Abstract
Rationale
The interaction between dieting and stress is a key factor for triggering binge episodes on palatable food in human binge eaters. Corticotropin releasing factor (CRF) mechanisms are known to play a pivotal role in the regulation of this maladaptive behavior.
Objective
The present study evaluated the effect of systemic injection of the CRF1 receptor antagonist R121919, the corticosterone synthesis inhibitor metyrapone and central amygdala (CeA) injections of the nonselective CRF antagonist D-Phe-CRF(12– 41) in rats in which binge eating was evoked by stress and cycles of food restriction.
Method
Female rats were subjected or not to repeated cycles of regular chow food restriction/refeeding during which they were also given limited access (2 h) to palatable food. On the test day, rats were either exposed or not to the sight of the palatable food for 15 min without allowing access, before assessing food consumption.
Results
Systemic injections of R121919, but not of the metyrapone, blocked binge-like eating behavior. Restricted and stressed rats showed up-regulation of crh1 receptor mRNA signal in the bed nucleus of the stria terminalis and CeA but not in basolateral amygdala (BLA) or in the paraventricular nucleus. Injection D-Phe-CRF(12– 41) in CeA but not in the BLA blocked binge-like eating behavior.
Discussion
These findings demonstrate that extra-hypothalamic CRF1 receptors, rather than those involved in endocrine functions, are involved in binge eating and the crucial role of CRF receptors in CeA. CRF1 receptor antagonism may represent a novel pharmacological treatment for binge-related eating disorders.
Keywords: Binge eating, HPA axis, stress, CRF1 receptor antagonist, BNST, CeA
Binge eating episodes represent a core behavioral feature of binge eating disorder (BED), bulimia nervosa, and binge/purge subtype anorexia nervosa (American Psychiatric Association, 2013). Episodes of binge-eating in humans are characterized by compulsive, non-homeostatic consumption of an unusually large quantity of highly palatable food in a short period of time. Binge eating is also an additional feature of obese individuals, significantly contributing to their high caloric intake and finally being overweight (Hudson, Hiripi, Pope, & Kessler, 2007; Swanson, Crow, Le Grange, Swendsen, & Merikangas, 2011). Even in the absence of hunger, some subjects exhibit episodes of rapid eating beyond satiation accompanied by an uncontrollable urge to obtain and consume food. This condition resembles behavioural phenomena described in drug dependence and similarities between binge eating and addiction have been proposed (Avena, 2011; Avena, Bocarsly, Hoebel, & Gold, 2011; Corwin & Grigson, 2009; Cottone et al., 2009; D’Addario et al., 2014; Johnson & Kenny, 2010; Volkow, Wang, Fowler, Tomasi, & Baler, 2012).
A large body of evidence suggests that dieting, stress and negative affect represent possible triggers of binge eating in patients suffering from BED or bulimia nervosa (Freeman & Gil, 2004). Indeed, dieting periods are a common finding in the history of binge eaters, although hunger per se appears to be insufficient to induce binge eating in the absence of stress and negative affective state (Polivy, Zeitlin, Herman, & Beal, 1994). The important role of stress in the etiology of binge eating is emphasized by the finding that obese individuals with BED exhibit activation of the hypothalamic-pituitary-adrenal (HPA) axis, and their cortisol levels are higher in comparison to those of obese individuals without BED (Gluck, Geliebter, Hung, & Yahav, 2004). Moreover, higher blood cortisol levels in response to stress predict greater intake of sweets (Epel, Lapidus, McEwen, & Brownell, 2001) and salivary cortisol levels are positively correlated with binge eating severity (Coutinho, Moreira, Spagnol, & Appolinario, 2007). Thus the increase of corticosterone levels represent a hormonal marker of binge eating induced by cyclic food restrictions and stress (Artiga et al., 2007; C. Cifani et al., 2010; C. Cifani, Polidori, Melotto, Ciccocioppo, & Massi, 2009) and palatable food intake has been shown to blunt activation of the HPA axis (Christiansen, Dekloet, Ulrich-Lai, & Herman, 2011; Kinzig, Hargrave, & Honors, 2008) suggesting that it may represent a kind of self-medication in conditions of stress. The CRF system initiates the neuroendocrine response to stress via the HPA axis, and coordinates several behaviors via actions on extra hypothalamic neural substrates. At extra hypothalamic sites, including the central amygdala (CeA) and the basolateral amygdala (BLA), CRF coordinates the affective reactions to stress (Gray, 1993; Wang et al., 2011). CRF1 is the most abundantly expressed CRF receptor in the brain and high density has been reported in the hypothalamus, in the ventral tegmental area, amygdala and various cortical structures. Expression of CRF2 receptors is more restricted and is primarily detected in the hypothalamus, dorsal raphe and lateral septum (Bale & Vale, 2004; Fekete & Zorrilla, 2007; Schank, Ryabinin, Giardino, Ciccocioppo, & Heilig, 2012).
It is well known the involvement of CRF system on CRF-induced anorexia (Cabanac & Richard, 1995; Ciccocioppo et al., 2002; Ciccocioppo, Martin-Fardon, Weiss, & Massi, 2001; Heinrichs et al., 1996; Heinrichs, Li, & Iyengar, 2001; Heinrichs & Richard, 1999; Richard, Lin, & Timofeeva, 2002) and stress-induced anorexia (Epstein et al., 2016; Harris, 2017; Stengel & Tache, 2014) or hypophagia for palatable food (Cottone et al., 2009; Fedeli et al., 2009; Iemolo et al., 2013; Zorrilla et al., 2004).
In particular CRF1 receptor antagonists have been reported to reduce stress-induced palatable food-seeking (Ghitza, Gray, Epstein, Rice, & Shaham, 2006) and to reduce withdrawal symptoms in conditions of intermittent access to palatable food (Cottone et al., 2009) in rats, and also to reduce palatable food craving and eating in restrained eaters (Epstein et al., 2016).
We recently reported that systemic injections of the CRF1 receptor antagonist R121919 and bed nucleus of the stria terminalis (BNST) injections of the CRF receptor antagonist D-Phe-CRF(12–41) decreased frustration stress-induced binge eating in rats with a history of food restriction (Micioni Di Bonaventura et al., 2014).
These data suggest the possibility that CRF induced modulation of binge eating is not only linked to peripheral corticosteroid hormonal release subsequent to HPA activation but also with the involvement of extra hypothalamic mechanisms.
