Abstract
Objective
Subjective memory change (SMC) in older individuals may represent a harbinger of cognitive decline. This study examined the factors associated with SMC in older African Americans (AA), who have greater risk of developing dementia. We predicted that symptoms of depression and anxiety, as well as the total number of cerebrovascular risk factors (tCVRFs), but not performances on objective memory measures, would be positively associated with SMC.
Methods
Ninety-six AA completed brief cognitive testing and answered questions about mood and memory at their primary care appointment. Vascular data were obtained from medical records.
Results
Symptoms of depression and anxiety, but not performances on objective memory measures, were positively associated with SMC, t(χ2(1) = 16.55 and 12.94, respectively, both P < .001). In nondepressed participants, the tCVRF was important in distinguishing between those with and without SMC.
Conclusions
In older AA, symptoms of depression or anxiety were associated with SMC. In nondepressed AA, the tCVRFs were important in distinguishing between those with and without SMC.
Keywords: subjective memory, African American, depression, anxiety, memory
Introduction
Subjective memory change (SMC) is common among the elderly, with community-based epidemiological studies showing that they occur in at least 1 out of 4 older individuals.1 The frequency of SMC increases with age, from 43% in those between the ages of 65 and 74, to 88% in individuals 85 and older.2 Older adults possess a varying degree of concern about their perceived memory deficits.3
The causes of SMC are unclear. Although some cross-sectional studies have found SMC to be associated with objective memory performance in nondemented older individuals,4,5 many others have failed to demonstrate similar findings and have instead shown stronger associations between depression, anxiety, and personality traits, most notably feelings of inferior self-worth or competence.6-13 Longitudinal studies have also consistently demonstrated an increased risk of cognitive decline1,14,15 and conversion to mild cognitive impairment (MCI) or dementia15 in previously cognitively healthy individuals with SMC. Such findings suggest potential self-awareness of an early degenerative process, even in the absence of objective cognitive dysfunction. Indeed, studies have shown that in cognitively normal older adults, a positive relationship exists between SMC and multiple biomarkers of Alzheimer disease (AD) and neurodegeneration, including increased accumulation of amyloid-b and neurofibrillary tangles,16 smaller hippocampal/ entorhinal volumes,17 and alterations in glucose metabolism in AD-vulnerable regions.18
Cerebrovascular risk factors (CVRFs) have long been associated with cognitive decline and an increased risk of dementia and AD.19 It is therefore reasonable to believe that CVRF may also be associated with SMC. To date, there have been minimal studies examining this potential association and of those completed, there have been mixed findings. Neither Jorm et al20 nor Stewart et al21 found direct correlations between individual CVRF and SMC. In contrast, Chen et al22 reported a significant association between hypertension and SMC across age-groups and showed that smoking increases the risk of SMC in young adults. Others have also showed positive associations between stroke, heart disease, and the presence of cerebral microbleeds and white matter lesions and SMC.23-26 Associations between diabetes, smoking, and receiving treatment for hypercholesterolemia and SMC have also been described, although these relationships may be attenuated by psychological and demographic factors.27 Finally, a strong negative association between high-density lipoprotein (HDL) and the 40-amino acid form of amyloid β protein (Aβ40) in those with, but not without SMC, has been reported.28
Beyond examining the influence of individual CVRF and SMC, there is evidence that having multiple chronic diseases negatively impacts self-perceived health status29 and self-perceptions of memory7 and cognitive efficacy.30 Chen et al22 specifically demonstrated that the odds of SMC significantly increase, as the number of modifiable risk factors an individual has rises. The degree to which an individual worries about SMC is also negatively impacted by multimorbidity,31 which is in line with broader evidence that consistently demonstrates positive associations between multimorbidity and the prevalence of psychiatric symptoms, including psychological distress and depression.32-35 Although SMC represents a risk factor for cognitive decline and poor psychological health, little attention has been paid to the examination of SMC in racially diverse populations, including in older African Americans (AA). This is concerning, given that the prevalence of CVRF and risk of developing dementia and AD is disproportionately greater for AA relative to Caucasians and other ethnic groups36 and that AA with SMC have been shown to have a significantly heightened risk of developing dementia.28 Given their higher rates of medical, and in particular vascular comorbidities, AA may also be subject to chronically heightened stress that independently presents as a risk factor for cognitive decline.37
This study examined the factors associated with SMC in older community-dwelling AA who had not been previously identified as having cognitive or memory problems. Of particular interest was the relationship between SMC, symptoms of depression and anxiety, and CVRF. We hypothesized that in older AA, who are at higher risk of cognitive decline, SMC would be positively associated with symptoms of anxiety and depression, but not objective measures of cognition. In addition, given the evidence pointing to an impact of multimorbidity on the prevalence of psychiatric symptoms,25,32-34 self-perceptions of cognitive efficacy30 and the degree to which individuals worry about SMC,31 we also hypothesized that the total number of CVRF (tCVRF) in any given individual would act as a proxy for poor perceived health status and increased stress, and/or potentially cerebrovascular burden, and therefore also be an important factor in distinguishing between those with and without SMC.
