Abstract
Cancer is associated with a reduction in immature and mature circulating dendritic cells (DCs), and with an impaired migratory capacity, compared with healthy donors. Therefore, modern approaches to the in vitro generation of DCs loaded with tumor antigens and their use for inducing antitumor immune responses in vivo are being investigated. The purpose of the present study was to investigate the phenotypic and functional characteristics of peripheral blood DC subsets in patients with non-small cell lung cancer (NSCLC), and the development of an antitumor cytotoxic response by mononuclear cells (MNCs) from patients using in vitro generated antigen-primed DCs. Heparinized peripheral venous blood samples were obtained from 10 healthy donors and 20 patients with a histologically verified diagnosis of NSCLC. The ability of antigen-activated DCs to stimulate the activity of MNCs against autologous tumor cells was evaluated using a cytotoxic test. Peripheral blood DC subsets from patients with NSCLC were identified to be decreased and to exhibit an impaired ability to mature, compared with healthy donors. Furthermore, DCs generated from MNCs from patients with NSCLC were able to stimulate a specific cytotoxic response when loaded with autologous tumor lysates or RNA and matured, in vitro. A perforin and granzyme B-dependent mode of cytotoxicity was primarily induced. The ability of DCs loaded with tumor antigens to increase the cytotoxic activity of MNCs against NSCLC cells in vitro indicates the effective induction and co-stimulation of T lymphocytes by the generated DCs.
Keywords: dendritic cells subsets, antigen-primed dendritic cells, antitumor cytotoxic response, non-small cell lung cancer
Introduction
Antigen-presenting cells (APCs), in particular dendritic cells (DCs), regulate immune responses and serve key functions in the induction of antitumor activity (1). DCs exhibit immunomodulatory functions using major histocompatibility complex (MHC) class I and II molecules, co-stimulatory molecules [cluster of differentiation (CD)80, CD86 and CD40] and molecules required for migration and for the capture and processing of antigens (2). Primed CD4+ and CD8+ T-lymphocytes secrete cytokines, including interferon gamma (IFN-γ) and tumor necrosis factor α (TNF-α), which promote the proliferation of cytotoxic lymphocytes, the destruction of tumor tissue and the control and elimination of tumor cells (3). DCs present antigens, in complex with MHC class I and II molecules, to T and B cells, and demonstrate a high capacity to present tumor-associated antigens in vitro and in vivo (4). However, the functional activity of DCs in patients with cancer is significantly decreased (5). Antigen-specific activation of dendritic cells with the formation of an antitumor cytotoxic immune response is considered a promising potential method of combating cancer (6). The purpose of the present study was to assess the phenotypic and functional characteristics of peripheral blood DC subsets in patients with non-small cell lung cancer (NSCLC) and the development of an antitumor cytotoxic response by mononuclear cells (MNCs) derived from patients using in vitro-generated antigen-primed DCs. It was hypothesized that cancer is associated with a decreased proportion of immature and mature DCs in the peripheral blood, and with an impaired migratory capacity, as compared with healthy donors. Tumor-derived molecules may affect the maturation of DCs, preventing them from becoming functionally active (7). DCs are crucial for generating antitumor immunity; thus, a large number of studies have aimed to generate methods for loading DCs with tumor antigens for use in vivo (6,8). These approaches aim to mobilize the patient's immune system and circumvent any tumor-derived inhibition of DC maturation.
Materials and methods
Patients and specimens
In the present study, heparinized peripheral venous blood samples were obtained from 10 healthy donors, 20 patients with histologically verified non-small cell lung cancer (NSCLC) at stages IIA, IIB and IIIA, and tumor biopsy material obtained during surgery (17 males, 3 females; mean age, 60.7±1.5 years). Clinical material was obtained perioperativelyin the Regional Clinical Oncology Center (Novosibirsk, Russia) and City Clinical Hospital No. 1 (Novosibirsk, Russia) from August 2013 to September 2014. The inclusion criterion was the lack of chemotherapy and/or radiotherapy prior to surgery. The exclusion criteria were as follows: Pregnancy; receiving immunocorrective drugs for concomitant pathology; rapid progression of the underlying disease, such that the use of immunotherapy is deontologically unjustified; individual intolerance to the components of the vaccine and/or the development of severe side effects in response to any of the components; refusal of the patient to participate in the study either orally or in writing; and patient involvement in any other clinical study. All patients provided written informed consent for the study and all protocols were approved by the Local Ethics Committee at the Research Institute of Fundamental and Clinical Immunology (Novosibirsk, Russia).
Phenotypic and functional characterization of peripheral blood DC subtypes of patients with NSCLC
The relative proportion of myeloid and plasmacytoid DCs, and their phenotype, were evaluated in the whole peripheral blood of patients with NSCLC and healthy donors. Flow cytometry of labeled monoclonal antibodies was performed on a BD FACSVerse device (BD Biosciences, Franklin Lakes, NJ, USA). Data analysis was performed using the BD FACSDiva Software v.6.1.3 (BD Biosciences, Franklin Lakes, NJ, USA). Peripheral blood dendritic cell subtypes were characterized by the following phenotype: Myeloid dendritic cells (mDC) [cluster of differentiation (CD)3−, CD14−, CD19−, CD45+, Human Leukocyte Antigen-Antigen D-Related (HLA-DR)+, BDCA1+ and BDCA2−] and plasmacytoid dendritic cells (pDC; CD3−CD14− CD19−CD45+ HLA-DR+ BDCA1− BDCA2+). For cytometric analysis, the following antibodies were used: CD3-fluorescein isothiocyanate (FITC; Sorbent, Moscow, Russia), CD19-FITC (Sorbent), HLA-DR-phycoerythrin (PE) (Sorbent); CD14-FITC (BD Biosciences), CD45-V450 (BD Biosciences); BDCA1-APC (BioVision, Inc., Milpitas, CA, USA), BDCA2-PerCP-C5.5 (BioVision, Inc.).
To evaluate the maturation ability and migration potential, DCs of the two subsets (myeloid and plasmacytoid DCs) were analyzed using marker expression of CD83, CD86, C-C chemokine receptor 7 (CCR7; BD Biosciences) prior to and following stimulation of maturation by agonists of specific toll-like receptors (TLR) R848 (20 ng/ml; Resiquimod; BioVision, Inc.) lipopolysaccharide (LPS; 25 ng/ml) [E. coli 0114:B4 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)].
