Abstract
Purpose
With cancer survivors now numbering over 13 million in the United States, and expected to continue to increase, it is important to consider the needs of this growing population. In the literature, one of the most common complaints by cancer survivors is perceived cognitive dysfunction. Since the preponderance of the research has focused on breast cancer survivors, the purpose of the present study was to explore the prevalence and correlates of perceived cognitive dysfunction in a large sample of cancer survivors with representation across a wide range of different types of cancer.
Methods
A sample of 3108 post-treatment cancer survivors completed the 2010 LIVESTRONG survey as part of a larger study of cancer survivorship. Respondents completed standardized questions regarding current and past perceived cognitive dysfunction, as well as depressive symptoms, and demographic and medical variables.
Results
Current perceived cognitive dysfunction was reported by nearly half of respondents (45.7 %), across a wide range of cancer types, with the highest prevalence among survivors of central nervous system cancers. Receiving chemotherapy and current report of depressive symptoms were both strongly associated with current perceived cognitive dysfunction.
Conclusion
These findings contribute to a growing appreciation of the high prevalence of perceived cognitive dysfunction in survivors of a wide range of cancer types and the potential interactive effect of concurrent symptoms of depression. These findings highlight a need to develop more effective means of preventing or reducing cognitive dysfunction in cancer survivors.
Implications for Cancer Survivors
Perceived cognitive dysfunction was reported in a wide range of cancer survivors. The potential interactive effect of symptoms of depression suggests the need to develop interventions targeting both cognitive dysfunction and depression to achieve improvements in cognitive functioning.
Keywords: Perceived cognitive dysfunction, Depression, Survivors, LIVESTRONG, Chemo-brain
Introduction
The number of cancer survivors continues to grow each year, as a result of advances in treatments and better screening technology leading to earlier disease detection [1]. In 2012, there were about 13.7 million cancer survivors in the United States, and this number is expected to increase to 18 million by 2022 [2]. With this growing cancer survivor population, the burden of disease- and treatment-related survivorship issues increases proportionally [3]. Cognitive dysfunction has been a common complaint among cancer survivors for many years and was initially reported by breast cancer survivors who complained of mental fogginess during and after chemotherapy treatments [4–7]. The term “chemo-brain” was first used by breast cancer patients to describe a constellation of perceived cognitive limitations occurring during and after chemotherapy treatments, and “chemo-brain” is increasingly recognized to be a major complaint of cancer survivors [4, 5]. Concerns reported by patients complaining of “chemo-brain” can include difficulties in memory, concentrating and staying focused, word finding, executive function (e.g., time management, decision-making), and slower processing speed; however, the areas of cognitive functioning affected are variable between patients [8]. While much of the research on cognitive dysfunction has focused on survivors after completion of chemotherapy [9, 10], cognitive concerns have also been reported in survivors treated after radiotherapy [5] and after hormonal therapy [11, 12].
Many of these studies assessed cognitive dysfunction using self-report measures [13, 14], while others have used objective cognitive tests [15, 16] often with inconsistent results between the two assessment approaches [15, 17–19]. Given this disparity, perceived cognitive dysfunction may be more likely to represent interference in daily functioning and quality of life in the cancer survivor [20, 21]. The preponderance of research on this topic has focused on breast cancer [8]. Perceptions of cognitive dysfunction and its impact on daily functioning for survivors of other types of cancer have begun to receive more recent research attention including colon cancer [22], testicular cancer [23], and patients undergoing allogeneic hematopoietic cell transplantation [24]. Particularly lacking are comparisons of the severity of these issues across survivors of different types of cancer.
Depression and depressive symptoms are also common problems for cancer survivors and may be associated with severity of cognitive dysfunction [8, 21]. This association has been reported in survivors assessed just after completion of adjuvant therapies [4], as well as more than 5 years post-treatment [21]. Again however, existing research has largely focused on breast cancer [25, 26]. A better understanding of the prevalence and impact of perceived cognitive dysfunction among survivors of different types of cancer and cancer treatments, as well as associations with depressive symptoms, would have important implications for interventions to improve the lives of cancer survivors.
The goal of the present paper is to explore the prevalence, characteristics, and correlates of perceived cognitive dysfunction in a large sample of post-treatment cancer survivors from the 2010 LIVESTRONG Survey Data and to explore associations with depressive symptoms. We hypothesized that the severity of perceived cognitive dysfunction among survivors will differ across different types of cancer and will be positively associated with a history of chemotherapy, as well as with higher levels of depressive symptoms.
Methods
Procedure
The LIVESTRONG 2010 survey was fielded online from June 2010 through March 2011 to assess post-treatment cancer survivors’ experiences (see Rechis et al. [27] for details on administration and a copy of the survey instrument). The survey was available on LIVESTRONG.org, and LIVESTRONG constituents (e.g., those who either received LIVESTRONG services or who participated in a prior LIVESTRONG event) were notified about the survey through emails and Twitter and Facebook posts. Additionally, LIVESTRONG reached out to partner organizations (e.g., the American Cancer Society) and state cancer coalitions who shared information about the survey with their constituents. Finally, LIVESTRONG worked with seven National Cancer Institute Comprehensive Cancer Centers to share the survey with patients.
Participants
A total of 4286 post-treatment cancer survivors responded to the 2010 LIVESTRONG survey. Post-treatment cancer survivors were defined as respondents who reported being finished with their initial regimen of curative cancer treatment. Respondents taking endocrine medication (e.g., tamoxifen) to reduce risk of recurrence were included. Respondents were excluded from the current analyses if they reported a recurrence of their cancer after completion of all adjuvant therapies for their primary diagnosis or if they reported a second primary cancer (n=861). Childhood cancer survivors (diagnosed at or before age 14) were also excluded (n=139), as were any respondents missing the items assessing perceived cognitive dysfunction or depression (n=182). The final sample for analyses included 3108 cancer survivors.
