Abstract
Background and study aims
Endoscopic radiofrequency ablation (RFA) is an established therapy for Barrett’s esophagus. Preliminary reports, limited by low patient numbers, suggest a possible role for RFA for early esophageal squamous cell neoplasia (ESCN), as well. The aim of this study was to evaluate the safety and effectiveness of RFA for early ESCN [moderate-grade/high-grade intraepithelial neoplasia (MGIN/HGIN) and early flat-type esophageal squamous cell carcinoma (ESCC)].
Patients and methods
In this prospective cohort study, patients had ≥1 flat (type 0-IIb) unstained lesion (USL) on Lugol’s chromoendoscopy and a consensus diagnosis of MGIN, HGIN, or early ESCC. RFA was used at baseline to treat all USLs, then biopsy (and focal RFA if USL(s) persisted) was performed every 3 months until all biopsies were negative for MGIN, HGIN and ESCC. The main outcome measurements were complete response (CR) at 3 and 12 months (absence of MGIN, HGIN, and ESCC), neoplastic progression, and adverse events.
Results
96 patients participated (MGIN 45, HGIN 42, early ESCC 9). At 3 and 12 months, respectively, 73% (70/96) and 84% (81/96) were CR. Two patients (2%) progressed (MGIN to HGIN; HGIN to T1m2 ESCC); both were treated endoscopically and achieved CR. Stricture occurred in 20 patients (21%), all after circumferential RFA. Lugol’s + RFA 12 J/cm2 (single application, no cleaning) was the favored baseline circumferential RFA technique (82% 12-month CR, 6% stricture).
Conclusion
In patients with early ESCN, RFA is associated with a high CR rate and acceptable safety profile.
Introduction
Esophageal cancer is the eighth most common cancer and sixth most common cause of cancer death worldwide, with an estimated 482,000 cases and 407,000 deaths in 2008. Most esophageal cancers (>80%) occur in developing countries; most of these (90%) are esophageal squamous cell carcinoma (ESCC). China, central Asia, northeastern Iran, eastern and southern Africa, and northern France have the highest ESCC incidence rates [1]. Approximately half of worldwide ESCC cases occur in China, where ESCC is the fourth leading cause of cancer-related death [1–3].
Several factors may influence the development of ESCC, including tobacco use; alcohol consumption; drinking hot liquids; exposure to nitrosamines, acetaldehyde, mycotoxins, and polycyclic aromatic hydrocarbons; nutritional deficiencies; poor oral health; achalasia; and low socioeconomic status, but differences in etiologic factors likely exist between high-risk and low-risk regions [1–3]. Evidence also supports a complex genetic milieu precipitating ESCC. In Chinese populations, susceptibility loci have been identified on several chromosomes, including 10q23 and 12q24, with 12q24 possessing gene-environmental interactions with tobacco and alcohol [4].
In China, esophageal squamous intraepithelial neoplasia is graded according to the proportion of epithelial involvement: 1/3 (low-grade intraepithelial neoplasia, LGIN), 2/3 (moderate-grade, MGIN), or 3/3 (high-grade, HGIN) [5]. The progression rate to invasive ESCC varies according to baseline neoplasia grade, with incidence rates at 3.5 and 13.5 years in LGIN, MGIN, and HGIN being 5/24%, 27/50%, and 65/75%, respectively [6–7]. Guidelines regarding the management of LGIN, MGIN and HGIN have not been established. Nonetheless, based on neoplastic progression rates, LGIN is generally managed with endoscopic surveillance and biopsies, and MGIN and HGIN are managed more aggressively with endoscopic resection (MGIN and HGIN), endoscopic submucosal dissection (MGIN and HGIN) or esophagectomy (HGIN) [7–8].
Endoscopic radiofrequency ablation (RFA) for esophageal neoplasia comprises delivery of a 465 KHz energy waveform via a bipolar electrode array to the diseased tissue. The electrode is mounted on the outside of a balloon, on an articulating platform on the distal end of an endoscope, or on a through-the-scope flexible catheter. RFA has been shown to safely, effectively, and durably eradicate early neoplasia in Barrett’s esophagus (BE), the precursor to esophageal adenocarcinoma [9]. A randomized, sham-controlled trial showed that RFA resulted in a high rate of complete eradication of neoplasia and a reduction in the rate of neoplastic progression [10]. We previously reported findings of our first 29 patients with early esophageal squamous cell neoplasia (ESCN) and early ESCC treated with RFA. These data suggested that RFA had similar outcomes in early ESCN as in Barrett’s neoplasia [11]. We presently report the continuation of our prior study, including outcomes of all patients (n=96) undergoing endoscopic RFA for MGIN, HGIN, and early flat-type (type 0-IIb) ESCC.
Patients and Methods
This prospective cohort study was conducted at the Cancer Institute and Hospital, Chinese Academy of Medical Sciences (CICAMS) from October 2008 to October 2012. The CICAMS Institutional Review Board approved the protocol and patient informed consent form. The reading of coded biopsy slides was exempted from review by the Office of Human Subjects Research of the National Institutes of Health. All study participants signed an informed consent form. An independent data and safety monitoring committee monitored the trial.
Patients
Inclusion criteria were: 1) a baseline qualifying high resolution Lugol’s chromoendoscopy within 3 months prior to primary ablation showing ≥1 unstained lesion (USL) measuring ≥3 cm and containing MGIN, HGIN or early flat (type 0-IIb) ESCC (limited to T1m2 invasion); 2) total length of USL-bearing esophagus ≤12 cm; 3) endoscopic ultrasound (EUS) without submucosal invasion and lymphadenopathy; 4) chest and upper abdomen CT scan (HGIN and ESCC patients) without metastasis and lymphadenopathy.
Exclusion criteria included: 1) esophageal stricture preventing endoscope passage; 2) prior endoscopic resection (ER) or ablation; 3) any non-flat esophageal mucosa; 4) any history of esophageal non-squamous cell cancer, or history of ESCC (any stage) prior to 3 months before primary RFA; 5) ESCC with T1m3 or worse invasion, G3 or worse differentiation, or lymphatic or vascular invasion; 6) N or M positive status in ESCC patients.
Study Devices
The Barrx™ Ablation System (Covidien GI Solutions, Sunnyvale, California, USA) comprises a radiofrequency generator and circumferential and focal ablation catheters, previously described in detail [11]. Circumferential RFA is delivered via a balloon-based catheter at a power density of 40 W/cm2 and energy density of 10–12 J/cm2. Focal RFA is delivered at 40 W/cm2 and 12 J/cm2 via a 13 × 20 mm articulating electrode platform brought into contact with target tissue.
Endoscopy procedures were performed with Olympus GIF-H260, GIF-H260Z, or GIF-H260J (Olympus, Tokyo, Japan) high-resolution endoscopes (Lucera systems). EUS was performed using a mechanical radial ultrasonic gastrovideoscope and miniprobes (GF-UM2000, UM-2R/3R, Olympus, Tokyo, Japan).
