Abstract
Background
Tumor grade is an important predictor of survival in gastroenteropancreatic (GEP) neuroendocrine tumors (NETs), as determined by Ki-67 expression and mitotic rate. NETs generally grow indolently, but some cells may acquire traits facilitating metastasis. It is unclear how frequently metastases differ in grade from their primary tumors, and whether increasing grade in metastases affects prognosis.
Methods
Ki-67 immunohistochemistry was performed on resected GEPNET specimens and cases with results for both primary tumors and concurrent metastases were identified. Grade was determined using a modified WHO classification (Ki-67:G1= 0–2%; G2 >2–20%; G3 >20%).
Results
Ki-67 was performed on both the primary tumor and metastases in 103 patients. Tumor grade was higher in metastases from 25 (24%) patients, 24 increased from G1 to G2, and 1 from G2 to G3; 68 (66%) patients had no change in grade (42 G1 and 26 G2) and 10 (10%) decreased from G2 to G1. No clinicopathologic factors were predictive of higher grade in metastases. The 5-year PFS was 55 % for patients with stable grade vs. 8% with increased grade, while 5-year OS was 92% and 54%, respectively. The 5-year OS of patients who had stable grade with G1 and G2 primaries was 92% and 64%, respectively.
Conclusions
Nearly one-third of patients had metastases with a different grade than their primary, and when grade increased, both PFS and OS significantly decreased. Determining the grade in both the primary tumor and a metastasis is important for estimating prognosis and to help inform decisions regarding additional therapies.
Introduction
The incidence of gastroenteropancreatic neuroendocrine tumors (GEPNETs) is increasing, with rates as high as 21.3 cases per 100,000, amounting to a 60–130% rise during the two decades preceding 2010 (1, 2). This is due in part to increased detection, but there are likely other, as yet undetermined, factors contributing to this increase. As more people are found to have GEPNETs, it has become evident that not all of these tumors follow the same clinical course, and that there are some inherent biological characteristics that lead them to behave differently.
Two markers used to help infer the biologic behavior of tumors are those involved in determining differentiation or grade, namely mitotic index and Ki-67 immunohistochemical (IHC) staining. Mitotic index is performed using hematoxylin-eosin stained slides of tumors and identifying the number of mitotic figures within a high-powered field (HPF). Ki-67 is a nuclear antigen expressed only during proliferation. IHC for Ki-67 requires staining of the tumor with the monoclonal antibody MIB1, which targets human Ki-67. A total of 500–2000 tumor cells are examined, and the percentage of cells staining positive is reported.
Increasing tumor grade correlates with a decrease in overall and progression-free survival (PFS) in GEPNETs(3, 4), with one study reporting 5-year overall survival (OS) of 89%, 48% and 0% for grades 1, 2 and 3 respectively(3). Thus, grade is often used as a surrogate for the biological aggressiveness of these tumors, and it helps to predict disease which might advance more rapidly. Mitotic index and Ki-67 percentage are both currently used to determine grade, but they don’t always agree(5, 6). Ki-67 has been shown to be a more reliable marker, with one study demonstrating that Ki-67 provided better prognostic information in cases where mitotic index and Ki-67 grades were discrepant(5). The World Health Organization (WHO) and the European Neuroendocrine Tumor Society use these indices to stratify GEPNETs into three grades. Using the Ki-67 percentage, both systems divide GEPNETs into the following grades: G1 is <2%, G2 is 3–20%, and G3 is >20% (7, 8). This classification scheme leaves some uncertainty for tumors falling in the 2–2.9% range, which has been interpreted differently at various institutions(7).
The current practice in histopathology laboratories is to report the grade of primary tumors in GEPNETs, but few routinely report the grade of metastases. However, if survival is ultimately determined by the behavior of the most aggressive tumors present, then it could be prognostic to know the grade of metastatic tumors as well. In this study, we set out to determine if tumor grade differed between primary GEPNETs and metastases, and whether increasing grade in metastases was associated with differences in patient survival.