Preparation of autologous tumor cells and tumor antigens
Tumor cells were isolated from surgical biopsy samples using mechanical homogenization (Potter homogenizer) and a metal filter to separate the large detritus. The cells were washed three times with serum-free RPMI-1640 medium (Biolot, St. Petersburg, Russia) containing 160 µg/ml gentamicin (Samson-Med, St. Petersburg, Russia), 200 µg/ml ampicillin and 5 µg/ml amphotericin B. The resulting cells were divided into three equal aliquots containing 300,000–1,000,000 cells, depending on the volume of the tumor. The first aliquot was cultured in complete RPMI-1640 medium containing 10% fetal calf serum (FCS; PAA Laboratories, Pasching, Austria), 2 mM L-glutamine (Vector, Novosibirsk, Russia), 10 mM HEPES buffer (Sigma-Aldrich; Merck KGaA), 5×10−4 M 2-mercaptoethanol (Sigma-Aldrich; Merck KGaA), 80 µg/ml gentamicin (Samson) and 100 µg/ml ampicillin (Sintez) at 37°C in an atmosphere containing 5% CO2 for 10 days. A total of half the supernatant was replaced following 3 and 7 days at 37°C with 5% CO2. The second aliquot was used to prepare a mixture of tumor antigens produced by three freeze-thaw cycles, followed by filtration through a 0.45-µm filter (EMD Millipore, Billerica, MA, USA). The third aliquot of tumor cells was used to prepare total RNA by centrifuging at 300xg for 7 min. The supernatant was removed and 1 ml TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was added and incubated at room temperature for 5 min to disrupt the nucleoprotein complex. Subsequently, 200 µl chloroform (Himreactiv, Nizhny Novgorod, Russia) was added and incubated for 3 min at room temperature, followed by centrifugation at 10,000 × g at 4°C for 15 min. Subsequently, 500 µl from the upper phase was transferred to a new tube and 500 µl isopropanol (ZAO Himreactiv) was added and incubated for 10 min at room temperature. The resulting mixture was centrifuged at 10,000 × g at 4°C for 10 min. The pellet was resuspended in 1 ml ethanol (70%) and centrifuged at 10,000 × g for 5 min at room temperature. The supernatant was discarded and the tube was left with the lid open for between 15 and 20 min to enable the sample to dry. The precipitate was resuspended in between 20 and 100 µl double distilled water. The total protein lysate end RNA concentration was determined using a NanoDrop device (Thermo Fisher Scientific, Inc.) with an absorbance ratio of 260/280 nm.
Isolation of mononuclear cells from the peripheral blood of patients with NSCLC and preparation of an adherent fraction of mononuclear cells (MNCs)
MNCs were isolated by centrifugation of the whole blood in the Ficoll-Urografin density gradient (ρ=1.077 g/l). For this purpose, 6 ml whole blood was layered onto 3 ml Ficoll-Urografin and centrifuged at 300 × g for 30 min at room temperature. Cells forming an interphase ring were selected, washed twice in RPMI-1640 medium (Vector) and resuspended in 5 ml complete RPMI-1640 medium containing 10% FCS (PAA), 2 mM L-glutamine (Vector), 10 mM HEPES buffer (Sigma-Aldrich; Merck KGaA), 5×10−4 M 2mercaptoethanol (Sigma-Aldrich; Merck KGaA), 80 µg/ml gentamicin (Samson) and 100 µg/ml ampicillin (Sintez), transferred to a Petri dish (90 mm; Nalge Nunc International, Penfield, NY, USA), and incubated at 37°C in an atmosphere containing 5% CO2 for 2 h. The medium with non-adherent MNCs was removed and cells were pelleted by centrifugation at 300 × g for 10 min at room temperature and stored until use at 37°C in an atmosphere containing 5% CO2. The surfaces of Petri dishes were rinsed twice with water and cells were selected using a cell scraper (BD Biosciences).
Generation of mature antigen-primed DCs
To produce immature DCs, isolated cells of the adherent MNC fraction were cultured (1×105 cells/ml) in complete medium supplemented with recombinant human (rh) granulocyte-macrophage colony-stimulating factor (GM-CSF; 50 ng/ml) and rh interleukin (IL-) 4 (100 ng/ml; PeproTech, Inc., Rocky Hill, NJ, USA) in 48-well plates (Linbro, San Rafael, CA, USA) for 72 h. DC populations were identified by evaluating the expression of CD14, HLA-DR, and CD86 markers at the stage of immature and mature DCs and the ability of dendritic cells for phagocytosis of FITC-dextran. After 72 h of culturing, the immature DC culture was divided into three parts. Antigens in the form of a tumor cell lysate (with protein concentration of 100 µg/ml, for 18 h) were added to the first part. In the second part, immature DCs were transfected with tumor cell RNA using Promokine reagents. DCs from the third part were not exposed to any antigens and were used as control group [DCs(0) without any antigens]. After 24 h, immature DCs were added with 25 ng/ml of rh tumor-necrosis factor (TNF)-α and 10 ng/ml of rhIL1-β for 24 h to produce mature DCs. To generate DCs transfected with tumor cell RNA, we performed magnetic transfection using Promokine reagents (PromoCell GmbH, Heidelberg, Germany), according to the manufacturer's protocol. RNA was dissolved in Dulbecco's Modified Eagle's Medium (DMEM; Vector), added with a MATra-A reagent at the ratio of 0.3 µg RNA/0.3 µl reagent, and incubated at room temperature for 20 min. During incubation, the medium in wells with dendritic cells was changed: RPMI-1640 medium was removed, and 250 µl DMEM was added. Subsequently, the RNA-MATra-A complex was added to cells in wells in the amount of 25 µl/well, and a plate was placed on a magnetic board for 15 min. The medium in wells was replaced following transfection: DMEM was removed and 300 µl RPMI-1640 medium supplemented with 10% FCS (PAA), 2 mM L-glutamine (Vector), 10 mM HEPES (Sigma, USA), 5×10−4 M 2-mercaptoethanol (Sigma-Aldrich; Merck KGaA), 80 µg/ml gentamicin and 100 µg/ml ampicillin was added. Transfected cells were incubated at 37°C in an atmosphere containing 5% CO2 for 24 h, prior to the addition of maturation factors (25 ng/mlrhTNF-α and 10 ng/ml rhIL1-β).
Analysis of phenotypic and functional indicators of generated DCs
The phenotypic characteristics of DCs were studied using fluorochrome labeled monoclonal antibodies analyzed on a FACSAria flow cytometer (BD Biosciences). Cells were incubated with antibodies at 37°C for 1 h, washed in PBS, and fixed in a 1% formalin solution. The following primary antibodies were used to evaluate the phenotypic and functional characteristics: CD3-FITC (Sorbent), CD19-FITC (Sorbent), HLA-DR-phycoerythrin (PE; Sorbent); CD14-FITC (cat. no. 555937; BD Biosciences), CD45-V450 (cat. no. 560367; BD Biosciences); CD83-APC (cat. no. 551073; BD Biosciences), CD86-PerCp-Cy5.5 (cat. no. 561129; BD Biosciences), C-C chemokine receptor 7-PE (CCR7; cat. no. 552176; BD Biosciences). All antibodies were used according to the manufacturer's protocol.
To evaluate the capability of produced DCs for antigen capturing, the receptor-mediated endocytosis efficiency was determined using FITC-dextran (Sigma-Aldrich; Merck KGaA). For this purpose, cells were incubated with FITC-dextran (1 µg/ml) in complete medium containing 10% FCS (PAA), 2 mM L-glutamine (Vector), 10 mM HEPES buffer (Sigma-Aldrich; Merck KGaA), 5×10−4 M 2-mercaptoethanol (Sigma-Aldrich; Merck KGaA), 80 µg/ml gentamicin (Samson) and 100 µg/ml ampicillin (Sintez) at 4 and 37°C for 30 min each. At 4°C, dextran binds to surface receptors, and at 37°C the bound dextran enters the cell (direct endocytosis). The analysis was performed on a FACSAria flow cytometer (BD Biosciences), on the basis of the fluorescence intensity in a FITC channel. The results are presented as the difference in the fluorescence intensity of cells capturing dextran at 4 and 37°C, described in the following equation: ΔMean fluorescence intensity (MFI)=[MFI (37°C)-MFI (4°C)].