Methods
Participants
We recruited community-dwelling AA aged 60 or older via convenience sampling, at a university-affiliated primary care center. Additional inclusion criteria included being an English speaker at an early age, having greater than 3 years of education, and possessing adequate visual and auditory acuity to complete cognitive testing. Exclusion criteria included having a history of schizophrenia, bipolar disorder, or schizoaffective disorder. Additional exclusion criteria included having a history of electroconvulsive therapy, a documented diagnosis of dementia, or evidence of intellectual disability. At the time of recruitment, all participants were being seen by their primary care physician for reasons unrelated to cognition. Per their medical records, no participant had previously reported memory problems to their physician, been diagnosed with dementia or MCI, or been diagnosed with major depression. This study was approved by the institutional review board of University of Virginia.
Procedures
Following their visit with their primary care physician and providing informed consent, participants completed the Mini-Mental Status Examination (MMSE),38 followed by a demographic questionnaire, and then the Computer Assessment of Mild Cognitive Impairment (CAMCI).39 At the beginning of the CAMCI, participants responded to several self-report questions, which included questions related to mood and SMC. Participants also consented to having their medical records accessed for the purposes of this study.
Measures
Cognition, mood, and subjective memory
Cognitive functioning was assessed with the MMSE and the CAMCI. The CAMCI is a validated battery of short, self-administered tests, presented on a portable tablet. At the beginning of the CAMCI, participants were asked (1) Have you been feeling sad or fearful in the past week? (2) Have you been feeling anxious in the past week? and (3) Is your memory worse now than it was 1 year ago? Cognitive performances on the CAMCI were assessed in terms of accuracy rates and measured in 5 domains: attention, executive functioning, verbal memory, functional memory, and incidental memory. For each participant, an overall accuracy rate was computed by taking the mean of the accuracy rates in each domain.
Cerebrovascular risk factors
Predefined CVRFs of interest were smoking, obesity, hypertension, diabetes mellitus, hyperlipidemia, and histories of stroke, coronary heart disease, heart failure, deep vein thrombosis, vascular heart disease, and arterial peripheral embolus. Smoking risk was considered in current smokers and/or those who had quit smoking less than 10 years ago. Obesity was calculated as body mass index ≥30. All other CVRFs were first defined by their corresponding International Classification of Diseases, Ninth Revision codes as documented in their medical records. For each participant, the tCVRF was computed by summing the number of documented CVRFs. We then obtained the most recent documented measurements of participants' systolic and diastolic blood pressure, hemoglobin A1c, and lipoproteins.
Statistical Analyses
Bivariate relations between participants' SMC and variables of interest were examined using Pearson χ2 test for independence or Fisher exact test (for categorical variables), Poisson regression models (for count variables), and linear regression models (for continuous variables). Classification tree models were used to examine the importance of factors that were associated with SMC. Classification tree models were adopted, as they allow simultaneous exploration of linear and nonlinear relations between variables and provide a set of decision rules that are easily interpreted. The classification tree models were conducted using the “rpart” package40 in R, the optimum tree models were derived using 10-fold cross-validation, and 20 minimum cases in parent node. All statistical analyses were performed using R version 3.2.3.41
Results
Descriptive Statistics
Ninety-six AA (37 men and 59 women) participated. Participants were, on average, 69.0 (standard deviation [SD] = 6.8) years old, with approximately 10.6 (SD = 3.3) years of education (Table 1). Twenty-five percent of all participants endorsed SMC, whereas near one-third endorsed symptoms of depression (31.3%) or anxiety (34.4%). Nearly 67% of participants with symptoms of both anxiety and depression also endorsed SMC. The median and maximum number of CVRF in this sample were 4 and 7, respectively. No participants had documented histories of deep vein thrombosis, vascular heart disease, and/or arterial peripheral embolus.
Table 1.
Demographic Characteristics.
| Demographics | Total, (N = 96) |
|---|---|
| Age | 69.03 (6.81) |
| Education | 10.6 (3.27) |
| Depression (%) | 31.25 |
| Anxiety (%) | 34.38 |
| SMC (%) | 25 |
| CVRFa | 4 [0-7] |
| MMSE | 25.49 (3.51) |
Abbreviations: CVRF, cerebrovascular risk factors; MMSE, Mini-Mental Status Examination; SMC, subjective memory changes.
Median [range].
