Abstract
Background
This study was designed to assess the ability of fed male Dermacentor reticulatus ticks to transmit Babesia canis to dogs after being detached from previous canine or ovine hosts.
Methods
The study was an exploratory, parallel group design conducted in two trials. All the animals were sero-negative for babesiosis prior to enrolment. In a first trial, donor dogs and donor sheep were infested with Babesia canis infected male and uninfected female ticks for 72 h. The ticks were detached and the second group of host dogs were infested for 24 h before tick removal. In a second trial, the experiment was repeated but the donor animals were infested for 88 h and the second group of host dogs were infested for 8 h prior to tick removal. After infestation, the dogs were maintained under clinical surveillance and blood samples were collected for blood smear, IFA and PCR analysis. A dog was considered infected if any of these tests were positive.
Results
All of the dogs (6 out of 6) were infected after being exposed to pre-activated male ticks for 24 h. Half of the dogs were infected after being exposed to pre-activated ticks for 8 h: 1 out of 3 dogs infested with ticks removed from sheep and 2 out of 3 dogs infested with ticks removed from dog. All the infected dogs were positive to blood smear, IFA and PCR. Three of these dogs exhibited elevated body temperature (> 39.4 °C).
Conclusions
This study demonstrates the ability of male D. reticulatus to transmit B. canis to dogs. The study also illustrates for the first time that, regardless of the first host on which ticks may attach and start feeding, Babesia canis can be transmitted to dogs within 8 h of infestation. Since no minimal transmission time can be established for all possible natural situations, a strategy of prevention based on anti-attachment or repellency is recommended.
Keywords: Infection, Tick-borne disease, Babesiosis, Piroplasmosis, Companion animals
Background
Babesia canis is a protozoan pathogen infecting red blood cells of canids after being transmitted by ticks. The ornate dog tick, Dermacentor reticulatus is the main competent vector tick species of this pathogen and it is widely distributed in Europe [1]. Because of the 48 h minimal duration required for sporogony [2, 3], Babesia spp. are generally considered as pathogens with a slow transmission compared to other tick-borne pathogens like Powassan virus, for which a transmission within 15 min of tick attachment in rodents was documented [4]. No minimal transmission time of Babesia spp. has been determined so far in dogs. In hamsters, transmission of B. microti by Ixodes dammini was reported to be more successful after 54 h of attachment than after 36 or 48 h [5]. Consequently, recent models used to assess the efficacy of parasiticides or repellents against the transmission of Babesia spp. in dogs allow the adult ticks to feed on the animals for at least 4 days [6]. However, faster transmission of tick-borne pathogens can occur in various situations such as partially-engorged ticks questing [7, 8] or younger life-stages initiating feeding faster [9]. The frequency of these situations is unknown, but tick movements were documented after attachment of Rhipicephalus sanguineus between co-housed dogs [10] and partially-engorged questing Ixodes ricinus nymphs were observed in the field [11]. For Ehrlichia canis, intrastadial transmission by male R. sanguineus during multiple feedings on dogs was demonstrated [12]. For Babesia spp., immediate transmission after a partial blood meal of moving male D. reticulatus ticks has been suggested [13] but never documented. The vectorial competency of male D. reticulatus is also unknown. This study was therefore designed to investigate the ability of partially-fed male D. reticulatus ticks to transmit B. canis to dogs within 24 or 8 h of infestation after being detached from a previous host.
Methods
The study was an exploratory, parallel group design, randomized, unicentre trial that was approved by an ethics committee prior to commencement. The study was performed in two sequential steps (experiments 1 and 2) and the design and schedule are described in Fig. 1.
Fig. 1.
