Abstract
Recent evidence has suggested that downregulation of the Wnt/β-catenin signaling pathway may contribute to the development and growth of HCC. Consequently, elements of this pathway have begun to emerge as potential targets for improving outcomes of anti-HCC. Thus, the present study sought to examine the effects of Wnt-1 blockade using the classical diethylnitrosamine (DEN)-induced chemical carcinogenesis mouse model of HCC. The depletion of Wnt-1 using neutralizing antisera was done for ten consecutive days at the age of 9 months and mice were examined for the following 20 days. At that time, DEN-treated mice had multiple variably-sized hepatic cell adenomas. Anti-Wnt-1 was particularly potent in suppressing the expression of critical elements of the Wnt/β-catenin signaling pathway, such as β-catenin and Frizzled-1 receptor, however, not Dickkopf-related protein 1. This effect co-existed with the suppression of Cyclin D1, FOXM1, NF-κΒ and c-Jun commensurate with proliferation and apoptosis blockade in hepatocellular adenomas, and reduced Bcl-2 and c-Met in the serum of mice. Nonetheless, tumor size and multiplicity were found to be unaffected, suggesting that apoptosis may be equally important to proliferation in the context of counteracting DEN induced hepatocellular adenomas of mice.
Keywords: hepatocellular cancer, diethylnitrosamine, Wnt-1, β-catenin, mice
Introduction
Hepatocellular carcinoma (HCC) is the most common type of liver cancer and among leading causes of cancer-related death of humans (1–3). Rodent models of HCC have been proven useful in revealing aspects of its multistep pathogenesis and preclinical testing of anti-HCC treatments (1,2). Mice have been shown to be particularly useful in that regard and a wide variety of genetically engineered, xenograft and chemically induced models are available for HCC research (1,2,4,5). Among them, the chemically induced model that utilizes diethylnitrosamine (DEN) for HCC initiation is widely used and well-characterized. This model recapitulates aspects of liver injury and fibrosis and hepatitis, which both are the basis of human HCC (1,2,4,6). For that, and because it is comparable to its human counterpart in terms of cancer-associated gene expression patterns and carcinogenetic pathways, it is considered among the best-fit experimental models of HCC (5).
Common molecular pathways of HCC pathogenesis include phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR), c-MET, AMP-activated protein kinase (AMPK), insulin growth factor 1 (IGF-1), H-Ras and vascular endothelial growth factor (VEGF)-mediated angiogenesis (4,7). Recently, the Wnt/β-catenin signaling pathway, mostly known for its contributions in mucosal epithelia cancers, has been added to the list of HCC pathways of carcinogenesis (8–13). In humans, the percentage of HCCs showing activation of this pathway have been reported to range from 20 to 90% (8–13).
Wnt proteins encode a large family of secreted glycoproteins that act as extracellular cell signaling molecules. Their binding to the transmembrane Frizzled (FZD) receptors activates the Wnt/β-catenin pathway that eventually results in the cytoplasmic accumulation and nuclear translocation of the β-catenin protein (11–13). Intranuclear β-catenin binding to T-cell factor 4 (Tcf4) consequently upregulates the expression of many different cancer-related genes, including c-myc and Cyclin-D1 (3,14).
Activating mutations of the β-catenin gene (CTNNB1), loss-of-function mutations in APC and Axin, as well as deregulation of other Wnt/β-catenin pathway elements [ligands, such as Wnt-1; receptors and co-receptors, such as Frizzled-1 (FZL-1), and inhibitors, such as DKK1] have all been implicated in HCC (3,8–13). Wnt/β-catenin as well as other molecular pathways of HCC interrelate with important inflammation, proliferation and apoptosis molecules, such as Forkhead box M1 (FOXM1), NF-κB, c-Jun and B-cell lymphoma 2 (Bcl-2) with important roles in HCC evolution and growth (15–19).
Although blocking of the Wnt/β-catenin pathway has emerged as potential anti-HCC treatment, blocking of the Wnt secreted ligands and especially Wnt-1 has not been adequately tested (9,10). A small number of studies, however, tested the depletion of Wnt-1 on HCC cell cultures and tumor transplant mouse models grafted with HCC cells. These studies show preliminary evidence of tumor suppressive effects (20–23).
In the light of this evidence, the present study aimed to test the effects and the outcome of Wnt-1 blockade in the DEN mouse model of chemically-induced spontaneous hepatocellular carcinogenesis.
Materials and methods
Animals
C56BL/6 male mice weighing 25–27 gr were purchased by the Hellenic Pasteur Institute. Mice were kept in stainless cages at constant 22 to 24°C temperature and allowed free access to food and water during the 24-h day/night cycle. All experimental procedures were performed according to the guide for care and use of laboratory animals (24), and ethical approval and licensing (License reference no. 4956) were provided by the competent National Veterinary Administration Authorities according to Greek legislative (Decree no. 2015/92, 160/91) and European Communities Council directive (no. 86/609/EEC).
Experimental design
A total of 28 male mice were used. At the age of 14 days, mice were injected with a single i.p. injection of the carcinogen N-nitrosodiethylamine (DEN; 5 mg/kg of BW) for the induction of hepatocellular carcinoma (n=22). Ten carcinogen-injected mice were further treated at the age of 9 months with daily i.p. anti-WNT-1 antibody (Abcam, Cambridge, UK; 50 mg/kg of bw) injections for ten consecutive days. Mice were killed with an overdose of ketamine and xylazine during anaesthesia at ten months of age (n=24) with the exception of four mice from the DEN-treated experimental group that were killed at the age of 12 months (n=4) Blood was collected for ELISA and liver tissues were fixed in neutral-buffered formalin 10% for histopathology and immunohistochemistry (IHC).
Histopathology, IHC and morphometry
Formalin-fixed livers were embedded in paraffin, cut at 5 µm, and stained with hematoxylin and eosin or IHC. Primary antibodies for IHC included rabbit antibodies against Ki-67, Cyclin D1, Wnt-1, DKK1 (Abcam), cleaved caspase-3, NF-κB p65, c-Jun (Cell Signaling Technology, Inc., Beverly, MA, USA), β-catenin (Thermo Fisher Scientific/Lab Vision, Fremont, CA, USA), Frizzled 1/Wnt receptor and FOXM1/HFH 11 (Bioss Inc., Woburn, MA, USA). Heat-induced antigen retrieval was performed with citrate buffer, pH 6, for c-Jun, Cyclin D1, cleaved caspase-3, NF-κB p65 and β-catenin or with EDTA buffer, pH 8, for Ki-67, DKK1, FOXM1, Wnt-1 and Frizzled 1/Wnt receptor. Rabbit primary antibody binding was detected with goat anti-rabbit polymer HRP (ZytoChem Plus, Berlin, Germany). Color was developed with Diaminobenzidine substrate-chromogen (Thermo Fisher Scientific/Lab Vision) and tissues were counterstained with hematoxylin.
