, Choi AM. Caveolae compartmentalization of heme oxygenase-1 in endothelial cells. FASEB J. 2004;18:1080–1089. doi: 10.1096/fj.03-1391com. [DOI] [PubMed] [Google Scholar]
- 52.Riwaldt S, et al. The Importance of Caveolin-1 as Key-Regulator of Three-Dimensional Growth in Thyroid Cancer Cells Cultured under Real and Simulated Microgravity Conditions. Int. J. Mol. Sci. 2015;16:28296–28310. doi: 10.3390/ijms161226108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riwaldt S, et al. Identification of proteins involved in inhibition of spheroid formation under microgravity. Proteomics. 2015;15:2945–2952. doi: 10.1002/pmic.201500067. [DOI] [PubMed] [Google Scholar]
- 54.Xie Y, Wang Y, Zong C, Cheng J. Transforming growth factor-Beta inhibits heme oxygenase-1 expression in lung fibroblast through nuclear factor-kappa-B-dependent pathway. Pharmacology. 2014;93:185–192. doi: 10.1159/000360638. [DOI] [PubMed] [Google Scholar]
- 55.Park SY, et al. Induction of heme oxygenase-1 expression by cilostazol contributes to its anti-inflammatory effects in J774 murine macrophages. Immunol. Lett. 2011;136:138–145. doi: 10.1016/j.imlet.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Qi YF, et al. Adrenomedullin induces heme oxygenase-1 gene expression and cGMP formation in rat vascular smooth muscle cells. Peptides. 2005;26:1257–1263. doi: 10.1016/j.peptides.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 57.Katoh Y, et al. Methionine adenosyltransferase II serves as a transcriptional corepressor of Maf oncoprotein. Mol. Cell. 2011;41:554–566. doi: 10.1016/j.molcel.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 58.Hideshima H, et al. IKKbeta inhibitor in combination with bortezomib induces cytotoxicity in breast cancer cells. Int. J. Oncol. 2014;44:1171–1176. doi: 10.3892/ijo.2014.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tafani M, et al. Modulators of HIF1alpha and NFkB in Cancer Treatment: Is it a Rational Approach for Controlling Malignant Progression? Front. Pharmacol. 2013;4:13. doi: 10.3389/fphar.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zerfaoui M, et al. Nuclear translocation of p65 NF-kappaB is sufficient for VCAM-1, but not ICAM-1, expression in TNF-stimulated smooth muscle cells: Differential requirement for PARP-1 expression and interaction. Cell. Signal. 2008;20:186–194. doi: 10.1016/j.cellsig.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanchez-Lopez E, et al. CTGF promotes inflammatory cell infiltration of the renal interstitium by activating NF-kappaB. J. Am. Soc. Nephrol. 2009;20:1513–1526. doi: 10.1681/ASN.2008090999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hirata N, et al. Sphingosine-1-phosphate promotes expansion of cancer stem cells via S1PR3 by a ligand-independent Notch activation. Nat Commun. 2014;5:4806. doi: 10.1038/ncomms5806. [DOI] [PubMed] [Google Scholar]
- 63.Zaremba T, et al. Poly(ADP-ribose) polymerase-1 polymorphisms, expression and activity in selected human tumour cell lines. Br. J. Cancer. 2009;101:256–262. doi: 10.1038/sj.bjc.6605166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gilardini Montani MS, et al. ATM-depletion in breast cancer cells confers sensitivity to PARP inhibition. J. Exp. Clin. Cancer Res. 2013;32:95. doi: 10.1186/1756-9966-32-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim H, Tarhuni A, Abd Elmageed ZY, Boulares AH. Poly(ADP-ribose) polymerase as a novel regulator of 17beta-estradiol-induced cell growth through a control of the estrogen receptor/IGF-1 receptor/PDZK1 axis. J. Transl. Med. 2015;13:233. doi: 10.1186/s12967-015-0589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khan S, Lopez-Dee Z, Kumar R, Ling J. Activation of NFkB is a novel mechanism of pro-survival activity of glucocorticoids in breast cancer cells. Cancer Lett. 2013;337:90–95. doi: 10.1016/j.canlet.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 67.Chang MM, Juarez M, Hyde DM, Wu R. Mechanism of dexamethasone-mediated interleukin-8 gene suppression in cultured airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L107–115. doi: 10.1152/ajplung.2001.280.1.L107. [DOI] [PubMed] [Google Scholar]
