SUMMARY
Understanding the organizational logic of neural circuits requires deciphering the biological basis of neuronal diversity and identity, but there is no consensus on defining a neuron type. We analyzed single cell transcriptomes of a set of anatomically and physiologically characterized cortical GABAergic neurons and conducted a computational genomic screen for transcriptional profiles that distinguish them. We discovered that cardinal GABAergic neuron types are delineated by a transcriptional architecture that encodes their synaptic communication patterns. This architecture comprises 6 categories of ~40 gene families including cell adhesion molecules, transmitter-modulator receptors, ion channels, signaling proteins, neuropeptides and vesicular release components, and transcription factors. Combinatorial expression of select members across families shapes a multi-layered molecular scaffold along cell membrane that may customize synaptic connectivity patterns and input-output signaling properties. This molecular genetic framework of neuronal identity integrates cell phenotypes along multiple axes and provides a foundation for discovering and classifying neuron types.
Graphical Abstract

INTRODUCTION
Since the discovery that individual neurons are basic building blocks of the nervous system (Cajal, 1892), the diversity and heterogeneity of nerve cells have remained a challenge for deciphering the organization of neural circuits (Armananzas and Ascoli, 2015). Recent technical advances in anatomical, physiological and functional studies increasingly reveal multi-modal and multi-dimensional variations of neuronal phenotypes (Huang and Zeng, 2013). A fundamental question is whether these variations are largely subjective to measurements and can only be managed by operational grouping, or whether multiple distinct and congruent cell features can be integrated to define discrete “cells types” that reflect biological reality and mechanisms. The problem of neuronal diversity is unlikely to be solved without solving the equally if not more fundamental problem of neuronal identity, the flip side of the cell type coin (Seung and Sumbul, 2014). However, the biological basis of neuronal identity is poorly understood and cell definition and classification schemes in the brain remain contentious (DeFelipe et al., 2013).
As individual neurons constitute basic units of gene regulation, a major determinant of each neuron’s phenotype and function likely lies in its transcription programs. Recent studies classify neurons using high-throughput single cell RNA sequencing (scRNAseq) (Tasic et al., 2016; Zeisel et al., 2015). A major challenge is to map transcriptome-based statistical clusters, which are prone to technical noise and methodological bias, to the biological ground truth - their anatomical and physiological properties that constrain and contribute to cell function in neural circuits. In the retina, where cell types are among the best understood in the mammalian nervous system, scRNAseq has identified distinct markers that correlate to known types and suggested novel types (Shekhar et al., 2016). In the cerebral cortex, where cell type definition is often ambiguous and controversial, scRNAseq analyses have parsed multiple “transcriptional types” (Tasic et al., 2016; Zeisel et al., 2015), but their correlations to bona-fide biological types jointly defined by anatomical and physiological features remain unclear. Thus although scRNAseq allows comprehensive and quantitative measurements of gene expression, a fundamental unresolved issue is how transcription profiles might contribute to the molecular genetic basis of neuron types. Discovering the transcriptional basis of neuronal identity is prerequisite to deciphering neuronal diversity and enumerating cell census. Furthermore, high-resolution transcriptional portraits that mechanistically explain and predict the multi-faceted yet functionally congruent cell phenotypes have yet to be achieved.
Here, we have explored the transcriptional architecture underlying the core identity of GABAergic neurons in the cerebral cortex. Unlike recent studies that classify neurons using unsupervised statistical clustering of transcriptomes from unbiased (Zeisel et al., 2015) or relatively broad populations (Tasic et al., 2016), we analyzed single cell transcriptomes of 6 genetically labeled and phenotypically well characterized GABAergic types or subpopulations to discover their core molecular features. Using these anatomically and physiologically defined subpopulations as an assay, we designed a computational genomics strategy to screen through the ~620 HGNC (Human Genome Nomenclature Committee) gene families for those whose differential expression reliably distinguish these subpopulations. Remarkably, approximately 40 gene families implicated in regulating synaptic communication best distinguish these subpopulations. These gene families constitute 6 functional categories that include cell adhesion molecules, neurotransmitter and modulator receptors, ion channels, regulatory components of membrane-proximal signaling pathways, neuropeptides and vesicular release components, and transcription factors. Combinatorial and coordinated expression of select family members across these categories shapes a molecular scaffold along the cell membrane that appears to customize the pattern and property of synaptic communication for each cell population. We further provide evidence that transcription factor profiles register the developmental history of GABAergic neurons and contribute to concerted gene expression patterns that shape cell phenotypes. These findings suggest that neuronal identity is encoded in a transcriptional architecture that orchestrates functionally congruent expression across multiple gene families to customize the patterns and properties of their input-output communication.
RESULTS
Single cell transcriptomes of phenotype characterized GABAergic cells
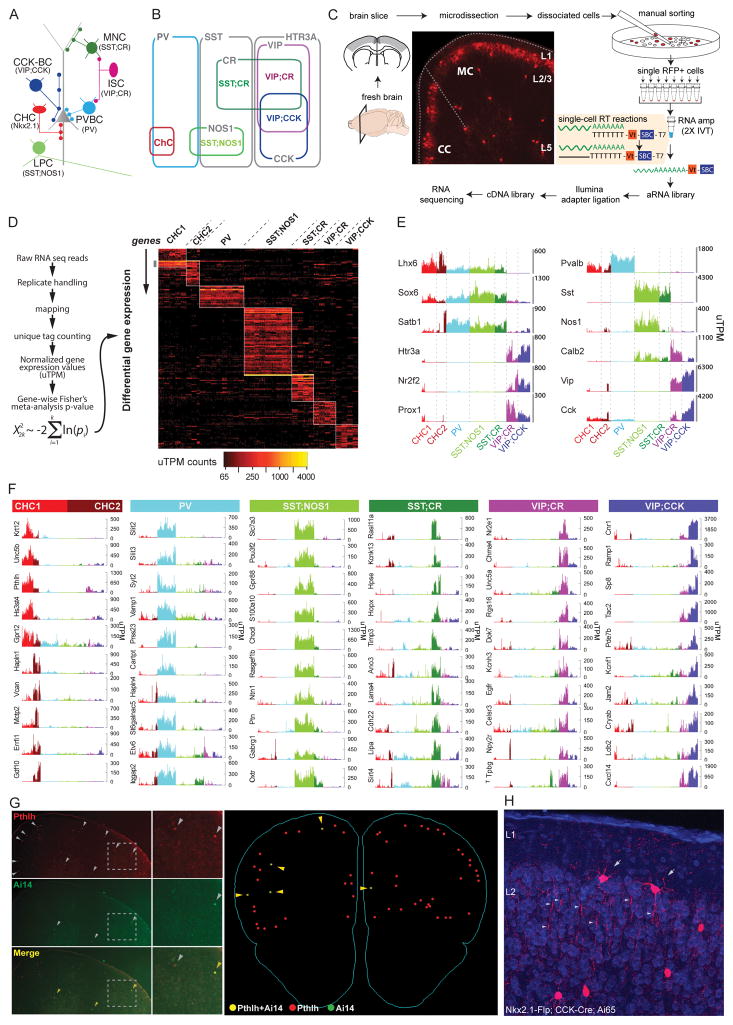
Our overall strategy is to compare high resolution transcription profiles of a set of well characterized GABAergic neurons defined by their anatomical, physiological and developmental attributes. We developed combinatorial recombinase driver lines to capture 6 GABAergic subpopulations (He et al., 2016) (Figure 1A–B; see Methods): 1) The Nkx2.1-CreER driver allows lineage and birth timing based targeting of chandelier cells (CHC) that target the axon initial segment (AIS) of pyramidal neurons (Taniguchi et al., 2013), 2) the PV-Cre driver labels fast-spiking basket cells (PVBC) that innervate the perisomatic region (Hu et al., 2014), 3) the SST-Flp;NOS1-CreER drivers target a unique type of long-projecting GABAergic cells (LPC) (Kilduff et al., 2011), 4) the SST-Flp;CR-Cre drivers include Martinotti cells (MNC) that target distal dendrites (Silberberg and Markram, 2007) and likely another cell type, 5) the VIP-Flp;CR-Cre drivers include interneuron-selective cells (ISC) (Staiger et al., 2004) and likely other types, 6) the VIP-Flp;CCK-Cre drivers include CCK basket cells (CCKC) (Armstrong and Soltesz, 2012) and likely other types. Accumulated anatomical, physiological, and molecular evidence indicate that these are non-overlapping subpopulations, and CHC, LPC and PVBC are considered cardinal types (He et al., 2016). We define these 6 populations as Phenotype Characterized Populations, or PCPs.
Figure 1. Transcriptomic analysis of GABAergic PCPs.
(A) Schematic of 6 PCPs with characteristic innervation patterns.
(B) Molecular markers parsing cortical GABA neurons into 3 non-overlapping populations and 6 PCPs.
(C) Experimental workflow.
(D) Bioinformatics pipeline (left) and DE genes across PCPs (right).
(E) Validation of known PCP markers; uTPM: unique transcripts per million.
(F) Novel PCP markers.
(G) Pthlh mRNA (grey arrowheads) co-localizes with RFP-labeled CHCs (yellow arrowheads) shown by FISH (left). Serial 3D reconstruction shows >95% RFP cells are Pthlh+ (right).
(H) Cck+ CHCs labeled in Nkx2.1-Flp;CCK-Cre;Ai65 cortex. Red: RFP; blue: DAPI; arrow: CHC; arrowhead: CHC axon boutons.
Using manual sorting of single RFP-labeled cells from microdissected motor and somatosensory cortex of 6-weeks old mice (Figure 1C), we obtained high-resolution transcriptome dataset of ~584 cells (CHC1=80, CHC2=52, PVBC=127, LPC=136, MNC=62, ISC=63 and CCKC=64 cells) from the 6 PCPs (Figure S1D; STAR Methods). Compared with previous methods (Shekhar et al., 2016; Tasic et al., 2016; Zeisel et al., 2015), our linear amplification with 10bp unique molecular identifiers (UMIs) achieved more comprehensive and quantitative transcriptome measurements (detecting ~9K genes/cell; Figure S1H; Star Methods); this facilitated more in-depth analysis of molecular profiles that contribute to the phenotypes and identity of PCPs.
Our analysis revealed 190 genes differentially expressed (DE) among PCPs with each gene expressed at >50uTPM (unique transcript per million), >4 folds enrichment and with p-value <5×10−4 (Figure 1D and Table S1). We confirmed known markers for MGE- and CGE- derived interneurons and the 6 PCPs (Figure 1E). We further profiled CHCs from upper (L2/3, CHC1) and deeper (L5/6, CHC2) layers and identified ~11 genes enriched in CHC2 over CHC1 (Figure 1F and Table S1). We validated PCP specific expression of ~10 transcripts by mRNA in-situ hybridization (Figure 1G and Figure S2A). We discovered a putative pan-CHC transcript Pthlh: ~95% of RFP-labeled CHCs were positive for Pthlh (136/143 cells) and their laminar distribution recapitulate CHC pattern in frontal cortex (Figure 1G, Figure S2A). To validate CCK expression in CHCs (Figure 1E), we demonstrate that an intersection of Nkx2.1-Flp and CCK-Cre labeled CHCs at L1, L2 border (Figure 1H).
A computation genomics screen identifies gene families and categories that distinguish PCPs
Cellular properties emerge from operations of macromolecular machineries comprising interacting components, each often implemented as one of multiple variants encoded by a gene family. Thus variations of cell properties often result from differential expression of select gene family members with characteristic biochemical and biophysical properties that confer customized features to cellular machines (Hartwell et al., 1999). We hypothesized that phenotypic differences among PCPs result from systematic differential transcription across multiple gene families. Our experimental design, whereby single cell transcriptomes derive from 6 PCPs, provided an effective assay to systematically screen for such gene ensembles that distinguish PCPs.
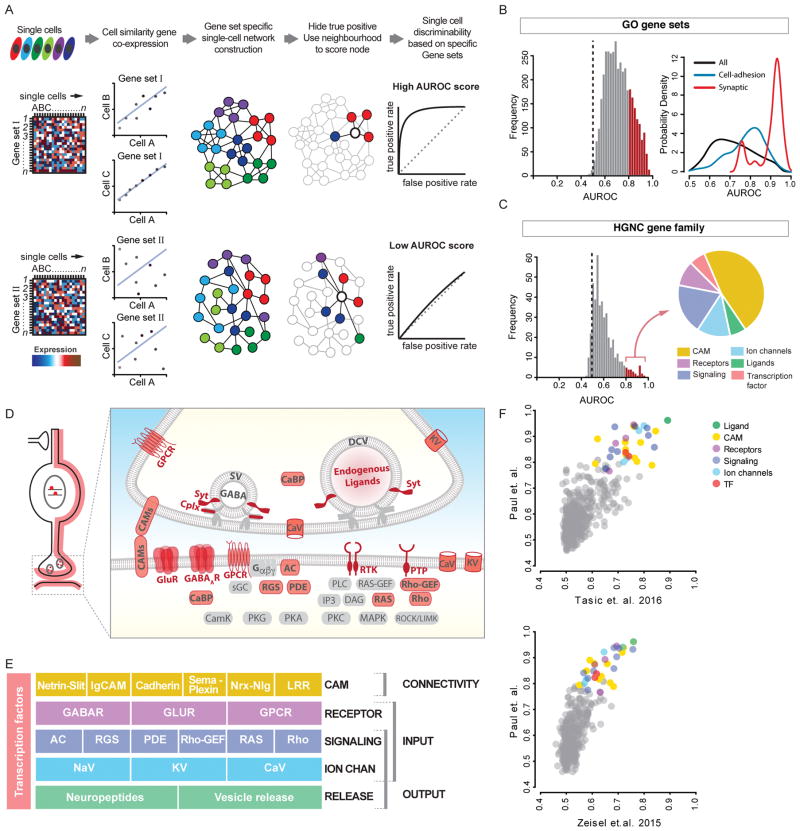
The essence of our computational screen, driven by a supervised, machine learning based algorithm - MetaNeighbour (Crow et al., 2017) is to detect whether a given set of genes shows correlated expression among cells of the same identity (Figure 2A). As our single cell transcriptomes derive from 6 PCPs, this data structure allowed characterizing the similarity between all cell pairs using co-variation of expression level in many known gene sets and measure whether a given set correctly links cells of known identity. In a network formalism, cells are linked as probabilistically related based on the similarity of their transcriptional profiles across a given set of genes (Figure 2A). This network classifies cells based on their proximity: closely linked neighbors are predicted to share an identity (see Methods). A subset of PCP labels is first applied to cells, giving a sub-network with known identities which can classify unlabeled cells. We then hold back the PCP identity of some cells and attempt to predict their identities using this sub-network of known identities. The efficacy of this test, reported as mean area under the receiver operator characteristic curve (AUROC), maps to the probability of a correct assignment when making a single binary choice (Figure 2A). Having constructed a computational assay for cell identity, we vary the transcriptomic features (e.g. gene families) used to characterize cells as neighbors. This computation screen thus selects gene ensemble features which jointly distinguish cell identities.
Figure 2. Gene families and categories that distinguish PCPs.
(A) MetaNeighbor schematic. scRNAseq values for gene sets are used to construct cell networks such that cells similar in gene expression space are close neighbors (connected by lines). PCP identity labels (colors) are then withheld and its identity inferred based on connectivity to immediate neighbors. The probability of being identified as the correct PCP is reported as AUROC score (0.5 is at chance).
(B) Left: AUROC value distribution of ~3800 GO terms. Red: AUROC>0.8. Right: GO-term probability density by keyword; “synaptic” and “cell-adhesion” are skewed with AUROC>0.8.
(C) AUROC distribution of 442 HGNC gene families. ~40 families (red bars) in 6 categories (pie chart) are highly predictive of PCP identities (AUROC≥0.8).
(D) Schematic showing that high-performance gene families (except TFs) encode proteins that primarily localize along cell and synaptic membrane.
(E) High-performance gene families constitute 5 layers of functional categories that organize synaptic connectivity and input-output signaling.
(F) MetaNeighbor analysis of two independent scRNAseq datasets yields similar rank order of gene families.
Also see Tables S2, S3, S4, S5 & S7; gene name abbreviations in Methods.