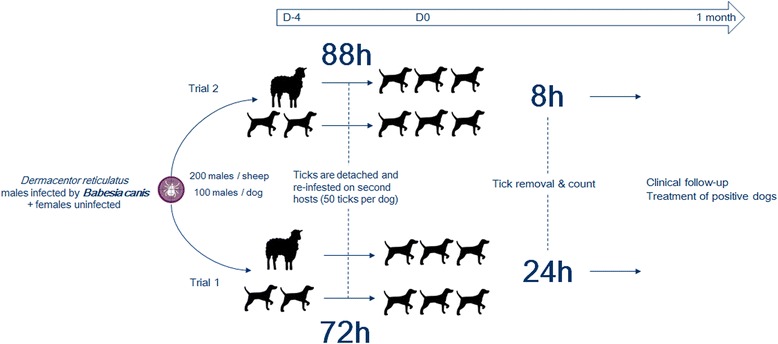
Experimental design
Animals
Each experiment of the study was conducted on one adult male Merino sheep (group 1, tick host donor) and 3 groups of adult mongrel and Beagle dogs (n = 8). All the animals were acclimated to their environment for at least 7 days prior to the start of the study. The sheep were individually identified with a numbered ear tag and were fed hay and a commercial pelleted maintenance concentrate. The sheep were kept individually. The dogs were individually identified by a microchip and were fed commercial dog food once daily. Water was available ad libitum for all the animals. From day -7 to day 8, the dogs were single housed in an indoor kennel. Starting from day 9, the dogs were housed in an outdoor kennel and communally housed within their study groups. All the animals were tested sero-negative for B. canis on day -7. In each experiment, the dogs were allocated to groups 2 (n = 2, tick donor), 3 (n = 3) or 4 (n = 3) according to gender and body weight. Clinical examinations were scheduled on dogs on study days -7, 7, 14, 21 and 28. General health observations were performed on a daily basis for the duration of the study.
Ticks and interrupted feeding
An Irish strain of D. reticulatus, enriched with ticks sampled from the Netherlands, was used in this study. All the ticks were adults, 4 months and 5.5 months since the molt, and bred in laboratory conditions. Only the male ticks used for infestation were infected with B. canis. These male ticks were taken from a batch of ticks infected with B. canis by feeding adult ticks until repletion on a dog with confirmed acute babesiosis. These ticks were subsequently used to propagate a next generation of infected adult ticks. An aliquot of 50 individual male ticks was tested for B. canis DNA by PCR to determine the percentage infection.
The sheep (group 1) were infested with 200 (± 16) male D. reticulatus infected with B. canis and 100 (± 8) female uninfected D. reticulatus. The ticks were kept on the sheep in a bag sealed on an area of shaved skin. The dogs (group 2) were infested with 100 (± 8) male D. reticulatus infected with B. canis and 50 (± 4) female uninfected D. reticulatus. The ticks were released on the dogs under light sedation (Domitor®, intramuscular injection at 0.06 ml/kg BW) and maintained in a chamber for up to 1 h after infestation. Tick feeding was interrupted on study day 0, by tick removal with forceps from group 1 and 2 hosts. In experiment 1, tick feeding was interrupted 72 ± 1 h after infestation. In experiment 2, tick feeding was interrupted 88 ± 1 h after infestation. Special care was taken to avoid damage to ticks during removal. At removal from each individual animal, the ticks were counted and categorised as live or dead, attached or free. The sheep from group 1 were euthanized. After tick removal, the dogs from group 2 were treated with diminazine aceturate (Berenil RTU, intramuscular injection of 1.0 ml/20 kg BW) on day 0 and imidocarb dipropionate (Forray 65, subcutaneous injection of 1.2 ml/20 kg BW) against B. canis infection.
Tick challenges
Within 2 h of the tick removal, the dogs from group 3 were infested with ticks taken from sheep (group 1) and the dogs from group 4 were infested with ticks taken from dogs (group 2). Only the male ticks found attached on the groups 1 and 2 animals were used to infest the dogs in groups 3 and 4. Each dog from groups 3 and 4 was exposed individually to 50 ± 3 male ticks infected with B. canis and 25 female D. reticulatus uninfected ticks on day 0 of the study. The ticks were released on the dogs under light sedation (Domitor®, intramuscular injection at 0.06 ml/kg BW) and maintained in an chamber for up to 1 h after infestation. In experiment 1, tick removal occurred 24 ± 1 h after infestation. In experiment 2, tick removal occurred 8 h ± 15 min after infestation. At removal from each individual animal, the ticks were counted and categorised as live or dead, attached or free.
