Abstract
Translesion synthesis is the process by which non-classical DNA polymerases bypass DNA damage during DNA replication. Cells possess a variety of non-classical polymerases, each one specific for incorporating nucleotides opposite one or more closely related DNA lesions, called its cognate lesions. In this article we discuss a variety of approaches for probing the catalytic activities and the protein-protein interactions of non-classical polymerases. With respect to their catalytic activities, we discuss polymerase assays, steady state kinetics, and pre-steady state kinetics. With respect to their interactions, we discuss qualitative binding assays such as enzyme-linked immunosorbent assays and coimmunoprecipitation; quantitative binding assays such as isothermal titration calorimetry, surface plasmon resonance, and nuclear magnetic resonance spectroscopy; and single-molecule binding assays such as total internal reflection fluorescence microscopy. We focus on how non-classical polymerases accommodate their cognate lesions during nucleotide incorporation and how the most appropriate non-classical polymerase is selected for bypassing a given lesion.
1. Introduction
DNA synthesis by classical DNA polymerases (i.e., those involved in normal DNA replication and repair) is blocked by DNA damage. Thus cells utilize several strategies for bypassing DNA damage during DNA replication. Our understanding of the mechanisms by which cells bypass damage emerged from studies of radiation sensitive mutants of yeast. The Rad6 epistasis group comprises a series of genes that encode the proteins that carry out damage bypass (Lawrence, 1994; Prakash et al., 1993). For example, Rad6 and Rad18 catalyze the mono-ubiquitylation of proliferating cell nuclear antigen (PCNA), the sliding clamp replication accessory factor (Hoege et al., 2002). Similarly, Mms2-Ubc13 and Rad5 catalyze the poly-ubiquitylation of PCNA (Hoege et al., 2002). PCNA mono-ubiquitylation facilitates damage bypass by translesion synthesis, and PCNA poly-ubiquitylation facilitates damage bypass by template switching. In this article, we will focus on damage bypass by translesion synthesis.
Translesion synthesis is a damage bypass strategy in which non-classical DNA polymerases are used to incorporate nucleotides opposite damaged DNA templates (Friedberg et al., 2002; Lawrence, 2002; Lehmann et al., 2007; Prakash et al., 2005; Prakash and Prakash, 2002; Pryor et al., 2014; Sale et al., 2012; Washington et al., 2010; Waters et al., 2009). Non-classical DNA polymerases differ from their classical counterparts in that they are able to synthesize DNA on both damaged and non-damaged templates. Cells possess a variety of non-classical polymerases to carry out translesion synthesis. For example, Rad30 is DNA polymerase eta (pol η), which functions in the bypass of cis-syn thymine dimers and 8-oxoguanines (Haracska et al., 2000; Johnson et al., 1999). Likewise, Rev3-Rev7 is DNA polymerase zeta (pol ζ), which participates in the bypass of a wide range of DNA lesions (Lawrence, 2002). Rev1 works in the bypass of abasic sites as well as major-groove and exocyclic guanine adducts (Nelson et al., 1996; Pryor and Washington, 2011; Washington et al., 2004). By convention, Rev1 is not given a Greek letter designation. However, in terms of its structure and enzymatic mechanism, Rev1 closely resembles other non-classical polymerases (Haracska et al., 2002; Nair et al., 2005). Thus Rev1 is a polymerase in every way except its name.
The presence of multiple non-classical polymerases with different substrate specificities has led to the notion of cognate lesions (Friedberg et al., 2002; Lehmann et al., 2007; Pryor and Washington, 2011; Waters et al., 2009). While the precise definition of a cognate lesion varies among researchers, the general idea is that a cognate lesion for a given polymerase is one that the polymerase has evolved to bypass. In this review, we will discuss biochemical approaches to understanding the enzymatic mechanism of non-classical polymerases as well as the protein-protein interactions in which they engage. Particular emphasis will be placed on how one can determine which types of DNA damage are cognate lesions for a given polymerase and how the most appropriate polymerase might be selected for bypassing a given lesion. Here we will use the yeast non-classical polymerases as examples, although these methods are equally applicable (and have been equally applied) to bacterial and mammalian non-classical polymerases.
2. Purification and structures of non-classical polymerases
Eukaryotic non-classical polymerases have structured and unstructured regions (Figure 1A) (Ohmori et al., 2009; Pryor et al., 2014). For example, pol η has a structured catalytic domain (residues 1 to 513) and an intrinsically disordered C-terminal region (residues 514 to 634). Within the C-terminal region is a small, folded ubiquitin-binding/zinc-binding (UBZ) motif (residues 552 to 574) and a PCNA-interacting protein (PIP) motif (residues 621 to 628). Similarly, Rev1 has a structured catalytic domain (residues 305 to 738), an intrinsically disordered N-terminal region (residues 1 to 304), and an intrinsically disordered C-terminal region (residues 739 to 985). Within the N-terminal region is a small, folded BRCA1 C-terminal (BRCT) domain (residues 164 to 250). Within the C-terminal region is a small, folded ubiquitin-binding motif (UBM; residues 809 to 837) and a small, folded C-terminal domain (CTD; residues 872 to 976). These non-catalytic domains and motifs are involved in protein-protein interactions.
Figure 1. Structural models of full-length pol η and Rev1 and their catalytic domains.

(A) Disorder plots showing the disorder probability calculated using PrDOS (Ishida and Kinoshita, 2007) graphed as a function of residue number for pol η (blue) and for Rev1 (red). The catalytic core, the UBZ, and the PIP motif of pol η are highlighted, and the BRCT, catalytic core, UBM, and CTD of Rev1 are highlighted. (B) Crystal structure of the catalytic core of pol η (blue) is shown with an inset detailing the binding of a dATP opposite the 3′T of a thymine dimer (PDB ID: 3MFI) (Silverstein et al., 2010b). Crystal structure of the catalytic core of Rev1 (red) is shown with an inset detailing the binding of dCTP to Arg-324 of Rev1 (PDB ID: 3OSP) (Nair et al., 2011).
Because these polymerases contain extended regions of intrinsic disorder, they are generally overexpressed and purified in yeast. Usually, they are produced as N-terminal glutathione-s-transferase (GST) fusion proteins and are purified by affinity chromatography using glutathione sepharose. Both full-length polymerases and the isolated catalytic domains can be produced and purified by this approach. Typical yields are ~1 mg of pure pol η or Rev1 per 40 L of culture. The overexpression and purification of yeast and mammalian non-classical polymerases have been reviewed in more detail previously (Johnson et al., 2006).
Multiple X-ray crystal structures of the catalytic domains of yeast pol η and Rev1 have been determined (Figure 1B) (Nair et al., 2005, 2008, 2011; Silverstein et al., 2010a; Silverstein et al., 2010b; Trincao et al., 2001). Both catalytic domains have four sub-domains: a “palm” subdomain, which forms the catalytic site; a “fingers” subdomain, which interacts with the incoming deoxynucleoside triphosphate (dNTP); a “thumb” subdomain, which interacts with the duplex region of the DNA substrate; and a “little fingers” subdomain (also known as a polymerase-associated domain, PAD), which also interacts with the DNA substrate. The active site is comprised of several acidic amino acid residues, which bind Mg2+ ions that catalyze nucleotide incorporation by deprotonating the 3′hydroxyl group of the primer strand and stabilizing the negatively charged penta-coordinate transition state.
These X-ray crystal structures have provided extensive insight into the structural basis of the specificity of these polymerases. Pol η, for instance, has a larger active site that allows it to accommodate both bases of a template cis-syn thymine dimer without perturbations of the active site (Silverstein et al., 2010b; Trincao et al., 2001). Thus pol η is able to correctly incorporate adenines opposite both thymine residues of the lesion by utilizing the intrinsic Watson-Crick base pairing ability of the dimer. By contrast, Rev1 flips the template base out of the DNA double helix so that it cannot pair with the incoming dNTP. Instead an arginine side chain of the protein acts as the template by forming hydrogen bonds with the incoming dNTP (Nair et al., 2005, 2008, 2011).