We first screened for gene ensembles according to GO terms, using randomized labels (AUROC~0.5) as controls. GO terms containing the keyword “synaptic” gave the highest AUROC score (0.91–0.98), suggesting that genes implicated in synaptic connectivity and function are most discriminating for PCPs (Figure 2B, Table S2). To identify more specific gene categories, we screened through all ~620 gene families annotated in the HGNC database (see Methods). We identified ~40 gene families (i.e. 7% of all gene families) with AUROC scores >0.75, generally regarded as a stringent threshold (Figure 2C, Table S3, S4). Strikingly, these gene families all fell into only 6 functional categories (Figure 2C–D): 1) cell adhesion molecules, 2) receptors for neurotransmitters and modulators, 3) voltage-gated ion channels, 4) regulatory signaling proteins, 5) neuropeptides and vesicle release machinery, 6) transcription factors. It is immediately evident from this list that except transcription factors (TFs), all gene categories encode proteins that localize along or close to cell and synaptic membrane (Figure 2D) and contribute to a singular aspect of neuronal biology - synaptic communication, which is implemented through synaptic connectivity and input-output signaling properties (Figure 2E).
To validate this discovery, we applied MetaNeighbor to two independent scRNAseq datasets (Tasic et al., 2016; Zeisel et al., 2015). Despite differences in experimental design and methods our meta-analysis yielded high replicability on PCP-distinguishing gene families (Figure 2F). Nearly identical gene families best discriminated equivalent cell populations (i.e. 6 PCPs) in the three datasets, and the AUROC values of these families (Figure 2C) were well correlated in pair wise comparisons even though the scores from the other two datasets were modestly lower (Figure 2F).
We then examined gene families that were not predictive of PCP identity. ~243 families with AUROC<0.6 (Table S5) were mostly implicated in generic cell functions ranging from DNA replication to energy conversion etc. This analysis also revealed gene families that a priori might be assumed to predict PCP identity but did not. These include 1) a substantial set of cytoplasmic signaling kinases (e.g. the entire mitogen-activated kinase cascade), phosolipases (30 members); 2) most cytoskeleton components (actins, myosin, dyneins, kinesins); 3) core components of vesicle release machinery (Table S5). These non-predictive gene families contrasted the top 40 predictive families in two major properties: they were all cytosolic instead of membrane or membrane proximal; and they often made up the generic scaffold of macromolecular complexes or pathways instead of the key regulatory components.
Differential expression of cell adhesion molecules and carbohydrate modifying enzymes suggests large capacity for cell surface and extracellular matrix labels
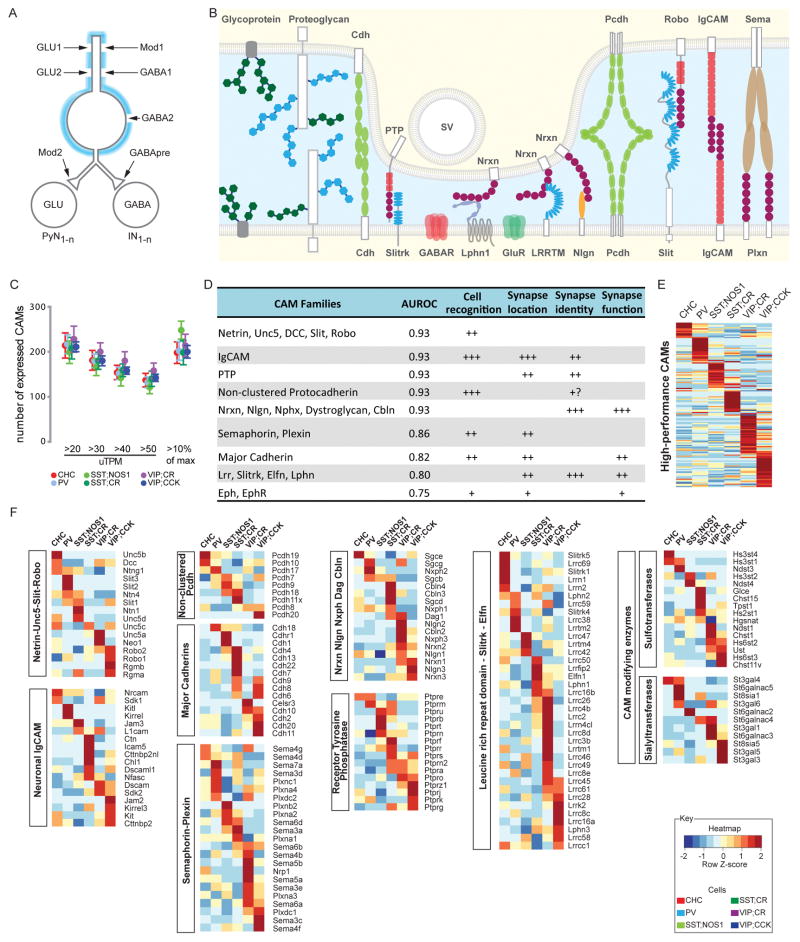
Each GABAergic neuron receives inputs from and extends output to diverse pre- and postsynaptic neurons, respectively (Figure 3A). The cell adhesion molecules (CAMs) that regulate their morphology, connectivity, synaptic transmission and plasticity are largely unknown. Our computational screen identified multiple CAM families that effectively discriminate PCPs (Figure 3B–E). Based on broadly annotated HGNC families and the literature (de Wit and Ghosh, 2016; Kolodkin and Tessier-Lavigne, 2011; Takahashi and Craig, 2013), we selected ~275 genes encoding all major neuronal CAMs and organized them into 12 adhesion groups according to sequence homology and receptor-ligand relationships (Figure 3B; Table S4). Nearly all major groups of neuronal CAMs implicated in different aspects of neural development are expressed in PCPs, and each PCP expresses ~200 CAM genes (Figure 3C). This was an underestimate of CAM diversity as our RNAseq method does not detect splicing variants. Among the ~275 CAM genes, 130 show highly distinct subpopulation profiles (Figure 3E, Table S6). Strikingly, multiple CAM families each manifests differential expression among PCPs (Figure 3F, Figure S3A–D). For example, UNC5 members and their ligand netrin1 are differentially expressed; UNC5b is highly specific to CHCs (Figure S2B, S3C); these receptor-ligand pairs might mediate cell-cell recognition (Figure S3A).
Figure 3. Differential expression of cell adhesion molecules and carbohydrate modifying enzymes among PCPs.
(A) A single GABAergic neuron receives multiple sources of glutamatergic, GABAergic and modulatory (Mod) inputs and innervates large sets of pyramidal neurons (PyN) and interneurons (IN). Blue shading: extracellular matrix.
(B) Multiple families of CAMs and glycoproteins provide extracellular coating, cell surface and synaptic labels.
(C) ~200 different CAM genes are expressed in each PCP estimated using sliding expression values or 10% of maximum expression value as thresholds.
(D) Major ligand-receptor cell adhesion systems and their roles in synaptic connectivity; all are highly discriminative of PCPs. “+” denotes the degree of involvement in the listed function.
(E) Differential expression (DE) of 136 CAM genes across 6 PCPs.
(F) DE of 8 cell adhesion systems and 2 carbohydrate modifying enzymes families among PCPs.
Also see Figure S3 and Table S6; gene name abbreviations in Methods.
Each of the major synaptic adhesion families (e.g. neurexin, neuroligin, protein tyrosine phosphatases, leucine rich repeat proteins, Slitrks) is differentially expressed among PCPs (Figure 3D–F, Figure S3E). Cell specific expression of LRRs might contribute to post- and trans-synaptic specializations that customize the property of synapse types defined by pre- and post-synaptic neuron identities. Together, cell and synaptic adhesion families likely constitute a comprehensive mosaic of multi-faceted cell surface code throughout the neuronal membrane.
We further discovered prominent differential expression in two families of carbohydrate modifying enzymes, sulfotransferases (AUROC=0.88) and sialyltransferases (AUROC=0.85), that may increase the molecular diversity of glycosylated CAMs and proteoglycans on cell membrane and in extracellular matrix (Figure 3F; Figure S3D). This suggests that each PCP might produce a characteristic cell coat through distinct carbohydrate modification patterns to diversify proteoglycans that facilitates or prevents cell interaction at a distance.
Differential expression of transmitter and modulator receptors shapes input properties of PCPs
Ionotropic glutamate receptors (iGluRs)
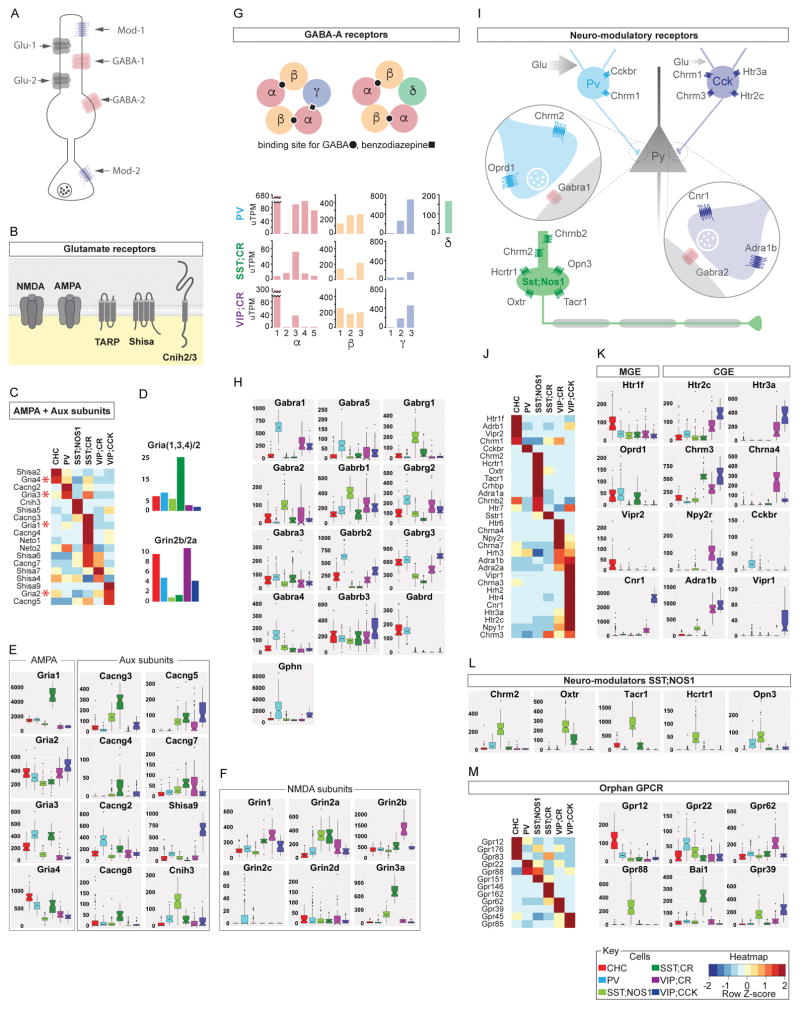
iGluRs play key roles in excitatory synaptic signaling and plasticity. Glutamatergic synapses in GABAergic interneurons often contain higher proportions of CP-AMPARs and GluN2B-NMDARs, although the ratio between the two types of AMPARs and NMDARs vary significantly among different cell populations (Akgul and McBain, 2016). Substantiating previous results from hippocampal interneurons (Akgul and McBain, 2016), we found that the mRNA levels and relative ratio of CP- vs CI-AMPAR subunits in PCPs vary in a highly cell type-dependent pattern (Figure 4A–D, Figure S4A). These results suggest cell type-dependent composition and correlation of AMPA and NMDA receptor pore-subunits, especially with regard to the relative abundance and ratio of CP- vs CI- AMPARs and 2B- vs 2A- NMDARs.
Figure 4. Differential expression of transmitter and modulatory receptors among PCPs.
(A) Schematic of transmitter and modulatory receptors on a generic GABAergic neuron.
(B) Schematic of glutamate receptor core subunits and auxiliary proteins that form native receptors.
(C) DE of AMPAR core subunits and auxiliary proteins across PCPs; SST;CR cells express the greatest diversity of AMPARs.
(D) Top: SST;CR cells show highest Gria1,3,4 (GluA1,A3A4)/Gria2 (GluA2) ratio among PCPs. Bottom: Most GABAergic neurons have more Grin2b (GluN2B) than Grin2a (GluN2A) receptors; the reverse is true in SST neurons.
(E) AMPAR core and auxiliary subunits shows striking differences among PCPs.
(F) DE of NMDAR subunits; glycine-activated Grin3a (GluN3A) is highly expressed in SST;CR cells.
(G) Top: Schematic of GABAAR and ligand binding sites. Bottom: DE of α, β and γ subunits within a PCP; PVBC and SST/CR cells have the most and least diversity, respectively.
(H) GABAAR subunit level differences among PCPs. PVBCs have the highest levels of α1, α4, α5 and also the inhibitory postsynaptic scaffolding protein Gphn (Gephrin).
(I) Schematic comparison of neuromodulatory receptors among PVBC, CCKC and LPCs.
(J) DE of neuromodulatory receptors among PCPs; LPCs and CCKC shows the highest diversity.
(K) CGE-derived interneurons express more neuromodulatory receptors types than MGE-derived interneurons.
(L) Select neuromodulatory receptors specific to or enriched in LPCs.
(M) DE of orphan GPCRs among PCPs shown as heatmap (left) and boxplots (right).
All boxplots y-axis in uTPM. Also see Figure S4.
In addition to pore-forming subunits, native AMPARs incorporate multiple auxiliary subunits that regulate AMPAR membrane trafficking, synaptic targeting, gating and signaling (Haering et al., 2014). TARP, SHISA and CNIH family auxiliary subunits show striking cell specific expression patterns (Figure 4C–E). TARPγ2 is enriched in PV cells, TARPγ3, γ8 and SHISA6 are enriched in SST/CR cells, TARPγ3 and SHISA9 are enriched in VIP/CCK cells. While PV cells predominantly express one auxiliary subunit (TARPγ2), SST/CR cells express at least 6 types (TARPγ2, γ3, γ8, γ5, γ7, SHISA6). Whereas pore-subunits differ in expression levels, auxiliary subunits often show ON/OFF expression among PCPs (Figure 4E). Thus different GABAergic neurons may assemble a specific set of native AMPARs with distinct pore and auxiliary subunit compositions, postsynaptic distribution patterns and biophysical properties. This large repertoire of native AMPARs may achieve cell type- and synapse- specific transmission and plasticity of glutamatergic inputs according to different presynaptic sources.
Ionotropic GABA receptors (GABAARs)
GABAA receptors mediate fast inhibitory neurotransmission and are assembled as heteropentameric chloride channels from 19 subunits, typically consisting of 2α, 2β, and 1γ subunits (Olsen and Sieghart, 2008) (Figure 4G–H). The vast majority of possible subunit combinations remain tentative as previous studies do not achieve cellular resolution of subunit co-expression. Whereas γ2 is regarded as the ubiquitous obligatory subunit of most if not all synaptic GABAaRs that mediate phasic inhibition, γ3 is sparsely expressed in cortical neurons of unknown identity and can assemble with α and β to form synaptic receptors with slowly decaying IPSCs (Kerti-Szigeti et al., 2014). We found that, surprisingly, γ3 is not only prevalent but also transcribed at much higher levels than γ2 subunits in all 6 PCPs (Figure 4H). This suggests that γ3 might contribute to the assembly of a class of slower decaying, longer duration synaptic GABAARs in GABAergic neurons. Different PCPs show specific subunit profiles and levels (Figure 4G, H). PVBCs express the largest variety (all except α2, α6, γ1) and overall highest levels of subunits, and uniquely high level of the GABAAR clustering/scaffolding protein gephyrin. In contrast, SST/CR cells express the least variety (mainly α3, β1/3, γ2/3) and lowest overall levels. SST/NOS1 cells are distinguished by predominant expression of slow kinetics α2-containing GABAARs and, surprisingly, the exceedingly rare γ1 subunit which is thought to assemble extra- or non- synaptic GABAaRs (Dixon et al., 2014). PVBCs and CHCs express the δ subunit, known to assemble extra-synaptic GABAaRs that possibly localize to presynaptic terminals.
These cell resolution profiles, when considered with the well-characterized connectivity patterns among PCPs, suggest that distinct GABAAR subtypes with specific subunit combinations are likely targeted to specific connections that match the presynaptic terminals to optimize inhibitory transmission properties (Figure S4B). Cell type specific subunit expression may assemble GABAAR subtypes with distinct biophysical and pharmacological properties, subcellular localization and thus might customize inhibitory transmission between cell types.