Co-culturing of antigen-primed DCs and MNCs of the non-adherent fraction from peripheral blood of patients with NSCLC
Produced antigen-primed DCs were co-cultured with MNCs of the non-adherent fraction in complete RPMI 1640 medium (ratio of DC: MNC, 1:10) for 5 days. Non-adherent MNCs cultured in the presence of DCs (0), not exposed to tumor antigens, [MNC+DC (0) group] were used as a control.
Analysis of the impact of antigen-primed DCs on the cytotoxic activity of MNCs against autologous NSCLC cells
Following the co-culture of MNCs of the non-adherent fraction and DCs primed with tumor antigens for 5 days, the cell suspension was centrifugedat 300 × g for 10 min at room temperature. Subsequently, the resulting cells and autologous tumor cells were co-cultured at a ratio of 10:1 ratio, respectively, in 96-well round bottom plates (TPP Techno Plastic Products AG, Trasadingen, Switzerland), 100 µl culture/well, with the cell concentration of 1×106 cells/ml for 18 h. The analysis was conducted using a CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega Corporation, Madison, WI, USA), according to the manufacturer's protocol. The cytotoxic effect was calculated using the formula proposed by the assay manufacturer and presented as the cytotoxicity percentage.
Investigation of the effector potential of MNCs induced by antigen-primed DCs
Following the co-culture of MNCs of the non-adherent fraction and antigen-primed DCs, and culture of control cells, for 5 days, the cell suspension was washed by centrifugation with PBS at300 × g for 10 min at room temperature. Subsequently, the level of MNCs expressing surface markers, including CD178, CD107a, CD253and the intracellular protein perforin or granzyme B, was determined in the total lymphocyte population and in CD8+ lymphocytes using flow cytometry with a BD FACSVerse device (BD Biosciences). Data analysis was performed using BD FACSDiva Software v.6.1.3 (BD Biosciences). The following antibodies were used according to the manufacturer's protocol: CD178-PE (cat. no. 564261; BD Biosciences), CD107a-FITC (cat. no. 555800; BD Biosciences), CD253-PE (cat. no. 550516; BD Biosciences), perforin-FITC (cat. no. 556577; BD Biosciences) and granzyme В-PE (cat. no. 561142; BD Biosciences).
Statistical analysis
Statistical analysis of the data was performed using Statistica 6.0 software (StatSoft Ltd., Bedford, UK) and GraphPad Prism v.6.01 (GraphPad Software, Inc., La Jolla, CA, USA). Data were presented as a median and a quartile range of values (25 and 75%). A non-parametric Wilcoxon test was used to identify significant differences when comparing two groups. In the analysis of more than two groups, the statistical significance of differences was determined using Kruskal-Wallis ANOVA and multiple comparisons test. To perform the post hoc analysis, Tukey's test was used in GraphPad Prism software. P<0.05 was considered to indicate a statistically significant difference. In figure captions, the number of individuals in a group is denoted as n.
Results
Patients with NSCLC exhibit decreased levels of mDCs and pDCs
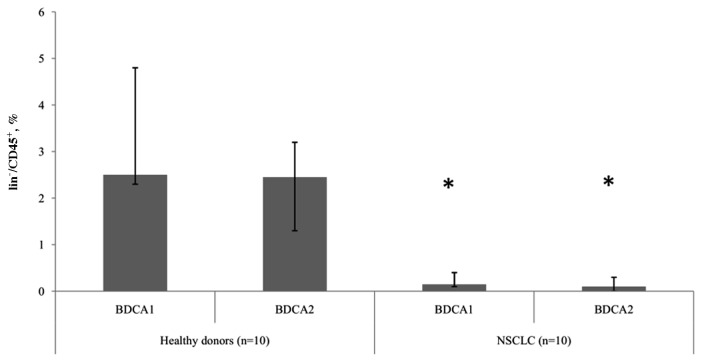
The present study revealed differences in the relative number of DC subtypes between groups of healthy donors and patients with NSCLC. In patients with tumors, significantly (P<0.05) decreased levels of mDCs and pDCs were observed, compared with healthy donors, indicating a depletion in the circulating DCs in NSCLC (Fig. 1). However, there were no statistically significant differences determined in the ratio of peripheral blood mDC and pDC subtypes between healthy donors and patients with NSCLC.
Figure 1.
Relative proportion of myeloid and plasmocytoid dendritic cells expressing BDCA1 and BDCA2 markers among lin−/CD45+ cells. Data are presented as the median and interquartile range. *P<0.05 vs. corresponding groups of healthy donors. NSCLC, non-small cell lung cancer; CD, cluster of differentiation; BDCA, blood dendritic cell antigen.
CD86 expression is decreased in patients with NSCLC
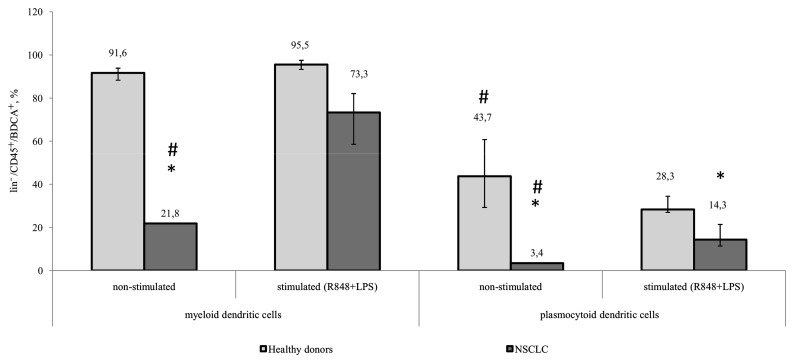
Decreased function of DCs in malignant diseases results from insufficient DC maturation. This is associated with phenotypic differences in the expression of membrane-bound markers and differences in their ability to capture an antigen, process it, and migrate to lymphoid organs for antigen presentation. While DCs are maturing, the expression of co-stimulatory molecules on their surface increases (8). Expression of CD86 begins early during maturation, whereas CD83 expression is typical of more mature DCs (9). To analyze the ability of DCs from patients with NSCLC to mature, TLR 4, 7, and 8 agonists, R848 and LPS were used as they are capable of inducing maturation in mDCs and pDCs. The results of the present study revealed that, prior to stimulation, the expression of CD86 on mDCs and pDCs in patients with NSCLC was significantly decreased, compared with that in healthy donors. A significant increase in the number of mDCs and pDCs expressing CD86 in response to stimulation with LPS and R848 was observed in healthy controls and tumor patients. Additionally, the pDC population from patients with NSCLC expressed significantly decreased CD86 following stimulation, compared with that in healthy donors (Fig. 2).
Figure 2.
Relative amount of myeloid and plasmocytoid dendritic cells expressing the CD86 marker among lin−/CD45+/BDCA+ cells. Healthy donors, n=10; patients with NSCLC, n=10. The data are presented as the median and interquartile range. *P<0.05 vs. corresponding groups of healthy donors; #P<0.05 vs. orresponding group of stimulated cultures. CD, cluster of differentiation; NSCLC, non-small cell lung cancer; BDCA, blood dendritic cell antigen; R848, Resiquimod; LPS, lipopolysaccharide serotype 0114:B4.