For quantitative histomorphometry, liver tumors in HE-stained sections were subscribed and their area was automatically measured in image pixels. The size of each tumor was recorded. The total tumor area per total liver area ratio was also calculated for each mouse liver section. IHC-positive cells or pixels were counted in hepatocellular adenoma images of ×20 representative high power fields and results were recorded as number of cells or pixels per image as previously described (25). The ImageJ image processing and analysis program (National Institutes of Health, Bethesda, MD, USA) was used for all histomorphometrical assessments.
Blood serum ELISA
Hepatocyte growth factor receptor (C-MET/HGFR) and Bcl-2 antagonist of cell death (BAD) serum levels were determined using a quantitative sandwich enzyme immunoassay technique of Cusabio Biotech Co., Ltd. (Wuhan, China). Standards and serum samples diluted 1:2 in Sample Diluent and assayed in duplicate in a 96-well microplate, pre-coated with an antibody specific for C-MET/HGFR and BAD, respectively. A 2-h incubation in 37°C was followed by the addition of the appropriate antibody, 1-h incubation in 37°C, washing, the addition of avidin conjugated horseradish peroxidase (HRP), incubation of 1 h in 37°C, washing and the addition of the appropriate substrate. Finally, the reaction was stopped and within 5 min the optical density was determined, using a microplate reader set to 450 nm. A standard curve was created and the concentration of the samples was calculated, taking into account the initial dilution of the samples. The inter-assay and intra-assay precision for both assays were <10% and <8%, respectively. The detection range for C-MET/HGFR was 0.078-5 ng/ml and for BAD was 31.2–2,000 pg/ml.
Statistical analyses
Histomorphometry and serum protein measurements data were compared between groups using Mann-Whitney U analysis. Statistical significance was set at P<0.05. All analyses were performed with the Graphpad Prism version 5.0 for windows (GraphPad Software, San Diego, CA, USA). Data representation was done with bar graphs depicting the mean and standard error of the parameter assessed for each experimental group.
Results
Hepatic cell tumors in DEN-challenged mice
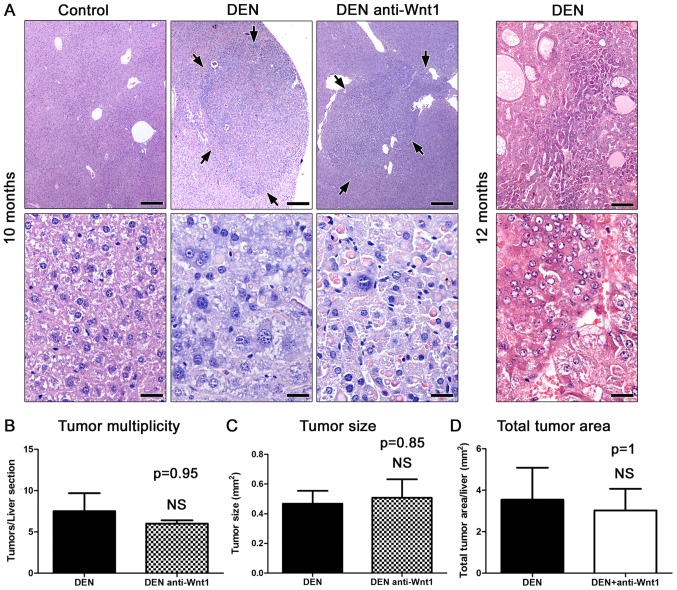
The carcinogen challenged male mice examined at 10 months after DEN administration (n=8) had multiple variably-sized hepatic cell tumors. Histologically, the tumors appeared as sharply demarcated, hypercellular, basophilic hepatic cell nodules. The lesions often compressed the adjacent liver tissue areas in a variable degree of severity. Consistently, the nodules showed loss of normal lobular architecture. The larger ones had peripheral liver plates that formed oblique angles with the corresponding plates of normal adjacent parenchyma. Neoplastic cells showed mild to moderate pleomorphism and atypia, and rare mitotic figures. In their majority tumors contained large amounts of spherical variably-sized eosinophilic hyaline inclusion bodies (Fig. 1A). According to recently published histopathological classification criteria (26), the tumors were diagnosed as hepatocellular adenomas rather than carcinomas, based on absent frank malignancy features, such as necrosis and hemorrhage, indistinct demarcation and trabeculae formation. Upon histological examination, the untreated mice used as controls (n=6) had normal livers.
Figure 1.
Anti-Wnt-1 treatment does not suppress DEN-induced liver tumors in mice. (A) Histopathology of liver tumors. By the age of 10 months DEN-treated mice had hepatocellular adenomas (arrows) appearing as well-circumscribed, hypercellular, slightly basophilic nodules in low-power magnification (upper row). In high-power magnification (bottom row) the liver tumors had typical histomorphology of hepatocellular adenomas including loss of lobular architecture, increased cellular pleomorphism and atypia and eosinophilic inclusion bodies. At the age of 12 months mice had large, highly infiltrative hepatocellular carcinoma masses. The area shown in low-power magnification (upper panel) contains areas with trabecular, solid or acinar growth patterns. Features of malignancy such as increased atypia, neoplastic hepatocellular cell necrosis and hemorrhage can be appreciated in high power magnification (bottom panel). (B-D) Analysis of histomorphometrical features of hepatocellular tumors in 10-month-old mice. The depletion of Wnt-1 applied from the age of 9 months onwards does not affect tumor multiplicity, tumor size and total tumor area at statistically significant levels. (A) Hematoxylin and Eosin. Scale bars: 250 µm (upper row) and 25 µm (bottom row). (B) Numbers on the y-axis of bar graphs correspond to the mean ± SEM of the parameter assessed; NS P>0.05. DEN, diethylnitrosamine.
To confirm the malignant potential of the DEN-induced liver lesions in our experiments, four additional mice (n=4) were examined at the more advanced time-point of 12 months post carcinogen administration. As expected, the liver of all 4 mice had notable well-sized typical HCC lesions. The tumors were highly infiltrative, had indistinct expanding borders and often contained areas of hemorrhage and necrosis. The neoplastic cells were highly pleomorphic and atypical. The degree of differentiation varied from tumor to tumor ranging from well to poorly differentiated. Several different histological types were recognized, including trabecular, solid, acinar and clear cell HHC (Fig. 1A).