Methods
A University of Iowa Institutional Review Board approved, prospective database was used to identify patients who underwent surgery for neuroendocrine tumors at a single tertiary medical center. Pathology reports were reviewed for patients who had pathology samples for at least two different tumor sites. The MIB-1 antibody was used for IHC of cross-sections of formalin-fixed, paraffin-embedded tumors in our central pathology laboratory. A database was created which included other clinicopathologic data combined with the results from the Ki-67 staining.
A total of 270 patients were identified with 232 primary tumor and 335 metastasis samples, which were stained with MIB-1 for determination of Ki-67 activity. Areas of high tumor density were identified on H&E stained slides and then these areas were specifically examined on corresponding Ki-67 stained slides. A minimum of 500 tumor cells per 400× field were counted. This threshold was lowered to 350 cells for a few samples where a single field containing 500 tumor cells could not be identified. Tumor cells with any MIB-1 activity were considered positive and it was this number divided by the total number of tumor cells in the HPF that was used to calculate the Ki-67 percentage. Tumor samples were then graded using the highest Ki-67 result recorded and a modified WHO classification system (G1 Ki-67=0–2%; G2 >2–20%; G3 >20%)(7, 8).
Upon completion of grading, patients who had grades for both a primary tumor and a metastasis were divided into groups based upon whether there was a change in grade between the primary tumor and the corresponding metastasis with the highest grade. Clinicopathologic characteristics of these groups were then compared using the Welch’s t-test, Kaplan-Meier method, Chi-Squared test, Log-Rank test, and Cox regression modeling with R2 analysis. Statistical analysis was performed using R v3.1.2 (Vienna, Austria). P-values less than 0.05 were considered significant.
Results
We identified 103 patients who had IHC results for both their primary tumor and at least one metastasis. Primary tumors were located in the small bowel (SBNETs, n=79), pancreas (PNETs, n=21), duodenum (2), and colon (1)(Table 1). Lymph nodes metastases were identified in 77/78 (99%) patients with SBNETS and 16/21 (76%) patients with PNETs, while liver metastases were noted in 64/79 (81%) SBNET cases and 14/21 (67%) PNET cases. All patients had their primary tumors excised and 60 underwent liver directed surgery. Other treatments included octreotide (n= 91), hepatic artery embolization (HAE, n=22), peptide receptor radionuclide therapy (PRRT, n=20) and chemo/targeted therapy (n=19). In addition to all primary tumors, Ki-67 was performed on lymph nodes in 90 patients and liver metastases in 60 patients (47 patients had Ki-67 staining in both liver and nodal metastases). The site of highest Ki-67 was the primary in 48 cases, the lymph nodes in 38 and liver in 17 (Table 1).
Table 1.
Tumor Sites and Grades
| n | |
|---|---|
| Site | |
| Primary | |
| Small Bowel | 79 |
| Pancreas | 21 |
| Duodenum | 2 |
| Colon | 1 |
| Metastasis | |
| Liver Only | 13 |
| Lymph Node Only | 43 |
| Liver and Lymph Node | 47 |
| Highest Ki-67 Location | |
| Primary Tumor | 48 |
| Liver Met | 17 |
| Lymph Node | 38 |
| Grade | |
| Stable | |
| G1->G1 | 42 |
| G2->G2 | 26 |
| Increased | |
| G1->G2 | 24 |
| G2->G3 | 1 |
| Decreased | |
| G2->G1 | 10 |
G1->G1: Grade 1 primary and metastasis;
G2->G2: Grade 2 primary and metastasis
G1->G2: Grade 1 primary and Grade 2 metastasis
G2->G3: Grade 2 primary and Grade 3 metastasis
G2->G1: Grade 2 primary and Grade 1 metastasis
In instances where individual patients had Ki-67 results for multiple primary tumors, lymph nodes or liver metastases, concordance of grade in primary tumors was found in 29/38 (76%) patients, 26/39 (67%) patients for lymph nodes and 12/20 (60%) patients for liver metastases. In the 47 patients who had Ki-67 staining performed on both lymph nodes and liver metastases, the grade was the same in 32 cases, lymph nodes had a higher grade than liver metastases in 10 cases, and the liver metastases had a higher grade than the lymph nodes in 5 cases.