Proportion of CD83+mDCs from patients with NSCLS is decreased
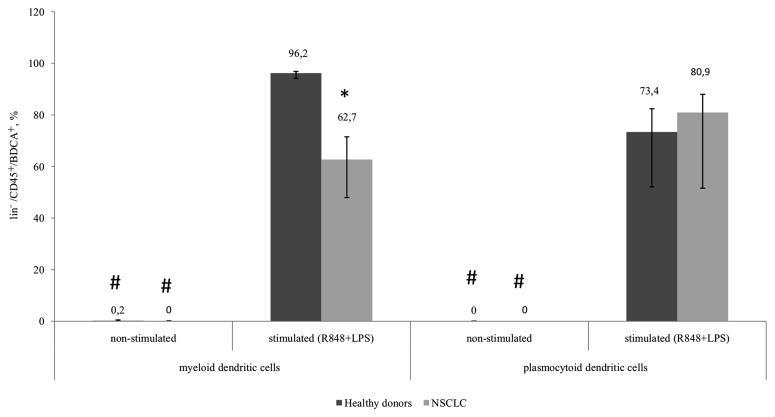
The expression of CD83 in the myeloid and plasmocytoid DC subsets in healthy donors and patients with NSCLC was not determined in unstimulated samples. However, a significant increase in the number of CD83-expressing cells was observed in all groups following stimulation. The number of CD83+mDCs from patients with NSCLC following TLR stimulation was significantly decreased compared with mDCs from healthy donors (Fig. 3).
Figure 3.
Relative amount of myeloid and plasmocytoid dendritic cells expressing the CD83 marker among lin−/CD45+/BDCA+ cells. Healthy donors, n=10; patients with NSCLC, n=10. The data are presented as the median and interquartile range. *P<0.05 vs. corresponding groups of healthy donors; #P<0.05 vs. corresponding group of stimulated cultures. CD, cluster of differentiation; NSCLC, non-small cell lung cancer; BDCA, blood dendritic cell antigen; Lin, lineage antigen, including СD3, CD14, CD19, CD20, CD16, CD56; CD, cluster of differentiation; R848, Resiquimod; LPS, lipopolysaccharide serotype 0114:B4.
The functional characteristics of tumor-derived DCs were additionally evaluated. The chemokine receptor, CCR7, was used as an indicator of migration ability. The number of CCR7-expressing cells from patients with NSCLC prior to and following stimulation was lower than the flow cytometer detection limit; whereas, a significant increase in the number of CCR7-expressing cells in response to stimulation with R848 and LPS occurred in healthy donors (data not presented).
DCs generated from patients with NSCLC are capable of endocytosis during early maturation stages
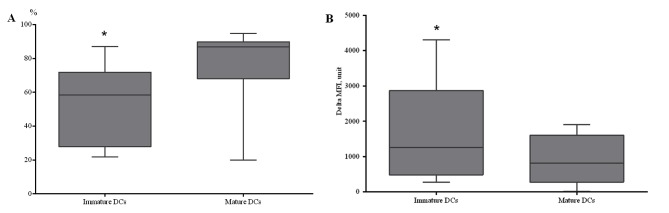
A potential approach to the immunotherapy of patients with NSCLC is the development of vaccines with in vitro induction of efficient antigen-presenting DCs. In the present study, the possibility of generating mature, functionally active DCs, from peripheral blood MNCs isolated from patients with NSCLC, was investigated. To prepare immature DCs, cells from the adherent MNC fraction were cultured in complete media supplemented with rhGM-CSF and rhIL-4 for 4 days. To generate mature DCs, the immature DCs were added with rhTNF-α and rhIL-1-β and cultured for 24 h. A statistically significant increase in CD86+ DCs was identified in DCs generated from patients with NSCLC and stimulated using TNF-α (Fig. 4A).
Figure 4.
Characterization of dendritic cells generated from patients with NSCLC. (A) The relative amount of CD86+ generated dendritic cells among monocytes in patients with NSCLC (n=7). (B) The capability of dendritic cells to capture the FITC-dextran antigen (n=8). Immature DCs are immature dendritic cells generated from the adherent fraction of mononuclear cells by addition of rhGM-CSF and rhIL-4 for 4 days. Mature DCs are mature dendritic cells generated from immature DCs by addition of rhTNF-α and rhIL1-β for 24 h. The error bars show a minimum and maximum of values, boxing is a quartile, and the inner line is a median. *P<0.05 vs. corresponding mature dendritic cells. CD, cluster of differentiation; NSCLC, non-small cell lung cancer; rh, human recombinant; GM-CSF, granulocyte macrophage colony-stimulating factor; IL, interleukin; DC, dendritic cells; MFI, mean fluorescence intensity.
To determine the capability of in vitro-derived DCs for antigen capturing, receptor-mediated endocytosis of dextran was evaluated at 4 and 37°C. A decreased endocytic ability was identified in mature DCs, compared with immature DCs, which indicated acquisition of the functional characteristics typical of the mature state of generated DCs (Fig. 4B). Therefore, it was demonstrated that DCs generated from the peripheral blood of patients with NSCLC are capable of endocytosis during early maturation stages, while during later maturation stages, DCs lose the ability to capture antigen.
Generated DCs increase the cytotoxic activity of MNCs against autologous NSCLC cells
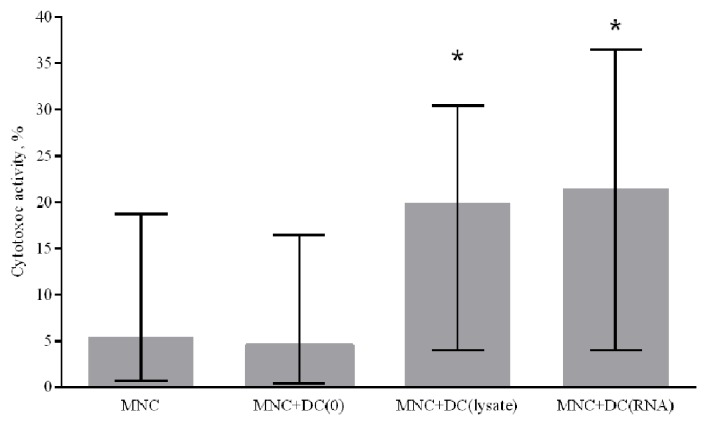
To assess the ability of in vitro-derived DCs to induce a specific antitumor immune response, a cytotoxic antitumor response was stimulated in the MNC culture in vitro. Immature DCs generated from patients with NSCLC were primed with a lysate of tumor cells or transfected with RNA from tumor cells. To determine the cytotoxic activity of MNCs against autologous tumor cells, antigen-primed mature DCs were co-cultured with peripheral blood MNCs of the non-adherent fraction from NSCLC patients for 5 days. Generated DCs primed with a tumor cell lysate and DCs transfected with tumor cell RNA were demonstrated to increase the cytotoxic activity of MNCs against autologous NSCLC cells by 4.3 and 4.7 times, respectively, compared with the control group where DCs were not primed with tumor antigens (Fig. 5).
Figure 5.
Effect of antigen-primed DCs on the cytotoxic activity of MNCs against autologous NSCLC cells in vitro, n=14. The error bars show a minimum and maximum of values, boxing is a quartile, and the inner line is a median. *P<0.05 vs. group MNC+DC(0) determined using Kruskal-Wallis ANOVA and multiple comparisons test. NSCLC, non-small cell lung cancer; DC, dendritic cells; MNC, mononuclear cells of the non-adherent fraction; MNC+DC(0), MNCs cultured with non-primed DCs; MNC+DC (lysate), MNCs cultured with lysate-treated DCs; MNC+DC(RNA), MNCs cultured with tumor RNA-treated DCs.