DEN-induced liver tumor formation does not require intact Wnt/β-catenin signaling
Accumulating data suggest that the Wnt/β-catenin pathway is involved in liver carcinogenesis (9). To test whether an exogenous disruption of the canonical Wnt/β-catenin signaling pathway might affect DEN-induced carcinogenesis, we treated DEN-challenged mice with neutralizing antibodies against Wnt-1 and examined their livers histologically at 10 months after DEN administration (n=10). By comparison with time-point-matched controls (n=8) the Wnt-1-depleted mice did not show a statistically significant difference in liver tumor multiplicity (Fig. 1B). Likewise, the size of liver tumors induced in the two experimental groups of mice did not differ at significant levels (Fig. 1A and B). To further confirm these findings, we also analyzed the total tumor area found in each liver histological section. As with previous comparisons no significant differences were found (Fig. 1B). The liver tumors of Wnt-1-depleted mice were comparable to control mouse tumors not only quantitatively but qualitatively as well. Indeed, the liver tumors of treated mice had the same typical histomorphological features of hepatocellular adenomas as those found in otherwise untreated mice at 10 months after DEN administration (Fig. 1A). These results suggest that lack of Wnt-1 does not affect hepatocellular tumor formation in the DEN mouse model of liver cancer.
Effects of Wnt-1 depletion on proliferation and apoptosis of liver tumor cells
Proliferation and apoptosis are key events in tumor evolution and progression. The Wnt/β-catenin pathway is involved together with other important signaling pathways in both proliferation and apoptosis of HCC cells (9). For that, we next sought to address whether the depletion of Wnt-1 affected proliferation or apoptosis in the hepatocellular adenomas found in the mice of our study.
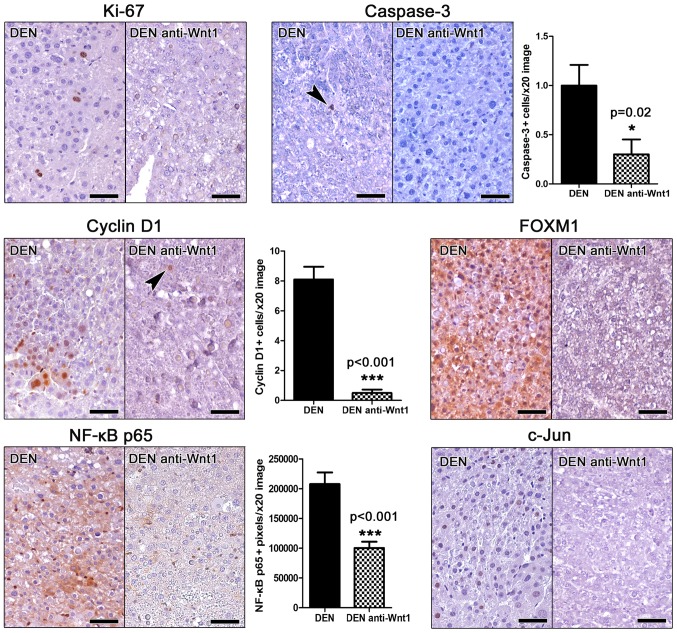
For assessing proliferation, we applied IHC to detect the proliferation marker ki-67 in liver sections. Unlike other mouse cancers stained simultaneously as stain controls, we find that hepatocellular adenomas of DEN-challenged mice contained very few (either none or up to six at the most per tumor) Ki-67-positive neoplastic cells (Fig. 2). Interestingly, we were able to detect none ki-67-positive neoplastic liver cell in the tumors of anti-Wnt-1-treated mice (Fig. 2), whereas other types of cells in the same liver sections showed Ki-67-positive immunostaining (internal stain control).
Figure 2.
Effects of Wnt-1 depletion on the in situ expression of tumor-associated factors. The qualitative or quantitative evaluation of immunohistochemically-stained liver sections shows that anti-Wnt-1 treatment suppresses both proliferation (Ki-67) and apoptosis (caspase-3) of hepatocellular adenoma cells. The cell-cycle regulators Cyclin D1 and FOXM1 and the tumor-associated proteins NF-κB p65 and c-Jun are evidently downregulated by treatment as well. Note that NF-κB p65 can be seen in the cytoplasm but not nucleus of neoplastic hepatocytes. Its nuclear expression restricts to non-hepatocyte stromal cells. IHC; Diaminobenzidine chromogen, hematoxylin counterstain. Scale bars: 50 µm. Numbers on the y-axis of bar graphs correspond to the mean ± SEM of the parameters assessed. *P<0.05, ***P<0.001. DEN, diethylnitrosamine; NF-κB, nuclear factor-κB.
Apoptosis in liver tumors was examined by means of Caspase-3-specific IHC. Similarly to proliferation, apoptosis was also in low levels, with occasional tumors cells showing Caspase-3 positive cytoplasmic signal. However, in Wnt-1-treated animal tumors the presence of apoptotic cells was particularly rare by comparison with liver tumors of untreated mice. To quantify this result we next assessed morphometrically Caspase-3 positive tumor cells. Indeed, we found that although in low numbers, the apoptotic tumor cell counts of Wnt-1-treated mice were lower than non-treated in statistically significant levels (Fig. 2).
These results suggest that DEN-induced liver tumor cells have low proliferative and apoptotic activity. Also, that the depletion of Wnt-1 suppresses further not only proliferation but apoptosis as well in DEN-induced hepatocellular adenomas.
Effects of Wnt-1 depletion on selected tumor markers
Having found that the intervention with neutralizing anti-Wnt-1 antibodies affects the proliferation and apoptosis of tumor cells, we next examined whether it modulated the expression of selected relevant tumor markers.
Cyclin D1 is a key regulator of cell cycle. Its upregulation is an early carcinogenesis event in many different tumor types including hepatocellular neoplasms (11). Wnt-1 blockade has been shown to suppress cyclin D1 expression in hepatocellular carcinoma cells in vitro (22). By applying Cyclin D1-specific IHC in mouse liver sections we found that hepatocellular adenomas in DEN-treated mice contained several Cyclin D1-positive tumor cells. Their anti-Wnt-1-treated counterpart tumors, however, contained significantly less as further confirmed by morphometric assessment (Fig. 2).
