Abstract
This Letter describes the development of a method for the Pd-catalyzed electrochemical acetoxylation of C–H bonds. The oxidation step of the catalytic cycle is probed through cyclic voltammetry and bulk electrolysis studies of a pre-formed palladacycle of 8-methylquinoline. A catalytic system for C–H acetoxylation is then developed and optimized with respect to the cell configuration, rate of oxidation, and chemistry at the counter electrode. This transformation is then applied to substrates containing various directing groups and to the acetoxylation of both C(sp2)–H and C(sp3)–H bonds.
Graphical abstract

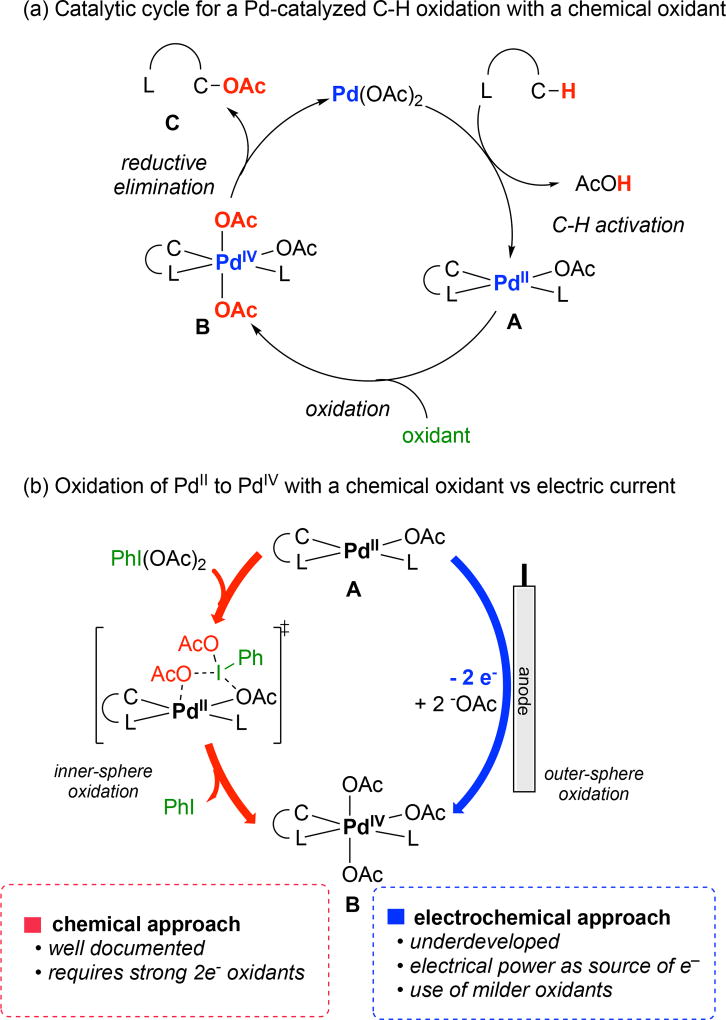
Our group has a long-standing interest in the development of palladium-catalyzed C–H bond oxygenation reactions.1,2 As shown in Figure 1a, these transformations are believed to proceed via a mechanism involving initial C–H bond activation at PdII to form an intermediate of general structure A. The two-electron oxidation of A with a chemical oxidant such as PhI(OAc)2 forms a transient, high valent Pd intermediate such as B.1,2,3 C–O bond-forming reductive elimination from B then releases the product (C) and regenerates the PdII catalyst. These transformations proceed with various C(sp2)–H and C(sp3)–H substrates and have been applied to the synthesis and diversification of natural products and pharmaceutical candidates.4 Nonetheless, they remain limited by the requirement for highly reactive stoichiometric chemical oxidants such as PhI(OAc)2.
Figure 1.
(a) Catalytic cycle for a Pd-catalyzed C–H bond oxidation. (b) Comparisons between chemical and electrochemical approaches for C–H oxidation.