Neuromodulatory and G-protein coupled receptors
Cortical GABAergic neurons received a range of modulatory inputs that convey diverse signals of brain states through a large family of G-protein coupled receptors (GPCRs). We found that whereas MGE-derived PCPs are characterized by higher levels and larger variety of iGluRs and GABAARs, CGE-derived PCPs express much larger variety of neuromodulatory receptors (Figure 4I–L). For example, although PV and VIP/CCK cells both innervate the perisomatic regions of pyramidal neurons, PVBCs enrich for only a few modulatory receptors (e.g. CCK2R, Oprd1) whereas VIP/CCK cells express multiple GPCRs for serotonin, acetylcholine, norepinephrin, endocannabinoid, adrenaline, NPY and VIP (Figure 4J–L). Considered with their iGluR and GABAAR profiles, the results suggest that, similar to their hippocampal homologs (Armstrong and Soltesz, 2012), cortical PVBCs are recruited by fast and precise excitatory and inhibitory inputs from local cortical sources, whereas CCKBCs are profoundly modulated by subcortical inputs that represent internal drive and behavioral states (Table S7)
As an exception among MGE-derived neurons, the long projection SST/NOS1 cells show lower levels of iGluRs and extra- or non-synaptic γ1-containing GABAARs but express a large and unusual set of modulatory receptors including hypocretin, oxytocin, neurokinin, Tacr1 (Figure 4L), which are released from hypothalamic centers that regulate global brain states (Kilduff et al., 2011). These results depict a cell type with weak phasic excitatory and inhibitory inputs but a wide range of tonic subcortical modulatory inputs, consistent with its activation by homeostatic sleep drive and speculated role in regulating global cortical networks (Kilduff et al., 2011) (Table S7).
PCPs are further characterized by their expression of orphan GPCRs for unknown or unproven ligands. Each PCP can be distinguished from all other by specific expression of at least 2 orphan GPCRs (Figure 4M). In particular, the metabotropic Zn2+ sensor GPR39/mZnR is specifically expressed in VIP/CCK and to a less extent SST/NOS1 cells. Upon Zn2+ binding, GPR39 promotes KCC2 membrane trafficking, thereby enhancing GABAAR mediated hyperpolarization (Chorin et al., 2011). Thus GPR39 in VIP/CCK cell might mediate activity-dependent modulation of their excitability.
Differential expression of voltage-gated ion channels and electrophysiological properties of PCPs
The ion homeostasis and sophisticated intrinsic and synaptic physiology properties are shaped by several families of voltage-gated ion channels (VGICs), each contains diverse family members with characteristic biophysical properties (Yu and Catterall, 2004). We demonstrate extensive differential transcription profiles within and across multiple VGICs families among PCPs (Figure S4). Within the Nav and Cav family, major pore-forming subunits are broadly expressed among PCPs, often with different expression levels (Figure S4C–E). Cav auxiliary subunits (β1–2, α2, δ1–4) show more distinct, often binary pattern (Figure S4D), suggesting cell specific regulation of the trafficking, gating, and kinetics of pore forming subunits. Within the Kv family, different subsets are differentially enriched among PCPs (Figure S4C); there is often a tight correlation between expression of principle subunits (e.g. Kcna1/Kv1.1 and Kcna2/Kv1.2) and their matching auxiliary subunits (Kvβ1–3, Kcnab1, b2 & b3) in specific PCPs (e.g. PVBCs; Figure S4), implying cell specific assembly of functional channel complex. These results suggest that differential and correlated expression across multiple families of VGIC subunits may customize electrical signaling among PCPs (Figure S4C–G).
Differential expression of signaling proteins in calcium, cyclic nucleotide and small GTPase 2nd messenger pathways in PCPs
As a conserved cell signaling scheme, a large repertoire of surface receptors transduce diverse extracellular signals into a small set of intracellular 2nd messengers, which trigger enzyme cascades that regulate excitability, transmitter release, metabolism, neurite motility and gene expression (Alberts et al., 2014) (Figure 5A). Superimposed upon these conserved 2nd messenger cascades, different cell types deploy a large set of regulatory signaling proteins to control the spatiotemporal dynamics of each signal transduction pathway to achieve appropriate responses. We discovered that whereas most kinase cascades and signal proteins are broadly expressed, a small set of regulatory protein families in the calcium, cyclic nucleotide and small GTPase pathways are highly differential among PCPs and may tailor specific signal transduction properties (Figure 5A).
Figure 5. Differential expression of regulatory proteins in 2nd messenger pathways customizes intracellular signaling in PCPs.
(A) A schematic showing that Ca, cAMP, cGMP, Ras and Rho signaling pathways are differentially configured among PCPs. While the core skeletons of transduction machineries, kinase cascades and effectors are common among PCPs (grey, low AUROC scores), a small set of regulatory proteins (red) are differentially expressed with high AUROC values.
(B) A GPCR signaling module illustrating that while multiple components (grey) are common among PCPs, different members of key regulatory proteins such as RGS, AC, PDE and AKAPs are differentially expressed.
(C) Different combinations of RGS, AC and PDE members are enriched in individual PCPs.
(D) DE of several classes of signaling proteins with high AUROC scores.
(E) Predicted NO-cGMP signaling in SST;NOS1 and CHC cells. The entire pathway of NO synthesis and cGMP production (guanylyl cyclase), degradation (PDE), kinase signaling (PKG) and putative phosphorylation targets are coherently and expressed or enriched in SST;NOS1 and CHC cells.
(F) DE of key components of NO-cGMP signaling (depicted in 5E) among PCPs; note ON/OFF patterns or dramatic level differences.
All boxplots y-axis in uTPM. Also see Figure S5.
Ca2+ binding proteins
Each PCP expresses a set of ~5–8 different Ca2+-binding proteins (CaBPs; Figure 5D, S5E), many of which are in fact signaling proteins (e.g. Rasgrp1 in CHCs). This result suggests that differential expression of multiple Ca2+ binding and signaling proteins might shape the spatiotemporal dynamics and specificity of Ca2+signaling among PCPs.
Adenylyl cyclase, phosphodiesterase and cAMP signaling
In mediating GPCR signaling, the synthesis, degradation and spatiotemporal dynamics of cAMP are stringently regulated at each step (Halls and Cooper, 2011). We found that, while the G protein subunits themselves are broadly expressed, regulators of G protein signaling (RGS) family members manifest highly differential expression, often with binary-ON/OFF patterns among PCPs (AUROC=0.93; Figure 5A–C); this suggests that the turning-off of Gα subunit, a crucial step of G protein regulation, is implemented in a cell-type specific manner. Downstream to G proteins, 7 of the 9 adenylate cyclase (ACs) members with different catalytic and regulatory properties are differentially expressed (AUROC=0.85; Figure 5A–C). More strikingly, phosphodiesterases (PDEs), which mediate rapid cAMP degradation (Maurice et al., 2014), is among the top differentially expressed gene families (AUROC=0.94): 15 of the 22 members are differentially expressed, often with ON/OFF patterns (Figure 5A–C, Figure S5A). The specific and correlated transcription of AC, PDE and their functional effectors in PCPs suggest possible mechanisms that craft the spatiotemporal patterns of a ubiquitous 2nd messenger to direct cell and receptor (i.e. input) specific signal transduction, in part through subcellular targeted “signalosomes” containing particular members of synthetic and degradation enzymes with distinct catalytic and regulatory properties.
cGMP signaling modules in LPC and CHC
cGMP signaling in the brain is predominantly triggered by nitric oxide (NO). In mature cortex, the synthetic enzyme NOS1 is expressed at high levels in a small set of SST+ long projection cells (LPCs) and much lower levels in several other GABA populations (Kilduff et al., 2011). We found that, in addition to NOS1, the neuronal L-arginine transporter Slc7a3 that supplies the substrate for NO synthesis (Friebe and Koesling, 2003) is also specific to LPC (Figure 5E); this tight co-expression may endow LPCs as the major source of cortical NO. As the key link from NO to cGMP production, the soluble guanylyl cyclase (sGC) functions as a strict heterodimer of α and β subunits (Friebe and Koesling, 2003). While Gucy1α2 is expressed at low levels across PCPs, Gucy1α3 and Gucy1β3 are highly enriched in CHC, PVBC and LPC cells but are nearly absent in SST/CR and VIP cells (Figure 5E). This suggests that cGMP signaling is prominent in the former three cell types but weak in the latter three populations. Consistently, cGMP-degrading Pde1a, 5a, 11a are also highly enriched in LPCs and CHCs (Figure 5E), which may regulate the spatiotemporal dynamics of cGMP in these cells. Among the two types of cGMP-dependent PKGs, Prkg1 is found in all PCPs but with major enrichment in CHCs (Figure 5E). Furthermore, several PKG-regulated ion channels are also differentially enriched in these two cell types (Figure 5F, Figure S5B). The stunning coordination in the expression of multiple (8–9) genes encoding almost the entire NO-cGMP pathway in LPCs and CHCs, from ligand synthesis and 2nd messenger signaling to potential effectors, suggests orchestration by a gene regulatory network.
Ras and Rho small GTPases
Many cell surface receptors signal through a large set of Ras superfamily small GTPases to activate multiple kinase cascades that engage effectors, including transcription factors that regulate gene expression and cytoskeleton proteins that regulate cell shape, motility, adhesion and intracellular transport (Alberts et al., 2014). The mammalian genome contains ~30 Ras-GTPases and ~ 20 Rho-GTPases, each is regulated by several dozens of guanine nucleotide exchange factors (GEFs) and inactivated by GTPase activating proteins (GAPs) (Cherfils and Zeghouf, 2013). Within the Ras family, 21 of the 32 members showed major enrichment in specific PCPs (AUROC=0.84). As different Ras family members might be activated by different upstream signals, have different cellular functions, and engage different downstream effectors (Alberts et al., 2014), PCPs might use Ras members to relay distinct external inputs and trigger appropriate transcription programs and other effectors that mediate long term cellular changes. Furthermore, both the Rho-GTPases and Rho-GEFs are differentially expressed. 37 of the 57 Rho-GEFs (AUROC=0.82) and 14 of the 19 Rho-GTPases (AUROC=0.72) are enriched in specific PCPs (Figure 5D, S5C-D). As different Rho members are often activated by designated GEFs (Cook et al., 2014), differential expression of Rho signaling and regulatory components might provide the mechanism and capacity to maintain diversity of GABAergic neuron morphology, connectivity, and to support different forms of neurite and synaptic motility and plasticity.
Together, our results suggest that, among the vast number of intracellular signaling proteins constituting myriad pathways that transduce major categories of extracellular inputs, a small number of regulatory components encoded by just a few gene families are differentially expressed among PCPs and likely customize a handful of 2nd messenger pathways. Superimposed upon the core common signaling scheme, these regulatory components likely shape the specificity and spatiotemporal dynamics of broadly acting 2nd messengers to translate specific inputs to appropriate cellular responses.
Differential expression of neuropeptides and vesicle release machinery shape distinct outputs
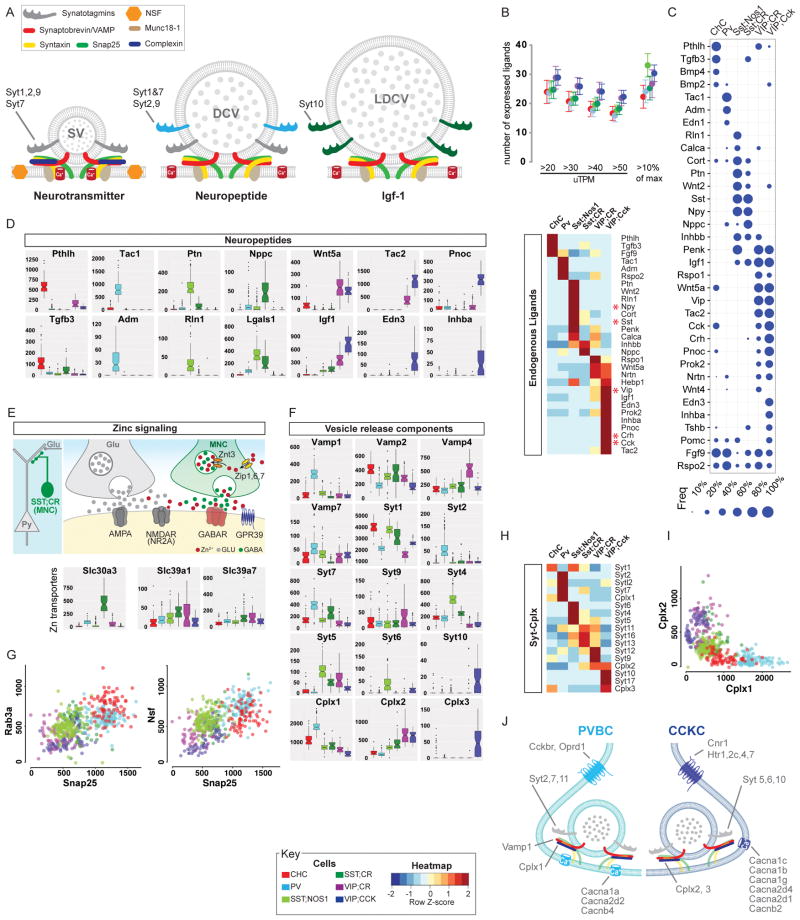
The single most important physiological action of a nerve cell is influencing the activity of its target cells through the release of appropriate neurochemicals in appropriate “styles”. We discovered a surprising diversity of neurochemical contents among PCPs and correlated differential expression of components of vesicular release machinery that may contribute to different release styles (Figure 6A).
Figure 6. Differential expression of neuropeptides and vesicle release machinery shape outputs and release styles in PCPs.
(A) Schematic of vesicular release machinery for synaptic vesicle (neurotransmitters), dense core vesicle (neuropeptides) and large dense core vesicle (protein/hormones) with putative Syt members.
(B) Top: Each PCP is estimated to express 20–30 peptides based on either a sliding threshold or dynamic threshold (10% of max expression value). Bottom: DE of endogenous ligands that constitute a neuropeptide code for PCPs.
(C) Fraction of individual cells expressing the most common neuropeptides among PCPs. Dot size represents fraction (see key).
(D) ON/OFF expression (uTPM) of specific neuropeptides/endogenous ligands, the gene family that best distinguishes PCPs (AUROC=0.96).
(E) Schematic showing that Zn may be co-released with GABA from SST;CR terminals. While GABA acts on GABAARs, Zn may act on nearby non-synaptic NMDARs and influence glutamatergic transmission. Boxplot: high level specific expression of the Zn vesicular transporter Slc30a3 in SST;CR cells, which also contain the Zn uptake importers Slc39a1 and Slc39a7.
(F) DE of vesicle release machinery components suggest different release styles in Ca2+ sensitivity and dynamics among PCPs.
(G) Scatter plots of mRNA levels (uTPMs) of Snap25 vs Rab3a (left) and Snap25 vs Nsf (right).
(H) Selective expression of Synaptotagmin and Complexin families in PCPs.
(I) Scatterplot of Cplx1 Vs Cplx2 levels shows that fast-release synapses of PV and CHC are biased towards Cplx1 whereas slow-release synapses of CCKCs mainly utilize Cplx2.
(J) Molecular correlates of fast-synchronous and slow-sustained vesicle release mechanisms in PVBC and CCKC with contrasting GABA release styles.
Also see Figure S6.
A neuropeptide code of PCPs
The release of different transmitters, peptides and hormones represent a fundamental distinction among neuron types as they produce categorically different outputs that elicit distinct physiological actions in target cells. We revealed a neuropeptide code of GABAergic neurons. Over 30 neuropeptides, hormones and secreted ligands are expressed in over 50% of single cells of the PCPs, and each expresses ~3–10 different endogenous ligands (Figure 6B, S6A); individual neurons express multiple peptide and protein ligands (Figure 6C). Importantly, differential expression of these ligands is the most discriminating gene family for PCPs (AUROC=0.96). Multiple PCPs are uniquely marked by individual ligands (Figure 6B–C; e.g. CHC: Pthlh, PV: Tac1, Adm, NOS1/SST: Ptn, Rln1, CR/SST: Nppc, VIP/CCK: Edn3, Pnoc). These results indicate that, beyond their morpho-physiological differences, PCPs are different neuroendocrine cells that produce distinct chemical outputs and elicit distinct physiological effects. Consistent with the demand for processing and packaging diverse neuropeptides, the granin gene family, which regulates pre-prohormone cleavage and biogenesis of DCVs, also shows differential expression (AUROC=0.81; Table S4). Further, based on the role Pleiotropin (PTN) in axon myelination and its exclusive expression in NOS1/SST cells, we discovered that the axons of these long projection GABAergic neurons are myelinated (Figure S6B–G).
