Abstract
Tumor-associated carbohydrate antigens (TACAs) are attractive targets for anticancer vaccine development. Due to the low immunogenicity of TACAs, a powerful carrier system is needed to boost immune responses. Virus-like particles (VLPs) are an exciting platform for delivering TACAs to the immune system. The high symmetry of VLPs enables the display of TACAs in an organized manner, which in turn can potently activate antibody secreting B cells, eliciting high titers of antiglycan IgG antibodies. In this chapter, the protocol for conjugating a prototypical TACA, the Tn antigen to a VLP, bacteriophage Qβ, is presented. On an average around 370 copies of Tn can be attached to each Qβ capsid. Immunization of mice with Qβ-Tn conjugate leads to over two orders of magnitude higher IgG antibodies compared to control mice receiving Qβ only without the Tn antigen. Antibodies induced by Qβ-Tn recognize Tn-expressing tumor cells strongly and protect mice from tumor-induced death. The techniques for evaluating antibody titers by enzyme-linked immunosorbent assay, antibody binding to tumor cells by flow cytometry, and the protection efficacy of the vaccine in a therapeutic model of tumor are discussed in this chapter.
1. INTRODUCTION
Due to their existence on many tumor types and their high expression levels on tumor cells, tumor-associated carbohydrate antigens (TACAs) are appealing for vaccine development (Buskas, Thompson, & Boons, 2009; Danishefsky & Allen, 2000; Guo & Wang, 2009; Liu & Ye, 2012; Monzavi-Karbassi, Pashov, & Kieber-Emmons, 2013; Yin & Huang, 2012). TACAs are recognized by B cell receptors (BCRs) on B cells, which are the only cell type in human bodies secreting antibodies. When administered alone, TACAs only weakly activate B cells producing low titers of IgM antibodies. To elicit strong and long-lasting IgG antibody responses, helper T cells need to be activated by the immunogen to provide costimulatory signals to B cells and induce antibody isotype switching from IgM to IgG (Goldsby, Kindt, & Osborne, 2000).
A popular strategy to overcome the low immunogenicity of TACAs is to covalently conjugate TACAs to a carrier containing epitopes for helper T cells (Goldsby et al., 2000; Yin & Huang, 2012). The most common carriers utilized are immunogenic proteins such as keyhole limpet hemocyanin (KLH) with multiple KLH-TACA constructs evaluated in clinical trials (Danishefsky & Allen, 2000; Gilewski et al., 2001; Huang et al., 2016; Livingston, 1995; Miles et al., 2011). However, for a prototypical TACA, the Tn antigen (N-acetyl galactosamine α-linked to serine or threonine), even after KLH conjugation, only low titers of anti-Tn antibodies were induced (Kagan et al., 2005). There is an urgent need to develop effective carriers to powerfully boost anti-TACA immune responses.
It is known that how B cell epitopes are presented by the immunogen is an important parameter influencing B cell activation (Bachmann, Hengartner, & Zinkernagel, 1995; Bachmann et al., 1993; Denis et al., 2007). A construct containing randomly displayed B cell antigens failed to generate high antibody responses, while the same epitope delivered to the immune system in an ordered pattern was able to elicit high antibody titers (Bachmann et al., 1993). This is attributed to the ability of antigens presented in an organized manner to effectively cross-link multiple BCRs leading to powerful B cell activation.
VLPs are an exciting platform for organized antigen display (Lua et al., 2014; Roldao, Mellado, Castilho, Carrondo, & Alves, 2010; Zeltins, 2013). VLPs are formed through self-assembly of multiple monomeric units, resulting in highly ordered structures. Crystal structures of many VLPs are known (Golmohammadi, Fridborg, Bundule, Valegard, & Liljas, 1996), which can enable structure-guided immunogen design. Additional potential advantages of VLPs include their stability in organic solvents and reagents for chemical modification, multiple functional groups available for bio-conjugation, and high thermal stability. Recently, VLPs have been applied as carriers for the development of carbohydrate-based anticancer vaccines (Kaltgrad et al., 2007; Miermont et al., 2008; Yin et al., 2013, 2016, 2012, 2015). In the following, we will discuss the protocols for conjugating the Tn antigen to a VLP, bacteriophage Qβ, and immunological evaluations of this conjugate.
2. CONJUGATION OF TN WITH VLP Qβ AND CHARACTERIZATION OF THE CONJUGATE
2.1 Tn Conjugation to Qβ VLP
The capsid of bacteriophage Qβ is composed of 180 copies of a 132-amino acid monomeric protein, which self-assemble into a 28-nm diameter icosahedral particle facilitated by the RNA sequences sequestered inside the capsid during expression in Escherichia coli (Golmohammadi et al., 1996). The inter-subunit disulfide network between cysteines at positions 74 and 80 makes the Qβ capsid stable toward a wide range of pH values, high temperature, and various chemical reagents. Each of the protein subunits has four amino groups, i.e., K2, K13, K16, and the N-terminus, on the external surface of the capsid, which can be potentially modified using N-hydroxysuccinimide (NHS)-activated ester chemistry.
Although α-amine of the N-terminus and the ε-amine of the lysine side chain require high pH to be completely deprotonated (pH>8) (Koniev & Wagner, 2015), the reaction of NHS esters with amines can occur at pH 7 in 10% dimethyl sulfoxide (DMSO) in PBS buffer with high recovery of the intact particles. The robustness of this chemistry for TACA conjugation has been tested with several carbohydrate antigens (Astronomo et al., 2010; Hovlid et al., 2014; Yin et al., 2013, 2016, 2015). In the following, the protocol for conjugating a Tn NHS ester (Yin et al., 2013, 2015) with Qβ capsid (Fig. 1A) is presented.