An attractive alternative would be to replace chemical oxidants with an anodic oxidation event.5,6 As shown in Figure 1b, this would require a change in mechanism from an inner-sphere chemical oxidation of the PdII intermediate A3 to an outer-sphere oxidation of A at an electrode surface. The supporting electrolyte would then serve as a ligand/nucleophile for the functionalization of the resulting high valent Pd intermediate B. A seminal report by Kakiuchi showed the viability of this approach by demonstrating the Pd-catalyzed electrochemical C(sp2)–H chlorination of 2-phenylpyridine derivatives with HCl as both the supporting electrolyte and the nucleophile.7a A related Pd-catalyzed C(sp2)–H chlorination of benzoquinoline was demonstrated by Gray using tetraethylammonium chloride as the nucleophile.7b More recently, Budnikova reported the Pd-catalyzed electrochemical C(sp2)–H oxygenation of 2-phenylpyridine, and conducted detailed investigations of the speciation and stoichiometric electrolysis of key cyclopalladated intermediates.8,9
Despite these advances, until very recently, general methods for Pd-catalyzed electrochemical C–H oxidation remained elusive. At the time that we initiated this work, the substrate scope of such transformations was limited to the oxidative functionalization of C(sp2)–H bonds in molecules containing pyridine-type directing groups. In addition, there was minimal data on the impact of different electrochemical conditions (most notably, the chemistry occurring at the counter electrode) on the outcome of these transformations. Finally, the complementary scope and side reactions of chemical versus electrochemical oxidation conditions had not been explored. This report describes our development of a Pd-catalyzed electrochemical acetoxylation of a variety of C(sp3)–H and C(sp2)–H substrates. The optimization of the electrochemical conditions as well as competing reactions are both explored, revealing complementarities between this approach versus the use of PhI(OAc)2. Notably, while these studies were underway, the Mei group reported several elegant examples of related Pd-catalyzed electrochemical C–H oxygenation reactions.10
Our initial studies focused on establishing the feasibility of the electrochemical oxidative functionalization of 8-methylquinoline (1). This substrate was selected as a model for assessing the feasibility of electrochemical C(sp3)–H acetoxylation. To decouple the C–H activation step from the electrochemical oxidation, we first examined the electrochemistry of palladacycle 2, the putative PdII intermediate formed from the C(sp3)–H activation of 8-methylquinoline. Cyclic voltammograms (CVs) of 2 were obtained in MeCN using tetramethylammonium tetrafluoroborate (TMABF4, a weak nucleophile) and tetramethylammonium acetate (TMAOAc, a stronger nucleophile) as the supporting electrolyte. When the experiment was performed with the TMABF4 support, two quasi-reversible peaks were observed with onset potentials for the oxidative peaks at ~0.21 V and ~0.66 V versus Ag/AgBF4 (Figure 2). In contrast, with TMAOAc as the support, the CV showed two irreversible oxidation peaks with onset potentials of ~0.04 V and ~0.33 V, respectively. This result is consistent with electrochemical oxidation of 2 followed by an irreversible chemical reaction with the TMAOAc electrolyte to form product 1a.
Figure 2.
CV of 2 in TMABF4 (black) in MeCN and TMAOAc (red) in MeCN.
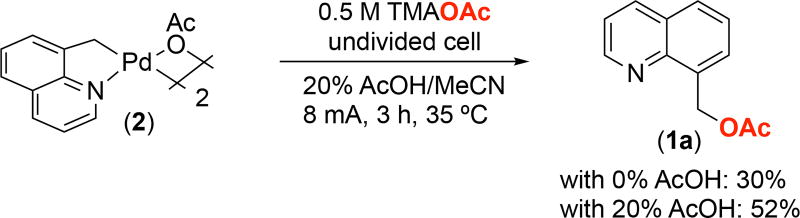
We next conducted bulk electrolysis (BE) in MeCN/TMAOAc to confirm the identity of the product(s) formed upon anodic oxidation of 2. The BE was performed in an undivided H-cell with 8 mA current for 3 h (4 Farads per equiv of complex 2). Under these conditions, the acetoxylated product 1a was formed in 30% yield as determined by 1H NMR spectroscopic analysis of the crude reaction mixture (Figure 3). We noted that 2 was not fully soluble in the MeCN/TMAOAc solution, and thus we next added 20% v/v of AcOH.11 Under these conditions, 1a was formed in 52% yield. These results demonstrate the feasibility of the electrochemical oxidative functionalization of this putative catalytic intermediate.