To explore this possibility, we used an animal model in female rats, in which binge-like eating was elicited by a history of cyclic food restriction and an acute frustration stress (exposure to a familiar palatable food, without being allowed access to it). This stress procedure has face validity as a model of the putative contributions of dieting and negative-valence states, to binge eating in humans (Association, 2013; Mathes, Brownley, Mo, & Bulik, 2009; Sanislow et al., 2010; Treasure, Claudino, & Zucker, 2010; Vannucci et al., 2015). Considering the high prevalence of binge eating in women (Hudson et al., 2007; Swanson et al., 2011), we used female rats, in which binge-like eating behavior varies across the estrus cycle (Alboni et al., 2017; Micioni Di Bonaventura et al., 2017), similarly to women (Culbert, Racine, & Klump, 2016; Edler, Lipson, & Keel, 2007; Klump et al., 2013; Schoofs, Chen, Braunig, Stamm, & Kruger, 2011) and this result increases the validity of the model that can be used in translational studies of the mechanism of binge eating behavior.
Here, using this rat model, we studied the role of CRF system in stress vs non stress conditions, in food restricted vs non food restricted conditions and both stress plus restriction that induce binge eating. We investigate the effect of exogenous corticosterone administration and metyrapone-induced corticosterone synthesis inhibition and the effect of systemic injection of the selective CRF1 antagonist R121919. Finally, guided by the analysis of crh1 receptor (crhr1) mRNA transcripts in the brain, using the non-selective CRF1/2 peptidergic antagonist D-Phe-CRF(12– 41), we evaluated the effect of blockade of CRF receptors in the CeA and the BLA on binge eating.
Method
Experiment 1. Effect of the CRF1 receptor antagonist R121919 or metyrapone on binge eating
We determined the effect of systemic injection of R121919 on frustration stress-induced binge-like eating to compare its effect with that of metyrapone, tested in the same rats.
We used 108 female Sprague-Dawley rats that were divided into 12 groups (n = 9 per group) in a 2 (history of intermittent food restriction: no, yes) x 2 (stress during testing: no, yes) x 3 (R121919 dose: 0, 10, 20 mg/kg) factorial design. We injected the rats with vehicle or R121919, 60 min before of the 2 h palatable food access.
These rats were exposed (or not exposed) for 24 days (d) to three 8-d cycles of food restriction (66% of chow intake on d 1–4 and free feeding on d 5–8 of each cycle) during which they were given access to palatable food (prepared by mixing of Nutella (Ferrero, Italy) chocolate-hazelnut cream, chow and water as described in Supplementary Methods) for 2 h during the light cycle on d 5–6 and 13–14 of the first 2 cycles (total of 4 exposures). On the binge intake test day, we assessed palatable food intake for 2 h immediately after exposure or not to frustration stress (Fig. 1A). This stress procedure was adopted to generate a mild stressful condition triggered by the view of palatable food and causes a significant increase in serum corticosterone levels (C. Cifani et al., 2010; Carlo Cifani, Di Bonaventura, Ciccocioppo, & Massi, 2013; Micioni Di Bonaventura, Vitale, Massi, & Cifani, 2012).
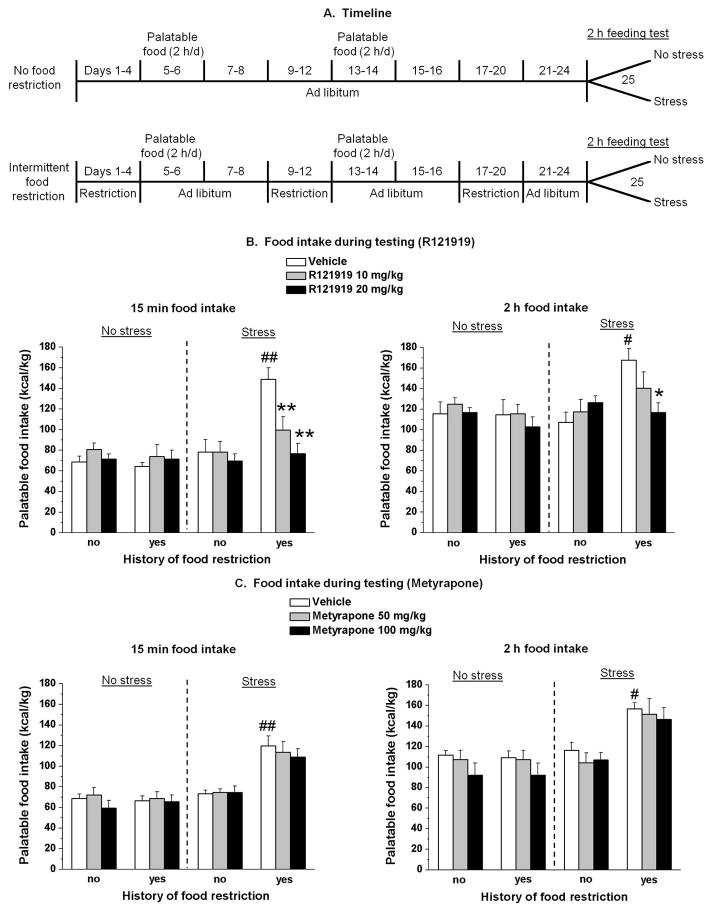
Fig. 1. Experimental timeline, body weight, and feeding test data.
Systemic injections of the CRF1 receptor antagonist R121919 but not metyrapone decreased frustration stress-induced binge eating in rats with a history of intermittent food restriction. (A) Timeline of the experimental procedures for the food non-restricted (top) and restricted (bottom) rats. (B) Mean ± SEM palatable food intake (kcal/kg) during the first 15 min (left) and the cumulative 2 h (right) test session. *p < 0.05; **p < 0.01, different from the vehicle condition; #p < 0.05; ##p < 0.01, different from the other three vehicle groups. n = 6–8 per group. (C) Mean ± SEM palatable food intake (kcal/kg) during the first 15 min (left) and the cumulative 2 h (right) test. #p < 0.05; ##p < 0.01, different from the non-restricted and non-stressed group. n = 6–8 per group.
In previous studies we observed that in the estrous phase of the ovarian cycle, female rats do not exhibit binge eating using our model (Alboni et al., 2017; Micioni Di Bonaventura et al., 2017) while in all other three phases, the rats exhibit binge eating without significant differences in intensity. Therefore, to control for the estrous cycle, immediately after the test on d 25, vaginal smears were collected and analysed under microscope by an experimenter blind to treatment conditions. Data from rats in the estrous phase were not included in the statistical analysis.