Vesicular zinc transporter in SST/CR cells suggests co-release of GABA and zinc
The divalent cation zinc is enriched in cerebral hemisphere and acts as a potent modulator of neuronal signaling (Marger et al., 2014). The vesicular transporter ZnT3 in certain glutamatergic neurons enables zinc co-release with glutamate. Synaptic source of zinc modulates multiple ion channels (e.g. inhibiting extra-synaptic NMDA receptors) (Marger et al., 2014). Surprisingly, we discovered that ZnT3 (Slc30a3) is highly and specifically expressed in SST/CR cells (Figure 6E), along with Zip1 (Slc39a1) and Zip7a (Slc39a7) transporters that uptake extracellular zinc into the cytosol. These results suggest that SST/CR cells co-release zinc and GABA. As most SST/CR cells are Martinotti cells that target the distal dendrites and spines of pyramidal neurons with abundant NMDARs (Silberberg and Markram, 2007), their powerful dendritic inhibition might be mediated through two parallel mechanisms: synaptic activation of GABAARs by GABA and extra-synaptic inhibition of NMDARs by zinc.
Synaptotagmin members correlate with vesicular contents
The molecular components of vesicle fusion machinery are encoded by several multi-gene families (Sudhof, 2013), but it is not clear how different gene family members shape transmitter and neuropeptide release properties (Moghadam and Jackson, 2013). We revealed a comprehensive molecular profiles of vesicle release machinery in PCPs (Figure 6F–I). Several core components of the fusion complex and active zone are broadly expressed, including Syntaxins (AUROC=0.5), SNAP complex (AUROC=0.614), RIMs and RIM binding proteins (AUROC=0.57) (Table S4). Yet even among these core components, VAMP (synaptobrevins) and SNAP members are significantly enriched in specific PCPs (Figure 6F–H). The vesicular Ca2+ sensor synaptotagmins (Syt) show more distinct patterns: 14 of the 17 Syts are differentially expressed among PCPs (Figure 6G–I; AUROC=0.78); individual neurons express 6–9 Syts. In particular, VIP/CCK cells specifically express Syt10 that mediates the release of Igf1 (Cao et al., 2011), which is also highly enriched in the same cells (Figure 6F, D). These results suggest that each PCP might deploy a specific set of Syts with different sensitivity to spatiotemporal Ca2+ signals that trigger particular types of fusion reactions, thereby shaping the specificity and properties in parallel exocytosis pathways.
Molecular signatures of vesicular release styles
The release sites (e.g. synaptic vs non-synaptic), temporal characteristics (e.g. fast-synchronous vs slow-asynchronous) and short term dynamics (facilitating vs depressing) of GABA and neuropeptides produce distinct spatiotemporal patterns of receptor activation and postsynaptic cell firing that impact circuit computation (Armstrong and Soltesz, 2012; Markram et al., 2015). Our analyses begin to reveal molecular distinctions of fast-synchronous (in PVBCs) vs slow-sustained release (in CCKBCs) machinery, which manifests even at the level of core fusion complex. Vamp1, Snap25, Nsf and Rab3a are highly enriched in PV compared to VIP/CCK cells (Figure 6G–H); co-expression of all three components in PVBCs supports their fast release properties (Parpura and Mohideen, 2008). Among the Ca2+-binding Syts localized to SVs (Syt1, 2, 9), both PV and VIP/CCK cells (and other PCPs) express Syt1, but only PVBCs express Syt2, which exhibits the fastest onset and decline in release. PVBCs express highest level of Cplx1 but lowest level of Cplx2, while VIP/CCK celld show the opposite (Figure 6H–J). This Cplx profile is highly congruent with the Syt profile, as Cplx1 is implicated in fast and synchronous release and in clamping spontaneous release (Yang et al., 2013). These results suggest that fast-synchronous release is supported by high levels of VAMP1, SNAP25, NSF, SYT2, CPLX1, whereas slow-asynchronous release is shaped with low levels of these components and high levels of CPLX2. Together with co-expression of matching properties of Ca2+ channels and CaBPs, these results suggest that PCPs might transmit multiple neurochemicals in multiple release styles through differential and coordinated expression of gene families that customize the vesicle fusion machinery (Table S7).
In addition, Syt4, 5, 6 are highly or uniquely enriched in NOS1/SST long projection neurons, which express over 11 peptides (Figure 6B–D, F). It is possible that these uncharacterized Syts might mediate synaptic release of peptide-containing DVCs or their endocrine/paracrine release along the axon-dendritic membrane.
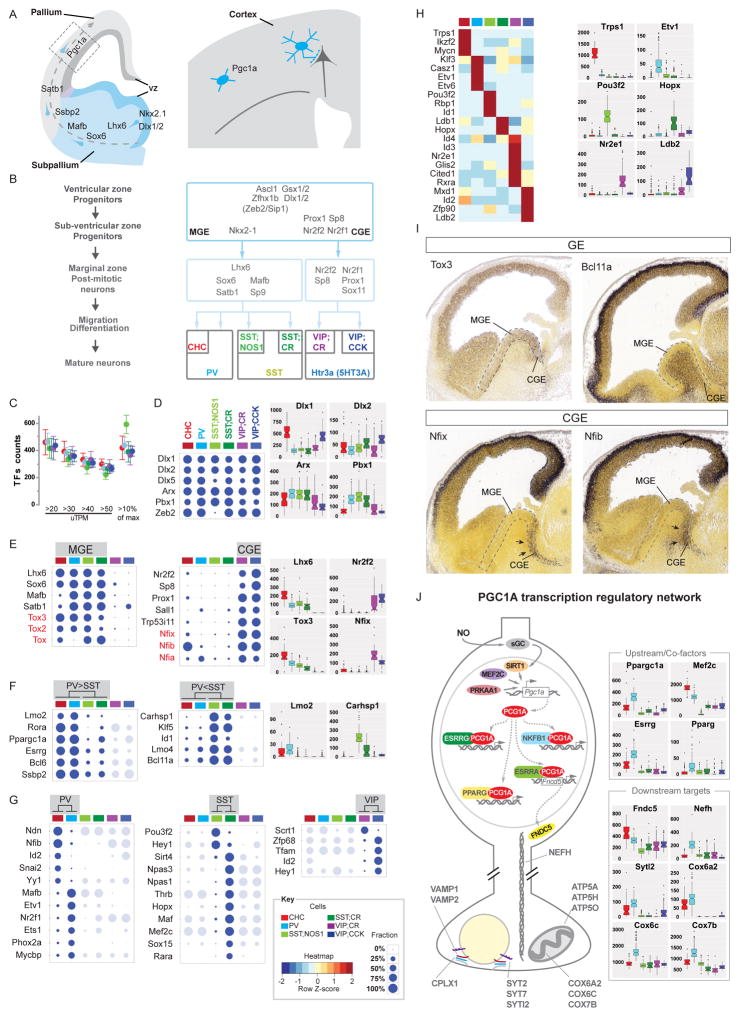
Transcription factors register the developmental history and contribute to the maintenance of PCP phenotypes
GABAergic neurons retain a transcription resume that registers their developmental history
Previous studies suggest that neuronal identities can be maintained by sustained expression of the same set of transcription factors that initiate terminal differentiation during development (Dalla Torre di Sanguinetto et al., 2008; Deneris and Hobert, 2014). In embryonic subpallium, transcriptional cascades orchestrate the specification and differentiation of major clades of GABAergic neurons (Kepecs and Fishell, 2014; Nord et al., 2015) (Figure 7A–B). We found that each PCP expresses ~350–400 TFs and over 300 TFs are expressed in an individual cell (Figure 7C). Among ~34 TF classes, basic-helix-loop-helix proteins, nuclear hormone receptors, POU-homeoboxes, and kruppel-like transcription factors are most differentially expressed among PCPs (Table S4, S3), and multiple TFs individually marks each PCP (Figure 7H, S7B).
Figure 7. Transcription factor profiles register the developmental history and contribute to maintenance of PCP phenotypes.
(A) Schematic developmental trajectory of cortical GABAergic neurons (PVBC as example), with TFs expressed at different stages.
(B) Schematic of MGE and CGE transcription cascades that regulate the development of different clades of GABAergic neurons, including PCPs
(C) Each PCP is estimated to express ~400 TFs.
(D–G) Fraction of cells expressing a given TF (10% of max level); boxplots show expression levels of selected TFs. (D) TFs in subpallium progenitors and GABA neuron precursors maintain their expression in PCPs in adult.
(E) TFs expressed in early postmitotic MGE- and CGE- derived neurons maintain expression within same clade of PCPs. Embryonic expression of Tox and Nfi family TFs were deduced from transcriptome analysis of PCPs and confirmed in (I).
(F) Among MGE-derived PCPs, subsets of TFs are preferentially expressed in PV (PV>SST) or SST (PV<SST) groups; CGE groups are not compared and shown in light shade; selected boxplots are shown.
(G) Within the PV, SST and VIP group, subsets of TFs are enriched in one or the other PCP; PCPs that are not compared are shown in light shade.
(H) DE of TFs is largely exclusive to each PCP (left); examples of ON/OFF expression in individual PCPs (right).
(I) Retrospective screen of Allen Developmental Mouse Brain in-situ database reveals that several TFs that express in MGE- or CGE- derived PCPs identified by transcriptome analysis indeed begin their expression in the corresponding embryonic germinal zone.
(J) Schematic of the Ppargc1α (PGC1α) transcription regulatory network highly enhanced in PVBCs. Multiple PGC1α upstream TFs activators, cofactors and large fraction of (>75%) of downstream effectors are enriched over 1.5 folds in PVBCs (p<5.0^−07). Boxplots show different expression levels of select sets of PGC1α co-factors and targets and putative targets among PCPs.
Also see also Figure S7.
As the 6 PCPs are embedded in 3 non-overlapping populations (PV, SST, VIP) derived from 2 separate developmental origins (MGE vs CGE) (Figure 7B), this data structure establishes a link between TF profiles in mature neurons to those in their embryonic precursors with cell type and single cell resolution. Almost all well-studied TFs in embryonic precursors maintain expression within the same clade of mature PCPs (Figure 7D, E): whereas Lhx6, Sox6, Mafb, Satb1 are expressed in PV and SST populations (the MGE clade), Coup-TF2, Sp8, Prox1, Npas1, Npas3; are expressed in VIP populations (the CGE clade). We further discovered additional TFs with similar patterns: whereas Tox family members (Tox, Tox2, Tox3) (Artegiani et al., 2015) are restricted to the MGE clade, Nfi family members (Nfia’, Nfib’, Nfix) (Piper et al.), Sall1 and Trp53i11 are restricted to the CGE clade. Importantly, by “reverse tracking” of their developmental history through screening the Allen Developmental Mouse Brain Atlas, we found that each of these TFs is indeed expressed in the embryonic MGE or CGE, consistent with their clade relationship (Figure 7I, Figure S7C).
Furthermore, by hierarchical and pair-wise comparison, we defined multiple sets of TFs that distinguish PV vs SST population (Figure 7F), and the PV, SST and VIP pairs of PCPs (Figure 7G). Again, we found evidence for developmental continuity of TF expression from embryonic precursors to mature neurons (Figure S7C). In particular, multiple PVBC-enriched TFs initiate expression at different stages of development, from Lhx6 and its downstream cascade (Figure 7A, D–E) to Ssbp2, Nfib and PGC1α (Figure 7E–G; (Batista-Brito et al., 2008), indicating that they maintain sequentially acquired transcriptional factors (Figure 7A).
These results suggest that PCPs register the developmental history of their transcription program, i.e. a “transcription resume”, through sustained expression of sequentially accumulated transcription factors. Conditional deletions of several of these TFs in adult cortex result in the loss of markers and physiological properties characteristic to their identity (e.g. (Close et al., 2012; Touzot et al., 2016), suggesting their requirement in mature neurons to maintain aspects of cell phenotypes and identity.
The PGC1a transcription program coordinates the release and metabolic properties of PV cells
The fast input-output transformation of PVBCs requires specialized energy metabolism and mitochondria features (Lucas et al., 2014). The transcription coactivator PGC1α cooperates with multiple transcription factors to regulate mitochondria biogenesis and energy metabolism (Lin et al., 2005). In the brain, it is largely restricted to cortical PVBCs and may directly regulate genes involved in mitochondria function and transmitter release (Lucas et al., 2014). Several PGC1a co-factors (e.g. Rora, Esrrg, Pparg) and all of its potential targets (PV, Syt2, Nefh, Cplx1, Atp50, Atp5a1) (Lucas et al., 2014) are substantially enriched in PVBCs (Figure 7J, S7D). We further found an extended set of PVBC enriched mRNAs associated with metabolic and mitochondria pathways (e.g. Fndc5, Cox6a2, Cox6c, Cox7b; Figure 7J). These results suggest that PGC1α might organize a transcription module that coordinates the release and metabolic properties in PVBCs.
Molecular portraits of GABAergic cell types
We have discovered highly correlated and congruent gene expression across multiple gene families and functional categories in each PCP. While coordinated expression within a functional category may shape a specific cell property, those across categories may jointly shape a set of congruent cell properties that together characterize cell phenotypes and identity (Table S7).
DISCUSSION
Transcriptional signatures of input-output communication define neuronal cell types
The biological basis and mechanistic framework of neuronal identity and diversity have been elusive. A communication-based approach to defining neuron types operationally by input/output relationships has been proposed (Lerner et al., 2016). Here, through single cell transcriptome analysis of phenotype-characterized GABAergic neurons, we have discovered that transcriptional architecture of input/output (I/O) synaptic communication may underlie neuronal identity. This overarching and mechanistic definition of neuronal identity integrates cell phenotypes along multiple axes and provides a foundation for understanding neuronal diversity and achieving biological (i.e. beyond operational) classification.
Although morphology is a common and intuitive description of neurons, it reflects and serves the more fundamental purpose of achieving proper connectivity. Thus morphological variability likely belies the co-variation of pre- and post-synaptic neurites that preserves connectivity patterns (Seung and Sumbul, 2014). Indeed, morphological types can be reliably identified from dense connectomes by computational algorithms (Jonas and Kording, 2015). Beyond anatomical connectivity, the physiological operation of a neuron type transforms information contents embedded in its synaptic inputs (e.g. transmitter and modulator types, strength, spatiotemporal dynamics) to appropriate outputs (Kepecs and Fishell, 2014), which are often characterized by cell intrinsic style of neurochemical release (e.g. vesicle contents and release speed, dynamics, plasticity). Although highly valuable, most electrophysiological measurements at cell soma regions, often in artificial conditions, provide a limited window into the elaborate subcellular biophysical, signaling and metabolic processes. Our comprehensive transcription overview of the synaptic, intrinsic and release machineries reveals strikingly coherent molecular ensemble properties congruent with well characterized physiological, biophysical and release properties of PCPs. They further predict multiple novel physiological features that can be experimentally verified. Thus transcriptional signatures of synaptic I/O machineries may begin to harmonize and extend the hitherto often limited, disparate and technically challenging electrophysiological measurements. Furthermore, neuron types defined by connectivity pattern and I/O styles may represent distinct structural and physiological motifs, with characteristic sets of dynamic properties that support and constrain their roles in circuit operations. Task-dependent recruitment of these motifs into brain networks may engage their systems level information processing and function. Finally, although transcription is influenced by cellular milieu including neural activity, core features of transcriptomes are outputs of cellular epigenomes customized primarily through developmental programming of the genome. Therefore, transcriptional signatures of synaptic I/O communication may integrate anatomical, physiological, functional and developmental genetic features that together define neuron types.
Computation genomics screen of gene ensembles that contribute to cell phenotypes
Previous studies often identify molecular markers of “transcriptomic types” that do not readily inform cell phenotypes. We instead focused on analyzing gene ensemble profiles (i.e. gene families) encoding proteins that constitute cellular modules (Hartwell et al., 1999) (i.e. macromolecular machines, signaling complexes), which more readily explain and predict cell phenotypes. Leveraging substantial phenotypic information of PCPs as an assay, MetaNeighbor allowed us to systematically screen all gene families and rank their ability to discriminate PCPs. This enabled us to discover a rather small set of functionally related gene families, which likely shape the I/O communication patterns of PCPs.
Transcription resume may reflect the developmental accumulation of gene regulatory programs that maintain cell phenotypes
Transcriptional control of “gene batteries” has been shown to coordinate effector gene expression and cellular properties (Hobert et al., 2010). Our study substantiates the role PCG1α transcription program in coordinating multiple physiological and metabolic properties in PVBCs (Figure 7J). Together with results of highly congruent expression of functional gene ensembles in other PCPs (e.g. NO-cGMP signaling pathway in LPCs), the cumulative evidence precludes piece meal, coincidental mechanisms of co-expression and suggests transcriptional programs that maintain cell phenotypes and identity.