Monitoring for babesiosis
Prior to day 0, blood samples were collected for PCR and for serum analysis from dogs in groups 3 and 4. Any abnormal sign reported from daily observations and scheduled veterinary clinical examination led to further inspection of the animals. Rectal body temperature of dogs from groups 3 and 4 was measured at least 3 times per week from day 5 to 28 and at each clinical examination. Animals with abnormally high temperature (> 39.4 °C) or with clinical signs commonly associated with babesiosis were examined and 2 blood smears modified-giemsa stained (Differential Quik Stain Kit, Kyron Laboratories (PTY) Ltd., Johannesburg, South Africa) were assessed for B. canis merozoites. If a dog was positive for B. canis on a blood smear, approximately 3 ml of whole blood was collected in EDTA tubes for PCR analysis [6] targeting 18S rRNA ITS-1 gene regions [14] prior to treatment. The dogs positive for B. canis were immediately treated with diminazine aceturate (Berenil RTU, intramuscular injection of 1.0 ml/20 kg BW) followed 24 h later by imidocarb dipropionate (Forray 65, subcutaneous injection of 1.2 ml/20 kg BW). The dogs were also administered prednisolone 1% (1 ml/10 kg BW id 3 days) and a vitamin mix (Kyro B + Liver, 2 ml sc id 3 days). For serologic analysis, approximately 3 ml of blood sample from all dogs in groups 3 and 4 were collected in dry tubes on days 0 (prior to tick infestation), 14, 21 and 28. During experiment 1, two additional blood samples were collected from 2 dogs (groups 3 and 4) on days 35 and 42. Serum was recovered after centrifugation of blood (25 °C, 3000× rpm for 10 min). Serum analysis for B. canis antibodies was performed using immunofluorescence antibody assay (IFA, Megaflow® Babesia canis; 1/50 dilution).
Statistics
Considering the small sample size, no statistical analysis was conducted and the individual data were reported.
Guidelines
This study was carried in compliance with Good Clinical Practice requirements [15].
Results
24 h transmission (experiment 1)
The BW of dogs on day -7 ranged from 12.7 to 20.3 kg. The proportion of infected male D. reticulatus ticks was 16.3% (8 positive ticks out of 49). None of the male ticks (n = 206, live attached) were found free or dead on the donor sheep after 72 h of infestation (Table 1). Live male (58–84) and female (21–38) ticks were found successfully attached on donor dogs (group 2). Live male ticks (12–46 per dog) were found attached on all dogs in groups 3 and 4. The average number of live male tick per dog was 33.5 ± 12.9. All the dogs from groups 3 and 4 were positive to all tests for babesiosis (blood smear, PCR and IFAT) and infected by B. canis (Table 2). Within 5 to 6 days after being exposed to infected ticks, all the dogs started to exhibit clinical signs typical of babesiosis: enlarged lymph nodes, pale mucous membranes, splenomegaly, panting. The body temperature did not increase above the threshold of 39.4 °C in 4 out of the 6 positive dogs.
Table 1.
Tick counts and category on animals
| Experiment | Group | Animal | Live male free | Live male attached | Live female free | Live female attached | Dead male | Dead female |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | CVS2087 | 0 | 206 | 0 | 90 | 0 | 6 |
| 2 | 86A86D | 0 | 58 | 0 | 21 | 2 | 0 | |
| B2B7D0 | 0 | 84 | 0 | 38 | 0 | 0 | ||
| 3 | 5BBA23 | 0 | 34 | 0 | 20 | 10 | 1 | |
| 5D39FA | 0 | 41 | 0 | 19 | 1 | 0 | ||
| E181CA | 0 | 43 | 0 | 11 | 2 | 0 | ||
| 4 | 5E0CAF | 0 | 12 | 0 | 13 | 8 | 2 | |
| CC1369 | 0 | 46 | 0 | 20 | 1 | 0 | ||
| EA19B5 | 0 | 25 | 0 | 15 | 16 | 3 | ||
| 2 | 1 | CVS2540 | 0 | 192 | 3 | 89 | 1 | 1 |
| 2 | 2892BE | 0 | 63 | 0 | 32 | 6 | 2 | |
| 5C6EF0 | 0 | 78 | 0 | 30 | 3 | 2 | ||
| 3 | 2851BE | 0 | 10 | 0 | 12 | 1 | 0 | |
| 5C81F4 | 0 | 29 | 0 | 7 | 2 | 1 | ||
| 697FD6 | 0 | 37 | 0 | 13 | 4 | 2 | ||
| 4 | 1FBF12 | 0 | 21 | 0 | 11 | 0 | 2 | |
| 5BF6DC | 0 | 23 | 0 | 13 | 8 | 4 | ||
| B2CA65 | 0 | 29 | 0 | 14 | 1 | 1 |
Table 2.