Baseline Qualifying Examination
Within 3 months prior to primary ablation, baseline qualifying high-resolution chromoendoscopy using 1.25% Lugol’s solution was performed to identify esophageal USLs. If USLs met study criteria, tattoos were placed 1 cm above and below the USL-bearing portion of the esophagus, defining the treatment area. The location and size of each USL was recorded, followed by biopsies of the USLs. The worst neoplasia grade in biopsies from this visit determined the patient’s entry diagnosis.
Patient Flow
Within 3 months of the baseline qualifying exam, participants underwent primary RFA of the entire treatment area (Appendix 1). Participants returned 3 months later for high-resolution chromoendoscopy. Biopsies were obtained from USLs and stained mucosa, followed by focal RFA if USLs were present. A finding of MGIN or worse prompted repeat endoscopy at 3-month intervals. If no MGIN or worse was detected, patients returned for the 12-month visit. At the 12-month primary endpoint visit, patients underwent high-resolution chromoendoscopy with biopsy of USLs and stained mucosa in the treatment area.
Ablation Procedures
For the primary circumferential RFA session, several circumferential techniques were evaluated. Lugol’s solution (1.25%) was applied before some circumferential RFA procedures (depending on group) and before all focal RFA procedures to aid in confirmation of USL extent and location. Circumferential ablation included 1–2 energy applications (10 or 12 J/cm2 at 40 W/cm2) encompassing the entire treatment area. In some patients, coagulum was cleaned from the ablation sites between the first and second ablation passes. Focal ablation was performed on USLs within the treatment area using 3 applications (12 J/cm2 at 40 W/cm2) with no cleaning. Patients took high-dose PPIs for one month post-RFA.
Esophageal Tissue Categorization and Specimen Procurement
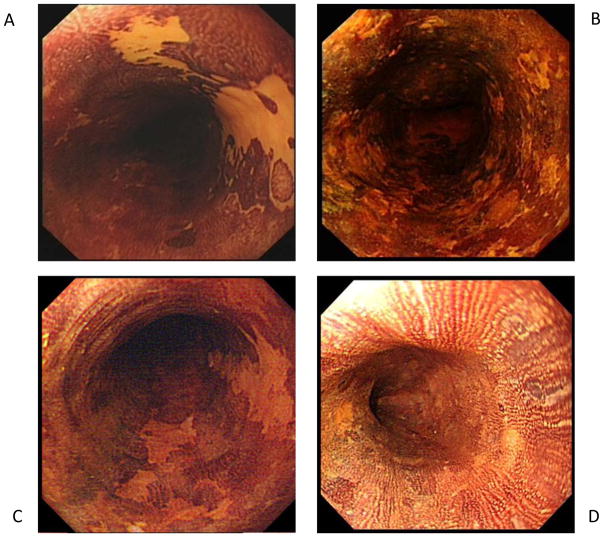
Stained tissue and USLs within the treatment area were recorded based on location and staining characteristics, which were graded as: unstained area (stain code 1), mosaic staining-intermixed stained and unstained areas (stain code 2), lightly stained area (stain code 3), and completely stained (stain code 4) (Figure 1). Stain codes 1–3 were considered USLs. Esophageal specimens were procured using large capacity biopsy forceps (FB-25k-1, 4.8 mm cup opening, Olympus, Japan). Each specimen was removed from the forceps using a toothpick, spread flat on a gloved finger, attached to filter paper, and placed flat into a specimen jar labeled with specimen location (maximum 2 specimens per jar). At the baseline qualifying exam and all follow-up visits, each USL was sampled with 1 biopsy from every 1 cm of the greatest USL dimension. At follow-up visits, normal staining treatment area mucosa was also sampled with 1–2 biopsies from every 2 cm of linear length. At the 12-month visit, treatment area biopsies were obtained with at least 4 biopsies from every 2 cm of linear length.
Figure 1.
Representative endoscopic images of the four stain codes used to categorize tissue appearance after application of 1.25% Lugol’s iodine stain. Designation of stain codes 1, 2 or 3 qualified a lesion as unstained.
A. Unstained area (stain code 1)
B. Mosaic staining-intermixed stained and unstained areas (stain code 2)
C. Lightly stained area (stain code 3)
D. Completely stained (stain code 4)
Histopathology Interpretation
Two of 3 expert GI pathologists (NL or LX, SMD) independently reviewed all specimens from each baseline qualifying exam and 12-month primary endpoint high-resolution chromoendoscopy. The pathologists were blinded to patient history, circumferential technique group, and prior histology results. Each specimen was graded as either: squamous epithelium without intra-epithelial neoplasia, or, squamous epithelium with LGIN, MGIN, HGIN, or invasive ESCC (depth and grade determined based upon biopsy depth). The most advanced diagnosis from a single visit was designated as the histology status for that visit. If the independent reviews were concordant for the most advanced diagnosis, no further review was performed. Discordant results were resolved by a joint review by both pathologists. Only the study site GI pathologists (NL, XL) interpreted specimens from the 3-, 6-, or 9-month visits.
Outcome Measures
The primary endpoint of the study was the proportion of patients with a histological complete response (CR) at 12 months, defined as absence of MGIN, HGIN and ESCC from any biopsy in the treatment area regardless of endoscopic staining characteristics. Secondary endpoints were: 1) proportion of patients with CR after primary RFA, defined as absence of MGIN, HGIN, and ESCC in the treatment area at the 3-month visit; 2) proportion of patients demonstrating neoplastic progression defined as detection of a more severe histological grade than the study entry grade at any follow-up visit; and 3) adverse events.
Statistical Analysis
Data analysis was performed using SAS software, version 9.3 (SAS Institute, Cary, North Carolina, USA). Means (± standard deviation) were computed for continuous variables and frequencies and percentages were computed for categorical variables. Continuous variables were compared between successes and failures by Student’s t test. Continuous variables were compared between circumferential technique groups (A, B C and D) by ANOVA. Fisher’s Exact Test was used for all categorical data. P values <0.05 were considered statistically significant. We conducted univariate analysis for baseline patient characteristics associated with CR at 12 months using logistic regression. For each dichotomous response of interest, a multivariate model was then constructed to determine the best set of predictors for CR. A forward stepwise procedure was performed to build the model, with no variables being forced into the model. A criterion of p<0.05 was used to determine if a new variable would enter the model at each step.
Results
Patients
Ninety-six patients were enrolled (MGIN 45, HGIN 42, ESCC 9) (Table 1). Most primary ablations were circumferential RFA (97%, 93/96) and most secondary ablations were focal RFA (98%, 87/89). Three patients were lost to follow-up: one withdrew consent and another suffered an unrelated cerebral hemorrhage before the 3-month visit; the third withdrew consent after the 3-month visit.
Table 1.
