Abstract
Neurotransmitters including catecholamines and serotonin play a crucial role in maintaining homeostasis in the human body. Studies on these neurotransmitters mainly revolved around their role in the “fight or flight” response, transmitting signals across a chemical synapse and modulating blood flow throughout the body. However, recent research has demonstrated that neurotransmitters can play a significant role in the gastrointestinal (GI) physiology. Norepinephrine (NE), epinephrine (E), dopamine (DA), and serotonin have recently been a topic of interest because of their roles in the gut physiology and their potential roles in gastrointestinal and central nervous system pathophysiology. These neurotransmitters are able to regulate and control not only blood flow, but also affect gut motility, nutrient absorption, gastrointestinal innate immune system, and the microbiome. Furthermore, in pathological states such as inflammatory bowel disease (IBD) and Parkinson’s disease, the levels of these neurotransmitters are dysregulated, therefore causing a variety of gastrointestinal symptoms. Research in this field has shown that exogenous manipulation of catecholamine serum concentrations can help in decreasing symptomology and/or disease progression. In this review article, we discuss the current state-of-the-art research and literature regarding the role of neurotransmitters in regulation of normal gastrointestinal physiology, their impact on several disease processes, and novel work focused on the use of exogenous hormones and/or psychotropic medications to improve disease symptomology.
INTRODUCTION
It has recently become evident that the gut microbiota has the ability to influence physiological aspects of the body, including a direct communication to the brain from the gut (O’Mahony et al., 2015). In that regard, the gut microbiota has demonstrated unique functions associated with behavior, mood, and cognition that are currently being explored. The gut microbiota can interact with the chemical messengers involved in the transmission of information including monoamines such as 5-hydroxytryptamine (5-HT), also known as serotonin. Monoamines are not only synthesized in neural cells, but are also produced within the gastrointestinal system. Traditionally, it was thought that these monoamines functioned only in the central nervous system (CNS) as neurotransmitters and neuromodulators. However, it is now believed that monoamines influence a wide range of effects throughout the body. Several studies have suggested their fundamental roles in the gut microbiome and their indirect function in regulating the brain and cognitive processes.
The enteric nervous system (ENS), also known as the intrinsic nervous system, governs the function of the GI system. It can be found from the beginning of the esophagus to the anus embedded in the lining of the GI system. Therefore, although being in direct contact with the central nervous system (CNS) through innervation by the autonomic nervous system (i.e. sympathetic and parasympathetic), the GI tract has its own independent reflex activity. The interaction between the ENS and CNS, often described as the gut-brain axis, has been sparking researchers’ interest for many years.
The gastrointestinal tract’s impact on brain function has been recognized since the 19th century and in recent history, research on the gut-brain axis has largely been focused on digestive function. A plethora of studies now are exploring other possible physiological roles of the gut-brain axis and how dysfunction of this axis can cause various human diseases (Table 1). High co-morbidities exist between certain psychiatric symptoms and gastrointestinal disorders, a well-known example being anxiety and irritable bowel syndrome (IBS) (Reber, 2012). These connections indicate the relevance of the gut-brain axis in pathophysiology and therefore, modulators within the axis are appealing targets for novel therapeutic developments (Table 2). Neurotransmitters including serotonin, norepinephrine, epinephrine, and dopamine can play an important role in regulating gut-brain axis. Additionally, recent studies have suggested that the gut microbiota is a contributor to the pathophysiological effects of the gut-brain axis (Rhee et al., 2009).
Table 1. Diseases arising from or involved with neurotransmitter dysfunction in the gastrointestinal tract.
Summary of diseases for which evidence has linked the pathology to the gut-brain axis. Included is the role played by the neurotransmitter in the pathology, the change in neurotransmitter levels systemically and in the mucosa, and therapies for the pathologies that influence neurotransmitter levels.
| Disease/Symptom | Etiology of Disease | Neurotransmitter levels | Neurotransmitter based therapeutic agents | References |
|---|---|---|---|---|
| Ulcerative colitis |
|
|
None that target neurotransmitter signaling | Baumgart and Carding, 2007; Sartre, 2006; Coates et al., 2004; Magro et al., 2002 |
| Crohn’s disease |
|
|
None that target neurotransmitter signaling | Baumgart and Carding, 2007; Sartre, 2006; Kidd et al., 2009; Magro et al., 2002; Linden et al., 2003 |
| Irritable bowel syndrome |
|
|
5-HT3 receptor antagonists: alosetron or ondansetron 5-HT4 receptor agonists: lubiprostone or prucalopride 5-HT4 receptor partial agonist: tegaserod | Camilleri, 2012; Coates et al., 2004; Crowell, 2004 |
| Diverticulitis |
|
|
None that targets neurotransmitter signaling | Costedio et al., 2008; West, 2008; Jeyarajah et al., 2012 |
| Celiac Disease |
|
|
None | Di Sabatino et al., 2014 |
| Gastro-esophageal reflux disease |
|
|
SRI’s and serotonin agonists are being tested for use in restoring the esophageal sphincter | Ostovaneh et al., 2014 |
| Parkinson’s disease |
|
|
Deep brain stimulation of the subthalamus results in improvement of GI dysfunction | Sugama and Kakinuma, 2016; Natale et al., 2008; Toti and Travagli, 2014; Singaram et al. 1995; Krygowska-Wajs et al., 2016; Corbillé et al., 2016 |
| Depression |
|
|
Agonists and reuptake inhibitors of serotonin, norepinephrine, and dopamine | O’Mahony et al, 2015; Collins et al., 2012; |
| Anxiety |
|
|
GABA potentiation relieves anxiety. Serotonin increase through SSRI’s or tryptophan is shown to relieve symptoms. Corticotropin releasing hormone antagonists decrease anxiety. | Liang et al., 2015; Desbonnet et al., 2015 |
| Autism Spectrum Disorders (ASD) |
|
Neurotransmitter levels are widely varied and poorly understood in ASD | Cognitive and behavioral therapy | De Angelis et al, 2015; Rosenfeld, 2015; Moos et al., 2016 |
| Esophageal Motility disorders |
|
|
Administration of SRI’s and serotonin agonists have shown some success in the treatment of esophageal motility disorders | Karamanolis et al., 2015; Scheerens et al., 2015 |
| Hypersensitive esophagus |
|
|
Administration of SRI’s and serotonin agonists have shown some success in the treatment of Hypersensitive esophagus in select patients | Dickman et al., 2014; Viazis et al., 2011; Viazis et al., 2012 |
| Gastrointestina l bleed |
|
|
Care should be taken when administering SRI’s to patients with ulcers from H. pylori. Aspirin should be avoided in conjunction with SRI’s for patients who have ulcers from H. pylori, but still must take SRI’s | Dall et al., 2011; Wang et al., 2014 |
Table 2. List of receptors and the molecules that activate them.
Summary of the receptors known to be relevant to the gut-brain axis including inhibitors, agonists, and the locations in which the receptors may be found.
| Receptor | Inhibitors | Agonists | Location | References |
|---|---|---|---|---|
| α1 - adrenoreceptors |
|
|
|
Luo et al., 2016, Kim and Jwa, 2015, Manoharan et al., 2016, Hirst and Silinsky, 1975, Grayson and Oyebola, 1983 |
| α2- adrenoreceptors | N/A |
|
|
Shelkar et al., 2016 |
| β1-adrenoreceptors |
|
|
|
Manivasagam et al., 2016, Grayson and Oyebola, 1983 |
| β2-adrenoreceptors |
|
|
|
Edgell et al., 2016, Arcaro et al., 2016, Cortese et al., 2016, Blais et al., 2016, Grayson and Oyebola, 1983 |
| D1 |
|
|
|
Borcherding et al., 2015, Sclafani, 2001 |
| D2 |
|
|
|
##Ji and Wu, 2016, Jiang et al., 2016, Li et al., 2015, Lladó-Pelfort et al., 2016, Sclafani, 2001 |
| D3 |
|
|
|
Watson et al., 2015, Sander et al., 2016, Sclafani, 2001 |
| D4 |
|
|
|
Sclafani, 2001 |
| D5 |
|
|
|
Sclafani, 2001 |
| 5 - HT1 |
|
|
|
Kishi et al., 2013, Araldi et al., 2016, Gershon et al., 1990 |
| 5-HT2 |
|
|
|
Karaki et al., 2014, Higgins et al., 2016, Gershon et al., 1990 |
| 5-HT3 | • Ondansentron | N/A |
|
Neal and Bornstein, 2006 |
| 5-HT4 | N/A |
|
|
Lefebvre et al., 2016, Neal and Bornstein, 2006 |
This review article aims at discussing our most recent understanding of the interactions between neurotransmitters composed of catecholamines and serotonin, and the gut-brain axis. Here we highlight their synthesis, receptors, and relevant modulation of the human microbiota.
SEROTONIN
The brain-gut axis describes a network that communicates bi-directionally between the two organs where serotonin is a critical signaling regulator that modulates complex physiological functions, including gastric secretion or body temperature control (O’Mahony et al., 2015). Consequently, a serotonin dysfunction in the gastrointestinal system could result in impairments in brain function, such as those involved in mood, sleep, and behavior (Delgado et al., 1990; Berger et al., 2009).
Synthesis
Tryptophan is a precursor in the biosynthesis of serotonin (Figure 1). Tryptophan is an essential amino acid, found in dietary proteins, including meats, dairy, and fruits (Friedman et al., 2012). Once it has been processed, tryptophan is able to cross the blood-brain barrier and metabolize into serotonin in the raphe nuclei within the brain stem (Le Floc’h et al., 2010). However, it is now known that much of serotonin synthesis occurs in the enterochromaffin cells (EC) and enteric nerves found within the gastrointestinal tract, where the transient receptor potential (TRP) cation channel TRPA1 activity critically control serotonin release (Mawe and Hoffman, 2013; Nozawa et al., 2009). The synthesis of serotonin is identical in the CNS and in the gut, where tryptophan is first converted to 5-hydroxytryptophan (5-HTP) via tryptophan hydroxylase (TPH), the rate-limiting enzyme in the biosynthesis of enzyme. Then, aromatic amino acid decarboxylase (AAAD) almost immediately converts 5-HTP to 5-HT (Berger et al., 2009). Additionally, tryptophan could enter a separate, more dominant metabolic pathway, ultimately being converted to kynurenine via action by tryptophan-2, 3-dioxygenase (TDO) or the more readily available indoleamine-2,3-dioxygenase (IDO) (Schwarcz et al., 2012; Vécsei et al., 2013). The activities of these two enzymes are uniquely induced by different stimuli – for instance, TDO is prevalent in the presence of glucocorticoids and IDO is dominant during inflammatory events (Ruddick et al., 2006). One of the end products of kynurenine metabolism is kynurenic acid, an alpha 7 nicotinic acetylcholine receptor antagonist and N-methyl-d-aspartate (NMDA) receptor agonist at the glycine site, where kynurenic acid (KYNA) has been implicated to play a neuroprotective role (Vecsei et al., 2013). The other metabolite is quinolinic acid, an NMDA receptor and a known neurotoxin (Braidy et al., 2009). Due to the opposing roles of these two metabolites, their balance directly affects healthy and pathological states. Furthermore, it is important to note that when tryptophan is diverted to produce kynurenic acid and quinolic acid, the amino acid is then less available for serotonin synthesis. Only approximately 1% of available tryptophan goes on to metabolize into serotonin, and it has recently been demonstrated that 5-HT and kynurenine compete for available tryptophan, which leads to serotonin dysfunction during acute stress (Bender 1983; Keszthelyi et al., 2012). However, the complex role of kynurenine is beyond the scope of this review. There is a growing amount of evidence indicating that the gut microbiota regulates which metabolic pathway tryptophan will go, eventually affecting cognitive and local GI functions (O’Mahony et al., 2015).
Figure 1. Biochemical pathway for serotonin synthesis with inhibitors of serotonin receptors.