We found 25 patients (24%) who had a metastasis with increased grade, with 24 increasing from G1 to G2, and 1 from G2 to G3 (Table 1). The patient with a G3 metastasis was the only patient with a high-grade tumor and was excluded from survival analyses. Sixty-eight patients (66%) had stable grade in their primary tumor and metastases, 42 with G1 tumors, and 26 with G2 tumors. There were 10 patients who had decreased grade in their metastases, all with G2 primaries and G1 metastases.
The groups with G1 primaries and stable vs. increased grade in metastases were statistically similar in most categories, including mean age at surgery, mean number of positive lymph nodes and mean Ki-67 value of the primary tumor (Table 2). There was a difference in the mean Ki-67 value for metastases, 4.73% in the increased group versus 1.07% in the stable group, (p<0.01).
Table 2.
Patient and tumor characteristics of patients with G1 primary tumors
| Stable Grade | Increased Grade | P-value | |
|---|---|---|---|
| Mean Age at Surgery (yrs.) | 62.1 | 59.4 | 0.40 |
| Mean Size of Primary (cm) | 2.12 | 2.59 | 0.25 |
| Mean Size of largest LN (cm) | 2.21 | 1.71 | 0.34 |
| Mean Number of Positive Nodes | 4.15 | 6.74 | 0.37 |
| Mean Ki-67 of primary | 1.10 | 1.22 | 0.28 |
| Mean Ki-67 of met | 1.07 | 4.73 | <0.01 |
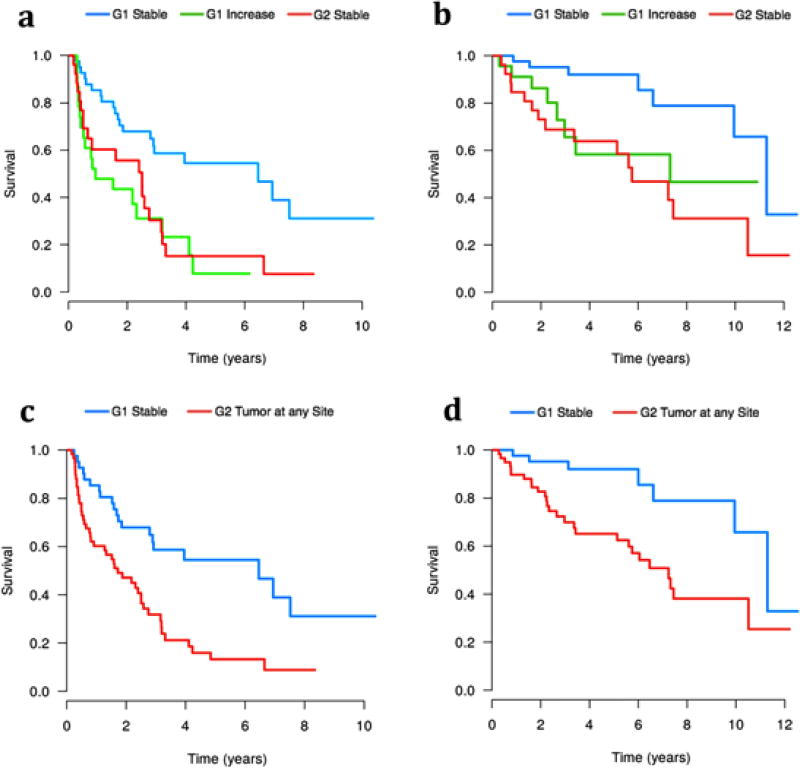
A significant survival difference existed between patients with G1 primary tumors who had the same (stable) grade between the primary tumor and metastases and those with increased grade in their metastases. PFS was significantly decreased for patients with G1 primaries and G2 metastases, with a median PFS of 0.9 years versus 6.5 years in those with stable grade (p<0.01). The majority of the G1 stable group remained progression-free at 5 years (54%), compared to only 7.8% in the increased grade group (Figure 1a). The increased grade group also had a shorter OS with a median of 7.3 years vs. 11.3 years in the stable group (p=0.02; Figure 1b). The 5 year OS was 92.1% for the G1 stable group, which fell to 58.4% for the increased group.
