Abstract
According to the social motivation theory of autism, children who develop Autism Spectrum Disorder (ASD) have early deficits in social motivation, which is expressed by decreased attention to social information. These deficits are said to lead to impaired socio-cognitive development, such as theory of mind (ToM). There is little research focused on the relation between social motivation and ToM in this population. The goal of the present study was to investigate the link between one aspect of social motivation, social orienting, and ToM in preschoolers with ASD. It was expected that, in contrast to typically developing (TD) children, children with ASD would show impaired performance on tasks measuring social orienting and ToM. It was also expected that children’s performance on the social orienting tasks would be correlated with their performance on the ToM task. A total of 17 children with ASD and 16 TD children participated in this study. Participants completed two social orienting tasks, a face preference task and a biological motion preference task, as well an implicit false belief task. Results reveal that TD children, but not children with ASD, exhibited social preference as measured by a preference for faces and biological motion. Furthermore, children with ASD tended to perform worse on the ToM task compared to their TD counterparts. Performance on the social motivation tasks and the ToM task tended to be related but only for the TD children. These findings suggest that ToM is multifaceted and that motivational deficits might have downstream effects even on implicit theory of mind.
Keywords: Autism, Social Motivation, Theory of Mind
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder characterized by deficits in social communication and social interaction evident in multiple contexts, as well as restricted repetitive patterns of behaviour, interests, or activities (American Psychiatric Association, 2013). Since its identification by Leo Kanner in 1943, an extensive body of research has generated many candidate theories that could explain ASD. One prominent theory posits that children’s social and communicative impairments can be explained by a Theory of Mind (ToM) deficit (Baron-Cohen, 1995; Leslie, 1987). This view argues that individuals with ASD have difficulty understanding another person’s perspective, which has downstream effects on social abilities (Kimhi, 2014). However, the idea that ToM is a core deficit in ASD has been criticized at many levels, including the lack of universality of this deficit in the ASD population, as well as its lack of specificity (Tager-Flusberg, 2007).
An alternative account for the development of the social deficits observed in ASD is the social motivation theory (Chevallier, Kohls, Troiani, Brodkin, & Schultz, 2012; Dawson, Meltzoff, Osterling, Rinaldi, & Brown, 1998; Kaiser, Delmolino, Tanaka, & Shiffrar, 2010; Klin, Jones, Schultz, Volkmar, & Cohen, 2002; Klin, Lin, Gorrindo, Ramsay, & Jones, 2009). As described by Chevallier and colleagues (2012), social motivation is “a set of psychological dispositions and biological mechanisms biasing the individual to preferentially orient to the social world (social orienting), to seek and take pleasure in social interactions (social reward), and to work to foster and maintain social bonds (social maintaining)” (p. 231). This theory suggests that children who go on to develop ASD have early deficits in social motivation, which prevents them from attending to and learning from socially relevant information in their environment. This deficit is proposed to explain the socio-cognitive deficits observed in ASD, including ToM deficits (Broekhof et al., 2015; Chevallier et al., 2012; Senju & Johnson, 2009). This theory is not specific to individuals with ASD and asserts that individual differences in social motivation relate to social cognitive abilities across typically developing (TD) and ASD populations (Chevallier et al., 2012).
As introduced previously, three types of motivational deficits have been documented as support for the social motivation theory of autism: social orienting, social reward, and social maintaining (Chevallier et al., 2012). Our focus is on social orienting, which is commonly tested with preferential looking tasks, whereby a social and a non-social stimulus are presented simultaneously. The participants’ looking behaviour is recorded to determine whether they show a preference for either stimulus. A significant body of research has demonstrated such social orienting deficits in the ASD population (Chevallier et al., 2012; Falck-Ytter, Rehnberg, & Bölte, 2013; Sasson, Elison, Turner-Brown, Dichter, & Bodfish, 2011; Wright, Kelley, & Poulin-Dubois, 2016). However, other research suggests that individuals with ASD develop these abilities by adolescence (Cleary, Looney, Brady, & Fitzgerald, 2014; Flanagan, Brodeur, & Burack, 2015) or adulthood (Rutherford & Troje, 2012). Social orienting is typically tested by contrasting social stimuli such as human faces, bodies or voices with non-social stimuli such as artefacts (Annaz, Campbell, Coleman, Milne, & Swettenham, 2012; Curtin & Vouloumanos, 2013; Sasson, Turner-Brown, Holtzclaw, Lam, & Bodfish, 2008). Two types of non-social stimuli have been identified and differentiated (Sasson et al., 2008): high-autism interest (HAI) images (e.g., circumscribed interests such as clocks or trains) and low-autism interest (LAI) images (e.g., plants, clothing). In a study conducted by Sasson and colleagues (2008), children with ASD aged 6 to 17 years looked less at the social stimuli if it was paired with an HAI object. These results were replicated in a later study with 2- to 5-year-olds with ASD (Sasson et al., 2011). Together, these findings demonstrate that children with ASD do not orient toward social stimuli when paired with a highly salient non-social contrast. Importantly, these results provide greater specificity surrounding the contexts in which social motivation deficits in children with ASD are readily observed.
At the biological level, social motivation is subserved by a network of brain areas (e.g., orbitofrontal-striatal-amygdala circuit), which plays an important role in guiding attention to social information, including biological motion (Chevallier et al., 2012). Deficits in detection and preference for biological motion have been shown among children with ASD. Using a split-screen showing human biological motion and phase-scrambled motion, Annaz and colleagues (2012) showed that TD children preferred the human biological motion stimulus, while children with ASD did not demonstrate a preference for either stimulus. Although children with ASD have often been shown to lack a preference for biological motion (Annaz et al., 2012; Falck-Ytter et al., 2013; Klin & Jones, 2008; Wright et al., 2016), recent results indicate that deficits in detection and visual preference for biological motion diminish with age (Annaz et al., 2010; Jones et al., 2011). Surprisingly, to our knowledge, no research has investigated whether different measures of social orienting are related within the same individuals. Testing the coherence of social motivation measures is one of the main goals of the current study.
