Abstract
Children who grow up in poverty are more likely to experience chronic stressors that generate “wear” on stress regulatory systems including the hypothalamus– pituitary–adrenal (HPA) axis. This can have long-term consequences for health and well-being. Prior research has examined the role of proximal family and home contributions to HPA axis functioning. However, there is evidence to suggest that more distal levels of context, including neighborhoods, also matter. Prior evidence has primarily focused on adolescents and adults, with little evidence linking the neighborhood context with HPA activity in infancy and toddlerhood. We tested whether neighborhood disadvantage (indexed by US Census data) was associated with basal salivary cortisol levels at 7, 15, and 24 months of child age in a large sample of families (N = 1,292) residing in predominately low-income and rural communities in the United States. Multilevel models indicated that neighborhood disadvantage was positively associated with salivary cortisol levels and that this effect emerged across time. This effect was moderated by the race/ethnicity of children such that the association was only observed in White children in our sample. Findings provide preliminary evidence that the neighborhood context is associated with stress regulation during toddlerhood, elucidating a need for future work to address possible mechanisms.
Research in child development is now converging on the idea that the origins of adult disease may lie in children’s experiences in very early life (Shonkoff, Boyce, & McEwen, 2009). In particular, exposure to chronic adversity during childhood has been shown to shape the neural and physiologic systems involved in the body’s stress response in ways that are injurious to lifelong physical and mental health (Cohen, Janicki-Deverts, Chen, & Matthews, 2010; Evans & Kim, 2012; McEwen, 2000, 2007). The hypothalamus–pituitary–adrenal (HPA) axis and its chemical output cortisol have been a main focus of research in this domain, and the effects of proximal psychosocial, sociodemographic, and family stress on children’s HPA axis functioning have been recently documented (e.g., Blair, Raver, et al., 2011; Gunnar & Donzella, 2002; Loman & Gunnar, 2010; Miller, Chen, & Zhou, 2007).
What is less well understood is the extent to which more distal environments that children are embedded within, like neighborhoods and communities, are also associated with HPA axis functioning and development. Child development research more broadly has shown that the neighborhoods in which children and families reside are linked to many domains of development (Brooks-Gunn, Duncan, & Aber, 1997), but very little prior research has addressed relations with stress physiology specifically. The limited studies conducted in this domain have primarily investigated adolescents and adults. Neighborhood effects, however, may be present much earlier. To explore this question, this study tests the extent to which neighborhood disadvantage, net of family-level disadvantage, is associated with individual differences in HPA axis functioning in a sample of infants and toddlers residing in primarily low-income and rural communities in the United States.
Biological Embedding of Early Life Adversity
The HPA axis is one of the body’s primary stress response systems. In response to perceived environmental threat, the HPA axis releases the glucocorticoid hormone cortisol from the adrenal glands into the bloodstream where it mobilizes a bodily response to stress (Gunnar & Quevedo, 2007). This physiological cascade is important for survival and constitutes a primary mechanism through which organisms are able to flexibly respond to different forms of stress in their environments. Repeated or overactivation of the HPA axis in the context of chronic stress, however, is associated with long-term alterations to the functioning of this system, and overexposure to circulating glucocorticoids is associated with metabolic and immune processes that when sustained can damage organ systems and tissues (a state known as allostatic load; McEwen, 2000, 2007). Indices of allostatic load have been shown to predict the onset of hypertension, diabetes, cardiac disease, and overall mortality (Juster, Mc-Ewen, & Lupien, 2010; Karlamangla, Singer, & Seeman, 2006; McEwen & Seeman, 1999). Chronic exposure to poverty-related stress is associated with allostatic load and hypothesized to be a mechanism in the relation between poverty and poor health (Seeman, Epel, Gruenewald, Karlamangla, & McEwen, 2010).
The effects of poverty-related adversity are evident early in life. Several recent studies have shown relations between early adversity and allostatic load in adolescents and young children (Brody, Lei, Chen & Miller, 2014; Evans, 2003; Evans & Schamberg, 2009; Merkin et al., 2009; Robinette, Charles, Almeida, & Gruenewald, 2016), suggesting that stress response systems including the HPA axis are malleable and amenable to environmental experience in early life. However, the specific aspects of young children’s broader environment that matter for early HPA axis functioning remain less well known. The context in which child development takes place can be considered at multiple hierarchical levels ranging from the most proximal family environment to more distal contexts, such as schools, neighborhoods, and communities in which children and families are embedded (Bronfenbrenner & Morris, 2006). These multiple levels of context represent likely pathways through which stress exposure “tunes” the early development of stress response physiology.
Proximal family-level stress and disadvantage
Much of the research that contributes to our understanding of relations between poverty-related stress and children’s stress response physiology concerns the proximal processes that occur between children and their immediate home environment. Children in families of low socioeconomic status (SES) are more likely to be exposed to chronic poverty-related stressors inside the home, including violence, neglect, household chaos, and food insecurity (experiences that may explain the origins of HPA dysregulation in the context of poverty). Studies have shown that young children from low SES families evidence heightened cortisol levels compared to children from higher SES families (Evans, 2003; Evans & English, 2002; Lupien, King, Meaney, & McEwen, 2000, 2001), which indicates a pattern of physiological hyperactivity among young children in the context of poverty. Aspects of the proximal caregiving environment are thought to partially mediate this association (Blair & Raver, 2012a; Gunnar & Donzella, 2002). For instance, one prior study with the current sample has shown that instability in the number of care-givers in the home as well as parents’ perceptions of financial insecurity are each uniquely associated with heightened salivary cortisol levels in infants and toddlers (Blair, Raver, et al., 2011). That is, infants and toddlers residing in unstable and financially insecure homes evidence a more reactive as opposed to reflective physiological profile, which is to be expected in a context characterized by uncertainty and adversity (Blair & Raver, 2012b). Furthermore, a second analysis with this same sample indicated that parenting behavior mediated the association between poverty and cortisol levels in children in the infant and toddler period with implications for children’s cognitive development (Blair, Granger, et al., 2011).
In contrast to studies indicating patterns of hypercortisolism or overactivity of the HPA axis in the context of adversity, others have observed patterns of hypocortisolism or blunted cortisol levels in these contexts (e.g., Gunnar & Vazquez, 2001). The experiential and individual factors associated with hyper- versus hypocortisolism are complex (Miller et al., 2007). Hypocortisolism is thought to reflect the chronicity and severity of stress exposure as seen in the instance of chronic neglect and maltreatment by caregivers (Cicchetti, Rogosch, Gunnar, & Toth, 2010; Gunnar & Vazquez, 2001; Tarullo & Gunnar, 2006). Hypocortisolism may result from overactivity of the HPA axis under conditions of long-term and severe stress leading to reduced rather than increased activation of the HPA axis in response to stress (Tarullo & Gunnar, 2006).
Distal neighborhood-level stress and disadvantage
In addition to the immediate family and home environments of children, the neighborhoods that children reside in may also constitute a context pertinent to HPA functioning. For instance, studies of diurnal HPA activity have found that neighborhood poverty (Do et al., 2011; Hajat et al., 2015) and perceptions of neighborhood stress (Karb, Elliott, Dowd, & Morenoff, 2012) are associated with flatter diurnal slopes across the day. Studies have also found that disadvantage at the neighborhood level is associated with HPA reactivity to stress (Brenner, Zimmerman, Bauermeister, & Caldwell, 2013; Hackman, Betancourt, Brodsky, Hurt, & Farah, 2012) and with measures of allostatic load that included cortisol levels among other indicators of physiological regulation (Brody et al., 2014; Robinette et al., 2016).
However, the findings regarding neighborhood effects on basal or resting cortisol levels have been mixed. For instance, one prior study of African American young adults showed that neighborhood disadvantage was associated with increased basal cortisol levels controlling for individual-level SES and perceived stress (Brenner et al., 2013). A similar effect was shown by Rudolph et al. (2014), who found in a large nationally representative and ethnically diverse sample of adolescents that those living in disadvantaged neighborhoods had higher baseline cortisol levels than those living in more advantaged neighborhoods. Acute stress exposure is associated with increased HPA activity (e.g., Gunnar & Quevedo, 2007), and individuals residing in poor communities often experience more negative life events and daily stressors, and are at higher risk of being exposed to crime and violence (Brooks-Gunn et al., 1997). It may be these and other related characteristics that account for the positive association found in prior studies between neighborhood disadvantage and cortisol levels. At the same time, however, other studies have found evidence that neighborhood disadvantage is associated with hypocortisolism or lower levels of cortisol in children and adolescents (Chen & Paterson, 2006; Dulin-Keita, Casazza, Fernandez, Goran, & Gower, 2010). As mentioned above, hypocortisolism is likely associated with longer and more chronic experiences of stress. Exposure to high neighborhood disadvantage may be associated with chronic stress processes that promote reduced as opposed to more reactive physiological profiles (Chen & Paterson, 2006).
