Abstract
Misfolded α-synuclein (A-syn) is widely recognized as the primal cause of neurodegenerative diseases including Parkinson’s disease and dementia with Lewy bodies. The normal cellular function of A-syn has, however, been elusive. There is evidence that A-syn plays multiple roles in the exocytotic pathway in the neuron, but the underlying molecular mechanisms are unclear. A-syn has been known to interact with negatively charged phospholipids and with vesicle SNARE protein VAMP2. Using single-vesicle docking/fusion assays, we find that A-syn promotes SNARE-dependent vesicles docking significantly at 2.5 μM. When phosphatidylserine (PS) is removed from t-SNARE-bearing vesicles, the docking enhancement by A-syn disappears and A-syn instead acts as an inhibitor for docking. In contrast, subtraction of PS from the v-SNARE-carrying vesicles enhances vesicle docking even further. Moreover, when we truncate the C-terminal 45 residues of A-syn that participates in interacting with VAMP2, the promotion of vesicle docking is abrogated. Thus, the results suggest that the A-syn’s interaction with v-SNARE through its C-terminal tail and its concurrent interaction with PS in trans through its amphipathic N-terminal domain facilitate SNARE complex formation, whereby A-syn aids SNARE-dependent vesicle docking.
Introduction
α-Synuclein (A-syn) is a small presynaptic protein of vaguely defined normal function [1]. But its medical implication is colossal because its misfolded products are the prime suspect of causing synucleinopathies including Parkinson’s disease and dementia with Lewy bodies [2–4]. Several missense mutations and gene multiplications are often found to bring about the early onset of the familial Parkinson’s disease [5–9]. Moreover, A-syn is a major component of the abnormal deposits found in the patients’ brains [10–12].
As inferred from its abundant presence at the presynapse [13,14], there is evidence that normal A-syn is broadly involved in vesicle trafficking pathways. Deletion of A-syn in cultured neurons leads to the depletion of the synaptic vesicle pool [15,16], while overexpression results in the defects in vesicle recycling [17–20]. It has also been shown, both in vivo and in vitro, that A-syn plays a role in clustering synaptic vesicles [19,21,22]. In chromaffin cells, however, A-syn appears to be involved in the late steps in the vesicle fusion, implying its direct involvement in vesicle fusion [18]. All in all, it seems that A-syn has its influence on nearly all steps in the exocytotic pathway. What would then be the molecular origin of A-syn’s such broad relevance in exocytosis?
Some insights into the A-syn’s broad implications in vesicle trafficking might be found in its capacity to bind to membranes [23,24]. Structurally, A-syn has a basic amino acid-rich, apolipoprotein-like N-terminal sequence [25], the hydrophobic core region that is responsible for aberrant aggregation [26], and the hydrophilic C-terminal region (Figure 1A). NMR and EPR studies have shown that the first 100 residues bind to the membrane in a helical conformation in either an extended or hairpin-like conformation with a break in the middle, whereas C-terminal region extends freely toward the solution phase [27–30]. A-syn’s promiscuous binding to negatively charged membranes may partly be responsible for its broad relevance on many aspects of vesicle trafficking. However, it does not explain why A-syn is specific to exocytotic membranes.
Figure 1. A-syn promotes vesicle docking at low concentrations but inhibits vesicle docking at high concentrations.
(A) Structural model of micelle-bound A-syn, with the electrostatic potential surface shown in color (red, negative; blue, positive). The letters N and C in square brackets indicate the N- and C-terminus of A-syn, respectively. The N-terminal region, which ends around residue 100 as indicated in square bracket, folds as a hairpin-like conformation, which consists of two α-helices with a break in the middle. The C-terminal region is predominantly unstructured. (B) Gel filtration profile and immunoblotting characterization of recombinant A-syn. (C) Schematic diagram of the single-vesicle docking/fusion assay. The t-vesicles bearing t-SNARE were immobilized on the biotin-PEG-coated surface of the flow cell. The v-vesicles reconstituted with VAMP2 or VAMP2 with syt1 were flown into the flow cell with or without A-syn. (D) Representative TIRF microscopic images of docked v-vesicles in the absence or presence of A-syn at different concentrations. (E) Vesicle docking probability versus concentration of A-syn. Error bars denote the standard deviation (SD) from three independent experiments. Movies recorded from more than five randomly selected screens were analyzed and averaged in each experiment. (F) Histograms of the effects of A-syn on the decay rate of Ca2+ triggered content mixing (left) and the cumulative content-mixing probability over the first 80 s period after Ca2+ injection (right). Cumulative plots of content-mixing events are shown in Supplementary Figure S2. Error bars denote the SD of three independent experiments.