One key prediction of the social motivation theory is that reduced social interest is expected to deprive children of critical social inputs that will ultimately lead to diminished expertise in social cognition. Importantly, the question of whether early social motivation deficits relate to children’s concurrent or subsequent socio-cognitive abilities, such as false belief understanding, has not been explored in ASD children. To our knowledge, only two studies have reported such a link and have typically compared the ability to detect biological motion and theory of mind tasks in TD school-aged children and adults (Miller & Saygin, 2013; Rice, Anderson, Velnoskey, Thompson, & Redcay, 2016). Individuals who were better able to perceive biological motion when masked by noise were better able to infer mental states from eye gaze or verbal information. Another main goal of the current study was to examine the link between performance on social orienting tasks (biological motion and face preference) and an implicit measure of false belief.
Although children with ASD have been shown to disproportionately fail standard false-belief tasks compared to TD counterparts (Baron-Cohen, Leslie, & Frith, 1985), as mentioned before, mentalizing deficits are not universal among individuals with ASD. A significant proportion of children and adults with ASD succeed on these tasks once they reach a certain verbal mental age (Frith, 2012; Happé, 1995; Peterson, 2014; Senju, 2012). It has been suggested that children with ASD fail the false belief task because it requires advanced pragmatic and inhibitory skills (Senju, 2012). For example, when asked where the protagonist will look for the marble, children may process the “where” and look where the marble is actually located, thus failing the explicit false belief task (Csibra & Southgate, 2006). Moreover, the explicit false belief task involves several executive functions, such as working memory to remember the story sequence, and inhibitory control to prevent oneself from pointing to the actual location of the marble (Kimhi, 2014). For this reason, implicit versions of the false belief task have been designed, wherein the child was not required to explicitly state (or point to) the correct answer. Such implicit false belief tasks use the violation of expectation (VOE) or anticipatory looking paradigms, were designed to test young children or preverbal infants (Baillargeon, Scott, & He, 2010; Clements & Perner, 1994), and have more recently been used to test children and adults with ASD (Schuwerk, Vuori & Sodian, 2015; Senju, 2012).
Schneider, Slaughter, Bayliss, and Dux (2013) investigated both implicit (anticipatory looking paradigm) and explicit ToM (ToM Scale and Strange Stories) abilities in adults diagnosed with ASD. They reported that adults with ASD, who performed similarly to neurotypical adults on the explicit ToM measure, did not demonstrate implicit ToM understanding. These results suggest that implicit and explicit ToM are separate processes and that implicit ToM appears to be impaired throughout the lifespan in individuals with ASD. Senju and colleagues (2010) used anticipatory looking as an implicit measure of false belief and showed that children with ASD (aged 6 to 8) failed to anticipate where the protagonist would look while the TD children correctly anticipated the protagonist’s future actions. In another study, children with ASD also failed to anticipate the correct location of the protagonist (Ruffman, Garnham, & Rideout, 2001). Thus, the performance of ASD children on standard false belief tasks cannot fully be explained by pragmatic and executive functioning deficits as the research using implicit false belief paradigms continues to show deficits in mind-reading skills.
In summary, the objectives of the present study were to 1) conduct an exploratory study on the association between social motivation and ToM abilities (i.e., implicit false belief), 2) explore whether different tasks assumed to all measure social motivation are correlated (i.e., construct validity), and 3) replicate recent findings that reported poorer performance of ASD children on social motivation and ToM abilities. The ToM task used was adapted from Thoermer, Sodian, Vuori, Perst, and Kristen’s (2012) location-change implicit false belief task and served as the socio-cognitive measure for the first objective. Social motivation was assessed using two types of stimuli: a biological motion visual preference task adapted from Annaz and colleagues (2012), and a static visual measure adapted from Sasson, Dichter, and Bodfish (2012). The static visual measure encompassed two types of non-social stimuli: HAI objects and LAI objects (Sasson et al., 2012).
Consistent with previous research, we expected that TD children would demonstrate a preference for socially relevant static and dynamic stimuli, whereas children with ASD would fail to show this same level of attention toward social stimuli. We also expected that, in comparison to TD controls, children with ASD would demonstrate significantly poorer performance on the implicit false belief ToM task. Given that individual differences in social motivation are hypothesized to relate to an individual’s relative social-cognitive abilities we also predicted that across both groups, performance on the ToM and social orienting tasks would be significantly correlated. Finally, we tested the hypothesis that since biological motion and face preference tasks are assumed to both measure social orienting, the scores on these two tasks will be related.
Method
Participants
Seventeen children diagnosed with ASD and sixteen TD children participated in the study. Participants in the ASD group were recruited from a University database, a hospital with specialized ASD diagnostic services, as well as referrals from specialized centers treating children with ASD. All participants in the ASD group had previously received a clinical diagnosis on the autism spectrum by satisfying the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV-TR; American Psychiatric Association, 2000) criteria, as well as the diagnostic thresholds on the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2012). One participant with ASD was excluded due to difficulty following the instructions required to complete the test of non-verbal cognitive ability. The final ASD sample consisted of sixteen English- (n = 8) and French-speaking (n = 8) participants (16 boys; mean age = 5.22 years, range = 3.08–7.08). Participants with ASD were excluded from the analyses of individual tasks in two instances due to technical difficulties on the biological motion task (n = 1), and static visual task (n = 1); none were excluded from the ToM task. Sixteen TD children participated in this experiment as a control group (8 boys and 8 girls; mean age = 3.98 years, range 2.08–7.50). Thirteen participants spoke English and three spoke French. Participants in the TD comparison group were recruited from a University database, had no other neurological or developmental disorders (e.g., language delay, epilepsy), and did not have any known first-degree relative with an ASD diagnosis. The TD children in this sample were selected from a larger sample to match the ASD sample on non-verbal mental age, which was measured using the non-verbal composite of the Differential Abilities Scale, Second Edition (DAS-II; Elliott, 2007; see Table 1). The two groups did not statistically differ in their receptive verbal abilities as measured by the Peabody Picture Vocabulary Test, Fourth Edition (PPVT-IV; Dunn, & Dunn, 2007). Given that there were no gender differences in performance in the TD group across tasks, this variable was ignored in all final analyses.
Table 1.