It is possible that such neighborhood effects manifest very early in life considering the significant development of the HPA axis during early infancy and toddlerhood (Gunnar, Brodersen, Krueger, & Rigatuso, 1996; Watamura, Donzella, Kertes, & Gunnar, 2004). If an effect of neighborhoods on cortisol is present this early in life, it is likely that neighborhood disadvantage would be associated with hypercortisolism at this age. This is because neighborhood disadvantage captures stress exposure that is within the normative as opposed to the more severe range. Furthermore, heightened as opposed to lowered cortisol levels would be expected to be associated with neighborhood disadvantage at this age because stress exposures in infancy and toddlerhood may not be prolonged enough to produce the more severe “wear” associated with hypocortisolism or blunted cortisol levels seen at later ages.
Interactions between stress regulation and race
A growing number of studies suggest that the relation between neighborhood disadvantage and HPA axis activity may vary as a function of children’s race/ethnicity. Studies concerning racial disparities in health have yielded evidence of race/ethnicity differences in levels of allostatic load. In a large study of adults, for instance, African Americans were shown to have higher indices of allostatic load relative to match-paired Whites, which in turn was linked to higher cardiovascular- and diabetes-related mortality (Duru, Harawa, Kermah, & Norris, 2012). Similarly, Rainisch and Upchurch (2013) found that African American adolescents had higher rates of allostatic load across all ages (from 12 to 19) relative to White and Mexican American youth. Prior analyses with the current sample indicate elevated cortisol in African American relative to White participants in infancy and early childhood over and above individual level risk factors (Blair et al., 2008; Blair, Granger, et al., 2011). Regarding neighborhood factors specifically, one study found that among African Americans, the odds of having high allostatic load were 200% greater for those who lived in the lowest SES neighborhoods compared to those living in the highest SES neighborhoods. Among Whites, however, the odds of having high allostatic load were only 30% greater for those living in the lowest SES neighborhoods compared to those living in the highest SES neighborhoods (Merkin et al., 2009). This suggests that the relation between neighborhood characteristics and health may vary as a function of race/ethnicity. Consistent with this, a longitudinal study of children starting at approximately 7 years of age found that neighborhood disadvantage was associated with lower cortisol levels in African American children, but not in White children (Dulin-Keita et al., 2010). African American families are more likely to reside in poorer and more disadvantaged neighborhoods, which is likely to account for some of these disparities, the impacts of discrimination, racial isolation, and economic segregation may also be hypothesized to drive these results (Merkin et al., 2009).
The Current Study
While prior studies on the effects of neighborhood disadvantage suggest that neighborhoods may be associated with individual differences in HPA axis functioning in adolescents and adults, very little is known about the emergence of this relationship across very early development. With the exception of Dulin-Keita et al. (2010), who looked at relations in a sample of children, all previous studies conducted in this domain have been with adolescents and adults, and none to our knowledge have been conducted with infants and toddlers. Therefore, it is not known how early in life the relation between neighborhood disadvantage and cortisol can be detected. To address this gap in the literature, the current study analyzed relations between neighborhood disadvantage and cortisol levels across three time points of study occurring in very early life (7, 15, and 24 months of child age) in a sample for which associations between proximal risk factors and cortisol have been previously demonstrated.
Our first research question concerned the extent to which neighborhood disadvantage is associated with infant and toddler’s resting cortisol levels across time. We addressed this question by considering zero-order correlations between neighborhood disadvantage and cortisol levels at each time point of study and, within a multivariate multilevel growth framework, by assessing the extent to which neighborhood disadvantage was associated with the intercept and slope of children’s cortisol levels across time. We hypothesized that neighborhood disadvantage would be associated with higher resting cortisol levels for infants and toddlers in the sample, and consistent with allostatic load theory and with prior analysis of these data in which levels of cortisol were shown to exhibit an normative pattern of decline across time (Blair, Raver, et al., 2011), we hypothesized that neighborhood disadvantage would attenuate this decline.
Our second research question concerned the extent to which child race/ethnicity moderated the relation between neighborhood disadvantage and children’s cortisol levels. In particular, we were interested in whether the effects of neighborhood disadvantage on the intercept and slope of children’s cortisol levels varied as a function of child race/ethnicity. Given the higher levels of neighborhood disadvantage and poverty experienced by African American families, and consistent with prior research indicating stronger relations between neighborhood SES and allostatic load in African Americans compared to Whites, we hypothesized that the relation between neighborhood disadvantage and the intercept and slope of children’s cortisol levels would be stronger among African American children in the sample.
Method
Participants
The Family Life Project was designed to study young children and families in two of the four major geographical areas of the United States with high poverty rates (Dill, 1999). Specifically, three counties in Eastern North Carolina (NC) and three counties in Central Pennsylvania (PA) were selected to be indicative of African American and White families in the South and White families in Appalachia, respectively. The Family Life Project adopted a developmental epidemiological design in which sampling procedures were employed to recruit a representative sample of 1,292 children whose families resided in one of the six counties at the time of the child’s birth. Low-income families in both states and African American families in NC were oversampled (African American families were not oversampled in PA because the African American populations of these counties was <5%). A comprehensive description of the sampling plan and recruitment procedures are provided by Vernon-Feagans, Cox, and Family Life Project Key Investigators (2013). In this manuscript, we analyze data from 1,184 families who were seen at 7-, 15-, and 24-month home visits. Families in the analysis sample were seen on at least one occasion (M number of visits = 2.51, range = 1–3). Fifty-eight percent of infants in the analysis sample were White and approximately 60% of families resided in NC. Descriptives of the analysis variables are presented in Table 1.
Table 1.
Descriptives of the analysis variables (N = 1,184)
| 7 Months
|
15 Months
|
24 Months
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Time-Varying Variables | N | M | SD | N | M | SD | N | M | SD |
| Cortisol (ln) | 1088 | −1.87 | 0.71 | 967 | −1.99 | 0.75 | 920 | −2.07 | 0.72 |
| Child age (months) | 1155 | 7.71 | 1.44 | 1129 | 15.72 | 1.35 | 1104 | 24.90 | 1.96 |
| Time of day | 1139 | 13.53 | 2.88 | 1113 | 13.97 | 2.91 | 1058 | 13.83 | 3.21 |
| Neighborhood disadvantage | 1154 | −0.01 | 0.76 | 1127 | −0.01 | 0.77 | 1102 | −0.01 | 0.76 |
| Cumulative risk | 1155 | 0.00 | 0.69 | 1129 | 0.00 | 0.69 | 1104 | 0.00 | 0.67 |
| Sensitive parenting | 1100 | 2.89 | 0.79 | 1065 | 2.79 | 0.79 | 1024 | 2.90 | 0.80 |
| Moved (1 = yes, 0 = no) | 1155 | 18.5% | 1129 | 24.3% | 1104 | 28.3% | |||
|
| |||||||||
| Time-Invariant Variables | |||||||||
|
| |||||||||
| Black NC | 1184 | 41.9% | |||||||
| White NC | 1184 | 19.2% | |||||||
| White PA | 1184 | 38.9% | |||||||
| Female (1 = female, 0 = male) | 1184 | 49.5% | |||||||
Note: ln, natural log transformation; NC, North Carolina; PA, Pennsylvania.
Procedures
Data collection took place at home visits when children were 7, 15, and 24 months of age. Visits lasted approximately 2–3 hr, during which time the child’s primary caregiver responded to questionnaires, primary caregivers and children participated in structured interactions, child assessments were conducted, and saliva samples were collected. Saliva samples were collected near the conclusion of each home visit after data collectors had been in the home for at least 1 hr. To assess basal levels of cortisol, unstimulated whole saliva was collected using either cotton or hydrocellulose absorbent material and expressing sample into 2-ml cryogenic storage vials using needleless syringe (cotton) or by centrifugation (hydrocellulose). Two prior studies indicated no differences in cortisol concentrations associated with the two collection techniques (Granger et al., 2007; Harmon, Hibel, Rumyantseva, & Granger, 2007).