Figure 3.
Bulk electrolysis of 2 in an undivided cell with and without added AcOH. Yields determined by 1H NMR spectroscopic analysis of crude reaction mixture.
We next sought to translate these stoichiometric studies to the Pd-catalyzed electrochemical C(sp3)–H acetoxylation of 8-methylquinoline. Notably, in the catalytic transformation, the C–H activation step to form 2 must occur prior to oxidation. As such, it is critical to optimize the reaction conditions to promote this step (which is often rate-determining)12 while minimizing competing decomposition of the Pd catalyst. We first performed the experiment under conditions analogous to the stoichiometric bulk electrolysis of 2: in an undivided cell with 10 mol % of the Pd(OAc)2 catalyst. However, under these conditions no product was formed and the precipitation of Pd-black was observed (Table 1, entry 1).
Table 1.
Optimization of catalytic electrochemical C–H acetoxylation of 1a

| ||||
|---|---|---|---|---|
|
| ||||
| entry | mA or V | time/h | oxidant | 1a (%) |
| 1b | 8 mA | 6 | - | 0 |
| 2c | 8 mA | 6 | - | 54 |
| 3c,d | 8 mA | 6 | BQ | 70 |
| 4c,e | 8 mA | 6 | BQ | 73 |
| 5c,e | 6 mA | 8 | BQ | 71 |
| 6c,e | 12 mA | 4 | BQ | 58 |
| 7c,e | 1.2 V | 12 | BQ | 30 ± 16 |
Reaction conditions: 0.4 mmol of 1, 10 mol % of Pd(OAc)2, 0.5 M TMAOAc, 20% AcOH in MeCN. Yield determined by 1H NMR spectroscopic analysis of crude reaction mixture.
Conducted in an undivided cell.
Conducted in a divided H-cell.
0.5 M (5 equiv) of benzoquinone (BQ) used as oxidant at counter electrode.
0.1 M (1 equiv) of benzoquinone (BQ) used as oxidant at counter electrode.
We reasoned that the Pd catalyst was undergoing competitive reduction at the cathode which would lead to the formation of Pd black (and thus catalyst deactivation). Hence, we next employed a divided H-cell with a physical barrier (a fine frit) between the working (W) and counter (C) electrodes. This resulted in the formation of la in 54% yield (Table 1, entry 2). However, a Pd-black precipitate was still observed on the working anode side at the completion of this reaction, indicating that some catalyst reduction is occurring under these conditions. This result was unexpected because the working side of this cell should always be under oxidizing conditions.
We hypothesized the Pd was being reduced chemically by products generated on the cathode side of the cell. Under these conditions, the electrochemical reaction is balanced by proton reduction to form H2 at the cathode. This H2 gas can then diffuse to the anode half cell, leading to the chemical reduction of PdII to Pd0. To address this issue, we introduced an alternative mild chemical oxidant on the cathode side of the cell. Benzoquinone (BQ) was selected as an electrochemically recyclable oxidant that is incapable of oxidizing 2 on its own.13 Consistent with our hypothesis, the use of BQ resulted in a significant increase in yield to 70%; furthermore, no palladium black was observed under these conditions (Table 1, entry 3).14
Several other parameters were optimized in this system, including solvent, cell configuration, electrode material, identity of the sacrificial oxidant at the counter electrode, and electrochemical settings (i.e., constant current versus constant potential). Selected details are discussed below, and more complete information is provided in the Supporting Information (p. S5). We hypothesized that to achieve productive oxidation, the rate of current discharge would need to be slower than that of C–H activation. Consistent with this proposal, increasing the rate of current discharge into the reaction resulted in a decrease in yield, while decreasing the rate led to a similar yield over 8 h (Table 1, entries 6 and 5, respectively). Conducting this reaction using a controlled potential of 1.2 V rather than a controlled current led to lower yields and poor reproducibility (Table 1, entry 7). Ultimately, the optimal conditions for the C(sp3)–H acetoxylation of 8-methylquinoline were identified as follows: 0.5 M TMAOAc, 20% AcOH v/v in MeCN, 0.4 mmol of benzoquinone on the counter side as a sacrificial oxidant, and 8 mA current passed over 6 h.