Lysate-primed DCs and RNA-transfected DCs increase the relative level of perforin-bearing cells
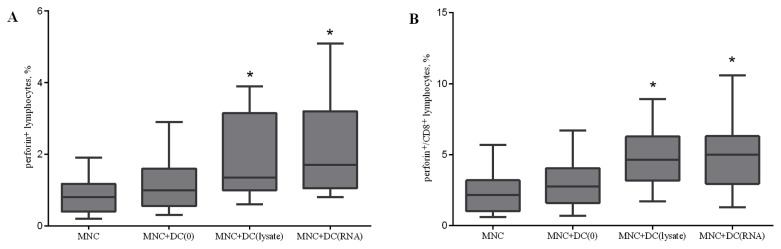
To validate the mechanism of the cytotoxicity induced during the co-culture of MNCs and DCs, the number of CD178+, CD107a+, CD253+, perforin+, and granzyme B+ cells in the total lymphocyte population and CD8+ cells were determined on the fifth day of incubation. There were no statistically significant changes in the expression of CD178, CD107a, and CD253, which are markers of the Fas ligand and TNF-related apoptosis inducing ligand (TRAIL)-dependent cytotoxic pathways and direct cytolysis of target cells (data not presented). Effector cells are capable of producing lytic granules containing perforin and granzymes. The accumulation of perforin in the total lymphocyte population and CD8+ cells following co-culture demonstrated that lysate-primed DCs and RNA-transfected DCs increase the relative level of perforin-bearing cells in the lymphocyte population by 1.6 and 2 times, respectively, and in CD8 cells by 2 times compared with the control group (Fig. 6).
Figure 6.
Effect of antigen-primed DCs on the relative amount of (A) perforin+ lymphocytes; and (B) perforin+/CD8+ lymphocytes in the co-culture of MNCs from patients with NSCLC, n=16. The error bars show a minimum and maximum of values, boxing is a quartile, and the inner line is a median. *P<0.05 vs. the group MNC determined using Kruskal-Wallis ANOVA and multiple comparisons test. NSCLC, non-small cell lung cancer; DC, dendritic cells; CD, cluster of differentiation; MNC, mononuclear cells of the non-adherent fraction; MNC+DC(0), MNCs cultured with non-primed DCs; MNC+DC(lysate), MNCs cultured with lysate-treated DCs; MNC+DC(RNA), MNCs cultured with tumor RNA-treated DCs.
Co-culturing MNCs with antigen-primed DCs increases the relative level of granzyme B positive cells
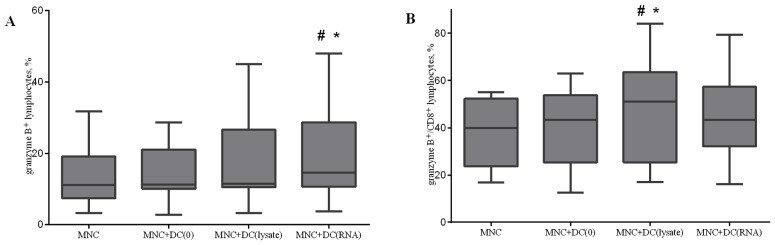
The accumulation of granzyme B in lytic granules was investigated. Co-culturing MNCs with antigen-primed DCs led to an increase in the relative level of granzyme B positive cells in the total lymphocyte population and in CD8+ cytotoxic T lymphocytes (Fig. 7). Similar results were observed when analysis of the relative level of double perforin and granzyme B positive cells after co-culturing of MNCs and DCs was performed (data not presented).
Figure 7.
Effect of antigen-primed DCs on the relative amount of (A) granzyme B+ lymphocytes; and (B) granzyme B+/CD8+ lymphocytes in the co-culture of MNCs from NSCLC patients, n=16. The error bars show a minimum and maximum of values, boxing is a quartile, and the inner line is a median *P<0.05 vs. group MNC and #P<0.05 vs. group MNC+DC(0), determined using Kruskal-Wallis ANOVA and multiple comparisons test. NSCLC, non-small cell lung cancer; DC, dendritic cells; CD, cluster of differentiation; MNC, mononuclear cells of the non-adherent fraction; MNC+DC(0), MNCs cultured with non-primed DCs; MNC+DC(lysate), MNCs cultured with lysate-treated DCs; MNC+DC(RNA), MNCs cultured with tumor RNA-treated DCs.
The results of the present study demonstrated that patients with NSCLC exhibited a decrease in mDCs and pDCs, compared with healthy donors. Additionally, DCs from patients with NSCLC exhibited impaired function, resulting in poor induction of an antitumor immune response. However, stimulation with agonists of TLR 4, 7, and 8 (R848 and LPS) resulted in partial recovery of the ability of DCs to reach a mature state. The use of antigen-primed DCs, which were generated from peripheral blood MNCs of patients with NSCLC for 5 days, to produce an antitumor cytotoxic response in vitro led to activation of effector cells, which resulted in an increased cytotoxic activity of MNCs and increased perforin+ and granzyme B+ lymphocytes and CD8+ cells. Despite patients with NSCLC exhibiting decreased mDCs and pDCs, compared with healthy donors, and impaired functional capabilities of DCs, it was possible to generate mature, functionally and phenotypically normal DCs, in vitro, capable of inducing a cytotoxic immune response upon culture with autologous MNCs.
Discussion
A number of oncological diseases, including NSCLC, are associated with a decrease in the absolute and relative number of immature and mature peripheral blood circulating DCs, compared with healthy donors (10–12). Additionally, there are various impairments to the ability of DCs to migrate and interact with other cells (13). There is insufficient data regarding peripheral blood DC subsets in NSCLC at present. The present study revealed differences in the relative number of DC subtypes between groups of healthy donors and patients with NSCLC. A significant decrease in the level of mDCs and pDCs was observed in the patients, compared with healthy donors, indicating a depletion of the total pool of circulating DCs in patients with NSCLC, which was observed by Domingues et al (14). In the present study, there were no significant differences in the ratio of peripheral blood mDCs and pDCs between healthy donors and patients with NSCLC. DCs are required to express appropriate co-stimulatory markers on the membrane to efficiently present an antigen to cytotoxic T lymphocytes (15,16). To assess the potential of circulating DCs for maturation, agonists of TLR 4, 7, and 8 (R-848 and LPS) were used which are capable of inducing maturation of mDCs and pDCs, with subsequent evaluation of the expression level of co-stimulatory molecules. In blood samples prior to LPS and R848 stimulation for the two DC subsets, the number of CD86-expressing cells in patients with NSCLC was significantly decreased, compared with that in healthy donors. A significant increase in the expression of CD86 in mDCs and pDCs, in response to stimulation with LPS and R848, was observed in all groups. In the present study, the number of CD86-bearing cells in the pDC population from patients with NSCLC, following stimulation, was decreased, compared with that in healthy donors. Expression of CD83 in DC subsets, in healthy donors and in tumor patients, was not detected. However, a significant increase in the expression level of CD8, following after stimulation with R848 and LPS, was observed in all groups. The relative amount of CD83+ cells among mDCs in patients with NSCLC following stimulation was significantly decreased, compared with that in the healthy donors, indicating the different stages of DC differentiation in NSCLC.