3. Catalytic activities of non-classical polymerases
In this section, we will review some of the most common biochemical methods for studying the catalytic activities of non-classical polymerases. Because of space constraints, we are not going to examine all the experimental approaches one must perform to obtain a full understanding of the catalytic mechanism. Other review articles cover some of these approaches (Creighton et al., 1995; Goodman et al., 1993; Johnson, 1995, 2010). Instead, we will focus here on the approaches one can use to understand the damage-bypass abilities of non-classical polymerases. In particular, we will focus on the experiments that one uses to draw conclusions about what types of DNA damage are the cognate lesions for a given non-classical polymerase.
3.1. Polymerase assays
One of the simplest and most common approaches to examine the ability of non-classical polymerases to synthesize DNA on damaged and non-damaged templates is a polymerase assay (Figure 2A). In this assay, the DNA substrate is made by annealing a short, 32P-end labeled primer strand (usually 25 to 30 nucleotides in length) to a longer template strand (usually 50 to 75 nucleotides in length). Depending on the location of the damage in the DNA substrate, these assays may be either “standing start” or “running start” experiments. In standing start experiments, the damaged template is the first available template residue. In running start experiments, several non-damaged residues in the template are used before the enzyme encounters the damage.
Figure 2. Analyzing the catalytic activity of non-classical polymerase.
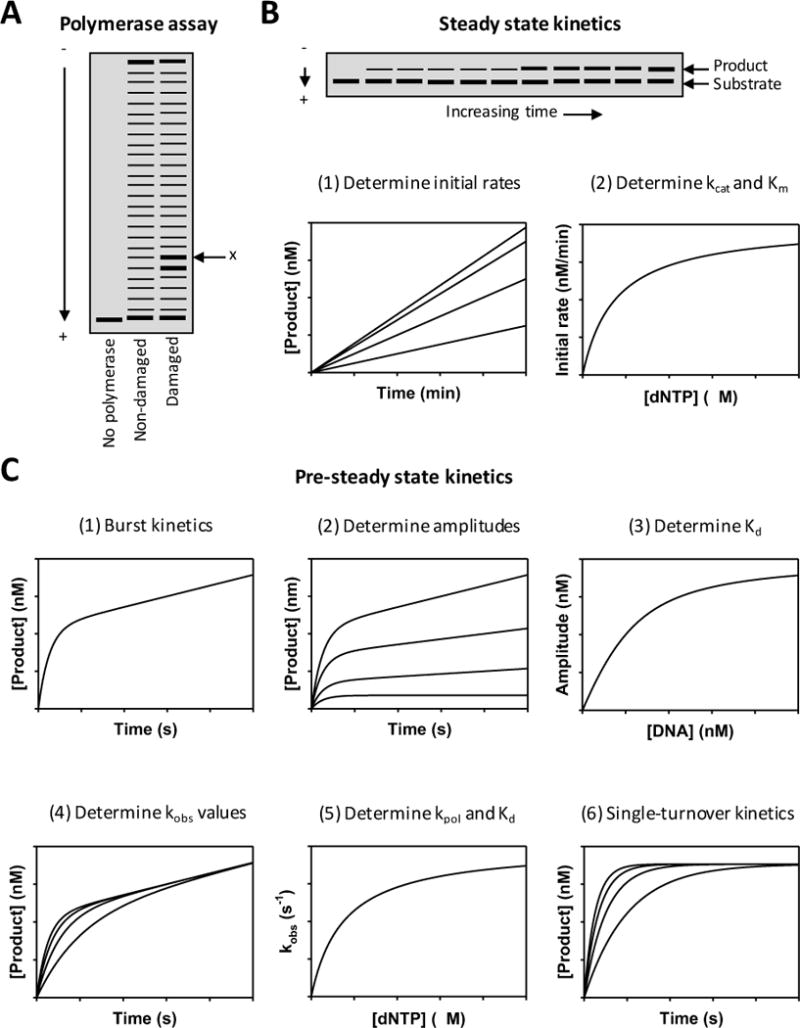
(A) A hypothetical gel image of a running start DNA polymerase assay is shown. The ‘x’ indicates the gel band corresponding to incorporation opposite the lesion. (B) A hypothetical gel image of nucleotide-incorporation reaction is shown. In steady state kinetics, the initial rates of nucleotide incorporation at various dNTP concentrations are determined from the linear slopes when the concentrations of product are graphed as a function of time. The kcat and Km parameters are determined from the best fit of the data to the Michaelis-Menten equation when the initial rates are graphed as a function of dNTP concentration. (C) In pre-steady state kinetics, bi-phasic (i.e., burst) kinetics can be observed when the concentrations of product are graphed as a function of time. Amplitudes of the burst phase at various concentrations of DNA are determined from the best fit of the data to EQ 2 when the concentrations of product are graphed as a function of time. The Kd for DNA binding is determined from the best fit of the data to EQ 3 when the amplitudes are graphed as a function of DNA concentration. The observed rate constants of the burst phase (kobs) at various dNTP concentrations are determined from the best fit of the data to EQ 2 when the concentrations of product are graphed as a function of time. The Kd for dNTP binding and the maximal rate constant for polymerization (kpol) are determined from the best fit of the data to EQ 4 when the kobs values are graphed as a function of dNTP concentration. In single-turnover experiments, the kobs values are determined from the best fit of the data to EQ 5 when the concentrations of product are graphed as a function of time.
In polymerase assays, the non-classical polymerase (usually 1 to 10 nM concentration) is pre-incubated with the DNA substrate (usually 10 to 50 nM concentration). The reactions are initiated with the addition of all four dNTPs (usually 10 to 20 μM each). Reactions are quenched after various time intervals (usually 2 to 30 minutes). The substrate and products of the reaction are then visualized on a denaturing polyacrylamide gel with single nucleotide resolution – in other words, an old-fashioned sequencing gel. The pattern of gel bands contains useful information about the ability of non-classical polymerases to bypass specific forms of DNA damage and provides potentially important clues about where kinetic barriers to nucleotide incorporation exist.
Classic examples of the use of polymerase reactions come from studies of the ability of yeast DNA polymerase h to bypass two types of ultraviolet radiation-induced DNA lesions. In the case of cis-syn thymine-thymine dimers, polymerase h-catalyzed DNA synthesis was indistinguishable on the damaged and non-damaged DNA substrates (Johnson et al., 1999). No gel bands accumulated in experiments with damaged DNA that did not also accumulate in control experiments with non-damaged DNA. This suggested that the cis-syn thymine-thymine dimer poses no additional kinetic barrier to DNA synthesis by polymerase h. By contrast, in the case of the (6–4) photoproduct, a distinct gel band accumulated at a position corresponding to incorporation opposite the 3′T of the photoproduct with no further extension products observed (Johnson et al., 2001). This indicated that the 6–4 photoproduct imposes a strong kinetic barrier to incorporating opposite the 5′T and that DNA synthesis terminates after incorporation opposite the 3′T.
Another use of polymerase assays is to determine the processivity of non-classical polymerases (Von Hippel et al., 1994; Washington et al., 1999). Processivity is a measure of how many nucleotides a DNA polymerase incorporates before dissociating from the DNA. When measuring processivity, it is necessary that the experiment be performed under “single hit” conditions so that when a polymerase dissociates from the DNA template, the template will not be engaged by another polymerase. This can be accomplished by ensuring that the DNA substrate is in large molar excess over the polymerase and that the reaction is stopped before more than 20 percent of the DNA substrates are extended. Quantification of the gel band intensities can be used to calculate P(n), which is the probability that a polymerase that has incorporated at least n nucleotides will incorporate additional nucleotides rather than dissociate. This is calculated using the following equation (EQ 1):
where In is the intensity of the gel band corresponding to n nucleotides incorporated, In+1 is the intensity of the gel band corresponding to n+1 nucleotides incorporated, and so on. Enzymes with high processivity will have average P(n) values very close to 1, and enzymes with low processivity will have average P(n) values less than 1. In the case of yeast DNA polymerase eta, its processivity is low with an average P(n) value around 0.7 (Washington et al., 1999).