Patient characteristics
| Variable | Value |
|---|---|
|
| |
| Patients – no. | 96 |
|
| |
| Age - years | |
| Mean ± SD | 59.9 ± 6.6 |
| Range | 43 – 77 |
|
| |
| Sex – no. (%) | |
| Male | 52 (54) |
| Female | 44 (46) |
|
| |
| Chinese Ethnicity – no. (%) * | 96 (100) |
|
| |
| Grade of esophageal neoplasia at study entry – no. (%) | |
| MGIN | 45 (47%) |
| HGIN | 42 (44%) |
| T1m2 ESCC | 9 (9%) |
|
| |
| Length of unstained lesions – cm | |
| Mean ± SD | 6.5 ± 2.9 |
| Range | 2 – 13† |
|
| |
| Length of treatment area – cm | |
| Mean ± SD | 8.6 ± 2.9 |
| Range | 4 – 15† |
|
| |
| Initial RFA - no. (%) | |
| Circumferential | 93 (97%) |
| Focal | 3 (3%) |
| Endo time (hr:min), median (IQR) | 0:28 (0:16–0:42) |
| RFA time (hr:min) only, median (IQR) | 0:15 (0:07–0:23) |
|
| |
| Follow-up RFA | |
| Patients receiving follow-up RFA - no. | 64 |
| Number of follow-up RFAs - no. | 89 |
| Circumferential - no. (%) | 1 (1%) |
| Focal - no. (%) | 87 (98%) |
| Circumferential and Focal - no. (%) | 1 (1%) |
| Endo time (hr:min), median (IQR) | 0:25 (0:21–0:31) |
| RFA time (hr:min) only, median (IQR) | 0:10 (0:06–0:13) |
|
| |
| Total RFA | |
| Patients - no. | 96 |
| Mean ± SD | 1.9 ± 0.8 |
| Range (min – max) | 1 – 4 |
|
| |
| Total RFA procedures in study | 185 |
Ethnicity was self-reported
After enrollment, one patient was determined to have a total length of unstained lesions of 13 cm and total length of treatment area of 15 cm; this was noted as a protocol deviation
MGIN: moderate-grade intraepithelial neoplasia
HGIN: high-grade intraepithelial neoplasia
ESCC: esophageal squamous cell carcinoma
hr:min: hour:minute duration of endoscopy procedure or RFA portion of procedure
Outcomes
In the assessment of all patients from all dosimetry groups, overall CR rates at 3 and 12 months were 73% (70/96) and 84% (81/96), respectively, (Table 2) by intention-to-treat (ITT) analysis. At 3 and 12 months, 17% (12/70) and 20% (16/81) of patients achieving CR had residual LGIN, respectively. Of the 12 patients with LGIN at 3 months (who thereby did not meet criteria for additional RFA treatment), 2 progressed to MGIN, 1 remained LGIN and 9 had less than LGIN at the 12-month primary outcome visit. Figure 2 shows the photomicrograph and endoscopic images of a study patient before (A–C), during (D–H), and after (I–J) circumferential and focal RFA who achieved CR. The 12 failures had baseline pathology of MGIN (n=1), HGIN (n=8) and ESCC (n=3), and residual disease was MGIN (n=3) or HGIN (n=9). On univariate analysis, likelihood of 12-month CR was associated with less severe baseline grade of neoplasia and shorter baseline total USL length and treatment area length (Table 3). Multivariable analysis demonstrated that baseline grade of neoplasia, specifically ESCC T1m2 (ESCC T1m2 vs. MGIN: odds ratio [OR], 0.05 [95% CI 0.01–0.65]) and USL length (OR, 0.79 [95% CI 0.64–0.98]) were independent predictors of CR at 12 months. Two patients (2%) demonstrated neoplastic progression, one from MGIN to HGIN at 3 months and one from HGIN to T1m2 ESCC at 6 months. Both achieved CR after treatment with 1–3 additional focal RFA sessions.
Table 2.
Patient outcomes - efficacy endpoints
| Outcome and Analysis | N/total number (%) | 95% CI |
|---|---|---|
|
| ||
| 3-month Complete Response | ||
| Intention to Treat | 70/96 (73%) | 63%–81% |
| Per Protocol | 70/94 (74%) | 66%–82% |
| MGIN | 40/43 (93%) | 81%–98% |
| HGIN | 24/42 (57%) | 42%–71% |
| ESCC | 6/9 (67%) | 35–88% |
|
| ||
| 12-month Complete Response | ||
| Intention to Treat | 81/96 (84%) | 76%–90% |
| Per Protocol | 81/93 (87%) | 79%–92% |
| MGIN | 41/42 (98%) | 88%–100% |
| HGIN | 34/42 (81%) | 67%–91% |
| ESCC | 6/9 (67%) | 35%–88% |
|
| ||
| Progression at any time | 2/96 (2%) | 0.6%–7.3% |
Complete Response is the proportion of patients with histological complete response at interim or 12-month visit, defined as no MGIN, HGIN, or ESCC on biopsy of treatment area.
MGIN: moderate-grade intraepithelial neoplasia
HGIN: high-grade intraepithelial neoplasia
ESCC: esophageal squamous cell carcinoma
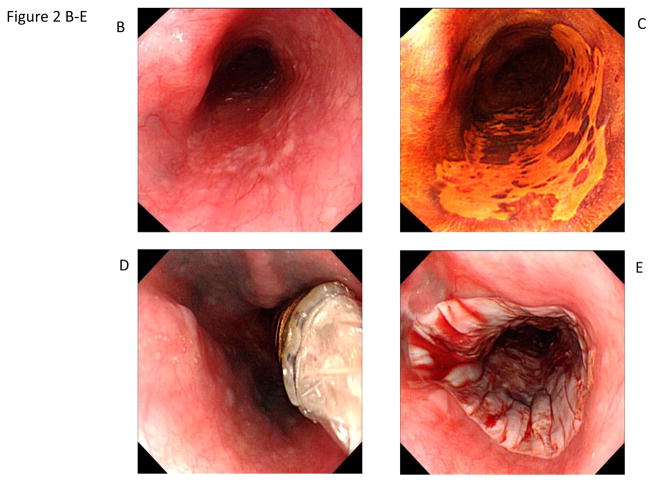
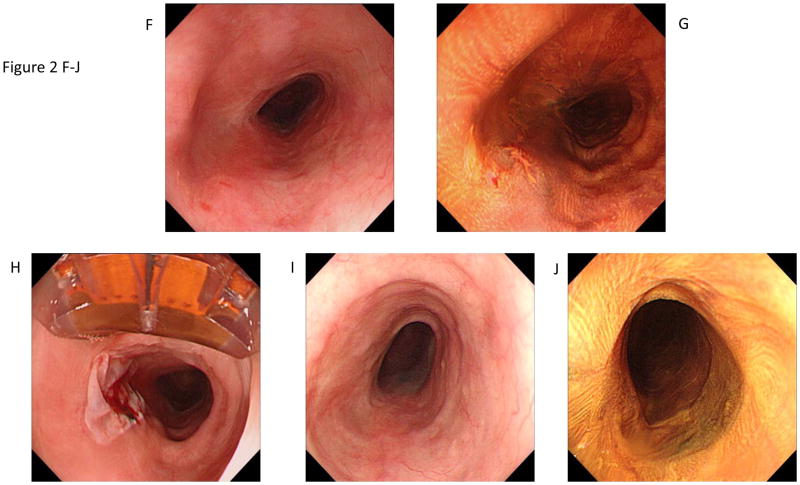
Figure 2.