In addition to saliva collection, mothers and their children engaged in a 10-min semistructured free-play task at the 7-and 15-month assessments. Mothers were provided with a standardized set of toys and were instructed to play with their child as if they had some free time. At the 24-month assessment, mothers and their children engaged in a puzzle task, which involved presenting the child with a jigsaw puzzle to complete and asking the mother to assist the child in any way that she chose. After a puzzle was completed, another puzzle of increased complexity was presented to the child (up to a total of three standardized puzzles).
Measures
Salivary cortisol
After collection at each assessment (7, 15, and 24 months), saliva samples were immediately placed on ice, then stored frozen at –20 °C and subsequently stored at –80 °C until they were assayed for salivary cortisol using a highly sensitive enzyme immunoassay US FDA 510k cleared for use as an in vitro diagnostic measure of adrenal function (Salimetrics, Carlsbad, CA). The samples were assayed in duplicate. Inter- and intra-assay coefficients of variation were, on average, less than 15% and 10%, respectively. The cortisol distributions were subjected to natural log transformation to correct for positive skew. Outliers greater than ±3 SD after transformation were treated as missing (n = 9, 16, 12 saliva samples at 7, 15, and 24 months, respectively). Visual inspection of histograms and skewness statistics indicated that the natural log transformed mean-composite cortisol data were normally distributed.
Time of day of saliva collection was inversely associated with cortisol at each time point (rs = −.18 to −.32, p < .001). Saliva samples collected later in the day had lower cortisol concentrations, on average, than saliva samples collected earlier in the day.
Thus, consistent with much prior literature showing the diurnal pattern of cortisol, it was important that we control for time of day in all analyses. We controlled for infants’ and toddlers’ diurnal rhythm by including time of day (24-hr clock) as a covariate in all analyses.
Neighborhood disadvantage
Families’ home addresses at 7-, 15-, and 24-month visits were linked with US Census data from the year 2000, and measures of neighborhood disadvantage were compiled at the Census block-group level. The indicators of neighborhood disadvantage considered in the present analysis included the percentage of individuals in the families’ block group reporting annual income below the federal poverty level, the percentage of households that were female headed, the percentage of the labor force that was unemployed, the percentage of the population that was less than 18 years of age, and the percent of the population who was over the age of 25 and had graduated high school (reverse-scored). Correlation coefficients among the five indicators ranged from r = .29 to .76 ( ps < .01). A continuous index of neighborhood disadvantage was generated by standardizing each of the five indicators and averaging the standardized indicators.
Cumulative risk
Based on prior work with these data (Vernon-Feagans et al., 2013), we used a cumulative risk composite of six variables that were measured at 7-, 15-, and 24-month visits: family income to needs ratio (INR), maternal education, constant spouse/partner living in the home, hours of employment, occupational prestige, and household density. A continuous cumulative risk index was generated by reverse-scoring the positively framed indicators, standardizing each risk measure, and averaging the standardized risk variables. Three cumulative risk scores were generated, one for each study wave, with higher scores reflecting higher levels of risk. At 7 months, correlation coefficients among the seven indicators included in the cumulative risk index ranged from r = –.12 to .52 ( ps < .01). At 15 months, correlation coefficients among the seven cumulative risk indicators ranged from r = –.15 to .55 ( ps < .01). At 24 months, correlation coefficients among the cumulative risk indicators ranged from r = –.08 to .53 ( ps < .01).
Maternal sensitivity
Free play and puzzle interactions were video recorded, and the following seven aspects of parenting were coded at the three time points: sensitivity, detachment, intrusiveness, stimulation, positive regard, negative regard, and animation in interacting with the child (Cox & Crnic, 2002; NICHD Early Child Care Research Network, 1999). Ratings were made on a scale ranging from 1 (not at all characteristic) to 5 (highly characteristic) at the 7- and 15-month assessments and on a scale ranging from 1 (not at all characteristic) to 7 (highly characteristic) at the 24-month assessment. To maintain consistency across time, scores at the 24-month visit were rescaled to range from 1 to 5 (score of 7 recoded to 5, scores of 6 and 5 recoded to 4, 4 recoded to 3, 3 and 2 recoded to 2, and 1 coded as 1; Mills-Koonce et al., 2011). Principal component analysis conducted with oblique rotation (i.e., Promax) at each time point indicated distinct dimensions of parenting behaviors. Maternal sensitivity included five maternal characteristics: sensitivity, detachment (reversed), stimulation of development, positive regard, and animation, which were averaged together to create the maternal sensitivity index used in the current analyses. Coders were trained and certified by a master coder, and reliability was determined by calculating the intraclass correlation coefficients (range = 0.74–0.89) for ratings made by pairs of trained coders for each scale of the maternal sensitivity dimension. A minimum of 30% of all observations were double coded, and discrepant codes were resolved by conferencing.
Covariates
Covariates included a dummy code for child sex (1 = female, 0 = male), and as mentioned previously, timing of saliva collection at 7, 15, and 24 months (24-hr clock) was controlled in each model to adjust for diurnal variation in cortisol levels. Whether the family’s home had changed addresses since the previous visit was coded at 7, 15, and 24 months (moved = 1, did not move = 0). Parent report of child race/ethnicity as either Black or White and the family’s state of residence (NC or PA) were used to categorize children as members of the following categories: Black children from PA, Black children from NC, White children from PA and White children from NC. Because so few PA-residing children were identified as Black or African American (n = 34), these children were excluded from the following analyses. Two ethnicity/region categories were then created using dummy codes (0 or 1) in order to separately estimate the role of race/ ethnicity for children living in NC (i.e., NC Black coded as 1, all other categories coded as 0) on the dependent variables. In addition, this coding scheme allowed for the control of state of residence for children identified as White (PA White coded as 1 vs. all other categories coded as 0). NC-residing children identified as White were therefore the reference group. All continuous variables were mean centered.
Missing data
The total sample at study entry was 1,292, with 1,204 families seen at 7 months, 1,169 at 15 months, and 1,144 at 24 months. Nine percent of children seen at 7 months were missing cortisol data. At 15 and 24 months, 17% and 20% of children were missing cortisol data, respectively. Children who had cortisol data on at least one occasion from 7 to 24 months were included in the current analysis sample n = 1,184. Missing cortisol data were not associated with household income, cumulative family-level risk, or neighborhood disadvantage. Five percent of cases in the analysis sample were missing maternal sensitivity data, the only predictor variable in the current analysis to have any missing data. We used full information maximum likelihood estimation to reduce the potential for bias in the analysis related to missing data (Enders, 2010). Full information maximum likelihood estimation uses all of the information in the independent variables to estimate the covariance matrix, thus allowing us to include all children who had any cortisol data, regardless of their amount of missingness on the independent variables.
Data analysis plan
Descriptive analyses included zero-order correlations between the primary variables of interest at 7, 15, and 24 months. To address our primary research aim, the prediction of basal cortisol levels at 7, 15, and 24 months of age, we used multilevel modeling using Mplus 7 software (Muthén & Muthén, 1998–2012) including maximum likelihood estimation with robust standard errors to predict the intercept and slope of salivary cortisol levels in children from 7 to 24 months of age. Satorra–Bentler scaled chi-square difference tests (Satorra & Bentler, 2001) were used to compare the fit of nested models. Random intercepts were included in all models. The data for this analysis were unbalanced and not time structured (i.e., the number of study visits varied across families in the sample and the timing of each study visit varied across families and reflected the actual age of each child). Time was centered at the between-person group mean of the 7-month visit (which was 7.71 months as shown in Table 1) and rescaled into years. Thus, the coefficient for time reflects change in cortisol levels across 1 year. Fixed linear effects for time were included in all models.
Model 1 included simultaneous entry of fixed effects for neighborhood disadvantage, cumulative family-level risk, and sensitive parenting, as well as covariates including indicators for child race/state of residence, sex, time of day of saliva collection, and whether or not the family had moved since the last home visit as predictors of cortisol levels. To address whether neighborhood effects on the intercept of cortisol varied as a function of child race/ethnicity, Model 1 also included two-way interactions between neighborhood disadvantage and Black NC and between neighborhood disadvantage and White PA. Model 2 included all the predictors of Model 1 with the addition of a linear trend for neighborhood disadvantage (two-way interaction between neighborhood disadvantage and time), which allowed us to examine the extent to which any neighborhood effects vary as a function of child age. Model 2 also included interaction terms between Black NC and time and between White NC and time. Model 3 tested the extent to which any effects of neighborhood disadvantage on the slope of cortisol levels across time varied as a function of child race/ethnicity. To do this, Model 3 included two three-way interactions that were between neighborhood disadvantage, Black NC, and time, and between neighborhood disadvantage, White PA, and time.