The major advantage of polymerase assays is their ease relative to other approaches. The major disadvantage is that they are only semi-quantitative. One often sees the percentage of primer extension or the percentage of lesions bypass reported in the literature. These values are frequently used to compare the abilities of an enzyme to utilize various DNA substrates. This can be problematic. For example, consider a scenario where one wants to compare the ability of a non-classical polymerase on damaged versus non-damaged DNA substrates. If the Km values for the two cases are significantly different, if the kcat values for these cases are comparable, and if one uses dNTP concentrations that are significantly greater than the Km values (which is nearly always the case in such assays), then the percentage of lesion bypass will be similar in these two cases despite significant differences in the efficiency of damage bypass between the two reactions. Thus extracting meaningful information about the efficiency of damage bypass from these semi-quantitative assays is highly problematic. The easiest way to compare the efficiencies of two reactions is to use steady state kinetics.
3.2. Steady state kinetics assays
The most straightforward way to meaningfully quantify the activity of non-classical DNA polymerases is through steady state kinetics (Figure 2B) (Creighton et al., 1995; Goodman et al., 1993). In these assays, a relatively small amount of the non-classical polymerase (usually about 1 nM concentration) is pre-incubated with the DNA substrate (usually about 50 nM concentration). The reactions are initiated by adding various concentrations of only one of the four possible incoming dNTPs (usually ranging from 1 to 1,000 μM). For each dNTP concentration, the reaction is stopped after various time points. Extended products are separated from non-extended substrates by denaturing polyacrylamide electrophoresis. The initial linear rates of nucleotide incorporation are plotted as a function of dNTP concentration. The data are fit to the Michaelis-Menten equation to obtain kcat and Km values, which are then used to determine the catalytic efficiency (kcat/Km).
Catalytic efficiencies are important parameters for comparing the ability of a non-classical polymerase to utilize different dNTP substrates. One use of catalytic efficiencies is to measure the fidelity of nucleotide incorporation by non-classical DNA polymerases. Fidelity is generally reported as a frequency of incorporating incorrect nucleotides (finc), which is the ratio of the catalytic efficiencies of incorporating incorrect versus correct dNTPs (Creighton et al., 1995). For example, in the case of yeast DNA polymerase η, the fidelity of nucleotide incorporation opposite non-damaged templates and thymine-thymine dimers is low with finc values ranging from 10−2 to 10−3 (Washington et al., 1999, 2000).
Another use of catalytic efficiencies is to probe the basis of polymerase specificity using nucleotide analogs. For example, difluoroacetone is an isosteric analog of thymine that lacks the ability to form stable Watson-Crick hydrogen bonds with adenine. Unlike classical DNA polymerases, which efficiently utilize dNTPs containing difluoroacetone, yeast DNA polymerase η does not efficiently utilize these nucleotide analogs (Washington et al., 2003a). This suggests that nucleotide incorporation by yeast DNA polymerase η is more reliant on Watson-Crick hydrogen bonding than is incorporation by classical DNA polymerases.
Catalytic efficiencies can also be used to compare the ability of non-classical polymerases to utilize different DNA substrates. Here one must assume that the polymerase binds both of the DNA substrates with the same efficiency, an assumption that is often correct. Presteady state kinetics (discussed below) can be used to directly verify whether this is true for any particular case. One application of this approach is to determine the efficiency of extending from different primer terminal base pairs (Creighton et al., 1995; Goodman et al., 1993). This is generally reported as a frequency of extending from mispaired primer termini (fext), which is the ratio of the catalytic efficiencies of extension from paired versus mispaired primer termini. For example, yeast DNA polymerase ζ was shown to be an efficient extender from aberrant (mispaired or damage-containing) primer terminal base pairs with fext values ranging from 10−1 to 10−2 (Johnson et al., 2000).
Another important use of this approach is to compare the efficiency of nucleotide incorporation on non-damaged versus damaged templates. Many publications report the ability of various non-classical DNA polymerase to incorporate nucleotides opposite some lesion or other. It is absolutely critical that these abilities be quantified using steady state kinetics and compared to incorporation opposite non-damaged templates. For example, yeast DNA polymerase η incorporates nucleotides opposite thymine dimers and 8-oxoguanines with the same catalytic efficiency as it does opposite non-damaged DNA (Haracska et al., 2000; Washington et al., 2000). This is strong evidence that these types of damage are cognate lesions for polymerase η. By contrast, this enzyme incorporates nucleotides opposite other lesions, such as abasic sites, with much lower catalytic efficiencies than it does opposite non-damaged templates (Haracska et al., 2001c). Thus, despite the fact that it can incorporate nucleotides opposite these lesions whereas many other polymerases cannot, it is unlikely that these lesions are genuine cognate lesions for polymerase η. This is a point that unfortunately is too often overlooked in the field.
Recently, steady state kinetics has been used for assessing the impact of other protein factors on the activity of non-classical polymerases. Proliferating cell nuclear antigen (PCNA) is a replication accessory factor that plays an important role in translesion synthesis. During this process, PCNA is mono-ubiquitylated. The catalytic efficiency of yeast DNA polymerase η is greater in the presence of PCNA than in its absence. Moreover, it is even greater in the presence of ubiquitin-modified PCNA than it is by unmodified PCNA (Dieckman and Washington, 2013; Freudenthal et al., 2010; Garg and Burgers, 2005). The mechanistic basis for this increased catalytic efficiency in the presence of PCNA and ubiquitin-modified PCNA is currently unclear.
Steady state kinetics, however, has limitations. While it is appropriate to draw conclusions about the efficiency of reactions, one must be careful not to draw mechanistic conclusions. The Km and kcat parameters are a complicated function of the equilibrium constants and rate constants of the elementary steps of the nucleotide incorporation reaction (Johnson, 1995). These elementary steps include the DNA-binding step, the dNTP-binding step, the chemical step of nucleotide incorporation, the pyrophosphate-release step, the DNA-release step, and any conformational change steps that occur during the reaction. Changes in Km and kcat cannot be interpreted in terms of any of these steps without making many (likely unjustified) assumptions. If one wants to understand the mechanistic basis of nucleotide incorporation and polymerase specificity, one should use pre-steady state kinetics.
3.3. Pre-steady state kinetics assays
Non-classical DNA polymerases utilize the same overall minimal mechanism as classical DNA polymerases do. Step 1: the polymerase binds the DNA substrate to form a polymerase-DNA binary complex in the pre-insertion configuration. Step 2: this binary complex binds the incoming dNTP to form a polymerase-DNA-dNTP ternary complex. Step 3: the dNTP is incorporated at the 3′ end of the primer strand to form the polymerase-DNA-pyrophosphate ternary complex. Step 4: this ternary complex releases the pyrophosphate to form the polymerase-DNA binary complex in the post-insertion configuration. At this point, the pathway splits into two directions described by step 5 and step 6, respectively. Step 5: the polymerase dissociates from the DNA and can catalyze another round of nucleotide incorporation starting again at step 1. Step 6: the polymerase moves ahead one nucleotide along the DNA converting the binary complex in the post-insertion configuration to one in the pre-insertion configuration in order to catalyze another round of nucleotide incorporation starting at step 2. Additional conformational change steps including the opening or closing of the enzyme may also occur during the course of the reaction. Using pre-steady state kinetics, it is possible in principle to measure the equilibrium constants and rate constants of each of these elementary steps. Here we will focus on measurements of the first three steps: DNA binding, dNTP binding, and nucleotide incorporation.