Figure 1.
a: Progression-free survival for patients with G1 primary and stable grade compared to those patients with G1 primaries and increased grade and those patients with G2 primaries and stable grade. b: Overall Survival of patients with G1 primary and stable grade compared to those patients with G1 primaries and increased grade and those patients with G2 primaries and stable grade. c: Progression-free survival of patients with G1 primary and stable grade versus all patients with any G2 tumor. d: Overall survival of patients with G1 primary and stable grade versus all patients with any G2 tumor.
Patients with G2 primaries and metastases behaved similarly to those with G1 primaries and G2 metastases, both with significantly worse PFS (Figure 1a) and OS (Figure 1b) than the G1 stable group. The median OS was 7.3 years versus 5.6 years for the G1 primaries>G2 metastases and G2 stable groups, respectively (p=0.32). A survival comparison was made between patients with G1 stable tumors and those patients with a G2 tumor at any site. This comparison demonstrated a significantly worse PFS (Figure 1c) and OS (Figure 1d) for patients with G2 tumors at any site, with a median OS of 7.2 years versus 11.3 years for patients with G1 stable tumors (p<0.01).
The G1/G2 cutoff used was 2%, but cutoffs of 3% and 5% were also tested for differences in survival. The results for the 3% cutoff mirrored those of the 2% cutoff, showing a significant decrease in PFS and OS for patients with G1 primaries and increased grade as compared with patients with G1 primaries and stable grade. A significant survival difference was not seen when using a 5% cutoff.
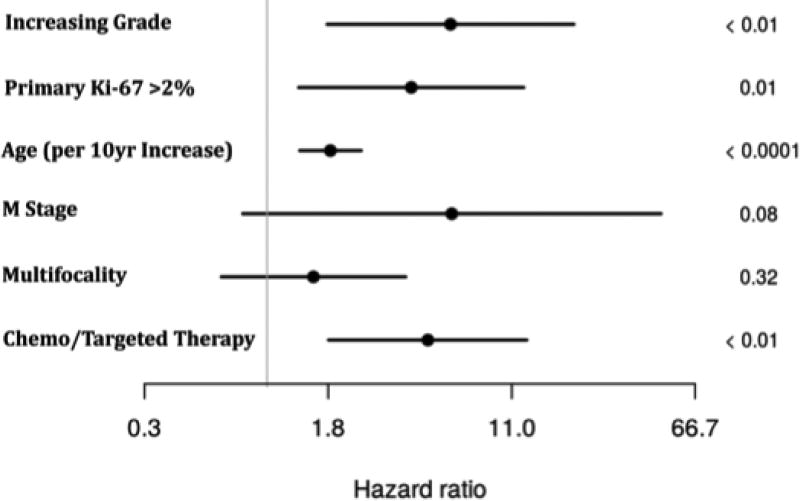
In order to further examine the role of Ki-67 based grading as it relates to OS, a multivariate regression model was created from variables that were found to be significant or approaching significance on univariate analysis and had no missing data points. The model took into account age, M stage, multifocality, administration of chemo/targeted therapy, primary tumor Ki-67 value, and increasing grade in metastasis. Liver directed surgery and PRRT were also considered but neither reached significance on univariate analysis and were excluded from multivariate calculations. On multivariate analysis, multifocality and M stage did not reach significance but all other factors remained as independent predictors of survival (Table 3). Increased grade in metastasis was the independent factor with the highest hazard ratio (HR=6.1; p< 0.01), followed by having undergone chemo/targeted therapy (HR=4.8; p<0.01), and primary Ki-67 value >2% (HR=4.1; p=0.01),while age had the lowest independent risk (HR=1.9; p<0.01; Figure 2).