Impaired function of peripheral blood DCs was observed in patients with NSCLC compared with healthy donors in the present study. In the present study, the number of cells expressing CCR7 was analyzed, which is associated with the ability of cells to migrate to the areas of antigen presentation and co-stimulation of T cells because its ligand CCL19 is expressed in T cell areas of secondary lymphoid organs (17). The number of CCR7-expressing cells in the patients, prior to and subsequent to stimulation, was below the flow cytometer detection limit, whereas a significant increase in the number of CCR7-expressing cells, in response to R848 and LPS stimulators, occurred in healthy donors. These results indicated an alteration in the migration ability of antigen-presenting cells into naive T cell areas within the lymph nodes, and may be the cause of failure or impairment of the antitumor immune response in NSCLC. Thus, the study of peripheral circulating DCs in patients with NSCLC revealed a number of features that may explain a high immunosuppression level accompanying the development of malignant lung tumors. A decrease in the number of mDCs and pDCs, compared with healthy donors, and a disturbance in the differentiation and functional activity of the DCs were identified in the present study. These results may prompt the generation of functionally active DCs from the peripheral blood of patients with NSCLC for use in immunotherapy regimes. The stimulation-induced recovery of DC maturation enables DCs to be a target for therapeutic intervention and indicates that DCs themselves may be a treatment option. To date, there are a number of approaches for generating DCs in vitro, including preparation of DCs from peripheral blood monocytes and bone marrow progenitors (CD34+ cells) (18). Furthermore, a wide range of factors may be used for differentiation and maturation of DCs (GM-CSF, IL-4, interferon-α, IL-2, IL-6, IL-15, TGF-α, stem cell factor, FLT-3 ligand and prostaglandin E2) that may facilitate, in a combination or individually, the generation of DCs from monocytes or CD34+ cells (19–23). The present study suggested that there is some danger in using numerous factors for differentiation and maturation of DCs that can lead to the production of DC subsets with a variety of phenotypes and functions. In the present study, DCs were generated from peripheral blood MNCs from patients with NSCLC. To produce immature DCs, the adherent fraction of MNCs was cultured with rhGM-CSF and rhIL-4. To generate mature DCs, rhTNF-α and rhIL1-β were added to immature DCs. This approach resulted in a DC culture with phenotypic and functional characteristics typical of mature DCs. The main advantage of using DCs in developing anticancer vaccines is the possibility to generate, in vitro, antigen presenting cells with certain characteristics required for a normal antitumor immune response (24). Additionally, an important feature of DCs that distinguishes them from other antigen-presenting cells is their ability to cross-present antigens (25). It was previously demonstrated that treatment of a patient with cancer with a DC-based vaccine led to a considerable reduction in the functional activity of DCs (26). Therefore, loading DCs with tumor antigens and induce maturation was required, as this would promote activation of an antigen-specific immune response and prevent the immunosuppressive influence of the tumor.
The source of tumor antigens serves an important function in their immunogenicity (27–29). Tumor cell lysates, tumor cell RNA, recombinant proteins of tumor-associated antigens, and DNA constructs encoding tumor-associated antigens have been used as antigens (21,30,31). Loading DCs with a tumor lysate enables presentation of a range of tumor antigens from a patient (32). The use of a tumor lysate as a tumor associated antigen source may have the advantage in stimulating a polyclonal immune response, as they may stimulate CD4+ T helpers and CD8+ cytotoxic T lymphocytes, thereby reducing the likelihood for tumor escape (33). This method decreases the time and effort required for identification and synthesis of certain immunodominant peptide epitopes, allowing DCs to process naturally occurring tumor antigens and stimulate a natural cytotoxic response (34,35). The use of a tumor lysate as a source of antigens for loading DCs is more efficient than the use of separate peptides (33). Autologous tumor cells from the patient contain a range of antigens (36), which may lead to the generation of a wider range of cytotoxic T lymphocytes (37). However, the use of a tumor cell lysate as a source of antigens is associated with immunosuppression (38). The use of tumor cell RNA to transfect DCs may prevent this adverse effect (39,40).
One of the potential and promising areas in cancer immunotherapy is the use of T cells co-cultured, and thereby ‘trained’, in the presence of antigen-primed DCs. One mature differentiated DC is capable of efficiently activating ~100 T cells, whereupon they may implement an immune response (41). Therefore, activation of the cellular immune response may be achieved during in vitro co-culturing of DCs and T cells, thereby, avoiding the negative influence of tumor growth products depressing the functional activity of mature antigen-primed DCs administered to the patient (42,43). After co-culturing MNCs and DCs, a lactate dehydrogenase (LDH) cytotoxicity test, which is on the basis of the release of LDH from lysed tumor cells in vitro, to study the stimulating effect of antigen-primed DCs on the cytotoxic activity of tumor MNCs against NSCLC cells. Generated DCs primed with a tumor cell lysate and DCs transfected with tumor cell RNA were able to enhance the cytotoxic activity of MNCs against autologous NSCLC cells by 4.3 and 4.7 times, respectively, compared with the control group of DCs not primed with tumor antigens. In the present study, cytotoxic T lymphocytes induced apoptosis of target cells via the accumulation of perforin and granzyme granules. Perforin is a pore-forming protein that has homology with the C9 complement component and is synthesized as an inactive precursor cleaved at the C-terminus to yield the active form (44). Perforin incorporates into the membrane of the target cell and may promote cell entry of granzyme B and enhance the cytotoxic effect of T lymphocytes (45). Additionally, Fas- and TRAIL-ligands act as apoptotic inducers (46). The Fas-ligand-mediated mechanism of apoptosis is involved in the elimination of unintended cells, thereby reducing the risk of developing tumor cells (47). Cytotoxic T lymphocytes use Fas-ligand to activate caspases that directly induce apoptosis (48). TRAIL is a death receptor that belongs to the family of TNF receptors (49). Fas and TRAIL apoptosis responds to certain stimuli (e.g., an increase in the expression of the corresponding ligands) and causes DNA damage and protein p53 activation, which leads to the release of mitochondrial pro-apoptotic factors triggering caspases. The TRAIL mechanism may be induced by factors including nuclear factor k-light-chain-enhancer of activated B cells, mitogen-activated protein kinase and protein kinase B (50). To study the mechanisms of cytotoxic activity observed in the co-culture of MNCs and DCs, the relative level of CD178+, CD107a+, CD253+, perforin+, and granzyme+ cells in the total lymphocyte population and CD8+ cells was determined on the fifth day of incubation. There were no significant changes identified in the expression of CD178, CD107a, and CD253, which are markers of FasL- and TRAIL-dependent pathways of cytotoxicity. The accumulation of perforin in the total lymphocyte population and CD8+ cells, following co-culture, demonstrates a stimulating effect of lysate-primed DCs and RNA-transfected DCs. DCs treated with tumor cell RNA as a source of antigens have a stimulating effect, similar to that of lysate-primed DCs, on the cytotoxic activity as observed with an increase in perforin+ and granzyme B+ lymphocytes and CD8+ cells. These results suggested that tumor cell RNA may be used with tumor cell lysates, particularly in situations where antigenic material may be unavailable, undesirable because of a possible immunosuppressive effect, or insufficient in quantity for preparation of a tumor cell lysate.