With respect to cognition, participants' average MMSE score was 25.49 (SD = 3.51). Based solely upon MMSE scores, 47 participants could be classified as having normal cognition (MMSE >26), 39 as having a higher likelihood of MCI (MMSE ≥21 and ≤26), and 10 as having a higher likelihood of dementia (MMSE ≤20). It should be noted that the MMSE has been shown to have poor specificity in screening for cognitive impairment in AA43,44 and that a more in-depth assessment of functional capacities would be necessary to substantiate a clinical diagnosis of MCI or dementia.
Bivariate Associations Between SMC and Other Variables
Subjective memory changes were not associated with gender, χ2(1) = .13, P = .716, or age, b = −1.88, SE = 1.60, t = −1.17, P = .245. Participants with more education were less likely to endorse SMC than those with less education (b = −1.69, SE = .76, t = −2.24, P = .03). Participants who endorsed symptoms of depression or anxiety were more likely to endorse SMC, compared to those who did not (χ2(1) = 16.55 and 12.94, respectively, both P < .001). In fact, over half of participants who positively endorsed symptoms of depression also endorsed SMC (54%), whereas only 12% of participants without depressive symptoms did so. Participants with symptoms of anxiety endorsed SMC nearly half of the time (49%) and more often than participants without anxiety (13%). In contrast, SMCs were not related to participants' performances on objective cognitive or memory measures (Table 2). When the extent to which SMC were related to each diagnosed CVRF was examined, only histories of coronary heart disease and heart failure were found to be correlated with SMC (Table 3). When associations between obtained laboratory values and SMC were examined, the results were consistent with those reported for the dichotomous CVRF.
Table 2.
Multiple Linear Regression Models Estimating the Associations Between Subjective Memory Changes and Cognitive Functioning.a
| Cognitive Tests/Domains | b | SE | t | P |
|---|---|---|---|---|
| MMSE | −1.21 | .82 | −1.47 | .145 |
| CAMCI | ||||
| Attention span | −5.32 | 5.30 | −1.01 | .317 |
| Executive functions | −6.38 | 4.55 | −1.40 | .164 |
| Verbal memory | −3.61 | 7.23 | −.50 | .619 |
| Functional memory | −11.57 | 5.84 | −1.98 | .051 |
| Incidental memory | 2.02 | 4.98 | .41 | .686 |
| Overall accuracy | −6.11 | 3.66 | −1.67 | .099 |
Abbreviations: CAMCI, computerized-based assessment of mild cognitive impairment; b, unstandardized regression coefficient; MMSE, Mini-Mental Status Examination; SE, standard error.
Subjective memory change is a dichotomous variable coded as 0 = no changes; 1 = changes.
Table 3.
Multiple Models Assessing the Associations Between Subjective Memory Changes and Vascular Risk Factors.a
| CVRF (Laboratory Values) | Fisher Exact Testb | Linear Regression Modelsc | ||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | P | Estimate (SE) | P | |
| Smoking | 2.25 (.68, 7.13) | .154 | ||
| Obesity (BMI) | 1.76 (.34, 17.82) | .724 | −1.36 (4.16) | .745 |
| Hypertension (SB, DBP) | 1.26 (.29, 7.69) | 1.000 | .57 (5.61) −.17 (2.43) | .919 .945 |
| Diabetes mellitus (HGBA1C) | 1.19 (.42, 3.52) | .813 | −.37 (.31) | .243 |
| Hyperlipidemia (TC) | 1.00 (.33, 3.28) | 1.000 | −10.33 (10.73) | .338 |
| Hx of stroke | 3.05 (.75, 12.17) | .083 | ||
| Hx of CHD | 3.76 (.98, 14.56) | .039 | ||
| Hx of HF | 4.39 (.85, 24.44) | .041 | ||
Abbreviations: BMI, body mass index; CHD, coronary heart disease; CVRF, cerebrovascular risk factors; DBP, diastolic blood pressure; HGBA1C, Hemoglobin A1c; Hx, history; HF, heart failure; SBP, systolic blood pressure; TC, total cholesterol.
None of the participants had documented histories of deep vein thrombosis, vascular heart disease, and/or arterial peripheral embolus.
Fisher exact tests were used, as some of the cells in the contingency table are less than 5.
Linear regression models were used to examine the associations between subjective memory changes and the laboratory values of the corresponding vascular risk factors.
Classification Tree Models
A series of classification tree models were performed to identify factors associated with SMC (Table 4). The misclassification rate (%) of each model was computed as the proportion of participants who were misclassified by the model. In Model 1, only participants' demographic variables were used. This serves as a baseline model, assuming that no additional information about the respondents is available. The misclassification rate was 23.96%. Thus, 1 in every 4 participants was misclassified as endorsing SMC when they did not, or vice versa.
Table 4.