Babesiosis clinical signs and determination
| Experiment | Group | Animal | Body temperature (°C, min-max) | Days with temperature > 39.4 °C | Positive blood smear (day) | Positive IFA (day) | Positive PCR (day) |
|---|---|---|---|---|---|---|---|
| 1 | 3 | 5BBA23 | 38.1–40.1 | 5 | 5 | 28 | 5 |
| 5D39FA | 38.0–39.3 | None | 6 | 42 | 6 | ||
| E181CA | 37.4–38.9 | None | 6 | 28 | 6 | ||
| 4 | 5E0CAF | 37.8–39.9 | 5, 23 | 5 | 28 | 5 | |
| CC1369 | 37.3–38.6 | None | 6 | 35, 42 | 6 | ||
| EA19B5 | 37.5–38.6 | None | 6 | 28 | 6 | ||
| 2 | 3 | 2851BE | 38.0–38.7 | None | – | – | – |
| 5C81F4 | 37.2–39.1 | None | 7 | 14, 21, 28 | 7 | ||
| 697FD6 | 38.9–39.6 | 5, 19, 26 | – | – | – | ||
| 4 | 1FBF12 | 37.8–38.6 | None | 7 | 14, 21, 28 | 7 | |
| 5BF6DC | 37.8–38.4 | None | – | – | – | ||
| B2CA65 | 38.3–38.9 | None | 7 | 14, 21, 28 | 7 |
8 h transmission (experiment 2)
The BW of dogs on day -7 ranged from 12.0 to 21.4 kg. The proportion of infected male D. reticulatus ticks was 13.7% (7 positive ticks out of 51). Only one dead male tick and 192 live male attached ticks were found on the donor sheep 88 h after infestation (Table 1). Live male (63–78) and female (30–32) ticks were found successfully attached on donor dogs (group 2). Live male ticks (10–37) were found attached on all dogs in groups 3 and 4. The average number of live male tick per dog was 24.8 ± 9.2. One dog from group 3 and two dogs from group 4 were positive to all tests for babesiosis (blood smear, PCR and IFAT) and therefore infected with B. canis (Table 2). Within 7 days after being exposed to infected ticks, these dogs started to exhibit clinical signs such as vomiting, tense abdomen, listless, panting. The body temperature did not increase above the threshold in any of the infected dogs.
Discussion
Methodological considerations
Ticks successfully re-attached to dogs after the interrupted feeding on the first hosts and the transmission of the pathogens occurred whatever the host species (dog or sheep) used as a tick host donor. The tick attachment rate of male ticks was numerically higher on sheep hosts (206 and 192 male ticks found attached from about 200 male ticks infested) compared to dogs (58 to 84 male ticks attached for about 100 male ticks infested on dogs). The use of the restrained infestation areas used for sheep vs free infestation on the total body of dogs may have contributed to these differences in attachment rates.
In the present study, male ticks were chosen for infection because they are more likely to move spontaneously between host in natural conditions, as shown for D. andersoni [16]. The vectorial competency of the male D. reticulatus ticks for B. canis was demonstrated prior to these experiments (J. Fourie, personal communication). Babesia canis-infected male ticks were already found questing [17]. Male D. reticulatus ticks were reported to feed for significant duration and already identified as competent vectors of rickettsial pathogens [18] and Anaplasma spp. [19]. Although both sexes can be found on dogs in various proportions [20], the ability of each sex to transmit B. canis was not documented.
After the first infestation on sheep or dogs, the female ticks were obviously semi-engorged or engorged with a massive visual enlargement of the aloscutum and changes in coloration. However, the engorgement of male ticks was much less obvious and only visible on the ventral view of the ticks with a moderate increase in the width of the abdomen and a slight change in colour. These observations are in line with previous reports of D. reticulatus collected from dogs in natural situations: semi-engorged or engorged female D. reticulatus were reported [21] while 48% of the male ticks were categorized as slightly engorged [20]. This lack of visual markers for feeding in male ticks will make them harder to detect by pet owners while the risk of rapid parasite transmission will be increased.
Transmission times
Despite the moderate percentage infection of ticks (below 20% in both experiments), transmission of the pathogen to the second hosts occurred in both experiments within 24 and 8 h of infestation. No earlier time-points were assessed and it is therefore not possible to determine the actual minimum duration required for transmission, if any. The first infestation obviously provided the necessary stimuli and conditions for the development of Babesia into their infective stage within the ticks. Various mechanisms can be involved but are still unknown [22].