Patients with NSCLC were identified to exhibit a decreased number of all peripheral blood DC subsets, compared with healthy donors. Additionally, DCs revealed an impaired ability to undergo maturation. DCs generated from peripheral blood MNCs of patients with NSCLC and primed by a lysate and tumor cell RNA were able to stimulate the cytotoxic activity of MNCs against autologous tumor cells in vitro. The observed ability of DCs loaded with tumor antigens (lysate or RNA) to increase the cytotoxic activity of MNCs against NSCLC cells in vitro indicates effective activation of T lymphocytes by the generated DCs. The effector reactions induced by antigen-primed DCs occurred primarily through a perforin-granzyme B-dependent cytotoxic pathway, which was demonstrated by an increase in perforin+ and granzyme B+ CD8+cells, following co-culture of MNCs and antigen-primed DCs and by the lack of statistically significant changes in markers of Fas- and TRAIL-dependent cytotoxic pathways.
To conclude, the disturbances to the phenotype and functional capabilities of peripheral blood DC subsets in patients with NSCLC could be overcome by TLR stimulation. Thus, autologous DCs may be considered for use in patients with NSCLC as a target for therapeutic strategies, and as the basis for cellular immunotherapy and induction of an antitumor cytotoxic immune response.
Acknowledgements
The present study was supported by the Federal Target Program ‘Research and development in priority areas of the Russian scientific and technological complex in 2014–2020’, agreement no. 14.607.21.0043, unique identifier: RFMEFI60714X0043.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Randolph GJ, Ochando J, Patrida-Sánchez S. Migration of dendritic cell subsets and their precursors. Annu Rev Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- 3.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 4.Lipscomb MF, Masten BJ. Dendrititc cells: Immune regulators in health and disease. Physiol Rev. 2002;82:97–130. doi: 10.1152/physrev.00023.2001. [DOI] [PubMed] [Google Scholar]
- 5.Sennikov SV, Obleukhova IA, Kurilin VV, Kulikova EV, Khristin AA. Features of functional activity of dendritic cells in tumor growth. Vopr Onkol. 2015;61:556–62. [PubMed] [Google Scholar]
- 6.Bol KF, Schreibelt G, Gerritsen WR, de Vries IJ, Figdor CG. Dendritic cell-based immunotherapy: State of the art and beyond. Clin Cancer Res. 2016;22:1897–1906. doi: 10.1158/1078-0432.CCR-15-1399. [DOI] [PubMed] [Google Scholar]
- 7.Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66:5527–5536. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 8.deVries IJ, Eggert AA, Scharenborg NM, Vissers JL, Lesterhuis WJ, Boerman OC, Punt CJ, Adema GJ, Figdor CG. Phenotypical and functional characterization of clinical grade dendritic cells. J Immunother. 2002;25:429–38. doi: 10.1097/00002371-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Koski GK, Cohen PA, Roses RE, Xu S, Czerniecki BJ. Reengineering dendritic cell-based anti-cancer vaccines. Immunol Rev. 2008;222:256–276. doi: 10.1111/j.1600-065X.2008.00617.x. [DOI] [PubMed] [Google Scholar]
- 10.Huang A, Gilmour JW, Imami N, Amjadi P, Henderson DC, Allen-Mersh TG. Increased serum transforming growth factor-beta1 in human colorectal cancer correlates with reduced circulating dendritic cells and increased colonic Langerhans cell infiltration. Clin Exp Immunol. 2003;134:270–278. doi: 10.1046/j.1365-2249.2003.02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lissoni P, Malugani F, Bonfanti A, Bucovec R, Secondino S, Brivio F, Ferrari-Bravo A, Ferrante R, Vigoré L, Rovelli F, et al. Abnormally enhanced blood concentrations of vascular endothelial growth factor (VEGF) in metastatic cancer patients and their relation to circulating dendritic cells, IL-12 and endothelin-1. J Biol Regul Homeost Agents. 2001;15:140–144. [PubMed] [Google Scholar]
- 12.Vetsika EK, Koinis F, Gioulbasani M, Aggouraki D, Koutoulaki A, Skalidaki E, Mavroudis D, Georgoulias V, Kotsakis A. Сirculating subpopulation of monocytic myeloid-derived suppressor cells as an independent prognostic/predictive factor in untreated non-small lung cancer patients. J Immunol Res. 2014;2014:659294. doi: 10.1155/2014/659294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Zhang H, Su L, Yang P, Xin Z, Zou J, Ren S, Zuo Y. Low expression of dendritic cell-specific intercellular adhesion molecule-grabbing nonintegrin-related protein in lung cancer and significant correlations with brain metastasis and natural killer cells. Mol Cell Biochem. 2015;407:151–160. doi: 10.1007/s11010-015-2465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domingues D, Turner A, Silva MD, Marques DS, Mellidez JC, Wannesson L, Mountzios G, de Mello RA. Immunotherapy and lung cancer: Current developments and novel targeted therapies. Immunotherapy. 2014;6:1221–1235. doi: 10.2217/imt.14.82. [DOI] [PubMed] [Google Scholar]
- 15.deVries IJ, Lesterhuis WJ, Scharenborg NM, Engelen LP, Ruiter DJ, Gerritsen MJ, Croockewit S, Britten CM, Torensma R, Adema GJ, et al. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res. 2003;9:5091–5100. [PubMed] [Google Scholar]
- 16.Stagg AJ, Hart AL, Knight SC, Kamm MA. The dendritic cell: Its role in intestional inflammation and relationship with gut bacteria. Gut. 2003;52:1522–1529. doi: 10.1136/gut.52.10.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Aït-Yahia S, Brière F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarnjak-Jankovic S, Hammerstad H, Sabbøe-Larssen S, Kvalheim G, Gaudernack G. A full scale comparative study of methods for generation of functional Dendritic cells for use as cancer vaccines. BMC Cancer. 2007;7:119. doi: 10.1186/1471-2407-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bykovskaia SN, Buffo M, Zhang H, Bunker M, Levitt ML, Agha M, Marks S, Evans C, Ellis P, Shurin MR, Shogan J. The generation of human dendritic and NK cells from hemopoietic progenitors induced by interleukin-15. J Leukoc Biol. 1999;66:659–666. doi: 10.1002/jlb.66.4.659. [DOI] [PubMed] [Google Scholar]
- 20.Dauer M, Obermaier B, Herten J, Haerle C, Pohl K, Rothenfusser S, Schnurr M, Endres S, Eigler A. Mature dendritic cells derived from human monocytes within 48 h: A novel strategy for dendritic cell differentiation from blood precursors. J Immunol. 2003;170:4069–4076. doi: 10.4049/jimmunol.170.8.4069. [DOI] [PubMed] [Google Scholar]
- 21.Kulikova EV, Kurilin VV, Shevchenko JA, Obleukhova IA, Khrapov EA, Boyarskikh UA, Filipenko ML, Shorokhov RV, Yakushenko VK, Sokolov AV, Sennikov SV. Dendritic cells transfected with a DNA construct encoding tumor-associated antigen epitopes induce a cytotoxic immune response against autologous tumor cells in a culture of mononuclear cells from colorectal cancer patients. Scand J Immunol. 2015;82:110–117. doi: 10.1111/sji.12311. [DOI] [PubMed] [Google Scholar]