Recently, an important discovery has been made, which might provide some clues to the function of A-syn in exocytosis: A-syn interacts with v-SNARE synaptobrevin 2 or VAMP2 [31], which is a vesicle protein that is involved in mediating fusion of vesicle to the plasma membrane [32,33]. It is shown that this interaction is essential for vesicle clustering [21]. Moreover, for misfolded A-syn oligomers, this specific interaction plays an essential role in hampering SNARE-dependent vesicle fusion [34]. Interestingly, it is also shown, both in vivo and in vitro, that this interaction promotes SNARE complex formation [31]. However, the mechanism in a molecular level, whereby A-syn promotes SNARE assembly, is unclear.
Here, we explore the possible cooperation of A-syn’s membrane binding with its v-SNARE binding in the pathway of SNARE-dependent membrane fusion using the single-vesicle docking/fusion assay [35–37]. Our results show that A-syn promotes vesicle docking at low concentrations (<2.5 μM) although it inhibits docking at high concentrations (>4 μM). For the promotion of vesicle docking, A-syn’s interaction with v-SNARE on v-SNARE proteoliposome and its concurrent interaction with negatively charged lipids on t-SNARE proteoliposome are both required. Thus, the results provide a hypothetical mechanism whereby A-syn may cross-bridge two membranes, which would promote SNARE complex formation and vesicle docking
Materials and methods
Plasmid constructs and site-directed mutagenesis
DNA sequences encoding syntaxin 1a (amino acids 2–288, three native cysteines C145, C271, and C272 were replaced by alanines), VAMP2 (amino acids 1–116, C103 was replaced by alanine), SNAP-25 (amino acids 1–206, four native cysteines C85, C88, C90, and C92 were replaced by alanines), α-synuclein (A-syn, amino acids 1–140), and C-terminus truncated α-synuclein (A-syn_1–95, amino acids 1–95) were inserted into the pGEX-KG vector as N-terminal glutathione S-transferase (GST) fusion proteins. Full-length rat synaptotagmin-1 (syt1, amino acids 50–421, four native cysteines C74, C75, C77, and C79 were replaced by alanines and another C82 was replaced by serine) was inserted into pET-28b vector as a C-terminal His-tagged protein. All DNA sequences were confirmed by the Iowa State University DNA Sequencing Facility.
Protein expression and purification
Recombined proteins were expressed and purified as recently described [38,39]. Briefly, all recombinant proteins were expressed in Escherichia coli BL21 (DE3) by growing the cells to OD600 of 0.6–0.8 at 37°C, and then the cells were induced with 0.5 mM IPTG (isopropyl β-D-1-thiogalactopyranoside) at 18°C for 16 h. The cells were harvested and resuspended in PBS or PBS with 0.2% (w/v) Triton X-100 (PBST) for membrane proteins, supplemented with 1 mM AEBSF [4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride]. After sonication, the cell lysate was centrifuged at 15 000 rpm for 40 min using a Beckman JA-25.50 rotor. For GST-tagged proteins, syntaxin 1a, SNAP-25, VAMP2, A-syn, and A-syn_1-95, the supernatants were bound to Glutathione Agarose beads (Sigma–Aldrich). After thoroughly washing, recombined proteins were cleaved from beads with thrombin (0.02 unit μl−1, Sigma–Aldrich) in PBS or PBS with 0.8% (w/v) octyl β-D-glucopyranoside (PBS-OG) for membrane proteins. For the His-tagged protein syt1, the supernatant was bound to Ni-NTA agarose beads (Qiagen), and the sample was eluted with buffer containing 25 mM HEPES–KOH (pH 7.4), 400 mM KCl, 250 mM imidazole, 0.8% OG, and 1 mM EDTA after washing.
Gel filtration and western blotting
To characterize the property of recombinant A-syn, the thrombin-cleaved A-syn was subjected to gel filtration chromatography on 10/300 GL Superdex 200 column (GE healthcare) using the biologic Duoflow system (Bio-Rad). The monomeric A-syn was collected and confirmed by another gel filtration. The elution volume of A-syn was determined using standard molecular mass markers (thyroglobulin, 670 kDa; γ-globulin, 158 kDa; ovalbumin, 44 kDa; myoglobin, 17 kDa; and vitamin B12, 1.35 kDa; Bio-Rad) on the same column. Western blotting of A-syn was carried out as previously described [39].
Lipid mixture preparation
The lipid molecules used in the present study are 1,2-dioleoyl-sn-glycero-3-phospho-L-serine (DOPS), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), phosphatidylinositol-4,5-bisphosphate (PIP2, from porcine brain), cholesterol, and 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamin-N-(biotinyl) (biotin-DPPE). All lipids were obtained from Avanti Polar Lipids. 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate (DiD), and sulforhodamine B were obtained from Invitrogen. The desired amounts of lipids were mixed in a glass tube. The mixture was first dried under the nitrogen flow to form a thin lipid film on the well of tube and then completely dried under vacuum.