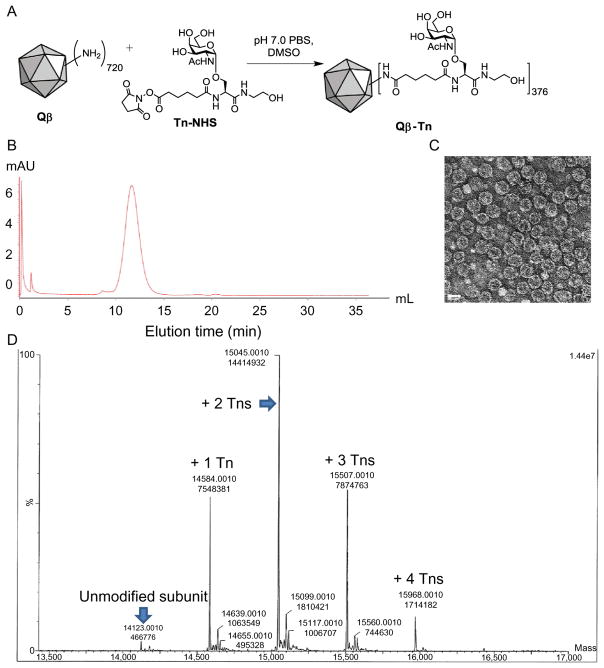
Fig. 1.
(A) Incubation of Qβ with Tn-NHS form Qβ-Tn conjugate. (B) FPLC chromatogram of Qβ-Tn. (C) TEM image of Qβ-Tn showing the capsid is intact after functionalization. The scale bar is 30nm. (D) LCMS QTOF ESI mass spectroscopy with data processing by Maximum Entropy deconvolution algorithm (MaxEnt™ 1) (Da Ren, Raimville, Wheat, Russell, & Mazzeo, 2004). Based on the MS data, the number of Tn is estimated to be 376 per capsid (Table 1).
2.2 Equipment
Nutating mixer
AKTApure 25L system, equipped with Superose 6 Increase 10/300 GL column and UV detector at wavelength 280nm
Millipore 100k MWCO centrifugal filter tube
Centrifuge tubes (15 and 50mL)
2.3 Buffers and Reagents
0.1M potassium phosphate buffer (PBS), pH 7.0
Coomassie Plus Protein Reagent (Pierce) for protein concentration determination with bovine serum albumin (BSA) as the standard.
DMSO
2.4 Procedure for Conjugating Qβ to Tn-NHS
VLP Qβ (13.2mg, 5.1nmol of particle corresponding to 0.9μmol of the subunit and 3.6μmol of reactive amines) suspended in PBS (0.1M, pH 7, 5.5mL) is added into a 15-mL centrifuge tube.
DMSO (0.35mL) is slowly dropped into the solution.
Tn-NHS (20mg/mL in 0.35mL, 0.017mmol, 4.7equiv. per reactive amine) is added into the reaction tube.
The reaction mixture is rotated on a nutating mixer at room temperature overnight.
The reaction is transferred into a 50-mL centrifuge tube and then diluted with 0.1M PBS, pH 7 to total volume 50mL.
The excess Tn-NHS is removed by filtration through Millipore 100k MWCO centrifugal filter tube and washed thoroughly with PBS.
The purity of the recovered Qβ conjugates is characterized by FPLC on Superose 6 Increase 10/300 GL column (Fig. 1B). The intact capsid is eluted from the column at around 11–13mL. Total protein concentration of the product is measured using the Coomassie Plus Protein Reagent (Pierce) with BSA as the standard. Typically, the yield of Qβ-Tn from this reaction is over 90%. The Qβ-Tn is also imaged via transmission electron microscopy showing intact particles with diameters of 28nm (Fig. 1C).
The average number of conjugated Tn on each viral capsid is calculated from the intensities of peaks in the deconvoluted mass spectrum from LCMS analysis together with Maximum Entropy deconvolution algorithm MaxEnt™ 1 (Da Ren et al., 2004). From the processed MS spectrum (Fig. 1D), the number of Tn added to each subunit is determined based on the increase of m/z values of the peaks compared to the m/z value of unmodified subunit (addition of one Tn to each subunit leads to an increase of 461 in m/z value) (Table 1). The total number of Tn per capsid is calculated by: relative intensity of each peak×number of Tn for each peak×180 (the number of subunits per capsid).
Table 1.
Calculations to Determine the Average Number of Tn on Each Qβ-Tn Capsid
| m/z of the Peaks | Δ m/z | Number of Tn/Subunit | MS Peak Intensity | Relative Intensity %=Peak Intensity/Total Intensity×100% | # Tn for Each Peak=Relative Intensity×Number of Tn for the Peak×180 |
|---|---|---|---|---|---|
| 14,123 | 0 | 466,776 | 1.4 | 0 | |
| 14,584 | 461 | 1 | 7,548,381 | 23.6 | 42 |
| 15,045 | 461 | 2 | 14,414,932 | 45.0 | 162 |
| 15,507 | 462 | 3 | 7,874,763 | 24.6 | 133 |
| 15,968 | 461 | 4 | 1,714,182 | 5.3 | 39 |
| Total | 32,019,034 | 100 | 376 |
3. VACCINATION OF MICE WITH Qβ-TN
With the successful synthesis of the immunogen Qβ-Tn, mouse immunization is performed. Due to the low immunogenicity of TACAs, multiple injections are administered with the primary vaccination on day 0 followed by two boost injections on days 14 and 28, respectively. To potentiate immune responses to TACAs, an immune adjuvant is added to the vaccine formulation. Due to its high potency, complete Freund’s adjuvant (CFA) has been used for the primary injection with incomplete Freund’s adjuvant (IFA) as part of the formulation for boost injections. There are multiple potential routes of vaccination, including subcutaneous, intradermal, intravenous, intranasal, and intraperitoneal administration. Subcutaneous administration is a convenient method for vaccination, which can lead to strong antibody responses.