Measures
Perceived Cognitive Dysfunction (PCD)
Questions about PCD were presented as a series of items starting with the following statement: “Since completing treatment, have any of the following statements been true for you as a result of your experience with cancer?” The statements included five items (coded yes, no, or don’t know) assessing perceived cognitive dysfunction (i.e., “I have had difficulties doing activities that require concentration,” “I have been bothered by having a short attention span,” “I had trouble remembering things,” “I have been bothered by forgetting what I started to do,” and “I have had ‘chemo-brain’”). If the respondent endorsed any of the items, then the respondent was considered to have been or currently be experiencing PCD. “Don’t know” responses were infrequent and were coded as “no.” The first four items are from the Quality of Life in Adult Cancer Survivors (QLACS) questionnaire and make up the “cognitive problems” subscale [28]. The final item regarding “chemo-brain” was added based on several years of work on the part of the LIVESTRONG Foundation with experts and survivors [27].
Current status of PCD was operationally defined by responses to questions asking if cognitive dysfunction had been experienced (a) “before my experience with cancer”; (b) “since completing treatment for cancer, and in the last six months”; and (c) “since completing my treatment for cancer, but not in the last six months.” Respondents were instructed to check any applicable category. Respondents were also asked how much PCD has limited activities over the past 7 days by choosing “a lot,” “a little,” “not at all,” or “I don’t know.”
Depressive symptoms
Questions about depressive symptoms were also presented as a series of items starting with the same statement as the perceived cognitive dysfunction items: “Since completing treatment, have any of the following statements been true for you as a result of your experience with cancer?” This was followed by a series of statements including “I have felt blue or depressed” and “I have been told by a doctor that I am suffering from depression.” If the respondent endorsed either of these two statements, then the respondent was considered to have been or to be currently experiencing depressive symptoms. The first item is from the QLACS questionnaire [28]. The second item was added based on several years of work on the part of the LIVESTRONG Foundation with experts and survivors [27].
Current status of depressive symptoms was operationally defined by a positive response to questions assessing if depression had been experienced (a) “before my experience with cancer”; (b) “since completing treatment for cancer, and in the last six months”; and (c) “since completing my treatment for cancer, but not in the last six months.” Respondents were instructed to check any applicable category.
The survey items described above were developed over several years of work on the part of the LIVESTRONG Foundation during which experts and survivors were consulted in a process of formative research to arrive at a series of items that reflected the challenges people affected by cancer were facing when they presented to LIVESTRONG for services [27].
Demographic and cancer-related variables
Demographic variables included age, gender, race/ethnicity, education, marital status, employment status, and household income. Cancer-related medical variables included type of cancer, treatment received (i.e., chemotherapy, radiation, surgery, hormonal therapy, no treatment), time since diagnosis, and time since completing initial curative treatment.
Analytic approach
Descriptive statistics were used to characterize the presence and history of PCD and depression, as well as demographic and clinical characteristics of the sample. Time since diagnosis was log-transformed in order to normalize its skewed distribution. Other variables met standard normality criteria.
Bivariate statistics (correlation, t tests, analysis of variance (ANOVA), and chi-square tests) descriptively characterized relationships between PCD and each of the demographic and clinical variables.
Logistic regression was used to model odds of reporting PCD. All study variables were included in the logistic regression based on a priori hypotheses. Potential predictors were entered in categories as follows: demographic, cancer-related, pre-cancer history of perceived cognitive dysfunction and/or depression, and current depression. Data analyses were conducted using IBM SPSS Version 20.
Results
Descriptive statistics
Participants were, on average, 48.8 (sd=12.1, range=18–94) years old and 4.6 (sd=5.5, range 0–43, skewness=2.28) years post-diagnosis. More than half (62.4 %) of the sample were female, most were married or partnered (69.0 %), most were White (86.9 %), more than half had a college education (52.1 %), and more than half were employed full-time (59.2 %). Household income ranged from less than $40,000 to over $120,000 annually, and most had health insurance (82.9 %).
The primary cancer types reported with the greatest prevalence were as follows: breast (29.1 %), testicular (9.1 %), prostate (7.4 %), colorectal (5.8 %), non-Hodgkin’s lymphoma (5.5 %), Hodgkin’s lymphoma (5.2 %), and head and neck (3.1 %). One quarter of participants (25.7 %) did not receive any treatment for their disease or only had surgery, while 15.6 % had radiation or radiation and surgery. Over half of participants received chemotherapy (58.7 %), many in addition to radiation (7.6 %), surgery (17.3 %), or both (24.2 %). Hormonal therapy was received by 14.8 % of participants.
Current PCD was reported by 45.7 % of the sample (n= 1419). Among the five items used to assess PCD, 8.8 % endorsed one item, 9.8 % endorsed two items, 11.9 % endorsed three items, 15.9 % endorsed four items, and 27.0 % endorsed all five items. The item “I had trouble remembering things” was endorsed the most frequently (65.4 %). Perceived cognitive dysfunction after cancer diagnosis, but not in the last six months, was reported by 11.2 % of participants, while 6.1 % reported PCD prior to cancer diagnosis. Only those participants that reported PCD in the last six months were included in the final sample for further analyses.
Participants described limitations on daily activities over the past 7 days from PCD as “a lot” (11.5 %), “a little” (48.9 %), “not at all” (38.5 %), and “I don’t know” (1.1 %). Current depression was reported by 66.8 % of the sample. Depression after cancer diagnosis, but not in the last six months, was reported by 13.0 % of participants, while 16.3 % reported depression prior to cancer diagnosis.
Bivariate analyses
Bivariate associations between PCD and demographic characteristics are shown in Table 1. Younger age (t= 2.50, p=0.012), female gender (χ2=166.30, p<0.001), being separated, divorced, or widowed (χ2=6.67, p=0.036), working part-time or being unemployed (χ2=43.35, p<0.001), and having a lower household income (χ2= 31.43, p<0.001) were all associated with PCD in bivariate analyses. Race was also significantly associated with PCD (χ2 = 166.30, p = 0.039) with those participants not reporting race less likely to report PCD. Education was not significantly associated with PCD (χ2=3.51, p=0.476).
Table 1.