Classification Tree Models.
| Model | Variables | Misclassification (%) |
|---|---|---|
| 1 | Age, gender, education | 23.96 |
| 2 | Age, gender, education, depression, anxiety, vascular risk factors | 14.58 |
| 3 | Age, gender, education, depression, anxiety, vascular risk factors, MMSE | 14.58 |
| 4 | Age, gender, education, depression, anxiety, vascular risk factors, CAMCI | 14.58 |
Abbreviations: CAMCI, computer-based assessment of mild cognitive impairment; MMSE, Mini-Mental Status Examination.
When participants' mental and physical health variables (ie, symptoms of depression, symptoms of anxiety, and the tCVRF) were added in model 2, the misclassification rate decreased to 14.58%. Model 2 resulted in a 2-level classification tree, with depression presenting as the most important variable, as reflected by its use in the first level of the tree model (Figure 1). Participants who endorsed symptoms of depression were classified into the group with SMC (“SMC: yes”; right node), whereas those who did not were classified into the group without SMC (“SMC: no”; left node). Symptoms of anxiety and the tCVRF were the next most important variables in model 2. Participants who endorsed symptoms of anxiety (and depression) were classified into the group with SMC, whereas those without anxiety (but with depression) were classified to the group without SMC. Patients without depression and less than 6 tCVRF were classified into the group without SMC, whereas those without depression and at least 6 tCVRF were classified into the group who endorsed SMC.
Figure 1.
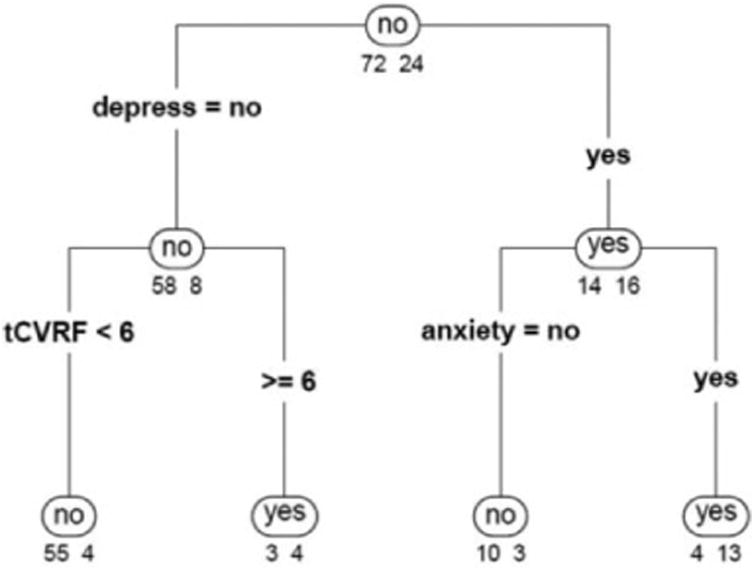
Classification tree categorizing patients with and without subjective memory changes using demographic and health characteristics (age, gender, education, depression, anxiety, and number of vascular risk factors). The numbers below each node indicate the proportion of patients with (right) and without (left) subjective memory changes for that classification outcome. For example, the “.88/.12” values on the left branch in the second level indicate that the majority of participants without symptoms of depression did not endorse subjective memory changes (88%), compared to a small proportion of those who did so (12%). depress indicates depression; tCVRF, total number of vascular risk factors.
Next, we examined whether model 2 could be improved by including objective measures of cognition and memory. When the MMSE and CAMCI scores were added in models 3 and 4, respectively, the misclassification rates did not improve. These results indicate that performances on objective cognitive or memory measures were not important determinants of endorsement of SMC.
Discussion
In this sample of older AA, SMCs were present in 25% of participants and not significantly associated with age. This percentage is less than what has been previously documented in other primarily Caucasian or AA groups similar in age.2,45 The relatively low endorsement of SMC in this sample is itself clinically relevant, given evidence that some AA may have worse memory than their subjective accounts would suggest.46 From a demographic standpoint, our results also indicate that AA with less education were more likely to endorse SMC, suggesting that those with less education and potentially less cognitive reserve have a smaller buffer before subtle changes in memory manifest.
Nearly a third of participants in our study endorsed symptoms of anxiety or depression. Consistent with previous research,6-8 our results show that in older AA, SMC were more closely linked to affective symptoms than objective measures of cognition or memory. In fact, older AA with symptoms of depression or anxiety were 4.5 and 3.8 times more likely to present with SMC, respectively, and the presence or absence of depressive symptoms proved to be the most significant variable differentiating between participants with or without SMC. Having comorbid symptoms of depression and anxiety further increased the likelihood of endorsement of SMC to 67%.