Proteoliposome reconstitution
To mimic a favorable charge distribution for Ca2+-triggered vesicle fusion as described previously [40], the molar ratios of lipids were set at 15: 62: 20: 2: 1: 0.1 (DOPS: POPC: cholesterol: PIP2: DiD: Biotin-DPPE) for the t-SNARE-reconstituted (t-) vesicles and 5: 74: 20: 1 (DOPS: POPC: cholesterol: DiI) for the v-SNARE-reconstituted (v-) vesicles. For the t- and v-vesicles with 20% PS or without DOPS, PIP2 was replaced with an equal amount of POPC, and equivalent amounts of POPC were removed or added to the lipid mixture. For the single-vesicle docking assay, the proteoliposome reconstitution was performed following the procedure described recently [38]. Briefly, the completely dried lipid film was hydrated with dialysis buffer (25 mM HEPES (pH 7.4) and 100 mM KCl). After five freeze–thaw cycles, the protein-free large unilamellar vesicles (~100 nm in diameter) were prepared by extrusion through a 100 nm polycarbonate filter (Whatman) for at least 30 times. To make t-vesicles, preassembled t-SNAREs (syntaxin: SNAP-25 = 1: 1.5) were mixed with protein-free vesicles at the protein to lipid molar ratio of 1: 200 for syntaxin 1A (this ratio was kept for all experiments including the single-vesicle content-mixing assay) in the presence of 0.8% OG in the dialysis buffer at 4°C for 15 min. The liposome/protein mixture was diluted two times with dialysis buffer for t-vesicles, and then the diluted t-vesicles were dialyzed in 2 liters of dialysis buffer at 4°C overnight. The v-vesicles were prepared by following the same procedure with VAMP2 or the mixture of VAMP2 and syt1. For v-vesicles with syt1, the mixture was diluted twice with dialysis buffer containing 1 mM EDTA and dialyzed in 2 liters of dialysis buffer with EDTA at 4°C overnight.
Proteoliposomes for the single-vesicle content-mixing assay, using the small sulforhodamine B (SRB) content indicator, were prepared as described in our recent work [38,41]. Briefly, the lipid compositions were the same as those used in the single-vesicle docking assay with the asymmetric charge distribution, except that the fluorescent lipid dyes (DiI and DiD) were replaced by the equal amount of POPC. The proteoliposome reconstitution was the same as that used for the single-vesicle docking assay, but the dried lipid film, intended for v-vesicles, was hydrated in the presence of 20 mM SRB which was kept throughout the sample preparation steps prior to the dialysis overnight. Remaining free SRB was removed using the PD-10 desalting column (GE healthcare) after dialysis.
Single-molecule total internal reflection fluorescence microscope setup
The single-vesicle docking/fusion assays were carried out on the prism-type TIRF (total internal reflection fluorescence) microscope as described in our recent work [38]. Briefly, a 532 nm laser was used to excite the DiI-labeled v-vesicles and measure FRET, and a 635 nm laser was used to check the presence of the DiD-labeled t-vesicles. The movies were analyzed by smCamera (kindly provided by the Dr Taekjip Ha’s group) and the Matlab programs developed in our laboratory to calculate the ratio of docked v-vesicles to immobilized t-vesicles.
Single-vesicle docking assay
The single-vesicle docking assay was described recently with some modifications [38]. The t-vesicles with the final lipid concentration of 1 μM were flown into the chamber and immobilized on the PEG-coated surface through the streptavidin-to-biotin lipid conjugation. After 30-min incubation at room temperature (~25°C), the unbound t-vesicles were removed by two rounds of washing with 200 μl of dialysis buffer. The v-vesicles (3 μM) with or without A-syn were flown into the chamber and the sample was incubated for another 30 min at 37°C. After washing off free v-vesicles using dialysis buffer containing A-syn or equal volume of dialysis buffer, movies were acquired by taking 100 consecutive frames with the 100 ms exposure time from five randomly chosen imaging areas. The first 60 frames were taken using 532 nm laser excitation for DiI-labeled v-vesicles, and these data were used to calculate the FRET efficiency, while the final 40 frames were taken by using 635 nm laser excitation to verify the presence of DiD-labeled t-vesicles. The nonspecifically bound v-vesicles were excluded from the analysis.
Single-vesicle content-mixing assay
The modified single-vesicle docking assay was described recently [38]. The t-SNARE vesicles (100 μM) were immobilized on the PEG-coated surface through the streptavidin-to-biotin lipid conjugation for 20 min. Unbound t-vesicles were washed off by several rounds of washing with 200 μl of dialysis buffer. The v-vesicles (with syt1), in the presence of 1 mM EDTA (10 μM) with or without A-syn, were injected into the flow chamber, and the sample was incubated at room temperature for 10 min. Then, the unbound v-vesicles were washed out with the dialysis buffer with or without A-syn, supplemented with 1 mM EDTA, and the samples were incubated for the additional 20 min, followed by the injection of 500 μM Ca2+ using the motorized syringe pump. Movies were acquired using the same TIRF setup as described above with 532 nm excitation for SRB, and the stepwise jump in the fluorescence emission intensity due to fluorescence dequenching of SRB was recorded as the signal for content mixing. TIRF microscope imaging and the data analysis of the single-vesicle content-mixing assay were described in detail elsewhere [42].