Mean chronological age, non-verbal mental age, verbal mental age and social communication questionnaire scores for the ASD and TD children, as well as the matching statistics.
| Group | CA (in years) | DAS-NVMA (age equivalent in years) | PPVT-VMA (age equivalent in years) | SCQ Scores | |
|---|---|---|---|---|---|
| TD | Mean | 3.98 | 4.28 | 4.72 | 4.13 |
| SD | 1.49 | 1.56 | 1.79 | 3.90 | |
| Range | 2.08–7.50 | 2.58–7.42 | 1.83–7.42 | 0–11 | |
| ASD | Mean | 5.22 | 4.36 | 4.06 | 16.67 |
| SD | 1.36 | 1.87 | 1.85 | 6.85 | |
| Range | 3.08–7.08 | 2.58–7.83 | 2.17–7.25 | 7–27 | |
|
| |||||
| t | −2.46 | −.120 | .991 | −6.32 | |
| df | 30 | 29 | 28 | 29 | |
| Sig. (2-tailed) | .020 | .906 | .330 | <.001 | |
Note. Scores above 15 on the SCQ are typically indicative of significant concern relating to ASD symptoms. However, it has been suggested that a lower cut-off score (e.g., 13 or 11) improves the sensitivity and specificity of the SCQ (Corsello et al., 2007; Snow & Lecavalier, 2008). Six participants diagnosed with ASD scored under 15 on the SCQ.
Materials and Procedure
The biological motion, static visual, and ToM tasks were all administered on a 23-inch laptop monitor (HP TouchSmart 520 PC) that housed an embedded camera.
Testing of each child took place across two visits to the laboratory. During the first visit, the participants’ caregivers completed a demographics questionnaire as well as the current version of the Social Communication Questionnaire (SCQ; Rutter, Bailey, & Lord, 2003), which was developed to screen for symptoms and behaviours associated with ASD. Each participant completed a total of five tasks: two social motivation tasks (static and dynamic), one socio-cognitive task (implicit false belief ToM), and both verbal and non-verbal measures of cognitive ability. The order of the tasks was counterbalanced across each visit. Tasks requiring that children be seated in front of a computer monitor were administered in one block and the cognitive tasks were administered in another block. This was done in order to avoid breaking the participants’ concentration in transitioning from the screen to the tabletop. The order of the tasks within each block was counterbalanced and the order in which the blocks were presented was randomized. To code participants’ looking time during the video tasks participants were filmed using a camera that was embedded in the center of the computer monitor.
Biological motion1
This task was adapted from Annaz and colleagues (2012). Point-light displays of a walking human and of a phase-scrambled human were presented on a split-screen. Eight trials were presented for 6 seconds each. Each display consisted of 13 point-light dots, which were placed on major parts of the human (e.g., one head, two shoulders, two elbows, two hands, two hips, two knees, and two feet). The phase-scrambled display was created by making the motion trajectories play temporally out of phase. Half of the trials displayed motion towards the right. The side on which the human was presented and the order of each video (right/left walking) was counterbalanced. A central fixation-cross accompanied by a chime sound was presented prior to each trial in order to orient children’s attention to the screen.
Static visual
This task was adapted from Sasson and colleagues (2012). Pictures of humans and objects were presented on a split screen. Twenty trials were presented for 5 seconds each. The 20 pictures of humans consisted of 10 males and 10 females, ranging in age (infants to elderly) and ethnicity. The 20 pictures of objects consisted of 10 HAI objects (two vehicles, two signs, two sets of blocks, two electronic devices, and two clocks) and 10 LAI objects (two pieces of clothing, two instruments, two plants, two tools, and two pieces of furniture). The side on which the human was presented and the order of male/female human and HAI/LAI object was counterbalanced. A central fixation-cross accompanied by a chime sound was presented prior to each trial in order to orient children’s attention to the center of the screen.
Implicit false-belief ToM
This task was adapted from Thoermer and colleagues (2012). Three short videos were shown in succession: two familiarization videos (26 seconds each) and a test video (35 seconds). The familiarization videos showed a protagonist watching a car moving from a garage on one end of the screen to the other garage at the other end. Following this, a chime signalled the two doors above each garage turning bright red for three seconds, serving as the anticipatory looking period. The protagonist then came out of the door above the garage containing the car and retrieved the object. A passing score was defined according to the direction of the participants’ first look (i.e., on the side of the screen where the car is located). The test trial showed the protagonist watching the car move across the screen. However, a phone ringing distracted the protagonist so that she did not see the car backing up and disappearing on the other side of the screen. Following the anticipatory period, the protagonist came out of the door above the garage where she had last seen the car. A passing score was defined according to whether the participants’ first look was to the side of the screen where the protagonist last saw the car. An attractive attention-getter (a green circle) paired with a chime sound was presented prior to each trial to orient children’s attention to the screen.
Coding
An experimenter blind to the location of the stimuli coded the participants’ looking behaviour from the recorded videos of the dynamic (biological) motion, static visual stimuli, and ToM tasks. A second experimenter coded 30% of the videos to establish reliability for each task. For each trial of the biological motion task, the experimenters calculated the total duration that each participant looked at the biological (social) and at the phase-scrambled (non-social) video. Cohen’s kappa inter-rater reliability was 0.84. From this calculation, the proportion of looking at the biological motion was calculated for each participant. For each trial of the static visual task, the experimenters coded the total looking time at the face (social), and at the HAI or LAI objects (non-social) images. Cohen’s kappa inter-rater reliability was 0.92. From this calculation, the proportion of looking at the human was calculated for each participant. For each trial of the theory of mind task, the experimenters coded the first look (correct or incorrect side) during the anticipatory looking period. The Cohen kappa inter-rater reliability was 0.82. Kappa values of above 0.80 are considered “almost perfect” (McHugh, 2012). Given that the Kappa values obtained were all above this cut-off, the scores of the primary coder were used in the analyses.
Results
Chronological age, non-verbal mental age, verbal mental age, and SCQ scores were normally distributed in both groups and did not contain outliers. Performances on the biological motion task, static visual task, and ToM task were also normally distributed and did not contain outliers.
All sixteen TD participants were included in the analyses for the biological motion task and the static visual task. Fifteen ASD participants were included in the biological motion task and fifteen participants were also included for the static visual task. A repeated measures 2 × 2 ANOVA [group (TD vs. ASD) and social motivation tasks (biological motion vs. static visual)] was conducted to determine whether children with ASD showed less social motivation than TD children. There were no main effects nor interactions found: both groups performed similarly on both social motivation tasks (see Figure 1).
Figure 1.

Proportion of looking time at the social stimuli on the social motivation tasks for TD and ASD children.