It is possible that SMC in older AA represent a benign byproduct of anxious or depressive symptoms and should not signal concern for future cognitive decline. Alternatively, higher rates of anxious and depressive symptoms in individuals with SMC may stem from legitimate concern and warrant attention. Indeed, others have shown that SMC should not be overlooked in otherwise cognitively healthy individuals, as they may represent the earliest preclinical signs of MCI or dementia.15,14 Significant correlations between SMC and biomarkers of AD have been described,16 further suggesting that SMC may be an early clinical marker of impending cognitive decline. Moreover, studies have shown that AD pathology may be more pronounced in individuals with lifetime histories of depression47 and that depression itself is associated with reduced hippocampal volume and cortical thinning in temporal and parietal regions.48 Thus, SMC and depressive symptoms, both of which have a high comorbid presence in our sample, have been shown to be independent risk factors for cognitive decline and AD.
It is well known that as groups, older adults and AA are at increased risk of developing CVRF and cognitive impairment.4,49 Alarmingly, the median prevalence of the tCVRF in our study was 4. Eighty-four percent of participants were classified as overweight or obese, whereas 82% had hypertension, 68% hyperlipidemia, and 57% diabetes. Consistent with our initial hypothesis and population-based studies demonstrating an association between multimorbidity and SMC,31 in nondepressed AA, the tCVRF was an important factor in distinguishing between those with and without SMC. This finding held true despite the absence of a positive association between the tCVRF and objective measures of global cognition, memory, attention, or executive functioning, the latter of which are particularly vulnerable to CVRF.50
It is well established that CVRFs increase the odds of AD or mixed dementia.49 The hippocampus has been shown to be particularly vulnerable to atrophy as part of degenerative or vascular processes,51 while a cluster of CVRF known as “metabolic syndrome” (ie, abnormalities in insulin, glucose and lipoprotein metabolism, hypertension, and abdominal obesity) have been associated with AD.19 Given the cross-sectional nature of this study, it is not known whether SMC in those with a higher tCVRF represent a harbinger of cognitive decline. However, the high frequency of CVRF in our sample suggests that a percentage of participants may have clinically significant ischemic disease. It is possible that the measure of tCVRF represents a marker for underlying white matter disease that subsequently contributes to early cognitive change and SMC. The severity of white matter lesions has been previously associated with subjective cognitive decline, even in the absence of objective cognitive impairment.52 Thus, our participants with a higher tCVRF who endorsed SMC may be more keenly aware of intrapersonal cognitive changes secondary to increasing disease burden, prior to detection on cognitive testing.
An alternative hypothesis to explain the relationship between the tCVRF and SMC is that the tCVRF acted as a proxy for self-perceived overall health. In this view, an increase in health problems (ie, CVRF) may have contributed to increased stress and subsequently a greater likelihood of endorsing SMC. Stress has been associated with SMC in older adults53 and specifically in older AA.54 Moreover, in older individuals, poorer self-perceived health has been shown to be predictive of SMC, greater anxiety in memory-demanding situations, and less control of one's memory.55 Ultimately, as suggested by Sims et al,54 in our sample of older AA, less perceived control over one's health may have contributed to the endorsement of SMC.
This study had several limitations, including being a retrospective analysis of a cross-sectional sample. The resulting classification tree model illustrates the associations of the variables of interest in this sample and may not be generalizable to other samples. Further research with a larger, more representative sample is needed. Longitudinal studies are also necessary to further clarify whether SMC increases the risk of cognitive decline in older AA. To this end, we are currently conducting longitudinal assessments with this cohort. Neuroimaging and other biomarker analyses could prove useful in identifying known markers of AD and cerebrovascular disease. Future studies would also benefit from inclusion of validated and more comprehensive assessments of depression and anxiety. The questions asked about mood in this study are not equivalent to the broadly accepted and operationalized diagnoses of clinical depression or anxiety.56 That said, the questions used assessed common symptoms of depression and anxiety, and the demonstrated association between these symptoms and SMC in our sample are consistent with many other studies that have used validated measures of mood. Similarly, though future studies would benefit from using a more comprehensive measure of SMC, it is notable that previous studies of older adults have successfully used one question in evaluating the relationship between SMC and cognition.2 Our ongoing longitudinal assessments of this cohort have incorporated additional validated measures of mood and SMC in an effort to expand upon the findings from this study.
Conclusions
There is increasing evidence to suggest that SMC is not a benign feature of normal aging. As a group, older AA have greater risk of developing CVRF and dementia. Consistent with previous studies conducted in predominantly Caucasian populations, we demonstrated that in older AA, symptoms of depression and anxiety, but not objective performances on measures of cognition or memory, were positively associated with SMC. A novel finding was that in nondepressed older AA, the tCVRF was an important factor in distinguishing between those with and without SMC. Further investigations are needed to determine the cause of this association and whether SMC is a risk factor for cognitive decline in older AA.