Pre-steady state kinetics requires specialized equipment such as the rapid chemical quench flow instrument that allows reactions to be started and stopped on the millisecond timescale. Typically, one pre-incubates the polymerase (usually about 50 nM concentration) with a slight excess of the DNA substrate (usually about 200 nM concentration) in one syringe of the quench flow instrument. Reactions are initiated by mixing the pre-incubated polymerase-DNA solution with a solution containing one of the four dNTPs (usually about 50 μM concentration) from the other syringe of the quench flow instrument. Products are separated from substrates by denaturing polyacrylamide electrophoresis, and the amount of product is plotted as a function of reaction time. Often one observes biphasic kinetics (i.e., burst kinetics) of product formation (Figure 2C). This occurs because nucleotide incorporation is more rapid in the first enzyme turnover than it is in subsequent enzyme turnovers (which are in the steady state phase). Typically, this is interpreted to mean that the rate-limiting step in the overall reaction cycle occurs after the chemical step of phosphodiester bond formation. Thus nucleotide incorporation in the first turnover is not limited by this slow step. From these experiments, one can obtain the amplitude (A) of the fast phase, the observed rate constant (kobs) of the fast phase, and the rate (v) of the slow phase from the best fit of the data to the following equation (EQ 2):
where [P] is the concentration of product and t is the reaction time.
Because the amplitude of the fast phase is proportional to the amount of polymerase-DNA complex formed during the pre-incubation period, one can use these experiments to determine the dissociation constant (Kd) for the DNA binding step. To do this, one carries out pre-steady state experiments at a fixed amount of polymerase (usually about 50 nM concentration) and various amounts of DNA (usually ranging from 10 to 200 nM concentration). By fitting each data set to the burst equation, one can obtain the amplitude of the fast phase for each DNA concentration. These amplitudes (A) can be plotted as a function of DNA concentration ([DNA]), and the Kd for the DNA-binding step can be obtained from the best fit of the data to the following equation (EQ 3):
where Amax is the amplitude at saturated DNA concentration.
This experiment is often called an active site titration, because the Amax value is equal to the concentration of active enzyme present in the protein preparation. In many cases, the concentration of active enzyme approaches the concentration of total polymerase, which means that nearly all the polymerase molecules are active. In other cases, however, one often observes a concentration of active enzyme that is significantly less than the concentration of total polymerase. This often occurs with certain non-optimal substrates including one containing DNA lesions. Rev1, for example, has reduced concentrations of active enzyme with certain DNA substrates (Pryor and Washington, 2011). These reductions in concentration of active enzyme likely reflect the formation of some fraction of non-productive complexes, i.e. complexes of polymerase and DNA that are not capable of efficiently catalyzing nucleotide incorporation.
If one assumes that nucleotide binding to the polymerase achieves equilibrium rapidly relative to the nucleotide-incorporation step (which is a reasonable assumption for non-classical polymerases due to their generally slow rate of nucleotide incorporation), then the observed rate constant for the fast phase of nucleotide incorporation (kobs) is directly proportional to the amount of dNTP bound to the polymerase-DNA complex at equilibrium. Thus one can use pre-steady state kinetics experiments to determine the maximal rate constant for the nucleotide incorporation step (kpol) and the dissociation constant (Kd) for the dNTP binding step. To do this, one carries out experiments at fixed amounts of polymerase and DNA (usually about 50 nM and 200 nM concentration, respectively) and various amounts of dNTP (usually ranging from 1 to 50 μM concentration). By fitting each data set to the burst equation (EQ 2), one can obtain the kobs values of the fast phase for each dNTP concentration. These values can be plotted as a function of dNTP concentration ([dNTP]), and the kpol and Kd parameters can be obtained from the best fit of the data to the following equation (EQ 4):
As an alternative to the experiments described above where the concentration of the DNA substrate is in slight excess over the concentration of the polymerase, one can carry out experiments in which the concentration of the polymerase is equal to or greater than the concentration of the DNA substrate. These are single-turnover experiments, because all of the substrate is converted to product in one turnover. When the concentration of product is plotted as a function of time, one should only observe a single, fast phase of nucleotide incorporation. One can obtain a kobs value from the best fit of the data to the following equation (EQ 5):
where [P] is the concentration of product, t is the reaction time, and A is the amplitude of the fast phase.
While one cannot obtain a Kd for DNA binding from single-turnover experiments, one can still obtain the kpol and the Kd for dNTP binding. To do this, one performs these experiments at various concentrations of dNTP. The kobs values are then plotted as a function of the dNTP concentration, and the kpol and Kd parameters are obtained from the best fit of the data to equation EQ 4.
3.4. Criteria for cognate lesions
Perhaps the most important application of steady state and pre-steady state kinetics to non-classical polymerases is to determine which DNA lesions are likely cognate lesions for a given DNA polymerase. Originally the criterion for concluding that a given DNA lesion is a cognate lesion for a non-classical polymerase is that the non-classical polymerase incorporates the correct nucleotide across from the template lesion (Friedberg et al., 2002; Lehmann et al., 2007; Waters et al., 2009). We believe that this criterion is problematic for several reasons. First, it is prone to false positives and false negatives. For example, a polymerase may accurately bypass a lesion, but do so at very low efficiency. This would not support the notion that the polymerase has evolved to bypass the lesion; in other words, this would be a false positive. By contrast, some lesions such as abasic sites cannot be bypassed accurately because the information in the template is absent. This is one of the most common lesions formed in the genome and there is compelling evidence that some non-classical polymerases, particularly Rev1, have evolved to bypass it. This by definition would be a false negative. So one needs a better set of criteria for cognate lesions.
We proposed a set of two criteria for concluding that a given lesion is a cognate lesion for a non-classical polymerase (Pryor and Washington, 2011). First, one needs clear evidence from kinetics (preferably pre-steady state kinetics) that nucleotide incorporation opposite the lesion occurs efficiently. For example, the polymerase should incorporate nucleotides opposite the lesion with similar kpol and Kd values as those for incorporation opposite non-damaged DNA. Studies such as those described above would allow for such comparisons. Second, one should have cell-based evidence that the polymerase incorporates nucleotides opposite the lesion in vivo. There is compelling kinetic and genetic evidence that yeast polymerase eta has at least two cognate lesions: thymine dimers and 8-oxoguanines (Carlson and Washington, 2005; Haracska et al., 2000; Johnson et al., 1999; Washington et al., 2000; Washington et al., 2003b). Similarly, there is compelling kinetic and genetic data that yeast Rev1 has several cognate lesions, most notably abasic sites (Haracska et al., 2001b; Pryor and Washington, 2011).
4. Interactions of non-classical polymerases
Structural and kinetics studies such as those outlined above have provided a wealth of insight into the mechanism by which non-classical polymerases accommodate and utilize damaged DNA templates. Despite this, several important questions remain regarding how non-classical polymerases carry out translesion synthesis. First, each non-classical polymerase has one or more cognate lesions. How is the most appropriate non-classical polymerase selected for a given lesion? Second, the bypass of some types of DNA damage requires the sequential action of multiple non-classical polymerases (Johnson et al., 2000; Prakash and Prakash, 2002). For example, Rev1 incorporates nucleotides opposite abasic sites, but cannot extend further; pol ζ carries out this extension step to complete the bypass of this lesion (Haracska et al., 2001b). How do these non-classical polymerases switch on the DNA template?
Non-classical polymerases function within a large, dynamic, multi-protein complex that forms at stalled replication forks (Wojtaszek et al., 2012). The exact composition and architecture of the complex is not well understood. In addition to the damaged DNA substrate, it contains ubiquitin-modified PCNA and multiple non-classical polymerases. It may also contain classical polymerases such as polymerase delta (pol δ) and polymerase epsilon (pol ε); Rad6 and Rad18, which catalyze PCNA mono-ubiquitylation; and Mms2-Ubc13 and Rad5, which catalyze PCNA poly-ubiquitylation. Answering the questions raised above requires an understanding of how non-classical polymerases function within the context of these complexes. Therefore, it requires a better understanding of the protein-protein interactions in which the non-classical polymerases engage.