As reported in our previous studies (C. Cifani et al., 2009; Micioni Di Bonaventura et al., 2013; Piccoli et al., 2012), after 1 d off at the end of the first test, the same animals received an additional 8-d cycle: the non-restricted groups had 8 d of chow ad libitum, whereas the restricted groups had 4 d chow restricted to 66% of the normal intake followed by 4 d of chow ad libitum. In this additional cycle, all groups did not have access to palatable food. The following day, the stressed groups were exposed to stress while the non-stressed groups were not. On the test day, 1 h before access to palatable food, rats were treated i.p. with Metyrapone (0, 50, 100 mg/kg).
Experiment 2. Effect of corticosterone on rats with a history of repeated cycles of food restriction/refeeding
To assess whether exogenous corticosterone, injected in rats submitted to 3 cycles of food restriction but not exposed to stress evokes binge eating, 63 female rats were used.
They were divided into 2 groups, matched for body weight and daily food intake: 1) non-restricted and not exposed to stress group (n = 27); 2) restricted and not exposed to stress group (n = 27). A third group (restricted and stressed rats, n = 9), treated only with vehicle, was added to compare the binge eating response to that of the other groups treated with corticosterone.
Rats were submitted to 3 consecutive 8-d cycles followed by the final test on d 25 as described in exp. 1.
On the test day (d 25), non-stressed animals were divided into subgroups of 9 rats and treated i.p. with corticosterone (2.5 or 5 mg/kg), or its vehicle, 30 min before access to palatable food.
After 1 d off at the end of the first test, the same animals received an additional 8-d cycle as described above. On the test day, animals were divided into 3 subgroups of 9 rats and treated i.p. with corticosterone (10 or 15 mg/kg), or its vehicle, 30 min before access to palatable food.
Experiment 3. In situ hybridization analysis of crhr1 mRNA transcripts in the BNST, CeA, BLA and paraventricular nucleus (PVN)
For the in situ hybridization experiment, 36 rats were divided into 4 groups (n = 9/group) in a 2 (history of intermittent food restriction: no, yes) x 2 (stress during testing: no, yes) factorial design.
These rats were exposed (or not exposed) for 24 d to 3 8-d cycles of food restriction. On the binge intake test day, they were exposed or not to frustration stress as described above. On d 25, rats were sacrificed by decapitation.
Experiment 4. Effect of CeA and BLA injection of D-Phe-CRF(12– 41) on binge eating
We determined the effect of CeA and BLA injections of the CRF receptor antagonist D-Phe-CRF(12– 41) on binge eating.
For CeA microinjection we used 32 rats that were divided into 4 groups (n = 8/group). Two groups were not exposed to cycles of food restriction and frustration stress and were tested for the effect of D-Phe-CRF(12– 41) (0, 300 ng/side) on palatable food intake during the 2 h test on d 25. Two other groups were exposed to 3 cycles of food restriction and frustration stress and were tested for the effect of D-Phe-CRF(12– 41) (0, 300 ng/side) on stress induced expression of binge eating. We injected D-Phe-CRF(12– 41) 30 min before the 2 h palatable food access (15 min before the beginning of the frustration stress for the stressed groups).
For BLA microinjection we used another 32 rats divided into 4 groups (n = 8/group); the experimental procedure was the same described above for CeA microinjection experiment.
Additional methodological details are contained in Supplementary Methods.
Results
Experiment 1
As in our previous studies (C. Cifani et al., 2010; C. Cifani et al., 2009; Micioni Di Bonaventura, Cifani, et al., 2012; Micioni Di Bonaventura, Vitale, et al., 2012; Pucci et al., 2015), body weight of rats was reduced during the 4 days of food restriction, but immediately afterwards the animals increased their food intake and rapidly recovered their body weight to levels of controls by the end of each cycle. On the test day body weight of animals, as well as their food intake in the previous 24 h, were not significantly different among the groups (data not shown).
Thirty-two rats (of the 108) were excluded from the analysis because of estrous on the test day.
At 15 min, a three-way ANOVA, which included the between-subjects factors of history of intermittent food restriction (no, yes), stress during testing (no, yes), and R121919 dose (0, 10, 20 mg/kg), showed a significant interaction among the three factors [F(2,64) = 3.5, p < 0.05]. Post hoc test showed an increase of palatable food consumption during the first 15 min of the feeding test on day 25 (p < 0.01) (Fig. 1B left panel), only in vehicle rats with a history of intermittent food restriction and frustration stress (binge eating group). Post hoc test revealed also that systemic injections of R121919 in restricted and stressed rats decreased palatable food consumption during the first 15 min of the feeding test (p < 0.01) (Fig. 1B left panel). In contrast, R121919 injections had no effect on palatable food intake in the other three groups (p > 0.05).
ANOVA of the 2 h cumulative palatable food showed a three-way interaction (food restriction, stress and R121919 dose) [F(2,64) = 3.3, p < 0.05]. As shown in Fig. 1B (right panel), post hoc test revealed that 2 h cumulative palatable food intake was significantly increased in the vehicle restricted and stressed group in comparison to the other vehicle groups (p < 0.01). Moreover, post hoc test showed that the treatment with R121919 (20 mg/kg) significantly reduced palatable food intake in restricted and stressed group (p < 0.01) (Fig. 1B, right panel).
In the second test with metyrapone treatment, thirty-one female rats (of the 108 used in the experiment) were excluded from the analysis because of estrous on the test day.
At 15 min, overall ANOVA which included the between-subjects factors of history of intermittent food restriction (no, yes), stress during testing (no, yes), and metyrapone dose (0, 50, 100 mg/kg), showed a significant interaction between food restriction and stress [F(1,66) = 33.7, p < 0.01]. As shown in Fig. 1C (left panel), 15 min palatable food consumption in vehicle rats with a history of food restriction, and exposed to 15 min frustration stress was significantly higher in comparison to the other vehicle groups. Systemic injections of metyrapone did not reduce palatable food intake in any group of rats.
ANOVA of the 2 h cumulative palatable food showed a significant interaction between food restriction and stress [F(1,66) = 14.8, p < 0.01]. As shown in Fig. 1C (right panel), 2 h cumulative palatable food intake was significantly increased only in the vehicle restricted and stressed group in comparison to the other vehicle groups. The treatment with metyrapone did not affect feeding in any group.
Experiment 2
Twenty rats (of the 88 used in the experiment) were excluded from the analysis because of estrous on the test day.
The statistical analysis of the first 15 min palatable food intake, which included the between-subjects factors of history of intermittent food restriction (no, yes), and corticosterone dose (0, 2.5, 5.0 mg/kg), did not show a significant interaction between two factors [F(6,99) = 0.5, p > 0.05] (Fig. 2A left panel).