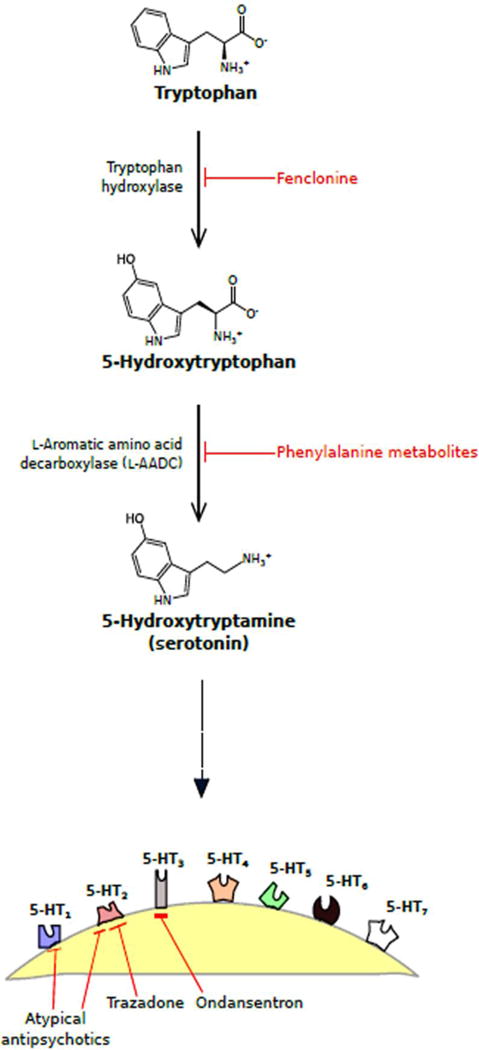
Synthesis pathway for serotonin (5-hydroxytryptamine, 5-HT) is depicted here with enzymes responsible for each step as well as known inhibitors of these enzymes. Serotonin will then bind to one of seven 5-HT receptors (5-HT1-7) at the nerve ending. A few known drug inhibitors of specific 5-HT receptors are also portrayed.
Receptors
Serotonin binds to specific receptors within the GI tract to produce a diversity of responses. Serotonin receptors have been divided into seven classes (5-HT1 to 5-HT7) and consist of a total of 14 known serotonin receptors (Hoyer et al., 2002; Wirth et al., 2016; Palacios, 2016). 5-HT1 has five receptor subclasses namely 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, and 5-HT1F, 5-HT2 has three subclasses, 5-HT2A, 5-HT2B, and 5-HT2C whereas 5-HT5 has two subclasses, 5-HT5A, and 5-HT5B (Hannon and Hoyer, 2008). Blockade of 5-HT1A receptors has been demonstrated to increase the severity of 2,4,6-Trinitrobenzene Sulfonic acid (TNBS)-induced colitis and exaggerated local and systemic neutrophil recruitment in a mouse model (Rapalli et al., 2016). On par with these findings, 5-HT1A agonist delayed and mitigated the severity of colitis, counteracting the increase in colonic 5-HT content. In contrast, blockade of 5-HT2A receptors improved global health conditions, reduced colonic morphological alterations, down-regulated neutrophil recruitment, inflammatory cytokines levels and colonic apoptosis. Antagonism of 5-HT3, 5-HT4, and 5-HT7 receptor sites did not remarkably affect the progression and outcome of the pathology or only slightly improved it. 5-HT4 agonists relieve visceral pain, as well as increase intestinal motility (Hoffman et al., 2012). The activation of 5-HT4 has also been found to prevent apoptosis of enteric neurons and inflammation caused by axon terminal degeneration and autophagy (Liu et al., 2009). Additionally, following ingestion of irritants, EC cells release more serotonin, which binds to 5-HT3 receptors in the GI to increase peristalsis and cause diarrhea as well as to 5-HT3 receptors in the chemoreceptor trigger zone within the brain stem to stimulate vomiting (Rang 2003). Furthermore, due to the presence of serotonin in both the brain and the gut, selective serotonin reuptake inhibitors (SSRIs) are not only able to treat depression and other neurological disorders/syndromes, but are also capable of decreasing pain and other symptoms associated with chronic GI disorders (Vanuytsel et al., 2014); these results were obtained independently to the gut-brain axis.
Serotonin in the GI tract: interaction with the microbiota
The gut microbiome refers to the population of microorganisms residing in the gut. Recently there has been increased interest in understanding how the gut microbiota influences the brain-gut axis. It has been demonstrated that patients suffering from IBS had significantly lower mucosal and higher systemic concentrations of both 5-HT and KYNA in comparison to healthy controls (Keszthelyi et al., 2013). This study disproved the hypothesis that increasing activation of the kynurenic pathway results in serotonin dysfunction; rather, it was suggested that both substances were released into the systemic compartment leading to complications in the gut. Interestingly, this study also uncovered a correlation between mucosal concentrations of KYNA and 5-HT in IBS patients and psychological state changes, evaluated using the Hospital Anxiety and Depression Scale and the Symptom Checklist-90. This indicates a possible secondary consequence due to changes in the stability and diversity of the microbiota found in IBS (Keszthelyi et al., 2013).
Serotonin synthesis not only has to compete for its precursor tryptophan from undergoing the kynurenic pathway, but the gut microbiota itself could directly utilize tryptophan, reducing its availability to the host. Bacterial species, such as Escherichia coli, Achromobacter liquefaciens, and Paracolobacturm coliforme, break down tryptophan into indole via the enzyme tryptophanase. By-products of this conversion include pyruvate, which can be utilized in other metabolic functions, such as in cellular respiration, and ammonia, which in large amounts is toxic to the intestinal epithelium (Smith and Macfarlane, 1997).
A balanced gut microbiota is necessary for the physiological function of the mucosa’s immune defense. Several studies have indicated that presence of bacteria is necessary to trigger normal immune responses. For instance, compromised expression of certain toll-like receptors (TLRs), responsible for differentiating pathogenic bacteria from harmless commensal ones and decreased IgA secretion were found in germ-free, gut microbiota-deficient, animals (Wostmann et al., 1970; Shanahan, 2002; Grenham et al., 2011). When the mucosa was allowed to colonize in germ-free animals, however, the mucosal immune system function is salvaged (Umesaki et al., 1995). Serotonin contributes to the immune response as 5-HT receptors have been found on lymphocytes, monocytes, macrophages, and dendritic cells (Cloez-Tayarani and Changeux, 2007). Factors that negatively affect serotonin’s immune-modulatory role could result in intestinal inflammation and the pathogenesis of gastrointestinal disorders, including Crohn’s disease, ulcerative colitis, celiac disease and diverticulitis (Shaw et al., 2010). In animal models, inducing inflammation with an infection resulted in a downregulation in sodium-dependent serotonin transporter, SERT, while the 5-HT and EC cell number increases (O’Hara et al., 2006; Wheatcroft et al., 2005). The precursor for serotonin (5-HTP) also promotes actin remodeling seen in microvilli development for phagocytic activity in immune cells and induces extracellular signal-regulated kinases (ERK) phosphorylation in macrophages (Nakamura et al., 2008).
Recent studies from our laboratory showed that increased levels of serotonin can enhance quorum sensing in Pseudomonas aeruginosa both in vitro and in vivo (Knecht et al., 2016). Quorum sensing is the process by which bacteria in close proximity are able to communicate with one another through chemical signaling (Castillo-Juárez et al., 2015). This bacterial communication is necessary for biofilm formation, swarming motility, induction of gene expression, exopolysaccharide production, and the exchange of virulence factors (Sauer, 2002; Liu et al., 2015; Abraham, 2016). Therefore, high serotonin levels resulted in increased P. aeruginosa pathogenicity by enhancing biofilm formation and elaboration of virulence factors, in vitro (Knecht et al., 2016). In a mouse model, we observed that animals treated with exogenous serotonin and infected with P. aeruginosa exhibited an increase in intestinal bacterial load and mortality compared to untreated animals. The administration of exogenous serotonin enhanced the production of pro-inflammatory cytokines, increased biofilm formation on mouse intestines and worsened intestinal pathological manifestations. Intriguingly, serotonin was able to restore the ability of avirulent quorum-sensing P. aeruginosa mutant to cause intestinal infection in mice. These discoveries call for further research in patients with high levels of serotonin whether due to physiological, pharmacological, or pathological reasons. Drugs such as SSRI’s used for the treatment of different mental disorders with the goal of increasing serotonin, despite being useful in the management of psychiatric issues, may also present negative side effects on patients. The action exerted by serotonin can be various and is dependent on the subtype of receptor with which the interaction occurs. Altogether, results suggest that serotonin plays both beneficial and detrimental roles, particularly in the gut. Therefore, therapeutic strategies targeting serotogenic system should be carefully designed considering the antithetical effects of these monoamines.
CATECHOLAMINES
Catecholamines are monoamines comprised of a catechol group and an amine side chain. The synthesis and degradation of these amines are well defined in the literature and have a wide impact on the human body. There are three main catecholamines: norepinephrine (noradrenaline), epinephrine (adrenaline), and dopamine; norepinephrine and epinephrine are also known as the “fight or flight” peripheral catecholamines while dopamine is a central acting catecholamine. It involves numerous neural pathways such as reward pathway via the nucleus accumbens. Dopamine is commonly associated with the ‘pleasure system’ of the brain, providing feelings of enjoyment and exhilaration.
Synthesis
Norepinephrine and epinephrine are mainly produced in two places; first being at the end of postganglionic sympathetic nerve fibers (local response), and second being synthesized by chromaffin cells of the adrenal medulla (systemic response). The local response is mainly controlled by the hypothalamus-pituitary adrenal axis while the systemic response is neural mediated. Dopamine, on the other hand, is synthesized mainly in the brain.
All catecholamines are derived from L-tyrosine. Using this base molecule, tyrosine hydroxylase, with co-factors, tetrahydrobiopterin and oxygen, convert L-tyrosine to L-dopa (Figure 3). Tyrosine hydroxylase is the rate limiting enzyme in the overall synthesis of catecholamines (Shiman et al., 1971). L-dopa is further manipulated into Dopamine with the enzyme DOPA decarboxylase and cofactors pyridoxal phosphate. Dopamine is the first catecholamine made and is found in the central nervous system. In peripheral tissues, dopamine is further manipulated by dopamine B-hydroxylase, with cofactors ascorbate and oxygen, to form norepinephrine and subsequently by phenylethanolamine N-methyltransferase, with cofactor S-adenosylmethionine, to finally make epinephrine. The last three products (dopamine, norepinephrine, and epinephrine) in this biosynthetic pathway are classified as catecholamines and each have specific properties and functions on various organ systems.
Figure 3. Neurotransmitters affect microbiota in the gut.
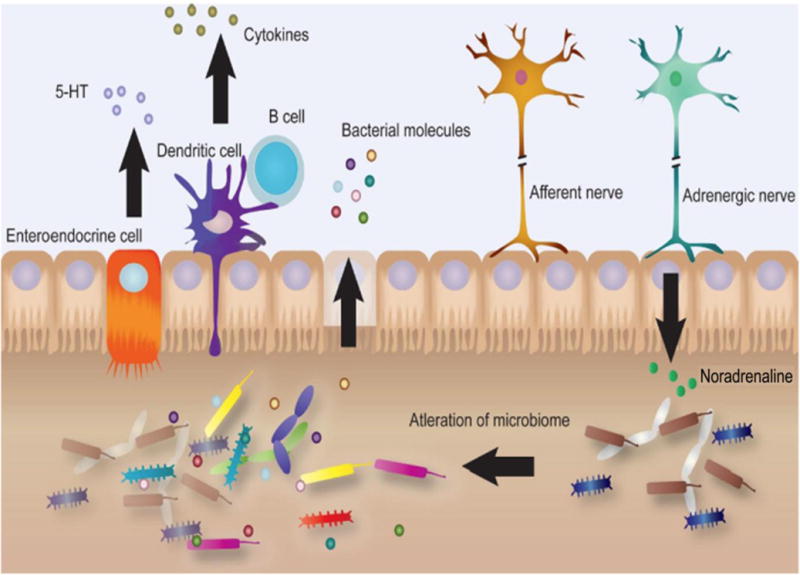
Neurotransmitters including serotonin alter the microbiota in the gut that can modulate the production of cytokines and bacterial byproducts leading to either heathy or diseased state.
The most important enzyme in catecholamine synthesis pathway is tyrosine hydroxylase. Extensive research has been conducted to determine regulation of this enzyme, which ultimately regulates the production of all three catecholamines. The main mechanism is through endogenous neuropeptide Y (NPY). The mechanism through which this works is complex, but mainly works by inhibiting Ca2+ influx through L-type Ca2+ channels through PKC pathway which subsequently inhibits neuronal depolarization and halts catecholamine synthesis (McCullough and Westfall, 1996). Other tyrosine hydroxylase inhibitors, such as alpha-methyl-p-tyrosine, has been shown to decrease overall synthesis of catecholamines (Thompson et al., 1983).