Results
Recombinant A-syn is predominantly monomeric
There has been controversy surrounding whether normal, non-aggregated A-syn is a monomer or a tetramer [43–45]. Recently, a consensus has been built around that it exists predominantly as an intrinsically disordered monomer although the transient presence of a metastable tetramer cannot be ruled out [46]. We confirm with gel filtration that our purified, recombinant A-syn behaves similar to a native A-syn purified from the mouse brain (Figure 1B) [46]. We, however, observe a small population of SDS-resistant oligomers in western blot (Figure 1B), similar to what was reported before [47]. But we do not detect the higher-order oligomers reminiscent of the dopamine-induced aggregation [34,48,49].
Low-level A-syn promotes SNARE-dependent vesicle docking
It was proposed by Burre et al. [31] that A-syn promotes SNARE complex formation. To investigate the mechanism whereby A-syn promotes SNARE assembly, we set up a single-vesicle docking/fusion assay. We immobilize t-SNARE-bearing proteoliposomes (t-vesicles) on the biotin-PEG-coated surface in a flow cell through the streptavidin-to-biotin lipid conjugation. Vesicles carrying VAMP2 (v-vesicles) are then added into the flow cell and vesicle docking is allowed in the absence or presence of A-syn (Figure 1C). We first vary the A-syn concentration up to 25 μM. Also, we use an asymmetric acidic lipid distribution on t- and v-vesicles (15 mol% PS plus 2 mol% PIP2 on t-vesicles and 5 mol% PS on v-vesicles, respectively), mimicking a favorable charge distribution for Ca2+-triggered vesicle fusion [40,50]. The results show that at concentrations below 2.5 μM, A-syn promotes vesicle docking by as much as three-fold (Figure 1D,E). However, vesicle docking is markedly reduced when the A-syn concentration is increased further (Figure 1D,E). We also find that A-syn enhances vesicle docking when a major Ca2+-sensor syt1 [51] is present on the v-vesicle although the effect comes out much smaller due to the fast saturation of docking in the presence of syt1 (Supplementary Figure S1). Reduced docking and aggregation of vesicles by A-syn have been extensively studied by Diao et al. [21]. Thus, our work is rather focused on a newly discovered promotion of vesicle docking by A-syn at low concentrations.
To address whether A-syn affects the downstream fusion steps after vesicle docking, we perform Ca2+-triggered content-mixing experiments. A-syn does not influence the kinetics and the efficiency of content mixing for docked vesicles within experimental errors (Figure 1F and Supplementary Figure S2), consistent with the results from Burre et al. [31]. Thus, the results show that A-syn’s effect is restricted to the vesicle docking step.
C-terminal domain of A-syn is essential for the promotion of vesicle docking
It has been shown that A-syn interacts with v-SNARE VAMP2 through its polar C-terminal domain [31]. To examine if the A-syn/VAMP2 interaction plays a role in promoting vesicle docking, we prepare the A-syn mutant (amino acids 1–95) that lacks the VAMP2-interacting C-terminal region. This mutant previously abolished the interaction with VAMP2 [31,34]. We find that the truncated A-syn is not capable of promoting vesicle docking (Figure 2A and Supplementary Figure S1). Thus, the result shows that the enhancement of docking requires the interaction of A-syn with VAMP2 through its C-terminal region. We also find that the truncated A-syn is not capable of inhibiting vesicle docking at high concentrations as wild-type A-syn is able to do (Figure 2B), also suggesting that the A-syn/VAMP2 interaction is essential for the inhibition of vesicle docking, consistent with the results by Diao et al. [21].
Figure 2. The C-terminal region of A-syn is required for the enhancement of SNARE-dependent vesicle docking.
(A) Histogram of the docking probabilities between t-vesicles and v-vesicles harboring VAMP2 (no syt1) in the absence or presence of 2.5 μM wild-type A-syn or its C-terminal truncation mutant A-syn_1–95. Error bars denote the SD of three independent experiments, and movies recorded from more than five randomly selected screens were analyzed and averaged in each experiment. (B) Vesicle docking probability versus concentration of A-syn_1–95. Error bars denote the SD of two independent experiments, and movies recorded from more than five randomly selected screens were analyzed and averaged in each experiment. The data present in (A) were collected independently of those in Figure 1E and in panel (B).
Acidic lipids on the t-SNARE side are essential for the promotion of vesicle docking by A-syn
Based on the membrane-bound structure, A-syn appears to poise as if it interacts with the negatively charged plasma membrane with its basic amphipatic N-terminal region and simultaneously reaches out and grabs VAMP2 on the vesicle through its hydrophilic C-terminal region, whereby it cross-bridges the vesicle and the plasma membrane in trans. We have shown that the interaction between A-syn and VAMP2 is essential for vesicle docking.