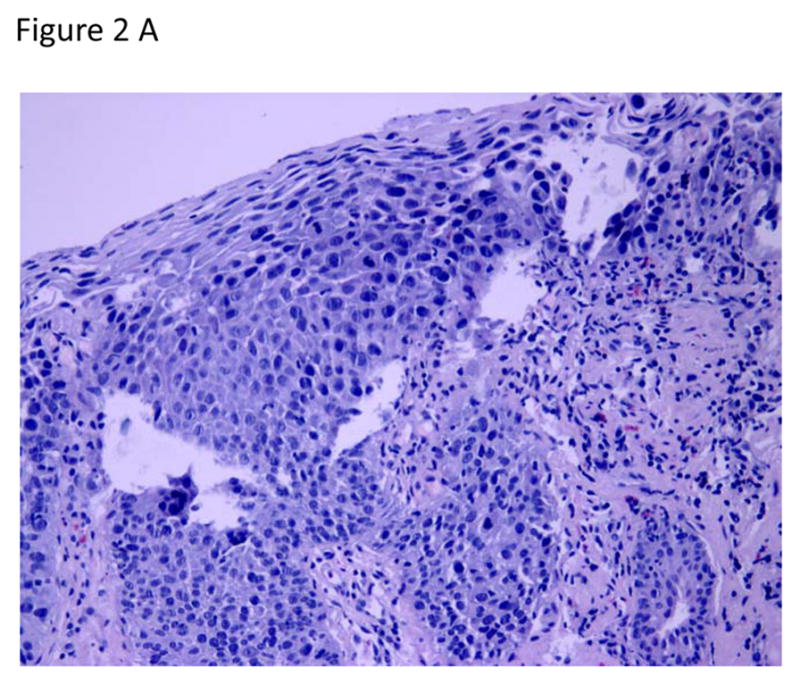
Circumferential and focal radiofrequency ablation of a 3-cm long flat-type early squamous cell neoplasia with high-grade intraepithelial neoplasia, treated with Lugol’s-10J/cm2-10J/cm2-no cleaning between ablation passes (circumferential technique Group D). The patient achieved a complete response (absence of moderate-grade intraepithelial neoplasia, high-grade intraepithelial neoplasia, and esophageal squamous cell carcinoma in the treatment area) at the 12-month primary outcome.
A. Photomicrograph of a pre-treatment esophageal biopsy specimen demonstrating HGIN (hematoxylin and eosin [H&E]; original magnification × 200).
B. Pretreatment white-light endoscopy image showing a reddish colored area from 4 o’clock to 7 o’clock.
C. Corresponding image with Lugol’s chromoendoscopy demonstrating a flat-type unstained lesion; biopsy samples showed HGIN.
D. Circumferential ablation catheter placed in the esophagus before the first ablation pass.
E. Appearance of the mucosa after the first circumferential ablation pass.
F. 3-month visit. White-light endoscopy image showing the treatment area.
G. Corresponding image with Lugol’s chromoendoscopy demonstrating an unstained lesion at 8 o’clock.
H. Appearance of the mucosa immediately after focal ablation of the unstained lesion; the ablation catheter can be seen at the top of the endoscopic image.
I–J.12-month primary endpoint visit. White-light endoscopy and Lugol’s high-resolution chromoendoscopy images demonstrate no evidence of residual squamous neoplasia. Biopsies confirmed complete response.
Table 3.
Baseline patient characteristics in 12-month CR vs.12-month failure groups with predictors of CR at 12 months
| Variable | 12-Month CR | 12-Month Failure | Univariable Analysis, OR (95% CI) | P Value† | Multivariable Analysis, OR (95% CI) | P Value† |
|---|---|---|---|---|---|---|
|
| ||||||
| Age (years) | ||||||
| Mean ± SD | 59.8 ± 6.6 | 61.9 ± 6.6 | 0.95 (0.87–1.04) | 0.29 | ||
| Range | 43–77 | 52–71 | ||||
|
| ||||||
| Sex n (%) | ||||||
| Male | 43 (53%) | 8 (67%) | 1.77 (0.49–6.34) | 0.38 | ||
| Female | 38 (47%) | 4 (33%) | ||||
|
| ||||||
| Baseline grade of ESCN | ||||||
| MGIN | 41 (51%) | 1 (8%) | ||||
| HGIN | 34 (42%) | 8 (67%) | 0.10 (0.01–0.87) | 0.04 | 0.13 (0.02–1.09) | 0.06 |
| ESCC T1m2 | 6 (7%) | 3 (25%) | 0.05 (0.004–0.55) | 0.01 | 0.05 (0.01–0.65) | 0.02 |
|
| ||||||
| Length of unstained lesions, cm | ||||||
| Mean ± SD | 6.2± 2.8 | 8.6 ± 3.0 | 0.77 (0.63–0.95) | 0.01 | 0.79 (0.64–0.98) | 0.04 |
| Range | 2–13 | 4–12 | ||||
|
| ||||||
| Length of treatment area, cm | ||||||
| Mean ± SD | 8.3 ± 2.8 | 10.5 ± 2.7 | 0.78 (0.63–0.96) | 0.02 | ||
| Range | 4–15 | 6–14 | ||||
CR: complete response
OR: odds ratio
ESCN: esophageal squamous cell neoplasia
MGIN: moderate-grade intraepithelial neoplasia
HGIN: high-grade intraepithelial neoplasia
ESCC: esophageal squamous cell carcinoma
P value < 0.05 considered statistically significant
Safety
There were no serious adverse events such as perforation, infection, or death. Four mucosal lacerations were observed during sizing (4% of patients); 2 delayed treatment but none required intervention. A mucosal bleb following the marking of treatment area borders with argon plasma coagulation occurred in one patient, which did not interfere with treatment. Stricture was observed in 20 patients (21%), all after circumferential RFA (none after focal RFA). All strictures resolved with dilation (median 4 sessions, IQR 2–6). Nineteen of the 20 stricture patients were CR at 12 months.
Safety and efficacy by circumferential technique group
The technique for circumferential RFA was adjusted over time in conjunction with amendments to the protocol and IRB approval, in an attempt to optimize outcomes of the primary intervention (improve efficacy, reduce stricture rate). A post-hoc sub-group analysis was performed, aggregating similar techniques into one of four groups (Table 4). Group A (n=35): Lugol’s, sizing, circumferential RFA, cleaning of ablation zone coagulum, and a second application of circumferential RFA. The 3- and 12-month CR rates were 74% (25/34) and 91% (31/34), respectively, with a 14% stricture rate. Group B (n=30): Sizing, circumferential RFA with 1 or 2 applications, without Lugol’s, without cleaning. The 3- and 12-month CR rates were 76% (22/29) and 86% (24/28), respectively, with a 43% stricture rate. Group C (n=17): Lugol’s, sizing, circumferential RFA (single application), no cleaning. The 3- and 12-month CR rates were 11/17 (65%) and 14/17 (82%), respectively, with a 6% stricture rate. Group D (n=10): Lugol’s, sizing, circumferential RFA (double application), no cleaning. The 3- and 12-month CR rates were 8/10 (80%), with a 10% stricture rate. There were no significant differences in patient characteristics among the groups and 3- and 12-month CR rates were similar. Group B demonstrated a higher stricture rate (p<0.01).