Acknowledgments
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by Award No. 13-4 from the Commonwealth of Virginia's Alzheimer's and Related Diseases Research Award Fund, administered by the Virginia Center on Aging, School of Allied Health, Virginia Commonwealth University, as well as by grants from The University of Virginia, Hoos for Memory. Siny Tsang is supported by the research training grant 5-T32-MH 13043 from the National Institute of Mental Health.
Footnotes
Authors' Note: This work was conducted at the University of Virginia, Charlottes-ville, Virginia.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Tobiansky R, Blizard R, Livingston G, Mann A. The Gospel Oak Study stage IV: the clinical relevance of subjective memory impairment in older people. Psychol Med. 1995;25(4):779–786. doi: 10.1017/s0033291700035029. [DOI] [PubMed] [Google Scholar]
- 2.Bassett SS, Folstein MF. Memory complaint, memory performance, and psychiatric diagnosis: a community study. J Geriatr Psychiatry Neurol. 1993;6(2):105–111. doi: 10.1177/089198879300600207. [DOI] [PubMed] [Google Scholar]
- 3.Commissaris CJ, Jolles J, Verhey FR, Ponds RW, Damoiseaux V, Kok GJ. Vergeetachtig of dement? Wiemaakt zich zorgen en waarom? [Forgetfulness or dementia? Who worries and why?] [in Dutch] Tijdschr Gerontol Geriatr. 1993;24(4):144–149. [PubMed] [Google Scholar]
- 4.Amariglio RE, Townsend MK, Grodstein F, Sperling RA, Rentz DM. Specific subjective memory complaints in older persons may indicate poor cognitive function. J Am Geriatr Soc. 2011;59(9):1612–1617. doi: 10.1111/j.1532-5415.2011.03543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snitz BE, Morrow LA, Rodriguez EG, Huber KA, Saxton JA. Subjective memory complaints and concurrent memory performance in older patients of primary care providers. J Int Neurop-sychol Soc. 2008;14(6):1004–1013. doi: 10.1017/S1355617708081332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanninen T, Reinikainen KJ, Helkala EL, et al. Subjective memory complaints and personality traits in normal elderly subjects. J Am Geriatr Soc. 1994;42(1):1–4. doi: 10.1111/j.1532-5415.1994.tb06064.x. [DOI] [PubMed] [Google Scholar]
- 7.Comijs HC, Deeg DJ, Dik MG, Twisk JW, Jonker C. Memory complaints; the association with psycho-affective and health problems and the role of personality characteristics. A 6-year follow-up study. J Affect Disord. 2002;72(2):157–165. doi: 10.1016/s0165-0327(01)00453-0. [DOI] [PubMed] [Google Scholar]
- 8.Smith GE, Petersen RC, Ivnik RJ, Malec JF, Tangalos EG. Subjective memory complaints, psychological distress, and longitudinal change in objective memory performance. Psychol Aging. 1996;11(2):272–279. doi: 10.1037//0882-7974.11.2.272. [DOI] [PubMed] [Google Scholar]
- 9.Kahn RL, Zarit SH, Hilbert NM, Niederehe G. Memory complaint and impairment in the age the effect of depression and altered brain function. Arch Gen Psychiatry. 1975;32(12):1569–1573. doi: 10.1001/archpsyc.1975.01760300107009. [DOI] [PubMed] [Google Scholar]
- 10.Minett TS, Da Silva RV, Ortiz KZ, Bertolucci PH. Subjective memory complaints in an elderly sample: a cross-sectional study. Int J Geriatr Psychiatry. 2008;23(1):49–54. doi: 10.1002/gps.1836. [DOI] [PubMed] [Google Scholar]
- 11.Minett TS, Dean JL, Firbank M, English P, O'Brien JT. Subjective memory complaints, white-matter lesions, depressive symptoms, and cognition in elderly patients. Am J Geriatr Psychiatry. 2005;13(8):665–671. doi: 10.1176/appi.ajgp.13.8.665. [DOI] [PubMed] [Google Scholar]
- 12.Schmand B, Jonker C, Geerlings MI, Lindeboom J. Subjective memory complaints in the elderly: depressive symptoms and future dementia. Br J Psychiatry. 1997;171:373–376. doi: 10.1192/bjp.171.4.373. [DOI] [PubMed] [Google Scholar]
- 13.Schofield PW, Marder K, Dooneief G, Jacobs DM, Sano M, Stern Y. Association of subjective memory complaints with subsequent cognitive decline in community-dwelling elderly individuals with baseline cognitive impairment. Am J Psychiatry. 1997;154(5):609–615. doi: 10.1176/ajp.154.5.609. [DOI] [PubMed] [Google Scholar]
- 14.Schmand B, Jonker C, Hooijer C, Lindeboom J. Subjective memory complaints may announce dementia. Neurology. 1996;46(1):121–5. doi: 10.1212/wnl.46.1.121. [DOI] [PubMed] [Google Scholar]
- 15.Geerlings MI, Jonker C, Bouter LM, Ader HJ, Schmand B. Association between memory complaints and incident Alzheimer's disease in elderly people with normal baseline cognition. Am J Psychiatry. 1999;156(4):531–537. doi: 10.1176/ajp.156.4.531. [DOI] [PubMed] [Google Scholar]
- 16.Barnes LL, Schneider JA, Boyle PA, Bienias JL, Bennett DA. Memory complaints are related to Alzheimer disease pathology in older persons. Neurology. 2006;67(9):1581–1585. doi: 10.1212/01.wnl.0000242734.16663.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Flier WM, van Buchem MA, Weverling-Rijnsburger AW, et al. Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. J Neurol. 2004;251(6):671–675. doi: 10.1007/s00415-004-0390-7. [DOI] [PubMed] [Google Scholar]