A repeated measures group (TD vs. ASD) X static visual condition (HAI vs. LAI) ANOVA was conducted to determine whether children’s social motivation varied depending on the type of non-social object was paired with the social images. A main effect of static visual group was found, F(29) = 9.92, p = .004, d = .255. A post-hoc paired samples t-test revealed that both groups looked longer at the social stimuli when it was paired with an LAI object, t(30) = −3.14, p = .004, d = .488.
Post-hoc analyses were conducted to determine whether TD children’s performance on each social motivation task was above chance (i.e., showing a social preference). On the biological motion task, participants looked at the social stimuli longer than at the non-social stimuli (M = .57, SD = .10; t(15) = 2.67, p = .017, 95% CI [.014, .120], d = .668; see Figure 1). On the static visual task, participants looked at the social stimuli longer than at the non-social stimuli (M = .56, SD = .10; t(15) = 2.29, p = .037, 95% CI [.004, .107], d = .573). Participants looked longer at the social stimuli when it was paired with an LAI object (M = .61, SD = .12; t(15) = 3.67, p = .002, CI [.045, .171], d = .917) but performed at chance when the social stimuli was paired with a HAI object (M = .51, SD = .09; t(15) = .25, p = .807, CI [−.044, .056], d = .062; see Figure 2). Given that HAI objects were selected because they were categorized as objects with which children with ASD tend to have circumscribed interest, this lack of social preference for the TD children was unexpected. To explore this effect, additional analyses were conducted to determine whether TD children’s performance on the HAI trials was related to their SCQ scores. The children who displayed a preference for the HAI objects (i.e., a social preference score below 0.50) did not have higher SCQ scores than children who displayed a social preference on these trials (t(14) = −.488, p = .633, CI [−5.31, 3.34], d = .241).
Figure 2.
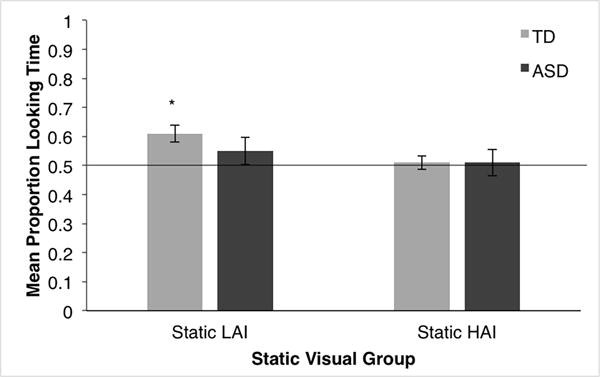
Proportion of looking time at the social stimuli on the static visual task when the social stimuli are paired with HAI or LAI objects for TD children and children with ASD.
Post hoc analyses were also conducted to determine whether the performance of children with ASD was above chance on the social motivation tasks. On the biological motion task, children with ASD performed at chance, indicating that they looked at the social and non-social stimuli equally (M = .53, SD = .16; t(14) = .79, p = .442, 95% CI [−.056, .121], d = .204; see Figure 1). On the static visual task, children with ASD also performed at chance (M = .52, SD = .16; t(14) = .58, p = .569, 95% CI [−.066, .115], d = .151). Whether social stimuli were presented with HAI or LAI objects was found to produce similar results [HAI object (M = .51, SD = .18; t(14) = .18, p = .860, CI [−.089, .106], d = .046); LAI object (M = .55, SD = .18; t(14) = .98, p = .344, CI [−.055, .146], d = .253); see Figure 2].
Chi-square analyses comparing group (TD vs. ASD) X ToM (pass vs. fail) was not statistically significant. As expected, there was a trend for TD children to perform better on the false-belief task (χ2 = 3.14, p = .077, V = .313). A total of 63% of the TD participants (10/16) passed the ToM task, a proportion not different than expected by chance (p = .454, d = .250). A total of 31% of the children with ASD passed the ToM task, a proportion also not statistically different from chance (p = .210, d = −.392).
In order to compare performance across the two social motivation tasks, and with the other measures, a series of zero-order correlations was computed for each group separately. In the case of TD children, performance on the biological motion task and performance on the static visual task, both measured by calculating the proportion of looking time to the social stimuli, were not correlated (see Table 2). However, the modest correlation (r = .319, p = .23) was in the expected direction. Similarly, although performance on the ToM task (pass vs. fail) was not statistically related to performance on any of the social orienting tasks, there was also a modest positive correlation between the social preference score (LAI) and ToM performance (rpb= .343, p = .19). Given that first look on the ToM task is a dichotomous variable, an independent samples t-test was also conducted. The TD children who passed the implicit ToM task did not orient to static social stimuli more than the children who failed the task (t(14) = −.621, p = .545, d = .344). Similarly, the TD children who passed the implicit ToM task did not orient to biological motion more than the children who failed the task (t(14) = .840, p = .415, d = −.430). Additionally, participants’ differential looking scores (DLS) were calculated (looking towards the correct side/total looking to both sides), with scores varying from 0 to 1 to examine if a continuous variable of children’s looking behaviour would yield different results. The TD children’s DLS scores were also not correlated with the social motivation tasks (see Table 2).
Table 2.
Zero-Order correlations between measures for TD children.
| DAS | PPVT | SCQ | Bio. Motion | Static HAI | Static LAI | Static Total | ToM First Look | ToM DLS | |
|---|---|---|---|---|---|---|---|---|---|
| DAS | 1 | .896* | .113 | −.263 | −.322 | −.031 | −.160 | .315 | .358 |
| PPVT | – | 1 | .154 | −.182 | −.048 | .022 | .011 | .049 | .080 |
| SCQ | – | – | 1 | .032 | .198 | .313 | .303 | .197 | .032 |
| Bio. Motion | – | – | – | 1 | .121 | .319 | .315 | −.219 | −.204 |
| Static HAI | – | – | – | – | 1 | .539 | .838* | −.082 | −.026 |
| Static LAI | – | – | – | – | – | 1 | .902* | .343 | .210 |
| Static Total | – | – | – | – | – | – | 1 | .164 | .113 |
| ToM First Look | – | – | – | – | – | – | – | 1 | .727* |
| ToM DLS | – | – | – | – | – | – | – | – | 1 |
Note.
indicates that the correlation is significant after the false discovery rate procedure (Benjamini & Hochberg, 1995) was applied, where the adjusted alpha is less than .05.