- 22.Leplina OY, Stupak VV, Kozlov YP, Pendyurin, Nikonov SD, Tikhonova MA, Sycheva NV, Ostanin AA, Chernykh ER. Use of interferon-alpha-induced dendritic cells in the therapy of patients with malignant brain gliomas. Bull Exp Biol Med. 2007;143:528–534. doi: 10.1007/s10517-007-0172-1. [DOI] [PubMed] [Google Scholar]
- 23.Obleukhova IA, Kurilin VV, Goncharov MA, Tarkhov AV, Krasil'nikov SE, Sennikov SV. Effect of mature dendritic cells primed with autologous tumor antigens from patients with epithelial ovarian cancer on stimulation of the cytotoxic immune response in culture of mononuclear cells. Bull Exp Biol Med. 2013;156:161–164. doi: 10.1007/s10517-013-2301-3. [DOI] [PubMed] [Google Scholar]
- 24.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–1766. [PubMed] [Google Scholar]
- 25.Ruben JM, Bontkes HJ, Westers TM, Hooijberg E, Ossenkoppele GJ, de Gruijl TD, van de Loosdrecht AA. Differential capacity of human interleukin-4 and interferon-α monocyte-derived dendritic cells for cross-presentation of free versus cell-associated antigen. Cancer Immunol Immunother. 2015;64:1419–1427. doi: 10.1007/s00262-015-1741-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almand В, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: A mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 27.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Türeci Ö, Vormehr M, Diken M, Kreiter S, Huber C, Sahin U. Targeting the heterogeneity of cancer with individualized neoepitope vaccines. Clin Cancer Res. 2016;22:1885–1896. doi: 10.1158/1078-0432.CCR-15-1509. [DOI] [PubMed] [Google Scholar]
- 29.Markiewicz MA, Kast WM. Progress in the development of immunotherapy of cancer using ex vivo-generated dendritic cells expressing multiple tumor antigen epitopes. Cancer Invest. 2004;22:417–434. doi: 10.1081/CNV-200029072. [DOI] [PubMed] [Google Scholar]
- 30.Sennikov SV, Kulikova E, Obleukhova IA, Shevchenko JA. Technologies of cellular antitumor immune response induction in vitro. Genes Cells. 2015;10:16–22. [Google Scholar]
- 31.Sennikov SV, Shevchenko JA, Kurilin VV, Khantakova JN, Lopatnikova JA, Gavrilova EV, Maksyutov RA, Bakulina AY, Sidorov SV, Khristin AA, Maksyutov AZ. Induction of an antitumor response using dendritic cells transfected with DNA constructs encoding the HLA-A*02:01-restricted epitopes of tumor-associated antigens in culture of mononuclear cells of breast cancer patients. Immunol Res. 2016;64:171–180. doi: 10.1007/s12026-015-8735-0. [DOI] [PubMed] [Google Scholar]
- 32.Robson NC, Hoves S, Maraskovsky E, Schnurr M. Presentation of tumour antigens by dendritic cells and challenges faced. Curr Opin Immunol. 2010;22:137–144. doi: 10.1016/j.coi.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Delirezh N, Moazzeni SM, Shokri F, Shokrgozar MA, Atri M, Kokhaei P. Autologous dendritic cells loaded with apoptotic tumor cells induce T cell-mediated immune responses against breast cancer in vitro. Cellular Immunol. 2009;257:23–31. doi: 10.1016/j.cellimm.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Galluzzi L, Senovilla L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, Zitvogel L, Kroemer G. Trial watch. Dendritic cell-based interventions for cancer therapy. OncoImmunology. 2012;1:1111–1134. doi: 10.4161/onci.21494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hargadon KM. Tumor-altered dendritic cell function: Implications for antitumor immunity. Front Immunol. 2013;4:192. doi: 10.3389/fimmu.2013.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang AE, Redman BG, Whitfield JR, Nickoloff BJ, Braun TM, Lee PP, Geiger JD, Mulé JJ. A phase i trial of tumor lysate-pulsed dendritic cells in the treatment of advanced cancer. Clin Cancer Res. 2002;8:1021–1032. [PubMed] [Google Scholar]
- 37.Win SJ, McMillan DG, Errington-Mais F, Ward VK, Young SL, Baird MA, Melcher AA. Enhancing the immunogenicity of tumour lysate-loaded dendritic cell vaccines by conjugation to virus-like particles. British J Cancer. 2012;106:92–98. doi: 10.1038/bjc.2011.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong B, Dai G, Xu L, Zhang Y, Ling L, Sun L, Lv J. Tumor cell lysate induces the immunosuppression and apoptosis of mouse Immunocytes. Mol Med Rep. 2014;10:2827–2834. doi: 10.3892/mmr.2014.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilboa E, Vieweg J. Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev. 2004;199:251–63. doi: 10.1111/j.0105-2896.2004.00139.x. [DOI] [PubMed] [Google Scholar]
- 40.Kreiter S, Diken M, Selmi A, Türeci Ö, Sahin U. Tumor vaccination using messenger RNA: Prospects of a future therapy. Curr Opin Immunol. 2011;23:399–406. doi: 10.1016/j.coi.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: Dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5:112–124. doi: 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- 42.Bernhard H, Neudorfer J, Gebhard K, Conrad H, Hermann C, Nährig J, Fend F, Weber W, Busch DH, Peschel C. Adoptive transfer of autologous, HER2-specific, cytotoxic T lymphocytes for the treatment of HER2-overexpressing breast cancer. Cancer Immunol Immunother. 2008;57:271–280. doi: 10.1007/s00262-007-0355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gelao L, Criscitiello C, Esposito A, De Laurentiis M, Fumagalli L, Locatelli MA, Minchella I, Santangelo M, De Placido S, Goldhirsch A, Curigliano G. Dendritic cell-based vaccines: Clinical applications in breast cancer. Immunotherapy. 2014;6:349–360. doi: 10.2217/imt.13.169. [DOI] [PubMed] [Google Scholar]
- 44.Cullen SP, Brunet M, Martin SJ. Granzymes in cancer and immunity. Cell Death Differ. 2010;17:616–623. doi: 10.1038/cdd.2009.206. [DOI] [PubMed] [Google Scholar]
- 45.Smyth MJ, Kelly JM, Sutton VR, Davis JE, Browne KA, Sayers TJ, Trapani JA. Unlocking the secrets of cytotoxic granule proteins. J Leukoc Biol. 2001;70:18–29. [PubMed] [Google Scholar]
- 46.Walczak H, Krammer PH. The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res. 2000;256:58–66. doi: 10.1006/excr.2000.4840. [DOI] [PubMed] [Google Scholar]
- 47.Poehlein CH, Hu HM, Yamada J, Assmann I, Alvord WG, Urba WJ, Fox BA. TNF plays an essential role in tumor regression after adoptive transfer of perforin/IFN-gamma double knockout effector T cells. J Immunol. 2003;170:2004–2013. doi: 10.4049/jimmunol.170.4.2004. [DOI] [PubMed] [Google Scholar]
- 48.Henkler F, Behrle E, Dennehy KM, Wicovsky A, Peters N, Warnke C, Pfizenmaier K, Wajant H. The extracellular domains of FasL and Fas are sufficient for the formation of supramolecular FasL-Fas clusters of high stability. J Cell Biol. 2005;168:1087–1098. doi: 10.1083/jcb.200501048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rahman M, Davis SR, Pumphrey JG, Bao J, Nau MM, Meltzer PS, Lipkowitz S. TRAIL induces apoptosis in triple-negative breast cancer cells with a mesenchymal phenotype. Breast Cancer Res Treat. 2009;113:217–230. doi: 10.1007/s10549-008-9924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stuckey DW, Shah K. TRAIL on trial: Preclinical advances for cancer therapy. Trends Mol Med. 2013;19:685–694. doi: 10.1016/j.molmed.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]