We found that numerous developmental transcription programs initiated at successive stages of postmitotic differentiation are maintained in the same clade of mature neurons (Figure 7A–G). It is possible that a hierarchy of transcription programs progressively restricts cell fate and establishes their competence in responding to subsequently initiated programs that guide the migration, differentiation and maturation of GABAergic neurons. Our results further suggest that most developmental programs persist in mature neurons and constitute a transcriptional resume that may maintain cell phenotypes and identity.
CHCs and PVBCs are implicated in the pathophysiology of schizophrenia (Gonzalez-Burgos et al., 2015), and mental disorders likely result from altered neural connectivity rooted in deficient cell types. Key transcription profiles of increasing number of neuron types will facilitate identifying homologous cell types across species through conserved genetic features, linking altered gene expression to aberrant cellular and circuit properties, and discovering therapeutic targets to ameliorate circuit deficits.
STAR METHODS
Contact for reagent and resource sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact Dr. Z. Josh Huang (huangj@cshl.edu).
Experimental model and subject details
Animals
Nkx2.1-CreER, Pv-ires-Cre animals were bred separately to Ai14 reporter to label CHC and PVBC in the cortex respectively. CHCs were enriched in frontal cortex with tamoxifen induction at E17.5. Intersectional labeling of PCPs were achieved by breeding (a) Sst-Flp, Nos1-CreER for LPN, (b) Sst-Flp, CR-Cre, for MNC (c) VIP-Flp, CR-Cre for ISC and (d) VIP-Flp, CCK-Cre for CCKC separately to Ai65 intersectional reporter that will label cells with tdTomato only when both the lox-Stop-lox and Frt-STOP-Frt cassettes are excised (Figure S1). For validation studies mice were also bred to Nkx-ires-FlpO (He et. al. 2016) to get Nkx2.1-Flp; Cck-Cre; Ai65. Ptn-CreER animals were generated as described in figure S6B and were induced postnatal at P5. Mice were bred and maintained according to animal husbandry protocols at Cold Spring Harbor Laboratory (Institutional Animal Care and Use Committee reference number 16-13-09-8) with access to food and water ad libitum and 12 h light-dark cycle.
Method details
Phenotype characterized GABAergic subpopulations
Cortical GABAergic neurons can be parsed into several non-overlapping populations and, in a few cases, bona-fide types based on developmental origin, innervation targets, and molecular markers (Kepecs and Fishell, 2014). The embryonic medial and caudal ganglionic eminences (MGE and CGE) give rise to two broad groups, the former is divided into parvalbumin (PV) and somatostatin (SST) populations and the latter is marked by HTR3a (Kepecs and Fishell, 2014) (Figure 1A–B). The PV population includes fast-spiking basket cells (PVBC) that innervate the perisomatic region (Hu et al., 2014) and chandelier cells (CHC) that target the axon initial segment (AIS) (Taniguchi et al., 2013). The SST population includes Martinotti cells (MNC) that target distal dendrites (Silberberg and Markram, 2007), long projection cells (LPC) (Kilduff et al., 2011) and multiple other types. The HTR3a group includes the Vassoactive intestinal peptide (VIP) and Reelin populations, and the VIP population comprises interneuron-selective dis-inhibitory cells (ISC) (Staiger et al., 2004), Cholecystokinin (CCK) small basket cells (CCKC) (Armstrong and Soltesz, 2012) and likely additional types. Accumulated anatomical, physiological, and molecular evidence indicate that these are non-overlapping subpopulations, and CHC, LPC and PVBC are considered cardinal types (He et al., 2016).
RNA double in-situ and imaging
RNA double in-situ was performed using Quantigene ViewRNA tissue ISH (Affymetrix, USA) following manufacturer’s recommended protocol. Fresh unfixed brain tissues were frozen in OCT blocks using dry-ice isopentane slurry. Brains can be stored in −80C until cryosectioning. Cryosectioning was done on Leica cryotome at 12um thickness, and sections collected on charged glass slides. Custom and off-the shelf branched-DNA oligo ISH probes were designed and synthesized by Affymetrix Quantigene ViewRNA. Sections on slides were postfixed just prior to ISH, and in-situ steps were followed according to manufacturer’s recommended protocol. For dual signal detection QuantiGene Type-1 and Type-6 probes were used. Fluorescent signals from Type-1 and Type-6 ISH probes were imaged on tile-scanning mode using Perkin Elmer spinning disk confocal at 10× magnification and auto-stitched using Volocity software. Stitched images were exported as TIFFs for further processing and adjustments to brightness and contrast in FIJI (Fiji is just ImageJ) and assembled in Adobe Illustrator.
Super-resolution microscopy
Longitudinal brain sections from Sst-Flp;Nos1-Cre;Ai65 animals were perfused and sectioned at 75um thickness then immunolabeled with anti-Caspr (EMD Millipore #MABN69, 1:500 dilution) and anti-RFP (Rockland #600-401-379, 1:1000 dilution). Super resolution images were acquired with GE Healthcare OMX V3 structured illumination microscopy system using: 488 and 593 nm solid state lasers; UPlanS Apochromat 100×1.4 NA objective lens (Olympus); 2 EM-CCD cameras (Cascade II 512, Photometrics). 3D structured illumination images were reconstructed with SoftWoRx® 6.5.2 software. 3D rendering was performed using Imaris (Bitplane) 7.6.5. exported as TIFF, processed in FIJI and assembled in Adobe Illustrator.
Manual cell sorting
To isolate individual RFP-labeled GABAergic neurons, we microdissected motor and somatosensory cortical slices from fresh brain tissues of mature (6 weeks old) mice, generated single cell suspension and manually purified single RFP-labeled cells (Sugino et al., 2006). Brains were sectioned at 300 μm thickness using a cooled stage vibratome (Microm, Model HM360) with circulating oxygenated artificial cerebrospinal fluid. Sections were blocked in AP5, CNQX, and TTX cocktail to prevent excitotoxic cell death and then treated with mild protease (Fraction IV protease Streptomyces, Sigma Cat#P5147-5G). Brain regions of interest were microdissected and triturated to dissociate the cells. Dissociated cells were put into in a Petri dish in low density for optimal cell-cell separation then purified progressively by transferring RFP cells to fresh plates 3 times. Finally single RFP-positive cells was collected using patch pipette capillary and dispensed individually into separate single tubes prefilled with RNAseOUT (Invitrogen), ERCC spike-in RNAs in 1:400 K dilution, sample specific RT primers for a total of 1 μL volume. Process was repeated to collect 32–64 cells in one manual cell sorting session. Cells were flash frozen in liquid nitrogen and stored at −80 °C until processed. Patch pipette was single use only and fresh pipettes were used for every single cell collected. Manual sorting resulted in negligible contaminants as shown by glial and excitatory neuronal transcripts in Fig-S1I.
Linear RNA amplification
Single cell mRNAs were converted to cDNAs through polyA primers containing a sample barcode and unique molecular identifiers (UMIs). We employed two rounds of in vitro transcription amplification (Eberwine et. al. 1992) followed by Illumina TrueSeq protocol to construct RNAseq libraries (Hashimshony et al., 2012).
Custom T7-polyA primers (N10B1 → N10B16, Integrated DNA Technologies, USA) were designed containing 9bp error correcting sequences for identifying single cells (sample barcode) and 10bp random nucleotide sequences (UMI/varietal tag) to label each mRNA molecule amplified with a unique barcode. The UMI allows for elimination of reads containing duplicate tags for the same mapped sequence and only tally up the total unique tags of all mapped sequence to a coding sequence. This primer also contained a 26bp flanking RA5 adapter sequence needed for downstream Illumina cDNA library step, which eliminates a rate limiting enzymatic 5′ ligation step of cDNA preparation increasing efficiency (Hashimshony et. al. 2012).
RNA was linearly amplified by T7 RNA polymerase using two rounds of in-vitro transcription (MessageAmp-II kit Life Technologies) according to the manufacturer’s recommended protocol with some modifications. Cells were lysed by repeated heating to 70C and snap cooling to 4C and first strand synthesis was carried out at 42C for 2hrs with first strand buffer, dNTP mix, RNase inhibitor and ArrayScript enzyme. Second strand synthesis was done at 16C for 2hrs with second strand buffer, dNTP mix, T4 DNA polymerase and RNAseH. cDNA was purified using columns and first round IVT was performed at 37C for 14hrs to make aRNA. For the 2nd round of linear amplification column purified aRNA from first IVT underwent another first strand synthesis at 42C for 2hrs using second round primers, followed by RNaseH digestion at 37C for 30mins and another 2nd round second strand synthesis at 16C with a T7-RA5 primer. The resulting double stranded cDNA underwent a final second IVT step at 37C for 14hrs to make aRNA. These two rounds of linearly amplified aRNA products now carried the 3′-end of the polyA transcripts for mapping to coding regions plus the sample barcode to indicate which PCP it came from, UMI sequence for counting unique cell-endogenous parent mRNA molecules and one of the flanking sequence (RA5 adapter) for Illumina sequencing. Second round aRNAs were fragmented chemically using NEBNext® Magnesium RNA Fragmentation Module (Cat#E6150S), column purified using RNA MinElute (Qiagen) for final Illumina cDNA library preparation steps.
cDNA library generation and sequencing
cDNA library was generated using Illumina TruSeq small RNA kit (Cat#RS-200-0012) and only 3′-adapter (RA3) need to be ligated enzymatically using truncated T4 RNA ligase (NEB M0242) on to the fragmented aRNA and the 5′ ligation step for RA5-adapter was skipped (Hashimshony et. al. 2012). Adapter ligated fragmented aRNA was reverse transcribed using SuperScriptIII reverse transcriptase (Invitrogen, USA) and PCR enriched using TruSeq indices (for multiplexing) for no more than 7–11 cycles. The resulting library was size-selected using SPRISelect magnetic beads (Agencourt) to select 350–450bp fragments and paired-end sequenced for 101bp in Illumina HiSeq. No more than 32 single cells were run in one lane of HiSeq2000 generating on average ~180–200 million reads per lane.
Quantification and Statistical Analysis
Mapping and tag counting
As in our previous work (Crow et al, 2017), Bowtie (v 0.12.7) was used for sequence alignment of read2 (polyA primed) to the mouse reference genome (mm9), while read1 sequences were used for UMI (varietal-tag) counting. A custom python script was used Bowtie (v 0.12.7) was used for sequence alignment of read2 (polyA primed) to the mouse reference genome (mm9), while read1 sequences were used for UMI (varietal-tag) counting. Multiple reads to the same gene with the same tag sequences were rejected and only counted as one, such that only mapped sequences with unique tags were retained and tallied for each mRNA for each cell.
We obtained ~ 4.8×105 (median, Avg=6.9×105) mapped reads per cell, each containing ~1.0×105 (median, Avg=1.4×105) unique reads that typically detected on average ~10,000 genes (range ~7,500 to ~12,000 genes median), with >95% of the single cells detecting >6,000 genes (Figure S1B–C). In each single cell ERCC spike-in RNA (Life Technologies) were used as internal controls, for which the absolute number of molecules that are added to sample can be calculated; this gave a linear relationship of input-output measures with a slope of 0.92 and adjusted R2=0.96 (Figure S1D). Following quality control screen, we obtained high depth transcriptome of ~584 cells from the 6 PCPs (Figure S1D). This unique dataset thus contains high-resolution transcriptomes of phenotype-defined cortical GABAergic PCPs.
For any given gene the absolute unique counts were normalized to the total unique counts across all genes in a single cell and are expressed as unique Transcripts Per Million (uTPM). To determine differential gene expression (DE) and calculate fold-change, gene-wise Fisher’s meta-analytic p-value was calculated on these normalized gene expression values without further batch effect correction.
Compared with previous UMI-based ((Zeisel et al., 2015); detects 1.8–4.7K genes/cell) and non-UMI based method ((Tasic et al., 2016), detects ~7.2K genes/cell), our linear amplification with 10bp UMIs improved gene detection and quantification (~9K genes/cell; Figure S1H). Compared with Dropseq which allows vast throughput at lower resolution (Shekhar et al., 2016), our complementary approach achieved more comprehensive and quantitative transcriptome measurement of targeted cell populations.
Fisher’s meta-analytic DE
To assess differential gene expression across PCPs, we took advantage of the replicate batches within each type and performed a meta-analysis across replicates based on non-parametric statistics. Briefly, for each PCP we performed one-tailed Mann-Whitney tests between individual batches within a cell type against all cells outside of that cell type. To ensure that significance would arise from replication rather than extreme p-values, prior to meta-analysis with Fisher’s method, p-values at FDR<=0.05 for an individual test were set to the maximum p-value meeting that criterion. Finally, meta-analytic p-values were FDR corrected. Differentially expressed gene sets were defined by FDR adjusted p-value <0.05 having log2 fold change >2.
MetaNeighbor (AUROC calculation)
To measure PCP identity we use the MetaNeighbor method as described in our companion paper (Crow et. al. 2017). In brief, MetaNeighbor requires the input of a set of genes, an expression matrix and two sets of labels: one set for labeling each experiment, and one set for labeling the cell types of interest. We perform a stratified cross-validation which allows us to explicitly block technical sources of variation in single-cell analysis, in close parallel to our meta-analytic evaluation of single-cell data (Crow et. al. 2017). Here, each batch was treated as an “experiment”, and we aimed to measure the replicability of cell identity across batches. Cell-type labels are held back from one experiment at a time and then predicted based on the others, to determine which gene sets functionally characterize cells across technical variation. For each gene set being used to evaluate a given cell-type, the method generates a network based on the Spearman correlation between all cells across the genes within the set. The correlation is rank standardized to provide network weightings between each pair of cells, and then a neighbor voting predictor scores cells as possessing a given annotation. The score is calculated as the sum of a given cell’s connectivity weighting to neighbors possessing a given cell annotation. For cross-validation, we permute through all possible combinations of leave-one-batch-out cross-validation, and report the degree to which cells of the same type are recovered as the mean area under the receiver operator characteristic curve (AUROC) across all folds. To improve speed, AUROCs are calculated analytically:
where “Ranks” are the ranks of the hidden positives, Npos is the number of true positives, and NNeg is the number of true negatives.
Notched Boxplots
Notched boxplots shown in main figures 3–7 and supplement figures S1, S3–S5: box represent interquantile range (IQR) 50 percent of data, notch is 95% confidence interval of the median, horizontal line inside box is median, upper whisker is lesser of 75 percentile or maximum value, lower whisker is greater of 25 percentile or minimum value, dots show outliers. According to Graphical Methods for Data Analysis (Chambers, 1983) although not a formal test the, if two boxes’ notches do not overlap there is ‘strong evidence’ (95% confidence) their medians differ. Package “ggplot2” (https://cran.r-project.org/web/packages/ggplot2/ggplot2.pdf) was used to plot notched boxplots (Wickham, 2009)
Data and software availability
A Github repository containing R scripts and parsed data can be found online (see Key Resources Table). Raw data files, parsed data, and metadata have been uploaded to GEO (accession GSE92522).