Regarding the ASD group, performance on the biological motion task was not correlated with performance on the static visual task (see Table 3). Children’s performance on the ToM task was also unrelated to their performance on any of the other tasks. In contrast to the TD children, all observed correlations were close to 0 (r = .11 to .14). The children with ASD who passed the implicit ToM task did not orient to the social static images more than the children with ASD who failed the task (t(13) = −.152, p = .881, d = .083). Children with ASD who passed the implicit ToM task did not orient to biological motion more than the children with ASD who failed the task (t(13) = .507, p = .621, d = −.316). DLS scores were also calculated for the children with ASD and were also not correlated with the social motivation scores (see Table 3).
Table 3.
Zero-order correlations between measures for children with ASD.
| DAS | PPVT | SCQ | Bio. Motion | Static HAI | Static LAI | Static Total | ToM First Look | ToM DLS | |
|---|---|---|---|---|---|---|---|---|---|
| DAS | 1 | .875* | −.285 | −.316 | −.212 | −.099 | −.169 | −.226 | −.272 |
| PPVT | – | 1 | −.252 | −.242 | .314 | −.271 | −.321 | −.130 | .053 |
| SCQ | – | – | 1 | .570* | .089 | −.092 | −.008 | −.285 | .131 |
| Bio. Motion | – | – | – | 1 | .165 | −.218 | −.041 | −.139 | −.060 |
| Static HAI | – | – | – | – | 1 | .686* | .913* | .180 | .283 |
| Static LAI | – | – | – | – | – | 1 | .922* | −.112 | .012 |
| Static Total | – | – | – | – | – | – | 1 | .042 | .163 |
| ToM First Look | – | – | – | – | – | – | – | 1 | .650* |
| ToM DLS | – | – | – | – | – | – | – | – | 1 |
Note.
indicates that the correlation is significant after the false discovery rate procedure (Benjamini & Hochberg, 1995) was applied, where the adjusted alpha is less than .05.
Discussion
The primary goal of this study was to explore whether social motivation is related to concurrent socio-cognitive abilities in TD children and children with ASD (Chevallier et al., 2012). The first step in testing this hypothesis was to investigate whether these two constructs, when measured concurrently, were related to each other. The social motivation theory would predict that reduced visual attention to social stimuli in young children with ASD would relate to the well-known social-cognitive deficits in ToM. According to this theory, one would expect a positive relation between performance on social motivation tasks and ToM for both the TD and ASD groups. This hypothesis, however, was not fully supported by the present data. Specifically, two different measures of social orienting were found to be unrelated to ToM performance in either group, suggesting that individual differences in social orienting are not associated with concurrent performance on an implicit false belief task based on anticipatory looking. This provides additional insight on the social motivation theory when tested in young children with ASD.
At first glance, this lack of association does not lend support to the social motivation theory. However, it is possible that the expected association was not found due to limited variability in the ToM score as it is a measure with a pass/fail outcome. To address this limitation, DLS scores were also computed but neither group’s DLS scores were correlated with the social motivation tasks. Additionally, the small sample of children in each group may mean that the analyses were underpowered to detect a less robust effect. A recent study conducted using the same design on a larger TD sample (n = 30) revealed a moderate, albeit non-significant, positive correlation between ToM and the Static LAI condition. This finding provides preliminary support for the social motivation theory in the TD population (Burnside, Wright, & Poulin-Dubois, 2016).
It has been suggested that ToM is composed of two distinct systems, with one being an automatic, precocious, efficient, but inflexible system while the other is flexible, inefficient, and develops later (Low, Apperly, Butterfill & Rakoczy, 2016). This theory is currently the topic of a hot debate, as some researchers argue that it is not an adequate conceptualization of ToM understanding (Michael & Christensen, 2016; Carruthers, 2016). The implicit false belief task used in this study is designed to measure the efficient ToM system. However, it has recently been reported that performance on implicit false belief tasks varies across studies in both preschoolers and adults (Schuwerk et al., 2015; Schuwerk, Jarvers, Vuori, & Sodian, 2016; Southgate, Senju, & Csibra, 2007; Thoermer et al., 2012). The anticipatory looking false belief task was initially developed to measure ToM understanding in toddlers and infants, and infants’ performance on implicit false belief tasks tend to be stable. Therefore, it is possible that the lack of association between implicit false belief and social orienting in the present study may be accounted for by the fact that implicit false belief is not robust in childhood, at least when measured using an anticipatory looking paradigm (Burnside, Azar, & Poulin-Dubois, 2017; Burnside, Ruel, Azar, & Poulin-Dubois, 2017).
The hypothesis that both static and dynamic measures of social orienting would show coherence was supported by the data. That is, TD children showed a significant preference for social stimuli on both tasks, while children with ASD responded at chance on both measures, indicating a lack of visual preference for social stimuli. Although the correlations between the measures of social orienting failed to reach statistical significance, they were in the expected direction, at least among the TD children. Again, preliminary results conducted on a larger TD sample (n = 29) revealed a significant correlation between the composite score of the static visual and the biological motion score (r = .449, p = .015; Burnside et al., 2016).
Consistent with the social motivation theory, we hypothesized differences in social orienting across ASD and TD children. As expected, TD children showed visual preference for the social stimuli when paired with an object of low autism interest (LAI), although they did not show this pattern when paired with an object of high autism interest (HAI). Furthermore, the TD children who displayed a preference for HAI objects did not have higher scores on the SCQ compared with children who displayed a preference for social stimuli. Thus, the preference for the HAI objects does not appear to be driven by ASD traits that could be present in the TD group. These results suggest that the HAI objects are more salient for all children, not just children with ASD. The fact that children with ASD did not show a social preference on either the biological motion or the static visual tasks provides support for the social motivation hypothesis. However, despite the fact that TD children performed above chance on these same measures, the analysis for group differences between ASD and TD children was not statistically significant. This absence of this between-group difference is likely due to lack of power since both groups had small sample sizes.
Chevallier and colleagues (2015) tested the predictive ability of static, dynamic, and interactive social preference stimuli with regards to children’s ASD diagnostic status. Their results suggested that the ecological validity of the stimuli (e.g., stimuli that move in an interactive manner) significantly increases the ability to predict diagnostic status. Given that the stimuli used in the present study showed either degraded social representations (e.g., point-light display) and/or lack of dynamic and interactive cues (e.g., static faces and object), it is possible that the present stimuli were not ecologically valid enough to detect the differences in social motivation between TD children and children with ASD. As such, the current design only provides a stringent test of the social motivation hypothesis and future work may include interactive tasks in order to increase the ability to discriminate groups.