Table 4.
Outcomes by circumferential RFA technique group
| Variable or Outcome | A | B | C | D | P Value† |
|---|---|---|---|---|---|
| Grouping | Lugol’s RFA Clean RFA | No Lugol’s RFA No Clean ±RFA | Lugol’s RFA No Clean | Lugol’s RFA No Clean RFA | |
| Dose and Technique | L-12-CL-12 (n =19) L-12-CL-10 (n=16) |
10-10 (n=14) 12-12 (n=12) 12 (n=4) |
L-12 (n=17) | L-10-10 (n=2) L-12-12 (n=7) L-12-10 (n=1) |
|
| N | 35 | 30 | 17 | 10 | |
| USL (cm) Mean ± SD | 6.7 ± 3.0 | 6.1 ± 2.7 | 6.2 ± 3.2 | 6.9 ± 3.1 | 0.85 |
| TA (cm) Mean ± SD | 8.9 ± 3.1 | 8.1 ± 2.6 | 8.7 ± 3.1 | 8.8 ± 3.0 | 0.87 |
| Stricture n (%) | 5 (14%) | 13 (43%) | 1 (6%) | 1 (10%) | <0.01 |
| 3 mo CR (PP) n (%) | 25/34 (74%) | 22/29 (76%) | 11/17 (65%) | 8/10 (80%) | 0.84 |
| 12 mo CR (PP) n (%) | 31/34 (91%) | 24/28 (86%) | 14/17 (82%) | 8/10 (80%) | 0.70 |
Not Shown: 12-CL-12 (n=1), L-Barrx™90 (n=3). These 4 patients did not fit into any of the aforementioned groups and did not have sufficient numbers for a meaningful analysis.
L: Lugol’s iodine solution (1.25%)
CL: cleaning of coagulum from treatment area after first ablation pass
Energy density was either 10J/cm2 or 12J/cm2 (designated as 10 or 12 in Dose and Technique descriptors)
USL: unstained lesion
TA: treatment area
CR: complete response, the proportion of patients with histological complete response at interim or 12-month visit, defined as no moderate-grade intraepithelial neoplasia, high-grade intraepithelial neoplasia, or esophageal squamous cell carcinoma on biopsy of treatment area.
ANOVA for comparing means; Fisher’s Exact Test for comparing percentages. P value < 0.05 considered statistically significant.
Assessment of Lugol’s mucosal staining pattern for detecting neoplasia after RFA
In follow-up visits, the sensitivity, specificity and positive predictive value (PPV) of USL biopsy specimens for ≥MGIN were 99%, 73% and 48%, respectively, and the negative predictive value (NPV) of completely staining tissue for ≥MGIN was 100% (Table 5). Comparable values for criterion of ≥LGIN are sensitivity 93%, specificity 79%, PPV 63% and NPV 97%. Post-RFA, a higher proportion of USLs were categorized as lightly staining versus pre-RFA (Table 6). For biopsy specimens from these lightly staining USLs (stain code 3), as well as for those from the unstained area category (stain code 1), the PPV for ≥MGIN decreased post-RFA. Overall, for biopsy specimens from USLs (inclusive of all 3 staining sub-categories), the PPV for ≥MGIN also decreased after ≥1 RFA session (Table 7).
Table 5.
Assessment of Lugol’s chromoendoscopy staining and histopathology, by biopsy specimen, after ≥ 1 RFA session
| Stain Category | Biopsy Specimens with ≥ MGIN n (%)* |
Biopsy Specimens with < MGIN n (%)* |
Total |
|---|---|---|---|
| Unstained mucosa | 511 (48%) | 552 (52%) | 1063 |
| Stained mucosa | 3 (0.2%) | 1488 (99.8%) | 1491 |
| Total | 514 | 2040 | 2554 |
| Outcome | |||
| Sensitivity | 99% | ||
| Specificity | 73% | ||
| Positive Predictive Value (PPV) | 48% | ||
| Negative Predictive Value (NPV) | 100% | ||
MGIN: moderate-grade intraepithelial neoplasia
Percentages are row percentages
Table 6.
Proportion of USLs in each Lugol’s chromoendoscopy stain sub-category, pre-RFA and after ≥ 1 RFA session
| Stain Category | Pre-RFA* | After ≥ 1 RFA* | P Value† |
|---|---|---|---|
|
| |||
| Total USLs (includes stain codes 1–3) n | 167 | 398 | |
|
| |||
| Unstained area (stain code 1) | 134 (80%) | 250 (63%) | <0.0001 |
| Mosaic staining (stain code 2) | 23 (14%) | 50 (12%) | |
| Lightly staining (stain code 3) | 10 (6%) | 98 (25%) | |
USL: unstained lesion
Percentages are column percentages
P value < 0.05 considered statistically significant
Table 7.
Positive predictive value of USLs for detecting ≥MGIN, by biopsy specimen, pre-RFA and after ≥ 1 RFA session
| Stain Category | PPV Pre-RFA | PPV After ≥ 1 RFA | P Value† |
|---|---|---|---|
|
| |||
| USL (includes stain codes 1–3) | 64% (349/549) | 48% (511/1063) | <0.0001 |
|
| |||
| Unstained area (stain code 1) | 71% (316/448) | 64% (450/706) | 0.02 |
| Mosaic staining (stain code 2) | 35% (25/71) | 27% (34/126) | 0.26 |
| Lightly staining (stain code 3) | 27% (8/30) | 12% (27/231) | 0.04 |
USL: unstained lesion
PPV: positive predictive value
P value < 0.05 considered statistically significant
Discussion
We report the outcomes of endoscopic RFA for early ESCN in 96 patients with MGIN, HGIN or flat-type (type 0-IIb) ESCC. At 12 months, CR was achieved in 84% of patients after a mean of 1.9 RFA treatments. More advanced baseline neoplasia grade and longer baseline total USL length were associated with a decreased likelihood of CR at 12 months. Two patients (2%) exhibited neoplastic progression (MGIN to HGIN at 3 months, HGIN to T1m2 ESCC at 6 months). Both had subsequent RFA and achieved CR. There were no serious adverse events. Stricture was the most common adverse event (all after circumferential RFA), affecting 21% of patients. A post-hoc analysis of different circumferential RFA technique groups showed that Group C (Lugol’s, sizing, circumferential RFA at 12 J/cm2, no cleaning) had the lowest stricture rate (6%) and similar 3- and 12-month CR compared to the other groups.