Comparison of perceived cognitive dysfunction with demographic and clinical characteristics
| With PCD (n=1419) | Without PCD (n=1689) | p* | |
|---|---|---|---|
| Age in years (t) | 48.2 | 49.3 | 0.012 |
| (SD) | (11.6) | (12.6) | |
| Gender (%) | |||
| Male | 30.6 | 69.4 | <0.001 |
| Female | 54.4 | 45.5 | |
| Marital status (%) | |||
| Married/partnered | 45.0 | 55.0 | 0.036 |
| Single | 44.6 | 55.4 | |
| Sep/div/widowed | 52.0 | 48.0 | |
| Race (%) | |||
| White | 46.1 | 53.9 | 0.039 |
| Non-white | 47.9 | 52.1 | |
| Did not report | 37.0 | 63.0 | |
| Employment (%) | |||
| Full-time | 42.6 | 57.4 | <0.000 |
| Part-time | 52.0 | 48.0 | |
| Student/caregiver/unemployed | 51.0 | 49.0 | |
| Retired | 41.6 | 58.4 | |
| Other | 67.2 | 32.8 | |
| Household income (%) | |||
| $0–40,000 | 53.3 | 46.7 | <0.000 |
| $41,000–60,000 | 50.4 | 49.6 | |
| $61,000–80,000 | 48.5 | 51.5 | |
| $81,000–100,000 | 45.0 | 55.0 | |
| $101,000–120,000 | 47.9 | 52.1 | |
| ≥$120,000 | 39.9 | 60.1 | |
| Prefer not to answer | 41.1 | 59.9 | |
| Time since diagnosis (t) | 0.55 | 0.61 | <0.000 |
| (Log-transformed) | (0.36) | (0.40) | |
| Tumor type (%) | |||
| Central nervous system | 81.0 | 19.0 | <0.000 |
| Breast | 57.6 | 42.4 | |
| Non-Hodgkin’s lymphoma | 50.0 | 50.0 | |
| Hodgkin’s lymphoma | 49.7 | 50.3 | |
| Colorectal | 45.9 | 54.1 | |
| Head and neck | 44.2 | 55.8 | |
| Other | 42.7 | 57.3 | |
| Testicular | 30.9 | 69.1 | |
| Prostate | 18.3 | 81.7 | |
| Surgery (%) | 45.7 | 54.3 | 0.985 |
| Radiation (%) | 51.8 | 48.2 | <0.000 |
| Chemotherapy (%) | 59.2 | 40.8 | <0.000 |
| Hormonal therapy (%) | 61.3 | 38.7 | <0.000 |
| Current depression (%) | 62.9 | 37.1 | <0.000 |
PCD perceived cognitive dysfunction, SD standard deviation
χ2 test for categorical variables and t test for continuous variables
Bivariate associations between PCD and clinical characteristics are also shown in Table 1. More recent diagnosis (t=4.54, p<0.001), primary cancer of the central nervous system (χ2=172.87, p<0.001), having received radiotherapy (χ2=43.09, p<0.001), having received chemotherapy (χ2=325.79, p<0.001), and having received hormonal therapy (χ2=52.28, p<0.001) were all associated with PCD in bivariate analyses. Undergoing surgical intervention for cancer was not associated with PCD. Finally, current depression was strongly associated with PCD (χ2=311.37, p<0.001).
Logistic regression
Logistic regression was used to model the odds of reporting PCD, as shown in Table 2 and Fig. 1. Demographic factors associated with greater odds of PCD included the following: female gender, declining to report household income, and having health insurance. Demographic factors not associated with greater odds of PCD were as follows: race, marital status, education, and employment status.
Table 2.
Logistic regression model of perceived cognitive dysfunction
| Odds of reporting PCD aR2=0.246 χ2=802.01; p<0.001 OR (95 % CI) | p | ||
|---|---|---|---|
| Age | Years | 1.00 (0.92, 1.01) | 0.820 |
| Gender | Female | Reference | <0.001 |
| Male | 0.50 (0.391, 0.645) | ||
| Race | White | Reference | 0.167 |
| Non-white | 1.23 (0.88, 1.74) | 0.233 | |
| Did not report | 0.76 (0.51, 1.12) | 0.170 | |
| Marital status | Married/partnered | Reference | 0.101 |
| Single | 0.78 (0.61, 0.99) | 0.044 | |
| Sep/div/widowed | 1.04 (0.78, 1.38) | 0.788 | |
| Education | High school or less | Reference | 0.061 |
| Some college | 1.51 (0.84, 1.59) | 0.391 | |
| College | 1.14 (0.82, 1.59) | 0.444 | |
| Graduate degree | 1.04 (0.73, 1.48) | 0.816 | |
| Other | 2.91 (1.39, 6.06) | 0.004 | |
| Annual income | $0–40,000 | Reference | 0.017 |
| $41,000–60,000 | 1.20 (0.86, 1.68) | 0.272 | |
| $61,000–80,000 | 1.07 (0.76, 1.52) | 0.695 | |
| $81,000–100,000 | 0.98 (0.68, 1.40) | 0.900 | |
| $101,000–120,000 | 1.15 (0.79, 1.68) | 0.468 | |
| ≥$120,000 | 0.92 (0.66, 1.30) | 0.649 | |
| Prefer not to answer | 0.70 (0.51, 0.96) | 0.026 | |
| Employment | Full-time | Reference | 0.162 |
| Part-time | 1.10 (0.81, 1.47) | 0.548 | |
| Student/caregiver/unemployed | 1.21 (0.94, 1.57) | 0.143 | |
| Retired | 1.07 (0.75, 1.48) | 0.667 | |
| Other | 1.71 (1.08, 2.70) | 0.023 | |
| Health insurance | Missing/did not report | Reference | 0.006 |
| Yes | 1.50 (1.16, 1.95) | 0.002 | |
| No | 1.73 (0.98, 3.04) | 0.058 | |
| Time since diagnosis | Years | 0.56 (0.44, 0.71) | <0.001 |
| Type of cancer | Breast | Reference | 0.002 |
| Central nervous system | 6.80 (2.58, 17.92) | <0.001 | |
| Colorectal | 0.68 (0.46, 1.03) | 0.067 | |
| Non-Hodgkin’s lymphoma | 0.82 (0.53, 1.27) | 0.370 | |
| Head and Neck | 0.81 (0.47, 1.38) | 0.431 | |
| Other | 1.11 (0.85, 1.44) | 0.447 | |
| Prostate | 1.06 (0.65, 1.74) | 0.822 | |
| Testicular | 1.04 (0.66, 1.63) | 0.883 | |
| Hodgkin’s lymphoma | 1.01 (0.64, 1.61) | 0.960 | |
| Depression history | Yes (reference: no) | 1.22 (0.96, 1.54) | 0.106 |
| Cognitive dysfunction history | Yes (reference: no) | 1.75 (1.22, 2.53) | 0.003 |
| Surgery | Yes (reference: no) | 0.96 (0.77, 1.19) | 0.679 |
| Radiation | Yes (reference: no) | 1.19 (0.99, 1.43) | 0.072 |
| Chemotherapy | Yes (reference: no) | 4.88 (3.98, 5.99) | <0.001 |
| Hormonal therapy | Yes (reference: no) | 1.70 (1.29, 2.25) | <0.001 |
| Depression currently | Yes (reference: no) | 3.54 (2.96, 4.23) | <0.001 |
PCD perceived cognitive dysfunction, OR odds ratio, CI confidence interval
Cox & Snell
Fig. 1.