- 18.Scheef L, Spottke A, Daerr M, et al. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79(13):1332–1339. doi: 10.1212/WNL.0b013e31826c1a8d. [DOI] [PubMed] [Google Scholar]
- 19.Vanhanen M, Koivisto K, Moilanen L, et al. Association of metabolic syndrome with Alzheimer disease: a population-based study. Neurology. 2006;67(5):843–847. doi: 10.1212/01.wnl.0000234037.91185.99. [DOI] [PubMed] [Google Scholar]
- 20.Jorm AF, Butterworth P, Anstey KJ, et al. Memory complaints in a community sample aged 60-64 years: associations with cognitive functioning, psychiatric symptoms, medical conditions, APOE genotype, hippocampus and amygdala volumes, and white-matter hyperintensities. Psychol Med. 2004;34(8):1495–1506. doi: 10.1017/s0033291704003162. [DOI] [PubMed] [Google Scholar]
- 21.Stewart R, Russ C, Richards M, Brayne C, Lovestone S, Mann A. Depression, APOE genotype and subjective memory impairment: a cross-sectional study in an African-Caribbean population. Psychol Med. 2001;31(3):431–440. [PubMed] [Google Scholar]
- 22.Chen ST, Siddarth P, Ercoli LM, Merrill DA, Torres-Gil F, Small GW. Modifiable risk factors for Alzheimer disease and subjective memory impairment across age groups. PLoS One. 2014;9(6):e98630. doi: 10.1371/journal.pone.0098630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langballe E, Tambs K, Saltvedt I, Midthjell K, Holmen J. The association between vascular factors and subjective memory impairment in older people: the HUNT study, Norway. Norsk Epidemiol. 2012;22(2):209–215. [Google Scholar]
- 24.de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and subjective cognitive dysfunction. The Rotterdam scan study. Neurology. 2001;56(11):1539–1545. doi: 10.1212/wnl.56.11.1539. [DOI] [PubMed] [Google Scholar]
- 25.Uiterwijk R, Huijts M, Staals J, et al. Subjective cognitive failures in patients with hypertension are related to cognitive performance and cerebral microbleeds. Hypertension. 2014;64(3):653–657. doi: 10.1161/HYPERTENSIONAHA.114.03621. [DOI] [PubMed] [Google Scholar]
- 26.Stewart R, Dufouil C, Godin O, et al. Neuroimaging correlates of subjective memory deficits in a community population. Neurology. 2008;70(18):1601–1607. doi: 10.1212/01.wnl.0000310982.99438.54. [DOI] [PubMed] [Google Scholar]
- 27.Paradise MB, Glozier NS, Naismith SL, Davenport TA, Hickie IB. Subjective memory complaints, vascular risk factors and psychological distress in the middle-aged: a cross-sectional study. BMC Psychiatry. 2011;11:108. doi: 10.1186/1471-244X-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abner EL, Kryscio RJ, Caban-Holt AM, Schmitt FA. Baseline subjective memory complaints associate with increased risk of incident dementia: the PREADVISE trial. J Prev Alzheimers Dis. 2015;2(1):11–16. doi: 10.14283/jpad.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cutler SJ, Grams AE. Correlates of self-reported everyday memory problems. J Gerontol. 1988;43(3):S82–S90. doi: 10.1093/geronj/43.3.s82. [DOI] [PubMed] [Google Scholar]
- 30.Lachman ME, Leff R. Perceived control and intellectual functioning in the elderly: a 5-year longitudinal study. Dev Psychol. 1989;25(5):722–728. [Google Scholar]
- 31.Aarts S, van den Akker M, Hajema KJ, et al. Multimorbidity and its relation to subjective memory complaints in a large general population of older adults. Int Psychogeriatr. 23(4):616–624. doi: 10.1017/S1041610210002024. 201. [DOI] [PubMed] [Google Scholar]
- 32.Kessler RC, Berglund P, Demler O, et al. National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 33.Evans DL, Charney DS, Lewis L, et al. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58(3):175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2013;31:105–114. doi: 10.1016/j.bbi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wells KB, Stewart A, Hays RD, et al. The functioning and well-being of depressed patients results from the medical outcomes study. JAMA. 1989;262(7):914–919. [PubMed] [Google Scholar]
- 36.Gurland BJ, Wilder DE, Lantigua R, et al. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry. 1999;14(6):481–493. [PubMed] [Google Scholar]
- 37.McDougall GJ, Holston EC. Black and white men at risk for memory impairment. Nurs Res. 2003;52(1):42–46. doi: 10.1097/00006199-200301000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 39.Saxton J, Morrow L, Eschman A, Archer G, Luther J, Zuccolotto A. Computer assessment of mild cognitive impairment. Postgrad Med. 2009;121(2):177–185. doi: 10.3810/pgm.2009.03.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atkinson B, Ripley B. Rpart: Recursive Partitioning and Regression Trees. R package version 4. 2015:1–9. http://CRAN.R-project.org/package=rpart.