Results
Sociodemographic characteristics of the families and neighborhoods
Neighborhood characteristics
Families in the analysis sample lived in 425 unique Census block groups across the 7-, 15-, and 24-month visits. According to 2000 Census data, the poverty rate in the block groups that families in the sample resided in at 7 months was 16.95%, on average, although this estimate varied considerably across block groups in the analysis sample (poverty rates ranged from 0% to 62.81% at the 7-month visit and ranged from 0% to 80.47% at 15- and 24-month visits). Unemployment levels in the block groups that families resided in at 7 months varied as well (M = 7.46%, SD = 5.67), suggesting that exposure to concentrated neighborhood disadvantage varied across families in the sample. African American children resided in neighborhoods that were more disadvantaged, on average, than White children at 7 months t (703.14) = –18.86, p < .001, and this pattern was consistent across the 15- and 24-month visits. Table 2 displays descriptive statistics for each indicator included in the neighborhood disadvantage composite. Table 3 displays the means and standard deviations of each indicator of neighborhood disadvantage and tests the differences in means between child race/ethnicity groups. As shown in Table 3, African American families resided in more disadvantaged neighborhoods according to each individual disadvantage indicator.
Table 2.
Sociodemographic characteristics of the families and the neighborhoods in the full sample
| 7 Months
|
15 Months
|
24 Months
|
||||
|---|---|---|---|---|---|---|
| Variables | M (SD) | Range (Min–Max) |
M (SD) | Range (Min–Max) |
M (SD) | Range (Min–Max) |
| Neighborhood disadvantage composite | ||||||
| Individuals below poverty line (%) | 16.95 (11.79) | 0–62.81 | 17.14 (12.19) | 0–80.47 | 16.85 (12.04) | 0–80.47 |
| Female-headed households (%) | 33.00 (13.56) | 5.78–84.41 | 32.93 (13.63) | 2.68–84.41 | 32.78 (13.44) | 2.68–77.54 |
| Unemployed (%) | 7.46 (5.67) | 0–42.99 | 7.48 (5.72) | 0–43.01 | 7.38 (5.60) | 0–43.01 |
| <18 years of age (%) | 26.66 (4.96) | 10.09–46.66 | 26.66 (5.19) | 7.67–46.66 | 26.53 (5.12) | 9.59–45.27 |
| >25 years old with HS graduation (%) | 75.00 (12.09) | 24.49–99.05 | 74.96 (12.33) | 24.49–99.44 | 75.32 (12.19) | 24.49–99.44 |
| Cumulative family-level risk composite | ||||||
| Income to needs ratio | 1.94 (1.71) | 0–16.49 | 1.89 (1.69) | 0–16.76 | 1.84 (1.61) | 0–16.24 |
| Maternal education | 14.46 (2.82) | 6.00–22.00 | 14.58 (2.75) | 6.00–22.00 | 14.78 (2.70) | 6.00–22.00 |
| Constant spouse/partner | 62% | 57% | 54% | |||
| Hours of employment | 32.18 (21.66) | 0–104.00 | 27.59 (21.41) | 0–95.00 | 29.05 (21.40) | 0–120.00 |
| Occupational prestige | 40.13 (12.09) | 16.78–86.05 | 40.28 (11.60) | 16.78–86.05 | 40.50 (11.70) | 16.78–86.05 |
| Household density | 0.88 (0.35) | 0.36–3.33 | 0.86 (0.25) | 0.38–2.27 | 0.85 (0.32) | 0.31–3.14 |
Note: HS, high school.
Table 3.
Sociodemographic characteristics of African American and White families
| 7 Months
|
15 Months
|
24 Months
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| African American |
White
|
African American |
White
|
African American |
White
|
||||
| Variables | M (SD) | M (SD) | Mean Diff. | M (SD) | M (SD) | Mean Diff. | M (SD) | M (SD) | Mean Diff. |
| Neighborhood disadvantage composite | 0.45 (0.84) | −0.34 (0.49) | 0.80*** | 0.44 (0.84) | −0.34 (0.50) | 0.78*** | 0.44 (0.84) | −0.33 (0.50) | 0.77*** |
| Individuals below poverty line (%) | 23.03 (12.72) | 12.69 (8.89) | 10.33*** | 23.17 (13.25) | 12.76 (9.14) | 10.41*** | 22.71 (13.21) | 12.70 (9.09) | 10.01*** |
| Female-headed households (%) | 40.07 (14.61) | 28.05 (10.21) | 12.01*** | 39.68 (15.08) | 28.02 (9.91) | 11.65*** | 39.78 (14.60) | 27.82 (9.93) | 11.95*** |
| Unemployed (%) | 9.24 (5.94) | 6.22 (5.12) | 3.02*** | 9.20 (5.79) | 6.23 (5.34) | 2.96*** | 9.04 (5.86) | 6.20 (5.09) | 2.84*** |
| <18 years of age (%) | 28.99 (5.11) | 25.02 (4.14) | 3.96*** | 28.90 (5.40) | 25.02 (4.36) | 3.87*** | 28.73 (5.34) | 24.97 (4.34) | 3.75*** |
| >25 years old with HS graduation (%) | 68.15 (12.55) | 79.79 (9.09) | −11.63*** | 68.07 (12.65) | 79.98 (9.30) | −11.91*** | 68.55 (12.64) | 80.12 (9.22) | −11.57*** |
| Cumulative family-level risk composite | 0.36 (0.55) | −0.25 (0.66) | 0.62*** | 0.33 (0.55) | −0.24 (0.68) | 0.57*** | 0.30 (0.54) | −0.21 (0.68) | 0.52*** |
| Income to needs ratio | 1.16 (1.0) | 2.43 (1.87) | −1.27*** | 1.18 (1.09) | 2.37 (1.86) | −1.18*** | 1.21 (1.06) | 2.29 (1.78) | −1.08*** |
| Maternal education | 13.72 (2.54) | 14.98 (2.89) | −1.26*** | 13.83 (2.50) | 15.12 (2.80) | −1.29*** | 14.10 (2.52) | 15.25 (2.73) | −1.14*** |
| Constant spouse/partner | 0.35 | 0.81 | −0.46*** | 0.3 | 0.77 | −0.46*** | 0.26 | 0.73 | −0.46*** |
| Hours of employment | 24.90 (21.31) | 37.28 (20.43) | −12.37*** | 24.42 (20.90) | 29.9 (21.49) | −5.48*** | 26.21 (21.29) | 31.05 (21.26) | −4.83*** |
| Occupational prestige | 36.84 (9.50) | 42.31 (13.10) | −5.47*** | 37.07 (9.54) | 42.49 (12.36) | −5.42*** | 37.27 (9.71) | 42.66 (12.41) | −5.39*** |
| Household density | 0.99 (0.40) | 0.80 (0.29) | 0.19*** | 0.95 (0.28) | 0.79 (0.22) | 0.15*** | 0.94 (0.35) | 0.79 (0.28) | 0.14*** |
Note: HS, high school.
p < .05.
p < .01.
p < .001.
Family characteristics
The average INR of families was 1.90 from 7 to 24 months, and 63% percent or nearly two-thirds of the families had an average household income below 200% of the federal poverty line (i.e., INR < 2.0). Fifty percent of mothers had a high school education or less, and 16% of mothers had completed at least 4 years of postsecondary education. At 7 months, 62% of primary caregivers were married or constantly partnered. Table 2 displays descriptive statistics for each indicator included in the cumulative risk composite. Descriptives of the cumulative risk variable have been published elsewhere (Vernon-Feagans et al., 2013). Table 3 displays the means and standard deviations of each indicator of family-level cumulative risk by child race/ethnicity group. As shown in Table 3, African American families were at higher risk according to each individual indicator of cumulative risk.