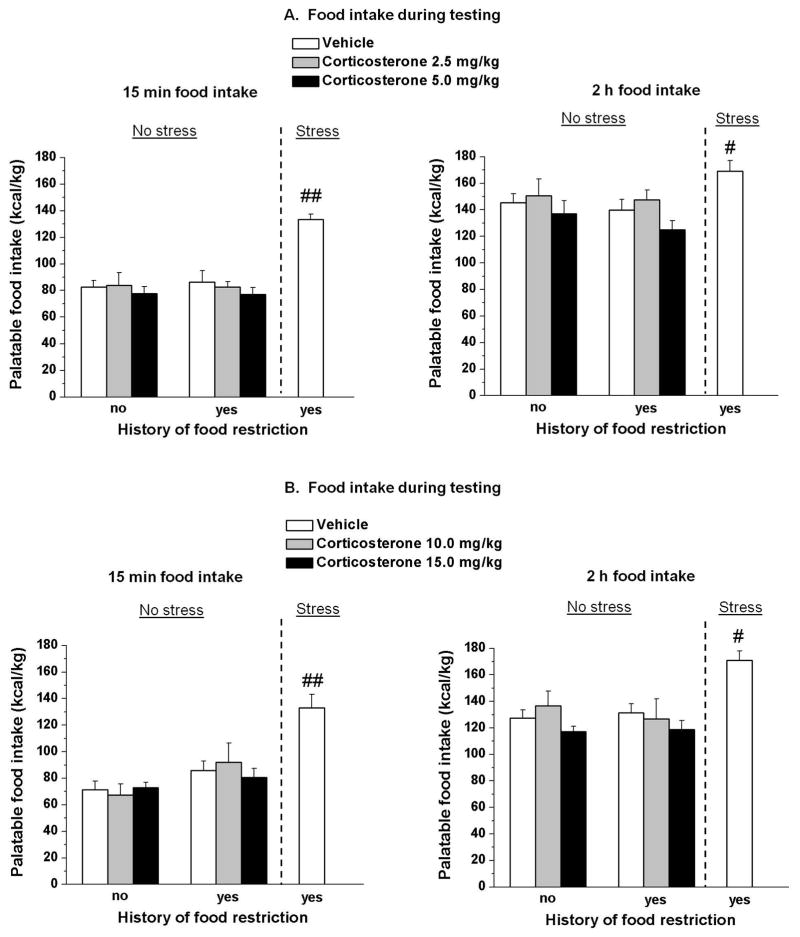
Fig. 2. Systemic injections of Corticosterone.
Systemic injection of corticosterone failed to elicit binge eating. (A) Mean ± SEM palatable food intake during the first 15 min (left) and the cumulative 2 h (right) test session. #p < 0.05; ##p < 0.01, different from the other two vehicle groups. n = 6–8 per group. (B) Mean ± SEM palatable food intake during the first 15 min (left) and the cumulative 2 h (right) test session. #p < 0.05; ##p < 0.01, different from the non-restricted and non-stressed group. n = 6–8 per group.
ANOVA of the 2 h cumulative palatable food did not show a two-way interaction (food restriction, and corticosterone dose) [F(2,33) = 0.13, p > 0.05] (Fig. 2A right panel).
After an additional food restriction/refeeding cycle, higher doses of corticosterone (10, 20 mg/kg) also did not produce significant interactions between the two factors during the first 15 min [F(6,105) = 1.9, p > 0.05] (Fig. 2B, left panel) or cumulatively through the 2 h session [F(2,35) = 0.32, p > 0.05] (Fig. 2B, right panel).
Overall ANOVA comparing the vehicle group (non-restricted and non-stressed; restricted and non-stressed; restricted and stressed) showed a significant group interaction in the first experiment [15 min: F(2,16) = 21.2, p < 0.01] [2 h: F(2,16) = 4.2, p < 0.05] and in the second experiment [15 min: F(2,15) = 15.3, p < 0.01] [2 h: F(2,16) = 13.1, p < 0.01]. Post hoc test showed that the palatable food intake of restricted and stressed rats (binge eating group) treated with vehicle was significantly higher in comparison to the other two vehicle groups both in the first test (15 min (p < 0.05) and cumulative 2 h (p < 0.01)) and in the second test (15 min (p < 0.05) and cumulative 2 h (p < 0.01)).
Experiment 3
For the in situ hybridization twelve rats were not used because of estrous on the test day.
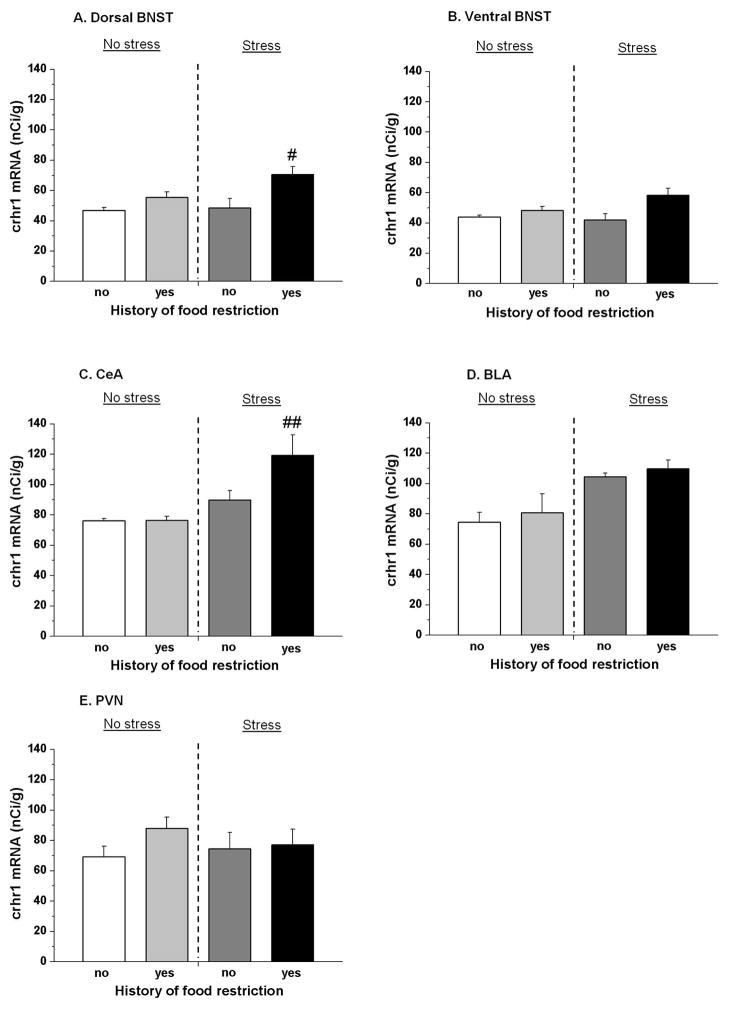
For crhr1 mRNA levels, overall two-way ANOVA with history of intermittent food restriction (no, yes), and stress during testing (no, yes) as between factors, showed a significant interaction in dorsal BNST [F(1,20) = 3.2, p < 0.05], but not in ventral BNST [F(1,20) = 2.9, p > 0.05]. Post-hoc test showed that in dorsal BNST crhr1 mRNA levels were significantly higher (p < 0.05) in restricted and stressed rats compared to the non-restricted and non-stressed rats (Fig. 3A).