Receptors
A number of studies have been carried out to determine the receptor of choice for norepinephrine and epinephrine. Their affinities do not change in the gut. Epinephrine mainly exerts its effects on α1 and β2 while norepinephrine exerts its effect on α1 and α2 with minimal, if any, effect on β2. This subtle difference determines each catecholamine’s specific effect on the entire gut, in terms of absorption, blood flow and motility. Dopamine on the other hand, despite being the precursor to epinephrine and norepinephrine, has its own receptors (D1 to D5). As of late, dopamine has been accepted as another major catecholamine that is involved with gut homeostasis. In terms of receptor location, D4 receptors are found only in the mucosal layer, whereas D1, D3, and D5 receptors can be found in both the nerve ending layer of the intestinal wall as well as the gut mucosa, and lastly D2 receptors are found only in the nerve ending layer of the intestinal wall. Studies have pointed towards the fact that the D2 receptor is the major mediator of the endogenous effects of dopamine (Sclafani, 2001).
Catecholamines in the GI tract: blood flow
The major blood supply to the splanchnic region comes from the celiac trunk, superior mesenteric artery, and inferior mesenteric artery and all three sources eventually flow into the portal system aimed into the liver. Hemodynamics with the administration of catecholamines is a complex subject and many variables come into play such as blood volume, organ failure, and concurrent hormones. Broadly, norepinephrine mainly acts on the alpha receptors at all concentration ranges which results in vasoconstriction, increasing vascular resistance, and a decrease in overall blood flow to the intestines. Norepinephrine is a last resort drug for patients with hemodynamic instability for which stability cannot be reached with the other catecholamines. Compared to its counterpart, epinephrine stimulates beta receptors leading to vasodilation at low doses, which would increase blood flow, and vasoconstriction at high doses similar to norepinephrine. These effects are seen in patients with a normal cardiac output and changes in heart function or during times of stress often skew these effects. Many studies have been performed on septic patients, patients with renal failure as well as heart failure and conflicting results have been found (Thompson et al., 1983; Abrass et al., 1985; Richardson and Withrington, 1982).
Although dopamine has five receptors, the main vasoactive receptor is the D1 receptor, which stimulates adenylyl cyclase and increases protein kinase C activity. Dopamine receptors are primarily found in the mesenteric, coronary, cerebral, gastric, and hepatic vasculature. Dopamine is a unique catecholamine because its receptor affinity is concentration dependent. At low doses (i.e. 1–3 μg/kg), it preferentially binds to its D1 receptors. At medium doses (i.e. 3–10 μg/kg) it additionally binds to β1 adrenergic receptors. At high doses (> 10 μg/kg) it additionally binds to α1 adrenergic receptor. Overall, low dose dopamine is known to cause vasodilation (similar to beta adrenergic receptors) which increases splanchnic blood flow through interaction with D1 receptor. At high doses, it can be considered similar to other catecholamines and be classified as a vasoconstrictor and decrease splanchnic blood flow (Grayson and Oyebola, 1983).
Catecholamines in the GI tract: nutrient absorption
Epinephrine and norepinephrine play a major role in changing absorption rates as per the needs of human body. In a study comparing glucose concentrations between jejunum arterial flow versus venous flow before and after administration of intravenous epinephrine, a significant hyperglycemic response has been demonstrated post-injection compared to controls. To localize the specific receptor subtype, they co-administered either propranolol, a beta receptor antagonist, and/or prazosin, an alpha receptor antagonist, with the catecholamine. The researchers concluded that simulation of the beta receptor was the cause of the hyperglycemic response (Grayson and Oyebola, 1983). Furthermore, epinephrine co-administered with thyroid hormone caused a significant overall increase in glucose concentrations compared to thyroid hormone alone in a dose dependent manner (Olaleye and Elegbe, 2005). This shows that epinephrine has the ability to potentiate the actions of other endogenous hormones.
Besides glucose absorption, epinephrine also affects absorption of several other molecules and ion transporters. Using rat jejunums, epinephrine showed increased rates of Dextran absorption (4000 Da) via paracellular route. Interestingly, absorption rates varied depending on the stimulation of specific receptor. Alpha and beta stimulation showed an increased rate of absorption compared to controls. Beta stimulation alone (co-administration with alpha antagonists) showed an increase rate of absorption but not to the extent seen in of alpha stimulation alone or with alpha and beta stimulation (Kamio et al., 2005). In addition, histological examination of jejunum mucosa showed no change.
A similar phenomenon occurs with the transport of oligopeptides in the gut. Epinephrine increases the concentration of oligopeptide transporters on the apical surface of mucosal intestinal cells to augment the uptake of oligopeptides from the lumen (Berlioz et al., 2000). Epinephrine has also been shown to increase transporter rates in transporters such as: Na+/H+ antiporter (Isom et al., 1987), Na+/K+/2Cl− cotransporter (Haas et al., 1995), Na+/glucose cotransporter (Ishikawa et al., 1997), and benzyl-penicillin transporter via transcellular movement (Skowronski et al., 2000). Taken overall, the alpha receptor subtype is the predominant receptor that governs intestinal absorption rates. In terms of beta receptors, conflicting evidence has been shown in which concurrent beta receptor antagonism and alpha receptor stimulation result in a higher absorption rate than alpha and beta receptor stimulation.
Compared to the other catecholamines, dopamine plays a small role in regulating electrolyte absorption in the GI tract. First, studies using rabbit ileum showed that dopamine stimulation increased Na+ and Cl− influx with concurrent decrease in Ca2+ influx. The researchers in this study concluded that dopamine’s cause of increased absorption acted indirectly through cross reaction with α2 receptors which can happen at medium to high doses of dopamine (Donowitz, 1983). A conflicting study showed an opposite reaction with dopamine stimulation using rat jejunums who were fed a high salt diet. An inverse correlation was found with Na+/K+ ATPase activity and dopamine concentration; this relationship was abolished with the administration of dopamine synthesis antagonists (Vieira-Coelho et al., 1998). Lastly, dopamine synthesis blockers has been found to play a role in mucosal protection against ulcer formation. Administration of catechol-O-methyl transferase (COMT) inhibitors with and without concurrent administration of adrenergic receptor blockers, both alpha and beta blockers, showed a dose dependent increase in bicarbonate secretion in rat duodenum via stimulation of peripheral D1 receptors (Flemstrom et al., 1993). Dopamine is likely to play a role in nutrient-derived post ingestive signaling and reward (Ren et al., 2010). It is already known that oral stimulation with glucose produces a significant increase in dopamine release; interestingly, a significant increase in glucose was also seen in ageusic animals (de Araujo et al., 2008). Furthermore, intragastric infusions of glucose stimulated significantly higher levels of dopamine levels in the nucleus accumbens compared to isocaloric intragastric infusions of L-serine (Ren et al., 2010). Taken together, these studies provide evidence that dopamine efflux in the reward pathway is produced by direct stimulation of the gastrointestinal tract.
Catecholamines in the GI tract: interaction with the innate immune system
The gut hosts a wide variety of bacteria that are essential for human well-being. They help in digestion, produce vitamins, and protect against harmful bacteria. However, because of the constant interaction with bacteria, the gut must have a large immune system and innate properties to protect the host from invading organisms. The majority of catecholamines that interact with the enteric system is mediated through postganglionic sympathetic neurons located in the celiac, superior mesenteric, and inferior mesenteric ganglia. These neurons interact with the intestines at the serosal surface and innervate the vascular beds as well as the enteric nervous system. These post-ganglionic sympathetic axons project to almost every lymphoid tissue throughout the body. Specifically, within the gut the majority of these axons terminate in the Peyer’s patches, intestinal aggregates of lymphoid follicles, while a few of these fibers terminate in the lamina propia (Janig, 2014). Interestingly, these axons do not make the traditional synaptic contacts as they would in the majority of the body (Vizi and Elenkov, 2002). Instead, the norepinephrine that is released from axon terminals must diffuse through a network of tunnels, sometimes travelling up to 1 μm, before interacting with postsynaptic receptors. Therefore, sympathetic innervation of the local sections of lymphoid tissue can be activated with relatively few sympathetic postganglionic axons (Dunn et al., 1999). In addition, serotonin and catecholamines released from the neurons can influence the microbiota present in the gut leading to altered release of cytokines and bacterial molecules (Figure 3).
The gut innate immune system provides the first line of defense against foreign pathogens. Macrophages constitute an integral part of gut innate immune system that circulate and provide constant surveillance of GI pathogens. Recent research has shown that depending on the location of the macrophages, they express a high degree of gene-expression specialization. Mucosal macrophages (Farache et al., 2013) are located in close proximity to the intestinal lumen while their counterparts, muscularis macrophages (Bogunovic et al., 2009) are located in the region between the two gut muscle layers alongside enteric neurons. It is found that these two distinct group of macrophages play different roles in both protection and regulation of gut mucosa and motility. The mucosal macrophages are involved in constant surveillance for pathogenic bacteria and play a role in tolerance to dietary antigens (Cervi et al., 2014). On the other hand, the muscularis macrophages regulate the enteric neurons and peristalsis. Furthermore, it is found that subpopulations of B-cells, neutrophils, and macrophages, specifically muscularis macrophages, express a large number of β2 adrenergic receptors (Cervi et al., 2014). It is also well described in the literature that alpha adrenergic signaling boosts inflammation while beta adrenergic signaling suppresses both innate and adaptive immunity (del Rey and Besedovsky, 2008; Guereschi et al., 2013). Taken together, it is postulated norepinephrine can mitigate the local pro-inflammatory state induced by mucosal macrophages via the muscularis macrophages. In addition, due to the close proximity of muscularis macrophages to enteric neurons, the local concentration of norepinephrine can lead to local suppression of inflammation and not have systemic effects (Farache et al., 2013; Abrass et al., 1985). This immune suppression occurs by downregulation of the Th1 immune response as evident by a decrease in INF-γ, TNF-α and IL-2 production (Kin and Sanders, 2006).
In terms of immunoglobulins, secretory IgA are constantly secreted transluminally across epithelial cells to provide protection along the mucosa. Adrenergic receptors in proximity to the IgA secretory B-cells and immunoglobulin receptors can be stimulated to release IgA and increase the concentration of IgA receptors, via mRNA expression (Mestecky and Russell, 2009), in both porcine large and small intestines (Macpherson and Slack, 2007). Rats in which were chronically stressed showed increased IgA positively stained intestinal cells. When these rats underwent sympathectomy with 6-hydroxydopamine, the number of IgA immunoreactive cells were significantly decreased (Reyna-Garfias et al., 2010). Further evidence of stress hormones affecting IgA secretion were shown in marathon runners where biopsies of their intestines confirmed the increased concentration of IgA (Nilssen et al., 1998).
Interestingly, newer evidence is suggesting that catecholamines not only suppress the host immune system, but also augments and stimulates bacterial virulence factors and pathogenesis of bacterial disease. In a non-immune manner, stress hormones can also interact with the bacteria and the environment to aid bacteria to thrive rather than affecting the host immune system (Lyte, 2004). Gram-negative bacteria incubated in nutrient deficient environments to mimic the intestinal environment showed a several log-fold increase in growth compared to control group following exposure to catecholamines (Lyte and Ernst, 1992; Lyte et al., 1997). Many bacteria have shown this response including Campylobacter jejuni (Cogan et al., 2007), Escherichia coli (Lyte et al., 1996; Diard et al., 2009), Helicobacter pylori (Doherty et al., 2009; Lyte, 2010), Pseudomonas aeruginosa (Li et al., 2009; Hegde et al., 2009), and Salmonella enterica spp (Bailey et al., 1999). The mechanism of this action is not quite fully understood but it is postulated that the structural presence of hydroxyl groups at the 3 and 4 position rather than receptor stimulation at the bacterial level causes this effect (Bailey et al., 1999). Studies aimed at determining whether alpha and beta blockers co-administered with catecholamines affect bacterial growth, observed no significant variation therefore further supporting the fact that the structure of these catecholamines is the mechanism through which they exert their effects.
Survival of bacteria depends partly on their ability to sequester iron. Pathogenic bacteria secrete siderophores to combat the human sequestration of iron via lactoferrin/transferrin glycoproteins (Freestone et al., 2000). These siderophores have similar structural resemblance to catecholamines with the 3,4 dihydroxyl group (Bailey et al., 1999). Catecholamines were found to facilitate the removal of iron from human lactoferrin and transferrin in a dose and time dependent manner which also correlated with bacterial growth. The mechanism by which this happens occurs through the formation of direct complexes with Fe3+ and the subsequent reduction of Fe3+ to Fe2+ which therefore releases the iron to be available to bacteria (Freestone et al., 2000).
In addition to iron release, catecholamines are able to stimulate the expression of various virulence factors which further facilitate bacterial invasion. Norepinephrine has been shown to increase the expression of K99 pillus adhesions (Lyte et al., 1997), release of shiga-like toxins (Lyte et al., 1996) of E. coli which then allowed more bacteria to adhere to the colonic wall. Norepinephrine also increase the expression of flagella and type III secretion systems in Salmonella (Moreira et al., 2010). Furthermore, iron can also increase the expression of flagella and type III secretions in Salmonella, norepinephrine therefore aids in Salmonella’s growth via an indirect manner (Bearson et al., 2010; Ellermeier and Slauch, 2008).