For our last hypothesis, we expected to replicate previous findings showing poorer performance of ASD children on an implicit task of false belief based on anticipatory looking. This hypothesis was partly supported; a total of 63% of the TD children passed the implicit false belief task. This was not statistically significantly above chance. In contrast, only 31% of the children with ASD passed this task; the difference between the TD children and the children with ASD on the implicit false belief task was a statistical trend. This suggests that, despite the fact that TD children are not performing statistically above chance level, children with ASD tend to perform worse than TD children on this task. Furthermore, due to difficulty obtaining a large sample of children with ASD, the low statistical power should be taken into consideration when interpreting the present results. Future studies with larger sample sizes would shed light on both TD children’s implicit false belief understanding and the potential relation between ToM and social orienting as suggested by the social motivation theory.
As previously mentioned, past studies using anticipatory looking paradigms to measure implicit false belief in young children have had varying success rates; key studies reported that between 55% and 85% of the participants correctly anticipated the protagonist’s actions (Senju, 2012; Southgate et al., 2007; Thoermer et al., 2012). Therefore, not all TD children will pass the implicit false belief task and this variability is typical in ToM research. A recent study conducted by Schuwerk and colleagues (2016) compared neurotypical children and children with ASD on the same false belief task used in the present study. Although the neurotypical children’s first look was directed to the correct door during the first test trial, they performed at chance on the second trial. The authors of this study administered two trials to investigate learning effects in the ASD group. These findings indicate that implicit false belief is not robust when measured with anticipatory looking.
Despite the varying success rates reported in the use of the implicit false belief ToM task used in the present experiment, performance of ASD children was still appreciably poorer than the performance of TD children. These results are consistent with previous research on implicit or anticipatory false belief in children with ASD (Kimhi, 2014; Senju, 2012; Schuwerk, et al., 2016; Slaughter, 2015). Some researchers have even found this to be true in adults with ASD, where the participants failed to spontaneously anticipate the protagonist’s actions in a false belief task (Senju, Southgate, White, & Frit, 2009). For example, Schuwerk and colleagues (2015) reported that adults with ASD passed some explicit false belief tasks, but performed significantly worse than the TD group on the implicit false belief tasks. In other words, the implicit and spontaneous attribution of mental states to others has been shown to remain impaired in individuals with ASD from childhood through adulthood. Individuals with ASD do perform better on the explicit version of the tasks, which may be due to improved ability to draw on other cognitive skills to compensate (Frith, 2012; Senju, 2012). These findings suggest that future research should use larger samples as well as improvements on the design, such as using other implicit ToM tasks that are not based on anticipatory looking.
To conclude, the present study is among the first to directly investigate the social motivation theory in preschoolers with ASD. Despite the methodological challenges that were encountered, we believe that the present findings provide important information about the hypothesis that diminished social motivation constitutes one of the core impairments of ASD. In line with previous research showing an audience effect in children with ASD, the theory of mind task that was administered using a non-social medium may have diminished the “social” component of the theory of mind task (Chevallier et al, 2014). Given that the anticipatory looking paradigm designed to test false belief tracking is very much affected by task demands (e.g., executive functions) that vary with the stimuli and scenarios, future studies will require the inclusion of additional theory of mind tasks, including those requiring spontaneous and elicited responses. An interactive false belief task, such as the one used in the study conducted by Buttelmann, Carpenter, and Tomasello (2009), based on a helping paradigm, requires a more “explicit” response than anticipatory looking without requiring verbal responses. Moreover, other ToM abilities that can be measured implicitly, such as intention and desire (Yott & Poulin-Dubois, 2016) may provide additional information on how different ToM abilities relate to social orienting. Given that most false belief paradigms have limitations, an exploratory study including several false belief measures is needed in order to determine whether there is a relation with a range of social motivation measures (i.e., social orienting, social reward, and social maintaining). Once these limitations are addressed, the next step, to determine if social motivation is related to ToM abilities, would be to examine if early social orienting predicts later ToM abilities. Given that social motivation theory posits that individual differences in social motivation have downstream effects on socio-cognitive abilities, a developmental pattern should be observed. A longitudinal design initiated during infancy would shed light on this potential mechanism for ASD symptomatology.
Lay Summary.
The goal of the present study was to examine the link between poor attention to social information and mindreading abilities in children with autism spectrum disorder (ASD). Results demonstrated that children with ASD tended to perform worse than neurotypical children on both social orienting and theory of mind tasks. Preference for human faces and motion tended to be related but only for the neurotypical children. These findings provide partial support for the social motivation theory.
Acknowledgments
This research was supported by research grants from the Social Sciences and Humanities Research Council of Canada (#435-2012-1403) and the National Institute of Child Health and Human Development (NICHD; #R01HD068458) to Diane Poulin-Dubois. The authors would like to gratefully thank Dr. Mayada Elsabbagh for providing assistance with recruitment of the children with ASD who participated in this study, as well as Dr. Elizabeth Kelley for her contribution to the design of the study. The authors also acknowledge the contribution of Melissa Lazo and Vivianne Severdija in data collection and coding and express their gratitude to the research participants whose contribution made this project possible.
Grant sponsor SSHRC; Grant number 465-2012-1403
Footnotes
The materials used in the present study can be obtained by contacting the senior author of the papers cited for each task.
Contributor Information
Kimberly Burnside, Department of Psychology, Concordia University, 7141 Sherbrooke St. West, PY-276-1, Montréal, Québec H4B 1R6, Canada.
Kristyn Wright, Department of Psychology, Concordia University, 7141 Sherbrooke St. West, PY-276-1, Montréal, Québec H4B 1R6, Canada.