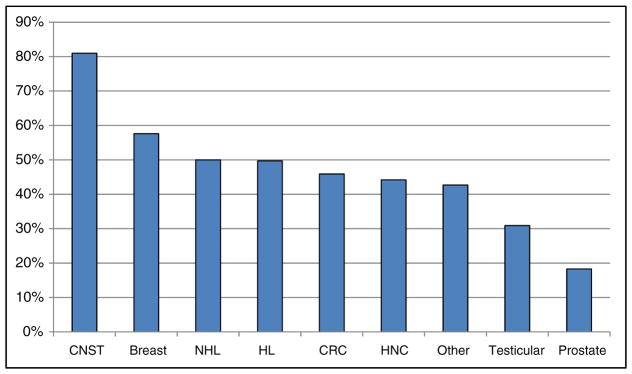
Prevalence of perceived cognitive dysfunction by cancer tumor type. CNST central nervous system tumor, NHL non-Hodgkin’s lymphoma, HL Hodgkin’s lymphoma, CRC colorectal cancer, HNC head and neck cancer
Abbreviations: CNST, central nervous system tumor; NHL, non-Hodgkin’s lymphoma; HL, Hodgkin’s lymphoma; CRC, colorectal cancer; HNC, head and neck cancer.
Clinical factors associated with greater odds of PCD included the following: more recent diagnosis, being diagnosed with cancer of the central nervous system (e.g., primary brain tumor), and reporting a pre-cancer history of perceived cognitive dysfunction. Figure 1 shows prevalence of PCD by cancer tumor type. Clinical interventions associated with greater odds of PCD included the following: receiving chemotherapy and receiving hormonal therapy. Current report of depression was also strongly associated with greater odds of PCD. Clinical factors not associated with greater odds of PCD were as follows: surgical intervention, receiving radiation, and reporting a pre-cancer history of depression.
Discussion
The transition from cancer patient to cancer survivor is a bumpy road with many challenges that are still not well understood by health-care professionals [29]. Cognitive dysfunction is increasingly recognized as an ongoing cancer and treatment-related survivorship issue and was among the most frequently reported survivorship concerns on the 2006 LIVESTRONG survey [3]. In the present study of 3108 survivors from the 2010 LIVESTRONG survey [27], nearly half of the participants reported PCD, showing that perceived cognitive dysfunction continues to be a significant survivorship concern. Of those reporting PCD in the present study, over 60 % described some degree of limitations on daily activities from PCD.
Other studies have found similar rates of post-treatment cognitive dysfunction after completion of treatment [21, 30]; however, much of this literature has been limited to breast cancer survivors [5, 8]. The present study included survivors of many different types of cancer and provides a unique look at the incidence of PCD, assessed with a common set of questions applied to a large sample of respondents. Study participants with a primary diagnosis of central nervous system cancer were the most likely to report PCD (81 %). This percentage is likely high due to direct tumor impact in survivors of primary brain tumors [31, 32], and may also be due to treatment-related factors such as the known side effects of bevacizumab and anti-epileptic drugs that are normally prescribed for CNS cancer patients [33, 34]. However, PCD was widely reported among survivors of all cancer types with about half of the study participants with breast, lymphoma, colorectal, and head and neck cancers reporting PCD (44.2–57.6 %, see Fig. 1). Interestingly, the odds of reporting PCD in breast cancer participants were not significantly different than for other cancers, with the exception of central nervous system tumors (see Table 2).
The extant literature on cognitive dysfunction in cancer survivors is limited regarding associations with demographic and clinical characteristics outside of research with breast cancer survivors, although this trend has started to change in the past few years (please see [22–24]). In the present study, participants reporting PCD tended to be younger, female, employed other than full-time, separated/divorced/widowed, and have lower household income. These findings are consistent with prior studies showing an association between age and cognitive dysfunction in survivors [35–37]; however, please note that these studies focused on accelerated cognitive decline in older cancer survivors [38] while the sample in the present study is younger with a broad age range. One possible reason for the association between age and PCD found in the present study sample may be that younger survivors are often trying to manage typical young adult concerns such as early career obligations and raising children while also coping with cancer diagnosis and treatment; thus, even minor changes in cognitive functioning are noticed and can impact daily functioning and life balance. While age and being separated/divorced/widowed were associated with report of PCD in the bivariate models here, in the logistic regression, age was not predictive of PCD nor was partner status.