In this section, we will review some of the most common biochemical methods for studying the protein-protein interactions of non-classical polymerases. We are focusing on biochemical methods because we believe that they are the most appropriate approaches for studying the interactions of non-classical polymerases and their organization within larger, dynamic multi-protein complexes. Unfortunately, many studies in the field have relied on nuclear foci formation, which has often been used as a proxy for direct interactions and function. It has become clear in recent years that nuclear foci formation is neither necessary nor sufficient for translesion synthesis (Gueranger et al., 2008; Sale et al., 2012). Moreover, it is clear that one cannot determine whether an interaction is direct or indirect from nuclear foci formation studies.
4.1. Qualitative binding assays
Qualitative binding assays are those that allow one to detect protein-protein interactions, but do not allow one to accurately determine the dissociation constant (Kd) for the protein complex. These assays are usually rapid and do not require a large amount of protein. Consequently, they are useful for screening a large number of mutant proteins and for mapping interactions sites. The two most common qualitative binding assays are enzyme-linked immunosorbent assays (ELISAs) and co-immunoprecipitation assays.
Enzyme-linked immunosorbent assays (ELISAs) are a quick and convenient way to monitor protein-protein interactions. One of the interacting partners (usually about 1 μg) is immobilized in the wells of a 96-well plate. After washing and blocking with bovine serum albumin (BSA), various amounts of the second protein are added (usually ranging from 1 to 20 μg). After another round of washing, the second protein is detected either directly with a horseradish peroxidase (HRP)-conjugated primary antibody or with a primary antibody and an HRP-conjugated secondary antibody. Formation of the complex is detected by an increase in absorbance at 450 nm compared to control experiments in which BSA was immobilized in the well rather than the protein of interest. The use of ELISAs to study the formation of binary and ternary complexes has been discussed in more detail in a recent review (Biesiadecki and Jin, 2011).
One must use care when comparing the results of ELISAs. For example, the absorbance signal intensity depends on several factors including the affinity of the primary antibody for its protein and the accessibility of the epitope within the immobilized complex. For this reason, we believe that it is only appropriate to compare ELISA results from two experiments if the same immobilized protein, the same binding partner protein, and the same primary antibody are used in both experiments. Thus the ability to compare ELISA results is strictly limited to cases where one is comparing the relative binding of wild type versus mutant proteins.
Co-immunoprecipitation assays are another useful way to monitor protein-protein interactions. These experiments are widely used across many fields, and so we will only discuss these approaches briefly. Co-immunoprecipitation experiments can be done in one of three ways. First, two purified proteins can be added together and the complex can be precipitated by adding an antibody to one of the purified proteins. Second, one purified protein can be added to a cell extract and the complex can be precipitated by adding an antibody to the purified protein. Third, the complex can be precipitated from a cell extract by adding an antibody to one of the proteins. Typically, the presence of both proteins in the precipitated complex is observed by western blot. When using cellular extracts, typically the presence of the protein in the non-precipitated sample is also observed by western blot.
4.2. Quantitative binding assays
Quantitative binding assays are those that can be used to determine a Kd for the protein complex. A variety of such assays are widely used. Here we will focus on three approaches that have been useful for examining the binding of non-classical polymerases with their interacting partners. These are isothermal titration calorimetry, surface plasmon resonance, and nuclear magnetic resonance spectroscopy. Each of these approaches provides additional thermodynamic, kinetic, or structural information about the interaction.
Isothermal titration calorimetry (ITC) requires large quantities of protein (often milligrams) and requires specialized equipment. One of the proteins is placed in the sample cell of the ITC instrument (usually 10 to 30 μM) and its interacting partner is placed in the syringe of the instrument (usually 100 to 300 μM). As the interacting partner is injected into the sample cell, the amount of heat absorbed or given off as the proteins interact with each other is recorded. Analysis of the ITC data using software accompanying the instrument provides not only the Kd for the interaction, but also the stoichiometry of the interaction, the change in enthalpy (ΔH) of the interaction, and the change in entropy (ΔS) of the interaction. Thus, using ITC, one can analyze the energetics of the interaction in terms of their enthalpic and entropic components and perhaps gain important information about the structural basis and nature of the interaction (i.e., whether it is driven by electrostatics, hydrogen bonding, or the hydrophobic effect). One possible limitation of ITC is that is difficult to obtain a strong signal when the ΔH of binding is very small. Nevertheless, ITC has been used to examine the interactions between the PIP motif of pol η and both PCNA and the Rev1 CTD (Boehm et al., 2016a). It has also been used to study the interactions between the UBZ motif of human pol η and ubiquitin (Bomar et al., 2007).
Surface plasmon resonance (SPR) is an optical technique for studying protein-protein interactions that requires specialized equipment. Unlike ITC, it does not require a large amount of protein. One of the proteins is immobilized on the surface of the SPR chip. The SPR instrument reflects light off the metallic surface of the chip at a critical angle such that the optical properties of the reflected light are sensitive to changes occurring at the surface of the chip. When a solution containing the interacting partner (usually 1 to 3 μM) is flowed across the surface of the chip, the proteins will form a complex. This causes an increase in the SPR response units (which are related to the changes in the optical properties of the reflected light) allowing the kinetics of complex formation to be measured. When a solution without the interacting partner is then flowed across the surface of the chip, the complex will dissociate. This causes a decrease in the response units allowing the kinetics of complex dissociation to be measured. Thus, using SPR, one can obtain the second-order rate constant of association (kon) and the first-order rate constants of dissociation (koff) for the complex. In fact, SPR can also be used when there are multi-step binding reactions such as those involving conformational change steps following complex formation. From the kon and koff values, one can determine the Kd for the complex. When using SPR, one must be cognizant of the potential for artifacts arising from protein immobilization and of potential mass transfer effects on the binding kinetics. Despite these potential pitfalls, SPR has been used to determine the affinities of PIP motifs of multiple non-classical polymerases for binding PCNA (Hishiki et al., 2009).
Nuclear magnetic resonance (NMR) titrations are another means of determining the Kd for protein complexes. This specialized and sophisticated technique is beyond the scope of this review, so we will only briefly outline how it can be applied to non-classical polymerases. First, one of the proteins must be isotopically labeled with 15N. This allows one to take heteronuclear single quantum coherence (HSQC) spectra of the labeled protein alone and in the presence of various concentrations of its interacting partner. The HSQC spectra give a set of cross-peaks, each corresponding to an individual backbone amide group of the protein. Shifts in the positions of cross-peaks in the presence of the interacting partner indicate changes in the chemical environment of the backbone amide group upon complex formation. One then plots the change in chemical shift of each peak as a function of interacting partner concentration, and the Kd of the complex can be obtained from the best fit of the data to the quadratic form of the binding equation (EQ 3, see above). Moreover, if the HSQC peaks have been assigned, one is able to determine which amino acids are responsive to the binding of the interacting partner. Thus one can map onto the structure the amino acid residues involved in the binding surface. NMR titrations have been used to measure the affinity of the UBZ of pol η for ubiquitin and the affinity of the BRCT domain of Rev1 for PCNA (Bomar et al., 2007; Pustovalova et al., 2013).