Table 3.
Univariate and Multivariate Cox Modeling
| Variable | Univariate Analysis
|
Multivariate Analysis
|
|||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P- value |
HR | 95% CI | P- value |
||
| Survival by Group: | G1->G1 | 1.0 | - | - | - | - | - |
| G1->G2 | 3.2 | 1.1 – 9.4 | 0.03 | - | - | - | |
| G2->G2 | 4.0 | 1.6 – 9.9 | <0.01 | - | - | - | |
| G2->G1 | 3.2 | 0.8 – 12.0 | 0.10 | - | - | - | |
| Increased Grade in Metastasis* | True vs. False | - | - | - | 6.1 | 1.8 – 20.3 | <0.01 |
| M Stage | 1 vs. 0 | 1.7 | 1.0 – 3.0 | 0.02 | 6.1 | 0.8 – 47.7 | 0.08 |
| Primary Ki-67 | >2% vs. ≤2% | 2.5 | 1.3 – 5.0 | <0.01 | 4.1 | 1.4 – 12.4 | 0.01 |
| Primary Size | Continuous | 1.1 | 0.8 – 1.5 | 0.71 | - | - | - |
| Age | 10 yr increments | 1.5 | 1.1 – 2.2 | 0.02 | 1.9 | 1.4 – 2.5 | <0.01 |
| Multifocality | True vs. False | 0.8 | 0.4 – 1.6 | 0.45 | 1.6 | 0.6 – 3.9 | 0.32 |
| Primary Site | Small Bowel vs. Pancreas | 1.0 | 0.4 – 2.1 | 0.93 | - | - | - |
| Chemo/Targeted therapy | Yes vs. No | 5.9 | 2.8 – 12.1 | <0.01 | 4.8 | 1.8 – 12.8 | <0.01 |
| PRRT | Yes vs. No | 0.5 | 0.2 – 1.3 | 0.12 | - | - | - |
| Liver Directed Surgery | Yes vs. No | 1.0 | 0.5 – 2.0 | 0.95 | - | - | - |
The multivariate model focused on whether increased grade in the metastasis was an independent predictor of survival, regardless of grouping.
PRRT = peptide receptor radionuclide therapy.
Figure 2.
Hazard ratio plot with confidence intervals generated from multivariate regression analysis of factors affecting overall survival.
Discussion
Our results demonstrate that it is not uncommon for metastases of neuroendocrine tumors to differ in Ki-67 value from their primary tumors. Heterogeneity of Ki-67 values between tumors has also been reported by other groups(9, 10). Grillo et al. described these differences when examining primary tumors, where they found 3/60 (5%) patients had Ki-67 differences between different primary tumor sections that were large enough to change grade. This increased to 23% (11/47) when comparing primary tumors and synchronous metastases(9). We found a similar rate of change in the current study, with 24% of patients having an increase in grade, while another 10% had a decrease in grade of their metastases relative to their primary tumors.
Most of our patients with increased grade in metastases changed from G1 to G2 (24/25), both of which are considered to be well-differentiated, raising the question of whether these changes would have clinical significance. We found that patients with G1 primary tumors and G2 metastases did significantly worse than patients with G1 primary tumors and stable grade. Increased grade was associated with a decrease in both OS (58% versus 92% in stable grade patients at 5 years) and PFS (8% versus 54% at 5 years), despite the two groups having similar clinicopathologic characteristics. Our patients with G1 primaries and stable metastases had a similar 5 year OS rate to that reported by Grillo et al. for G1 tumors (92% versus 89%, respectively), and our patients whose metastases increased to G2 had a 5 year OS rate comparable to their patients with G2 primaries (58% versus 48%, respectively)(3). We compared our patients with G1 primary tumors and G2 metastases with our patients who had both G2 primaries and metastases. Although the patients who had increased grade in their metastases did somewhat worse, there was no significant difference in PFS (p=0.55) or OS (p=0.32). These findings suggest that survival correlates best with the highest grade tumor found in each patient. This was supported by the Kaplan-Meier comparison of patients who had only G1 tumors (G1 stable group), with those patients who had a G2 tumor at any location. This demonstrated a decreased OS for those with any G2 tumors (Figure 1d), reinforcing the hypothesis that the highest grade at any site is the predominant determinant of survival when using grade as a prognostic marker.