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| IF: chicken anti-RFP | Rockland | Cat# 600-901-379 RRID:AB_10704808 |
| IF: rabbit anti-RFP | Rockland | Cat# 600-401-379 RRID:AB_2209751 |
| IF: anti-CASPR (Clone K65/35) | EMD Millipore | Cat# MABN69 RRID:AB_10806491 |
| IF: anti-Sst | Peninsula laboratories | Cat# T-4103.0050 RRID:AB_518614 |
| IF: anti-Nos1 | Thermo Fisher | Cat# 61-7000 RRID:AB_2533937 |
| Bacterial and Virus Strains | ||
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| ERCC RNA Spike-In Control Mixes | Thermo Fisher | Cat# 4456740 |
| SuperScript III | Thermo Fisher | Cat# 18080093 |
| RNaseOUT Recombinant Ribonuclease Inhibitor | Thermo Fisher | Cat# 10777019 |
| RNA fragmentation buffer | New England Biolabs | Cat# E6105S |
| RNA MinElute kit | Qiagen | Cat# 74204 |
| Antarctic phosphatase | New England Biolabs | Cat# M0289 |
| Poly nucleotide kinase | New England Biolabs | Cat# M0201 |
| T4 RNA ligase2, truncated | New England Biolabs | Cat# M0242 |
| Ampure XP beads | Beckman Coulter | Cat# A63880 |
| SPRIselect size selection beads | Thermo Fisher | Cat# B23317 |
| DL-AP5 | Tocris | Cat# 0105 |
| CNQX | Tocris | Cat# 1045 |
| TTX | Tocris | Cat# 1078 |
| Protease from Streptomyces griseus | Sigma-Aldrich | Cat# P5147 |
| Critical Commercial Assays | ||
| Message Amp II kit | Thermo Fisher | Cat# AM1751 |
| Illumina TrueSeq smallRNA kit | Illumina | Cat# RS-200-0012 |
| Bioanalyzer RNA Pico chip | Agilent | Cat# 5067-1513 |
| Bioanalyzer High Sensitvity DNA chip | Agilent | Cat# 5067-4626 |
| Panomics ViewRNA ISH Tissue 2-Plex Assay kit | Affymetrix | Cat# QVT0012 |
| Deposited Data | ||
| Raw scRNA-seq data | This paper | NCBI GEO accession number GSE92522 |
| Experimental Models: Cell Lines | ||
| Experimental Models: Organisms/Strains | ||
| Mouse: Nkx2-1 tm1.1(cre/ERT2)Zjh/J | Jackson laboratory | Stock No. 014552 |
| Mouse: Nkx2-1 tm2.1(flpo)Zjh/J | Jackson laboratory | Stock No: 028577 |
| Mouse: B6.129P2-Pvalb tm1(cre)Arbr/J | Jackson laboratory | Stock No: 017320 |
| Mouse: Sst tm3.1(flpo)Zjh/J | Jackson laboratory | Stock No: 028579 |
| Mouse: B6;129S-Nos1 tm1.1(cre/ERT2)Zjh/J | Jackson laboratory | Stock No: 014541 |
| Mouse: B6(Cg)-Calb2 tm2.1(cre/ERT2)Zjh/J | Jackson laboratory | Stock No: 013730 |
| Mouse: Vip tm2.1(flpo)Zjh/J | Jackson laboratory | Stock No: 028578 |
| Mouse: Cck tm1.1(cre)Zjh/J | Jackson laboratory | Stock No: 012706 |
| Mouse: Ptn-CreER | This paper | N/A |
| Oligonucleotides | ||
| N10B1: CGATTGAGGCCGGTAATACGACTCACTATAGGGGTTCAGAGTTCTACAGTCCGACGATCNNNNNNNNNNTGTAACCACTTTTTTTTTTTTTTTTTTTTTTTTTTTTTVN | This paper | N/A |
| N10B2: CGATTGAGGCCGGTAATACGACTCACTATAGGGGTTCAGAGTTCTACAGTCCGACGATCNNNNNNNNNNTAGGAGCACTTTTTTTTTTTTTTTTTTTTTTTTTTTTTVN | This paper | N/A |
| N10B3: CGATTGAGGCCGGTAATACGACTCACTATAGGGGTTCAGAGTTCTACAGTCCGACGATCNNNNNNNNNNGCACAGGACTTTTTTTTTTTTTTTTTTTTTTTTTTTTTVN | This paper | N/A |
| N10B4: CGATTGAGGCCGGTAATACGACTCACTATAGGGGTTCAGAGTTCTACAGTCCGACGATCNNNNNNNNNNACTCTGGACTTTTTTTTTTTTTTTTTTTTTTTTTTTTTVN | This paper | N/A |
| N10B5: CGATTGAGGCCGGTAATACGACTCACTATAGGGGTTCAGAGTTCTACAGTCCGACGATCNNNNNNNNNNCCTGACGACTTTTTTTTTTTTTTTTTTTTTTTTTTTTTVN | This paper | N/A |
| N10B6: CGATTGAGGCCGGTAATACGACTCACTATAGGGGTTCAGAGTTCTACAGTCCGACGATCNNNNNNNNNNGACGTGGACTTTTTTTTTTTTTTTTTTTTTTTTTTTTTVN | This paper | N/A |
| N10B7: CGATTGAGGCCGGTAATACGACTCACTATAGGGGTTCAGAGTTCTACAGTCCGACGATCNNNNNNNNNNCTTCTGCACTTTTTTTTTTTTTTTTTTTTTTTTTTTTTVN | This paper | N/A |
| N10B8: CGATTGAGGCCGGTAATACGACTCACTATAGGGGTTCAGAGTTCTACAGTCCGACGATCNNNNNNNNNNAAGATCGACTTTTTTTTTTTTTTTTTTTTTTTTTTTTTVN | This paper | N/A |
| N10B9: CGATTGAGGCCGGTAATACGACTCACTATAGGGGTTCAGAGTTCTACAGTCCGACGATCNNNNNNNNNNCGTGACCACTTTTTTTTTTTTTTTTTTTTTTTTTTTTTVN | This paper | N/A |
| N10B10: CGATTGAGGCCGGTAATACGACTCACTATAGGGGTTCAGAGTTCTACAGTCCGACGATCNNNNNNNNNNCTTATGGACTTTTTTTTTTTTTTTTTTTTTTTTTTTTTVN | This paper | N/A |
| N10B11: CGATTGAGGCCGGTAATACGACTCACTATAGGGGTTCAGAGTTCTACAGTCCGACGATCNNNNNNNNNNTGGTACCACTTTTTTTTTTTTTTTTTTTTTTTTTTTTTVN | This paper | N/A |
| N10B12: CGATTGAGGCCGGTAATACGACTCACTATAGGGGTTCAGAGTTCTACAGTCCGACGATCNNNNNNNNNNGTCTACGACTTTTTTTTTTTTTTTTTTTTTTTTTTTTTVN | This paper | N/A |
| N10B13: CGATTGAGGCCGGTAATACGACTCACTATAGGGGTTCAGAGTTCTACAGTCCGACGATCNNNNNNNNNNCCTAAGCACTTTTTTTTTTTTTTTTTTTTTTTTTTTTTVN | This paper | N/A |
| N10B14: CGATTGAGGCCGGTAATACGACTCACTATAGGGGTTCAGAGTTCTACAGTCCGACGATCNNNNNNNNNNCGCGTCGACTTTTTTTTTTTTTTTTTTTTTTTTTTTTTVN | This paper | N/A |
| N10B15: CGATTGAGGCCGGTAATACGACTCACTATAGGGGTTCAGAGTTCTACAGTCCGACGATCNNNNNNNNNNCAGTTGCACTTTTTTTTTTTTTTTTTTTTTTTTTTTTTVN | This paper | N/A |
| N10B16: CGATTGAGGCCGGTAATACGACTCACTATAGGGGTTCAGAGTTCTACAGTCCGACGATCNNNNNNNNNNAGCAAGCACTTTTTTTTTTTTTTTTTTTTTTTTTTTTTVN | This paper | N/A |
| T7-RA5: CGATTGAGGCCGGTAATACGACTCACTATAGGGGTTCAGAGTTCTACAGTCCGACGATC | This paper | N/A |
| Second round primer: NNNNNN | This paper | N/A |
| Recombinant DNA | ||
| Software and Algorithms | ||
| Bowtie2 | Langmead and Salzberg, 2012 | http://bowtie-bio.sourceforge.net/bowtie2/index.shtml |
| Samtools | Li et al., 2009 | http://samtools.sourceforge.net/ |
| Custom Python script to count tags | Crow et. al. 2016 | https://github.com/maggiecrow/scCoexp |
| MetaNeighbor in R | Crow et. al. 2017 https://doi.org/10.1101/150524 | https://github.com/maggiecrow/MetaNeighbor |
| R and RStudio | The R Foundation | https://www.r-project.org/; https://www.rstudio.com/ |
| Fiji | Schindelin, J.; Arganda-Carreras, I. & Frise, E. et al. (2012) | https://fiji.sc |
| Volocity Software | Perkin Elmer | http://cellularimaging.perkinelmer.com/downloads/ |
| SoftWoRx® 6.5.2 software | GE Healthcare | http://incelldownload.gehealthcare.com/bin/download_data/SoftWoRx/6.5.2/SoftWoRx.html |
| Imaris 7.6.5. | Bitplane | http://www.bitplane.com/releasenotes.aspx |
| Other | ||
| FISH ViewRNA probe: Cdh1 NM_009864.2, type1 | Affymetrix | Cat# VB1-15084 |
| FISH ViewRNA probe: Gpr88 NM_022427.2, type1 | Affymetrix | Cat# VB1-13597 |
| FISH ViewRNA probe: Hapln1 NM_013500, type6 | Affymetrix | Cat# VB6-13436 |
| FISH ViewRNA probe: Hhip NM_020259.4, type1 | Affymetrix | Cat# VB1-18918 |
| FISH ViewRNA probe: Htr7 NM_008315.2, type1 | Affymetrix | Cat# VB1-15758 |
| FISH ViewRNA probe: Pthlh NM_008970.3.4, type1 | Affymetrix | Cat# VB1-12560 |
| FISH ViewRNA probe: Ptn NM_008973.2, type1 | Affymetrix | Cat# VB1-18915 |
| FISH ViewRNA probe: Slc7a3 NM_007515.3, type1 | Affymetrix | Cat# VB1-18914 |
| FISH ViewRNA probe: Tacr1 NM_009313.5, type1 | Affymetrix | Cat# VB1-12918 |
| FISH ViewRNA probe: Unc5b NM_029770, type6 | Affymetrix | Cat# VB6-13432 |
| FISH ViewRNA probe: Td-Tomato, type6 | Affymetrix | Cat# VB6-13925 |
| FISH ViewRNA probe: Td-Tomato, type1 | Affymetrix | Cat# VF1-14985 |
Ethics
Mice were bred and maintained according to animal husbandry protocols at Cold Spring Harbor Laboratory (Institutional Animal Care and Use Committee reference number 16-13-09-8).
Supplementary Material
(A) Intersectional, lineage and birth time dependent labeling (top row) of 6 PCPs with characteristic laminar distribution (middle row). Representative single cell reconstruction depicted characteristic morphology in each PCP (bottom row).
(B) Histogram of total mapped reads per single cell with a median read depth of 5.7×105 reads per cell.
(C) Histogram of unique reads per single cell with a median of 5.0×105 counts.
(D) Plot of ERCC observed unique reads versus the numbers of molecules expected for each species from the ERCC cocktail shows linearity and slope close to 1; unity line shown as dotted grey line, blue shaded region shows 95% confidence interval.
(E) Number of genes detected in single cells versus total mapped reads
(F) Unique reads shows that gene detectability is correlated to read counts but there are no gene detection bias towards any of the PCPs (color code as in A).
(G) Genes detected across PCPs range between 7.5 – 12 thousands (median).
(H) Higher levels of genes detected in single cells compared to GSE60361 (Zeisel et al., 2015) and GE71585 (Tasic et al., 2016) dataset.
(I) Negligible glial transcripts observed; such as Gfap, Vim, Sox10 and excitatory neuronal mRNAs such as Tbr1, Emx1, Slc17a7, Slc17a6 and Slc17a8. In contrast, high expression of typical GABAergic observed transcripts such as Gad1, Gad2 and Slc32a1.
(A) Top left: representative sections from 3D render. 136/143 tdTomato+ (~95%) co-express Pthlh. Laminar distribution of Pthlh signal was similar to those reported for CHC distribution pattern (Taniguchi et. al. 2013). Right:Partial 3D reconstruction render from serial coronal sections (spanning 288um, rostro-caudal) of mouse forebrain showing double mRNA in situ of CHC enriched transcript Pthlh (red), Ai14/tdTomato (green) and co-localization (yellow). Only a subset of CHCs were labeled in Nkx2.1-CreER;Ai14 mice by tamoxifen induction at E17.
(B) Representative single images from double RNA in situ of Ai14/tdTomato (green) shows co-localization (yellow) with select transcripts enriched in CHC2 (Unc5b, Hapln1) SST;NOS1 cells (Ptn, Slc7a3, Tacr1, Gpr88, Hhip, Cdh1 and Hrt7). Dotted box represents area in higher magnification, arrowheads indicate co-localization.
(A) Approximately 20 immunoglobulin cell adhesion molecule are differentially expressed (also see Figure 3F) and may contribute to the cellular, subcellular and synaptic specificity of PCPs. Among them, CHL1 is particularly enriched in SST/CR population which includes dendrite-targeting Martinotti cells, consistent with its role in regulating subcellular synapse specificity; Kirrel, JAM2, 3, ICAM5 are each enriched in a specific subpopulation (also see Figure 3F).
(B) Each PCP enriches for a different set of 6–12 LRR proteins (also see Figure 3F). Among these, Elfn1 is prominent in SST/CR cells (mostly Martinotti cells), cortical homologue of hippocampal O-LM interneurons in which Elfn1 contributes to the synaptic facilitation of their glutamatergic inputs
(C) Schematic depiction of known attractive (+) and repulsive (-) interactions between the ligand Netrin (Ntn1) and its receptors - Unc5 family members and DCC. Boxplots show highly specific expression of Ntn1 and Unc5 family members among PCPs.
(D) Boxplots of CAM modifying enzymes Sulfo- and Sialyl-transferases enriched in individual PCPs. Six sulfotransferase are highly specific to different PCPs: Hs3st4 to ChC, Chst15 to SST/CR, Chst1 to VIP/CR, Hs3st5 to SST/NOS; Hs3st1 is enriched in ChC and PV cells and Hs6st3 is enriched in VIP/CCK cells.
(E) Representative in situ images from Allen Brain Atlas showing distinct laminar distribution patterns of some carbohydrate modifying enzymes in coronal brain sections. Many of these patterns are characteristic to the distribution of subsets of GABAergic neurons
(A) CGE-derived VIP cells have overall relatively low AMPARs and roughly similar GluA1 and GluA2 levels (GluA1:GluA2 = 1.4), and VIP/CR cells have relatively more NMDARs especially those containing GluN2B (GluN2B:GluN2A = 11.0). MGE-derived cells have much higher levels of GluA1 (average GluA1:GluA2 = 8.4), with striking cell type differences: GluA1:GluA2 ranges from 4.1 in SST/NOS1 cells to 20.4 in SST/CR cells. While PV cells have highest GluA3 levels and CHCs have highest GluA4 levels, SST/CR cells show highest levels of GluA1 and highest non-GluA2/GluA2 ratio (24.8). Interestingly, SST/CR cells also have relatively high GluN2A:GluN2B ratio and high level GluN3A for NMDARs among the PCPs.
(B) Distinct GABAAR subtypes with specific subunit combinations are likely targeted to specific connections that match the presynaptic terminals to optimize inhibitory transmission properties. PVBCs predominantly mediate self-inhibition (i.e. other PV cells) in addition to perisomatic inhibition of Py. They further receive inhibitory inputs from SST cells (mainly SST/CR positive Martinotti cells) and interneuron-selective VIP cells (mostly VIP/CR positive). PV-PV transmission is one of the fastest in the brain, mediated by the α1β2γ2 subtype. It thus can be inferred that γ3-containing slow kinetics receptors, likely abundant in PVBCs, are excluded from PV-PV synapses. The unique co-expression α4 and δ, a well-established combination for extrasynaptic and axonal GABAAR, suggest the presence of this subtype in PVBC terminals, likely activated by GABA spill-over during concerted GABA release from dense perisomatic synapses characteristic to PV axon terminals. These considerations further raise the possibility that other subunit combinations, such as those containing α3, α5, and γ3, might support inputs from SST/CR and VIP/CR cells, especially in the dendritic compartment of PVBCs. Following similar logic, SST/CR cells might receive VIP cell inputs through α3β1/3γ3 type GABAARs; VIP cells likely receive PV cell input through α1-containing GABAARs and Martinotti cell input through α3- containing GABAARs.
(C) An unrooted cladistic representation drawn using minimum evolution analysis of 143 members of structurally related voltage-gated ion channels based on their amino acid sequence of the minimal pore regions adopted without structural manipulations (modified from Yu and Catterall, 2004). Only three of these families, Kv, NaV and CaV are highly discriminative of PCPs as shown by their AUROC scores (boxed, red text shows high scoring families).
(D) Schematic of the subcellular distribution of voltage gated ion channels and their subunit compositions of pore forming and accessory regulatory proteins.
(E) Heatmap and selected boxplots showing differential expression of Kv channels and their regulatory subunits among PCPs. Fast-spiking PV basket cells express the highest levels and diversity of Kv channels.
(F) Heatmap and selected boxplots showing differential expression of CaV channels.
(G) Heatmap and selected boxplots showing differential expression of NaV channels. Fast-spiking PV basket cells express the highest levels and diversity of Nav channels. The relevance of ion channel transcription profiles to physiological properties is highlighted by the striking case of PVBCs. PVBCs convert an excitatory input to an inhibitory output within a millisecond, a stunning cell biology feat that appears to involve optimizing multiple aspects of electrical signaling across subcellular compartments, in part through specific expression and localization of a unique assortment of VGICs with highly tailored biophysical properties. The prominent enrichment of multiple Navs (Scn9a, Scn8a, Scn1a, Scn3a, Scn1b, Scn4b, Scn2b) likely underlie the “supercritical density” of Nav for ensuring fast signaling in PV cell axons, and the striking elevation of a large set of fast-kinetics Kv1–4 members may implement rapid repolarization and narrow AP duration at each subcellular domain. Expression of auxiliary subunits Kca, Kir and K2p further hints uncharacterized physiological properties.