Studies using these approaches have led to structural models of the interactions among pol η, Rev1, and PCNA. The BRCT domain of Rev1 interacts with the side of the PCNA ring (Pustovalova et al., 2013). Pol η has a single PIP motif in its disordered C-terminal region. This motif mediates interactions with PCNA by binding to a hydrophobic pocket on the front face of the PCNA ring (Figure 3A) (Boehm et al., 2016a; Haracska et al., 2001a). The pol η PIP motif also mediates interactions with Rev1 by binding to a hydrophobic pocket on the Rev1 CTD (Boehm et al., 2016a). Given that the interactions of the pol η PIP motif with PCNA and with the Rev1 CTD are mutually exclusive, the ternary complexes formed by these proteins can only have two possible architectures: PCNA tool belts and Rev1 bridges (Figure 3B). In PCNA tool belts, pol η and Rev1 both directly bind PCNA, but not each other. Pol η binds to PCNA via its PIP motif, and Rev1 binds to PCNA via its BRCT domain. In Rev1 bridges, pol η and PCNA both directly bind Rev1, but not each other. Pol η binds to Rev1 via its PIP motif, PCNA binds to Rev1 via the Rev1 BRCT domain. Determining whether such ternary complexes form required single-molecule binding assays.
Figure 3. Structural models of the interactions of pol η, Rev1, and PCNA.

(A) Homology model of the structure of the PIP motif of pol η (blue) bound to the front face of the PCNA (green). This model was based on PDB ID: 2OD8 (Vijayakumar et al., 2007). (B) Homology model of the structure of the PIP motif of pol η (blue) bound to the CTD of Rev1 (red). This model was based on PDB ID: 4FJO (Wojtaszek et al., 2012). (C) Model of the PCNA tool belt architecture in which the BRCT domain of Rev1 (red) binds to one subunit of the PCNA ring (green) and the PIP motif of pol η (blue) binds to another subunit of PCNA. (D) Model of the Rev1 bridge architecture in which the front face of the PCNA ring (green) binds to the BRCT domain of Rev1 (red) and the PIP motif of pol η (blue) binds to CTD of Rev1.
4.3. Single-molecule binding assays
Single molecule total internal reflection (TIRF) microscopy provides another means of measuring the interactions of non-classical polymerases (Figure 4A). This approach requires a very small amount of protein, but it also requires immobilization of one of the proteins. In single-molecule TIRF assays, one of the proteins (usually about 1 nM) is biotinylated and attached to the surface of a microscope slide sparsely coated with neutravidin. Various concentrations of the other protein are labeled with a fluorophore such as Cy3 or Cy5 and is added to the microscope slide chamber (usually ranging from 50 pM to 500 pM). An electron multiplying charge coupled device (EMCCD) camera records the fluorescence intensity from the location of each immobilized protein. Increases in fluorescence intensity above the baseline indicate the association of the labeled protein with the immobilized one, and decreases of fluorescence intensity back to the baseline indicate the dissociation of the labeled protein from the immobilized one (Figure 4B). The uses of single-molecule TIRF microscopy assays to study the formation and architecture of binary and higher-order protein complexes has been reviewed in detail recently (Boehm et al., 2016c).
Figure 4. Analyzing single-molecule TIRF microscopy binding assays.

(A) A diagram of a one-color single-molecule assay is shown. Pol η is immobilized to the slide, and PCNA is labeled with Cy3. When PCNA is not bound to pol η (off), it does not fluoresce; when PCNA is bound to pol η (on), it does fluoresce. The on-times and off-times are obtained from idealized fluorescence trajectories showing the fluorescence intensity from single immobilized pol η molecules. Histograms showing the number of binding events with given on-times are generated, and the koff is determined from the best fit of the data to EQ 6. Histograms showing the number of intervals between biding events with given off-times are generated, and the von is determined from the best fit of the data to EQ 6. The von parameters are determined for various concentrations of the labeled protein, and the kon is determined from the slope of the linear portion of the plot when von is graphed as a function of protein concentration. (B) A diagram of a two-color single-molecule assay is shown. Pol η is immobilized to the slide, PCNA is labeled with Cy3, and Rev1 is labeled with Cy5. When PCNA and Rev1 simultaneously bind to pol η, both fluoresce. The ternary complex can exist in two possible architectures: PCNA tool belts and Rev1 bridges. Idealized fluorescence trajectories for these two architectures are shown.
The amount of time that a binary complex persists before dissociation is the on time (i.e., dwell time), and one can obtain kinetic constants for the dissociation of the complex by examining the distribution of on times. One can create a histogram showing the number of binding events with various on times and obtain the first-order rate constant for the dissociation for the complex (koff) from the best fit of these data to an exponential decay (EQ 6):
Similarly, the amount of time between binding events is the off time, and one can obtain kinetic constants for the association to form the complex by examining the distribution of off times. Again one creates histograms showing the number of intervals with various off times and obtains observed rates of association (von) from the best fit of these data to an exponential decay. This is repeated at various concentrations of labeled protein in the microscope slide chamber, and the von values are plotted as a function of labeled protein concentration. The slope of the linear portion of this graph is the second-order association constant for the complex (kon). The Kd for the complex can then be determined from the kon and koff values.
Single-molecule TIRF microscopy assays were used to study the interactions between pol η and PCNA as well as pol η and Rev1 (Boehm et al., 2016a). These studies showed that the Kd for the pol η-PCNA and the pol η-Rev1 binary complexes have similar affinities. Further studies with mutant proteins also showed that the interaction between pol η and PCNA depends on the PIP motif of pol η and the pocket on the front face of PCNA near the IDCL. Likewise, the interactions between pol η and Rev1 depend on the PIP motif of pol η and the CTD of Rev1.
Similar single-molecules assays using two fluorescently labeled proteins were also used to study the formation of the ternary complexes comprised of pol η, PCNA, and Rev1 (Boehm et al., 2016b). In these experiments, pol η was immobilized on the slide, PCNA was labeled with Cy3, and Rev1 was labeled with Cy5. Ternary complexes were observed, in which both Cy3 and Cy5 fluorescence were increased simultaneously at a single immobilized pol η. Moreover, by examining which of the pol η-binding partners bound and released first, the architectures of these ternary complexes were determined. For example, when PCNA bound pol η before Rev1, this indicated the formation of a PCNA tool belt. Likewise, when Rev1 bound before PCNA, this indicated the formation of a Rev1 bridge. It was shown that tool belts and bridges both formed with nearly equal frequencies (Boehm et al., 2016b).
The clearest evidence for these architectures came when specific mutant proteins were used in these single-molecule studies. For example, a mutant Rev1 protein in which the CTD was disrupted is able to interact with PCNA, but not with pol η (Boehm et al., 2016b). Thus this mutant protein should be able to form PCNA tool belts, but not Rev1 bridges. Consistent with this, PCNA tool belts were formed at the same frequency as they did with wild-type Rev1, while the number of Rev1-bridges was substantially diminished.
5. Conclusions
The approaches described above have provided a wealth of information about the mechanism of nucleotide incorporation by non-classical polymerases and the protein-protein interactions in which these enzymes engage. They have shown that different non-classical polymerases have different cognate lesions opposite which they incorporate nucleotides efficiently and mostly accurately. They have shown that these non-classical polymerases form binary and higher-order protein complexes including PCNA tool belts and Rev1 bridges. Despite this great progress, there are still many more important things that we can learn using these approaches. We will conclude this article by discussing one important question that these approaches may help us to answer, the problem of non-classical polymerase selection.
Because non-classical polymerases usually incorporate nucleotides more accurately opposite their cognate lesions, cells can minimize the likelihood of misincorporation (and ultimately mutation) by ensuring that DNA lesions are bypassed by the most appropriate non-classical polymerases (i.e., the non-classical polymerase for which the given lesion is a cognate lesion). Very little is known about how the most appropriate non-classical polymerase is selected to carry out damage bypass. The findings reviewed in this paper, however, suggest a plausible model.
Kinetics studies of nucleotide incorporation by non-classical polymerases have shown that incorporation opposite a cognate lesion is significantly faster than is incorporation opposite non-cognate lesions (Pryor et al., 2014; Washington et al., 2010; Washington et al., 2003b). Binding studies have shown that non-classical polymerases form binary and higher-order protein complexes, including PCNA tool belts and Rev1 bridges (Boehm et al., 2016b). The conformations of these complexes are quite flexible owing to the large degree of intrinsic disorder among the components of the complexes. Taken together, it is likely that the multiple non-classical polymerases within the same complex can compete with one another for the DNA primer terminus.