The greatest HR for OS found in this study was associated with having increasing grade in a metastasis (HR=6.1; p< 0.01), which exceeded that of patients undergoing chemo/targeted therapy (HR=4.8; p<0.01). The significance of chemo/targeted therapy is likely secondary to selection bias as those patients recommended to undergo chemo/targeted therapy typically have progressive disease. Having Ki-67 value >2% in the primary tumor was also an independent prognostic factor for decreased OS (HR=4.1; p=0.01), as was increasing age. Multifocality and M stage did not significantly affect OS on multivariate analysis, the latter potentially due to the fact that the majority of patients in this study had distant metastases (85/103, 83%).
The change in grade of metastases and its effect on survival may alter discussions with patients regarding their prognosis, but its impact on treatment remains to be seen. Only one of our patients had an increase to grade 3, which limits our ability to discuss the impact of this change. Those patients with changes from grade 1 to grade 2 would still be considered well-differentiated and treatment might not necessarily change under current management strategies. It is common practice to give patients with poorly-differentiated grade 3 tumors cisplatin and etoposide(11–13), often combined with somatostatin analogues (SSA) such as octreotide or lanreotide(14–16). For patients with low grade GEPNETs and metastases, various combinations of removing the primary tumor, debulking of metastases(17, 18), and SSAs are the most frequently employed treatments. Other regimens for metastatic disease in patients with GEPNETs include everolimus(19–21), sunitinib(22), or temozolomide combined with capecitabine(23–25). The latter agents are often reserved for when progression is seen on SSA therapy, but their use might also be considered in patients whose metastases are found to have a higher grade, since this was associated with reduced survival in this study. It is our practice to follow our patients every 6 months to evaluate for progression by serum markers and imaging with the plan to modify therapy at progression (increase the dose or decrease the interval of SSAs, refer for PRRT, embolization, chemo/targeted therapy). Based upon the finding of reduced survival for those patients with increasing grade (G1>G2) in metastases, we would have a lower threshold for augmenting treatment in patients with G2 metastases.
The grading system we used was a modified WHO system, which classified tumors with Ki-67 of >2% and up to 20% as G2, rather than having a gap between >2% and 3% as proposed in the original WHO classification(7, 8). Others have proposed increasing the G1 upper bound to 3%(26), and some have proposed using even higher cutoffs of 5–9% for G2 tumors in order to better separate the biological behavior of NETs(27, 28). Richards-Taylor et al. demonstrated that when the G1/G2 cutoff was increased to 5%, the 5 year OS decreased from 70% to 51% in the G2 group, but remained at 89% in the G1 group(27). This suggested that the patients with Ki-67 values of less than 5% behave similarly, and implied that the G2 threshold should be revised. Hamilton et al. created their own divisions, <5% for G1, 5–9% for G2 and >9% for G3 and showed that these cutoffs resulted in significant differences in OS(28). We reanalyzed our G1/G2 cutoffs for primary tumors using 3% and 5%, and found statistically significant differences for OS and PFS when comparing the increased grade and stable grade groups using a 3% G1/G2 cutoff, but not 5%. R2 analysis demonstrated that a G1/G2 cutoff of ~2.25% was better than 3% or 4% (R = 0.23, 0.18 and 0.15, respectively). Larger studies need to be performed to identify the G1/G2 cutoff that provides the best prognostic information, as there is no consensus on which modified system represents the best solution. The current WHO grade classification of not specifying the numeric grade assigned to Ki-67 values >2 through 3% results in confusion when comparing studies, and it therefore should be revised.