Fig. 3. crhr1 mRNA levels.
Restricted and stressed rats showed up-regulation of crh1 receptor mRNA signal in the BNST and CeA but not in BLA or in PVN. (A) crhr1 mRNA levels in dorsal BNST, (B) ventral BNST, (C) CeA, (D) BLA and (E) PVN. #p < 0.05; ##p < 0.01, different from the non-restricted and non-stressed group. n = 6 per group.
In the CeA overall ANOVA revealed also a significant interaction between the two factors of history of intermittent food restriction and stress [F(1,20) = 3.9, p < 0.05]. As shown in Fig. 3C, post hoc test revealed a higher level (p < 0.01) of crhr1 mRNA in restricted and stressed rats compared to the non-restricted and non-stressed rats.
Two-way ANOVA showed that crhr1 mRNA levels in BLA were significantly affected by frustration stress [F(1,20) = 14.5, p < 0.01] but not by restriction [F(1,20) = 0.6, p > 0.05]. No interaction between these two factors was detected [F(1,20) = 0.002, p > 0.05] (Fig. 3D).
Two-way ANOVA showed that crhr1 mRNA levels in PVN were not significantly affected by frustration stress [F(1,20) = 1.4 p > 0.05] nor by restriction [F(1,20) = 0.08, p > 0.05]. No significant interaction between these two factors was detected [F(1,20) = 0.8, p > 0.05] (Fig. 3E).
Experiment 4
Fourteen female rats (of the 64 used in the experiment) were excluded from the statistical analysis because of estrous on the test day.
After histological evaluation for correct cannula placement 9 (3 were already excluded because in the estrous phase) of the 64 rats were excluded from the analysis because of placements outside the CeA or BLA. Representative brains with correct cannula placements in CeA and in BLA are shown in Fig. S1.
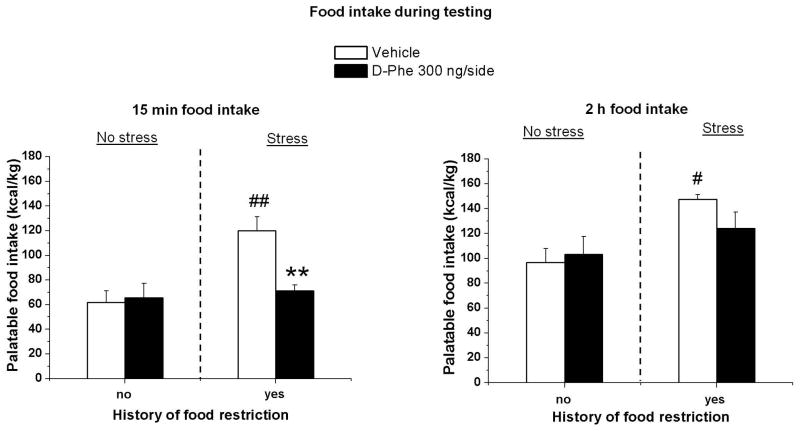
The statistical analysis for microinjection in CeA, which included the between-subjects factors of group condition (non-restricted and non-stressed and restricted and stressed groups) and D-Phe-CRF(12– 41) dose (0, 300 ng/side), showed a significant interaction between the two factors [F(1,19) = 7.5, p < 0.05], at 15 min.
ANOVA of the 2 h cumulative palatable food intake showed a significant interaction between the two factors [F(1,19) = 4.9, p < 0.05].
As shown in Fig. 4, post hoc test revealed a significant increase in palatable food consumption in the restricted and stressed groups during the first 15 min (p < 0.01) and at the end of the test (2 h cumulative food intake) (p < 0.05). Moreover, the treatment with D-Phe-CRF(12– 41) (300 ng/side) significantly reduced palatable food intake only in restricted and stressed group at 15 min (p < 0.01).
Fig. 4. CeA injections of D-Phe-CRF(12-41).
Injection D-Phe-CRF(12– 41) in CeA blocked binge-like eating behaviour. Mean ± SEM palatable food intake during the first 15 min (left) and the cumulative 2 h (right) test session. **p < 0.01, different from the vehicle condition #p < 0.05; ##p < 0.01, different from the other vehicle groups. n = 5–6 per group.
The statistical analysis for microinjection in BLA did not show a significant interaction between the two factors (group condition, and D-Phe dose) at 15 min [F(1,17) = 0.2, p > 0.05] and 2 h [F(1,17) = 0.02, p > 0.05] (data not shown).
Discussion
In previous work we found that systemic injections of the CRF1 receptor antagonist R121919 or intra-BNST administration of the CRF receptor antagonist D-Phe-CRF(12–41) selectively decreased frustration stress-induced binge eating in female rats with a history of food restriction. These compounds did not affect palatable food intake in control rats (Micioni Di Bonaventura et al., 2014).
It is known that binge eating is also associated with stimulation of HPA axis activity and elevation of peripheral corticosteroids (Artiga et al., 2007; C. Cifani et al., 2010; C. Cifani et al., 2009). Activation of CRF1 receptors in the PVN is a primary mechanism responsible for HPA axis activation by stress, whereas at extrahypothalamic sites (i.e, BNST, CeA, BLA etc) CRF1 modulates affective and emotional reactions.
We sought therefore, importantly, to determine whether the effect of CRF1 receptor antagonist on binge eating is linked to their ability to blunt the activity of HPA axis system.
To examine this possibility, we tested the effect of metyrapone, a corticosterone synthesis inhibitor, to inhibit binge eating, but it did not reduce palatable food intake in any experimental group. To confirm this finding, after three cycles of food restriction and refeeding, we replaced the frustration stress with multiple concentrations of corticosterone, but it failed to elicit binge eating. Taken together, these findings do not support a critical role for HPA axis in the expression of this food-related maladaptive behavior in female rats. Then we focused our attention to the regulation of CRF1 receptors at extrahypothalamic sites: we determined expression of the crhr1 mRNA in restricted and stressed rats showing binge eating behavior or their controls. Data revealed a significant up-regulation of crhr1 mRNA levels in the dorsal portion of the BNST and in the CeA only in restricted and stressed rats, whereas no changes were detected in the BLA and in the PVN in any groups of rats.