We now test if the negatively charged lipids, which are known to be essential for A-syn’s membrane binding [52], are required on t-vesicles to promote vesicle docking. In our aforementioned experiments (Figures 1 and 2), we used the lipids’ composition of 5 mol% PS in POPC for the v-vesicles, and 15 mol% PS and 2 mol% PIP2 in POPC for the t-vesicles. We now ask if the negatively charged lipids on t-vesicles are required for the promotion of vesicle docking by A-syn.
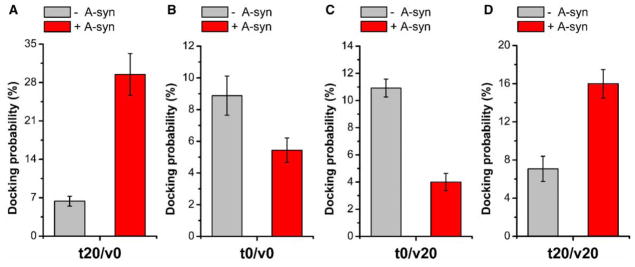
To answer this question, we first prepare t-vesicles of 20 mol% PS and v-vesicles of no PS. As it is shown in Figure 1, we observe the four-fold enhancement of vesicle docking with 2.5 μM A-syn (Figure 3A). However, when PS on t-vesicles is reduced to zero, A-syn becomes inhibited instead of promoting (30% inhibition) (Figure 3B). Moreover, when we reverse the charge asymmetry to have t-vesicle of no PS and v-vesicle of 20 mol% PS, we observe significant decrease, as much as a factor of three, of vesicle docking (Figure 3C). On the other hand, when we add 20 mol% PS on both sides, we still observe a factor of two enhancement of vesicle docking (Figure 3D), showing that PS on the t-vesicle is critical for the promotion of vesicle docking by A-syn. Thus, the results show that A-syn’s interaction with negatively charged PS on t-vesicles plays an essential role in promoting vesicle docking.
Figure 3. Negatively charged lipids on t-SNARE side are essential for the positive role of A-syn in vesicle docking.
Histograms of docking probabilities without (−A-syn) or with 2.5 μM A-syn (+A-syn) using t-vesicles containing 20 mol% DOPS and v-vesicles without DOPS (t20/v0) (A), both t- and v-vesicles without DOPS (t0/v0) (B), t-vesicles without DOPS and v-vesicles containing 20 mol% DOPS (t0/v20) (C), and both t- and v-vesicles containing 20 mol% DOPS (t20/v20) (D). Error bars denote the SD of three independent experiments, and movies recorded from more than five randomly selected screens were analyzed and averaged in each experiment.
A-syn is incapable of cross-bridging vesicles in the absence of t-SNAREs
The results suggest that A-syn promotes vesicle docking in VAMP2- and PS-dependent manners. It was also proposed that A-syn may connect the two opposite membranes directly in trans [53,54]. Therefore, it raises the possibility that A-syn could mediate vesicle docking by itself even in the absence of SNARE complex formation.
To test this possibility, we carry out the vesicle docking assay using the liposomes without t-SNAREs or VAMP2 in replacement of the corresponding SNAREs-carrying vesicle. We find that A-syn is not capable of mediating vesicle docking in the absence of SNARE complex formation (Figure 4). Thus, the results show that A-syn works as a sort of catalyst for SNARE-dependent vesicle docking which assists vesicle docking mediated by SNARE complex formation.
Figure 4. A-syn cross-bridges vesicles in an SNARE-dependent manner.

Histogram of the probability of docking, in the presence of 2.5 μM A-syn, between the v-vesicle (0 mol% PS) and the t-vesicle (20 mol% PS) (gray), between the v-vesicle (0 mol% PS) and the liposome (20 mol% PS) without t-SNAREs (red), and between the liposome (0 mol% PS) without VAMP2 and the t-vesicle (20 mol% PS) (blue). Error bars denote the SD of three or more independent experiments, and movies recorded from more than five randomly selected screens were analyzed and averaged in each experiment.
Discussion
On the basis of our results, we propose a hypothetical mechanistic model whereby A-syn cross-bridges vesicle and plasma membranes, which facilitate SNARE-dependent vesicle docking and subsequently, SNARE complex formation (Figure 5). The N-terminal amphipathic and hydrophobic regions (amino acids 1–100) bind to the plasma membrane in a helical conformation with a break in the middle. Acidic phospholipids in the plasma membrane, including highly negatively charged PIP2, would make A-syn’s binding to it favorable. Meanwhile, the hydrophilic C-terminal region (amino acids 101–140) reaches out to the solution phase and interacts with the N-terminal domain of VAMP2, thereby cross-linking the vesicle to the plasma membrane. We present the membrane-bound portion of A-syn to be a predominantly α-helical monomer. Recently, Südhof and co-workers proposed, based on their fluorescence study, that the N-terminal region of A-syn forms oligomers of helical hairpins upon binding to the membrane [55]. In either case, the molecular interactions that govern the A-syn-induced membrane cross-bridging would be the same; membrane binding via the N-terminal domain and trans-binding to VAMP2 via the C-terminal domain. The oligomerization might help A-syn molecules to work cooperatively in stimulating vesicle docking.