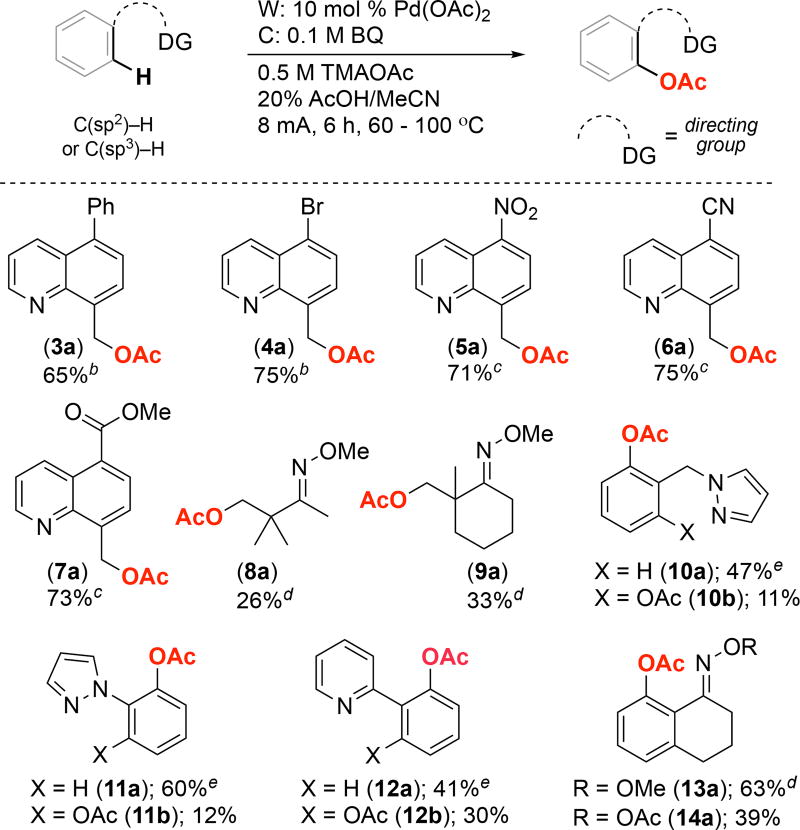
We next examined the scope of this electrochemical C– H oxidation reaction. As mentioned above, most previously reported electrochemical Pd-catalyzed C–H oxidation reactions were applied to a limited set of phenylpyridine substrates7,8,9 In contrast, the current transformation is significantly more general. A series of 8-methylquinoline derivatives with different functional groups at the 5-position underwent C(sp3)–H acetoxylation in moderate to good yields to form products 3a–7a (Figure 4). Pyridine, pyrazole, oxime ether, and O-acetyl oxime directing groups were tolerated. Both 5- and 6-membered palladacyclic intermediates underwent electrochemical C–H acetoxylation in moderate to high yields (for example, compare products 10a/b and 11a/b). Finally, both C(sp3)–H and C(sp2)–H bonds can be functionalized. Notably, while the yields for the acetoxylation of unactivated C(sp3)–H bonds (for example to form 8a and 9a) are relatively modest under our conditions, we note that Mei has recently reported a related method that is specifically optimized for this class of substrates.10,15
Figure 4.
Substrate scope. Reaction conditions: 0.4 mmol substrate, 10 mol % of Pd(OAc)2, 0.5 M TMAOAc, 20% AcOH v/v in MeCN. b 65 °C. c 75 °C. d Reaction conditions: 0.4 mmol substrate, 10 mol % of Pd(OAc)2, 0.5 M TMAOAc, 20% AcOH, 10% Ac2O in MeCN at 100 °C. e 100 °C.