Lack of changes in the PVN provides a further confirmation for a limited role of hypothalamic CRF1 receptor mediated mechanisms in binge eating expression, whereas it is extremely interesting to observe over expression of the of crhr1 transcripts both in the BNST and CeA. Considering these in situ hybridization data, nonselective CRF receptor antagonist D-Phe-CRF(12–41) was administered into the CeA and the BLA. Blockade of CRF receptors in the CeA decreased binge eating in restricted and stressed rats, whereas no effect was detected in the BLA. In this work we focused on the amygdala, because we already reported the important role of BNST in the expression of binge eating (Micioni Di Bonaventura et al., 2014): we found an enhancement of c-fos levels in binge female rats in this brain region and the D-Phe-CRF(12–41) administration completely blocked this behavior selectively in restricted and stressed rats.
The amygdala and BNST are brain regions anatomically and functionally connected (de Olmos & Heimer, 1999) and the amygdala is known to have CRF-containing projection to the BNST (Erb, Salmaso, Rodaros, & Stewart, 2001) and in particular CeA has emerged as an important area in the regulation of excessive consumption of palatable food (Blasio et al., 2013; de Olmos & Heimer, 1999; Iemolo et al., 2013; Micioni Di Bonaventura et al., 2017). The observation that blockade of CRF receptors in these brain areas completely reversed stress-induced binge eating in our model indicates a critical role of extrahypothalamic CRF systems. Moreover, both CRF antagonists tested, centrally and peripherally injected, selectively blocked frustration stress-induced binge eating after a history of food restriction, without affecting only stressed or only restricted rats.
In this regard it is also important to consider that the effect of CRF1 receptor antagonism on binge eating, appears to be unrelated to the regulation of satiety mechanism or caloric intake, as we detected an effect only when consumption was triggered by stress and associated with a loss of control. This finding is in agreement with Parylak et al. (2012) report, showing that systemic injections of R121919 has no effect on binge-like eating in female rats not exposed to stress in a different binge-eating model (Parylak, Cottone, Sabino, Rice, & Zorrilla, 2012). Taking into account this selective effect, it is also possible to exclude with these compounds and these doses malaise and taste aversion, that CRF antagonism can produce (Heinrichs et al., 1998) and influence satiety or feeding behavior per se.
This work extends to our previous results on the role of CRF receptors and supports further investigation to understand how CRF antagonists block feeding consumption in different animal conditions, such as intermittent access to chocolate flavored, high-sucrose diet (Cottone et al., 2009) or reduce stress-induced reinstatement of palatable food seeking (Ghitza et al., 2006).
Moreover, in humans, promising anti-craving properties were exhibited in the presence of the CRF1 antagonist, pexacerfont, especially in the presence of palatable food in restrained eaters (Gold, Frost-Pineda, & Jacobs, 2003). Unfortunately, this clinical study was interrupted due to only an administrative interpretation of US federal law and not for the course of study.
In conclusion, the results of the present study do not support a direct role of the HPA axis and corticosterone in the expression of binge eating in female rats. Eating disorders share important commonalities with substance abuse (Avena et al., 2011; Shepard, Barron, & Myers, 2000), for example, high circulating levels of glucocorticoids can sensitize the CRF systems in extrahypothalamic sites such as the CeA and norepinephrine systems in the BLA, that are known to be involved in behavioral responses to stressors (Imaki, Nahan, Rivier, Sawchenko, & Vale, 1991; Shepard et al., 2000). Thus, it may be hypothesized that activation of the HPA axis may lead to subsequent activation of extrahypothalamic brain stress systems, as described for drug addiction (G. F. Koob & Le Moal, 2005; G. Koob & Kreek, 2007; Kreek & Koob, 1998), and that CRF1 receptors may be involved in these effects.
Conclusion
The findings of the present study provide clear evidence that CRF is involved in the binge eating response to stress and food restrictions in female rats, and the crucial role of CRF receptors in BNST and CeA; this effect may be related to extrahypothalamic effects of CRF and CRF1 receptor antagonists may represent interesting tools for the pharmacotherapy of bingeing-related eating disorders.
Supplementary Material
Cannula placement (CeA and BLA).
Acknowledgments
The work was supported by the Italian Ministry of University and Research under grants FIRB-RBFR12DELS and PRIN-2012JTX3KL to CC. The work of the Drug Design and Synthesis Section, MTMDB, NIDA, and NIAAA was supported by the NIH Intramural Research Programs of the National Institute on Drug Abuse (NIDA) and the National Institute of Alcohol Abuse and Alcoholism (NIAAA). L’Oreal Italia per le Donne e la Scienza covered the post-doctoral fellowship of MVMDB.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
References
- Alboni S, Micioni Di Bonaventura MV, Benatti C, Giusepponi ME, Brunello N, Cifani C. Hypothalamic expression of inflammatory mediators in an animal model of binge eating. Behav Brain Res. 2017;320:420–430. doi: 10.1016/j.bbr.2016.10.044. [DOI] [PubMed] [Google Scholar]
- Artiga AI, Viana JB, Maldonado CR, Chandler-Laney PC, Oswald KD, Boggiano MM. Body composition and endocrine status of long-term stress-induced binge-eating rats. Physiol Behav. 2007;91(4):424–431. doi: 10.1016/j.physbeh.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association, American Psychiatric. Diagnostic and statistical manual of mental disorders (DSM-5®) American Psychiatric Pub; 2013. [Google Scholar]
- Avena NM. Food and addiction: implications and relevance to eating disorders and obesity. Curr Drug Abuse Rev. 2011;4(3):131–132. doi: 10.2174/1874473711104030131. [DOI] [PubMed] [Google Scholar]
- Avena NM, Bocarsly ME, Hoebel BG, Gold MS. Overlaps in the nosology of substance abuse and overeating: the translational implications of “food addiction”. Curr Drug Abuse Rev. 2011;4(3):133–139. doi: 10.2174/1874473711104030133. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Blasio A, Iemolo A, Sabino V, Petrosino S, Steardo L, Rice KC, … Cottone P. Rimonabant precipitates anxiety in rats withdrawn from palatable food: role of the central amygdala. Neuropsychopharmacology. 2013;38(12):2498–2507. doi: 10.1038/npp.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanac M, Richard D. Acute intraventricular CRF lowers the hoarding threshold in male rats. Physiol Behav. 1995;57(4):705–710. doi: 10.1016/0031-9384(94)00322-x. [DOI] [PubMed] [Google Scholar]