- 41.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. URL http://www.R-project.org/ [Google Scholar]
- 42.Wood RY, Giuliano KK, Bignell CU, Pritham WW. Assessing cognitive ability in research: use of MMSE with minority populations and elderly adults with low education levels. J Gerontol Nurs. 2006;32(4):45–54. doi: 10.3928/00989134-20060401-08. [DOI] [PubMed] [Google Scholar]
- 43.Fillenbaum G, Heyman A, Williams K, Prosnitz B, Burchett B. Sensitivity and specificity of standardized screens of cognitive impairment and dementia among elderly black and white community residents. J Clin Epidemiol. 1990;43(7):651–660. doi: 10.1016/0895-4356(90)90035-n. [DOI] [PubMed] [Google Scholar]
- 44.Stephenson J. Racial barriers may hamper diagnosis, care of patients with Alzheimer disease. JAMA. 2001;286(7):779–780. [PubMed] [Google Scholar]
- 45.Bazargan M, Barbre AR. Self-reported memory problems among the black elderly. Educ Gerontol. 1992;18(1):71–82. [Google Scholar]
- 46.Blazer DG, Hays JC, Fillenbaum GG, Gold DT. Memory complaint as a predictor of cognitive decline: a comparison of African American and White elders. J Aging Health. 1997;9(2):171–184. doi: 10.1177/089826439700900202. [DOI] [PubMed] [Google Scholar]
- 47.Rapp MA, Schnaider-Beeri M, Grossman HT, et al. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch Gen Psychiatry. 2006;63(2):161–167. doi: 10.1001/archpsyc.63.2.161. [DOI] [PubMed] [Google Scholar]
- 48.Lebedeva A, Westman E, Lebedev AV, et al. Structural brain changes associated with depressive symptoms in the elderly with Alzheimer's disease. Alzheimer's disease neuroimaging initiative. J Neurol Neurosurg Psychiatry. 2014;85(8):930–935. doi: 10.1136/jnnp-2013-307110. [DOI] [PubMed] [Google Scholar]
- 49.Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late-life dementia. Nat Rev Neurol. 2009;5(12):649–658. doi: 10.1038/nrneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- 50.Smith PJ, Blumenthal JA, Babyak MA, et al. Cerebrovascular risk factors, vascular disease, and neuropsychological outcomes in adults with major depression. Psychosom Med. 2007;69(6):578. doi: 10.1097/PSY.0b013e31812f7b8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zarow C, Sitzer TE, Chui HC. Understanding hippocampal sclerosis in the elderly: epidemiology, characterization, and diagnostic issues. Curr Neurol Neurosci Rep. 2008;8(5):363–370. doi: 10.1007/s11910-008-0057-3. [DOI] [PubMed] [Google Scholar]
- 52.Benedictus MR, van Harten AC, Leeuwis AE, et al. White matter hyperintensities relate to clinical progression in subjective cognitive decline. Stroke. 2015;46(9):2661–2664. doi: 10.1161/STROKEAHA.115.009475. [DOI] [PubMed] [Google Scholar]
- 53.Neupert SD, Almeida DM, Mroczek DK, Spiro A., III Daily stres-sors and memory failures in a naturalistic setting: findings from the VA normative aging study. Psychol Aging. 2006;21(2):424–429. doi: 10.1037/0882-7974.21.2.424. [DOI] [PubMed] [Google Scholar]
- 54.Sims RC, Whitfield KE, Ayotte BJ, Gamaldo AA, Edwards CL, Allaire JC. Subjective memory in older African Americans. Exp Aging Res. 2011;37(2):220–240. doi: 10.1080/0361073X.2011.555640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDougall GJ. Predictors of metamemory in older adults. Nurs Res. 1994;43(4):212–218. [PMC free article] [PubMed] [Google Scholar]
- 56.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]