- 68.Svejgaard B, et al. Common Effects on Cancer Cells Exerted by a Random Positioning Machine and a 2D Clinostat. PloS one. 2015;10:e0135157. doi: 10.1371/journal.pone.0135157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.El-Mowafy AM, Alkhalaf M. Resveratrol activates adenylyl-cyclase in human breast cancer cells: a novel, estrogen receptor-independent cytostatic mechanism. Carcinogenesis. 2003;24:869–873. doi: 10.1093/carcin/bgg015. [DOI] [PubMed] [Google Scholar]
- 70.Herve R, et al. The PDE4 inhibitor rolipram prevents NF-kappaB binding activity and proinflammatory cytokine release in human chorionic cells. J. Immunol. 2008;181:2196–2202. doi: 10.4049/jimmunol.181.3.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chira S, et al. CRISPR/Cas9: Transcending the Reality of Genome Editing. Mol Ther Nucleic Acids. 2017;7:211–222. doi: 10.1016/j.omtn.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choi K, Benveniste EN, Choi C. Induction of intercellular adhesion molecule-1 by Fas ligation: proinflammatory roles of Fas in human astroglioma cells. Neurosci. Lett. 2003;352:21–24. doi: 10.1016/j.neulet.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 73.Gorgoulis VG, et al. p53 activates ICAM-1 (CD54) expression in an NF-kappaB-independent manner. EMBO J. 2003;22:1567–1578. doi: 10.1093/emboj/cdg157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strell C, Lang K, Niggemann B, Zaenker KS, Entschladen F. Neutrophil granulocytes promote the migratory activity of MDA-MB-468 human breast carcinoma cells via ICAM-1. Exp. Cell Res. 2010;316:138–148. doi: 10.1016/j.yexcr.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 75.Chen L, Vicente-Manzanares M, Potvin-Trottier L, Wiseman PW, Horwitz AR. The integrin-ligand interaction regulates adhesion and migration through a molecular clutch. PloS one. 2012;7:e40202. doi: 10.1371/journal.pone.0040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van der Valk J, et al. Optimization of chemically defined cell culture media–replacing fetal bovine serum in mammalian in vitro methods. Toxicol. In Vitro. 2010;24:1053–1063. doi: 10.1016/j.tiv.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 77.Fawcett H, Mader JS, Robichaud M, Giacomantonio C, Hoskin DW. Contribution of reactive oxygen species and caspase-3 to apoptosis and attenuated ICAM-1 expression by paclitaxel-treated MDA-MB-435 breast carcinoma cells. Int. J. Oncol. 2005;27:1717–1726. [PubMed] [Google Scholar]
- 78.Ma X, et al. Genomic approach to identify factors that drive the formation of three-dimensional structures by EA.hy926 endothelial cells. PloS one. 2013;8:e64402. doi: 10.1371/journal.pone.0064402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pietsch J, et al. Three-dimensional growth of human endothelial cells in an automated cell culture experiment container during the SpaceX CRS-8 ISS space mission - The SPHEROIDS project. Biomaterials. 2017;124:126–156. doi: 10.1016/j.biomaterials.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 80.Buxant F, Kindt N, Laurent G, Noel JC, Saussez S. Antiproliferative effect of dexamethasone in the MCF-7 breast cancer cell line. Mol. Med. Rep. 2015;12:4051–4054. doi: 10.3892/mmr.2015.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Loon JJWA. Some history and use of the random positioning machine, RPM, in gravity related research. Adv. Space Res. 2007;39:1161–1165. doi: 10.1016/j.asr.2007.02.016. [DOI] [Google Scholar]
- 82.Magnusson NE, et al. Gene networks modified by sulphonylureas in beta cells: a pathway-based analysis of insulin secretion and cell death. Basic Clin. Pharmacol. Toxicol. 2012;111:254–261. doi: 10.1111/j.1742-7843.2012.00902.x. [DOI] [PubMed] [Google Scholar]
- 83.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wehland M, et al. The impact of altered gravity and vibration on endothelial cells during a parabolic flight. Cell. Physiol. Biochem. 2013;31:432–451. doi: 10.1159/000343380. [DOI] [PubMed] [Google Scholar]
- 85.Grosse J, et al. Short-term weightlessness produced by parabolic flight maneuvers altered gene expression patterns in human endothelial cells. FASEB J. 2012;26:639–655. doi: 10.1096/fj.11-194886. [DOI] [PubMed] [Google Scholar]
- 86.Kuhn M, et al. STITCH 4: integration of protein-chemical interactions with user data. Nucleic Acids Res. 2014;42:D401–407. doi: 10.1093/nar/gkt1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 and Supplemental Fig. 1