Treatments for cancer are aggressive and can include multiple interventions such as surgery, chemotherapies, radiation, immunotherapies, and hormonal therapies, and the vast improvements in the efficacy of these treatment modalities are the primary reason for the exponential growth in survivors [1]. However, cancer treatments can be highly toxic and are often non-specific, affecting normally functioning cells and bodily systems. Many prior studies of post-treatment cognitive dysfunction have focused on chemotherapy as the “source” for cognitive dysfunction, as assessed using standard neuropsychological tests as well as via self-report instruments of perceived cognitive dysfunction. Consistent with this view, in the present study, a history of chemotherapy treatment was found to be the strongest predictor of PCD. The central nervous system may be particularly sensitive to toxicities associated with chemotherapy treatments either directly after exposure to chemotherapy [39] or indirectly via chemotherapy-induced systemic inflammation or hormonal changes [40]. Results of the present study also show that these effects are comparable across most cancer types. Hormonal therapy was also found to be a predictor of PCD. These therapies have become standard treatment for some cancers (e.g., breast cancer, ovarian cancer, prostate cancer [41, 42]). Several recent studies provide evidence of cognitive dysfunction associated with these therapies with self-report assessments and via standard neuropsychological assessment [11, 43]. However, no association between hormonal therapy and cognitive dysfunction has also been found [44], suggesting the need for more research.
Participants that reported experiencing PCD prior to cancer diagnosis and treatment were also more likely to report PCD in the past 6 months as shown Table 2. This association has been found in several studies [45, 46] with cognitive dysfunction assessed even before receiving any treatments for cancer. However, the exact mechanisms are not well understood and have not been addressed directly [47]. Pre-treatment cognitive dysfunction may be associated with inflammatory responses to the disease which results in neurotoxic cytokines [40], as well as other risk factors for post-treatment cognitive dysfunction such as hormonal changes [48], diminished cognitive reserve [37, 49], and possibly depression [50, 51].
Current symptoms of depression were reported by well over half the study sample (66.8 %), consistent with previous studies suggesting that depression may be an under-recognized, under-reported, and under-treated issue for many cancer survivors [52–54]. Symptoms of depression may of course be heightened by the impact of other survivorship issues, including cognitive dysfunction. Interestingly, previous studies have suggested that declines in cognitive dysfunction after adjuvant treatments may be exacerbated by significant symptoms of depression in cancer survivors [4, 8, 21]. In the present study, participants reporting PCD also endorsed significant symptoms of concurrent depression in both bivariate and logistic regression analyses. Indeed, current symptoms of depression were the strongest correlates of PCD in logistic regression after chemotherapy.
Survivors experiencing significant PCD may potentially benefit from cognitive training and rehabilitation programs. Cognitive training and rehabilitation has proven effective in treating cognitive dysfunction in other medical populations such as stroke [55], traumatic brain injury [56], and more recently mild cognitive impairment [57]. The new generation of computerized cognitive training programs use repetitive hierarchical tasks designed to exercise and improve attention, memory, processing speed, and executive functioning. Research in medical populations is still being developed, and recent studies have demonstrated benefit of these programs on cognitive functioning in breast cancer survivors [58], childhood cancer survivors [59], and individuals with mild cognitive impairment [60]. These studies, however, did not intervene to reduce depressive symptoms [61, 62], which an emerging literature suggests may have beneficial effects on cognitive function as well [63, 64]. The association between heightened depressive symptoms and perceived cognitive dysfunction found in the present study raises the possibility that concurrent treatment of cognitive dysfunction and symptoms of depression may be a particularly powerful strategy for cancer survivors, with potential for synergistic enhancements in efficacy.
Additional research is needed to (1) improve identification of PCD in cancer survivors of all tumor types, (2) better educate survivors on potential ways to lessen the impact and interference of PCD on daily activities by providing self-care strategies, and (3) develop interventions to concurrently improve PCD and reduce comorbid symptoms of depression. Given the recent strong evidence of the benefit of concurrent mild exercise (e.g., brisk walking) with cognitive training on patients with mild cognitive impairment [65, 66], similar benefits may be found in cancer survivors.
Limitations
Several limitations of the study must be noted. First, the study relied on a cross-sectional, anonymous survey. Thus, the temporal and causal direction of the observed associations among the study variables cannot be determined. Prospective longitudinal studies and randomized intervention trials are needed to determine causal relationships among variables associated with perceived cognitive dysfunction in cancer survivors. The significance of this issue is particularly relevant regarding the relationship between symptoms of depression and current cognitive dysfunction, given our finding that current depression is strongly associated with current perceived cognitive dysfunction in the study sample. Second, the survey did not employ validated self-report measures of depression or cognitive function. Instead, face valid, consensus-derived items were used. The findings reported here thus need to be confirmed in future research using validated measures established to have strong psychometric properties. Third, cognitive dysfunction was not assessed objectively using well-established (though lengthy) cognitive testing approaches. Perceptions of cognitive dysfunction assessed via self-report measures, even validated instruments, have been inconsistent with results obtained using objective testing tools [67, 68]. It should be noted however that perceived cognitive dysfunction is more likely to capture the respondent’s experiences of interference in daily functioning and quality of life [68]. Fourth, two of the items used to assess PCD (“I have had difficulties doing activities that require concentration” and “I have been bothered by having a short attention span”) overlap with one of the diagnostic criteria for depression (DSM-V, APA); thus, study participants that endorsed one or both of these items may have been experiencing symptoms of depression. Finally, given the anonymous, online, and voluntary nature of the survey, respondents may not be representative of the general population of cancer survivors; thus, caution should be used when interpreting results.
Conclusion
The findings reported here contribute to a growing scientific understanding of the prevalence and correlates of cognitive dysfunction in cancer survivors and the potential interactive effects of concurrent symptoms of depression that may exacerbate the severity and impact of cognitive dysfunction. The high prevalence of perceived cognitive dysfunction found in the current study, with nearly half the study sample endorsing current cognitive dysfunction and interference in daily activities, underscores the importance of the problem and the need to develop and apply more effective interventions.
Acknowledgments
This research was supported by funding from the LIVESTRONG Foundation.
Footnotes
Conflict of interest The authors declare that they have no competing interests.