Catecholamines in the GI tract: gut Motility
The gut is highly sensitized by the sympathetic system and motility is highly effected. Epinephrine’s preference towards beta adrenergic receptors usually cause smooth muscle relaxation, therefore slowing the overall transit and decreasing the amount of migratory motor complexes. Interestingly, if a beta adrenergic receptor is blocked through phentolamine administration, the remaining alpha receptors can cause intestinal smooth muscle contraction, speeding up overall intestinal motility (Hirst and Silinsky, 1975). Studies using rat colon showed that administration of catechol-O-methyltransferase inhibitors, which inhibits degradation of catecholamines, inhibited longitudinal muscle contraction therefore decreasing the colonic transit. When co-administered with a β2 adrenergic receptor blocker, the colonic inhibition was abolished suggesting that beta adrenergic stimulation in the colon participate in inhibition of colonic transit. Further supporting this evidence, acute and chronic stressed rats showed increased circulating norepinephrine causing increase gene transcription of Ca2+ L-type channels in colonic smooth muscle which resulted in enhanced colonic motor function (Tache et al., 2001; Choudhury et al., 2009).
The effects of D2 receptors are specific and distinct. Comparing D2 to D3 knock-out mice, D2 knock-out mice showed increased total GI tract and regional colonic motility. This shows that D2 mediated effects slow GI overall transit time, similar to its catecholamine counterparts. In addition, the same study showed that D2 knock mice had severe problems in weight gain. These mice with faster intestinal tract time had ate and drank more but failed to gain weight normally when compared to D3 knockout mice and resulted in an increased dry weight and water content of the stools, suggesting possible absorption problems (Gagon, 1970; Costa and Furness, 1976). Furthermore, D2 knockout mice had an enhanced peristaltic reflex therefore it follows that D2 mediation inhibition occurs within the ganglia which affect the microcircuits that govern overall peristaltic and/or secretory function. This hypothesis is further supported by the aforementioned location of D2 receptor found at the neuritic process myenteric and submucosal neurons (Li et al., 2006). This location is of importance because it is in close proximity of acetylcholine releasing neurons. Dopamine and D2 agonist have been shown to reduce the release of acetylcholine from enteric neurons (Kusunoki et al., 1985).
It is also postulated that dopamine plays a role in gastric emptying. Gastric empting rates as well as dopamine efflux were measured comparing administration of high versus low caloric lipid emulsions. There was a direct correlation between the two, high caloric emulsion was related to high dopamine concentrations in the ventral and dorsal striatum as well as slower gastric emptying and vice versa. There are a lot of other factors that also come into play including gastric distension (Feifel et al., 2003), caloric density, gastric secretion of ghrelin, physical factors, and CCK (Ferreira et al., 2012).
NEUROTRANSMITTERS AND DISEASES
Serotonin and catecholamines are extensively involved in numerous physiological processes and as such, changes in their levels and/or activity are associated with a myriad of human diseases (Table 1) (Berger et al., 2009; O’Mahony et al., 2015). Recently there has been a renowned interest in understanding the role of neurotransmitters in a number of brain disorders including autism as patients suffering from these disorders exhibit profound gut dysbiosis (De Angelis et al, 2015; Rosenfeld, 2015; Moos et al., 2016). Future investigations will help in deciphering the contribution of neurotransmitters in modulating gut-brain axis during autism.
Catecholamine levels affect the whole body, from the brain with sensation of happiness to the regulation of the GI tract. It is therefore not surprising that diseases of the CNS can have a profound impact on the ENS. Parkinson disease (PD) is a prime example of what happens when the intricate connections between CNS and ENS are jeopardized. It is a neurodegenerative condition that is mainly characterized by the loss of dopaminergic neurons in the nigrostriatal and mesolimbic pathways including the ventral tegmental area (Sugama and kakinuma, 2016). As first described by James Parkinson, GI dysfunction is an important feature of PD. It represents the most common autonomic disorder of the disease. In the ascending colon of PD patients the levels of DA were found to be decreased, and thus it seems to be a major candidate for the impairment of GI function. DA has been shown in rats to modulate fluid absorption, motility, blood flow, exocrine secretion, and cytoprotection (Natale et al., 2008). Dopaminergic degeneration of the substantia nigra pars compacta in rats induced by injection of 6-hydroxy-dopamine is associated with neurofunctional and neuroanatomical changes of the brain-gut axis circuitry controlling but not limited to the stomach. The delayed gastric emptying associated with these alterations is similar to the gastric dysfunctions observed in PD patients (Toti and Travagli, 2014). Further down the GI tract, clinical studies comparing colonic tissue from 11 patients with advanced Parkinson disease to control subjects showed that patient with Parkinson disease had substantially fewer dopaminergic myenteric neurons (Singaram et al., 1995). Nonetheless, as a way to alleviate most of the symptoms associated with the neurological condition the use of subthalamic deep brain stimulation may be recommended for PD patients. This revolutionary modality has been suggested to improve gastric motility and reduce GI dysfunction (Krygowska-Wajs et al., 2016) amongst other benefits such as tremor reduction. As for diagnostic purposes, recent work demonstrated that peripheral neurons contained in the ENS are affected by Lewy pathology which can appear before first symptoms. Therefore, a GI biopsy can be a source of biomarkers in PD (Corbillé et al., 2016), underlying the importance of early detection.
Yet another intestinal disorder dependent on hormone levels, IBS causes abdominal pain, bloating, diarrhea, and constipation. Its cause is not well understood but symptoms can be controlled by managing diet, lifestyle, and stress. Medications and counseling may be required. Patients with IBS show evidence for increased noradrenergic activity consistent with downregulation of presynaptic inhibitory α2 adrenergic receptors. Activity within the central arousal circuits is biased toward greater excitability and reduced inhibition in IBS. These abnormalities could be explained by an early life trauma as explained by Berman and al. (Berman et al., 2012). Evidence suggests a great deal of differences in neuroendocrine levels in women during sleep between those suffering from constipation-predominant IBS and those suffering from diarrhea-predominant IBS. Indeed, when compared to controls, the constipation-predominant group demonstrated significantly increased levels of E and NE whereas the diarrhea-predominant group demonstrated significantly lower NE. Such results suggest that neuroendocrine profiles during sleep may help us understand symptom expression in IBS (Burr et al., 2009). Since a large factor of IBS is due to stress, enhanced stress responsiveness has been implicated as a possible mechanism. However, although dysregulation in the stress-response systems such as the hypothalamic-pituitary-adrenal axis and mucosal immune function have been demonstrated in IBS, they seem not to have a primary role and the severity of the disease and abdominal pain (Chang et al., 2009).
When inflammation of the intestine resulting from an autoimmune reaction occurs it is often referred to as IBD. There are two major types of IBD, ulcerative colitis (UC) and Crohn’s disease (CD). An interesting study looking at the two types, side by side, noted that NE tissue levels in both inflamed and non-inflamed colonic mucosa were markedly lower in CD, but not in UC. However, there were increased levels of L-dopa and decreased levels of DA in both diseases, resulting in a reduction in DA/L-DOPA tissue ratios indicating low L-amino acid decarboxylase activity (Magro et al., 2002). The treatment of UC with D-2 receptor agonists decreased its severity in animal models by attenuation of enhanced vascular permeability and prevention of excessive vascular leakage. The fact that dopamine agonists could rescue normal function is evidence that the impairment of the dopaminergic system is a feature of IBD pathogenesis (Tolstanova et al., 2015).
In the CNS, serotonin is a key player within cognitive spheres involved in mood and behavior, as found in pathological models (O’Mahony et al., 2015; Collins et al., 2012). Chronic stress in particular is well understood to cause adverse effects in behavior, cognition, as well as in gut microbiota. It has recently been shown that administration of probiotics can improve chronic stress-induced depression. Rats within the study were subjected to 21 days of restraint stress followed by behavioral testing and biochemical analysis, and every day, Lactobacillus helveticus NS8 was supplemented until the end of the experiment with SSRI citalopram serving as a positive control. The study determined that the addition of the daily probiotic improved anxiety, depression, and cognitive dysfunction comparable to the positive control. Plasma levels of hormones released from stress (corticosterone, adrenocorticotropic hormone, and norepinephrine) were also found to decrease (Liang et al., 2015). Another study determined that microbiota is necessary to initiate of behavioral despair and anxiety-like behavior, such that microbiota depletion resulted in decreased anxiety. Mice were treated with antibiotics from weaning onwards to assess potential changes in adulthood. Not only was decreased anxiety-like behavior was observed in the mice, but deficits in cognition were evident as well. In addition, neuromodulators, including tryptophan, monoamines, and neuropeptides, and brain-derived neurotrophic factor expression were greatly reduced when evaluated during adulthood. These results indicate that early life events can influence adult behaviors via neurochemical changes that resulted from altering the microbiota (Desbonnet et al., 2015).
The diseases linked to a dysregulation in serotonin levels can affect virtually any organ of the gastrointestinal tract. Increasing serotonin availability between neurons and their effector cells is the primary mechanism by which re-establishment of normal physiological function can be achieved. The relationship between esophageal disease and serotonin is one that is primarily centered on the use of serotonin reuptake inhibitors (SRIs) and serotonin agonists for treatment. At this time, SRIs and serotonin agonists are only sparsely used in the management of upper GI tract disorders. Nevertheless, studies are looking into their use to treat esophageal motility disorders (Karamanolis et al., 2015; Scheerens et al., 2015), gastroesophageal reflux disorders (Ostovaneh et al., 2014), and hypersensitive esophagus (Dickman et al., 2014; Viazis et al., 2011; Viazis et al., 2012). Recent research analyzing the effectiveness of the serotonin agonist Buspirone in the treatment of esophageal dysfunction associated with systemic sclerosis demonstrated an increase in esophageal motility in 30 patients (Karamanolis et al., 2015). Other investigations inspired by this study tested greater levels of Buspirone, amongst other similar compounds, and found that serotonin agonists produce enough effect to warrant greater study and consideration (Scheerens et al., 2015). A study by Wu et al. presented evidence that serotonin disrupts tight junctions in the esophagus by reducing the expression of their proteins (Wu et al., 2016). This study calls for greater investigation since the breakdown of tight junctions could result over time in dramatic consequences such as tumor progression and metastasis.
The effect of serotonin on the stomach is a widely discussed topic but very few studies have found reliable data. Nonetheless, a series of studies have demonstrated that serotonin may be an aggravating factor in dyspepsia. In addition, it has been proposed that SRIs may exacerbate upper gastrointestinal bleeds in patients with Helicobacter pylori infections (Dall et al., 2011). Furthermore, evidence shows that a combination of SRIs and aspirin significantly improves the likelihood of gastrointestinal bleed (Wang et al., 2014).
Also, elevated levels of mucosal serotonin in the small intestine, such as seen in celiac disease, can cause increased immune response in the gut and thus have negative effects on patients (Di Sabatino et al., 2014). Unwanted symptoms from serotonin excess can also be found in ulcerative colitis and Crohn’s Disease (Guseva et al. 2014; Kidd et al. 2009; Minderhoud et al. 2007; Yu et al. 2016).
Interestingly, in patients suffering from irritable bowel disease, as previously seen with opposed levels of catecholamines, an increase in serotonin is correlated with diarrhea whereas a decrease is correlated with constipation (Yu et al., 2016; Zang et al., 2016). Irregularities in serotonin levels have also been observed in diverticulitis where a dysfunction in serotonin transporters results in inflammation compromising the intestinal wall (Costedio et al., 2008).
The effects of serotonin on the GI tract are numerous, its benefits in the treatment of pathologies still seem uncertain. The fact that serotonin application results in opposite outcomes in various disease processes is contradictory. Therefore, care providers should proceed with caution when considering to prescribe medications that increase the stimulation of serotonin receptors.
ADENOSINE TRIPHOSPHATE
Adenosine triphosphate (ATP) is classically known as the major driving force for chemical reactions in the body, but ATP can also participate in the transduction of neurological signals by acting as a neurotransmitter or co-transmitter. As a neurotransmitter, ATP and adenosine act primarily on the purinergic receptors of class P1, P2X, and P2Y. P1 and P2Y are G-protein coupled receptors, while P2X is a ligand-gated ion channel. In the enteric nervous system, it is unclear what role purinergic receptors play, but it is known that the receptors can be found in the gut, particularly in the myenteric plexus. The data on these receptors has not yet provided a clear picture of the role these receptors play. On the one hand, mice born without purinergic receptors in the gut display no evident physiological abnormalities (Sun et al., 2001). Yet in tissues of organisms with purinergic receptors, ATP is found to have an activating effect on gut motility and is seen as an important element of signal transduction for the submucosal plexus of guinea pigs (Monro et al., 2004). In pathologies, ATP has been found to be elevated in inflamed regions of the gut, but no studies thus far have found what role ATP plays in gut inflammation (Diezmos et al., 2016). Outside of this, the role of ATP is not well understood in the gut, seemingly because the body is capable of adapting to states in which ATP is imbalanced (Ren and Bertrand, 2008).