- Christiansen AM, Dekloet AD, Ulrich-Lai YM, Herman JP. “Snacking” causes long term attenuation of HPA axis stress responses and enhancement of brain FosB/deltaFosB expression in rats. Physiol Behav. 2011;103(1):111–116. doi: 10.1016/j.physbeh.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Biondini M, Antonelli L, Wichmann J, Jenck F, Massi M. Reversal of stress- and CRF-induced anorexia in rats by the synthetic nociceptin/orphanin FQ receptor agonist, Ro 64-6198. Psychopharmacology (Berl) 2002;161(2):113–119. doi: 10.1007/s00213-002-1020-7. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F, Massi M. Nociceptin/orphanin FQ inhibits stress- and CRF-induced anorexia in rats. Neuroreport. 2001;12(6):1145–1149. doi: 10.1097/00001756-200105080-00019. [DOI] [PubMed] [Google Scholar]
- Cifani C, Micioni Di BMv, Vitale G, Ruggieri V, Ciccocioppo R, Massi M. Effect of salidroside, active principle of Rhodiola rosea extract, on binge eating. Physiol Behav. 2010;101(5):555–562. doi: 10.1016/j.physbeh.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Cifani C, Polidori C, Melotto S, Ciccocioppo R, Massi M. A preclinical model of binge eating elicited by yo-yo dieting and stressful exposure to food: effect of sibutramine, fluoxetine, topiramate, and midazolam. Psychopharmacology (Berl) 2009;204(1):113–125. doi: 10.1007/s00213-008-1442-y. [DOI] [PubMed] [Google Scholar]
- Cifani Carlo, Di Bonaventura, Micioni Maria Vittoria, Ciccocioppo Roberto, Massi Maurizio. Binge eating in female rats induced by yo-yo dieting and stress. Animal Models of Eating Disorders. 2013:27–49. [Google Scholar]
- Corwin RL, Grigson PS. Symposium overview--Food addiction: fact or fiction? J Nutr. 2009;139(3):617–619. doi: 10.3945/jn.108.097691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, … Zorrilla EP. CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci U S A. 2009;106(47):20016–20020. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho WF, Moreira RO, Spagnol C, Appolinario JC. Does binge eating disorder alter cortisol secretion in obese women? Eat Behav. 2007;8(1):59–64. doi: 10.1016/j.eatbeh.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Culbert KM, Racine SE, Klump KL. Hormonal Factors and Disturbances in Eating Disorders. Curr Psychiatry Rep. 2016;18(7):65. doi: 10.1007/s11920-016-0701-6. [DOI] [PubMed] [Google Scholar]
- D’Addario C, Micioni Di Bonaventura MV, Pucci M, Romano A, Gaetani S, Ciccocioppo R, … Maccarrone M. Endocannabinoid signaling and food addiction. Neurosci Biobehav Rev. 2014;47:203–224. doi: 10.1016/j.neubiorev.2014.08.008. [DOI] [PubMed] [Google Scholar]
- de Olmos JS, Heimer L. The concepts of the ventral striatopallidal system and extended amygdala. Ann N Y Acad Sci. 1999;877:1–32. doi: 10.1111/j.1749-6632.1999.tb09258.x. [DOI] [PubMed] [Google Scholar]
- Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychol Med. 2007;37(1):131–141. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26(1):37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Kennedy AP, Furnari M, Heilig M, Shaham Y, Phillips KA, Preston KL. Effect of the CRF1-receptor antagonist pexacerfont on stress-induced eating and food craving. Psychopharmacology (Berl) 2016;233(23–24):3921–3932. doi: 10.1007/s00213-016-4424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158(4):360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Fedeli A, Braconi S, Economidou D, Cannella N, Kallupi M, Guerrini R, … Ciccocioppo R. The paraventricular nucleus of the hypothalamus is a neuroanatomical substrate for the inhibition of palatable food intake by neuropeptide S. Eur J Neurosci. 2009;30(8):1594–1602. doi: 10.1111/j.1460-9568.2009.06948.x. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front Neuroendocrinol. 2007;28(1):1–27. doi: 10.1016/j.yfrne.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman LM, Gil KM. Daily stress, coping, and dietary restraint in binge eating. Int J Eat Disord. 2004;36(2):204–212. doi: 10.1002/eat.20012. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychopharmacology. 2006;31(10):2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck ME, Geliebter A, Hung J, Yahav E. Cortisol, hunger, and desire to binge eat following a cold stress test in obese women with binge eating disorder. Psychosom Med. 2004;66(6):876–881. doi: 10.1097/01.psy.0000143637.63508.47. [DOI] [PubMed] [Google Scholar]
- Gold Mark S, Frost-Pineda Kimberly, Jacobs William S. Overeating, binge eating, and eating disorders as addictions. Psychiatric Annals. 2003;33(2):117–122. [Google Scholar]
- Gray TS. Amygdaloid CRF pathways. Role in autonomic, neuroendocrine, and behavioral responses to stress. Ann N Y Acad Sci. 1993;697:53–60. doi: 10.1111/j.1749-6632.1993.tb49922.x. [DOI] [PubMed] [Google Scholar]
- Harris RB. Repeated restraint stress lowers the threshold for response to third ventricle CRF administration. Horm Behav. 2017;89:64–68. doi: 10.1016/j.yhbeh.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Klaassen A, Koob GF, Schulteis G, Ahmed S, De Souza EB. Corticotropin-releasing factor receptor blockade enhances conditioned aversive properties of cocaine in rats. Psychopharmacology (Berl) 1998;136(3):247–255. doi: 10.1007/s002130050563. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Lapsansky J, Behan DP, Chan RK, Sawchenko PE, Lorang M, … De Souza EB. Corticotropin-releasing factor-binding protein ligand inhibitor blunts excessive weight gain in genetically obese Zucker rats and rats during nicotine withdrawal. Proc Natl Acad Sci U S A. 1996;93(26):15475–15480. doi: 10.1073/pnas.93.26.15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Li DL, Iyengar S. Corticotropin-releasing factor (CRF) or CRF binding-protein ligand inhibitor administration suppresses food intake in mice and elevates body temperature in rats. Brain Res. 2001;900(2):177–185. doi: 10.1016/s0006-8993(01)02286-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Richard D. The role of corticotropin-releasing factor and urocortin in the modulation of ingestive behavior. Neuropeptides. 1999;33(5):350–359. doi: 10.1054/npep.1999.0047. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemolo A, Blasio A, St Cyr SA, Jiang F, Rice KC, Sabino V, Cottone P. CRF-CRF1 receptor system in the central and basolateral nuclei of the amygdala differentially mediates excessive eating of palatable food. Neuropsychopharmacology. 