3.1 Equipment
Vortex mixer
Sterile Eppendorf tubes (0.5 and 1.5mL)
Sterile disposable syringes (BD 1mL Syringe) and needles (25G 5/8 in.)
Blood collection tubes (Microvette® CB 300 Z)
3.2 Buffers and Reagents
Sterile PBS
Complete Freund’s adjuvant (Sigma-Aldrich, USA, F5881)
Incomplete Freund’s adjuvant (Sigma-Aldrich, F5506)
3.3 Procedures
3.3.1 Blood Collection and Serum Preparation
Sera (0.1mL per mouse) are collected from saphenous vein of mice on days 0, 7, and 35. The blood is collected following a published procedure (Parasuraman, Raveendran, & Kesavan, 2010).
The collected serum samples in blood collection tubes are centrifuged at 4°C, 4000rpm for 30min to separate the serum from red blood cells (RBCs).
The clear serum in the top layer is drawn from the tube using a micropipette.
The serum is transferred into a sterile Eppendorf tube and kept at −20°C till analysis.
3.3.2 Vaccine Emulsion Preparation
The vaccine is prepared by mixing 1:1 (v/v) of the solution of Qβ-Tn and CFA or IFA. Each mouse receives the vaccine dose corresponding to 1.93μg of the attached Tn. The vaccine is prepared at 150% of the calculated amounts to ensure there is enough sample for all injections accounting for sample losses due to transfer and dead volume of the syringes and needles. For example, for five mice receiving 0.1mL of the emulsion each for one injection, 0.75mL of the emulsion is prepared.
CFA or IFA is drawn into a sterile syringe and added to the Qβ-Tn solution in PBS slowly (1:1 (v/v)) in an Eppendorf tube.
The nonmiscible mixture is vigorously mixed on a vortex mixer in 4°C. The emulsion is ready when a drop of the white emulsion is put on water in a beaker, the droplet does not spread out on water surface. This process usually takes 1–2h. The Qβ-Tn capsid is stable under this mixing condition as confirmed by FPLC.
3.3.3 The Procedure for Subcutaneous Injections
The prepared emulsion is drawn into a 1-mL syringe slowly avoiding air bubbles and a needle (25G 5/8 in.) is put on the syringe.
The plunger of the syringe is slowly pushed forward to remove air bubbles and fill the needle with the emulsion.
The syringe is placed on an alcohol pad.
A mouse is firmly held on the scruff in the palm of the left hand of the operator. The thumb and the index finger of the left hand are used to pull the loose skin over the scruff of the mouse with the mouse tail held between the ring finger and the palm.
The prepared syringe is used to pierce through the skin on the scruff between the thumb and the index finger to inject the vaccine (0.1mL) slowly under the scruff.
-
The syringe is drawn out slowly after injection and the mouse is returned to the cage.
Note: Some vaccinated mice may develop skin irritation or less frequently ulceration at the injection site due to the side effect of CFA. This can render it difficult to restrain the mouse for boost injections. If this is the case, mouse can be anesthetized with isoflurane before injection.
4. EVALUATION OF ANTIBODY TITERS AND SUBTYPES BY ELISA
Antibodies elicited by vaccination can be first analyzed through ELISA by determining the amounts of antibodies in the postimmune sera that can bind antigens immobilized on ELISA plates. ELISA can also report on the subtypes of antibodies generated, providing valuable information on the mechanism of immune responses. As antibodies can be elicited against both the TACA and the carrier moiety through vaccination, it is important to use TACA conjugated to a different multivalent platform for ELISA to avoid the potential interference from anticarrier antibodies for the analysis. For evaluation of anti-Tn antibody responses by Qβ-Tn, BSA conjugated with Tn (BSA-Tn) containing 30–35 copies of Tn per BSA is used, which is superior to the alternative Tn-biotin/streptavidin system (Yin et al., 2012, 2015).
4.1 Equipment
Microplate absorbance reader (Bio-Rad)
96-well Nunc microtiter plates
Multichannel micropipette
Incubator at 37°C under 5% CO2
Vortex mixer
4.2 Buffers and Reagents
BSA-Tn (1mg/mL)
NaHCO3/Na2CO3 buffer (0.05M, pH 9.6): Na2CO3 (159mg), NaHCO3 (293mg), NaN3 (20mg), H2O (100mL)
PBS/0.5% Tween-20 (PBST)
1% (w/v) BSA
Horseradish peroxidase (HRP)-conjugated goat antimouse IgG, IgM, IgG1, IgG2b, IgG2c, or IgG3 (Jackson ImmunoResearch Laboratory)
3,3′,5,5′-Tetramethylbenzidine (TMB)
0.5M H2SO4
Citric acid buffer: Na2HPO4 (7.5g), citric acid monohydrate (4.57g) in distilled water (1L)
4.3 Procedure
A solution of BSA-Tn (1mg/mL) in NaHCO3/Na2CO3 buffer (10 μg/mL, 100μL/well) is added to a 96-well Nunc microtiter plate and incubated at 4°C overnight.
The plate is washed with PBST (4×200μL), followed by the addition of 1% (w/v) BSA in PBS (200μL) to each well and incubation at rt for 1h.