References
- 1.Cancer facts and figures 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 2.de Moor JS, Mariotto AB, Parry C, Alfano CM, Padgett L, Kent EE, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomark Prev. 2013;22(4):561–70. doi: 10.1158/1055-9965.EPI-12-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckjord EB, Reynolds KA, van Londen GJ, Burns R, Singh R, Arvey SR, et al. Population-level trends in posttreatment cancer survivors’ concerns and associated receipt of care: results from the 2006 and 2010 LIVESTRONG surveys. J Psychosoc Oncol. 2014;32(2):125–51. doi: 10.1080/07347332.2013.874004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vearncombe KJ, Rolfe M, Wright M, Pachana NA, Andrew B, Beadle G. Predictors of cognitive decline after chemotherapy in breast cancer patients. J Int Neuropsych Soc. 2009;15(6):951–62. doi: 10.1017/S1355617709990567. [DOI] [PubMed] [Google Scholar]
- 5.Jim HS, Donovan KA, Small BJ, Andrykowski MA, Munster PN, Jacobsen PB. Cognitive functioning in breast cancer survivors: a controlled comparison. Cancer. 2009;115(8):1776–83. doi: 10.1002/cncr.24192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brezden CB, Phillips KA, Abdolell M, Bunston T, Tannock IF. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol: Off J Am Soc Clin Oncol. 2000;18(14):2695–701. doi: 10.1200/JCO.2000.18.14.2695. [DOI] [PubMed] [Google Scholar]
- 7.Hurria A, Somlo G, Ahles T. Renaming “chemobrain”. Cancer Investig. 2007;25(6):373–7. doi: 10.1080/07357900701506672. [DOI] [PubMed] [Google Scholar]
- 8.Joly F, Rigal O, Noal S, Giffard B. Cognitive dysfunction and cancer: which consequences in terms of disease management? Psycho-Oncology. 2011;20(12):1251–8. doi: 10.1002/pon.1903. [DOI] [PubMed] [Google Scholar]
- 9.Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psycho-Oncology. 2009;18(2):134–43. doi: 10.1002/pon.1379. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal K, Onami S, Mortimer JE, Pal SK. Cognitive changes associated with endocrine therapy for breast cancer. Maturitas. 2010;67(3):209–14. doi: 10.1016/j.maturitas.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganz PA, Petersen L, Castellon SA, Bower JE, Silverman DH, Cole SW, et al. Cognitive function after the initiation of adjuvant endocrine therapy in early-stage breast cancer: an observational cohort study. J Clin Oncol: Off J Am Soc Clin Oncol. 2014;32(31):3559–67. doi: 10.1200/JCO.2014.56.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitsiades N, Correa D, Gross CP, Hurria A, Slovin SF. Cognitive effects of hormonal therapy in older adults. Semin Oncol. 2008;35(6):569–81. doi: 10.1053/j.seminoncol.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Myers JS. Chemotherapy-related cognitive impairment: the breast cancer experience. Oncol Nurs Forum. 2012;39(1):E31–40. doi: 10.1188/12.ONF.E31-E40. [DOI] [PubMed] [Google Scholar]
- 14.Pullens MJ, De Vries J, Roukema JA. Subjective cognitive dysfunction in breast cancer patients: a systematic review. Psycho-Oncology. 2010;19(11):1127–38. doi: 10.1002/pon.1673. [DOI] [PubMed] [Google Scholar]
- 15.Hermelink K, Kuchenhoff H, Untch M, Bauerfeind I, Lux MP, Buhner M, et al. Two different sides of ‘chemobrain’: determinants and nondeterminants of self-perceived cognitive dysfunction in a prospective, randomized, multicenter study. Psycho-Oncology. 2010;19(12):1321–8. doi: 10.1002/pon.1695. [DOI] [PubMed] [Google Scholar]
- 16.Lindner OC, Phillips B, McCabe MG, Mayes A, Wearden A, Varese F, et al. A meta-analysis of cognitive impairment following adult cancer chemotherapy. Neuropsychology. 2014;28(5):726–40. doi: 10.1037/neu0000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Von Ah D, Tallman EF. Perceived cognitive function in breast cancer survivors: evaluating relationships with objective cognitive performance and other symptoms using the functional assessment of cancer therapy-cognitive function instrument. J Pain Symptom Manag. 2015;49(4):697–706. doi: 10.1016/j.jpainsymman.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Hutchinson AD, Hosking JR, Kichenadasse G, Mattiske JK, Wilson C. Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat Rev. 2012;38(7):926–34. doi: 10.1016/j.ctrv.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Biglia N, Bounous VE, Malabaila A, Palmisano D, Torta DM, D’Alonzo M, et al. Objective and self-reported cognitive dysfunction in breast cancer women treated with chemotherapy: a prospective study. Europ J Cancer Care. 2012;21(4):485–92. doi: 10.1111/j.1365-2354.2011.01320.x. [DOI] [PubMed] [Google Scholar]
- 20.Boykoff N, Moieni M, Subramanian SK. Confronting chemobrain: an in-depth look at survivors’ reports of impact on work, social networks, and health care response. J Cancer Surviv: Res Pract. 2009;3(4):223–32. doi: 10.1007/s11764-009-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Von Ah D, Russell KM, Storniolo AM, Carpenter JS. Cognitive dysfunction and its relationship to quality of life in breast cancer survivors. Oncol Nurs Forum. 2009;36(3):326–36. doi: 10.1188/09.ONF.326-334. [DOI] [PubMed] [Google Scholar]
- 22.Cruzado JA, Lopez-Santiago S, Martinez-Marin V, Jose-Moreno G, Custodio AB, Feliu J. Longitudinal study of cognitive dysfunctions induced by adjuvant chemotherapy in colon cancer patients. Support Care Cancer: Off J Multinatl Assoc Support Care Cancer. 2014;22(7):1815–23. doi: 10.1007/s00520-014-2147-x. [DOI] [PubMed] [Google Scholar]