The expression of the proto-oncogene key cell cycle regulator Forkhead box M1 (FOXM1) (27) was in accordance to ki-67 and Cyclin D1 results. Indeed, by IHC the hepatocellular adenomas in DEN-treated mice showed ample FOXM1 expression. In contrast, adenomas of Wnt-1-depleted mice had FOXM1 in practically non-detectable levels (Fig. 2).
The activation of NF-κB and c-Jun signaling pathways in tumor cells correlate with increased tumor malignancy in hepatocellular tumors of both humans and mouse models (17). Using IHC and morphometric counts of positively stained image pixels (NF-κB p65), we found that hepatocellular adenomas of anti-Wnt-1-treated mice had significantly less NF-κB p65expression compared to their matched controls and c-Jun (Fig. 2). Also, that they had absent c-Jun-positive cells, whereas the detection of such cells in the tumors of control mice was consistent (Fig. 2).
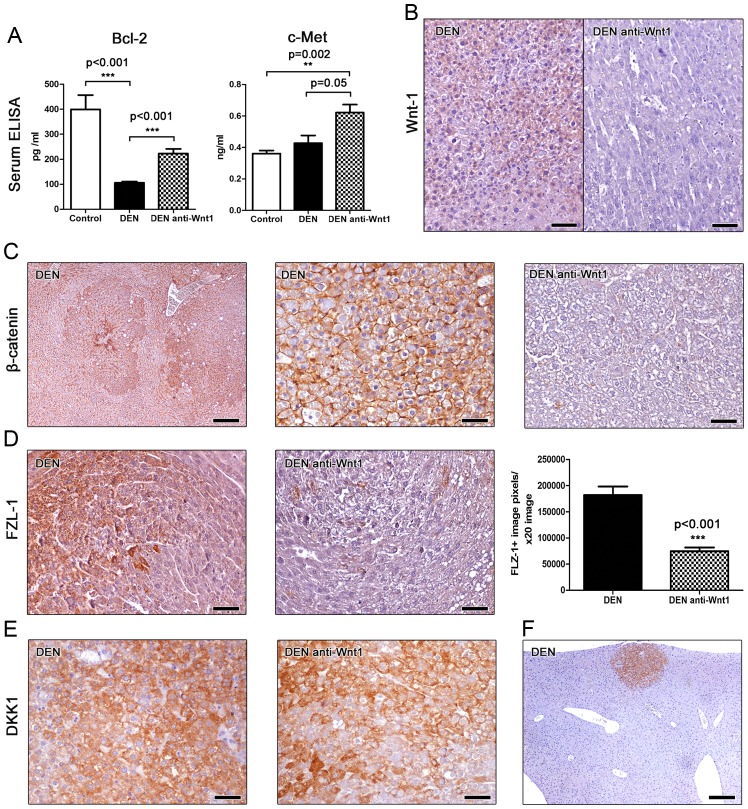
B-cell lymphoma 2 (Bcl-2) is a major anti-apoptotic signaling protein affecting the development of many different neoplasms, including hepatocellular cancer (28). For that, we next assessed Bcl-2 levels in the serum of mice using ELISA. We found that mice bearing liver tumors had significantly lower serum levels of Bcl-2 compared to cancer-free controls. However, treatment with Wnt-1 neutralizing antibodies had a statistically significant effect in increasing circulating levels of Bcl-2 (Fig. 3A). This result suggests that Wnt-1 blockade increases Bcl-2-associated anti-apoptotic signals in mice with DEN-induced liver tumors.
Figure 3.
Effects of Wnt-1 depletion on blood serum tumor markers and neoplastic cell Wnt/β-catenin signaling. (A) Mice with DEN-induced hepatocellular adenomas have significantly reduced Bcl-2 in their blood serum by comparison with tumor-free controls. The levels of Bcl-2 increase significantly after anti-Wnt-1 treatment. Serum c-met is upregulated in mice with liver tumors. Tis effect reaches statistical significance only after the neutralization of Wnt-1. (B) Treatment with Wnt-1-neutralizing antibodies diminishes Wnt-1-specific immunohistochemical signal in liver tumors. (C) Left panel: Well-demarcated hepatocellular adenomas show a more prominent β-catenin immunohistochemical signal than adjacent liver tissue. Middle panel: High-power magnification reveals that increased expression of β-catenin in hepatocellular adenomas does not co-exist with its aberrant cytoplasmic or nuclear stabilization. The protein retains its normal cellular distribution in cell membranes. Anti-Wnt-1 treatment blocks β-catenin expression (right panel of C) and (D) reduces significantly FLZ-1 in liver tumors. (E) By contrast, it does not affect tumoral cell DKK1 expression. (F) Small-sized early neoplastic lesion in the liver of a DEN-treated mouse shows prominent DKK1-specific immunohistochemical signal, allowing its easy microscopical detection. IHC; Diaminobenzidine chromogen, Hematoxylin counterstain. Scale bars: (C) and (H) 250 µm; remaining images, 50 µm. Numbers on the y-axis of bar graphs correspond to the mean ± SEM of the parameters assessed. **P<0.01, ***P<0.001. DEN, diethylnitrosamine; FLZ-1, Frizzled-1; DKK1, Dickkopf-related protein.
By using ELISA, we also measured c-Met serum protein levels in the mice of our study. c-Met, also called hepatocyte growth factor receptor (HGFR), is a tyrosine-protein kinase activator that is abnormally upregulated in liver cancer and has been shown to correlate with poor prognosis (7). We found that serum c-met is upregulated in mice with DEN-induced liver tumors by comparison with cancer-free controls, with the difference reaching statistical significance, however, only for the anti-Wnt-1-treated experimental group. This group showed a considerable statistical significance trend for higher c-met levels when compared to DEN-exposed mice that received no further treatment (Fig. 3A). The circulating c-met level assessment results match our histopathological findings showing that Wnt-1 blockade did not suppress DEN-induced liver tumor formation in mice.
Effects of Wnt-1 depletion on selected Wnt/β-catenin signaling molecules
Utilizing IHC we first tested the efficacy of our depletion strategy in the mouse liver sections. Non-tumoral liver tissue and hepatocellular adenomas of DEN-treated mice showed a mild diffuse Wnt-1-specific staining. The same areas of the livers of mice that were further subjected to Wnt-1 blockade, however, showed absent immunohistochemical signal. This result suggests that no particular Wnt-1 upregulation is observed in DEN-induced hepatocellular adenomas compared to the non-tumoral liver tissue. Also, that the depletion strategy we applied using anti-Wnt-1 antisera worked to bring down the presence of this protein in immunohistochemically non-detectable levels (Fig. 3B).