Preliminary analyses
Table 4 displays zero-order correlations among the primary analysis variables. Small significant correlations between salivary cortisol levels and neighborhood disadvantage were evident at 7 and 15 months of age (rs = .07, p < .05). At 24 months, toddlers’ cortisol levels and neighborhood disadvantage were correlated at r = .13, p < .001. Cumulative family-level risk was also associated with infants’ and toddlers’ cortisol levels although correlations were relatively small across time (rs = .10, .11, and .13, ps < .001, at 7, 15, and 24 months, respectively). On average, African American children had higher cortisol levels than White children in the sample (rs = .10 to .15, ps < .001). There was no association between child gender and cortisol levels at 7, 15, or 24 months. Neighborhood disadvantage and family-level cumulative risk were moderately correlated (rs = .42 to .43, ps < .001), suggesting some shared variance between the two risk indicators. African American children lived in families at higher cumulative risk than White children on average (rs = .38 to .44, ps < .001), and consistent with this, the neighborhoods that African American children resided in were more disadvantaged, on average, than the neighborhoods that White children resided in (rs = .49 to .51, ps < .001).
Table 4.
Zero order correlations among the analysis variables (N = 1,184)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Cortisol 7 | 1 | ||||||||||
| 2. Cortisol 15 | .02 | 1 | |||||||||
| 3. Cortisol 24 | .08* | .11** | 1 | ||||||||
| 4. Neighborhood dis. 7 | .07* | .07* | .07* | 1 | |||||||
| 5. Neighborhood dis. 15 | .08** | .07* | .10** | .85*** | 1 | ||||||
| 6. Neighborhood dis. 24 | .08** | .03 | .13*** | .67*** | .76*** | 1 | |||||
| 7. Cumulative risk 7 | .10*** | .11*** | .10** | .42*** | .44*** | .43*** | 1 | ||||
| 8. Cumulative risk 15 | .09** | .11*** | .11*** | .42*** | .43*** | .44 *** | .90*** | 1 | |||
| 9. Cumulative risk 24 | .08** | .11** | .13*** | .40*** | .42*** | .42*** | .86*** | .90*** | 1 | ||
| 10. Race (Black = 1, White = 0) | .15*** | .10** | .15*** | .51*** | .49*** | .49*** | .44*** | .41*** | .38*** | 1 | |
| 11. Female | −.03 | −.03 | −01 | .01 | .01 | .01 | .02 | .03 | .02 | .01 | 1 |
Note: Neighborhood dis., neighborhood disadvantage; Cumulative risk, cumulative family-level risk.
p < .05.
p < .01.
p < .001.
Main analyses
Table 5 displays the results from a series of multilevel models that assessed the extent to which neighborhood disadvantage, family-level cumulative risk, sensitive parenting, and covariates were associated with cortisol levels across the child’s first 2 years. The coefficient for time (b = −0.142, p < .001) in Model 1 indicates that children’s cortisol levels decreased across time, on average. Model 1 also indicated an inverse association between time of day of saliva collection and cortisol levels (b = −0.057, p < .001), indicating that, across study waves, samples collected later in the day tended to have lower cortisol concentrations than those collected earlier. African American children evidenced higher cortisol concentrations than White children in our sample, on average (b = 0.173, p < .001), and consistent with other papers that have been published using these data, sensitive parenting behavior was associated with lower cortisol levels, on average (b = −0.054, p < .01). Model 1 also included coefficients for cumulative family-level risk and neighborhood disadvantage. Neither the fixed main effect for cumulative family-level risk (b = 0.000, ns) nor for neighborhood disadvantage (b = 0.067, ns) was associated with the intercept of children’s cortisol levels. The relation between neighborhood disadvantage and children’s 7-month cortisol values (intercepts) did not vary as a function of children’s race/ ethnicity (bNeighborhood × BlackNC = −0.066, ns) nor state of residence (bNeighborhood × WhitePA = −0.073, ns).
Table 5.
Multilevel models predicting children’s cortisol levels at 7, 15, and 24 months of age (N = 1,184)
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Fixed effects | |||
| Intercept | −1.939 | −1.878 | −1.907 |
| Time | −0.142*** | −0.228*** | −0.191*** |
| Black NC | 0.173*** | 0.134* | 0.147** |
| White PA | 0.020 | −0.083 | −0.067 |
| Female | −0.040 | −0.040 | −0.039 |
| Time of day | −0.057*** | −0.058*** | −0.058*** |
| Moved | −0.007 | −0.008 | −0.008 |
| Sensitive parenting | −0.054** | −0.053** | −0.052** |
| Cumulative family-level risk | 0.000 | 0.000 | 0.000 |
| Neighborhood disadvantage | 0.067 | 0.040 | −0.076 |
| Neighborhood Disadvantage × Black NC | −0.066 | −0.073 | 0.076 |
| Neighborhood Disadvantage × White PA | −0.073 | −0.072 | 0.009 |
| Neighborhood Disadvantage × Time | 0.048 | 0.212** | |
| Black NC × Time | 0.057 | 0.042 | |
| White PA × Time | 0.159** | 0.147* | |
| Neighborhood Disadvantage × Black NC×Time | −0.210* | ||
| Neighborhood Disadvantage × White PA×Time | −0.098 | ||
| Random effects | |||
| Level 1 | 0.470 | 0.467 | 0.466 |
| Level 2 | 0.025 | 0.026 | 0.027 |
Note: NC, North Carolina; PA, Pennsylvania.
p < .05.
p < .01.
p < .001.
Results from Model 2 indicated no statistically significant effect of neighborhood disadvantage on the slope of cortisol levels for all children in the analysis sample (bNeighborhood × Time = 0.048, ns). The significant two-way White PA × Time interaction (b = 0.159, p < .01) suggests that the slope of cortisol across time varied by state of residence among White participants in the sample.
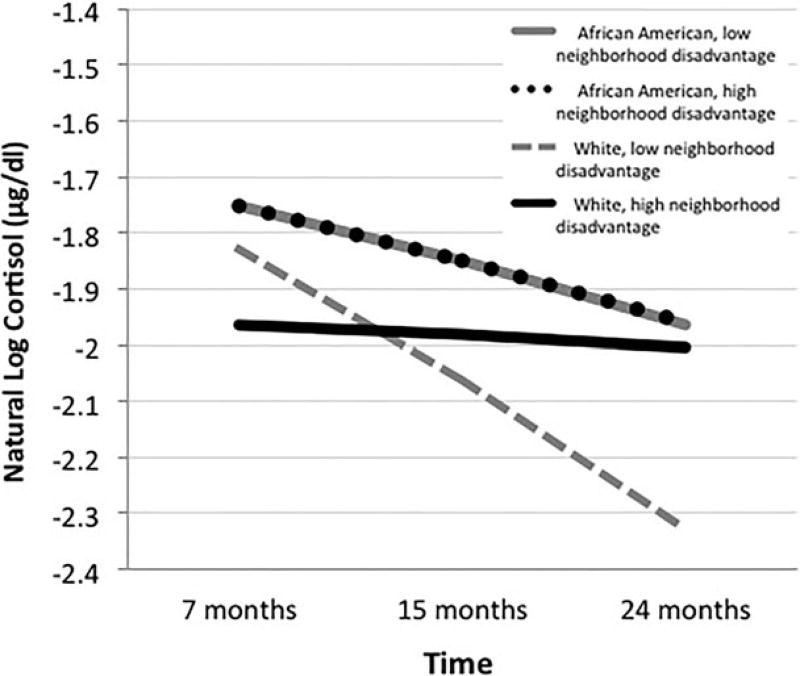
To test the extent to which any effects of neighborhood disadvantage on the slope of cortisol levels varied as a function of child race/ethnicity, Model 3 included two three-way interactions, Neighborhood Disadvantage × Black NC × Time and Neighborhood Disadvantage × White PA × Time. The significant three-way interaction Neighborhood Disadvantage × Black NC × Time suggests that the relation between neighborhood disadvantage and the slope of cortisol levels across time varied as a function of child race/ethnicity (bNeighborhood × BlackNC × Time = −0.210, p < .05). This effect, depicted in Figure 1, indicated that White children residing in highly disadvantaged neighborhoods displayed higher cortisol levels than their White counterparts living in more advantaged neighborhoods by 24 months of age. This pattern of effects is distinct from that of African American children in our sample who sustained overall higher levels of cortisol but showed no evidence of individual differences in basal cortisol levels as a function of exposure to neighborhood disadvantage.
Figure 1.
The relation between child cortisol and time varies as a function of both neighborhood disadvantage and child race/ethnicity. High and low neighborhood disadvantage indicate ± 1 SD from the mean of neighborhood disadvantage, respectively. Point estimates for African American low and high neighborhood disadvantage subgroups overlap one another.