Figure 5. A proposed mechanistic model for A-syn-assisted SNARE-mediated vesicle docking.

A-syn adsorbs to the surface of the plasma membrane through its N-terminal region and concurrently interacts with the VAMP2 on synaptic vesicles through its C-terminal region, thereby cross-bridging the synaptic vesicles with plasma membrane, which facilitates the SNARE complex formation that in turn stabilizes the docked vesicle.
We find that in the absence of SNAREs, A-syn is not capable of holding docked vesicles stably (Figure 4). Thus, we speculate that the cross-bridging of two membranes by A-syn is weak and perhaps transient. Yet, it is sufficient to facilitate SNARE complex formation, which would accelerate SNARE-dependent vesicle docking.
The idea of cross-bridging of the synaptic vesicle to the plasma membrane mediated by A-syn has been previously put forth by Snead and Eliezer [54]. However, their proposed molecular mechanism is quite different from ours. In their model, the N-terminal amphipathic region of A-syn binds to the plasma membrane, whereas the C-terminal hydrophobic core region interacts in trans with the vesicle membrane [53]. In contrast, our mechanistic model proposes the critical role of the specific interaction between A-syn and VAMP2 in vesicle binding.
There is evidence, both in vivo and in vitro, that A-syn has the capability of mediating clustering of vesicles [21,22]. Roy and coworkers showed that the metastable or transient tetrameric species might be involved in this process [22]. Then, the mechanism underlying vesicle clustering might be fundamentally different from our mechanistic model for the promotion of vesicle docking by A-syn (Figure 5).
We observed the inhibition of vesicle docking by A-syn when PS was removed from t-vesicles (Figure 3). The results might suggest that the A-syn-mediated attraction among v-vesicles is in competition with the A-syn-mediated attraction between v- and t-vesicles. Thus, when PS is removed from t-vesicles, the attraction among v-vesicles induced by A-syn surpasses the attraction between t- and v-vesicles, because A-syn no longer has the affinity to the t-vesicle membrane. This would result in reduced vesicle docking.
Although A-syn has the ability to promote vesicle docking, it does not change the efficiency or the kinetics of SNARE-dependent fusion pore opening among docked vesicles (Figure 1F), consistent with the results from electrophysiological measurements of cultured neurons [31,56]. Our results show that A-syn is likely to be involved in vesicle docking step prior to membrane fusion. For example, A-syn potentially plays a role in effectively replenishing the readily releasable pool of vesicles. The measurement of the rates of docking with the single-vesicle docking assay would provide further insights into A-syn’s role in vesicle trafficking.
In this work, we have examined the effect of A-syn on docking of two equally sized proteoliposomes of ~90 nm [36]. In reality, synaptic vesicles are smaller, whereas the plasma membrane is flatter than the proteoliposomes. Because A-syn has the preferred affinity to the highly curved membranes [57], the hypothetical cross-bridging of synaptic vesicles to the plasma membrane might be somewhat weaker than what has been observed in our in vitro study, warranting further work.
In summary, A-syn is structured in such a way that it could interact with the membrane via the amphipathic N-terminal region and concurrently with VAMP2 on the opposite membrane. The results suggest that such a cross-bridging could be the mechanism whereby A-syn promotes SNARE complex formation and vesicle docking.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Institutes of Health Grants R01 GM051290 (to Y.-K.S.), Membrane Protein Structure and Dynamics Consortium (to Y.-K.S.), and Roy J. Carver Professorship (to Y.-K. S.).
Abbreviations
- A-syn
α-synuclein
- biotin-DPPE
1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamin-N-(biotinyl)
- DiD
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- DOPS
1,2-dioleoyl-sn-glycero-3-phospho-L-serine
- FRET
fluorescence resonance energy transfer
- GST
glutathione S-transferase
- IPTG
isopropyl β-D-1-thiogalactopyranoside
- PBS-OG
PBS with octyl β-D-glucopyranoside
- PEG
polyethylene glycol
- PIP2
phosphatidylinositol-4,5-bisphosphate
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- PS
phosphatidylserine
- SNARE
soluble N-ethylmaleimide-sensitive fusion protein-attachment protein receptor
- SRB
sulforhodamine B
- syt1
synaptotagmin 1
- T-SNARE
target SNARE
- TIRF
total internal reflection fluorescence
- VAMP
vesicle-associated membrane protein
- V-SNARE
vesicle SNARE
Footnotes
Author Contribution
X.L. and Y.-K.S. designed research; X.L., B.J.H., and J.K. performed research; X.L. and Y.-K.S. analyzed data; and X.L. and Y.-K.S. wrote the paper.