The plate is washed with PBST (4×200μL) and incubated with serial dilutions of antisera from immunized mice in 0.1% BSA/PBS (100μL/well, four wells for each dilution). The plate is incubated for 2h at 37°C and then washed with PBST (4×200μL).
A 1:2000 dilution of HRP-conjugated goat antimouse IgG, IgM, IgG1, IgG2b, IgG2c, or IgG3 (Jackson ImmunoResearch Laboratory) in 0.1% BSA/PBS (100μL) is added to the wells, respectively, to determine the subtypes of antibodies generated. The plate is incubated for 1h at 37°C.
A solution of enzymatic substrate is prepared by dissolving TMB (5mg) in a mixture of DMSO (2mL) and citric acid buffer (18mL) in a 50-mL centrifuge tube covered with aluminum foil. H2O2 (20 μL) is added and the mixture is homogenized by vortexing.
The plate is washed with PBST (4×200μL) and a solution of enzymatic substrate is added (200μL). Color is allowed to develop for 15min and then 0.5M H2SO4 (50 μL) is added to quench the reaction.
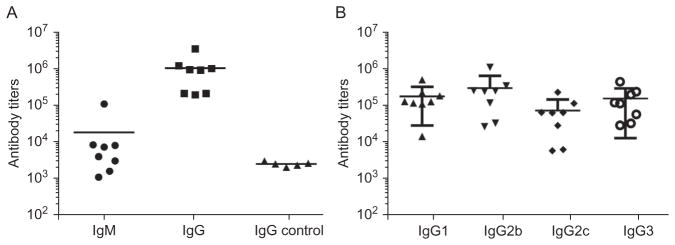
The absorbance is measured at 450nm using a microplate reader. The titer is determined by regression analysis with log10 dilution plotted with optical density. The titer is reported as the highest fold of dilution that gives OD=0.3. 35 days after the primary vaccination, mice immunized with Qβ-Tn produce high titers of anti-Tn IgG antibodies, which are more than two orders of magnitude higher than those from control mice receiving Qβ (Fig. 2A). The high IgG titers suggest helper T cells have been activated by the vaccine construct. In addition, all subtypes of IgG antibodies, i.e., IgG1, IgG2b, IgG2c, and IgG3 have been produced from vaccination (Fig. 2B), indicating balanced IgG antibody responses.
Fig. 2.
(A) High anti-Tn antibody responses are induced by immunization with Qβ-Tn. The primary antibody response is IgG due to significantly higher IgG titers than IgM. The IgG titers from Qβ-Tn are much higher than those from control mice receiving Qβ only. (B) Titers of IgG subtypes antibodies (IgG1, IgG2b, IgG2c, and IgG3) elicited by Qβ-Tn immunization. All major IgG subtypes are induced suggesting balanced IgG responses.
5. DETECTION OF ANTIBODY BINDING TO TUMOR CELLS BY FLOW CYTOMETRY
As ELISA measures antibody binding to antigens immobilized on the surface of a microtiter plate, it is important to test whether antibodies elicited could recognize antigens presented in its native environment, i.e., on the surface of tumor cells. This can be accomplished by incubating postimmune serum with Tn-expressing tumor cells. Upon removal of unbound antibodies, fluorescently labeled secondary antibodies are added to the cells. If antibodies produced can recognize Tn antigens on tumor cell surface, the cells would be fluorescently labeled by the secondary antibodies. The number of cells with enhanced fluorescence and fluorescence intensities can be readily quantified by flow cytometry. For anti-Tn vaccine studies, Tn-expressing human T cell leukemia Jurkat cells and mouse breast cancer cell TA3Ha are used for flow cytometry analysis.
5.1 Equipment
Flow cytometer LSR II (BD)
Corning falcon round-bottom polystyrene tubes (FACS tube, 5mL)
Cell culture dishes (Sigma-Aldrich)
Sterile cell culture CO2 incubator at 37°C
Microcentrifuge tubes (1.5mL) and conical sterile centrifuge tubes (15 and 50mL)
Sterile plastic pipette and pipette controller
Hemocytometer
5.2 Buffers and Reagents
Jurkat cell culture medium recipe: RPMI 1640, 10% (v/v) FBS, 2mM sodium pyruvate, minimal essential medium nonessential amino acid, 1% (v/v) Penicillin Streptomycin (Pen Strep) (100units/mL penicillin and 100units/mL streptomycin)
Dulbecco’s phosphate buffered saline (DPBS)
FACS buffer (PBS, pH 7.4, 0.1% NaN3, 1% FBS)
FITC-labeled goat antimouse IgG (minimal x-reactivity) antibody (0.5mg/mL, BioLegend, 405305)
Fresh TA3Ha cells are isolated from ascites of A/J mice (see Section 6.3 for detail)
5.3 Procedure for in vitro Culturing of TA3Ha and Jurkat Cells
A vial of frozen TA3Ha or Jurkat cells kept in liquid nitrogen storage tank is resuscitated by quickly warming up the vial in a 37°C water bath. The external surface of the tube is wiped with 70% ethanol before the tube is placed in a biological safety cabinet.
The whole content of the cell suspension in the vial is transferred into a 50-mL sterile tube.
Cell culture medium (10mL) is slowly added to the tube, which is gently agitated.
The cell suspension is centrifuged at 1600rpm for 5min at 4°C and the culture medium is then removed.
The culture medium (10mL) is added to resuspend the cells. The cells are counted to determine cell concentration with the cell density adjusted to 1×105 cells/mL.
The cell suspension (5mL) is seeded into a culture plate, which is placed in an incubator (37°C, 5% CO2). These cells grow nonadherently.