In 2011 we reported results from the first 29 patients enrolled in this study. At 12 months, after a mean of 1.7 RFA sessions, CR was achieved in 97% of patients, strictures occurred in 14% of patients, and there was no neoplastic progression [11]. Van Vilsteren et al. reported their experience in 13 ESCN patients with baseline HGIN and mucosal ESCC, 9 of whom underwent ER of non-flat mucosal abnormalities 2 months prior to RFA. All patients achieved CR after a median of 2 RFA sessions. RFA-related adverse events included 2 mucosal lacerations at ER scars, 1 intramural hematoma, and 3 strictures. There were no recurrences during a median follow-up of 17 months [12]. Becker and colleagues treated 6 patients with multifocal/recurrent superficial ESCC (maximum depth T1sm) using RFA ± ER or endoscopic submucosal dissection (ESD) (n=4/6). After a mean of 2.8 endoscopic procedures (mean 1.1 ER/ESD, 1.7 RFA), complete eradication of cancer was achieved in all patients with a mean follow-up of 14 months. No major adverse events occurred [13].
Results of these first studies of RFA for early ESCN should be considered vis-à-vis other available therapies, specifically ER, ESD and esophagectomy. ER and ESD, perhaps the most accepted endoscopic approaches for ESCN, are associated with high complete response rates, ranging from 71% to 96% for ER, and 78% to 97% for ESD [14–19]. However, both techniques have limitations when attempting to eradicate long segment and/or circumferential early ESCN, as treated in this protocol. ER has been associated with stricture and perforation in up to 80% and 2.4% of ESCN patients, respectively, and for lesions > 2 cm, piecemeal resection is required [20–22]. Compared to ER, ESD has higher rates of en bloc resection and decreased local recurrence rates, however, is technically demanding with more frequent and severe complications [16, 20, 23]. Perforation has been reported in up to 6.9% of patients, with several studies reporting rates > 5%, and stricture has been reported in up to 75% [17, 20, 22–25].
Esophagectomy is a curative intervention for most early ESCN patients, but is a major surgery with significant associated risks. In-hospital mortality at high-volume centers is 0–5% with several recent series reporting rates of 1–2%; in low-volume centers, the in-hospital mortality is even higher [26–28]. In addition, the post-procedure morbidity is 20–50% [28–29]. Minimally invasive surgical techniques have been applied in an attempt to decrease the associated morbidity and mortality rates. However, widespread adoption of these techniques, while increasing, has been limited secondary to the technical difficulty of the procedure and inconsistent improvements in surgical outcomes [29]. Overall, when considering the performance of these existing alternatives for the management of ESCN, the results of the present study, while early, suggest RFA, as a primary or complimentary therapy, is worthy of further exploration in these patients.
Considering endoscopic treatment for early ESCN requires careful patient selection. We included flat MGIN and HGIN, given their low likelihood of concurrent (occult) invasive disease and unacceptably high rate of progression to invasive cancer. We also included patients with flat type 0-IIb ESCC limited to T1m2 invasion, given their low likelihood of submucosal invasion or lymph node metastases (further confirmed with EUS and CT) [30–32]. With the availability of resective and surgical alternatives, inclusion of early ESCC patients may be seen by some as risky and premature. In our study, 67% (6/9) of these patients achieved CR at 12 months. All 3 patients who did not achieve CR were downgraded to HGIN and continue to undergo endoscopic therapy. Overall, in patients with ESCC T1m2, we caution that additional investigation is required, including that which explores a combined approach of ER followed by RFA as employed in advanced Barrett’s esophagus. Until this occurs, we cannot recommend RFA as an accepted treatment for ESCC T1m2 patients at this time.
In consideration of efficacy outcomes, the primary endpoint was the CR rate of MGIN and worse at 12 months. The threshold for re-treatment at preceding follow-up visits was also the presence of MGIN or worse. Notably, 20% of patients who achieved CR at the 12-month primary outcome had LGIN. In consideration of the limited safety data on RFA of ESCN and the desire to mitigate risks associated with possible over-treatment, as well as the mixed precedent with endoscopic excision treatment algorithms [i.e. some treating to and reporting elimination of entry disease grades and worse similar to the present study, and others doing so for disease grades lesser than required for entry (i.e. LGIN)], and in the absence of clear guidelines, we elected to proceed conservatively [8, 14–15, 19, 23, 33–35]. As a result, the study design and results preclude meaningful conclusions regarding the performance of RFA for all ESCN (i.e. LGIN and worse); future studies should consider if the follow-up visit treatment threshold and CR criteria should include the presence of any dysplasia, including LGIN.
The 21% overall stricture rate in our study was higher than those reported in RFA trials for BE [9]. All strictures occurred after circumferential RFA. The technique was adjusted during the course of the trial in an attempt to optimize efficacy and stricture rate. In a post-hoc sub-group analysis, we compared several circumferential technique groups to determine which factors may contribute to stricture formation. The groups were similar for 3- and 12-month CR rates. After Group A demonstrated a 14% stricture rate, we hypothesized that Lugol’s solution might augment an inflammatory response after RFA and cause a higher stricture rate than seen in RFA for BE. In Group B, Lugol’s (and cleaning) was eliminated to possibly lower the observed stricture rate, but this resulted in an even higher rate of 43%. In Group C, Lugol’s was reinstituted, and, as additional measures of caution, cleaning and the second ablation were eliminated, resulting in a 6% stricture rate. Group D included Lugol’s plus a double application of circumferential RFA without cleaning, resulting in a 10% stricture rate.
The stricture rate in Group C was comparable to published rates in BE and, in the context of ESCN’s severity and the adverse event profiles of existing therapies, was deemed acceptable [9]. There was no significant difference in 3- and 12-month CR rates between the four circumferential technique groups, and Group C’s regimen had the additional benefit of procedural simplicity. Nonetheless, while the treatment group data is suggestive, the post-hoc nature of the analysis limits meaningful conclusions and serves primarily to inform more definitive future study. While we focused on procedure technique as it relates to stricture development and efficacy outcomes, we cannot exclude the possibility that other factors inherent to ESCN or the patient population, such as cell-type specific responsiveness to thermal ablation or genetic factors played a role as well. Overall, our experience suggests ESCN may be more sensitive to RFA than BE, and that less ablation is required.
In ablation-naïve patients, Lugol’s chromoendoscopy is the gold standard for identification of ESCN with ≥96% sensitivity [36–37]. In our study, when considered with this historical data, the utility of Lugol’s after RFA persists, with 99% sensitivity for the detection of ≥MGIN in post-RFA USLs. The PPV for biopsies from USLs post-RFA was lower compared to pre-RFA, however, because of the presence of more lightly stained (stain code 3) non-neoplastic lesions. This finding likely represents focal areas of incomplete glycogen accumulation in regenerating neosquamous epithelium. Nonetheless, biopsy specimens from these post-RFA lightly stained USLs contain ≥MGIN frequently enough (12% of the time) to justify biopsy and focal RFA.
Strengths of this study include a prospective design, large number of participants, use of histological primary endpoints, and expert GI pathologists with blinded consensus review for baseline and primary endpoint histopathology.