Supplementary analyses
Given the confounding of child race/ethnicity and neighborhood disadvantage in this sample (see Table 3), robustness checks were undertaken. Models were rerun separately for each race/ethnicity group. Results from these separate models confirmed what was observed in the full model. That is, when modeling only the White subsample, there was a significant linear trend for neighborhood disadvantage on cortisol levels (bNeighborhood × Time = 0.13, p = .013), and when modeling only the African American subsample, there was no significant linear trend for neighborhood disadvantage (bNeighborhood × Time = 0.001, ns).
In an effort to decouple race/ethnicity and neighborhood disadvantage in this analysis, we reran the model in a subsample of all cases who resided within ± 1 SD of the sample mean of neighborhood disadvantage for White families. This allowed us to assess potential race/ethnicity differences of neighborhood disadvantage on cortisol within a smaller subsample of cases that were relatively similar in terms of neighborhood disadvantage. Doing so retained a subsample of children (n = 842, of which 285 were African American and 557 were White) residing in relatively advantaged neighborhoods (M neighborhood disadvantage = −0.34, SD = 0.48, range = −0.82 to 0.14) relative to the full sample mean (M = −0.01, SD = 0.76, range = −1.17 to 3.45). In this subsample of cases, the linear trend for neighborhood disadvantage on cortisol levels was reduced to trend-level statistical significance (bNeighborhood × Time = 0.17, p = .07). Subsequently allowing this effect to vary across race/ethnicity groups indicated that there was no statistically significant difference in this pattern across race/ethnic groups (bNeighborhood × BlackNC × Time = 0.02, p = .93). Results from this supplementary analysis in which the distribution of neighborhood disadvantage of the African American sample was constrained to be of lower risk than in the full sample suggest that the race/ethnicity differences found in our primary analyses may be due to the confounding of race and neighborhood disadvantage in the full sample.
Discussion
Early experience has been shown to have lifelong consequences for physical and mental health (Ganzel & Morris, 2011; McEwen, 2000). The effect of early experience on the development of stress response physiology is one mechanism through which such effects have been shown to transpire. Exposure to early life stress such as that associated with poverty is associated with increased cortisol output, leading to alterations in physiological and neural systems that increase risk for later adverse physical and mental health outcomes (McEwen & Gianaros, 2011). Although a number of studies have examined stressors in children’s proximal home and childcare environments, relatively little is known about stressors outside of the home. The current study contributes to the literature on poverty and stress by demonstrating relations among neighborhood disadvantage and cortisol levels in a population-based sample of infants and toddlers living in predominantly low-income and rural communities. The association between neighborhood disadvantage and cortisol emerged between child ages 7 and 24 months and was specific to White families in highly disadvantaged neighborhoods. For African American families, neighborhood disadvantage was unrelated to child cortisol levels. It is important to note, however, that African American families in the sample are more likely to reside in disadvantaged neighborhoods as well as to experience a greater number of proximal risk factors. Consequently, race/ethnicity and poverty-related risk cannot be effectively disentangled in our data. Our supplementary analysis with a subset of African American families living in neighborhoods with levels of disadvantage similar to White families indicated that the effect for race/ ethnicity was reduced when we restricted the range of neighborhood disadvantage for the African American sample. Given the reduction in sample size for this supplementary analysis and resulting change in statistical power, however, we do not consider it to be definitive.
To our knowledge, this is the first study to link neighborhood disadvantage to salivary cortisol levels in infants and toddlers. Our findings, however, are consistent with prior studies with adolescents and adults showing that neighborhood disadvantage is associated with markers of health including cardiometabolic risks (Brody et al., 2014; Merkin, 2009; Robinette et al., 2016), perinatal risks including low birth weight and preterm birth (Ncube, Enquobahriw, Albert, Herrick, & Burke, 2016), telomere length (Theall, Brett, Shirtcliff, Dunn, & Drury 2013), and with the activity of the HPA axis (Brenner et al., 2013; Chen & Paterson, 2006; Do et al., 2011; Dulin-Keita et al., 2010; Hackman et al., 2012; Hajat et al., 2015; Karb et al., 2012; Rudolph et al., 2014). Our findings build on the small number of prior studies providing preliminary evidence for the role of neighborhoods in individual differences in HPA functioning, extending it to very young children.
As noted, however, prior findings are mixed as to whether neighborhood disadvantage is associated with a pattern of hypo- rather than hypercortisol. Consistent with the idea that neighborhood disadvantage captures distal stress processes that are for the most part within the normative range, we observed that neighborhood disadvantage was associated with heightened cortisol levels in toddlerhood. Here, our findings are consistent with two prior studies with adolescents showing neighborhood disadvantage to be associated with increased basal cortisol levels (Brenner et al., 2013; Rudolph et al., 2014). Because toddlerhood represents a distinct developmental period from later childhood and adolescence, it is unclear how divergent our findings are from other previous studies that found that neighborhood disadvantage was associated with lower levels of cortisol in children and adolescents (Chen & Paterson, 2006; Dulin-Keita et al., 2010). It may be, for instance, that chronic and prolonged exposure to high neighborhood disadvantage is eventually associated with blunted cortisol in later childhood or adolescence in this sample, but this was impossible to test in the current study.
The idea that stress response systems are sensitive to environmental influence is well established (McEwen & Gianaros, 2011; Meaney, 2001). Theory suggests that the plasticity of the HPA axis represents a form of conditional adaptation that, in an evolutionary sense, increases an individual’s fitness within the environment by enabling individuals to use information from prior experience to anticipate their future environments (Boyce & Ellis, 2005). Heightened activation of stress response physiology in highly disadvantaged and threatening contexts may benefit an individual’s survival goals, but not without certain costs that can accumulate over time and negatively affect cognitive and self-regulatory processes (Blair & Raver, 2012b) as well as physical health and disease risk (e.g., McEwen, 2000). Understanding how distal and proximal features of the environment may be influencing these psychobiological processes and what long-term consequences they have for child development is therefore important.
A central feature of the results is that the significant association found between neighborhood disadvantage and basal salivary cortisol levels in toddlerhood was present over and above indicators of the proximal home environment that included a cumulative family risk variable and an observed measure of sensitive parenting. Recent work suggests that neighborhood segregation by family income is increasing in the United States (Bischoff & Reardon, 2014), and this may be particularly salient in rural contexts where decades of economic change have caused large amounts of outmigration from rural communities (Vernon Feagans, Burchinal, & Mokrova, 2015). Concentrated disadvantage within communities may be conceptualized as a type of environmental exposure that is related to but distinct from stressors within the immediate family environment (Brooks-Gunn et al., 1997). How neighborhood characteristics associated with disadvantage, including pollution and crime (Evans, 2004), for instance, affect children at the physiological level and what effect any such relations have on later health and functioning are questions that remain largely unanswered.
Finally, our findings suggest that how neighborhood disadvantage influences cortisol could vary as a function of race. Among White children in the sample, we observed that exposure to high neighborhood disadvantage attenuated the normative pattern of decline in cortisol levels from 7 to 24 months, potentially implying an allostatic process in which chronic exposure to environmental stressors shifts the resting activity of toddlers’ HPA axis toward a more reactive profile. However, this pattern was not present among African American children in the sample who showed an attenuated pattern over 7–24 months but no variation in this decline as a function of neighborhood disadvantage. Given that the African American children in the sample resided in neighborhoods that were more disadvantaged, on average, than White children, these findings likely reflect differences in stress exposure overall. Specifically, sustained heightened cortisol levels among African American children may reflect the overall higher amounts of disadvantage faced by their families in this sample; they ranked higher on each indicator included in the neighborhood disadvantage and cumulative risk composites. It is possible that higher and relatively more flat slopes represent very early stress-regulatory responses to the environment that make it more difficult to detect changes over time in the role of neighborhood disadvantage amid other (and higher) stressors.
Limitations
The primary goal of this study was to explore relations between neighborhood characteristics and cortisol levels in young children. With little previous research on this link, findings from the current study represent a necessary proof-of-concept step that requires a cautious interpretation. For instance, the confounding of race/ethnicity with both family-level and neighborhood-level risk represents a limitation of the current analysis. African American families ranked higher on each individual indicator of cumulative risk as well as on each indicator of neighborhood disadvantage. This was true for both the entire sample and for the subsample of participants residing in North Carolina only. This is problematic for this analysis because fewer African American families residing in more advantaged neighborhoods may have reduced our ability to detect any positive correlates of neighborhoods within this subgroup. Differences in the distribution of disadvantage across race/ethnicity groups in our sample make comparisons between race/ethnicity groups and the interpretation of our findings more complex. This may also be a reason that findings from the current study stand in contrast to findings from previous studies that have found associations between neighborhood disadvantage and HPA regulation in African Americans (Brenner et al., 2013; Hackman et al., 2012).