(A) Selected RNA in-situ images from Allen Brain showing that multiple phosphodiesterases are expressed in restricted neuronal populations in adult mouse cortex with patterns characteristic to GABAergic interneurons.
(B) Barplots showing coordinated expression of multiple genes in SST/NOS1 cells encoding almost the entire NO-cGMP pathway. These include the substrate L-arginine transporter (Slc7a3), synthetic enzyme (NOS1), 2nd messenger synthesis (Gucy1α3 and Gucy1β3), degradation (Pde1a) and signaling (Prkg). Furthermore, two Prkg targets, members of the transient receptor potential channels (Trpc5) and BK-type potassium channels (α1 core subunit and β4 auxiliary subunits of Kcnma) are also differentially enriched.
(C–E) Boxplots for Ras (B), Rho-GEF (C), and EF-hand/CaBP (D) family members among PCPs.
(A) Barplot of neuropeptides and endogenous ligands expressed in ON/OFF pattern in individual cells of each PCP (colors).
(B) Left: Schematic role of PTN in promoting differentiation of oligodendrocyte precursors for axonal myelination. PTN expression in LPCs predicts myelination of their axons. Right: Confirmation of myelination of LPC axons. 3D rendering of a deep layer LPC axon (red) with paranodal CASPR expression (green).
(C) Genetic knock-in strategy to generate Ptn-CreER mouse line. Inset shows Southern blot of the targeted locus with band at expected ~6.5Kb; clone C4 was used for blastocyst injection. Clones were also confirmed by PCR of 3′ and 5′ arms (data not shown).
(D–E) As predicted from Fig-6D and FigS6A, Ptn labels LPCs. Coronal sections of Ptn-CreER;Ai14 brain upon sparse (low-dose) Tamoxifen induction at P14 labels Sst positive LPCs in L2/3 (C) and L6 (D). P14 induction in Ptn-CreER driver also labeled some pyramidal neurons (arrowheads); this is expected as Ptn is known to be expressed in pyramidal neurons (Fame et al., 2017). CC= corpus callosum, st= striatum.
(F) Layer 6 Ptn-CreER;Ai14 LPC is also immunopositive for Nos1; arrow shows long axon entering corpus callosum (CC) (He et. al. 2016).
(G) Left: 63× super-resolution image shows SST/NOS1 axons traversing deep cortical layers, among a field of myelin nodes labeled by CASPR immunostaining. Dotted box is enlarged in the right 3 panels, which show 3D rendering of higher magnification images and coaxially apposed CASPR signal over tdTomato-labeled axons of SST/NOS1 at three angular rotations (white arrows).
(A) Top: Venn diagram of all TFs expressed in each PCP; TFs were assigned to a set if it is expressed in ≥75% of cells within a PCP at a level of ≥30uTPM in single cells. Common TFs in MGE and CGE derived PCPs are further intersected to generate a list of common GABAergic TFs. Bottom: Table of TFs in each set, green fonts indicate TFs known to be expressed as computed from the Venn diagram.
A comparison between MGE vs CGE neurons revealed that ~65 TFs are common between the two populations with ~90 TFs enriched in MGE and ~110 TFs enriched in CGE populations. Among the 4 MGE-derived and 2 CGE-derived PCPs, each expresses between 150–220 TFs (Figure S7)
(B) Barplots of TFs expressed in ON/OFF pattern in individual cells of PCPs
(C) mRNA in situ Images from Allen Developmental Mouse Brain Atlas at E13.5 show expression of transcripts in the MGE that are expressed in PV<SST and SST/CR and SST/NOS1 from Fig-7F and G.
(D) Table showing that >75% of PGC1α targets identified by Lucas et. al. 2014 are also significantly (p<1×10^−5) enriched in PVC transcriptome data.
Table S1. Related to Figure1D. DE table of genes in Figure1D, FC>4, uTPM>50, q value<5.0 × 10−4, batches>80%
Table S2. Related to Figure 2B. AUROCs values for ~3888 GO gene sets
Table S3. Related to Figure 2C. AUROC scores of ~450 HGNC gene families from 3 studies (Paul et. al., Tasic et. al. 2016 and Zeisel. et. al. 2015)
Table S4. Related to Figures 2D, 2E and 3D. AUROC scores of custom gene sets used
Table S5. Related to Figure 3D and 3E. Low AUROC gene family list
Table S7. Related to Figure 2. Molecular portraits of GABAergic Phenotype Characterized Populations.
Acknowledgments
We thank M. Wigler for UMI, J. Kendall for coding, N. El-Amine for microscopy; L. Luo, C. McBain, A. Zador, L. Van Aelst, and G. Fishell for comments. Supported by: NIH 5R01MH094705-04, R01MH109665-01 and CSHL Robertson Neuroscience Fund to Z.J.H.; NARSAD Postdoctoral Fellowship to A.P; NIH 1R01MH113005 to J.G.; 1F32MH114501 to M.C.
Glossary of terms and abbreviations
- Neighbor voting
A method to classify cells into known types based on the similarity of the gene expression profiles for a given gene set.
- Cross-validation
A method to estimate how well the results of an analysis will generalize. The main purpose of cross-validation is to avoid overfitting to a particular dataset, or in this case, library batch. There are many ways to implement cross-validation; in this study we use a batch-stratified cross-validation. In this analysis, we hide labels from one batch at a time, making predictions as each label is hidden, until we have tested all of the batches within a particular cell type.
- Performance
The metric used to quantify cell identity. This refers to the area under the receiver operating characteristic curve (AUROC).
- PCP
Phenotype Characterized Population
- PV
Parvalbumin
- SST
Somatostatin
- CCK
Cholesytokinin
- VIP
Vasoactive Intestinal Peptide
- CR
Calretinin (Calbindin2)
- MGE
Medial Ganglionic Eminence
- CGE
Caudal Ganglionic Eminence
- PVBC
Parvalbumin positive basket cell
- CHC
Chandelier cell
- LPC
Long Projecting cell
- MNC
Martinotti cell
- ISC
Interneuron selective cell
- CCKC
CCK positive basket cell
- UMI
Unique molecule identifier
- DE
Differentially expressed
- AUROC
area under the receiver operator characteristic curve
- CAMs
cell adhesion molecules
- GPCRs
G-protein coupled receptors
- Tac1
Tachykinin Precursor 1 encodes four products of the tachykinin peptide hormone family, substance P and neurokinin A, as well as the related peptides, neuropeptide K and neuropeptide gamma. These hormones are thought to function as neurotransmitters which interact with nerve receptors and smooth muscle cells.
- Adm
Adrenomedullin is a preprohormone which is cleaved to form two biologically active peptides, adrenomedullin and proadrenomedullin N-terminal 20 peptide. Adrenomedullin is a 52 aa peptide with several functions, including vasodilation, regulation of hormone secretion, promotion of angiogenesis, and antimicrobial activity.
- Ptn
Plieotropin is a secreted growth factor that induces neurite outgrowth and myelination
- Rln1
Relaxin1; Relaxins are known endocrine and autocrine/paracrine hormones, belonging to the insulin gene superfamily.
- Nppc
Natriuretic Peptide C; hormone specifically binds and stimulates the cGMP production
- Pnoc
Prepronociceptin; a preproprotein that is proteolytically processed to generate nociceptin, nocistatin, and orphanin
- SV
synaptic vesicle
- DCV
Dense core vesicles
- Syt
synaptotagmins
Abbreviations
- GPCR
G-protein coupled receptor
- SV
synaptic vesicle
- DCV
dense core vesicle
- KV
voltage-gated K+ chan
- CaV
voltage-gated Ca+2 chan
- Syt
Synaptotagmins
- Cplx
Complexins
- CaBP
Ca+2 binding protein
- CAMs
cell adhesion molecules
- GluR
glutamate receptor
- GABAAR
GABA-A receptor
- Gα,β,γ
trimeric G-protein
- AC
Adenylate cyclase
- PTP
Protein tyrosine phosphatase, sGC, soluble Guanylate cyclase
- RGS
Regulator of G-protein signaling
- PDE
Phosphodiesterase
- PLC
Phospholipase C, RAS-GEF, Ras Guanine nucleotide exchange factor
- Rho-GEF
Rho Guanine nucleotide exchange factor
- IP3
Inositol triphosphate
- DAG
Diacyl glycerol
- RAS
Ras GTPase
- Rho
Rho GTPase
- CamK
Ca+2/Calmodulin dependent protein kinase
- PKG
Protein kinase G
- PKA
Protein kinase A
- MAPK
Mitogen activated protein kinase, ROCK/LIMK, Rho/LIM kinase
- Cdh
Cadherin
- PTP
protein tyrosine phosphatase
- Nrxn
Neurexin
- Pcdh
Protocadherin
- Robo
Roundabout
- IgCAM
immunoglobulin CAMs
- Sema
Semaphorin
- Slitrk
SLIT And NTRK Like Family Member
- GABAR
GABA receptor
- Lphn1
Laterophilin1
- LRRTM
Leucine Rich Repeat Transmembrane
- Nlgn
Neuroligin
- Slit
Slit Guidance Ligand
- Plxn
Plexin.
Footnotes
AUTHOR CONTRIBUTIONS
Z.J.H. and A.P. conceived the study. Z.J.H. organized the study. A.P. performed scRNAseq experiments. A.P. and R.R. performed mRNA in situ and histology experiments. A.P., Z.J.H., J.G., M.C. analyzed the data. M.C. and J.G. developed MetaNeighbour and performed DE, AUROC analysis. M.H. contributed to driver lines. Z.J.H. and A.P. wrote the manuscript with contributions from J.G. and M.C.
References
- Akgul G, McBain CJ. Diverse roles for ionotropic glutamate receptors on inhibitory interneurons in developing and adult brain. The Journal of physiology. 2016;594:5471–5490. doi: 10.1113/JP271764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, Walter P. Cell Signaling Molecular Biology of the Cell. 2014;Chapter 15 [Google Scholar]
- Armananzas R, Ascoli GA. Towards the automatic classification of neurons. Trends in neurosciences. 2015;38:307–318. doi: 10.1016/j.tins.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C, Soltesz I. Basket cell dichotomy in microcircuit function. The Journal of physiology. 2012;590:683–694. doi: 10.1113/jphysiol.2011.223669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artegiani B, de Jesus Domingues AM, Bragado Alonso S, Brandl E, Massalini S, Dahl A, Calegari F. Tox: a multifunctional transcription factor and novel regulator of mammalian corticogenesis. The EMBO journal. 2015;34:896–910. doi: 10.15252/embj.201490061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Machold R, Klein C, Fishell G. Gene expression in cortical interneuron precursors is prescient of their mature function. Cerebral cortex. 2008;18:2306–2317. doi: 10.1093/cercor/bhm258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal SRy. El nuevo concepto de la histologia de los centros nerviousos. Rev Cienc Med. 1892;18:457–476. [Google Scholar]
- Cao P, Maximov A, Sudhof TC. Activity-dependent IGF-1 exocytosis is controlled by the Ca(2+)-sensor synaptotagmin-10. Cell. 2011;145:300–311. doi: 10.1016/j.cell.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiological reviews. 2013;93:269–309. doi: 10.1152/physrev.00003.2012. [DOI] [PubMed] [Google Scholar]
- Chorin E, Vinograd O, Fleidervish I, Gilad D, Herrmann S, Sekler I, Aizenman E, Hershfinkel M. Upregulation of KCC2 activity by zinc-mediated neurotransmission via the mZnR/GPR39 receptor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:12916–12926. doi: 10.1523/JNEUROSCI.2205-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close J, Xu H, De Marco Garcia N, Batista-Brito R, Rossignol E, Rudy B, Fishell G. Satb1 is an activity-modulated transcription factor required for the terminal differentiation and connectivity of medial ganglionic eminence-derived cortical interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:17690–17705. doi: 10.1523/JNEUROSCI.3583-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DR, Rossman KL, Der CJ. Rho guanine nucleotide exchange factors: regulators of Rho GTPase activity in development and disease. Oncogene. 2014;33:4021–4035. doi: 10.1038/onc.2013.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow M, Paul A, Ballouz S, Huang ZJ, Gillis J. Quantifying neuronal identity through meta-analysis. 2017 Submitted. [Google Scholar]
- Dalla Torre di Sanguinetto SA, Dasen JS, Arber S. Transcriptional mechanisms controlling motor neuron diversity and connectivity. Current opinion in neurobiology. 2008;18:36–43. doi: 10.1016/j.conb.2008.04.002. [DOI] [PubMed] [Google Scholar]
- de Wit J, Ghosh A. Specification of synaptic connectivity by cell surface interactions. Nature reviews Neuroscience. 2016;17:22–35. doi: 10.1038/nrn.2015.3. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Lopez-Cruz PL, Benavides-Piccione R, Bielza C, Larranaga P, Anderson S, Burkhalter A, Cauli B, Fairen A, Feldmeyer D, et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nature reviews Neuroscience. 2013;14:202–216. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneris ES, Hobert O. Maintenance of postmitotic neuronal cell identity. Nature neuroscience. 2014;17:899–907. doi: 10.1038/nn.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon C, Sah P, Lynch JW, Keramidas A. GABAA receptor alpha and gamma subunits shape synaptic currents via different mechanisms. The Journal of biological chemistry. 2014;289:5399–5411. doi: 10.1074/jbc.M113.514695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberwine J, Yeh H, Miyashiro K, Cao Y, Nair S, Finnell R, Zettel M, Coleman P. Analysis of gene expression in single live neurons. Proc Natl Acad Sci USA. 1992;89:3010–3014. doi: 10.1073/pnas.89.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebe A, Koesling D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circulation research. 2003;93:96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biological psychiatry. 2015;77:1031–1040. doi: 10.1016/j.biopsych.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haering SC, Tapken D, Pahl S, Hollmann M. Auxiliary subunits: shepherding AMPA receptors to the plasma membrane. Membranes. 2014;4:469–490. doi: 10.3390/membranes4030469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halls ML, Cooper DM. Regulation by Ca2+-signaling pathways of adenylyl cyclases. Cold Spring Harbor perspectives in biology. 2011;3:a004143. doi: 10.1101/cshperspect.a004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell LH, Hopfield JJ, Leibler S, Murray AW. From molecular to modular cell biology. Nature. 1999;402:C47–52. doi: 10.1038/35011540. [DOI] [PubMed] [Google Scholar]
- Hashimshony T, Wagner F, Sher N, Yanai I. CEL-Seq: single-cell RNA-Seq by multiplexed linear amplification. Cell reports. 2012;2:666–673. doi: 10.1016/j.celrep.2012.08.003. [DOI] [PubMed] [Google Scholar]
- He M, Tucciarone J, Lee S, Nigro MJ, Kim Y, Levine JM, Kelly SM, Krugikov I, Wu P, Chen Y, et al. Strategies and Tools for Combinatorial Targeting of GABAergic Neurons in Mouse Cerebral Cortex. Neuron. 2016;91:1228–1243. doi: 10.1016/j.neuron.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O, Carrera I, Stefanakis N. The molecular and gene regulatory signature of a neuron. Trends in neurosciences. 2010;33:435–445. doi: 10.1016/j.tins.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Gan J, Jonas P. Interneurons. Fast-spiking, parvalbumin(+) GABAergic interneurons: from cellular design to microcircuit function. Science. 2014;345:1255263. doi: 10.1126/science.1255263. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Zeng H. Genetic approaches to neural circuits in the mouse. Annual review of neuroscience. 2013;36:183–215. doi: 10.1146/annurev-neuro-062012-170307. [DOI] [PubMed] [Google Scholar]
- Jonas E, Kording K. Automatic discovery of cell types and microcircuitry from neural connectomics. eLife. 2015;4:e04250. doi: 10.7554/eLife.04250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature. 2014;505:318–326. doi: 10.1038/nature12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerti-Szigeti K, Nusser Z, Eyre MD. Synaptic GABAA receptor clustering without the gamma2 subunit. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:10219–10233. doi: 10.1523/JNEUROSCI.1721-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilduff TS, Cauli B, Gerashchenko D. Activation of cortical interneurons during sleep: an anatomical link to homeostatic sleep regulation? Trends in neurosciences. 2011;34:10–19. doi: 10.1016/j.tins.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harbor perspectives in biology. 2011;3 doi: 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner TN, Ye L, Deisseroth K. Communication in Neural Circuits: Tools, Opportunities, and Challenges. Cell. 2016;164:1136–1150. doi: 10.1016/j.cell.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell metabolism. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Lucas EK, Dougherty SE, McMeekin LJ, Reid CS, Dobrunz LE, West AB, Hablitz JJ, Cowell RM. PGC-1alpha provides a transcriptional framework for synchronous neurotransmitter release from parvalbumin-positive interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:14375–14387. doi: 10.1523/JNEUROSCI.1222-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marger L, Schubert CR, Bertrand D. Zinc: an underappreciated modulatory factor of brain function. Biochemical pharmacology. 2014;91:426–435. doi: 10.1016/j.bcp.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Markram H, Muller E, Ramaswamy S, Reimann MW, Abdellah M, Sanchez CA, Ailamaki A, Alonso-Nanclares L, Antille N, Arsever S, et al. Reconstruction and Simulation of Neocortical Microcircuitry. Cell. 2015;163:456–492. doi: 10.1016/j.cell.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Maurice DH, Ke H, Ahmad F, Wang Y, Chung J, Manganiello VC. Advances in targeting cyclic nucleotide phosphodiesterases. Nature reviews Drug discovery. 2014;13:290–314. doi: 10.1038/nrd4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadam PK, Jackson MB. The functional significance of synaptotagmin diversity in neuroendocrine secretion. Frontiers in endocrinology. 2013;4:124. doi: 10.3389/fendo.2013.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord AS, Pattabiraman K, Visel A, Rubenstein JL. Genomic perspectives of transcriptional regulation in forebrain development. Neuron. 2015;85:27–47. doi: 10.1016/j.neuron.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacological reviews. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Mohideen U. Molecular form follows function: (un)snaring the SNAREs. Trends in neurosciences. 2008;31:435–443. doi: 10.1016/j.tins.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Piper M, Barry G, Harvey TJ, McLeay R, Smith AG, Harris L, Mason S, Stringer BW, Day BW, Wray NR, et al. NFIB-mediated repression of the epigenetic factor Ezh2 regulates cortical development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:2921–2930. doi: 10.1523/JNEUROSCI.2319-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seung HS, Sumbul U. Neuronal cell types and connectivity: lessons from the retina. Neuron. 2014;83:1262–1272. doi: 10.1016/j.neuron.2014.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar K, Lapan SW, Whitney IE, Tran NM, Macosko EZ, Kowalczyk M, Adiconis X, Levin JZ, Nemesh J, Goldman M, et al. Comprehensive Classification of Retinal Bipolar Neurons by Single-Cell Transcriptomics. Cell. 2016;166:1308–1323. e1330. doi: 10.1016/j.cell.2016.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron. 2007;53:735–746. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Staiger JF, Masanneck C, Schleicher A, Zuschratter W. Calbindin-containing interneurons are a target for VIP-immunoreactive synapses in rat primary somatosensory cortex. The Journal of comparative neurology. 2004;468:179–189. doi: 10.1002/cne.10953. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron. 2013;80:675–690. doi: 10.1016/j.neuron.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino K, Hempel CM, Miller MN, Hattox AM, Shapiro P, Wu C, Huang ZJ, Nelson SB. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nature neuroscience. 2006;9:99–107. doi: 10.1038/nn1618. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Craig AM. Protein tyrosine phosphatases PTPdelta, PTPsigma, and LAR: presynaptic hubs for synapse organization. Trends in neurosciences. 2013;36:522–534. doi: 10.1016/j.tins.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, Lu J, Huang ZJ. The spatial and temporal origin of chandelier cells in mouse neocortex. Science. 2013;339:70–74. doi: 10.1126/science.1227622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasic B, Menon V, Nguyen TN, Kim TK, Jarsky T, Yao Z, Levi B, Gray LT, Sorensen SA, Dolbeare T, et al. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nature neuroscience. 2016;19:335–346. doi: 10.1038/nn.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzot A, Ruiz-Reig N, Vitalis T, Studer M. Molecular control of two novel migratory paths for CGE-derived interneurons in the developing mouse brain. Development. 2016;143:1753–1765. doi: 10.1242/dev.131102. [DOI] [PubMed] [Google Scholar]
- Yang X, Cao P, Sudhof TC. Deconstructing complexin function in activating and clamping Ca2+-triggered exocytosis by comparing knockout and knockdown phenotypes. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20777–20782. doi: 10.1073/pnas.1321367110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Catterall WA. The VGL-chanome: a protein superfamily specialized for electrical signaling and ionic homeostasis. Science’s STKE : signal transduction knowledge environment. 2004;2004:re15. doi: 10.1126/stke.2532004re15. [DOI] [PubMed] [Google Scholar]
- Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, Marques S, Munguba H, He L, Betsholtz C, et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–1142. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Intersectional, lineage and birth time dependent labeling (top row) of 6 PCPs with characteristic laminar distribution (middle row). Representative single cell reconstruction depicted characteristic morphology in each PCP (bottom row).