- 23.Wefel JS, Vidrine DJ, Marani SK, Swartz RJ, Veramonti TL, Meyers CA, et al. A prospective study of cognitive function in men with non-seminomatous germ cell tumors. Psycho-Oncology. 2014;23(6):626–33. doi: 10.1002/pon.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Syrjala KL, Artherholt SB, Kurland BF, Langer SL, Roth-Roemer S, Elrod JB, et al. Prospective neurocognitive function over 5 years after allogeneic hematopoietic cell transplantation for cancer survivors compared with matched controls at 5 years. J Clin Oncol: Off J Am Soc Clin Oncol. 2011;29(17):2397–404. doi: 10.1200/JCO.2010.33.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montazeri A. Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. J Experiment Clin Cancer Res: CR. 2008;27:32. doi: 10.1186/1756-9966-27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hegel MT, Moore CP, Collins ED, Kearing S, Gillock KL, Riggs RL, et al. Distress, psychiatric syndromes, and impairment of function in women with newly diagnosed breast cancer. Cancer. 2006;107(12):2924–31. doi: 10.1002/cncr.22335. [DOI] [PubMed] [Google Scholar]
- 27.Rechis R, Reynolds KA, Beckjord EB, Nutt S, Burns RM, Schaefer JS. “I learned to live with it” is not enough: challenges reported by post-treatment cancer survivors in the LIVESTRONG surveys. Austin: LIVESTRONG; 2011. [Google Scholar]
- 28.Avis NE, Smith KW, McGraw S, Smith RG, Petronis VM, Carver CS. Assessing quality of life in adult cancer survivors (QLACS) Qual Life Res: Int J Qual Life Asp treat, Care Rehab. 2005;14(4):1007–23. doi: 10.1007/s11136-004-2147-2. [DOI] [PubMed] [Google Scholar]
- 29.Hudson MM, Landier W, Ganz PA. Impact of survivorship-based research on defining clinical care guidelines. Cancer Epidemiol, Biomark Prevent: Public Am Assoc Cancer Res, Cospons Am Soc Prevent Oncol. 2011;20(10):2085–92. doi: 10.1158/1055-9965.EPI-11-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenkins V, Shilling V, Deutsch G, Bloomfield D, Morris R, Allan S, et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer. 2006;94(6):828–34. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meskal I, Gehring K, van der Linden SD, Rutten GJ, Sitskoorn MM. Cognitive improvement in meningioma patients after surgery: clinical relevance of computerized testing. J Neurooncol. 2015;121(3):617–25. doi: 10.1007/s11060-014-1679-8. [DOI] [PubMed] [Google Scholar]
- 32.van Nieuwenhuizen D, Ambachtsheer N, Heimans JJ, Reijneveld JC, Peerdeman SM, Klein M. Neurocognitive functioning and health-related quality of life in patients with radiologically suspected meningiomas. J Neurooncol. 2013;113(3):433–40. doi: 10.1007/s11060-013-1132-4. [DOI] [PubMed] [Google Scholar]
- 33.Klein M, Engelberts NH, van der Ploeg HM, Kasteleijn-Nolst Trenite DG, Aaronson NK, Taphoorn MJ, et al. Epilepsy in low-grade gliomas: the impact on cognitive function and quality of life. Ann Neurol. 2003;54(4):514–20. doi: 10.1002/ana.10712. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lange M, Rigal O, Clarisse B, Giffard B, Sevin E, Barillet M, et al. Cognitive dysfunctions in elderly cancer patients: a new challenge for oncologists. Cancer Treat Rev. 2014;40(6):810–7. doi: 10.1016/j.ctrv.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Lange M, Giffard B, Noal S, Rigal O, Kurtz JE, Heutte N, et al. Baseline cognitive functions among elderly patients with localised breast cancer. Europ J Cancer. 2014 doi: 10.1016/j.ejca.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 37.Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol: Off J Am Soc Clin Oncol. 2010;28(29):4434–40. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandelblatt JS, Hurria A, McDonald BC, Saykin AJ, Stern RA, VanMeter JW, et al. Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know? Semin Oncol. 2013;40(6):709–25. doi: 10.1053/j.seminoncol.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDonald BC, Conroy SK, Smith DJ, West JD, Saykin AJ. Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: a replication and extension study. Brain Behav Immun. 2013;30(Suppl):S117–25. doi: 10.1016/j.bbi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ganz PA, Bower JE, Kwan L, Castellon SA, Silverman DH, Geist C, et al. Does tumor necrosis factor-alpha (TNF-alpha) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun. 2013;30(Suppl):S99–108. doi: 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Institutes of Health Consensus Development P. National Institutes of Health Consensus Development Conference statement: adjuvant therapy for breast cancer, November 1–3, 2000. J Natl Cancer Inst Monograph. 2001;(30):5–15. [PubMed] [Google Scholar]
- 42.Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59(1):61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 43.Schilder CM, Seynaeve C, Beex LV, Boogerd W, Linn SC, Gundy CM, et al. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol: Off J Am Soc Clin Oncol. 2010;28(8):1294–300. doi: 10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- 44.Fan HG, Houede-Tchen N, Yi QL, Chemerynsky I, Downie FP, Sabate K, et al. Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1-and 2-year follow-up of a prospective controlled study. J Clin Oncol: Off J Am Soc Clin Oncol. 2005;23(31):8025–32. doi: 10.1200/JCO.2005.01.6550. [DOI] [PubMed] [Google Scholar]
- 45.Wefel JS, Lenzi R, Theriault R, Buzdar AU, Cruickshank S, Meyers CA. ‘Chemobrain’ in breast carcinoma?: a prologue. Cancer. 2004;101(3):466–75. doi: 10.1002/cncr.20393. [DOI] [PubMed] [Google Scholar]