While we included a vector of proximal-level covariates to ensure our finding is unlikely to be an artifact of proximal factors, there remain additional potential pathways not represented in our data that merit future investigation. This includes unmeasured features of the neighborhood environment such as pollution and chemical contamination, the relative lack of necessary services and infrastructure, and for rural areas in particular, matters of isolation and social support that may represent mechanisms through which disadvantage at the neighborhood level affects children and families. Another unmeasured but potentially important characteristic of the neighborhood environment for early HPA axis functioning may be violence exposure given research showing that residential proximity to recent homicides is linked with attention and self-regulation processes in very young children (McCoy, Raver, & Sharkey, 2015; Sharkey, 2010; Sharkey, Tirado-Strayer, Papachristos, & Raver, 2012).
Another limitation of our study is the reliance on Census block groups as the measure of neighborhoods. While these do represent the smallest geographic unit linked to Census data, they do not necessarily reflect the same community boundaries that residents associate with the neighborhood in which they live. This issue may be exacerbated in the rural context where the contact and shared experience of families within the same block group could vary greatly by how much geographic space separates them. One final limitation of the current analysis concerns the fact that time of day of saliva collection varied between and within participants across time. To address this concern, we controlled for time of day of saliva collection in all models. We acknowledge, however, that while statistical control for time of day may be able to address concerns relating to the diurnal rhythm of cortisol, it does not address more substantive issues related to the interpretation of elevations in morning versus afternoon or evening HPA activity.
Finding relations between neighborhood disadvantage and HPA axis functioning in the first months of childhood increases our understanding of how susceptible very early stress physiology might be to more distal environmental stimuli. With growing empirical support that early exposure to stress is associated with a wide array of adult disease morbidity, our results underscore the importance of understanding the mechanisms through which poverty-related stress at multiple contextual levels influences early development. Moreover, our finding that these relationships may operate differently depending on the racial/ethnic background of the child has important implications for the complex challenges faced by African American families who are often overrepresented in more disadvantaged neighborhoods and communities.
Implications for intervention
Establishing whether and how stressors outside of the home get under the skin to affect a child’s neurophysiology is an important and incremental step in our understanding of the health-related costs of growing up in poor, disadvantaged neighborhoods (Diez Roux & Mair, 2010). Findings from the current study relating neighborhood-level disadvantage with resting HPA activity in toddlerhood are consistent with prior research indicating that early life neighborhood environments may be especially important for health (Vartanian & Houser, 2010). The findings imply, at least preliminarily, that psychobiological processes in toddlerhood may be amenable to intervention at the community level. Given the many “downstream” health consequences of chronic early life stress exposure (Shonkoff et al., 2009), and the intervening psychobiological processes thought to be, in part, responsible for these outcomes, early preventative efforts may offer the most effective means of buffering against the prospect of lifelong ill health associated with early adversity.
In addition to the relatively well-studied link between stress exposure and HPA axis functioning, there is also a need for continued research that explores relations between community-level stressors and the integrated functioning of multiple physiological systems in the body. For instance, research in child development would certainly benefit from increased collaboration between basic and applied work that extends questions regarding the extent to which contextual community-level risk factors are associated with functioning in sympathetic nervous and cardiovascular systems, along with other biomarkers of health, including markers of inflammation (Miller & Chen, 2013; Miller, Chen, & Parker, 2011). Very recent perspectives that take a multilevel developmental approach to link stress exposure and the neuroendocrine, nervous, and immune system to child and adult psychopathology (O’Connor, Moynihan, & Caserta, 2014) are promising. Applying these types of integrative developmental health frameworks to community-level initiatives would present novel opportunities for program evaluation focused on child development at multiple levels.
Converging with this emphasis on multiple levels of development is evidence from community development work suggesting that some of the most successful community-level interventions for improving the life chances of children from highly disadvantaged communities are those that simultaneously target the improvement of multiple aspects of community experience (e.g., child and family health and functioning, the built environment and physical infrastructure, social cohesion, and investments in human capital; Jutte, Miller, & Erickson, 2015). Recent calls from leaders at the interface of policy and health research emphasize the need for collaboration between health researchers, medical practitioners, and professionals from the community development sector, to design, implement, and evaluate novel evidence-based community-level interventions that aim to improve living conditions at scale (Jutte et al., 2015). Innovative perspectives on intervention at the community level targeting improvements to the whole community will likely support and extend research and initiatives in the field of child development targeting improvements to the whole child, which emphasize the need to understand developmental processes across multiple levels and contexts.
Acknowledgments
The Family Life Project was partially supported by a grant from the National Institute of Child Health and Human Development (Grant P01 HD39667), with cofunding from the National Institute on Drug Abuse.
References
- Bischoff K, Reardon SF. Residential segregation by income, 1970–2009. In: Logan JR, editor. Diversity and disparities: America enters a new century. New York: Russell Sage Foundation; 2014. pp. 208–233. [Google Scholar]
- Blair C, Granger DA, Kivlighan KT, Mills-Koonce R, Willoughby M, Greenberg MT, Family Life Project Investigators Maternal and child contributions to cortisol response to emotional arousal in young children from low-income, rural communities. Developmental Psychology. 2008;44:1095–1109. doi: 10.1037/0012-1649.44.4.1095. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger DA, Willoughby M, Mills-Koonce R, Cox M, Greenberg MT, Family Life Project Key Investigators Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development. 2011;82:1970–1984. doi: 10.1111/j.1467-8624.2011.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Raver CC. Child development in the context of adversity: Experiential canalization of brain and behavior. American Psychologist. 2012a;67:309–318. doi: 10.1037/a0027493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Raver CC. Individual development and evolution: Experiential canalization of self-regulation. Developmental Psychology. 2012b;48:647–657. doi: 10.1037/a0026472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Raver CC, Granger D, Mills-Koonce R, Hibel L, Family Life Project Key Investigators Allostasis and allostatic load in the context of poverty in early childhood. Development and Psychopathology. 2011;23:845–857. doi: 10.1017/S0954579411000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. 10.10170S0954579405050145. [DOI] [PubMed] [Google Scholar]
- Brenner AB, Zimmerman MA, Bauermeister JA, Caldwell CH. The physiological expression of living in disadvantaged neighborhoods for youth. Journal of Youth and Adolescence. 2013;42:792–806. doi: 10.1007/s10964-012-9838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Lei M, Chen E, Miller GE. Neighborhood poverty and allostatic load in African American youth. Pediatrics. 2014;134:e1362–e1368. doi: 10.1542/peds.2014-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfenbrenner U, Morris PA. The bioecological model of human development. In: Lerner RM, editor. Handbook of child psychology: Vol. 1. Theoretical models of human development. 6. Hoboken, NJ: Wiley; 2006. pp. 793–828. [Google Scholar]
- Brooks-Gunn J, Duncan GJ, Aber JL, editors. Neighborhood poverty: Context and consequences for children. New York: Russell Sage Foundation; 1997. [Google Scholar]
- Chen E, Paterson LQ. Neighborhood, family, and subjective socioeconomic status: How do they relate to adolescent health? Health Psychology. 2006;25:704–714. doi: 10.1037/0278-6133.25.6.704. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. Child Development. 2010;81:252–269. doi: 10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Chen E, &Matthews KA. Childhood socioeconomic status and adult health. Annals of the New York Academy of Sciences. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- Cox MJ, Crnic K. Qualitative ratings for parent-child interaction at 3–12 months of age. University of North Carolina at Chapel Hill; 2002. Unpublished manuscript. [Google Scholar]
- Diez Roux AV, Mair C. Neighborhoods and health. Annals of the New York Academy of Sciences. 2010;1186:125–145. doi: 10.1111/j.1749-6632.2009.05333.x. [DOI] [PubMed] [Google Scholar]
- Dill BT. Poverty in the rural US: Implications for children, families, and communities. Annie E. Casey Foundation; 1999. Unpublished manuscript. [Google Scholar]