These findings are in contradiction with general beliefs about tick-borne disease transmission and related risk. It is indeed most often considered that no transmission can occur for the first 24–48 h after attachment and/or feeding of ticks on their host [23]. For babesiosis, this so-called “grace period” was even longer with 48–72 h of time required after infestation for sporogony of Babesia canis in Dermacentor reticulatus as observed by microscopy [3] and 36 h Babesia microti in Ixodes dammini, although no shorter period of transmission was tested [5]. As a consequence, and as demonstrated in experimental situations using strictly unfed ticks [6, 24, 25], veterinarians and pet owners rely on acaricidal products that kill the ticks during this misleading time interval. However, if this concept is suitable to experimental and controlled conditions, it does not simulate real-life situations where the history of vectors is unknown. In natural situations, ticks may detach spontaneously from their host in case of death. They can also be detached by scratching behaviour of the host or detach spontaneously because of mating behaviour. Rhipicephalus sanguineus adult ticks were indeed shown to detach spontaneously from dogs [26] and to migrate between dog hosts after attachment [10]. Ticks were also shown to detach from naïve [27] and immunized hosts [28]. As demonstrated for Borrelia, the ticks would still be competent vectors, even if they fed on a previously immunized host [29]. The activation of the transmission of bacterial pathogen by interrupted-feeding was already demonstrated with Rickettsia rickettsii and nymphs or male Amblyomma aureolatum ticks infesting naïve rabbits and guinea pigs. An initial feeding phase of the male ticks of 48 h on rabbits, followed by an immediate transfer on the second host, was able to shorten the transmission time of the pathogen to guinea pigs from 12 h to 10 min [8].
The present experiment did not investigate the minimal transmission time for B. canis in dogs. A successful transmission occurred within 8 h of tick infestation but we did not explore shorter time intervals. Since the clock started at the second infestation with ticks being deposited on the ground, close to the dogs, the attachment time of ticks is unknown and expected to vary between individuals. The effect of the time interval between detachment from the first host and re-attachment on the second host is also unknown. In the present experiments, this time interval did not exceed 2 h. However, this time interval is expected to be highly variable in natural situations. No information is available about the maximum time during which the pathogens can be kept alive and in their infective stage. Further experiments should be designed to explore faster transmission of B. canis in dogs and the influence of the time interval between detachment from the first host and re-attachment on the second host.
Based on these results, in areas at risk for canine babesiosis or where the vector may spread, we recommend to revise and reinforce the tick-prevention strategy [30]. A multi-modal approach implementing several layers of protection such as reduction of D. reticulatus habitat, early removal of any tick found on dogs, prevention of tick attachment by repellent effect and vaccination when available are recommended to prevent the transmission and the development of tick-borne diseases such as babesiosis in dogs.
Conclusions
These experiments provide evidence of an early transmission of B. canis to dogs within 8 h of infestation when the tick vector has a history of interrupted-feeding from a previous host. The study also demonstrates the ability of male D. reticulatus to transmit B. canis to dogs. In natural conditions, ticks, and in particular males, can detach spontaneously from their host and re-attach. It is impossible to determine the history of individual ticks that may transmit pathogens. Since no minimal transmission time can be established for all possible natural situations, a multi-modal strategy of prevention implementing tick anti-attachment (repellency) is recommended.
Acknowledgements
The authors acknowledge the Clinvet and Qvative team members for their respective contributions in this experiment.
Funding
The study was funded by Ceva Santé Animale.
Availability of data and materials
The data supporting the conclusions of this article are included within the article.
Abbreviations
- BW
Body weight
- DNA
Deoxyribonucleic acid
- EDTA
Ethylenediamine tetraacetic acid
- IFA
Immunofluorescent assay
- PCR
Polymerase chain reaction
Authors’ contributions
MV formulated the idea and drafted the manuscript. JL wrote the protocol and directed the study under JF supervision. JF revised the manuscript. All authors provided relevant input at different stages of manuscript preparation. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
MV is an employee of Ceva Santé Animale. JL and JF are employees of Clinvet international, a research organization contracted by Ceva Santé Animale to perform the study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marie Varloud, Email: marie.varloud@ceva.com.