This result matched similar outcomes of our staining for β-catenin, which is a basic downstream target molecule of Wnt-1 protein (11). The livers of DEN-treated control mice showed normal β-catenin staining. Hepatocellular adenomas in these mice had a more pronounced β-catenin staining compared to non-tumoral liver tissue (Fig. 3C). However, the liver tumor cells did not show aberrant nuclear or cytoplasmic accumulation of β-catenin (Fig. 3C). By contrast, livers from Wnt-1-depleted mice had diminished presence of β-catenin throughout, including both non-tumoral and tumor areas. (Fig. 3C).
The Frizzled-1 (FZL-1) wnt receptor is a basic element of the β-catenin canonical signaling pathway. Upon binding with Wnt-1 the activation of disheveled proteins inhibits glycogen-synthase kinase-3 leading to an aberrant cytoplasmic and nuclear accumulation of β-catenin (11). The anti-FLZ-1 receptor immunohistochemical stain of DEN-treated mouse livers showed a multifocal positivity of hepatocytes, by contrast to untreated normal mouse livers, which had absent IHC signal. FLZ-1 receptor expressing hepatocytes located mostly in (without completely restricting to) the centrilobular zone of hepatic lobules. Neoplastic cells of hepatocellular adenomas, however, showed a more often and prominent positive immunohistochemical signal. The anti-Wnt-1 treatment appeared to reduce FLZ-1 expression in both non-tumoral and neoplastic areas of liver. To quantify FLZ-1 expression in hepatocellular adenomas we next performed morhometrical counts. We found that tumors from Wnt-1-depleted mice had significant less FLZ-1 compared to tumors from untreated controls (Fig. 3D).
Dickkopf-related protein 1 (DKK1) inhibits the WNT signaling pathway and reduces β-catenin expression (3). Immunohistochemically, DKK1 was found to be consistently upregulated in all hepatocellular adenomas found in both anti-Wnt-1-treated and untreated mice. The staining pattern and density was comparable between the two groups (Fig. 3E). Interestingly, upregulation of DKK1 was clearly evident not only in large tumors but in small-sized neoplastic and preneoplastic lesions as well (Fig. 3F). This result suggests that DKK1 upregulation is an early event in DEN-induced mouse liver carcinogenesis. Also, that this upregulation remains unaffected in the absence of Wnt-1.
Discussion
Recent evidence suggests that deregulations of the Wnt/β-catenin signaling pathway contribute to HCC development and growth (3,11–13). Consequently, elements of this pathway started to emerge as potential targets for improving outcomes of anti-HCC treatment (9,10). In the light of this evidence, the present paper examined the effects of Wnt-1 blockade in the classical DEN-induced chemical carcinogenesis mouse model of HCC (5). We found that the depletion of Wnt-1 using neutralizing antisera for ten consecutive days at the age of 9 months was particularly potent in suppressing the expression of critical elements of the Wnt/β-catenin pathway and selected tumor markers. Nonetheless, by examining mouse livers 20 days after the completion of treatment we found that tumor size and multiplicity were not affected.
In our study, mice treated with the chemical carcinogen DEN at 10 months of age had hepatocellular adenomas. The malignant potential of these adenomas was confirmed, since four mice that were kept for an additional period of two months developed well-sized hepatocellular carcinomas. The occurrence, growth and evolution of DEN-induced mouse hepatic proliferative and neoplastic lesions depends on several factors including the dose scheme and administration route of DEN and also age, strain and gender of mice (1,2). Our finding of hepatocellular adenomas in 10-months-old C56BL/6 male mice that were treated with 5 mg/kg BW of DEN matches the results of other published studies using mice of comparable genetic backgrounds and the same gender and similar experimental designs (6,29).
The hepatocellular adenomas found in the present study had unusually low neoplastic cell proliferation and apoptosis levels, which is not similar to what is typically observed in other types of tumors seen in the liver or elsewhere. This unexpected feature of DEN-induced hepatocellular adenomas, however, has been reported by others before. Comparisons between different studies using DEN and mice is difficult due to the usage of different strains of mice, and the application of divergent experimental designs and morphometrical approaches for accessing proliferation and apoptosis. One study that is in many ways comparable to the present one, however, reported that DEN-induced hepatocellular proliferating lesions including adenomas have only 11 PCNA-positive proliferating hepatocytes in every 100 abnormal hepatocytes counted (29). The relatively low proliferating index, which is a typical feature of human hepatocellular adenoma as well (30), probably reflects the low proliferation rate of hepatocytes that in normal conditions rest in the G0 phase of the cell cycle. It also explains the slow growth of hepatocellular tumors in DEN-treated rodent models, that, as in the present study, are not further manipulated to promote tumor initiation and growth (1,2). Similarly to proliferation, apoptosis in hepatocellular adenomas of mice has also been reported to be as low as 0.02 to 0.44% regardless of mouse strain and induction of carcinogenesis protocol (29,31–33). The results of caspase-3 specific IHC performed in the present study further confirm that apoptosis in mouse hepatocellular adenomas is rather rare.
The Wnt-1 glycoprotein is a secreted ligand of the Wnt/β-catenin pathway (34). Its binding to the transmembrane Frizzled (FZD) receptors of cells activates the Wnt/β-catenin pathway leading to cytoplasmic accumulation and nuclear translocation of the β-catenin protein (11–13). Intranuclear β-catenin binds to T-cell factor 4 (Tcf4) and thus activates an array of genes that regulate fundamental cellular functions including proliferation, apoptosis, differentiation and migration (3,9–13). The Wnt/β-catenin pathway plays a significant role in embryonic liver development and post-natal growth. In the adult normal liver, however, it remains inactive with β-catenin restricting to the cell membrane of hepatocytes; its activation, evidenced by cytoplasmic and nuclear stabilization of β-catenin, occurs only in the case of liver regeneration and disease, including hepatocellular cancer (11–13).
Along these lines, the depletion of Wnt-1 and the immunohistochemical stain of selected Wnt/β-catenin pathway proteins in the present study are informative at many different levels. In the context of hepatocellular cancer, Wnt-1 blockade testing is thus far restricted to cell culture studies (20,22,23) or studies using HCC xenograft mouse models (20,22). To the best of our knowledge, our study is the first examining the effects of blocking Wnt-1 in the mouse liver using a spontaneous HCC mouse model.