There are two major limitations of this study; the first is the temporal adjustment of the circumferential RFA technique in an attempt to optimize efficacy and stricture rates. Due to the relatively small numbers of patients in each circumferential technique subgroup and the variability of dose within these subgroups, this data must be interpreted with caution and the ability to draw robust conclusions about optimal circumferential RFA technique is limited. By virtue of having the lowest stricture rate, a comparable 12-month CR rate to the other dosimetry groups, and the benefit of being the most technically simple procedure amongst the groups, the current data reflects most favorably on the circumferential regimen that employs Lugols-12J/cm2 1×-no cleaning (Group C). Nonetheless, additional study is needed to confirm the optimized circumferential RFA dose and technique.
The second major limitation is that this data set represents relatively short-term RFA outcomes at one year. In ESCN patients treated with ER, longer term data indicate local recurrence rates of 0–26% with up to 16% of patients experiencing metachronous lesions over average follow-up periods ranging from 37–83 months [15, 34, 38]. For patients treated with ESD, local recurrence rates are 0–3.8% with 0–3.6% of patients experiencing metachronous lesions over average follow-up periods of 18–36 months [16, 18, 24, 38–39]. To better understand the role of RFA in ESCN, longer term RFA data and comparative data amongst these endoscopic modalities are needed. Note that this study has been extended with ongoing ethics committee approval for 5-year outcomes.
Additional limitations include the single-center nature of the study and the possible influence of biopsy sampling error on 12-month CR rates, although the high sensitivity of Lugol’s staining makes it less likely that occult disease was missed in completely staining tissue at follow-up. In addition, it is uncertain whether the two progressors were true progressors or initially under-staged due to biopsy sampling error. For the sake of analysis, we considered them true progressors to provide a conservative representation of possible outcomes. Finally, we were unable to compare sensitivity, specificity and NPV of Lugol’s chromoendoscopy before and after RFA due to the limited number of biopsies from completely staining tissue at baseline. However, because the literature for these outcomes in Lugol’s chromoendoscopy in similar (ablation-naïve) ESCN patients is well established, we believe these historical values act as a reasonable proxy for comparison [36–37]. Most important, sensitivity and NPV of Lugol’s chromoendoscopy post-RFA remain high at 99% and 100%, respectively, which is comparable to values reported in ablation-naïve ESCN patients.
Conclusion
In early ESCN patients, RFA is associated with a high rate of histological CR and an acceptable adverse event profile. Assessment of several circumferential technique groups resulted in identification of a technique favored for further evaluation (Lugol’s-sizing-circumferential RFA 12 J/cm2-no cleaning), which demonstrated similar 3-month CR and significantly lower stricture incidence compared to other groups. Focal RFA technique was standardized for all treatment sessions and was well tolerated (no stricture), allowing focal eradication of residual USLs. Additional data focused on long-term outcomes and head-to-head comparison with other resection techniques is needed to further clarify the role of RFA in these patients.
Acknowledgments
Funding: Covidien, GI Solutions (formerly BARRX Medical) provided the study devices. The study was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Division of Cancer Epidemiology and Genetics.
Abbreviations
- RFA
radiofrequency ablation
- ESCN
esophageal squamous cell neoplasia
- ESCC
esophageal squamous cell carcinoma
- USL
unstained lesion
- LGIN
low-grade intraepithelial neoplasia
- MGIN
moderate-grade intraepithelial neoplasia
- HGIN
high-grade intraepithelial neoplasia
- CR
complete response
- W/cm2
Watts per square centimeter
- J/cm2
Joules per square centimeter
- EUS
endoscopic ultrasound
- ER
endoscopic resection
- CRF
case report form
- ESD
endoscopic submucosal dissection
- PPV
positive predictive value
- NPV
negative predictive value
- CL
cleaning of coagulum from treatment area
- L
Lugol’s iodine solution
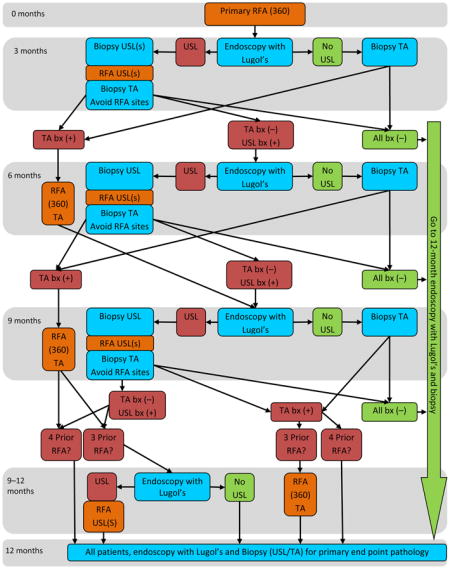
Appendix 1: Patient Flow
Patient flow. After enrollment, patients underwent primary circumferential radiofrequency ablation (RFA) of the treatment area (TA). Patients returned at 3-month intervals for endoscopy with Lugol’s chromoendoscopy to identify residual unstained lesions (USLs). The flow diagram depicts the decision tree at each visit based on endoscopic findings and subsequent histology results. Once a patient demonstrated a histological complete response defined as absence of moderate-grade intraepithelial neoplasia, high-grade intraepithelial neoplasia, and esophageal squamous cell carcinoma in all biopsy samples obtained at a follow-up visit, they were released to the 12-month primary endpoint endoscopy visit. bx, biopsy.