γ-AMINOBUTYRIC ACID
The neurotransmitter γ-aminobutyric acid (GABA) is the most known and essential inhibitory neurotransmitter of the CNS. Dysfunctions of GABA transmission and signaling have been implicated in a variety of mental illnesses, including anxiety and depression (Cryan, et al., 2005). Ongoing studies are revealing the complexity of the microbiota in modulating GABAergic signaling in the CNS. In addition, there has been an extensive amount of research that has concluded that GABA mediates the enteric nervous system and therefore, is involved in gastrointestinal function. Contrary to its role within the CNS, GABA is involved in neuronal excitability within the ENS, particularly the GABA-GABAA receptor system (Seifi et al., 2014). GABA contributes especially to GI motility from the stomach to the ileum as well as peristaltic reflex in the colon (Auteri et al., 2015). Furthermore, GABAA receptors in particular have been found to mediate inhibition of T cell responses, which would indicate another function of GABA as a natural immunomodulator of T lymphocytes (Tian et al., 1999; Bjurstöm et al., 2008). As a notable enteric immunomodulatory mediator, manipulating GABAergic signaling and GABA receptors have thus been subject to much research interest in their potential to alleviate inflammatory GI diseases, such as IBD. For example, it was found that enhancement of the γ2 subunit of the GABAA receptor on enteric corticotropin-releasing hormone (CRH) neurons with alprazolam, a benzodiazepine, reversed the increase in force of spontaneous colonic contractions in stress-induced mice (Seifi et al., 2014).
CONCLUSION
The gastrointestinal tract is a complex system that is intricately controlled by several modulators. Local mediators, central nervous system, enteric nervous system as well as hormones produced by other organs all influence catecholamine/serotonin concentrations and its end effect on gut physiology. Furthermore, research shows that this relationship is bi-directional, suggesting that changes in the GI system and enteric nervous system can affect the central nervous system, which is evident in human diseases such as Parkinson’s disease. Neurotransmitters namely epinephrine, norepinephrine, serotonin and dopamine, have shown that they play a major role in controlling and maintaining homeostasis within the gut system in terms of nutrient absorption, blood flow, gut microbiome, local immune system, and overall gut motility.
Neurotransmitters play a crucial part in maintaining homeostasis for the entire body. It is understandable that their full functions are not completely understood, because their endogenous concentrations are determined by physiology of the entire human body, making it impossible to isolate the gut. In addition, these catecholamines/serotonin activate different receptors at different concentrations making the picture even more confusing. Even though a vast amount of research is still required, our current working knowledge of catecholamines have already improved patient care; for example, these hormones are used as a last resort to restore blood flow to various organs in times of hemodynamic and septic shock.
The concentration of neurotransmitters is affected in multiple pathological states in which the local concentration can aid and decrease symptomology, secondarily change the gut microbiome, and potentially serve as a biomarker for both GI and CNS pathologies. For example, it is known that both the CNS and gut microbiome plays a significant role in diseases, such as irritable bowel syndrome. In addition, catecholamines and serotonin in the gut can affect both the gut microbiome and the CNS, thus providing a potential therapy method for such diseases. Preliminary research has already shown that they can impact the course of several diseases; further studies are needed to fully evaluate this effect on catecholamines as well as other neurotransmitters and hormones. Understanding molecular mechanisms through which neurotransmitters regulate gut-brain axis will open up avenues to design novel treatment modalities against human diseases.
Figure 2. Biochemical pathway for catecholamine synthesis with inhibitors of different dopaminergic and adrenergic receptors.
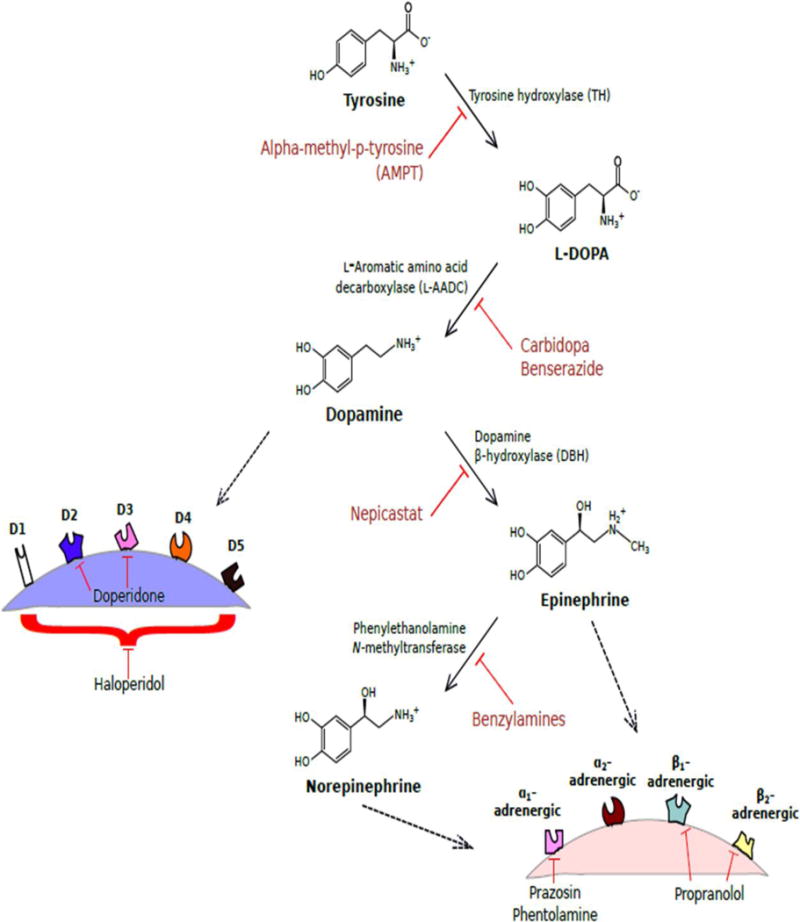
Synthesis pathway for catecholamines dopamine, norepinephrine, and epinephrine are depicted here with enzymes responsible for each step as well as known inhibitors of these enzymes. Dopamine will then bind to one of five dopaminergic receptors (D1 to D5) at the nerve ending. Norepinephrine and epinephrine will then bind one of four adrenergic receptors (α1, α2, β1, or β2). A few known drug inhibitors of some of these receptors are also portrayed.
Acknowledgments
We are thankful to Dr. Patricia Blackwelder and April Mann for critical reading of the manuscript. SKD would like to thank National institute of General Medical Sciences (R01GM047915). SD would like to thank the National Science Foundation (CHE-1506740) and the Miller School of Medicine of the University of Miami for the Lucille P. Markey Chair in Biochemistry and Molecular Biology.
Contract grant sponsor: National institute of General Medical Sciences; National Science Foundation Contract grant numbers: R01GM047915; CHE-1506740
Footnotes
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: [10.1002/jcp.25518]
Conflict of Interest
The authors declare no conflicts of interest.
References
- Abraham WR. Going beyond the control of quorum-sensing to combat biofilm infections. Antibiotics (Basel) 2016;5:3. doi: 10.3390/antibiotics5010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrass CK, O’Connor SW, Scarpace PJ, Abrass IB. Characterization of the beta-adrenergic receptor of the rat peritoneal macrophage. J Immunol. 1985;135:1338–1341. [PubMed] [Google Scholar]
- Araldi D, Ferrari LF, Levine JD. Gi-Protein coupled 5-HT1B/D receptor agonist sumatriptan Induces Type I hyperalgesic priming. Pain. 2016;157:1773–1782. doi: 10.1097/j.pain.0000000000000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro I, Fischer BL, Lascola KM, Clark-Price SC. Effects of intravenous terbutaline on heart rate, arterial pressure and blood gases in anesthetized horses breathing air. Vet Anaesth Analg. 2016 doi: 10.1111/vaa.12377. (In Press) [DOI] [PubMed] [Google Scholar]
- Auteri M, Zizzio MG, Serio R. GABA and GABA receptors in the gastrointestinal tract: from motility to inflammation. Pharmacol Res. 2015;93:11–21. doi: 10.1016/j.phrs.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Karaszewski JW, Lubach GR, Coe CL, Lyte M. In vivo adaptation of attenuated Salmonella typhimurium results in increased growth upon exposure to norepinephrine. Physiol Behav. 1999;67:359–364. doi: 10.1016/s0031-9384(99)00087-6. [DOI] [PubMed] [Google Scholar]
- Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- Bearson BL, Bearson SM, Lee IS, Brunelle BW. The Salmonella enterica serovar Typhimurium QseB response regulator negatively regulates bacterial motility and swine colonization in the absence of the QseC sensor kinase. Microb Pathog. 2010;48:214–219. doi: 10.1016/j.micpath.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Bender DA. Effects of a dietary excess of leucine on the metabolism of tryptophan in the rat: a mechanism for the pellagragenic action of leucine. Br J Nutr. 1983;50:24–32. doi: 10.1079/bjn19830068. [DOI] [PubMed] [Google Scholar]
- Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlioz F, Maoret JJ, Paris H, Laburthe M, Farinotti R, Roze C. alpha(2)-adrenergic receptors stimulate oligopeptide transport in a human intestinal cell line. J Pharmacol Exp Ther. 2000;294:466–472. [PubMed] [Google Scholar]
- Berman S, Suyenobu B, Naliboff BD, Bueller J, Stains J, Wong H, Mandelkern M, Fitzgerald L, Ohning G, Gupta A, Labus JS, Tillisch K, Mayer EA. Evidence for alterations in central noradrenergic signaling in irritable bowel syndrome. Neuroimage. 2012;4:1854–1863. doi: 10.1016/j.neuroimage.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjurstöm H, Wang J, Ericsson I, Bengtsson M, Liu Y, Kumar-Mendu S, Issazadeh-Navikas S, Birnir B. GABA, a natural immunomodulator of T lymphocytes. J Neuroimmunol. 2008;205:44–50. doi: 10.1016/j.jneuroim.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Blais AS, Nadeau G, Moore K, Genois L, Bolduc S. Prospective Pilot Study of Mirabegron in Pediatric Patients with Overactive Bladder. Eur Urol. 2016;16:182. doi: 10.1016/j.eururo.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borcherding DC, Tong W, Hugo ER, Barnard DF, Fox S, LaSance K, Shaughnessy E, Ben-Jonathan N. Expression and therapeutic targeting of dopamine receptor-1 (D1R) in breast cancer. Oncogene. 2016;35:3103–3113. doi: 10.1038/onc.2015.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braidy N, Grant R, Adams S, Brew BJ, Guillemin GJ. Mechanism for quinolinic acid cytotoxicity tin human astrocytes and neurons. Neurotox Res. 2009;16:77–86. doi: 10.1007/s12640-009-9051-z. [DOI] [PubMed] [Google Scholar]
- Burr RL, Jarrett ME, Cain KC, Jun SE, Heitkemper MM. Catecholamine and cortisol levels during sleep in women with irritable bowel syndrome. Neurogastroenterol Motil. 2009;11:1148–1197. doi: 10.1111/j.1365-2982.2009.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M. Mechanisms of disease: peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2012;367:1626–1635. doi: 10.1056/NEJMra1207068. [DOI] [PubMed] [Google Scholar]
- Castillo-Juárez I, Maeda T, Mandujano-Tinoco EA, Tomás M, Pérez-Eretza B, García-Contreras SJ, Wood TK, García-Contreras R. Role of quorum sensing in bacterial infections. World J Clin Cases. 2015;3:575–598. doi: 10.12998/wjcc.v3.i7.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervi AL, Lukewich MK, Lomax AE. Neural regulation of gastrointestinal inflammation: role of the sympathetic nervous system. Auton Neurosci. 2014;182:83–88. doi: 10.1016/j.autneu.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Chang L, Sundaresh S, Elliott J, Anton PA, Baldi P, Licudine A, Mayer M, Vuong T, Hirano M, Naliboff BD, Ameen VZ, Mayer EA. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil. 2009;2:149–159. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury BK, Shi XZ, Sarna SK. Norepinephrine mediates the transcriptional effects of heterotypic chronic stress on colonic motor function. Am J Physiol Gastrointest Liver Physiol. 2009;296:1238–1247. doi: 10.1152/ajpgi.90712.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloez-Tayarani I, Changeux JP. Nicotine and serotonin in immune regulation and inflammatory processes: a perspective. J Leukoc boil. 2007;81:599–606. doi: 10.1189/jlb.0906544. [DOI] [PubMed] [Google Scholar]
- Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Cogan TA, Thomas AO, Rees LE, Taylor AH, Jepson MA, Williams PH, Ketley J, Humphrey TJ. Norepinephrine increases the pathogenic potential of Campylobacter jejuni. Gut. 2007;56:1060–1065. doi: 10.1136/gut.2006.114926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nature Rev Microbiol. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- Corbillé AG, Clairembault T, Coron E, Leclair-Visonneau L, Preterre C, Neunlist M, Derkinderen P. What a gastrointestinal biopsy can tell us about Parkinson’s disease? Neurogastroenterol Motil. 2016 doi: 10.1111/nmo.12797. (In Press) [DOI] [PubMed] [Google Scholar]