Diane Poulin-Dubois, Department of Psychology, Concordia University, 7141 Sherbrooke St. West, PY-170, Montréal, Québec H4B 1R6, Canada.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: Author; 2010. text rev. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Washington, DC: Author; 2013. [Google Scholar]
- Annaz D, Campbell R, Coleman M, Milne E, Swettenham J. Young children with autism spectrum disorder do not preferentially attend to biological motion. Journal of Autism and Developmental Disorders. 2012;42(3):401–408. doi: 10.1007/s10803-011-1256-3. [DOI] [PubMed] [Google Scholar]
- Annaz D, Remington A, Milne E, Coleman M, Campbell R, Thomas MC, Swettenham J. Development of motion processing in children with autism. Developmental Science. 2010;13(6):826–838. doi: 10.1111/j.1467-7687.2009.00939.x. [DOI] [PubMed] [Google Scholar]
- Baillargeon R, Scott RM, He Z. False-belief understanding in infants. Trends in Cognitive Sciences. 2010;14(3):110–118. doi: 10.1016/j.tics.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S. Mindblindness: An essay on autism and theory of mind. Cambridge, MA, US: The MIT Press; 1995. [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a ‘theory of mind’? Cognition. 1985;21(1):37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57(1):289–300. http://www.jstor.org/stable/2346101. [Google Scholar]
- Broekhof E, Ketelaar L, Stockmann L, Zijp A, Bos MN, Rieffe C. The understanding of intentions, desires and beliefs in young children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2015;45(7):2035–2045. doi: 10.1007/s10803-015-2363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnside K, Azar N, Poulin-Dubois D. Dissociation between Anticipatory Looking and Theory of Mind Reasoning Tasks in Preschoolers. Poster presented at the Society for Research in Child Development (SRCD) Biennial Meeting; Austin, Texas. 2017. Apr, [Google Scholar]
- Burnside K, Ruel A, Azar N, Poulin-Dubois D. A Critical Look at Implicit False Belief Based on Anticipatory Looking. 2017 Manuscript submitted for publication. [Google Scholar]
- Burnside K, Wright K, Poulin-Dubois D. Social orienting and implicit false belief in typically developing children. 2016 doi: 10.1016/j.jecp.2018.05.015. Manuscript in under review. [DOI] [PubMed] [Google Scholar]
- Buttelmann D, Carpenter M, Tomasello M. Eighteen-month-old infants show false belief understanding in an active helping paradigm. Cognition. 2009;112(2):337–342. doi: 10.1016/j.cognition.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Carruthers P. Two systems for mindreading? Review of Philosophy and Psychology. 2016;7(1):141–162. doi: 10.1007/s13164-015-0259-y. [DOI] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends in Cognitive Sciences. 2012;16(4):231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C, Parish-Morris J, Tonge N, Le L, Miller J, Schultz RT. Susceptibility to the audience effect explains performance gap between children with and without autism in a theory of mind task. Journal of Experimental Psychology: General. 2014;143(3):972–979. doi: 10.1037/a0035483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C, Parish-Morris J, McVey A, Rump KM, Sasson NJ, Herrington JD, Schultz RT. Measuring social attention and motivation in Autism Spectrum Disorder using eye‐tracking: Stimulus type matters. Autism Research. 2015;8(5):620–628. doi: 10.1002/aur.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary L, Looney K, Brady N, Fitzgerald M. Inversion effects in the perception of the moving human form: A comparison of adolescents with autism spectrum disorder and typically developing adolescents. Autism. 2014;18(8):943–952. doi: 10.1177/1362361313499455. [DOI] [PubMed] [Google Scholar]
- Clements WA, Perner J. Implicit understanding of belief. Cognitive Development. 1994;9(4):377–395. doi: 10.1016/0885-2014(94)90012-4. [DOI] [Google Scholar]
- Corsello C, Hus V, Pickles A, Risi S, Cook EJ, Leventhal BL, Lord C. Between a ROC and a hard place: Decision making and making decisions about using the SCQ. Journal Of Child Psychology And Psychiatry. 2007;48(9):932–940. doi: 10.1111/j.1469-7610.2007.01762.x. [DOI] [PubMed] [Google Scholar]
- Csibra G, Southgate V. Evidence for infants’ understanding of false beliefs should not be dismissed. Trends in Cognitive Sciences. 2006;10(1):4–5. doi: 10.1016/j.tics.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Curtin S, Vouloumanos A. Speech preference predicts autistic-like behavior in 18-months-olds at risk for Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. 2013;43:2114–2020. doi: 10.1007/s10803-013-1759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Meltzoff A, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders. 1998;28(6):479–485. doi: 10.1023/A:1026043926488. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM. Peabody Picture Vocabulary Test. Fourth. Pearson; 2007. [Google Scholar]
- Elliott CD. Differential Abilities Scale. Second. Pearson; 2007. [Google Scholar]
- Falck-Ytter T, Rehnberg E, Bölte S. Lack of visual orienting to biological motion and audiovisual synchrony in 3-year-olds with autism. PLoS ONE. 2013;8(7):e68816. doi: 10.1371/journal.pone.0068816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan T, Brodeur DA, Burack JA. A Point of Departure in the Comparison of Social and Nonsocial Visual Orienting Among Persons With Autism Spectrum Disorders. Autism Research. 2015;8(5):575–582. doi: 10.1002/aur.1472. [DOI] [PubMed] [Google Scholar]
- Frith U. Why we need cognitive explanations of autism. The Quarterly Journal of Experimental Psychology. 2012;65(11):2073–2092. doi: 10.1080/17470218.2012.697178. [DOI] [PubMed] [Google Scholar]
- Happé FE. The role of age and verbal ability in the theory of mind task performance of subjects with autism. Child Development. 1995;66(3):843–855. doi: 10.2307/1131954. [DOI] [PubMed] [Google Scholar]
- Jones CG, Swettenham J, Charman T, Marsden AS, Tregay J, Baird G, Happé F. No evidence for a fundamental visual motion processing deficit in adolescents with autism spectrum disorders. Autism Research. 2011;4(5):347–357. doi: 10.1002/aur.209. [DOI] [PubMed] [Google Scholar]
- Kaiser MD, Delmolino L, Tanaka JW, Shiffrar M. Comparison of visual sensitivity to human and object motion in autism spectrum disorder. Autism Research. 2010;3(4):191–195. doi: 10.1002/aur.137. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Kimhi Y. Theory of mind abilities and deficits in autism spectrum disorders. Topics in Language Disorders. 2014;34(4):329–343. doi: 10.1097/TLD.0000000000000033. [DOI] [Google Scholar]