Provided that the amount of time a polymerase spends engaged with the primer terminus is short (less than a second or so), the kinetics of nucleotide incorporation will greatly favor incorporation by the most appropriate polymerases. According to this “kinetic selection” model, if an inappropriate polymerase engages the primer terminus, it will likely dissociate before it has a chance to incorporate a nucleotide. By contrast, if an appropriate polymerase engages the primer terminus, it will likely incorporate a nucleotide before dissociation. This trial and error strategy will ultimately ensure that the lesion will be bypassed as accurately as possible. We expect that in the years to come, kinetic studies, quantitative binding studies, and single-molecule binding studies will provide a better understanding of how non-classical polymerase selection occurs.
Acknowledgments
We thank Elizabeth Boehm, Lynne Dieckman, Melissa Gildenberg, Christine Kondratick, Brittany Ripley, and Maria Spies for valuable discussions. The project described was supported by award number GM081433 from the National Institute of General Medicine to M.T.W. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
References
- Biesiadecki BJ, Jin JP. A High-Throughput Solid-Phase Microplate Protein-Binding Assay to Investigate Interactions between Myofilament Proteins. Journal of Biomedicine and Biotechnology. 2011 doi: 10.1155/2011/421701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm EM, Powers KT, Kondratick CM, Spies M, Houtman JC, Washington MT. The Proliferating Cell Nuclear Antigen (PCNA)-interacting Protein (PIP) Motif of DNA Polymerase eta Mediates Its Interaction with the C-terminal Domain of Rev1. The Journal of biological chemistry. 2016a;291:8735–8744. doi: 10.1074/jbc.M115.697938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm EM, Spies M, Washington MT. PCNA tool belts and polymerase bridges form during translesion synthesis. Nucleic acids research. 2016b;44:8250–8260. doi: 10.1093/nar/gkw563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm EM, Subramanyam S, Ghoneim M, Washington MT, Spies M. Quantifying the Assembly of Multicomponent Molecular Machines by Single-Molecule Total Internal Reflection Fluorescence Microscopy. Methods Enzymol. 2016c;581:105–145. doi: 10.1016/bs.mie.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomar MG, Pai MT, Tzeng SR, Li SS, Zhou P. Structure of the ubiquitin-binding zinc finger domain of human DNAY-polymerase eta. EMBO Rep. 2007;8:247–251. doi: 10.1038/sj.embor.7400901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson KD, Washington MT. Mechanism of efficient and accurate nucleotide incorporation opposite 7,8-dihydro-8-oxoguanine by Saccharomyces cerevisiae DNA polymerase eta. Molecular and cellular biology. 2005;25:2169–2176. doi: 10.1128/MCB.25.6.2169-2176.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton S, Bloom LB, Goodman MF. Gel fidelity assay measuring nucleotide misinsertion, exonucleolytic proofreading, and lesion bypass efficiencies. Methods Enzymol. 1995;262:232–256. doi: 10.1016/0076-6879(95)62021-4. [DOI] [PubMed] [Google Scholar]
- Dieckman LM, Washington MT. PCNA trimer instability inhibits translesion synthesis by DNA polymerase eta and by DNA polymerase delta. DNA repair. 2013;12:367–376. doi: 10.1016/j.dnarep.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenthal BD, Gakhar L, Ramaswamy S, Washington MT. Structure of monoubiquitinated PCNA and implications for translesion synthesis and DNA polymerase exchange. Nature structural & molecular biology. 2010;17:479–484. doi: 10.1038/nsmb.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg EC, Wagner R, Radman M. Specialized DNA polymerases, cellular survival, and the genesis of mutations. Science. 2002;296:1627–1630. doi: 10.1126/science.1070236. [DOI] [PubMed] [Google Scholar]
- Garg P, Burgers PM. Ubiquitinated proliferating cell nuclear antigen activates translesion DNA polymerases eta and REV1. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18361–18366. doi: 10.1073/pnas.0505949102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MF, Creighton S, Bloom LB, Petruska J. Biochemical basis of DNA replication fidelity. Crit Rev Biochem Mol Biol. 1993;28:83–126. doi: 10.3109/10409239309086792. [DOI] [PubMed] [Google Scholar]
- Gueranger Q, Stary A, Aoufouchi S, Faili A, Sarasin A, Reynaud CA, Weill JC. Role of DNA polymerases eta, iota and zeta in UV resistance and UV-induced mutagenesis in a human cell line. DNA repair. 2008;7:1551–1562. doi: 10.1016/j.dnarep.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Haracska L, Kondratick CM, Unk I, Prakash S, Prakash L. Interaction with PCNA is essential for yeast DNA polymerase eta function. Molecular cell. 2001a;8:407–415. doi: 10.1016/s1097-2765(01)00319-7. [DOI] [PubMed] [Google Scholar]
- Haracska L, Prakash S, Prakash L. Yeast Rev1 protein is a G template-specific DNA polymerase. The Journal of biological chemistry. 2002;277:15546–15551. doi: 10.1074/jbc.M112146200. [DOI] [PubMed] [Google Scholar]
- Haracska L, Unk I, Johnson RE, Johansson E, Burgers PM, Prakash S, Prakash L. Roles of yeast DNA polymerases delta and zeta and of Rev1 in the bypass of abasic sites. Genes & development. 2001b;15:945–954. doi: 10.1101/gad.882301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haracska L, Washington MT, Prakash S, Prakash L. Inefficient bypass of an abasic site by DNA polymerase eta. The Journal of biological chemistry. 2001c;276:6861–6866. doi: 10.1074/jbc.M008021200. [DOI] [PubMed] [Google Scholar]
- Haracska L, Yu SL, Johnson RE, Prakash L, Prakash S. Efficient and accurate replication in the presence of 7,8-dihydro-8-oxoguanine by DNA polymerase eta. Nature genetics. 2000;25:458–461. doi: 10.1038/78169. [DOI] [PubMed] [Google Scholar]
- Hishiki A, Hashimoto H, Hanafusa T, Kamei K, Ohashi E, Shimizu T, Ohmori H, Sato M. Structural basis for novel interactions between human translesion synthesis polymerases and proliferating cell nuclear antigen. The Journal of biological chemistry. 2009;284:10552–10560. doi: 10.1074/jbc.M809745200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- Ishida T, Kinoshita K. PrDOS: prediction of disordered protein regions from amino acid sequence. Nucleic acids research. 2007;35:W460–464. doi: 10.1093/nar/gkm363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA. Rapid quench kinetic analysis of polymerases, adenosinetriphosphatases, and enzyme intermediates. Methods Enzymol. 1995;249:38–61. doi: 10.1016/0076-6879(95)49030-2. [DOI] [PubMed] [Google Scholar]
- Johnson KA. The kinetic and chemical mechanism of high-fidelity DNA polymerases. Biochimica etbiophysica acta. 2010;1804:1041–1048. doi: 10.1016/j.bbapap.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RE, Haracska L, Prakash S, Prakash L. Role of DNA polymerase eta in the bypass of a (6–4) TT photoproduct. Molecular and cellular biology. 2001;21:3558–3563. doi: 10.1128/MCB.21.10.3558-3563.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RE, Prakash L, Prakash S. Yeast and human translesion DNA synthesis polymerases: expression, purification, and biochemical characterization. Methods Enzymol. 2006;408:390–407. doi: 10.1016/S0076-6879(06)08024-4. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Prakash S, Prakash L. Efficient bypass of a thymine-thymine dimer by yeast DNA polymerase, Poleta. Science. 1999;283:1001–1004. doi: 10.1126/science.283.5404.1001. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Washington MT, Haracska L, Prakash S, Prakash L. Eukaryotic polymerases iota and zeta act sequentially to bypass DNA lesions. Nature. 2000;406:1015–1019. doi: 10.1038/35023030. [DOI] [PubMed] [Google Scholar]