- Do DP, Diez Roux AV, Hajat A, Auchincloss AH, Merkin SS, Ranjit N, Seeman T. Circadian rhythm of cortisol and neighborhood characteristics in a population-based sample: The multi-ethnic study of atherosclerosis. Health & Place. 2011;17:625–632. doi: 10.1016/j.healthplace.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulin-Keita A, Casazza K, Fernandez JR, Goran MI, Gower B. Do neighbourhoods matter? Neighbourhood disorder and long-term trends in serum cortisol levels. Journal of Epidemiology and Community Health. Advance online publication. 2010 doi: 10.1136/jech.2009.092676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duru OK, Harawa NT, Kermah D, Norris KC. Allostatic load burden and racial disparities in mortality. Journal of the National Medical Association. 2012;104:89–95. doi: 10.1016/S0027-9684(15)30120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK. Applied missing data analysis. New York: Guilford Press; 2010. [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology. 2003;39:924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Evans GW. The environment of childhood poverty. American Psychologist. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Evans GW, English K. The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Development. 2002;73:1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P. Childhood poverty and young adults’ allostatic load: The mediating role of childhood cumulative risk exposure. Psychological Science. 2012;23:979–983. doi: 10.1177/0956797612441218. [DOI] [PubMed] [Google Scholar]
- Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences. 2009;106:6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzel BL, Morris PA. Allostasis and the developing human brain: Explicit consideration of implicit models. Development and Psychopathology. 2011;23:955–974. doi: 10.1017/S0954579411000447. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, Whembolua G. Integration of salivary bio-markers into developmental and behaviorally-oriented research: Problems and solutions for collecting specimens. Physiology & Behavior. 2007;92:583–590. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Krueger K, Rigatuso J. Dampening of adrenocortical responses during infancy: Normative changes and individual differences. Child Development. 1996;67:877–889. doi: 10.1111/j.1467-8624.1996.tb01770.x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/S0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annur-ev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hackman DA, Betancourt LM, Brodsky NL, Hurt H, Farah MJ. Neighborhood disadvantage and adolescent stress reactivity. Frontiers in Human Neuroscience. 2012;6:1–11. doi: 10.3389/fnhum.2012.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat A, Moore K, Do DP, Merkin SS, Punjabi NM, Sáñchez BN, Diez-Roux AV. Examining the cross-sectional and longitudinal association between diurnal cortisol and neighborhood characteristics: Evidence from the multi-ethnic study of atherosclerosis. Health & Place. 2015;34:199–206. doi: 10.1016/j.healthplace.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon AG, Hibel LC, Rumyantseva O, Granger DA. Measuring salivary cortisol in studies of child development: Watch out—What goes in may not come out of saliva collection devices. Developmental Psychobiology. 2007;49:495–500. doi: 10.1002/dev.20231. [DOI] [PubMed] [Google Scholar]
- Juster R, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Jutte DP, Miller JL, Erickson DJ. Neighborhood adversity, child health, and the role for community development. Pediatrics. 2015;135:S48–S57. doi: 10.1542/peds.2014-3549F. [DOI] [PubMed] [Google Scholar]
- Karb RA, Elliott MR, Dowd JB, Morenoff JD. Neighborhood-level stressors, social support, and diurnal patterns of cortisol: The Chicago community adult health study. Social Science and Medicine. 2012;75:1038–1047. doi: 10.1016/j.socscimed.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, Seeman TE. Reduction in allostatic load in older adults in associated with lower all-cause mortality risk: MacArthur studies of successful aging. Psychosomatic Medicine. 2006;68:500–507. doi: 10.1097/01.psy.0000221270.93985.82. [DOI] [PubMed] [Google Scholar]
- Loman MM, Gunnar MR. Early experience and the development of stress reactivity and regulation in children. Neuroscience & Bio-behavioral Reviews. 2010;34:867–876. doi: 10.1016/j.neubiorev.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biological Psychiatry. 2000;48:976–980. doi: 10.1016/S0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Development and Psychopathology. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- McCoy DC, Raver CC, Sharkey P. Children’s cognitive performance and selective attention following recent community violence. Journal of Health and Social Behavior. 2015;56:19–36. doi: 10.1177/0022146514567576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Review. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress and allostasis-induced brain plasticity. Annual Review of Medicine. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress: Elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999;897:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Merkin SS, Basurto-Dávila R, Karlamangla A, Bird C, Lurie N, Escarce J, Seeman T. Neighborhoods and cumulative biological risk profiles by race/ethnicity in a national sample of U.S. adults: NHANES III. Annals of Epidemiology. 2009;19:194–201. doi: 10.1016/j.anne-pidem.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E. The biological residue of childhood poverty. Child Development Perspectives. 2013;7:67–73. doi: 10.1111/cdep.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Mills-Koonce WR, Garrett-Peters P, Barnett M, Granger DA, Blair C, Cox MJ, Family Life Project Key Investigators Father contributions to cortisol responses in infancy and toddlerhood. Developmental Psychology. 2011;47:388–395. doi: 10.1037/a0021066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutheén LK, Mutheén BO. Mplus user’s guide. 7. Los Angeles: Author; 1998–2012. [Google Scholar]
- Ncube CN, Enquobahrie DA, Albert SM, Herrick AL, Burke JG. Associations of neighborhood context with offspring risk of preterm birth and low birth weight: A systematic review and meta-analysis of population-based studies. Social Science & Medicine. 2016;153:156–164. doi: 10.1016/j.socscimed.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network. Chronicity of maternal depressive symptoms, maternal sensitivity, and child functioning at 36 months. Developmental Psychology. 1999;35:1297–1310. doi: 10.1037/0012-1649.35.5.1297. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Moynihan JA, Caserta MT. Annual Research Review: The neuroinflammation hypothesis for stress and psychopathology in children—Developmental psychoneuroimmunology. Journal of Child Psychology and Psychiatry. 2014;55:615–631. doi: 10.1111/jcpp.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainisch BKW, Upchurch DM. Sociodemographic correlates of allostatic load among a national sample of adolescents: Findings from the National Health and Nutrition Examination Survey, 1999–2008. Journal of Adolescent Health. 2013;53:506–511. doi: 10.1016/j.jadohealth.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinette JW, Charles ST, Almeida DM, Gruenewald TL. Neighborhood features and physiological risk: An examination of allostatic load. Health & Place. 2016;41:110–118. doi: 10.1016/j.healthplace.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KE, Wand GS, Stuart EA, Glass TA, Marques AH, Duncko R, Merikangas KR. The association between cortisol and neighborhood disadvantage in a U.S. population-based sample of adolescents. Health & Place. 2014;25:68–77. doi: 10.1016/j.healthplace.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satorra A, Bentler PM. A scaled difference chi-square test statistic for moment structure analysis. Psychometrika. 2001;66:507–514. doi: 10.1007/BF02296192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Annals of the New York Academy of Sciences. 2010;1186:223–239. doi: 10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- Sharkey P. The acute effect of local homicides on children’s cognitive performance. Proceedings of the National Academy of Sciences. 2010;107:11733–11738. doi: 10.1073/pnas.1000690107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey PT, Tirado-Strayer N, Papachristos AV, Raver CC. The effect of local violence on children’s attention and impulse control. American Journal of Public Health. 2012;102:2287–2293. doi: 10.2105/AJPH.2012.300789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities. Journal of the American Medical Association. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and Behavior. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Theall KP, Brett ZH, Shirtcliff EA, Dunn EC, Drury SS. Neighborhood disorder and telomeres: Connecting children’s exposure to community level stress and cellular response. Social Science & Medicine. 2013;85:50–58. doi: 10.1016/j.socscimed.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian TP, Houser L. The effects of childhood neighborhood conditions on self-reports of adult health. Journal of Health and Social Behavior. 2010;51:291–306. doi: 10.1177/0022146510378241. [DOI] [PubMed] [Google Scholar]
- Vernon-Feagans L, Burchinal M, Mokrova I. Diverging destinies in rural America. In: Amato PR, Booth A, McHale SM, Van Hook J, editors. National Symposium on Family Issues: Vol. 5. Families in an era of increasing inequality. Geneva: Springer; 2015. pp. 35–49. [Google Scholar]
- Vernon-Feagans L, Cox M, Family Life Project Key Investigators The family life project: An epidemiological and developmental study of young children living in poor rural communities. Monographs of the Society for Research in Child Development. 2013;78:1–150. doi: 10.1111/mono.12046. [DOI] [PubMed] [Google Scholar]
- Watamura SE, Donzella B, Kertes DA, Gunnar MR. Developmental changes in baseline cortisol activity in early childhood: Relations with napping and effortful control. Developmental Psychobiology. 2004;45:125–133. doi: 10.1002/dev.20026. [DOI] [PubMed] [Google Scholar]