The cell growth medium is changed every 2 days. To change the culture medium, or harvest the cells, the cell suspension is collected into a centrifuge tube. The cells are pelleted down by centrifuge to remove the medium.
Fresh medium is added to resuspend the cells by seeding 1×105 cells/mL (5mL) into a new plate for further passaging (Note: During cell culture, TA3Ha tend to lose its cell surface Tn-bearing glycoprotein epiglycanin (Thingstad & Hilkens, 2003; Thingstad, Vos, & Hilkens, 2001.). As a result, the cells with more in vitro passages can become increasingly adherent to cell culture plates. When cells become adherent, they should be passaged in vivo to regain the expression of epiglycanin (see Section 6.3).
5.4 Procedure for Flow Cytometry Analysis
Jurkat cells or TA3Ha cells are cultured at 37°C under 5% CO2 in cell growth medium until cells reach confluency.
The mixture of cells in cell growth medium is transferred to a conical centrifuge tube.
The centrifuge tubes with cells are centrifuged at 1600rpm for 5min at 4°C. The pellet is resuspended in growth medium (10mL). The number of cells is determined with a hemocytometer.
Suspensions of 3.0×105 cells are added to each FACS tube, which are centrifuged at 1600rpm for 5min to remove the supernatant.
The cells are washed with FACS buffer, and incubated with 1:20 dilution of antisera in FACS buffer (100μL) for 30min on ice.
The incubated cells are washed twice with FACS buffer, which is followed by incubation with FITC anti-mouse IgG (2μL) for 30min on ice.
The cells are washed twice and resuspended in FACS buffer before analysis by LSR II (BD Biosciences). Data are processed by FlowJo software. As shown in Fig. 3, the postimmune sera from mice immunized with Qβ-Tn bind much more strongly with both TA3Ha and Jurkat cells compared to sera from control mice receiving Qβ only.
Fig. 3.
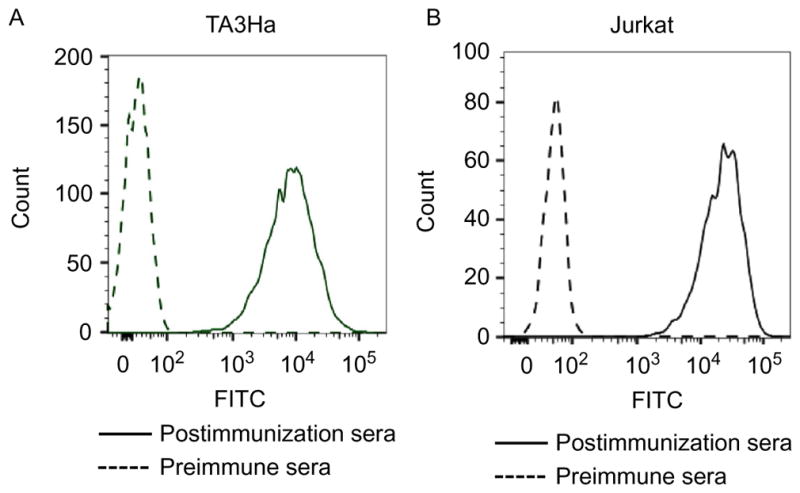
Recognition of (A) TA3Ha cells and (B) Jurkat cells by IgG antibodies in serum from mice before and after immunization with Qβ-Tn (for clarity, only one representative example of binding by postimmune serum is shown). Adapted with permission from Yin, Z., Wright, W. S., McKay, C., Baniel, C., Kaczanowska, K., Bentley, P., et al. (2015). Significant impact of immunogen design on the diversity of antibodies generated by carbohydrate-based anti-cancer vaccine. ACS Chemical Biology, 10, 2364–2372. http://dx.doi.org/10.1021/acschembio.5b00406. Copyright 2015 American Chemical Society.
6. VACCINATION WITH Qβ-TN CAN PROTECT MICE FROM DEATH DUE TO PREEXISTING TUMOR
With the strong recognition of Tn-expressing tumor cells by sera from mice immunized with Qβ-Tn, the abilities of Qβ-Tn in protecting mice from tumor-induced death can be evaluated using the TA3Ha murine mammary adenocarcinoma cells. This model has been used in investigations of Tn-based cancer vaccines in vivo (Lo-Man et al., 2001, 2004). TA3Ha cells are derived from A/J mouse strain (Thingstad & Hilkens, 2003), but can grow well in both C57BL/6 and BALB/c mice (Leyva, Littrell, Yin, Huang, & Haas, 2016; Lo-Man et al., 2001; Yin et al., 2015). To mimic clinical condition for tumor therapy, vaccination is performed after mice receive injection of tumor cells. In addition, the possibility of combining immunotherapy with chemotherapy has been investigated to establish potential synergistic effects in therapy.
6.1 Equipment
Allegra® X-15R centrifuge (Beckman Coulter)
15mL Sterile tubes
Sterile plates
Biological safety cabinet
Sterile cell culture CO2 incubator at 37°C
Sterile plastic pipette and pipette controller
6.2 Buffers and Reagents
TA3Ha cell culture medium recipe: RPMI 1640, 10% (v/v) FBS, 1% (v/v) Penicillin Streptomycin (Pen Strep) (100units/mL penicillin and 100units/mL streptomycin)
Sterile DPBS, pH 7.4
70% Ethanol in water
1× RBC lysis buffer
DMSO
6.3 Procedure for in vivo TA3Ha Cell Passage
A/J mice (7–10 weeks old) are injected intraperitoneally with TA3Ha tumor cells (5000 cells/mouse in 100μL PBS).