- Cortese S, Gatta A, Della Valle L, Mangifesta R, Di Giampaolo L, Cavallucci E, Petrarca C, Paganelli R, Di Gioacchino M. Fluticasone/formoterol association favors long-lasting decrease in bronchial reactivity to methacholine and weekly PEF variability. Int J Immunopathol Pharmacol. 2016 doi: 10.1177/0394632016650896. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Furness JB. The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn Schmiedebergs Arch Pharmacol. 1976;294:47–60. doi: 10.1007/BF00692784. [DOI] [PubMed] [Google Scholar]
- Costedio MM, Coates MD, Danielson AB, Buttolph TR, 3rd, Blaszyk HJ, Mawe GM, Hyman NH. Serotonin signaling in diverticular disease. J Gastrointest Surg. 2008;12:1439–1445. doi: 10.1007/s11605-008-0536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell MD. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol. 2004;141:1285–1293. doi: 10.1038/sj.bjp.0705762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Kaupmann K. Don’t worry ‘B’ happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci. 2005;26:36–43. doi: 10.1016/j.tips.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Dall M, Schaffalitzky de Muckadell OB, Moller Hansen J, Wildner-Christensen M, Touborg Lassen A, Hallas J. Helicobacter pylori and risk of upper gastrointestinal bleeding among users of selective serotonin reuptake inhibitors. Scand J Gastroenterol. 2011;46:1039–1044. doi: 10.3109/00365521.2011.580100. [DOI] [PubMed] [Google Scholar]
- De Angelis M, Francavilla R, Piccolo M, De Giacomo A, Gobbetti M. Autism spectrum disorders and intestinal microbiota. Gut Microbes. 2015;6:207–213. doi: 10.1080/19490976.2015.1035855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- del Rey A, Besedovsky HO. Sympathetic nervous system-immune interactions in autoimmune lymphoproliferative diseases. Neuroimmunomodulation. 2008;15:29–36. doi: 10.1159/000135621. [DOI] [PubMed] [Google Scholar]
- Delgado PL, Charney DS, Price LH, Aghajanian GK, Landis H, Heninger GR. Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry. 1990;47:411–418. doi: 10.1001/archpsyc.1990.01810170011002. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Traplin A, O’Sullivan I, Crispie F, Moloney RD, Cotter PD, Dinan TG, Cryan JF. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav Immun. 2015;48:165–173. doi: 10.1016/j.bbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Di Sabatino A, Giuffrida P, Vanoli A, Luinetti O, Manca R, Biancheri P, Bergamaschi G, Alvisi C, Pasini A, Salvatore C, Biagi F, Solcia E, Corazza GR. Increase in neuroendocrine cells in the duodenal mucosa of patients with refractory celiac disease. Am J Gastroenterol. 2014;109:258–269. doi: 10.1038/ajg.2013.426. [DOI] [PubMed] [Google Scholar]
- Diard S, Lievin-Le Moal V, Toribio AL, Boum Y, Vigier F, Servin AL, Bouvet O. Norepinephrine-dependently released Dr fimbriae of diffusely adhering Escherichia coli strain IH11128 promotes a mitogen-activated protein kinase ERK1/2-dependent production of pro-inflammatory cytokine, IL-8 in human intestinal Caco-2/TC7 cells. Microbes Infect. 2009;11:886–894. doi: 10.1016/j.micinf.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Dickman R, Maradey-Romero C, Fass R. The role of pain modulators in esophageal disorders - no pain no gain. Neurogastroenterol Motil. 2014;26:603–610. doi: 10.1111/nmo.12339. [DOI] [PubMed] [Google Scholar]
- Diezmos EF, Bertrand PP, Liu L. Purinergic Signaling in Gut Inflammation: The Role of Connexins and Pannexins. Front Neurosci. 2016;10:311. doi: 10.3389/fnins.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty NC, Tobias A, Watson S, Atherton JC. The effect of the human gut-signalling hormone, norepinephrine, on the growth of the gastric pathogen Helicobacter pylori. Helicobacter. 2009;14:223–230. doi: 10.1111/j.1523-5378.2009.00682.x. [DOI] [PubMed] [Google Scholar]
- Donowitz M. Ca2+ in the control of active intestinal Na and Cl transport: involvement in neurohumoral action. Am J Physiol. 1983;245:165–177. doi: 10.1152/ajpgi.1983.245.2.G165. [DOI] [PubMed] [Google Scholar]
- Dunn WR, Brock JA, Hardy TA. Electrochemical and electrophysiological characterization of neurotransmitter release from sympathetic nerves supplying rat mesenteric arteries. Br J Pharmacol. 1999;128:174–180. doi: 10.1038/sj.bjp.0702760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell H, Moore LE, Chung C, Byers BW, Stickland MK. Short-term cardiovascular and autonomic effects of inhaled salbutamol. Respir Physiol Neurobiol. 2016;231:14–20. doi: 10.1016/j.resp.2016.05.014. [DOI] [PubMed] [Google Scholar]
- Ellermeier JR, Slauch JM. Fur regulates expression of the Salmonella pathogenicity island 1 type III secretion system through HilD. J Bacteriol. 2008;190:476–486. doi: 10.1128/JB.00926-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farache J, Koren I, Milo I, Gurevich I, Kim KW, Zigmond E, Furtado GC, Lira SA, Shakhar G. Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity. 2013;38:581–595. doi: 10.1016/j.immuni.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Shilling PD, Kuczenski R, Segal DS. Altered extracellular dopamine concentration in the brains of cholecystokinin-A receptor deficient rats. Neurosci Lett. 2003;348:147–150. doi: 10.1016/s0304-3940(03)00767-5. [DOI] [PubMed] [Google Scholar]
- Ferreira JG, Tellez LA, Ren X, Yeckel CW, de Araujo IE. Regulation of fat intake in the absence of flavour signalling. J Physiol. 2012;590:953–972. doi: 10.1113/jphysiol.2011.218289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemstrom G, Safsten B, Jedstedt G. Stimulation of mucosal alkaline secretion in rat duodenum by dopamine and dopaminergic compounds. Gastroenterology. 1993;104:825–833. doi: 10.1016/0016-5085(93)91019-e. [DOI] [PubMed] [Google Scholar]
- Freestone PP, Lyte M, Neal CP, Maggs AF, Haigh RD, Williams PH. The mammalian neuroendocrine hormone norepinephrine supplies iron for bacterial growth in the presence of transferrin or lactoferrin. J Bacteriol. 2000;182:6091–6098. doi: 10.1128/jb.182.21.6091-6098.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M, Levin CE. Nutritional and medicinal aspects of D-amino acids. Amino Acids. 2012;42:1553–1582. doi: 10.1007/s00726-011-0915-1. [DOI] [PubMed] [Google Scholar]
- Gagon DJ. Intestinal smooth muscles: demonstration of catecholamines-induced contraction mediated through alpha-adrenergic receptors. Eur J Pharmacol. 1970;10:297–300. doi: 10.1016/0014-2999(70)90287-6. [DOI] [PubMed] [Google Scholar]
- Gershon MD, Wade PR, Kirchgessner AL, Tamir H. 5-HT receptor subtypes outside the central nervous system. Roles in the physiology of the gut. Neuropsychopharmacology. 1990;3:385–395. [PubMed] [Google Scholar]
- Grayson J, Oyebola DD. The effect of catecholamines on intestinal glucose and oxygen uptake in the dog. J Physiol. 1983;343:311–322. doi: 10.1113/jphysiol.1983.sp014894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guereschi MG, Araujo LP, Maricato JT, Takenaka MC, Nascimento VM, Vivanco BC, Reis VO, Keller AC, Brum PC, Basso AS. Beta2-adrenergic receptor signaling in CD4+ Foxp3+ regulatory T cells enhances their suppressive function in a PKA-dependent manner. Eur J Immunol. 2013;43:1001–1012. doi: 10.1002/eji.201243005. [DOI] [PubMed] [Google Scholar]
- Guseva D, Holst K, Kaune B, Meier M, Keubler L, Glage S, Buettner M, Bleich A, Pabst O, Bachmann O, Ponimaskin EG. Serotonin 5-HT7 receptor is critically involved in acute and chronic inflammation of the gastrointestinal tract. Inflamm Bowel Dis. 2014;20:1516–1529. doi: 10.1097/MIB.0000000000000150. [DOI] [PubMed] [Google Scholar]
- Haas M, McBrayer D, Lytle C. [Cl-]i-dependent phosphorylation of the Na-K-Cl cotransport protein of dog tracheal epithelial cells. J Biol Chem. 1995;270:28955–28961. doi: 10.1074/jbc.270.48.28955. [DOI] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Hegde M, Wood TK, Jayaraman A. The neuroendocrine hormone norepinephrine increases Pseudomonas aeruginosa PA14 virulence through the las quorum-sensing pathway. Appl Microbiol Biotechnol. 2009;84:763–776. doi: 10.1007/s00253-009-2045-1. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Altherr EB, MacMillan C, Fletcher PJ, Pratt WE. Lorcaserin and CP-809101 reduce motor impulsivity and reinstatement of food seeking behavior in male rats: Implications for understanding the anti-obesity property of 5-HT<sub>2C</sub> receptor agonists. Psychopharmacology (Berl) 2016 doi: 10.1007/s00213-016-4329-3. (In Press) [DOI] [PubMed] [Google Scholar]
- Hirst GD, Silinsky EM. Some effects of 5-hydroxytryptamine, dopamine and noradrenaline on neurones in the submucous plexus of guinea-pig small intestine. J Physiol. 1975;251:817–832. doi: 10.1113/jphysiol.1975.sp011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JM, Tyler K, MacEachern SJ, Balemba OB, Johnson AC, Brooks EM, Zhao H, Swain GM, Moses PL, Galligan JJ, Sharkey KA, Greenwood-Van Meerveld B, Mawe GM. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142:844–854. doi: 10.1053/j.gastro.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological adn functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Eguchi T, Ishida H. Mechanism of beta-adrenergic agonist-induced transmural transport of glucose in rat small intestine. Regulation of phosphorylation of SGLT1 controls the function. Biochim Biophys Acta. 1997;1357:306–318. doi: 10.1016/s0167-4889(97)00043-8. [DOI] [PubMed] [Google Scholar]
- Isom LL, Cragoe EJ, Limbird LE. Alpha 2-adrenergic receptors accelerate Na+/H+ exchange in neuroblastoma X glioma cells. J Biol Chem. 1987;262:6750–6757. [PubMed] [Google Scholar]
- Janig W. Sympathetic nervous system and inflammation: a conceptual view. Auton Neurosci. 2014;182:4–14. doi: 10.1016/j.autneu.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Jeyarajah S, Akbar N, Moorhead J, Haji A, Banerjee S, Papagrigoriadis S. A clinicopathological study of serotonin of sigmoid colon mucosa in association with chronic symptoms in uncomplicated diverticulosis. Int J Colorectal Dis. 2012;27:1597–1607. doi: 10.1007/s00384-012-1515-6. [DOI] [PubMed] [Google Scholar]