2013;38(12):2456–2466. doi: 10.1038/npp.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991;11(3):585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13(5):635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzig KP, Hargrave SL, Honors MA. Binge-type eating attenuates corticosterone and hypophagic responses to restraint stress. Physiol Behav. 2008;95(1–2):108–113. doi: 10.1016/j.physbeh.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Racine SE, Burt SA, Neale M, Sisk CL, … Hu JY. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. J Abnorm Psychol. 2013;122(1):131–137. doi: 10.1037/a0029524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8(11):1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164(8):1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51(1–2):23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Mathes WF, Brownley KA, Mo X, Bulik CM. The biology of binge eating. Appetite. 2009;52(3):545–553. doi: 10.1016/j.appet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micioni Di Bonaventura MV, Ciccocioppo R, Romano A, Bossert JM, Rice KC, Ubaldi M, … Cifani C. Role of bed nucleus of the stria terminalis corticotrophin-releasing factor receptors in frustration stress-induced binge-like palatable food consumption in female rats with a history of food restriction. J Neurosci. 2014;34(34):11316–11324. doi: 10.1523/JNEUROSCI.1854-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micioni Di Bonaventura MV, Cifani C, Lambertucci C, Volpini R, Cristalli G, Massi M. A2A adenosine receptor agonists reduce both high-palatability and low-palatability food intake in female rats. Behav Pharmacol. 2012;23(5–6):567–574. doi: 10.1097/FBP.0b013e3283566a60. [DOI] [PubMed] [Google Scholar]
- Micioni Di Bonaventura MV, Lutz TA, Romano A, Pucci M, Geary N, Asarian L, Cifani C. Estrogenic suppression of binge-like eating elicited by cyclic food restriction and frustrative-nonreward stress in female rats. Int J Eat Disord. 2017;50(6):624–635. doi: 10.1002/eat.22687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micioni Di Bonaventura MV, Ubaldi M, Liberati S, Ciccocioppo R, Massi M, Cifani C. Caloric restriction increases the sensitivity to the hyperphagic effect of nociceptin/orphanin FQ limiting its ability to reduce binge eating in female rats. Psychopharmacology (Berl) 2013;228(1):53–63. doi: 10.1007/s00213-013-3013-0. [DOI] [PubMed] [Google Scholar]
- Micioni Di Bonaventura MV, Vitale G, Massi M, Cifani C. Effect of Hypericum perforatum Extract in an Experimental Model of Binge Eating in Female Rats. J Obes. 2012;2012:956137. doi: 10.1155/2012/956137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parylak SL, Cottone P, Sabino V, Rice KC, Zorrilla EP. Effects of CB1 and CRF1 receptor antagonists on binge-like eating in rats with limited access to a sweet fat diet: lack of withdrawal-like responses. Physiol Behav. 2012;107(2):231–242. doi: 10.1016/j.physbeh.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli L, Micioni Di Bonaventura MV, Cifani C, Costantini VJ, Massagrande M, Montanari D, … Corsi M. Role of orexin-1 receptor mechanisms on compulsive food consumption in a model of binge eating in female rats. Neuropsychopharmacology. 2012;37(9):1999–2011. doi: 10.1038/npp.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polivy J, Zeitlin SB, Herman CP, Beal AL. Food restriction and binge eating: a study of former prisoners of war. J Abnorm Psychol. 1994;103(2):409–411. doi: 10.1037//0021-843x.103.2.409. [DOI] [PubMed] [Google Scholar]
- Pucci M, Micioni Di Bonaventura MV, Giusepponi ME, Romano A, Filaferro M, Maccarrone M, … D’Addario C. Epigenetic regulation of nociceptin/orphanin FQ and corticotropin-releasing factor system genes in frustration stress-induced binge-like palatable food consumption. Addict Biol. 2015 doi: 10.1111/adb.12303. [DOI] [PubMed] [Google Scholar]
- Richard D, Lin Q, Timofeeva E. The corticotropin-releasing factor family of peptides and CRF receptors: their roles in the regulation of energy balance. Eur J Pharmacol. 2002;440(2–3):189–197. doi: 10.1016/s0014-2999(02)01428-0. [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, … Cuthbert BN. Developing constructs for psychopathology research: research domain criteria. J Abnorm Psychol. 2010;119(4):631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Schank JR, Ryabinin AE, Giardino WJ, Ciccocioppo R, Heilig M. Stress-related neuropeptides and addictive behaviors: beyond the usual suspects. Neuron. 2012;76(1):192–208. doi: 10.1016/j.neuron.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoofs N, Chen F, Braunig P, Stamm T, Kruger S. Binge eating disorder and menstrual cycle in unmedicated women with bipolar disorder. J Affect Disord. 2011;129(1–3):75–78. doi: 10.1016/j.jad.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000;861(2):288–295. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- Stengel A, Tache Y. CRF and urocortin peptides as modulators of energy balance and feeding behavior during stress. Front Neurosci. 2014;8:52. doi: 10.3389/fnins.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson SA, Crow SJ, Le Grange D, Swendsen J, Merikangas KR. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry. 2011;68(7):714–723. doi: 10.1001/archgenpsychiatry.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treasure J, Claudino AM, Zucker N. Eating disorders. Lancet. 2010;375(9714):583–593. doi: 10.1016/S0140-6736(09)61748-7. [DOI] [PubMed] [Google Scholar]
- Vannucci A, Nelson EE, Bongiorno DM, Pine DS, Yanovski JA, Tanofsky-Kraff M. Behavioral and neurodevelopmental precursors to binge-type eating disorders: support for the role of negative valence systems. Psychol Med. 2015;45(14):2921–2936. doi: 10.1017/S003329171500104X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci. 2012;11:1–24. doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- Wang L, Goebel-Stengel M, Stengel A, Wu SV, Ohning G, Tache Y. Comparison of CRF-immunoreactive neurons distribution in mouse and rat brains and selective induction of Fos in rat hypothalamic CRF neurons by abdominal surgery. Brain Res. 2011;1415:34–46. doi: 10.1016/j.brainres.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Reinhardt LE, Valdez GR, Inoue K, Rivier JE, Vale WW, Koob GF. Human urocortin 2, a corticotropin-releasing factor (CRF)2 agonist, and ovine CRF, a CRF1 agonist, differentially alter feeding and motor activity. J Pharmacol Exp Ther. 2004;310(3):1027–1034. doi: 10.1124/jpet.104.068676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cannula placement (CeA and BLA).