Julian Liebenberg, Email: Julian.Liebenberg@clinvet.com.
Josephus Fourie, Email: Josephus.Fourie@clinvet.com.
References
- 1.Rubel F, Brugger K, Pfeffer M, Chitimia-Dobler L, Didyk YM, Leverenz S, et al. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick Borne Dis. 2016;7:224–233. doi: 10.1016/j.ttbdis.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Mehlhorn H, Schein E. The piroplasms: life cycle and sexual stages. Adv Parasitol. 1984;23:37–103. doi: 10.1016/S0065-308X(08)60285-7. [DOI] [PubMed] [Google Scholar]
- 3.Schein E, Mehlhorn H, Voigt WP. Electron microscopical studies on the development of Babesia canis (Sporozoa) in the salivary glands of the vector tick Dermacentor reticulatus. Acta Trop. 1979;36:229–241. [PubMed] [Google Scholar]
- 4.Ebel GD, Kramer LD. Short report: duration of tick attachment required for transmission of powassan virus by deer ticks. Am J Trop Med Hyg. 2004;71:268–271. [PubMed] [Google Scholar]
- 5.Piesman J, Spielman A. Human babesiosis on Nantucket Island: prevalence of Babesia microti in ticks. Am J Trop Med Hyg. 1980;29:742–746. doi: 10.4269/ajtmh.1980.29.742. [DOI] [PubMed] [Google Scholar]
- 6.Beugnet F, Halos L, Larsen D, Labuschagné M, Erasmus H, Fourie J. The ability of an oral formulation of afoxolaner to block the transmission of Babesia canis by Dermacentor reticulatus ticks to dogs. Parasit Vectors. 2014;7:283. doi: 10.1186/1756-3305-7-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shih CM, Spielman A. Accelerated transmission of Lyme disease spirochetes by partially fed vector ticks. 1993. J Clin Microbiol. 1993;31:2878–2881. doi: 10.1128/jcm.31.11.2878-2881.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saraiva DG, Soares HS, Soares JF, Labruna MB. Feeding period required by Amblyomma aureolatum ticks for transmission of Rickettsia rickettsii to vertebrate hosts. Emerg Infect Dis. 2014;20:1504–1510. doi: 10.3201/eid2009.140189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray J, Stanekb G, Kundic M, Kocianova E. Dimensions of engorging Ixodes ricinus as a measure of feeding duration. Int J Med Microbiol. 2005;295:567–572. doi: 10.1016/j.ijmm.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Little SE, Hostetler J, Kocan KM. Movement of Rhipicephalus sanguineus adults between co-housed dogs during active feeding. Vet Parasitol. 2007;150:139–145. doi: 10.1016/j.vetpar.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 11.van Duijvendijk G, Coipan C, Wagemakers A, Fonville M, Ersöz J, Oei A, et al. Larvae of Ixodes ricinus transmit Borrelia afzelii and B. miyamotoi to vertebrate hosts. Parasit Vectors. 2016;9:97. doi: 10.1186/s13071-016-1389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bremer WG, Schaefer JJ, Wagner ER, Ewing SA, Rikihisa Y, Needham GR, et al. Transstadial and intrastadial experimental transmission of Ehrlichia canis by male Rhipicephalus sanguineus. Vet Parasitol. 2005;131:95–105. doi: 10.1016/j.vetpar.2005.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heile C, Hoffmann-Köhler P, Wiemann A, Schein E. Transmission time of tick-borne disease agents in dogs: Borrelia, Anaplasma, Ehrlichia and Babesia. Praktische Tierarzt. 2007;88:584–590. [Google Scholar]
- 14.Duarte SC, Linhares GF, Romanowsky TN, da Silveira Neto OJ, Borges LM. Assessment of primers designed for the subspecies-specific discrimination among Babesia canis canis, Babesia canis vogeli and Babesia canis rossi by PCR assay. Vet Parasitol. 2008;152:16–20. doi: 10.1016/j.vetpar.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 15.VICH. Guideline on good clinical practices. GL9. 2009. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500004343.pdf. Accessed 20 Oct 2017.