- Lawrence C. The RAD6 DNA repair pathway in Saccharomyces cerevisiae: what does it do, and how does it do it? BioEssays : news and reviews in molecular, cellular and developmental biology. 1994;16:253–258. doi: 10.1002/bies.950160408. [DOI] [PubMed] [Google Scholar]
- Lawrence CW. Cellular roles of DNA polymerase zeta and Rev1 protein. DNA repair. 2002;1:425–435. doi: 10.1016/s1568-7864(02)00038-1. [DOI] [PubMed] [Google Scholar]
- Lehmann AR, Niimi A, Ogi T, Brown S, Sabbioneda S, Wing JF, Kannouche PL, Green CM. Translesion synthesis: Y-family polymerases and the polymerase switch. DNA repair. 2007;6:891–899. doi: 10.1016/j.dnarep.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Nair DT, Johnson RE, Prakash L, Prakash S, Aggarwal AK. Rev1 employs a novel mechanism of DNA synthesis using a protein template. Science. 2005;309:2219–2222. doi: 10.1126/science.1116336. [DOI] [PubMed] [Google Scholar]
- Nair DT, Johnson RE, Prakash L, Prakash S, Aggarwal AK. Protein-template-directed synthesis across an acrolein-derived DNA adduct by yeast Rev1 DNA polymerase. Structure. 2008;16:239–245. doi: 10.1016/j.str.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Nair DT, Johnson RE, Prakash L, Prakash S, Aggarwal AK. DNA synthesis across an abasic lesion by yeast REV1 DNA polymerase. Journal of molecular biology. 2011;406:18–28. doi: 10.1016/j.jmb.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JR, Lawrence CW, Hinkle DC. Deoxycytidyl transferase activity of yeast REV1 protein. Nature. 1996;382:729–731. doi: 10.1038/382729a0. [DOI] [PubMed] [Google Scholar]
- Ohmori H, Hanafusa T, Ohashi E, Vaziri C. Separate roles of structured and unstructured regions of Y-family DNA polymerases. Advances in protein chemistry and structural biology. 2009;78:99–146. doi: 10.1016/S1876-1623(08)78004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S, Johnson RE, Prakash L. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annual review of biochemistry. 2005;74:317–353. doi: 10.1146/annurev.biochem.74.082803.133250. [DOI] [PubMed] [Google Scholar]
- Prakash S, Prakash L. Translesion DNA synthesis in eukaryotes: a one- or two-polymerase affair. Genes & development. 2002;16:1872–1883. doi: 10.1101/gad.1009802. [DOI] [PubMed] [Google Scholar]
- Prakash S, Sung P, Prakash L. DNA repair genes and proteins of Saccharomyces cerevisiae. Annu Rev Genet. 1993;27:33–70. doi: 10.1146/annurev.ge.27.120193.000341. [DOI] [PubMed] [Google Scholar]
- Pryor JM, Dieckman LM, Boehm EM, Washington MT. Eukaryotic Y-Family Polymerases: A Biochemical and Structural Perspective. Nucleic Acids Mol Bi. 2014;30:85–108. [Google Scholar]
- Pryor JM, Washington MT. Pre-steady state kinetic studies show that an abasic site is a cognate lesion for the yeast Rev1 protein. DNA repair. 2011;10:1138–1144. doi: 10.1016/j.dnarep.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pustovalova Y, Maciejewski MW, Korzhnev DM. NMR mapping of PCNA interaction with translesion synthesis DNA polymerase Rev1 mediated by Rev1-BRCT domain. Journal of molecular biology. 2013;425:3091–3105. doi: 10.1016/j.jmb.2013.05.029. [DOI] [PubMed] [Google Scholar]
- Sale JE, Lehmann AR, Woodgate R. Y-family DNA polymerases and their role in tolerance of cellular DNA damage. Nature reviews Molecular cell biology. 2012;13:141–152. doi: 10.1038/nrm3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein TD, Jain R, Johnson RE, Prakash L, Prakash S, Aggarwal AK. Structural basis for error-free replication of oxidatively damaged DNA by yeast DNA polymerase eta. Structure. 2010a;18:1463–1470. doi: 10.1016/j.str.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein TD, Johnson RE, Jain R, Prakash L, Prakash S, Aggarwal AK. Structural basis for the suppression of skin cancers by DNA polymerase eta. Nature. 2010b;465:1039–1043. doi: 10.1038/nature09104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trincao J, Johnson RE, Escalante CR, Prakash S, Prakash L, Aggarwal AK. Structure of the catalytic core of S. cerevisiae DNA polymerase eta: implications for translesion DNA synthesis. Molecular cell. 2001;8:417–426. doi: 10.1016/s1097-2765(01)00306-9. [DOI] [PubMed] [Google Scholar]
- Vijayakumar S, Chapados BR, Schmidt KH, Kolodner RD, Tainer JA, Tomkinson AE. The C-terminal domain of yeast PCNA is required for physical and functional interactions with Cdc9 DNA ligase. Nucleic acids research. 2007;35:1624–1637. doi: 10.1093/nar/gkm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hippel PH, Fairfield FR, Dolejsi MK. On the processivity of polymerases. Ann NY Acad Sci. 1994;726:118–131. doi: 10.1111/j.1749-6632.1994.tb52803.x. [DOI] [PubMed] [Google Scholar]
- Washington MT, Carlson KD, Freudenthal BD, Pryor JM. Variations on a theme: eukaryotic Y-family DNA polymerases. Biochimica et biophysica acta. 2010;1804:1113–1123. doi: 10.1016/j.bbapap.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington MT, Helquist SA, Kool ET, Prakash L, Prakash S. Requirement of Watson-Crick hydrogen bonding for DNA synthesis by yeast DNA polymerase eta. Molecular and cellular biology. 2003a;23:5107–5112. doi: 10.1128/MCB.23.14.5107-5112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington MT, Johnson RE, Prakash S, Prakash L. Fidelity and processivity of Saccharomyces cerevisiae DNA polymerase eta. The Journal of biological chemistry. 1999;274:36835–36838. doi: 10.1074/jbc.274.52.36835. [DOI] [PubMed] [Google Scholar]
- Washington MT, Johnson RE, Prakash S, Prakash L. Accuracy of thymine-thymine dimer bypass by Saccharomyces cerevisiae DNA polymerase eta. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3094–3099. doi: 10.1073/pnas.050491997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington MT, Minko IG, Johnson RE, Haracska L, Harris TM, Lloyd RS, Prakash S, Prakash L. Efficient and error-free replication past a minor-groove N2-guanine adduct by the sequential action of yeast Rev1 and DNA polymerase zeta. Molecular and cellular biology. 2004;24:6900–6906. doi: 10.1128/MCB.24.16.6900-6906.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington MT, Prakash L, Prakash S. Mechanism of nucleotide incorporation opposite a thymine-thymine dimer by yeast DNA polymerase eta. Proceedings of the National Academy of Sciences of the United States of America. 2003b;100:12093–12098. doi: 10.1073/pnas.2134223100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters LS, Minesinger BK, Wiltrout ME, DSouza S, Woodruff RV, Walker GC. Eukaryotic translesion polymerases and their roles and regulation in DNA damage tolerance. Microbiology and molecular biology reviews : MMBR. 2009;73:134–154. doi: 10.1128/MMBR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtaszek J, Lee CJ, DSouza S, Minesinger B, Kim H, D’Andrea AD, Walker GC, Zhou P. Structural basis of Rev1-mediated assembly of a quaternary vertebrate translesion polymerase complex consisting of Rev1, heterodimeric polymerase (Pol) zeta, and Pol kappa. The Journal of biological chemistry. 2012;287:33836–33846. doi: 10.1074/jbc.M112.394841. [DOI] [PMC free article] [PubMed] [Google Scholar]