Using this model and neutralizing antibodies against Wnt-1 for ten consecutive days we found diminished expression of Wnt-1 in the liver of mice 20 days after the end of treatment. This result confirms the efficacy of the depletion treatment applied. It also demonstrates that in this experimental setting the rabbit polyclonal IgG antibody used remained in recipient mouse circulation in adequate numbers for Wnt-1 depletion for at least 20 days. This conclusion is further evidenced by the results of the β-catenin-specific IHC stain we have applied. Indeed, anti-Wnt-1 treatment was potent enough to switch off β-catenin expression in DEN-treated mouse livers, both in the hepatocellular adenomas and in the remaining non-tumoral liver tissue.
In the untreated controls of our study the normal liver showed a weak hepatocyte cell membrane β-catenin staining, which was clearly fainter compared to what has been previously described (11–13,35–37). Other studies in adult mice have reported either a complete lack of specific β-catenin IHC signal (38), or a rather non-specific one along liver sinusoids (38,39). The different primary antibodies and IHC protocols used in the various studies could contribute to these discrepant results, but not completely explain them. Especially, since in most of these studies the IHC assays applied yielded convincing β-catenin-specific IHC stain outcomes in mouse liver tumors (12,13,35–40). Therefore, it is possible that the differences observed may reflect inherent or temporal variances of the subclinical metabolic state of the adult mice examined in each case. Indeed, liver metabolism is influenced by genetic background, diet or even the gut microbiome (8,12,13,35,37,41,42).
Interestingly, by comparison with the normal liver of untreated controls, the non-tumoral liver tissue of DEN-treated mice showed increased expression of β-catenin and FZL-1, but unchanged expression of Wnt-1. The Wnt signaling inhibitor DKK1 had absent expression in the normal liver of untreated controls and the non-lesional liver of DEN-treated mice. In hepatocellular adenomas, the expression of β-catenin and FLZ-1 were further increased, DKK1 expression emerged, but Wnt-1 remained unaffected. The increased β-catenin in the hepatocellular adenomas observed in our study restricted to the cell membranes of hepatocytes, which is consistent with what others have observed in liver adenomas of mice treated with DEN (39) or other chemical carcinogens (43). By contrast, hepatocellular adenomas of certain genetically engineered mouse models of HCC contain cells with aberrant nuclear localization of β-catenin (36).
The depletion of Wnt-1 in our study effectively suppressed β-catenin and FLZ-1 but had no effect on DKK1 expression and failed to reduce size and multiplicity of DEN-induced hepatocellular adenomas. Although the role of Wnt-1, β-catenin and FLZ-1 in the early preneoplastic stages of DEN-induced liver cancer cannot be definitively excluded, our results do not confirm that Wnt-1 blockade counteracts hepatocellular tumors, by contrast to previous studies (20,22,23). It should be noted, however, that those studies base their conclusions on experiments using HCC cell cultures and tumor transplant mouse models grafted with HCC rather than hepatocellular adenoma cells (20,22,23). β-catenin which is an important downstream molecule of Wnt-1-FLZ1 binding is mutated in mice treated with DEN and phenobarbital but not in those treated with DEN alone (4,44). According to another study, β-catenin mutations are absent in DEN-treated mouse hepatocellular adenomas, but appear at the later stage of HCC (39).
Cyclin D1 is a major cell cycle regulator and a target molecule of the Wnt/β-catenin pathway and β-catenin nuclear translocation (14). Cyclin D1 is a proto-oncogene with important roles in the growth and evolution of many types of cancer including HCC (11,13,45). In the present study, hepatocellular adenomas showed no evidence of β-catenin nuclear translocation. Nonetheless, they had neoplastic cells with nuclear Cyclin D1 signal, which suggests that DEN-induced hepatocellular tumors of mice at the stage of adenoma do not require nuclear β-catenin for the initial stages of Cyclin D1 upregulation. On the other hand, however, the disruption of the Wnt/β-catenin pathway in the present study worked to suppress Cyclin D1 in the mouse tumors, which is similar to what has been frequently reported in studies using mouse models of HCC (4,13,20,22,36,46).
Another proto-oncogene key cell cycle regulator examined in our study is FOXM1. This molecule that has also been described to participate in apoptosis and DNA repair processes is overexpressed in various types of cancers, including HCC (27). In line with these findings, we found increased FOXM1 expression in DEN-induced hepatocellular adenomas of mice. We also observed that Wnt-1 blockade suppressed FOXM1 expression in neoplastic hepatocytes. In other types of cancer, such as glioma and lung, cervical and pancreatic cancers FOXM1 is involved in the activation of Wnt pathway and the nuclear translocation of β-catenin (19,47–49). In our study, overexpression of FOXM1, however, did not coincide with nuclear stabilization of β-catenin in neoplastic cells. Furthermore, the Wnt pathway blockade-associated downregulation of FOXM1 did not affect hepatocellular adenomas. Our result agrees with findings in the DEN-phenobarbital mouse model of HCC suggesting that this molecule has unremarkable effects on tumor evolution (33). On the other hand they contrast findings of others which use the same mouse model to demonstrate that FOXM1 is essential for the development of HCC (18).
NF-κB and c-Jun are important pleiotropic molecules that regulate fundamental cell processes including proliferation and apoptosis and their role in DEN-induced liver cancer has been reported (17,50). Specifically, it has been shown that the deletion of either IκB kinase β (IKKβ), which is required for NF-κB activation (17), or c-Jun (50) markedly affected the early stages of tumor development and reduced chemical carcinogenesis. In our study, however, the considerable reduction of both NF-κB and c-Jun in hepatocellular adenomas after Wnt-1 depletion, had no effect in hepatocellular adenomas. It is possible that a longer period of Wnt-1 blockade may have been required for counteracting hepatic tumors in this case.
The hepatocyte growth factor receptor (HGFR) c-Met has been shown to promote all major events of human HCC evolution including tumor cell growth and survival (7). Its tumor promoting role has also been demonstrated in the DEN mouse model of liver cancer (51). In our study, we find that c-met is increased in the blood serum of mice bearing hepatocellular adenomas and that its increased serum levels are not affected by anti-Wnt-1 treatment.