- 16.Lysyk TJ. Movement of male Dermacentor andersoni (Acari: Ixodidae) among cattle. J Med Entomol. 2013;50:977–985. doi: 10.1603/ME13012. [DOI] [PubMed] [Google Scholar]
- 17.Zając V, Wójcik-Fatla A, Sawczyn A, Cisak E, Sroka J, Kloc A, et al. Prevalence of infections and co-infections with 6 pathogens in Dermacentor reticulatus ticks collected in eastern Poland. Ann Agric Environ Med. 2017;24:26–32. doi: 10.5604/12321966.1233893. [DOI] [PubMed] [Google Scholar]
- 18.Földvári G, Rigó K, Lakos A. Transmission of Rickettsia slovaca and Rickettsia raoultii by male Dermacentor marginatus and Dermacentor reticulatus ticks to humans. Diagn Microbiol Infect Dis. 2013;76:387–389. doi: 10.1016/j.diagmicrobio.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Zivkovic Z, Nijhof AM, de la Fuente J, Kocan KM, Jongejan F. Experimental transmission of Anaplasma marginale by male Dermacentor reticulatus. BMC Vet Res. 2007;3:32. doi: 10.1186/1746-6148-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mierzejewska EJ, Welc-Faleciak R, Karbowiak G, Kowalec M, Behnke JM, Bajer A. Dominance of Dermacentor reticulatus over Ixodes ricinus (Ixodidae) on livestock, companion animals and wild ruminants in eastern and central Poland. Exp Appl Acarol. 2015;66:83–101. doi: 10.1007/s10493-015-9889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Földvári G, Farkas R. Ixodid tick species attaching to dogs in Hungary. Vet Parasitol. 2005;129:125–131. doi: 10.1016/j.vetpar.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 22.Antunes S, Rosa C, Couto J, Ferrolho J, Domingos A. Deciphering Babesia-vector interactions. Front Cell Infect Microbiol. 2017;7:429. doi: 10.3389/fcimb.2017.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honsberger NA, Six RH, Heinz TJ, Weber A, Mahabir SP, Berg TC. Efficacy of sarolaner in the prevention of Borrelia burgdorferi and Anaplasma phagocytophilum transmission from infected Ixodes scapularis to dogs. Vet Parasitol. 2016;222:67–72. doi: 10.1016/j.vetpar.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Jongejan F, Fourie JJ, Chester ST, Manavella C, Mallouk Y, Pollmeier MG, et al. The prevention of transmission of Babesia canis canis by Dermacentor reticulatus ticks to dogs using a novel combination of fipronil, amitraz and (S)-methoprene. Vet Parasitol. 2011;179:343–350. doi: 10.1016/j.vetpar.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 25.Fourie JJ, Stanneck D, Jongejan F. Prevention of transmission of Babesia canis by Dermacentor reticulatus ticks to dogs treated with an imidacloprid/flumethrin collar. Vet Parasitol. 2013;192:273–278. doi: 10.1016/j.vetpar.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Varloud M, Delcombel R, Karembe H, Fourie JJ. Kinetics of ticks (Rhipicephalus sanguineus sensu lato) spontaneous detachment from dogs after experimental infestations: a meta-analytical approach. Kuala Lumpur: 26th International Conference of the World Association for the Advancement of Veterinary Parasitology; 2017. p. 309.
- 27.Kim TK, Tirloni L, Pinto AFM, Moresco J, Yates JR, da Silva Vaz I, Jr, et al. Ixodes scapularis tick saliva proteins sequentially secreted every 24h during blood feeding. PLoS Negl Trop Dis. 2016;10:e0004323. doi: 10.1371/journal.pntd.0004323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alani AJ, Herbert IV. Effect of host resistance on the feeding and reproductive performance of Haemaphysalis punctata and Ixodes ricinus ticks. Res Vet Sci. 1987;42:238–243. [PubMed] [Google Scholar]
- 29.Kurtenbach K, Dizij A, Seitz HM, Margos G, Moter SE, Kramer MD, et al. Differential immune responses to Borrelia burgdorferi in European wild rodent species influence spirochete transmission to Ixodes ricinus L. (Acari: Ixodidae) Infect Immun. 1994;62:5344–5352. doi: 10.1128/iai.62.12.5344-5352.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solano-Gallego L, Sainz Á, Roura X, Estrada-Peña A, Miró G. A review of canine babesiosis: the European perspective. Parasit Vectors. 2016;9:336. doi: 10.1186/s13071-016-1596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusions of this article are included within the article.