- 46.Askren M, Jung M, Berman M, Zhang M, Therrien B, Peltier S, et al. Neuromarkers of fatigue and cognitive complaints following chemotherapy for breast cancer: a prospective fMRI investigation. Breast Cancer Res Tr. 2014;147(2):445–55. doi: 10.1007/s10549-014-3092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schagen SB, Klein M, Reijneveld JC, Brain E, Deprez S, Joly F, et al. Monitoring and optimising cognitive function in cancer patients: present knowledge and future directions. Eur J Cancer Suppl. 2014;12(1):29–40. doi: 10.1016/j.ejcsup.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schilder CMT, Seynaeve C, Linn SC, Boogerd W, Beex LVAM, Gundy CM, et al. Cognitive functioning of postmenopausal breast cancer patients before adjuvant systemic therapy, and its association with medical and psychological factors. Crit Rev Oncol/Hematol. 2010;76(2):133–41. doi: 10.1016/j.critrevonc.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosc Rep. 2012;12(3):267–75. doi: 10.1007/s11910-012-0264-9. [DOI] [PubMed] [Google Scholar]
- 50.Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. 2013;139(1):81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Farrell E, MacKenzie J, Collins B. Clearing the air: a review of our current understanding of “chemo fog”. Curr Oncol Rep. 2013;15(3):260–9. doi: 10.1007/s11912-013-0307-7. [DOI] [PubMed] [Google Scholar]
- 52.Spoletini I, Gianni W, Repetto L, Bria P, Caltagirone C, Bossu P, et al. Depression and cancer: an unexplored and unresolved emergent issue in elderly patients. Crit Rev Oncol/Hematol. 2008;65(2):143–55. doi: 10.1016/j.critrevonc.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 53.Williams S, Dale J. The effectiveness of treatment for depression/depressive symptoms in adults with cancer: a systematic review. Br J Cancer. 2006;94(3):372–90. doi: 10.1038/sj.bjc.6602949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hopko DR, Bell JL, Armento MEA, Robertson SMC, Hunt MK, Wolf NJ, et al. The phenomenology and screening of clinical depression in cancer patients. J Psychosoc Oncol. 2007;26(1):31–51. doi: 10.1300/j077v26n01_03. [DOI] [PubMed] [Google Scholar]
- 55.van Heugten C, Wolters Gregório G, Wade D. Evidence-based cognitive rehabilitation after acquired brain injury: a systematic review of content of treatment. Neuropsychologic Rehab. 2012;22(5):653–73. doi: 10.1080/09602011.2012.680891. [DOI] [PubMed] [Google Scholar]
- 56.Carney N, Chesnut RM, Maynard H, Mann NC, Patterson P, Helfand M. Effect of cognitive rehabilitation on outcomes for persons with traumatic brain injury: a systematic review. J Head Trauma Rehabil. 1999;14(3):277–307. doi: 10.1097/00001199-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Zelinski EM, Spina LM, Yaffe K, Ruff R, Kennison RF, Mahncke HW, et al. Improvement in memory with plasticity-based adaptive cognitive training: results of the 3-month follow-up. J Am Geriatr Soc. 2011;59(2):258–65. doi: 10.1111/j.1532-5415.2010.03277.x. [DOI] [PubMed] [Google Scholar]
- 58.Kesler S, Hadi Hosseini SM, Heckler C, Janelsins M, Palesh O, Mustian K, et al. Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clin Breast Cancer. 2013;13(4):299–306. doi: 10.1016/j.clbc.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kesler SR, Lacayo NJ, Jo B. A pilot study of an online cognitive rehabilitation program for executive function skills in children with cancer-related brain injury. Brain Injur: [BI] 2011;25(1):101–12. doi: 10.3109/02699052.2010.536194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Finn M, McDonald S. Computerised cognitive training for older persons with mild cognitive impairment: a pilot study using a randomised controlled trial design. Brain Impair. 2011;12(03):187–99. [Google Scholar]
- 61.Gehring K, Patwardhan S, Collins R, Groves M, Etzel C, Meyers C, et al. A randomized trial on the efficacy of methylphenidate and modafinil for improving cognitive functioning and symptoms in patients with a primary brain tumor. J Neuro-Oncol. 2012;107(1):165–74. doi: 10.1007/s11060-011-0723-1. [DOI] [PubMed] [Google Scholar]
- 62.Gehring K, Roukema J, Sitskoorn M. Review of recent studies on interventions for cognitive deficits in patients with cancer. Expert Rev Anticancer Ther. 2012;12(2):255–69. doi: 10.1586/era.11.202. [DOI] [PubMed] [Google Scholar]
- 63.Mackin RS, Nelson JC, Delucchi K, Raue P, Byers A, Barnes D, et al. Cognitive outcomes after psychotherapeutic interventions for major depression in older adults with executive dysfunction. Am J Geriatr Psychiatr: Off J Am Assoc Geriatr Psychiatr. 2013 doi: 10.1016/j.jagp.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Culang ME, Sneed JR, Keilp JG, Rutherford BR, Pelton GH, Devanand DP, et al. Change in cognitive functioning following acute antidepressant treatment in late-life depression. Am J Geriatr Psychiatr: Off J Am Assoc Geriatr Psychiatr. 2009;17(10):881–8. doi: 10.1097/jgp.0b013e3181b4bf4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Law LL, Barnett F, Yau MK, Gray MA. Effects of combined cognitive and exercise interventions on cognition in older adults with and without cognitive impairment: a systematic review. Ageing Res Rev. 2014;15:61–75. doi: 10.1016/j.arr.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 66.Barnes DE, Santos-Modesitt W, Poelke G, Kramer AF, Castro C, Middleton LE, et al. The Mental Activity and eXercise (MAX) trial: a randomized controlled trial to enhance cognitive function in older adults. JAMA Int Med. 2013;173(9):797–804. doi: 10.1001/jamainternmed.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Von Ah D, Carpenter JS, Saykin A, Monahan P, Wu JW, Yu MG, et al. Advanced cognitive training for breast cancer survivors: a randomized controlled trial. Breast Cancer Res Tr. 2012;135(3):799–809. doi: 10.1007/s10549-012-2210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hermelink K, Kuchenhoff H, Untch M, Bauerfeind I, Lux MP, Buhner M, et al. Two different sides of ‘chemobrain’: determinants and nondeterminants of self-perceived cognitive dysfunction in a prospective, randomized, multicenter study. Psycho-Oncology. 2010;19(12):1321–8. doi: 10.1002/pon.1695. [DOI] [PubMed] [Google Scholar]