7 Days after tumor inoculation, the ascites are collected and treated with 1 RBC lysis buffer to lyse the RBCs.
The remaining cell suspension is centrifuged, washed with DPBS for three times, and resuspended in the culture medium.
PBS is replaced by the culture medium. Cells are counted and the cell concentration is adjusted to 5×106 cells/mL. DMSO is added to cell suspension to achieve a final concentration of 5% (v/v). The cell suspension is aliquoted into cryovials and frozen in liquid nitrogen.
2–3 Days before in vivo administration of tumor cells, the frozen cells are thawed, cultured in the culture medium, and harvested following the in vitro cell culture procedure.
6.4 Procedure for Evaluating the Efficacy of Qβ-Tn for Protecting Mice From TA3Ha Tumor Challenge
In a therapeutic model, the tumor challenge and treatments are performed with the following schedule:
On day 0, to prepare for the tumor cell inoculation, suspension of 50,000 TA3Ha cells/mL (5mL) in pH 7.4 PBS is kept on ice until ready for injection. In a sterile hood, the cell culture tube is allowed to warm up to rt. Mice are sterilized by wiping with an alcohol pad at the injection sites. The cell suspension up to 0.6mL is drawn up into a 1-mL syringe. 100μL (5000 cells) of the suspension is injected intraperitoneally into each mouse.
On day 1, three groups of mice are treated intraperitoneally with a chemotherapeutic drug, cyclophosphamide (CP), at a dose of 50mg/kg. One group of mice is immunized with the Qβ-Tn vaccine (corresponding to 8μg of Tn) intravenously with 20μg of MPLA as the adjuvant. The second group of mice receives Qβ with 20μg of MPLA and the third group of mice receives PBS only. In addition to these three groups, a fourth group of mice is immunized with the Qβ-Tn vaccine (corresponding to 8μg of Tn) intravenously with 20μg of MPLA as the adjuvant without CP treatment. A fifth group of mice receives PBS injection only as control.
On days 4 and 8, booster injections are performed. Groups 1–4 of mice receive the Qβ-Tn vaccine, Qβ only without Tn, PBS, and Qβ-Tn, respectively, intravenously with 20μg of MPLA as the adjuvant.
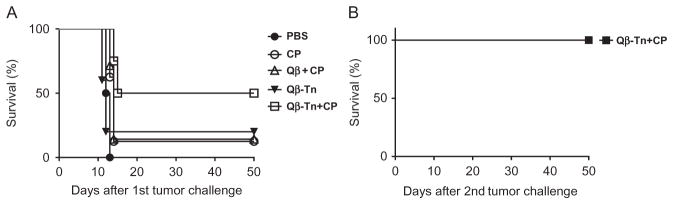
The survival of all mice is continuously monitored until day 50 and Kaplan–Meier survival curves are constructed to compare the protective efficacies of various treatment regimes. As shown in Fig. 4A, mice receiving PBS only all die within 13 days. The administration of CP improves the survival rate to 12.5%. Qβ-Tn vaccination alone helps improve the survival rate to 20%. The combination of Qβ-Tn vaccination and CP treatment protect 50% of the mice from tumor-induced death. There are significant statistical differences between Qβ-Tn +CP group and PBS group (P <0.0001) or all other treatment groups (P <0.05). Statistical analysis is performed using the log-rank test through the GraphPad Prism software.
The mice survived the first tumor challenge from the Qβ-Tn+CP group are reinjected with 5000 TA3Ha cells intraperitoneally. Their survival is continuously monitored. Without any further treatment, these mice all survive the tumor challenge (Fig. 4B). This suggests effective antitumor immunity is generated in these mice protecting them from tumor-induced death.
Fig. 4.
Kaplan–Meier survival curves of mice receiving various treatment regimes. (A) Groups of mice are intraperitoneally injected with 5000 TA3Ha cells on day 0, with or without intraperitoneal treatment of cyclophosphamide (50mg/kg). PBS buffer, Qβ particle plus MPLA, or Qβ-Tn plus MPLA are administrated on days 1, 4, and 8. The survival of mice is followed for 50 days. There is significant difference in survival rates between the Qβ-Tn+CP group and the PBS group (P <0.0001) or all other treatment groups (P <0.05). Statistical analysis of survival is performed with GraphPad Prism using the log-rank test. (B) Mice surviving tumor challenge from Qβ-Tn +CP group are rechallenged with 5000 TA3Ha cells. All mice survive without any further treatment. Adapted with permission from Yin, Z., Wright, W. S., McKay, C., Baniel, C., Kaczanowska, K., Bentley, P., et al. (2015). Significant impact of immunogen design on the diversity of antibodies generated by carbohydrate-based anti-cancer vaccine. ACS Chemical Biology, 10, 2364–2372. http://dx.doi.org/10.1021/acschembio.5b00406. Copyright 2015 American Chemical Society.
7. SUMMARY
VLPs are powerful platforms for organized antigen display. With the multiple functional groups available on the external surfaces, TACAs can be readily conjugated to VLPs and the conjugates are well characterized. Mouse immunization with the conjugate show that high titers of IgG antibodies can be generated against the TACA, as represented by a prototypical TACA, the Tn antigen. The antibodies generated can recognize Tn-expressing tumor cells strongly. Immunization with Qβ-Tn conjugate coupled with chemotherapy significantly protect mice from death induced by preexisting tumor.
Acknowledgments
We are grateful to the National Cancer Institute (R01CA149451-01A1) for financial support of our work.