In previous studies using B6C3F1 mice and DEN, Lee et al (29) have shown that the anti-apoptotic molecule Bcl-2 is increased in hepatocellular adenomas (29,52). In the serum of DEN-treated mice, however, we found that Bcl-2 was significantly decreased in comparison with cancer-free controls of the same age. Also, that anti-Wnt-1 treatment worked to increase the circulating levels of Bcl-2, which suggests increased anti-apoptotic signaling after disruption of the Wnt pathway. This suggests a pro-apoptotic function of Wnt-1, which differs from earlier findings of an in vitro study that used cell cultures to show that Wnt-1 signaling inhibits apoptosis (53).
Overall, the results of our study show that ani-Wnt-1 blockade affects an array of important regulators of proliferation and apoptosis. Indeed, immunohistochemical assessment of Ki-67 and caspase-3 showed that anti-Wnt-1 treatment suppressed both proliferation and apoptosis. This anti-proliferative and anti-apoptotic milieu, however, failed to reduce hepatocellular adenomas. This result suggests, that, at least for counteracting DEN-induced liver tumors of mice, reducing proliferation of neoplastic hepatocytes does not suffice. It appears that in this mouse model of cancer, hepatocellular apoptosis is equally important to proliferation in the critical stage of adenoma to carcinoma transition. Furthermore, the present study adds to existing preclinical data aiming to explore the roles of Wnt/β-catenin pathway in hepatocellular cancer. Further testing in mice involving longer periods of anti-Wnt-1 treatment of liver tumors compared to the 10-days-long period applied in the present study may be useful along these lines. A considerable amount of such data is needed before considering therapeutic interventions based on the most fundamental and pleiotropic biological pathways, such as the Wnt/β-catenin one.
Acknowledgements
This study was founded as Research Scholarship by the Experimental-Research Center ELPEN. Also, we would like to express our sincere thanks to Mr. E. Gerakis and Mr. S. Gerakis for their assistance in the implementation of the experiments.
References
- 1.Santos NP, Colaço AA, Oliveira PA. Animal models as a tool in hepatocellular carcinoma research: A review. Tumor Biol. 2017;39:1010428317695923. doi: 10.1177/1010428317695923. [DOI] [PubMed] [Google Scholar]
- 2.Heindryckx F, Colle I, Van Vlierberghe H. Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol. 2009;90:367–386. doi: 10.1111/j.1365-2613.2009.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giakoustidis A, Giakoustidis D, Mudan S, Sklavos A, Williams R. Molecular signalling in hepatocellular carcinoma: Role of and crosstalk among WNT/β-catenin, sonic hedgehog, notch and dickkopf-1. Can J Gastroenterol Hepatol. 2015;29:209–217. doi: 10.1155/2015/172356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim Y, Sills RC, Houle CD. Overview of the molecular biology of hepatocellular neoplasms and hepatoblastomas of the mouse liver. Toxicol Pathol. 2005;33:175–180. doi: 10.1080/01926230590522130. [DOI] [PubMed] [Google Scholar]
- 5.Lee JS, Chu IS, Mikaelyan A, Calvisi DF, Heo J, Reddy JK, Thorgeirsson SS. Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nat Genet. 2004;36:1306–1311. doi: 10.1038/ng1481. [DOI] [PubMed] [Google Scholar]
- 6.Goldfarb S, Pugh TD, Koen H, He YZ. Preneoplastic and neoplastic progression during hepatocarcinogenesis in mice injected with diethylnitrosamine in infancy. Environ Health Perspect. 1983;50:149–161. doi: 10.1289/ehp.8350149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bupathi M, Kaseb A, Meric-Bernstam F, Naing A. Hepatocellular carcinoma: Where there is unmet need. Mol Oncol. 2015;9:1501–1509. doi: 10.1016/j.molonc.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monga SP. Role of Wnt/β-catenin signaling in liver metabolism and cancer. Int J Biochem Cell Biol. 2011;43:1021–1019. doi: 10.1016/j.biocel.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pez F, Lopez A, Kim M, Wands JR, Caron De Fromentel C, Merle P. Wnt signaling and hepatocarcinogenesis: Molecular targets for the development of innovative anticancer drugs. J Hepatol. 2013;59:1107–1117. doi: 10.1016/j.jhep.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Dahmani R, Just PA, Perret C. The Wnt/β-catenin pathway as a therapeutic target in human hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2011;35:709–713. doi: 10.1016/j.clinre.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Thompson MD, Monga SP. WNT/β-catenin signaling in liver health and disease. Hepatology. 2007;45:1298–1305. doi: 10.1002/hep.21651. [DOI] [PubMed] [Google Scholar]
- 12.Nejak-Bowen KN, Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: Sorting the good from the bad. Semin Cancer Biol. 2011;21:44–58. doi: 10.1016/j.semcancer.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monga SP. β-catenin signaling and roles in liver homeostasis, injury and tumorigenesis. Gastroenterology. 2015;148:1294–1310. doi: 10.1053/j.gastro.2015.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway; Proc Natl Acad Sci USA; 1999; pp. 5522–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen Q, Fan J, Yang XR, Tan Y, Zhao W, Xu Y, Wang N, Niu Y, Wu Z, Zhou J, et al. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: A large-scale, multicentre study. Lancet Oncol. 2012;13:817–826. doi: 10.1016/S1470-2045(12)70233-4. [DOI] [PubMed] [Google Scholar]