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Bendor JT, Logan TP, Edwards RH. The function of α-synuclein. Neuron. 2013;79:1044–1066. doi: 10.1016/j.neuron.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M. α-Synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 3.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, et al. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 5.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 6.Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, et al. AlaSOpro mutation in the gene encoding α-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 7.Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, et al. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 8.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, et al. α-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 9.Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, et al. α-Synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 10.Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, et al. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg MS, Lansbury PT., Jr Is there a cause-and-effect relationship between α-synuclein fibrillization and Parkinson’s disease? Nat Cell Biol. 2000;2:E115–E119. doi: 10.1038/35017124. [DOI] [PubMed] [Google Scholar]
- 12.Lotharius J, Brundin P. Pathogenesis of Parkinson’s disease: dopamine, vesicles and α-synuclein. Nat Rev Neurosci. 2002;3:932–942. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- 13.Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clayton DF, George JM. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998;21:249–254. doi: 10.1016/S0166-2236(97)01213-7. [DOI] [PubMed] [Google Scholar]
- 15.Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yavich L, Tanila H, Vepsäläinen S, Jäkälä P. Role of α-synuclein in presynaptic dopamine recruitment. J Neurosci. 2004;24:11165–11170. doi: 10.1523/JNEUROSCI.2559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, et al. α-Synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006;26:11915–11922. doi: 10.1523/JNEUROSCI.3821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, et al. Increased expression of α-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott D, Roy S. α-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J Neurosci. 2012;32:10129–10135. doi: 10.1523/JNEUROSCI.0535-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diao J, Burré J, Vivona S, Cipriano DJ, Sharma M, Kyoung M, et al. Native α-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2. eLife. 2013;2:e00592. doi: 10.7554/eLife.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Das U, Scott DA, Tang Y, McLean PJ, Roy S. α-Synuclein multimers cluster synaptic vesicles and attenuate recycling. Curr Biol. 2014;24:2319–2326. doi: 10.1016/j.cub.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 24.Rhoades E, Ramlall TF, Webb WW, Eliezer D. Quantification of α-synuclein binding to lipid vesicles using fluorescence correlation spectroscopy. Biophys J. 2006;90:4692–4700. doi: 10.1529/biophysj.105.079251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varkey J, Isas JM, Mizuno N, Jensen MB, Bhatia VK, Jao CC, et al. Membrane curvature induction and tubulation are common features of synucleins and apolipoproteins. J Biol Chem. 2010;285:32486–32493. doi: 10.1074/jbc.M110.139576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giasson BI, Murray IVJ, Trojanowski JQ, Lee VMY. A hydrophobic stretch of 12 amino acid residues in the middle of α-synuclein is essential for filament assembly. J Biol Chem. 2001;276:2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- 27.Rao JN, Jao CC, Hegde BG, Langen R, Ulmer TS. A combinatorial NMR and EPR approach for evaluating the structural ensemble of partially folded proteins. J Am Chem Soc. 2010;132:8657–8668. doi: 10.1021/ja100646t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jao CC, Hegde BG, Chen J, Haworth IS, Langen R. Structure of membrane-bound α-synuclein from site-directed spin labeling and computational refinement. Proc Natl Acad Sci USA. 2008;105:19666–19671. doi: 10.1073/pnas.0807826105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulmer TS, Bax A, Cole NB, Nussbaum RL. Structure and dynamics of micelle-bound human α-synuclein. J Biol Chem. 2005;280:9595–9603. doi: 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- 30.Georgieva ER, Ramlall TF, Borbat PP, Freed JH, Eliezer D. Membrane-bound α-synuclein forms an extended helix: long-distance pulsed ESR measurements using vesicles, bicelles, and rodlike micelles. J Am Chem Soc. 2008;130:12856–12857. doi: 10.1021/ja804517m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. α-Synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumert M, Maycox PR, Navone F, De Camilli P, Jahn R. Synaptobrevin: an integral membrane protein of 18,000 daltons present in small synaptic vesicles of rat brain. EMBO J. 1989;8:379–384. doi: 10.1002/j.1460-2075.1989.tb03388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi BK, Choi MG, Kim JY, Yang Y, Lai Y, Kweon DH, et al. Large α-synuclein oligomers inhibit neuronal SNARE-mediated vesicle docking. Proc Natl Acad Sci USA. 2013;110:4087–4092. doi: 10.1073/pnas.1218424110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kyoung M, Zhang Y, Diao J, Chu S, Brunger AT. Studying calcium-triggered vesicle fusion in a single vesicle-vesicle content and lipid-mixing system. Nat Protoc. 