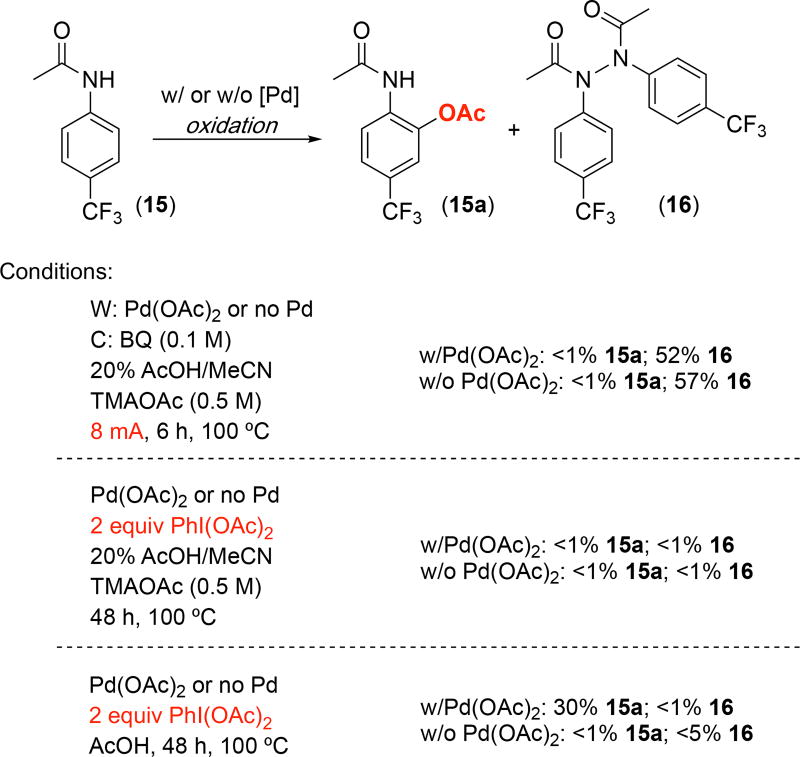
We also observed some key complementarities between electrochemical and chemical oxidation reactions. For example, as shown in Scheme 1, the acetanilide substrate 15 afforded none of the expected C(sp2)–H acetoxylation product 15a16 when subjected to our Pd-catalyzed electrochemical oxidation conditions. Instead, N–N coupling occurred to afford 16 in 52% yield. This type of reactivity has been reported in the literature in the absence of a Pd catalyst,17 and, indeed, conducting the electrochemical reaction without Pd under otherwise identical conditions afforded 16 in 57% yield. We next explored the chemical oxidation of 15 using PhI(OAc)2. Use of the electrochemical conditions (20% AcOH/MeCN, 0.5 M TMAOAc, 100 °C, 6 h), but with 1 equiv of PhI(OAc)2 in place of electrical current, resulted in no detectable formation of either 15a or 16 in the presence or absence of Pd(OAc)2.18 In contrast, conducting the Pd-catalyzed oxidation with PhI(OAc)2 under more standard chemical oxidation conditions (in AcOH/Ac2O at 100 °C for 6 h) afforded 30% yield of 15a. Only starting material was recovered under these conditions in the absence of Pd. Overall, these results demonstrate that there can be key differences in the relative rates of competing oxidation reactions under chemical versus electrochemical oxidation conditions, resulting in the formation of complementary products. Furthermore, the changes required to achieve an electrochemical reaction (often using different solvents as well as significant quantities of supporting electrolyte) can lead to fundamental changes in catalyst performance/reaction outcomes. Both of these points will be critical to consider for the future development and applications of electrochemistry in organic synthesis.
Scheme 1.
Electrochemical versus chemical oxidation of 15
In conclusion, this report describes an electrochemical, Pd-catalyzed oxygenation of C(sp2)–H and C(sp3)–H bonds. By decoupling the C–H activation and oxidation steps, we were able to study the oxidation of the palladacycle of 8-methylquinoline. These studies ultimately led to the development and optimization of parameters for a catalytic system. We have shown that the addition of a mild oxidant to the cathode half-cell improves the overall reaction yield. Furthermore, we have shown that the choice of oxidant (chemical versus electrochemical) as well as the reaction medium (solvent and electrolyte) can lead to different reaction outcomes.