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Kamangar F, Chow WH, Abnet CC, et al. Environmental causes of esophageal cancer. Gastroenterol Clin North Am. 2009;38:27–57. vii. doi: 10.1016/j.gtc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin Y, Totsuka Y, He Y, et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013;23:233–42. doi: 10.2188/jea.JE20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu C, Kraft P, Zhai K, et al. Genome-wide association analyses of esophageal squamous cell carcinoma in Chinese identify multiple susceptibility loci and gene-environment interactions. Nature Genetics. 2012;44:1090–7. doi: 10.1038/ng.2411. [DOI] [PubMed] [Google Scholar]
- 5.Dawsey SM, Lewin KJ, Liu FS, et al. Esophageal morphology from Linxian, China. Squamous histologic findings in 754 patients. Cancer. 1994;73:2027–37. doi: 10.1002/1097-0142(19940415)73:8<2027::aid-cncr2820730803>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Dawsey SM, Lewin KJ, Wang GQ, et al. Squamous esophageal histology and subsequent risk of squamous cell carcinoma of the esophagus. A prospective follow-up study from Linxian, China. Cancer. 1994;74:1686–92. doi: 10.1002/1097-0142(19940915)74:6<1686::aid-cncr2820740608>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 7.Wang GQ, Abnet CC, Shen Q, et al. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54:187–92. doi: 10.1136/gut.2004.046631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin XF, Sun Qy, Chai TH, et al. Clinical value of multiband mucosectomy for the treatment of squamous intraepithelial neoplasia of the esophagus. J Gastroenterol Hepatol. 2013;28:650–5. doi: 10.1111/jgh.12111. [DOI] [PubMed] [Google Scholar]
- 9.Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett’s esophagus: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1245–1255. doi: 10.1016/j.cgh.2013.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277–88. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 11.Bergman JJ, Zhang YM, He S, et al. Outcomes from a prospective trial of endoscopic radiofrequency ablation of early squamous cell neoplasia of the esophagus. Gastrointest Endosc. 2011;74:1181–90. doi: 10.1016/j.gie.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Vilsteren FG, Alvarez Herrero L, Pouw RE, et al. Radiofrequency ablation for the endoscopic eradication of esophageal squamous high grade intraepithelial neoplasia and mucosal squamous cell carcinoma. Endoscopy. 2011;43:282–90. doi: 10.1055/s-0030-1256309. [DOI] [PubMed] [Google Scholar]
- 13.Becker V, Bajbouj M, Schmid RM, Meining A. Multimodal endoscopic therapy for multifocal intraepithelial neoplasia and superficial esophageal squamous cell carcinoma - a case series. Endoscopy. 2011;43:360–364. doi: 10.1055/s-0030-1256310. [DOI] [PubMed] [Google Scholar]
- 14.Ciocirlan M, Lapalus MG, Hervieu V, et al. Endoscopic mucosal resection for squamous premalignant and early malignant lesions of the esophagus. Endoscopy. 2007;39:24–9. doi: 10.1055/s-2006-945182. [DOI] [PubMed] [Google Scholar]
- 15.Pech O, May A, Gossner L, et al. Curative endoscopic therapy in patients with early esophageal squamous-cell carcinoma or high-grade intraepithelial neoplasia. Endoscopy. 2007;39:30–5. doi: 10.1055/s-2006-945040. [DOI] [PubMed] [Google Scholar]
- 16.Ishihara R, Iishi H, Uedo N, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc. 2008;68:1066–72. doi: 10.1016/j.gie.2008.03.1114. [DOI] [PubMed] [Google Scholar]
- 17.Fujishiro M, Yahagi N, Kakushima N, et al. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol. 2006;4:688–94. doi: 10.1016/j.cgh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Repici A, Hassan C, Carlino A, et al. Endoscopic submucosal dissection in patients with early esophageal squamous cell carcinoma: results from a prospective Western series. Gastrointest Endosc. 2010;71:715–21. doi: 10.1016/j.gie.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Wen J, Linghu E, Yang Y, et al. Relevant risk factors and prognostic impact of positive resection margins after endoscopic submucosal dissection of superficial esophageal squamous cell neoplasia. Surg Endosc. 2014;28:1653–9. doi: 10.1007/s00464-013-3366-9. [DOI] [PubMed] [Google Scholar]
- 20.Sgourakis G, Gockel I, Lang H. Endoscopic and surgical resection of T1a/T1b esophageal neoplasms: a systematic review. World J Gastroenterol. 2013;19:1424–37. doi: 10.3748/wjg.v19.i9.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seewald S, Ang TL, Omar S, et al. Endoscopic mucosal resection of early esophageal squamous cell cancer using the Duette mucosectomy kit. Endoscopy. 2006;38:1029–31. doi: 10.1055/s-2006-944527. [DOI] [PubMed] [Google Scholar]
- 22.Oyama T. Endoscopic treatment for esophageal squamous cell carcinoma. Recent Results Cancer Res. 2012;196:143–54. doi: 10.1007/978-3-642-31629-6_9. [DOI] [PubMed] [Google Scholar]
- 23.Teoh AY, Chiu PW, Ngo DK, et al. Outcomes of endoscopic submucosal dissection versus endoscopic mucosal resection in management of superficial squamous esophageal neoplasms outside Japan. J Clin Gastroenterol. 2010;44:e190–4. doi: 10.1097/MCG.0b013e3181ce52fb. [DOI] [PubMed] [Google Scholar]
- 24.Ishii N, Horiki N, Itoh T, et al. Endoscopic submucosal dissection with a combination of small-caliber-tip transparent hood and flex knife is a safe and effective treatment for superficial esophageal neoplasias. Surg Endosc. 2010;24:335–42. doi: 10.1007/s00464-009-0560-x. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto S, Kobayashi M, Takeuchi M, et al. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc. 2011;74:1389–93. doi: 10.1016/j.gie.2011.07.070. [DOI] [PubMed] [Google Scholar]
- 26.Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg. 2012;256:95–103. doi: 10.1097/SLA.0b013e3182590603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markar SR, Karthikesalingam A, Thrumurthy S, et al. Volume-outcome relationship in surgery for esophageal malignancy: systematic review and meta-analysis 2000–2011. J Gastrointest Surg. 2012;16:1055–63. doi: 10.1007/s11605-011-1731-3. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto M, Weber JM, Karl RC, et al. Minimally invasive surgery for esophageal cancer: review of the literature and institutional experience. Cancer Control. 2013;20:130–7. doi: 10.1177/107327481302000206. [DOI] [PubMed] [Google Scholar]
- 29.Perry KA, Funk LM, Muscarella P, et al. Perioperative outcomes of laparoscopic transhiatal esophagectomy with antegrade esophageal inversion for high-grade dysplasia and invasive esophageal cancer. Surgery. 2013;154:901–7. doi: 10.1016/j.surg.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Endo M, Kawano T. Detection and classification of early squamous cell esophageal cancer. Dis Esophagus. 1997;10:155–8. doi: 10.1093/dote/10.3.155. [DOI] [PubMed] [Google Scholar]
- 31.Noguchi H, Naomoto Y, Kondo H, et al. Evaluation of endoscopic mucosal resection for superficial esophageal carcinoma. Surg Laparosc Endosc Percutan Tech. 2000;10:343–50. [PubMed] [Google Scholar]
- 32.Akutsu Y, Uesato M, Shuto K, et al. The overall prevalence of metastasis in T1 esophageal squamous cell carcinoma: a retrospective analysis of 295 patients. Ann Surg. 2013;257:1032–8. doi: 10.1097/SLA.0b013e31827017fc. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu M, Ban S, Odze RD. Squamous dysplasia and other precursor lesions related to esophageal squamous cell carcinoma. Gastroenterol Clin North Am. 2007;36:797–811. doi: 10.1016/j.gtc.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Kume K. The local recurrence and the metachronous cancer after EMR for early esophageal cancer confined within the lamina propria mucosae. Hepatogastroenterology. 2009;56:699–702. [PubMed] [Google Scholar]
- 35.Nakagawa K, Koike T, Iijima K, et al. Comparison of the long-term outcomes of endoscopic resection for superficial squamous cell carcinoma and adenocarcinoma of the esophagus in Japan. Am J Gastroenterol. 2014;109:348–56. doi: 10.1038/ajg.2013.450. [DOI] [PubMed] [Google Scholar]
- 36.Dawsey SM, Fleischer DE, Wang GQ, et al. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer. 1998;83:220–31. [PubMed] [Google Scholar]
- 37.Fornari F, Wagner R. Update on endoscopic diagnosis, management and surveillance strategies of esophageal diseases. World J Gastrointest Endosc. 2012;4:117–22. doi: 10.4253/wjge.v4.i4.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi H, Arimura Y, Masao H, et al. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video) Gastrointest Endosc. 2010;72:255–64. doi: 10.1016/j.gie.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 39.Ono S, Fujishiro M, Goto O, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc. 2009;70:860–6. doi: 10.1016/j.gie.2009.04.044. [DOI] [PubMed] [Google Scholar]