2013;8:1–16. doi: 10.1038/nprot.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon TY, Okumus B, Zhang F, Shin YK, Ha T. Multiple intermediates in SNARE-induced membrane fusion. Proc Natl Acad Sci USA. 2006;103:19731–19736. doi: 10.1073/pnas.0606032103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diao J, Su Z, Ishitsuka Y, Lu B, Lee KS, Lai Y, et al. A single-vesicle content mixing assay for SNARE-mediated membrane fusion. Nat Commun. 2010;1:54. doi: 10.1038/ncomms1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lou X, Shin J, Yang Y, Kim J, Shin YK. Synaptotagmin-1 is an antagonist for Munc18-1 in SNARE zippering. J Biol Chem. 2015;290:10535–10543. doi: 10.1074/jbc.M114.631341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai Y, Kim S, Varkey J, Lou X, Song JK, Diao J, et al. Nonaggregated α-synuclein influences SNARE-dependent vesicle docking via membrane binding. Biochemistry. 2014;53:3889–3896. doi: 10.1021/bi5002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai Y, Shin YK. The importance of an asymmetric distribution of acidic lipids for synaptotagmin 1 function as a Ca2+ sensor. Biochem J. 2012;443:223–229. doi: 10.1042/BJ20112044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai Y, Lou X, Diao J, Shin YK. Molecular origins of synaptotagmin 1 activities on vesicle docking and fusion pore opening. Sci Rep. 2015;5:9267. doi: 10.1038/srep09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diao J, Ishitsuka Y, Lee H, Joo C, Su Z, Syed S, et al. A single vesicle-vesicle fusion assay for in vitro studies of SNAREs and accessory proteins. Nat Protoc. 2012;7:921–934. doi: 10.1038/nprot.2012.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang W, Perovic I, Chittuluru J, Kaganovich A, Nguyen LT, Liao J, et al. A soluble α-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci USA. 2011;108:17797–17802. doi: 10.1073/pnas.1113260108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 45.Bartels T, Choi JG, Selkoe DJ. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burré J, Vivona S, Diao J, Sharma M, Brunger AT, Sudhof TC. Properties of native brain α-synuclein. Nature. 2013;498:E4–E6. doi: 10.1038/nature12125. discussion E6–E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwata A, Maruyama M, Akagi T, Hashikawa T, Kanazawa I, Tsuji S, et al. Alpha-synuclein degradation by serine protease neurosin: implication for pathogenesis of synucleinopathies. Hum Mol Genet. 2003;12:2625–2635. doi: 10.1093/hmg/ddg283. [DOI] [PubMed] [Google Scholar]
- 48.Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr Kinetic stabilization of the α-synuclein protofibril by a dopamine-α-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 49.Cappai R, Leck SL, Tew DJ, Williamson NA, Smith DP, Galatis D, et al. Dopamine promotes α-synuclein aggregation into SDS-resistant soluble oligomers via a distinct folding pathway. FASEB J. 2005;19:1377–1379. doi: 10.1096/fj.04-3437fje. [DOI] [PubMed] [Google Scholar]
- 50.Vennekate W, Schroder S, Lin CC, van den Bogaart G, Grunwald M, Jahn R, et al. Cis- and trans-membrane interactions of synaptotagmin-1. Proc Natl Acad Sci USA. 2012;109:11037–11042. doi: 10.1073/pnas.1116326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chapman ER. How does synaptotagmin trigger neurotransmitter release? Annu Rev Biochem. 2008;77:615–641. doi: 10.1146/annurev.biochem.77.062005.101135. [DOI] [PubMed] [Google Scholar]
- 52.Jo E, McLaurin J, Yip CM, St George-Hyslop P, Fraser PE. α-Synuclein membrane interactions and lipid specificity. J Biol Chem. 2000;275:34328–34334. doi: 10.1074/jbc.M004345200. [DOI] [PubMed] [Google Scholar]
- 53.Fusco G, Pape T, Stephens AD, Mahou P, Costa AR, Kaminski CF, et al. Structural basis of synaptic vesicle assembly promoted by α-synuclein. Nat Commun. 2016;7:12563. doi: 10.1038/ncomms12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snead D, Eliezer D. Alpha-synuclein function and dysfunction on cellular membranes. Exp Neurobiol. 2014;23:292–313. doi: 10.5607/en.2014.23.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burré J, Sharma M, Südhof TC. α-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci USA. 2014;111:E4274–E4283. doi: 10.1073/pnas.1416598111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, Liu X, et al. Double-knockout mice for α- and β-synucleins: effect on synaptic functions. Proc Natl Acad Sci USA. 2004;101:14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Middleton ER, Rhoades E. Effects of curvature and composition on α-synuclein binding to lipid vesicles. Biophys J. 2010;99:2279–2288. doi: 10.1016/j.bpj.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.