References
- Astronomo RD, Kaltgrad E, Udit A, Wang SK, Doores KJ, Huang CY, et al. Defining criteria for oligomannose immunogens for HIV using icosahedral virus capsid scaffolds. Chemistry & Biology. 2010;17:357–370. doi: 10.1016/j.chembiol.2010.03.012. http://dx.doi.org/10.1016/j.chembiol.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MF, Hengartner H, Zinkernagel RM. T helper cell-independent neutralizing B cell response against vesicular stomatitis virus: Role of antigen patterns in B cell induction? European Journal of Immunology. 1995;25:3445–3451. doi: 10.1002/eji.1830251236. http://dx.doi.org/10.1002/eji.1830251236. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. http://dx.doi.org/10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- Buskas T, Thompson P, Boons GJ. Immunotherapy for cancer: Synthetic carbohydrate-based vaccines. Chemical Communications (Cambridge, England) 2009;36:5335–5349. doi: 10.1039/b908664c. http://dx.doi.org/10.1039/b908664c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Ren HSG, Raimville P, Wheat T, Russell RJ, Mazzeo JR. Mass spectrometry quantification of protein mixtures. Milford, MA: Waters Corperation; 2004. http://www.waters.com/webassets/cms/library/docs/wa31808.pdf. [Google Scholar]
- Danishefsky SJ, Allen JR. From the laboratory to the clinic: A retrospective on fully synthetic carbohydrate-based anticancer vaccines. Angewandte Chemie, International Edition. 2000;39:836–863. doi: 10.1002/(sici)1521-3773(20000303)39:5<836::aid-anie836>3.0.co;2-i. http://dx.doi.org/10.1002/(SICI)1521-3773(20000303)39:5<836::AID-ANIE836>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Denis J, Majeau N, Acosta-Ramirez E, Savard C, Bedard MC, Simard S, et al. Immunogenicity of papaya mosaic virus-like particles fused to a hepatitis C virus epitope: Evidence for the critical function of multimerization. Virology. 2007;363:59–68. doi: 10.1016/j.virol.2007.01.011. http://dx.doi.org/10.1016/j.virol.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Gilewski T, Ragupathi G, Bhuta S, Williams LJ, Musselli C, Zhang XF, et al. Immunization of metastatic breast cancer patients with a fully synthetic Globo-H conjugate: A phase I trial. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3270–3275. doi: 10.1073/pnas.051626298. http://dx.doi.org/10.1073/pnas.051626298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsby RA, Kindt TJ, Osborne BA. Kuby immunology. New York, NY: Freeman; 2000. pp. 275–285. [Google Scholar]
- Golmohammadi R, Fridborg K, Bundule M, Valegard K, Liljas L. The crystal structure of bacteriophage Qβ at 3.5 Å resolution. Structure. 1996;4:543–554. doi: 10.1016/s0969-2126(96)00060-3. http://dx.doi.org/10.1016/S0969-2126(96)00060-3. [DOI] [PubMed] [Google Scholar]
- Guo ZW, Wang QL. Recent development in carbohydrate-based cancer vaccines. Current Opinion in Chemical Biology. 2009;13:608–617. doi: 10.1016/j.cbpa.2009.08.010. http://dx.doi.org/10.1016/j.cbpa.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovlid ML, Lau JL, Breitenkamp K, Higginson C, Laufer B, Manchester M, et al. Encapsidated atom-transfer radical polymerization in Qβ virus-like nanoparticles. ACS Nano. 2014;8:8003–8014. doi: 10.1021/nn502043d. http://dx.doi.org/10.1021/nn502043d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CS, Yu M, Tseng LM, Chow LWC, Hou MF, Hurvitz SA, et al. Randomized phase II/III trial of active immunotherapy with OPT822/OPT821 in patients with metastatic breast cancer. Journal of Clinical Oncology. 2016;34(Suppl):1003. abstr http://meetinglibrary.asco.org/content/168513-168176. [Google Scholar]
- Kagan E, Ragupathi G, Yi SS, Reis CA, Gildersleeve J, Kahne D, et al. Comparison of antigen constructs and carrier molecules for augmenting the immunogenicity of the monosaccharide epithelial cancer antigen Tn. Cancer Immunology, Immunotherapy: CII. 2005;54:424–430. doi: 10.1007/s00262-004-0584-y. http://dx.doi.org/10.1007/s00262-004-0584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltgrad E, Sen Gupta S, Punna S, Huang CY, Chang A, Wong CH, et al. Anti-carbohydrate antibodies elicited by polyvalent display on a viral scaffold. Chembiochem. 2007;8:1455–1462. doi: 10.1002/cbic.200700225. http://dx.doi.org/10.1002/cbic.200700225. [DOI] [PubMed] [Google Scholar]
- Koniev O, Wagner A. Developments and recent advancements in the field of endogenous amino acid selective bond forming reactions for bioconjugation. Chemical Society Reviews. 2015;44:5495–5551. doi: 10.1039/c5cs00048c. http://dx.doi.org/10.1039/C5CS00048C. [DOI] [PubMed] [Google Scholar]
- Leyva MA, Littrell CA, Yin Z, Huang X, Haas KM. PD-1 suppresses B cell responses to Tn antigen and protection against Tn-expressing tumors. Cancer Immunology Research. 2016;4:1027–1037. doi: 10.1158/2326-6066.CIR-16-0184. http://dx.doi.org/10.1158/2326-6066.CIR-16-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Ye XS. Carbohydrate-based cancer vaccines: Target cancer with sugar bullets. Glycoconjugate Journal. 2012;29:259–271. doi: 10.1007/s10719-012-9399-9. http://dx.doi.org/10.1007/s10719-012-9399-9. [DOI] [PubMed] [Google Scholar]