Supplementary Material
Acknowledgments
We acknowledge financial support from NIH NIGMS (GM073836). In addition, ML thanks the NSF for a graduate fellowship.
Footnotes
ASSOCIATED CONTENT
- Experimental details, characterization, and NMR data for isolated compounds (PDF)
References
- 1.For selected reviews on Pd-catalyzed C–H functionalization: He J, Wasa M, Chan KSL, Shao Q, Yu J-Q. Chem. Rev. 2017;117:8754. doi: 10.1021/acs.chemrev.6b00622.Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147. doi: 10.1021/cr900184e.Daugulis O, Do H-Q, Shabashov D. Acc. Chem. Res. 2009;42:1074. doi: 10.1021/ar9000058.
- 2.For selected examples of high valent Pd chemistry see: Powers DC, Lee E, Ariafard A, Sanford MS, Yates BF, Canty AJ, Ritter T. J. Am. Chem. Soc. 2012;134:12002. doi: 10.1021/ja304401u.Deprez NR, Sanford MS. J. Am. Chem. Soc. 2009;134:11234. doi: 10.1021/ja904116k.For reviews see: Powers DC, Ritter T. Acc. Chem. Res. 2012;45:840. doi: 10.1021/ar2001974.Hickman AJ, Sanford MS. Nature. 2012;484:177. doi: 10.1038/nature11008.Choy PY, Kwong FY. Org. Lett. 2013;15:270. doi: 10.1021/ol303088z.
- 3.Canty AJ, Ariafard A, Sanford MS, Yates BF. Organometallics. 2013;32:544. [Google Scholar]
- 4.For examples see: Sharpe RJ, Johnson JS. J. Am. Chem. Soc. 2015;137:4968. doi: 10.1021/jacs.5b02631.Ding C, Li J, Jiao M, Zhang A. J. Nat. Prod. 2016;79:2514. doi: 10.1021/acs.jnatprod.6b00370.Baudoin O. Acc. Chem. Res. 2017;50:1114. doi: 10.1021/acs.accounts.7b00099.Brady PB, Bhat V. Eur. J. Org. Chem. 2017;35:5179.
- 5.For selected reviews on anodic oxidation in organic chemistry: Moeller KD. Tetrahedron. 2000;56:9527.Jiao K-J, Zhao C-Q, Fang P, Mei T-S. Tetrahedron Lett. 2017;58:797.Dudkina YB, Gryaznova TV, Sinyashin OG, Budnikova YH. Russ. Chem. Bull. 2015;64:1713.Horn EJ, Rosen BR, Baran PS. ACS Cent. Sci. 2016;2:306. doi: 10.1021/acscentsci.6b00091.Yoshida J-i, Kataoka K, Horcajada R, Nagaki A. Chem. Rev. 2008;108:2265. doi: 10.1021/cr0680843.Sperry JB, Wright DL. Chem. Soc. Rev. 2006;35:605. doi: 10.1039/b512308a.Lund H. J. Electrochem. Soc. 2002;149:S21.
- 6.For selected examples on electroorganic synthesis: Badalyan A, Stahl SS. Nature. 2016;535:406. doi: 10.1038/nature18008.Kawamata Y, Yan M, Liu Z, Bao D-H, Chen J, Starr JT, Baran PS. J. Am. Chem. Soc. 2017;139:7448. doi: 10.1021/jacs.7b03539.Horn EJ, Rosen BR, Chen Y, Tang J, Chen K, Eastgate MD, Baran PS. Nature. 2016;533:77. doi: 10.1038/nature17431.Redden A, Perkins RJ, Moeller KD. Angew. Chem. Int. Ed. 2013;52:12865. doi: 10.1002/anie.201308739.Lips S, Wiebe A, Elser B, Schollmeyer D, Dyballa KM, Franke R, Waldvogel SR. Angew. Chem. Int. Ed. 2016;55:10872. doi: 10.1002/anie.201605865.Weibe A, Schollmeyer D, Dyballa KM, Franke R, Waldvogel SR. Angew. Chem. Int. Ed. 2016;55:11802. doi: 10.1002/anie.201604321.