The findings of this study suggest that staining of GEPNET metastases for Ki-67 should be performed when tissue is available to 1) provide better prognostic information and 2) identify patients more likely to progress and therefore have closer follow-up and potentially earlier intervention. This staining should be performed in addition to and not to the exclusion of primary tumor staining. The time required to classify tumors using Ki-67 has been described as 45–64 minutes per case(3), but only about 5 minutes of that is the Pathologist’s time to read the slide. Additional studies will help to clarify the full impact of increasing grade in GEPNET metastases, which will be further facilitated by developing a unified grading system without gaps in the 2–3% range. This unified system in conjunction with regular Ki-67 staining of metastases will give improved prognostic information and potentially help select patients who would benefit from more aggressive medical therapy.
Acknowledgments
T32: T32CA148062 (KK, JM) and SPORE: P50CA174521 (JH, JD, PB, GL, TO, AB).
References
- 1.Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121(4):589–97. doi: 10.1002/cncr.29099. [DOI] [PubMed] [Google Scholar]
- 2.Boyar Cetinkaya R, Aagnes B, Thiis-Evensen E, Tretli S, Bergestuen DS, Hansen S. Trends in Incidence of Neuroendocrine Neoplasms in Norway: A Report of 16,075 Cases from 1993 through 2010. Neuroendocrinology. 2015:1–10. doi: 10.1159/000442207. [DOI] [PubMed] [Google Scholar]
- 3.Grillo F, Albertelli M, Annunziata F, Boschetti M, Caff A, Pigozzi S, et al. Twenty years of gastroenteropancreatic neuroendocrine tumors: is reclassification worthwhile and feasible? Endocrine. 2015:58–62. doi: 10.1007/s12020-015-0734-3. [DOI] [PubMed] [Google Scholar]
- 4.Yang M, Ke NW, Zeng L, Zhang Y, Tan CL, Zhang H, et al. Survival Analyses for Patients With Surgically Resected Pancreatic Neuroendocrine Tumors by World Health Organization 2010 Grading Classifications and American Joint Committee on Cancer 2010 Staging Systems. Medicine (Baltimore) 2015;94(48):e2156. doi: 10.1097/MD.0000000000002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan MS, Luong TV, Watkins J, Toumpanakis C, Caplin ME, Meyer T. A comparison of Ki-67 and mitotic count as prognostic markers for metastatic pancreatic and midgut neuroendocrine neoplasms. Br J Cancer. 2013;108(9):1838–45. doi: 10.1038/bjc.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCall CM, Shi C, Cornish TC, Klimstra DS, Tang LH, Basturk O, et al. Grading of well-differentiated pancreatic neuroendocrine tumors is improved by the inclusion of both Ki67 proliferative index and mitotic rate. Am J Surg Pathol. 2013;37(11):1671–7. doi: 10.1097/PAS.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rindi G, Kloppel G, Couvelard A, Komminoth P, Korner M, Lopes JM, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2007;451(4):757–62. doi: 10.1007/s00428-007-0452-1. [DOI] [PubMed] [Google Scholar]
- 8.Rindi G, Arnold R, Bosman FT, Capella C, Klimstra DS, Kloppel G, et al. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. 4. Lyon: International Agency for Research on Cancer; 2010. pp. 13–4. [Google Scholar]
- 9.Grillo F, Albertelli M, Brisigotti MP, Borra T, Boschetti M, Fiocca R, et al. Grade Increases in Gastro-Entero-Pancreatic Neuroendocrine Tumor Metastases Compared to the Primary Tumor. Neuroendocrinology. 2015:452–9. doi: 10.1159/000439434. [DOI] [PubMed] [Google Scholar]