(B) Histogram of total mapped reads per single cell with a median read depth of 5.7×105 reads per cell.
(C) Histogram of unique reads per single cell with a median of 5.0×105 counts.
(D) Plot of ERCC observed unique reads versus the numbers of molecules expected for each species from the ERCC cocktail shows linearity and slope close to 1; unity line shown as dotted grey line, blue shaded region shows 95% confidence interval.
(E) Number of genes detected in single cells versus total mapped reads
(F) Unique reads shows that gene detectability is correlated to read counts but there are no gene detection bias towards any of the PCPs (color code as in A).
(G) Genes detected across PCPs range between 7.5 – 12 thousands (median).
(H) Higher levels of genes detected in single cells compared to GSE60361 (Zeisel et al., 2015) and GE71585 (Tasic et al., 2016) dataset.
(I) Negligible glial transcripts observed; such as Gfap, Vim, Sox10 and excitatory neuronal mRNAs such as Tbr1, Emx1, Slc17a7, Slc17a6 and Slc17a8. In contrast, high expression of typical GABAergic observed transcripts such as Gad1, Gad2 and Slc32a1.
(A) Top left: representative sections from 3D render. 136/143 tdTomato+ (~95%) co-express Pthlh. Laminar distribution of Pthlh signal was similar to those reported for CHC distribution pattern (Taniguchi et. al. 2013). Right:Partial 3D reconstruction render from serial coronal sections (spanning 288um, rostro-caudal) of mouse forebrain showing double mRNA in situ of CHC enriched transcript Pthlh (red), Ai14/tdTomato (green) and co-localization (yellow). Only a subset of CHCs were labeled in Nkx2.1-CreER;Ai14 mice by tamoxifen induction at E17.
(B) Representative single images from double RNA in situ of Ai14/tdTomato (green) shows co-localization (yellow) with select transcripts enriched in CHC2 (Unc5b, Hapln1) SST;NOS1 cells (Ptn, Slc7a3, Tacr1, Gpr88, Hhip, Cdh1 and Hrt7). Dotted box represents area in higher magnification, arrowheads indicate co-localization.
(A) Approximately 20 immunoglobulin cell adhesion molecule are differentially expressed (also see Figure 3F) and may contribute to the cellular, subcellular and synaptic specificity of PCPs. Among them, CHL1 is particularly enriched in SST/CR population which includes dendrite-targeting Martinotti cells, consistent with its role in regulating subcellular synapse specificity; Kirrel, JAM2, 3, ICAM5 are each enriched in a specific subpopulation (also see Figure 3F).
(B) Each PCP enriches for a different set of 6–12 LRR proteins (also see Figure 3F). Among these, Elfn1 is prominent in SST/CR cells (mostly Martinotti cells), cortical homologue of hippocampal O-LM interneurons in which Elfn1 contributes to the synaptic facilitation of their glutamatergic inputs
(C) Schematic depiction of known attractive (+) and repulsive (-) interactions between the ligand Netrin (Ntn1) and its receptors - Unc5 family members and DCC. Boxplots show highly specific expression of Ntn1 and Unc5 family members among PCPs.
(D) Boxplots of CAM modifying enzymes Sulfo- and Sialyl-transferases enriched in individual PCPs. Six sulfotransferase are highly specific to different PCPs: Hs3st4 to ChC, Chst15 to SST/CR, Chst1 to VIP/CR, Hs3st5 to SST/NOS; Hs3st1 is enriched in ChC and PV cells and Hs6st3 is enriched in VIP/CCK cells.
(E) Representative in situ images from Allen Brain Atlas showing distinct laminar distribution patterns of some carbohydrate modifying enzymes in coronal brain sections. Many of these patterns are characteristic to the distribution of subsets of GABAergic neurons
(A) CGE-derived VIP cells have overall relatively low AMPARs and roughly similar GluA1 and GluA2 levels (GluA1:GluA2 = 1.4), and VIP/CR cells have relatively more NMDARs especially those containing GluN2B (GluN2B:GluN2A = 11.0). MGE-derived cells have much higher levels of GluA1 (average GluA1:GluA2 = 8.4), with striking cell type differences: GluA1:GluA2 ranges from 4.1 in SST/NOS1 cells to 20.4 in SST/CR cells. While PV cells have highest GluA3 levels and CHCs have highest GluA4 levels, SST/CR cells show highest levels of GluA1 and highest non-GluA2/GluA2 ratio (24.8). Interestingly, SST/CR cells also have relatively high GluN2A:GluN2B ratio and high level GluN3A for NMDARs among the PCPs.
(B) Distinct GABAAR subtypes with specific subunit combinations are likely targeted to specific connections that match the presynaptic terminals to optimize inhibitory transmission properties. PVBCs predominantly mediate self-inhibition (i.e. other PV cells) in addition to perisomatic inhibition of Py. They further receive inhibitory inputs from SST cells (mainly SST/CR positive Martinotti cells) and interneuron-selective VIP cells (mostly VIP/CR positive). PV-PV transmission is one of the fastest in the brain, mediated by the α1β2γ2 subtype. It thus can be inferred that γ3-containing slow kinetics receptors, likely abundant in PVBCs, are excluded from PV-PV synapses. The unique co-expression α4 and δ, a well-established combination for extrasynaptic and axonal GABAAR, suggest the presence of this subtype in PVBC terminals, likely activated by GABA spill-over during concerted GABA release from dense perisomatic synapses characteristic to PV axon terminals. These considerations further raise the possibility that other subunit combinations, such as those containing α3, α5, and γ3, might support inputs from SST/CR and VIP/CR cells, especially in the dendritic compartment of PVBCs. Following similar logic, SST/CR cells might receive VIP cell inputs through α3β1/3γ3 type GABAARs; VIP cells likely receive PV cell input through α1-containing GABAARs and Martinotti cell input through α3- containing GABAARs.
(C) An unrooted cladistic representation drawn using minimum evolution analysis of 143 members of structurally related voltage-gated ion channels based on their amino acid sequence of the minimal pore regions adopted without structural manipulations (modified from Yu and Catterall, 2004). Only three of these families, Kv, NaV and CaV are highly discriminative of PCPs as shown by their AUROC scores (boxed, red text shows high scoring families).
(D) Schematic of the subcellular distribution of voltage gated ion channels and their subunit compositions of pore forming and accessory regulatory proteins.
(E) Heatmap and selected boxplots showing differential expression of Kv channels and their regulatory subunits among PCPs. Fast-spiking PV basket cells express the highest levels and diversity of Kv channels.
(F) Heatmap and selected boxplots showing differential expression of CaV channels.
(G) Heatmap and selected boxplots showing differential expression of NaV channels. Fast-spiking PV basket cells express the highest levels and diversity of Nav channels. The relevance of ion channel transcription profiles to physiological properties is highlighted by the striking case of PVBCs. PVBCs convert an excitatory input to an inhibitory output within a millisecond, a stunning cell biology feat that appears to involve optimizing multiple aspects of electrical signaling across subcellular compartments, in part through specific expression and localization of a unique assortment of VGICs with highly tailored biophysical properties. The prominent enrichment of multiple Navs (Scn9a, Scn8a, Scn1a, Scn3a, Scn1b, Scn4b, Scn2b) likely underlie the “supercritical density” of Nav for ensuring fast signaling in PV cell axons, and the striking elevation of a large set of fast-kinetics Kv1–4 members may implement rapid repolarization and narrow AP duration at each subcellular domain. Expression of auxiliary subunits Kca, Kir and K2p further hints uncharacterized physiological properties.
(A) Selected RNA in-situ images from Allen Brain showing that multiple phosphodiesterases are expressed in restricted neuronal populations in adult mouse cortex with patterns characteristic to GABAergic interneurons.
(B) Barplots showing coordinated expression of multiple genes in SST/NOS1 cells encoding almost the entire NO-cGMP pathway. These include the substrate L-arginine transporter (Slc7a3), synthetic enzyme (NOS1), 2nd messenger synthesis (Gucy1α3 and Gucy1β3), degradation (Pde1a) and signaling (Prkg). Furthermore, two Prkg targets, members of the transient receptor potential channels (Trpc5) and BK-type potassium channels (α1 core subunit and β4 auxiliary subunits of Kcnma) are also differentially enriched.
(C–E) Boxplots for Ras (B), Rho-GEF (C), and EF-hand/CaBP (D) family members among PCPs.
(A) Barplot of neuropeptides and endogenous ligands expressed in ON/OFF pattern in individual cells of each PCP (colors).
(B) Left: Schematic role of PTN in promoting differentiation of oligodendrocyte precursors for axonal myelination. PTN expression in LPCs predicts myelination of their axons. Right: Confirmation of myelination of LPC axons. 3D rendering of a deep layer LPC axon (red) with paranodal CASPR expression (green).
(C) Genetic knock-in strategy to generate Ptn-CreER mouse line. Inset shows Southern blot of the targeted locus with band at expected ~6.5Kb; clone C4 was used for blastocyst injection. Clones were also confirmed by PCR of 3′ and 5′ arms (data not shown).
(D–E) As predicted from Fig-6D and FigS6A, Ptn labels LPCs. Coronal sections of Ptn-CreER;Ai14 brain upon sparse (low-dose) Tamoxifen induction at P14 labels Sst positive LPCs in L2/3 (C) and L6 (D). P14 induction in Ptn-CreER driver also labeled some pyramidal neurons (arrowheads); this is expected as Ptn is known to be expressed in pyramidal neurons (Fame et al., 2017). CC= corpus callosum, st= striatum.
(F) Layer 6 Ptn-CreER;Ai14 LPC is also immunopositive for Nos1; arrow shows long axon entering corpus callosum (CC) (He et. al. 2016).
(G) Left: 63× super-resolution image shows SST/NOS1 axons traversing deep cortical layers, among a field of myelin nodes labeled by CASPR immunostaining. Dotted box is enlarged in the right 3 panels, which show 3D rendering of higher magnification images and coaxially apposed CASPR signal over tdTomato-labeled axons of SST/NOS1 at three angular rotations (white arrows).
(A) Top: Venn diagram of all TFs expressed in each PCP; TFs were assigned to a set if it is expressed in ≥75% of cells within a PCP at a level of ≥30uTPM in single cells. Common TFs in MGE and CGE derived PCPs are further intersected to generate a list of common GABAergic TFs. Bottom: Table of TFs in each set, green fonts indicate TFs known to be expressed as computed from the Venn diagram.
A comparison between MGE vs CGE neurons revealed that ~65 TFs are common between the two populations with ~90 TFs enriched in MGE and ~110 TFs enriched in CGE populations. Among the 4 MGE-derived and 2 CGE-derived PCPs, each expresses between 150–220 TFs (Figure S7)
(B) Barplots of TFs expressed in ON/OFF pattern in individual cells of PCPs
(C) mRNA in situ Images from Allen Developmental Mouse Brain Atlas at E13.5 show expression of transcripts in the MGE that are expressed in PV<SST and SST/CR and SST/NOS1 from Fig-7F and G.
(D) Table showing that >75% of PGC1α targets identified by Lucas et. al. 2014 are also significantly (p<1×10^−5) enriched in PVC transcriptome data.
Table S1. Related to Figure1D. DE table of genes in Figure1D, FC>4, uTPM>50, q value<5.0 × 10−4, batches>80%
Table S2. Related to Figure 2B. AUROCs values for ~3888 GO gene sets
Table S3. Related to Figure 2C. AUROC scores of ~450 HGNC gene families from 3 studies (Paul et. al., Tasic et. al. 2016 and Zeisel. et. al. 2015)
Table S4. Related to Figures 2D, 2E and 3D. AUROC scores of custom gene sets used
Table S5. Related to Figure 3D and 3E. Low AUROC gene family list
Table S7. Related to Figure 2. Molecular portraits of GABAergic Phenotype Characterized Populations.