- 7.(a) Kakiuchi F, Kochi T, Mutsutani H, Kobayashi N, Urano S, Sato M, Nishiyama S, Tanabe T. J. Am. Chem. Soc. 2009;131:11310. doi: 10.1021/ja9049228. [DOI] [PubMed] [Google Scholar]; (b) Durrell AC, Jackson MN, Hazari N, Gray HB. Eur. J. Inorg. Chem. 2013:1134–1137. [Google Scholar]
- 8.(a) Dudkina YB, Mikhaylov DY, Gryaznova TV, Tufatullin AI, Kataeva ON, Vicic DA, Budnikova YH. Organometallics. 2013;32:4785. [Google Scholar]; (b) Dudkina YB, Mikhaylov DY, Gryaznova TV, Sinyashin OG, Vicic DA, Budnikova YH. Eur. J. Org. Chem. 2012:2114. [Google Scholar]
- 9.Gryaznova TV, Dudkina YB, Islamov DR, Kataeva ON, Sinyashin OG, Vicic DA, Budnikova YH. J. Organomet. Chem. 2015;785:68. [Google Scholar]
- 10.(a) Yang QL, Li YQ, Ma C, Fang P, Zhang XJ, Mei TS. J. Am. Chem. Soc. 2017;139:3293. doi: 10.1021/jacs.7b01232. [DOI] [PubMed] [Google Scholar]; (b) Li YQ, Yang QL, Fang P, Mei TS, Zhang D. Org. Lett. 2017;19:2905. doi: 10.1021/acs.orglett.7b01138. [DOI] [PubMed] [Google Scholar]
- 11.The addition of 20% AcOH leads to some changes in the CV of 2, most notably a shift to higher potential. See SI (p. S3) for a CV under these conditions.
- 12.Powers DC, Geibel MAL, Klein JEMN, Ritter T. J. Am. Chem. Soc. 2009;131:17050. doi: 10.1021/ja906935c. [DOI] [PubMed] [Google Scholar]
- 13.A control chemical reaction was run under identical conditions using benzoquinone (1.1 equiv) as an oxidant instead of electric current. No reaction was observed and starting material was recovered at the end of the reaction.
- 14.Upon closer evaluation of the working-side and counter-side potentials during the reaction, we observed that the potential on the counter side was significantly lower (by almost 1 V) with the addition of BQ (see SI). The working side potential was also slightly reduced. We believe that both this reduction of overpotential on the working side as well as the prevention of H2 formation benefit the overall yield of the reaction. In addition, a control chemical reaction with BQ as an oxidant did not yield any acetoxylated product (see SI). This result substantiates the fact that BQ alone cannot affect the oxidation of Pd (II) to Pd (IV).
- 15.Mei has demonstrated that the use of a different counterion (nucleophile) in the supporting electrolyte and a different acid can lead to different functionalization reactions. See ref. 10a.
- 16.Wang G-W, Yuan T-T, Wu X-L. J. Org. Chem. 2008;73:4714. doi: 10.1021/jo8003088. [DOI] [PubMed] [Google Scholar]
- 17.(a) Gieshoff T, Schollmeyer D, Waldvogel SR. Angew. Chem. Int. Ed. 2016;55:9437. doi: 10.1002/anie.201603899. [DOI] [PubMed] [Google Scholar]; (b) Rosen BR, Werner EW, O’Brien AG, Baran PS. J. Am. Chem. Soc. 2014;136:5571. doi: 10.1021/ja5013323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The use of MeCN as a solvent and TMAOAc as an additive has a detrimental impact on the Pd-catalyzed C–H acetoxylation of substrate 15 under both electrochemical and chemical oxidation conditions. This may be due to changes in the speciation of the palladium catalyst or palladacycle intermediate as a function of solvent, pH, and/or concentration of acetate.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.