- Livingston PO. Approaches to augmenting the immunogenicity of melanoma gangliosides: From whole melanoma cells to ganglioside-KLH conjugate vaccines. Immunology Reviews. 1995;145:147–166. doi: 10.1111/j.1600-065x.1995.tb00080.x. http://dx.doi.org/10.1111/j.1600-065X.1995.tb00080.x. [DOI] [PubMed] [Google Scholar]
- Lo-Man R, Vichier-Guerre S, Bay S, Deriaud E, Cantacuzene D, Leclerc C. Anti-tumor immunity provided by a synthetic multiple antigenic glycopeptide displaying a tri-Tn glycotope. Journal of Immunology. 2001;166:2849–2854. doi: 10.4049/jimmunol.166.4.2849. http://dx.doi.org/10.4049/jimmunol.166.4.2849. [DOI] [PubMed] [Google Scholar]
- Lo-Man R, Vichier-Guerre S, Perraut R, Deriaud E, Huteau V, BenMohamed L, et al. A fully synthetic therapeutic vaccine candidate targeting carcinoma-associated Tn carbohydrate antigen induces tumor-specific antibodies in nonhuman primates. Cancer Research. 2004;64:4987–4994. doi: 10.1158/0008-5472.CAN-04-0252. http://dx.doi.org/10.1158/0008-5472.CAN-04-0252. [DOI] [PubMed] [Google Scholar]
- Lua LH, Connors NK, Sainsbury F, Chuan YP, Wibowo N, Middelberg AP. Bioengineering virus-like particles as vaccines. Biotechnology and Bioengineering. 2014;111:425–440. doi: 10.1002/bit.25159. http://dx.doi.org/10.1002/bit.25159. [DOI] [PubMed] [Google Scholar]
- Miermont A, Barnhill H, Strable E, Lu X, Wall KA, Wang Q, et al. Cowpea mosaic virus capsid: A promising carrier for the development of carbohydrate based anti-tumor vaccines. Chemistry: A European Journal. 2008;14:4939–4947. doi: 10.1002/chem.200. http://dx.doi.org/10.1002/chem.200800203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles D, Roche H, Martin M, Perren TJ, Cameron DA, Glaspy J, et al. Phase III multicenter clinical trial of the sialyl-Tn (STn)-keyhole limpet hemocyanin (KLH) vaccine for metastatic breast cancer. The Oncologist. 2011;16:1092–1100. doi: 10.1634/theoncologist.2010-0307. http://dx.doi.org/10.1634/theoncologist.2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzavi-Karbassi B, Pashov A, Kieber-Emmons T. Tumor-associated glycans and immune surveillance. Vaccine. 2013;1:174–203. doi: 10.3390/vaccines1020174. http://dx.doi.org/10.3390/vaccines1020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasuraman S, Raveendran R, Kesavan R. Blood sample collection in small laboratory animals. The Journal of Pharmacy and Pharmacology. 2010;1:87–93. doi: 10.4103/0976-500X.72350. http://dx.doi.org/10.4103/0976-500X.72350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldao A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. Virus-like particles in vaccine development. Expert Review of Vaccines. 2010;9:1149–1176. doi: 10.1586/erv.10.115. http://dx.doi.org/10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- Thingstad T, Hilkens J. Tumor-host interactions regulate glucocorticoid-mediated epiglycanin expression in TA3Ha murine mammary carcinoma cells. Tumor Biology. 2003;24:116–129. doi: 10.1159/000073841. http://dx.doi.org/10.1159/000073841. [DOI] [PubMed] [Google Scholar]
- Thingstad T, Vos HL, Hilkens J. Biosynthesis and shedding of epiglycanin: A mucin-type glycoprotein of the mouse TA3Ha mammary carcinoma cell. Biochemical Journal. 2001;353:33–40. http://dx.doi.org/10.1042/bj3530033. [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Comellas-Aragones M, Chowdhury S, Bentley P, Kaczanowska K, BenMohamed L, et al. Boosting immunity to small tumor-associated carbohydrates with bacteriophage Qβ capsids. ACS Chemical Biology. 2013;8:1253–1262. doi: 10.1021/cb400060x. http://dx.doi.org/10.1021/cb400060x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Dulaney S, McKay C, Baniel C, Kaczanowska K, Ramadan S, et al. Chemical synthesis of GM2 glycans, bioconjugation with bacteriophage Qβ and the induction of anti-cancer antibodies. Chembiochem. 2016;17:174–180. doi: 10.1002/cbic.201500499. http://dx.doi.org/10.1002/cbic.201500499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Huang X. Recent development in carbohydrate based anticancer vaccines. Journal of Carbohydrate Chemistry. 2012;31:143–186. doi: 10.1080/07328303.2012.659364. and references cited therein http://dx.doi.org/10.1080/07328303.2012.659364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Nguyen HG, Chowdhury S, Bentley P, Bruckman MA, Miermont A, et al. Tobacco mosaic virus as a new carrier for tumor associated carbogydrate antigens. Bioconjugate Chemistry. 2012;23:1694–1703. doi: 10.1021/bc300244a. http://dx.doi.org/10.1021/bc300244a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Wright WS, McKay C, Baniel C, Kaczanowska K, Bentley P, et al. Significant impact of immunogen design on the diversity of antibodies generated by carbohydrate-based anti-cancer vaccine. ACS Chemical Biology. 2015;10:2364–2372. doi: 10.1021/acschembio.5b00406. http://dx.doi.org/10.1021/acschembio.5b00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltins A. Construction and characterization of virus-like particles: A review. Molecular Biotechnology. 2013;53:92–107. doi: 10.1007/s12033-012-9598-4. http://dx.doi.org/10.1007/s12033-012-9598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]