- 10.Shi C, Gonzalez RS, Zhao Z, Koyama T, Cornish TC, Hande KR, et al. Liver metastases of small intestine neuroendocrine tumors: Ki-67 heterogeneity and World Health Organization grade discordance with primary tumors. Am J Clin Pathol. 2015;143(3):398–404. doi: 10.1309/AJCPQ55SKOCYFZHN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitry E, Baudin E, Ducreux M, Sabourin JC, Rufie P, Aparicio T, et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer. 1999;81(8):1351–5. doi: 10.1038/sj.bjc.6690325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moertel CG, Kvols LK, O'Connell MJ, Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68(2):227–32. doi: 10.1002/1097-0142(19910715)68:2<227::aid-cncr2820680202>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 13.Fjallskog ML, Granberg DP, Welin SL, Eriksson C, Oberg KE, Janson ET, et al. Treatment with cisplatin and etoposide in patients with neuroendocrine tumors. Cancer. 2001;92(5):1101–7. doi: 10.1002/1097-0142(20010901)92:5<1101::aid-cncr1426>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 14.Caplin ME, Pavel M, Cwikla JB, Phan AT, Raderer M, Sedlackova E, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–33. doi: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- 15.Frilling A, Clift AK. Therapeutic strategies for neuroendocrine liver metastases. Cancer. 2015;121(8):1172–86. doi: 10.1002/cncr.28760. [DOI] [PubMed] [Google Scholar]
- 16.Rinke A, Muller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656–63. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 17.Givi B, Pommier SJ, Thompson AK, Diggs BS, Pommier RF. Operative resection of primary carcinoid neoplasms in patients with liver metastases yields significantly better survival. Surgery. 2006;140(6):891–7. doi: 10.1016/j.surg.2006.07.033. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 18.Maxwell JE, Sherman SK, O'Dorisio TM, Bellizzi AM, Howe JR. Liver-directed surgery of neuroendocrine metastases: What is the optimal strategy? Surgery. 2016;159(1):320–35. doi: 10.1016/j.surg.2015.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387(10022):968–77. doi: 10.1016/S0140-6736(15)00817-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–23. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu E, Marincola P, Oberg K. Everolimus in the treatment of patients with advanced pancreatic neuroendocrine tumors: latest findings and interpretations. Therap Adv Gastroenterol. 2013;6(5):412–9. doi: 10.1177/1756283X13496970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–13. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 23.Abdel-Rahman O, Fouad M. Temozolomide-based combination for advanced neuroendocrine neoplasms: a systematic review of the literature. Future Oncol. 2015;11(8):1275–90. doi: 10.2217/fon.14.302. [DOI] [PubMed] [Google Scholar]
- 24.Claringbold PG, Price RA, Turner JH. Phase I-II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother Radiopharm. 2012;27(9):561–9. doi: 10.1089/cbr.2012.1276. [DOI] [PubMed] [Google Scholar]
- 25.Welin S, Sorbye H, Sebjornsen S, Knappskog S, Busch C, Oberg K. Clinical effect of temozolomide-based chemotherapy in poorly differentiated endocrine carcinoma after progression on first-line chemotherapy. Cancer. 2011;117(20):4617–22. doi: 10.1002/cncr.26124. [DOI] [PubMed] [Google Scholar]
- 26.Cherenfant J, Talamonti MS, Hall CR, Thurow TA, Gage MK, Stocker SJ, et al. Comparison of tumor markers for predicting outcomes after resection of nonfunctioning pancreatic neuroendocrine tumors. Surgery. 2014;156(6):1504–10. doi: 10.1016/j.surg.2014.08.043. discussion 10–1. [DOI] [PubMed] [Google Scholar]
- 27.Richards-Taylor S, Ewings SM, Jaynes E, Tilley C, Ellis SG, Armstrong T, et al. The assessment of Ki-67 as a prognostic marker in neuroendocrine tumours: a systematic review and meta-analysis. J Clin Pathol. 2015 doi: 10.1136/jclinpath-2015-203340. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton NA, Liu TC, Cavatiao A, Mawad K, Chen L, Strasberg SS, et al. Ki-67 predicts disease recurrence and poor prognosis in pancreatic neuroendocrine neoplasms. Surgery. 2012;152(1):107–13. doi: 10.1016/j.surg.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]