- Wu M, Ji XY. mRNA expression of dopamine receptor D2 and dopamine transporter in peripheral blood lymphocytes before and after treatment in children with tic disorder. Zhongguo Dang Dai Er Ke Za Zhi. 2016;4:297–300. doi: 10.7499/j.issn.1008-8830.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Lian A, He Q. Dopamine inhibits somatolactin gene expression in tilapia pituitary cells through the dopamine D2 receptors. Comp Biochem Physiol A Mol Integr Physiol. 2016;197:35–42. doi: 10.1016/j.cbpa.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Kamio Y, Saito Y, Utoguchi N, Kondoh M, Koizumi N, Fujii M, Watanabe Y. Epinephrine is an enhancer of rat intestinal absorption. J Control Release. 2005;102:563–568. doi: 10.1016/j.jconrel.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Karaki S, Becamel C, Murat S, Mannoury la Cour C, Millan MJ, Prézeau L, Bockaert J, Marin P, Vandermoere F. Quantitative phosphoproteomics unravels biased phosphorylation of serotonin 2A receptor at Ser280 by hallucinogenic versus nonhallucinogenic agonists. Mol Cell Proteomics. 2014;5:1273–1285. doi: 10.1074/mcp.M113.036558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamanolis GP, Panapoulos S, Karlaftis A, Denaxas K, Kamberoglou D, Sifakakis PP, Ladas SD. Beneficial effect of the 5-HT1A receptor agonist buspirone on esophageal dysfunction associated with systemic sclerosis: A pilot study. United European Gastroenterol J. 2015;3:266–271. doi: 10.1177/2050640614560453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keszthelyi D, Troost FJ, Jonkers DM, Kruimel JW, Leue C, Masclee AA. Decreased levels of kynurenic acid in the intestinal mucosa of IBS patients: relation to serotonin and psychological state. J Psychosom Res. 2013;74:501–504. doi: 10.1016/j.jpsychores.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Keszthelyi D, Troost FJ, Jonkers DM, van Donkelaar EL, Dekker J, Buurmna WA, Masclee AA. Does acute tryptophan depletion affect peripheral serotonin metabolism in the intestine? Am J Clin Nutr. 2012;95:603–608. doi: 10.3945/ajcn.111.028589. [DOI] [PubMed] [Google Scholar]
- Kidd M, Gustafsson BI, Drozdov I, Modlin IM. IL1beta- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn’s disease. Neurogastroenterol Motil. 2009;21:439–450. doi: 10.1111/j.1365-2982.2008.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Jwa CS. Effect of alpha-1-adrenergic agonist, midodrine for the management of long-standing neurogenic shock in patient with cervical spinal cord injury: a case report. Korean J Neurotrauma. 2015;2:147–150. doi: 10.13004/kjnt.2015.11.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kin NW, Sanders VM. It takes nerve to tell T and B cells what to do. J Leukoc Biol. 2006;79:1093–1104. doi: 10.1189/jlb.1105625. [DOI] [PubMed] [Google Scholar]
- Kishi T, Meltzer HY, Matsuda Y, Iwata N. Azapirone 5-HT1A receptor partial agonist treatment for major depressive disorder: systematic review and meta-analysis. Psychol Med. 2013;11:2255–2269. doi: 10.1017/S0033291713002857. [DOI] [PubMed] [Google Scholar]
- Knecht L, O’Connor G, Mittal R, Liu XZ, daftarian P, Deo SK, Daunert S. Serotonin activates bacterial quorum sensing and enhances the virulence of Pseudomonas aeruginosa in the host. EBio Medicine. 2016 doi: 10.1016/j.ebiom.2016.05.037. (in press). http://dx.doi.org/10.1016/j.ebiom.2016.05.037. [DOI] [PMC free article] [PubMed]
- Krygowska-Wajs A, Furgala A, Gorecka-Mazur A, Pietraszko W, Thor P, Potasz-Kulikowska K, Moskala M. The effect of subthalamic deep brain stimulation on gastric motility in Parkinson’s disease. Parkinsonism Relat Disord. 2016;26:35–40. doi: 10.1016/j.parkreldis.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Kusunoki M, Taniyama K, Tanaka C. Dopamine regulation of [3H]acetylcholine release from guinea-pig stomach. J Pharmacol Exp Ther. 1985;234:713–719. [PubMed] [Google Scholar]
- Le Floc’h N, Otten W, Merlot E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids. 2010;41:1195–1205. doi: 10.1007/s00726-010-0752-7. [DOI] [PubMed] [Google Scholar]
- Lefebvre RA, Van Colen I, Pauwelyn V, De Maeyer JH. Synergistic effect between 5-HT4 receptor agonist and phosphodiesterase 4-inhibitor in releasing acetylcholine in pig gastric circular muscle in vitro. Eur J Pharmacol. 2016;781:76–82. doi: 10.1016/j.ejphar.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Li B, Liu ML, Wu XP, Jia J, Cao J, Wei ZW, Wang YJ. Effects of ketamine exposure on dopamine concentrations and dopamine type 2 receptor mRNA expression in rat brain tissue. Int J Clin Exp Med. 2015;7:11181–11187. [PMC free article] [PubMed] [Google Scholar]
- Li W, Lyte M, Freestone PP, Ajmal A, Colmer-Hamood JA, Hamood AN. Norepinephrine represses the expression of toxA and the siderophore genes in Pseudomonas aeruginosa. FEMS Microbiol Lett. 2009;299:100–109. doi: 10.1111/j.1574-6968.2009.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZS, Schmauss C, Cuenca A, Ratcliffe E, Gershon MD. Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J Neurosci. 2006;26:2798–2807. doi: 10.1523/JNEUROSCI.4720-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Wang T, Hu X, Luo J, Li W, Wu X, Duan Y, Jin F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–577. doi: 10.1016/j.neuroscience.2015.09.033. [DOI] [PubMed] [Google Scholar]
- Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J PHysiol Gastrointest Liver Physiol. 2003;235:207–216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- Liu MT, Kuan YH, Wang J, Hen R, Gershon MD. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci. 2009;29:9683–9699. doi: 10.1523/JNEUROSCI.1145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YC, Chan KG, Chang CY. Modulation of Host Biology by Pseudomonas aeruginosa Quorum Sensing Signal Molecules: Messengers or Traitors. Front Microbiol. 2015;6:1226. doi: 10.3389/fmicb.2015.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lladó-Pelfort L, Troyano-Rodriguez E, van den Munkhof HE, Cervera-Ferri A, Jurado N, Núñez-Calvet M, Artigas F, Celada P. Phencyclidine-induced disruption of oscillatory activity in prefrontal cortex: Effects of antipsychotic drugs and receptor ligands. Eur Neuropsychopharmacol. 2016;3:614–625. doi: 10.1016/j.euroneuro.2015.11.015. [DOI] [PubMed] [Google Scholar]
- Luo XJ, Zheng M, Tian G, Zhong HY, Zou XJ, Jian DL. Comparison of the treatment effects of methoxamine and combining methoxamine with atropine infusion to maintain blood pressure during spinal anesthesia for cesarean delivery: a double blind randomized trial. Eur Rev Med Pharmacol Sci. 2016;3:561–567. [PubMed] [Google Scholar]
- Lyte M, Arulanandam B, Nguyen K, Frank C, Erickson A, Francis D. Norepinephrine induced growth and expression of virulence associated factors in enterotoxigenic and enterohemorrhagic strains of Escherichia coli. Adv Exp Med Biol. 1997;412:331–339. doi: 10.1007/978-1-4899-1828-4_54. [DOI] [PubMed] [Google Scholar]
- Lyte M, Ernst S. Catecholamine induced growth of gram negative bacteria. Life Sci. 1992;50:203–212. doi: 10.1016/0024-3205(92)90273-r. [DOI] [PubMed] [Google Scholar]
- Lyte M, Frank CD, Green BT. Production of an autoinducer of growth by norepinephrine cultured Escherichia coli O157:H7. FEMS Microbiol Lett. 1996;139:155–159. doi: 10.1111/j.1574-6968.1996.tb08196.x. [DOI] [PubMed] [Google Scholar]
- Lyte M. Microbial endocrinology and infectious disease in the 21st century. Trends Microbiol. 2004;12:14–20. doi: 10.1016/j.tim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Lyte M. Microbial endocrinology as a basis for improved L-DOPA bioavailability in Parkinson’s patients treated for Helicobacter pylori. Med Hypotheses. 2010;74:895–897. doi: 10.1016/j.mehy.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Slack E. The functional interactions of commensal bacteria with intestinal secretory IgA. Curr Opin Gastroenterol. 2007;23:673–678. doi: 10.1097/MOG.0b013e3282f0d012. [DOI] [PubMed] [Google Scholar]
- Magro F, Vieira-Coelho MA, Fraga S, Serrão MP, Veloso FT, Ribeiro T, Soares-da-Silva P. Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. 2002;1:216–224. doi: 10.1023/a:1013256629600. [DOI] [PubMed] [Google Scholar]
- Manivasagam S, Subramanian V, Tumala A, Vellaichamy E. Differential expression and regulation of anti-hypertrophic genes Npr1 and Npr2 during β-adrenergic receptor activation-induced hypertrophic growth in rats. Mol Cell Endocrinol. 2016 doi: 10.1016/j.mce.2016.06.010. (In Press) [DOI] [PubMed] [Google Scholar]
- Manoharan A, Morrison AE, Lipworth BJ. Effects of the inverse alpha-agonist doxazosin in allergic rhinitis. Clin Exp Allergy. 2016;5:696–704. doi: 10.1111/cea.12700. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Hoffman JM. Serotonin signaling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LA, Westfall TC. Mechanism of catecholamine synthesis inhibition by neuropeptide Y: role of Ca2+ channels and protein kinases. J Neurochem. 1996;67:1090–1099. doi: 10.1046/j.1471-4159.1996.67031090.x. [DOI] [PubMed] [Google Scholar]
- Mestecky J, Russell MW. Specific antibody activity, glycan heterogeneity and polyreactivity contribute to the protective activity of S-IgA at mucosal surfaces. Immunol Lett. 2009;124:57–62. doi: 10.1016/j.imlet.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minderhoud IM, Oldenburg B, Schipper ME, Ter Linde JJ, Samsom M. Serotonin synthesis and uptake in symptomatic patients with Crohn’s disease in remission. Clin Gastroenterol Hepatol. 2007;5:714–720. doi: 10.1016/j.cgh.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Monro RL, Bertrand PP, Bornstein JC. ATP Participates in Three Excitatory Postsynaptic Potentials in the Submucous Plexus of the Guinea Pig Ileum. J Physiol. 2004;556:571–584. doi: 10.1113/jphysiol.2004.060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos WH, Faller DV, Harpp DN, Kanara I, Pernokas J, Powers WR, Steliou K. Microbiota and Neurological Disorders: A Gut Feeling. Biores Open Access. 2016;5:137–145. doi: 10.1089/biores.2016.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira CG, Weinshenker D, Sperandio V. QseC mediates Salmonella enterica serovar typhimurium virulence in vitro and in vivo. Infect Immun. 2010;78:914–926. doi: 10.1128/IAI.01038-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Sato T, Ohashi A, Tsurui H, Hasegawa H. Role of a serotonin precursor in development of gut microvilli. Am J Pathol. 2008;172:333–344. doi: 10.2353/ajpath.2008.070358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale G, Pasquali L, Ruggieri S, Paparelli A, Fornai F. Parkinson’s disease and the gut: a well-known clinical association in need of an effective cure and explanation. Neurogastroenterol Motil. 2008;7:741–749. doi: 10.1111/j.1365-2982.2008.01162.x. [DOI] [PubMed] [Google Scholar]
- Neal KB, Bornstein JC. Serotonergic receptors in therapeutic approaches to gastrointestinal disorders. Curr Opin Pharmacol. 2006;6:547–552. doi: 10.1016/j.coph.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Nilssen DE, Oktedalen O, Lygren I, Opstad PK, Brandtzaeg P. Intestinal IgA- and IgM-producing cells are not decreased in marathon runners. Int J Sports Med. 1998;19:425–431. doi: 10.1055/s-2007-971940. [DOI] [PubMed] [Google Scholar]
- Nozawa K, Kawabata-Shoda E, Doihara H, Kojima R, Okada H, Mochizuki S, Sano Y, Inamura K, Matsushime H, Koizumi T, Yokoyama T, Ito H. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc Natl Acad Sci. 2009;106:3408–3413. doi: 10.1073/pnas.0805323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara JR, Skinn AC, MacNaughton WK, Sherman PM, Sharkey KA. Consequences of Citrobacter rodentium infection on enteroendocrine cells and the enteric nervous system in the mouse colon. Cell Microbiol. 2006;8:646–660. doi: 10.1111/j.1462-5822.2005.00657.x. [DOI] [PubMed] [Google Scholar]
- O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Olaleye SB, Elegbe RA. Catecholamines potentiate the effect of thyroid hormone on intestinal absorption of glucose in the rat. Afr J Med Med Sci. 2005;34:177–183. [PubMed] [Google Scholar]
- Ostovaneh MR, Saeidi B, Hajifathalian K, Farrokhi-Khajeh-Pasha Y, Fotouhi A, Mirbagheri SS, Emami H, Barzin G, Mirbagheri SA. Comparing Omeprazole with Fluoxetine for treatment of patients with heartburn and normal endoscopy who failed once daily proton pump inhibitors: double-blind placebo-controlled trial. Neurogastroenterol Motil. 2014;26:670–678. doi: 10.1111/nmo.12313. [DOI] [PubMed] [Google Scholar]