- Klin A, Jones W. Altered face scanning and impaired recognition of biological motion in a 15-month-old infant with autism. Developmental Science. 2008;11(1):40–46. doi: 10.1111/j.1467-7687.2007.00608.x. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie AM. Pretense and representation: The origins of ‘theory of mind’. Psychological Review. 1987;94(4):412–426. doi: 10.1037/0033-295X.94.4.412. [DOI] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. Autism diagnostic observation schedule. Second. Los Angeles: Western Psychological Services; 2012. (ADOS-2). Manual (Part I) [Google Scholar]
- Low J, Apperly IA, Butterfill SA, Rakoczy H. Cognitive architecture of belief reasoning in children and adults: A primer on the two-systems account. Child Development Perspectives. 2016;10(3):184–189. doi: 10.1111/cdep.12183. [DOI] [Google Scholar]
- McHugh ML. Interrater reliability: the kappa statistic. Biochemia Medica. 2012;22(3):276–282. doi: 10.11613/BM.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael J, Christensen W. Flexible goal attribution in early mindreading. Psychological Review. 2016;123(2):219–227. doi: 10.1037/rev0000016. [DOI] [PubMed] [Google Scholar]
- Miller LE, Saygin AP. Individual differences in the perception of biological motion: Links to social cognition and motor imagery. Cognition. 2013;128(2):140–148. doi: 10.1016/j.cognition.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Peterson C. Theory of mind understanding and empathic behavior in children with autism spectrum disorders. International Journal of Developmental Neuroscience. 2014;39:16–21. doi: 10.1016/j.ijdevneu.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Rice K, Anderson LC, Velnoskey K, Thompson JC, Redcay E. Biological motion perception links diverse facets of theory of mind during middle childhood. Journal of Experimental Child Psychology. 2016;146:238–246. doi: 10.1016/j.jecp.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Ruffman T, Garnham W, Rideout P. Social understanding in autism: Eye gaze as a measure of core insights. Journal of Child Psychology and Psychiatry. 2001;42(8):1083–1094. doi: 10.1111/1469-7610.00807. [DOI] [PubMed] [Google Scholar]
- Rutherford MD, Troje NF. IQ predicts biological motion perception in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2012;42(4):557–565. doi: 10.1007/s10803-011-1267-0. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social Communication Questionnaire (SCQ) Western Psychological Services; 2003. [Google Scholar]
- Sasson NJ, Dichter GS, Bodfish JW. Affective responses by adults with autism are reduced to social images but elevated to images related to circumscribed interests. PLoS ONE. 2012;7(8):e42457. doi: 10.1371/journal.pone.0042457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ, Elison JT, Turner-Brown LM, Dichter GS, Bodfish JW. Brief report: Circumscribed attention in young children with autism. Journal of Autism and Developmental Disorders. 2011;41(2):242–247. doi: 10.1007/s10803-010-1038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ, Turner‐Brown LM, Holtzclaw TN, Lam KL, Bodfish JW. Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism Research. 2008;1(1):31–42. doi: 10.1002/aur.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D, Slaughter VP, Bayliss AP, Dux PE. A temporally sustained implicit theory of mind deficit in autism spectrum disorders. Cognition. 2013;129(2):410–417. doi: 10.1016/j.cognition.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Schuwerk T, Jarvers I, Vuori M, Sodian B. Implicit mentalizing persists beyond early childhood and is profoundly impaired in children with autism spectrum condition. Frontiers in Psychology. 2016;7:1–9. doi: 10.3389/fpsyg.2016.01696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuwerk T, Vuori M, Sodian B. Implicit and explicit Theory of Mind reasoning in autism spectrum disorders: The impact of experience. Autism. 2015;19(4):459–468. doi: 10.1177/1362361314526004. [DOI] [PubMed] [Google Scholar]
- Senju A. Spontaneous theory of mind and its absence in autism spectrum disorders. The Neuroscientist. 2012;18(2):108–113. doi: 10.1177/1073858410397208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju A, Southgate V, White S, Frit U. Mindblind eyes: An absence of spontaneous theory of mind in Asperger syndrome. Science. 2009;325(5942):883–885. doi: 10.1126/science.1176170. [DOI] [PubMed] [Google Scholar]
- Senju A, Johnson MH. The eye contact effect: Mechanisms and development. Trends in Cognitive Sciences. 2009;13(3):127–134. doi: 10.1016/j.tics.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Senju A, Southgate V, Miura Y, Matsui T, Hasegawa T, Tojo Y, Csibra G. Absence of spontaneous action anticipation by false belief attribution in children with autism spectrum disorder. Development and Psychopathology. 2010;22(2):353–360. doi: 10.1017/S0954579410000106. [DOI] [PubMed] [Google Scholar]
- Slaughter V. Theory of mind in infants and young children: A review. Australian Psychologist. 2015;50(3):169–172. doi: 10.1111/ap.12080. [DOI] [Google Scholar]
- Snow AV, Lecavalier L. Sensitivity and specificity of the Modified Checklist for Autism in Toddlers and the Social Communication Questionnaire in preschoolers suspected of having pervasive developmental disorders. Autism. 2008;12(6):627–644. doi: 10.1177/1362361308097116. [DOI] [PubMed] [Google Scholar]
- Southgate V, Senju A, Csibra G. Action anticipation through attribution of false belief by 2-year-olds. Psychological Science. 2007;18(7):587–592. doi: 10.1111/j.1467-9280.2007.01944.x. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H. Evaluating the theory-of-mind hypothesis of autism. Current Directions in Psychological Science. 2007;16(6):311–315. doi: 10.1111/j.1467-8721.2007.00527.x. [DOI] [Google Scholar]
- Thoermer C, Sodian B, Vuori M, Perst H, Kristen S. Continuity from an implicit to an explicit understanding of false belief from infancy to preschool age. British Journal of Developmental Psychology. 2012;30(1):172–187. doi: 10.1111/j.2044-835X.2011.02067.x. [DOI] [PubMed] [Google Scholar]
- Wright K, Kelley E, Poulin-Dubois D. Biological motion and the animate–inanimate distinction in children with high-functioning Autism Spectrum Disorder. Research in Autism Spectrum Disorders. 2016;25:1–11. doi: 10.1016/j.rasd.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yott J, Poulin-Dubois D. Are infants’ theory-of-mind abilities well integrated? Implicit understanding of intentions, desires, and beliefs. Journal of Cognition and Development. 2016;17(5):683–698. doi: 10.1080/15248372.2015.1086771. [DOI] [Google Scholar]