- Palacios JM. Serotonin receptors in brain revisited. Brain Res. 2016 doi: 10.1016/j.brainres.2015.12.042. (in press) [DOI] [PubMed] [Google Scholar]
- Rang C, Galen JE, Kaper JB, Chao L. Fitness cost of the green fluorescent protein in gastrointestinal bacteria. Can J Microbiol. 2003;49:531–537. doi: 10.1139/w03-072. [DOI] [PubMed] [Google Scholar]
- Rapalli A, Bertoni S, Arcaro V, Saccani F, Grandi A, Vivo V, Cantoni AM, Barocelli E. Dual role of endogenous serotonin in 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Front Pharmacol. 2016;7:68. doi: 10.3389/fphar.2016.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber SO. Stress and animal models of inflammatory bowel disease - an update on the role of the hypothalamo-pituitary-adrenal axis. Psychoneuroendocrinology. 2012;37:1–19. doi: 10.1016/j.psyneuen.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Ren J, Bertrand P. Purinergic Receptors and Synaptic Transmission in Enteric Neurons. Purinergic Signal. 2008;4:255–266. doi: 10.1007/s11302-007-9088-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Yeckel CW, de Araujo IE. Nutrient selection in the absence of taste receptor signaling. J Neurosci. 2010;30:8012–8023. doi: 10.1523/JNEUROSCI.5749-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyna-Garfias H, Miliar A, Jarillo-Luna A, Rivera-Aguilar V, Pacheco-Yepez J, Baeza I, Campos-Rodriguez R. Repeated restraint stress increases IgA concentration in rat small intestine. Brain Behav Immun. 2010;24:110–118. doi: 10.1016/j.bbi.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implication of the brain-gut-enteric microbiota axis. Nature Rev Gastroentrol Hepatic. 2009;6:304–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PD, Withrington PG. Physiological regulation of the hepatic circulation. Annu Rev Physiol. 1982;44:57–69. doi: 10.1146/annurev.ph.44.030182.000421. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CS. Microbiome Disturbances and Autism Spectrum Disorders. Drug Metab Dispos. 2015;43:1557–1571. doi: 10.1124/dmd.115.063826. [DOI] [PubMed] [Google Scholar]
- Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA, Lowry CA. Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med. 2006;8:1–27. doi: 10.1017/S1462399406000068. [DOI] [PubMed] [Google Scholar]
- Sander CY, Hooker JM, Catana C, Rosen BR, Mandeville JB. Imaging agonist-induced D2/D3 receptor desensitization and internalization in vivo with PET/FMRI. Neuropsychopharmacology. 2016;5:1427–1436. doi: 10.1038/npp.2015.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartre RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J Bacteriol. 2002;4:1140–1154. doi: 10.1128/jb.184.4.1140-1154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheerens C, Tack J, Rommel N. Buspirone, a new drug for the management of patients with ineffective esophageal motility. United European Gastroenterol J. 2015;3:261–265. doi: 10.1177/2050640615585688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcsz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Reve Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A. Post-ingestive positive controls of ingestive behavior. Appetite. 2001;36:79–83. doi: 10.1006/appe.2000.0370. [DOI] [PubMed] [Google Scholar]
- Seifi M, Brown JF, Mills J, Bhandari P, Belelli D, Lambert JJ, Rudolph U, Swinny JD. Molecular and Functional Diversity of GABA-A Receptors in the Enteric Nervous System of the Mouse Colon. J Neurosci. 2014;34:10361–10378. doi: 10.1523/JNEUROSCI.0441-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan F. Gut flora in gastrointestinal disease. Eur J Surg Suppl. 2002;587:47–52. [PubMed] [Google Scholar]
- Shaw SY, Blanchard JF, Berstein CN. Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. Am J Gastroenterol. 2010;105:2687–2692. doi: 10.1038/ajg.2010.398. [DOI] [PubMed] [Google Scholar]
- Shelkar GP, Gakare SG, Chakraborty S, Dravid SM, Ugale RR. Nitric Oxide-α2 -adrenergic receptor interaction within locus coeruleus underlies facilitation of inhibitory avoidance memory by agmatine. Br J Pharmacol. 2016 doi: 10.1111/bph.13531. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiman R, Akino M, Kaufman S. Solubilization and partial purification of tyrosine hydroxylase from bovine adrenal medulla. J Biol Chem. 1971;246:1330–1340. [PubMed] [Google Scholar]
- Singaram C, Ashraf W, Gaumnitz EA, Torbey C, Sengupta A, Pfeiffer R, Quigley EM. Dopaminergic defect of enteric nervous system in Parkinson’s disease patients with chronic constipation. Lancet. 1995;8979:861–864. doi: 10.1016/s0140-6736(95)92707-7. [DOI] [PubMed] [Google Scholar]
- Skowronski MT, Ishikawa Y, Ishida H. Enhancement by epinephrine of benzylpenicillin transport in rat small intestine. J Pharmacol Exp Ther. 2000;293:128–135. [PubMed] [Google Scholar]
- Smith EA, Macfarlane GT. Dissimilatory amino acid metabolism in human colonic bacteria. Anaerobe. 1997;3:327–337. doi: 10.1006/anae.1997.0121. [DOI] [PubMed] [Google Scholar]
- Sugama S, Kakinuma Y. Loss of Dopaminergic neurons occurs in the ventral tegmental area and hypothalamus of rats following chronic stress: possible pathogenetic loci for depression involved in Parkinson’s disease. Neurosci Res. 2016;16:30048–30047. doi: 10.1016/j.neures.2016.04.008. [DOI] [PubMed] [Google Scholar]
- Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of Tubuloglomerular Feedback by Adenosine: Evidence from Mice Lacking Adenosine 1 Receptors. Proc Natl Acad Sci USA. 2001;98:9983–9988. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tache Y, Martinez V, Million M, Wang L. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am J Physiol Gastrointest Liver Physiol. 2001;280:173–177. doi: 10.1152/ajpgi.2001.280.2.G173. [DOI] [PubMed] [Google Scholar]
- Thompson MA, Woolley DE, Gietzen DW, Conway S. Catecholamine synthesis inhibitors acutely modulate [3H]estradiol binding by specific brain areas and pituitary in ovariectomized rats. Endocrinology. 1983;113:855–865. doi: 10.1210/endo-113-3-855. [DOI] [PubMed] [Google Scholar]
- Tian J, Chau C, Hales TG, Kaufman DL. GABA(A) receptors mediate inhibition of T cell responses. J Neuroimmunol. 1999;96:21–28. doi: 10.1016/s0165-5728(98)00264-1. [DOI] [PubMed] [Google Scholar]
- Tolstanova G, Deng X, Ahluwalia A, Paunovic B, Prysiazhniuk A, Ostapchenko L, Tarnawski A, Sandor Z, Szabo S. Role of Dopamine and D2 Dopamine Receptor in the Pathogenesis of Inflammatory Bowel Disease. Dig Dis Sci. 2015;10:2963–2975. doi: 10.1007/s10620-015-3698-5. [DOI] [PubMed] [Google Scholar]
- Toti L, Travagli RA, Am J. Gastric dysregulation induced by microinjection of 6-OHDA in the substantia nigra pars compacta of rats is determined by alterations in the brain-gut axis. Physiol Gastrointest Liver Physiol. 2014;10:1013–1023. doi: 10.1152/ajpgi.00258.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesaki Y, Okada Y, Matsumoto S, Imaoka A, Setoyama H. Segmented filamentous bacteria are indigenous intestinal bacteria that activate intraepithelial lymphocytes and induce MHC class II molecules and fucosyl asialo GM1 glycolipids on the small intestinal epithelial cells in the ex-germ-free mouse. Microbiol Immunol. 1995;39:555–562. doi: 10.1111/j.1348-0421.1995.tb02242.x. [DOI] [PubMed] [Google Scholar]
- Vanuytsel T, Tack JF, Boeckxstaens GE. Treatment of abdominal pain in irritable bowel syndrome. J Gastroenterol. 2014;49:1193–1205. doi: 10.1007/s00535-014-0966-7. [DOI] [PubMed] [Google Scholar]
- Vécsei L, Szalárdy L, Fülöp F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat Reve Drug Discov. 2013;12:64–82. doi: 10.1038/nrd3793. [DOI] [PubMed] [Google Scholar]
- Viazis N, Karamanolis G, Vienna E, Karamanolis DG. Selective-serotonin reuptake inhibitors for the treatment of hypersensitive esophagus. Therap Adv Gastroenterol. 2011;4:295–300. doi: 10.1177/1756283X11409279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viazis N, Keyoglou A, Kanellopoulos AK, Karamanoulis G, Vlachogiannakos J, Triantafyllou K, Ladas SD, Karamanolis DG. Selective serotonin reuptake inhibitors for the treatment of hypersensitive esophagus: a randomized, double-blind, placebo-controlled study. Am J Gastroenterol. 2012;107:1662–1667. doi: 10.1038/ajg.2011.179. [DOI] [PubMed] [Google Scholar]
- Vieira-Coelho MA, Teixeira VA, Finkel Y, Soares-Da-Silva P, Bertorello AM. Dopamine-dependent inhibition of jejunal Na+-K+-ATPase during high-salt diet in young but not in adult rats. Am J Physiol. 1998;275:1317–1323. doi: 10.1152/ajpgi.1998.275.6.G1317. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Elenkov IJ. Nonsynaptic noradrenaline release in neuro-immune responses. Acta Biol Hung. 2002;53:229–244. doi: 10.1556/ABiol.53.2002.1-2.21. [DOI] [PubMed] [Google Scholar]
- Wang YP, Chen YT, Tsai CF, Li SY, Luo JC, Wang SJ, Tang CH, Liu CJ, Lin HC, Lee FY, Chang FY, Lu CL. Short-term use of serotonin reuptake inhibitors and risk of upper gastrointestinal bleeding. Am J Psychiatry. 2014;171:54–61. doi: 10.1176/appi.ajp.2013.12111467. [DOI] [PubMed] [Google Scholar]
- Watson DJ, King MV, Gyertyá NI, Kiss B, Adham N, Fone KC. The dopamine D3-preferring D2/D3 dopamine receptor partial agonist, cariprazine, reverses behavioural changes in a rat neurodevelopmental model for schizophrenia. Eur Neuropsychopharmacol. 2015;2:208–224. doi: 10.1016/j.euroneuro.2015.12.020. [DOI] [PubMed] [Google Scholar]
- West AB. The pathology of diverticulitis. J Clin Gastroenterol. 2008;42:1137–1138. doi: 10.1097/MCG.0b013e3181862a9f. [DOI] [PubMed] [Google Scholar]
- Wheatcroft J, Wakelin D, Smith A, Mahoney CR, Mawe G, Spiller R. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil. 2005;17:863–870. doi: 10.1111/j.1365-2982.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- Wirth A, Holst K, Ponimaskin E. How serotonin receptors regulate morphogenic signalling in neurons. Prog Neurobiol. 2016 doi: 10.1016/j.pneurobio.2016.03.007. (in press) [DOI] [PubMed] [Google Scholar]
- Wostmann BS, Pleasants JR, Bealmear P, Kincade PW. Serum proteins and lymphoid tissues in germ-free mice fed a chemically defined, water soluble, low molecular weight diet. Immunology. 1970;19:443–448. [PMC free article] [PubMed] [Google Scholar]
- Wu L, Oshima T, Tomita T, Ohda Y, Fukui H, Watari J, Miwa H. Serotonin disrupts esophageal mucosal integrity: an investigation using a stratified squamous epithelial model. J Gastroenterol. 2016 doi: 10.1007/s00535-016-1195-z. (In Press) [DOI] [PubMed] [Google Scholar]
- Yu FY, Huang SG, Zhang HY, Ye H, Chi HG, Zou Y, Lv RX, Zheng XB. Comparison of 5-hydroxytryptophan signaling pathway characteristics in diarrhea-predominant irritable bowel syndrome and ulcerative colitis. World J Gastroetnerol. 2016;22:3451–3459. doi: 10.3748/wjg.v22.i12.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang KH, Shao YY, Zuo X, Rao Z, Qin HY. Oridonin alleviates visceral hyperalgesia in a rat model of postinflammatory irritable bowel syndrome: role of colonic enterochromaffin cell and serotonin availability. J Med Food. 2016 doi: 10.1089/jmf.2015.3595. (In Press) [DOI] [PubMed] [Google Scholar]