- 16.Yu B, Yang X, Xu Y, Yao G, Shu H, Lin B, Hood L, Wang H, Yang S, Gu J, et al. Elevated expression of DKK1 is associated with cytoplasmic/nuclear beta-catenin accumulation and poor prognosis in hepatocellular carcinomas. J Hepatol. 2009;50:948–957. doi: 10.1016/j.jhep.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKK beta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Kalinichenko VV, Major ML, Wang X, Petrovic V, Kuechle J, Yoder HM, Dennewitz MB, Shin B, Datta A, Raychaudhuri P, Costa RH. Foxm1b transcription factor is essential for development of hepatocellular carcinomas and is negatively regulated by the p19ARF tumor suppressor. Genes Dev. 2004;18:830–850. doi: 10.1101/gad.1200704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Li Y, Xue J, Gong A, Yu G, Zhou A, Lin K, Zhang S, Zhang N, Gottardi CJ, Huang S. Wnt-induced deubiquitination FoxM1 ensures nucleus β-catenin transactivation. EMBO J. 2016;35:668–684. doi: 10.15252/embj.201592810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang JG, Shi Y, Hong DF, Song M, Huang D, Wang C, Zhao G. miR-148b suppresses cell proliferation and invasion in hepatocellular carcinoma by targeting WNT1/β-catenin pathway. Sci Rep. 2015;5:8087. doi: 10.1038/srep08087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, Xu L, Liu P, Jairam K, Yin Y, Chen K, Sprengers D, Peppelenbosch MP, Pan Q, Smits R. Blocking wnt secretion reduces growth of hepatocellular carcinoma cell lines mostly independent of β-catenin signaling. Neoplasia. 2016;18:711–723. doi: 10.1016/j.neo.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei W, Chua MS, Grepper S, So SK. Blockade of Wnt-1 signaling leads to anti-tumor effects in hepatocellular carcinoma cells. Mol Cancer. 2009;8:76. doi: 10.1186/1476-4598-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahsani Z, Mohammadi-Yeganeh S, Kia V, Karimkhanloo H, Zarghami N, Paryan M. WNT1 gene from wnt signaling pathway is a direct target of mir-122 in hepatocellular carcinoma. Appl Biochem Biotechnol. 2017;181:884–897. doi: 10.1007/s12010-016-2256-8. [DOI] [PubMed] [Google Scholar]
- 24.National Research Council, corp-author. Guide for the Care and Use of Laboratory Animals. 8th. National Academies Press (US); Washington, DC: 2011. p. 118. [Google Scholar]
- 25.Ouzounidis N, Giakoustidis A, Poutahidis T, Angelopoulou K, Iliadis S, Chatzigiagkos A, Zacharioudaki A, Angelopoulos S, Papalois A, Papanikolaou V, Giakoustidis D. IL-18 Binding protein ameliorates ischemia/reperfusion-induced hepatic injury in mice. Liver Transpl. 2016;22:237–246. doi: 10.1002/lt.24359. [DOI] [PubMed] [Google Scholar]
- 26.Thoolen B, Maronpot RR, Harada T, Nyska A, Rousseaux C, Nolte T, Malarkey DE, Kaufmann W, Küttler K, Deschl U, et al. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol Pathol. 2010;38(7 Suppl):5S–81S. doi: 10.1177/0192623310386499. [DOI] [PubMed] [Google Scholar]
- 27.Koo CY, Muir KW, Lam EWF. FOXM1: From cancer initiation to progression and treatment. Biochim Biophys Acta. 2012;1819:28–37. doi: 10.1016/j.bbagrm.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Mott JL, Gores GJ. Piercing the armor of hepatobiliary cancer: Bcl-2 homology domain 3 (BH3) mimetics and cell death. Hepatology. 2007:1–911. doi: 10.1002/hep.21812. [DOI] [PubMed] [Google Scholar]
- 29.Lee GH, Ooasa T, Osanai M. Mechanism of the paradoxical, inhibitory effect of phenobarbital on hepatocarcinogenesis initiated in infant B6C3F1 mice with diethylnitrosamine. Cancer Res. 1998;58:1665–1669. [PubMed] [Google Scholar]
- 30.Kanel GC. Pathology of Liver Diseases. John Wiley & Sons, Ltd.; Hoboken, NJ: 2017. Hepatic Tumors Benign; pp. 266–88. [DOI] [Google Scholar]
- 31.Bursch W, Chabicovsky M, Wastl U, Grasl-Kraupp B, Bukowska K, Taper H, Schulte-Hermann R. Apoptosis in stages of mouse hepatocarcinogenesis: Failure to counterbalance cell proliferation and to account for strain differences in tumor susceptibility. Toxicol Sci. 2005;85:515–529. doi: 10.1093/toxsci/kfi129. [DOI] [PubMed] [Google Scholar]
- 32.Chabicovsky M, Wastl U, Taper H, Grasl-Kraupp B, Schulte-Hermann R, Bursch W. Induction of apoptosis in mouse liver adenoma and carcinoma in vivo by transforming growth factor-beta1. J Cancer Res Clin Oncol. 2003;129:536–542. doi: 10.1007/s00432-003-0460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalinina OA, Kalinin SA, Polack EW, Mikaelian I, Panda S, Costa RH, Adami GR. Sustained hepatic expression of FoxM1B in transgenic mice has minimal effects on hepatocellular carcinoma development but increases cell proliferation rates in preneoplastic and early neoplastic lesions. Oncogene. 2003;22:6266–6276. doi: 10.1038/sj.onc.1206640. [DOI] [PubMed] [Google Scholar]
- 34.Nusse R, Brown A, Papkoff J, Scambler P, Shackleford G, McMahon A, Moon R, Varmus H. A new omenclature for int-1 and related genes: The wnt gene family. Cell. 1991;64:231. doi: 10.1016/0092-8674(91)90633-A. [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Mowry LE, Nejak-Bowen KN, Okabe H, Diegel CR, Lang RA, Williams BO, Monga SP. β-catenin signaling in murine liver zonation and regeneration: A Wnt-Wnt situation. Hepatology. 2014;60:964–976. doi: 10.1002/hep.27082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calvisi DF, Factor VM, Loi R, Thorgeirsson SS. Activation of beta-catenin during hepatocarcinogenesis in transgenic mouse models: Relationship to phenotype and tumor grade. Cancer Res. 2001;61:2085–2091. [PubMed] [Google Scholar]
- 37.Benhamouche S, Decaens T, Godard C, Chambrey R, Rickman DS, Moinard C, Vasseur-Cognet M, Kuo CJ, Kahn A, Perret C, Colnot S. Apc tumor suppressor gene Is the ‘Zonation-Keeper’ of mouse liver. Dev Cell. 2006;10:759–770. doi: 10.1016/j.devcel.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 38.Salleng KJ, Revetta FL, Deane NG, Washington MK. The applicability of a human immunohistochemical panel to mouse models of hepatocellular neoplasia. Comp Med. 2015;65:398–408. [PMC free article] [PubMed] [Google Scholar]
- 39.Ogawa K, Yamada Y, Kishibe K, Ishizaki K, Tokusashi Y. Beta-catenin mutations are frequent in hepatocellular carcinomas but absent in adenomas induced by diethylnitrosamine in B6C3F1 mice. Cancer Res. 1999;59:1830–1833. [PubMed] [Google Scholar]
- 40.Colnot S, Decaens T, Niwa-Kawakita M, Godard C, Hamard G, Kahn A, Giovannini M, Perret C. Liver-targeted disruption of Apc in mice activates beta-catenin signaling and leads to hepatocellular carcinomas; Proc Natl Acad Sci USA; 2004; pp. 17216–17221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M. Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology. 2006;43:817–825. doi: 10.1002/hep.21131. [DOI] [PubMed] [Google Scholar]
- 42.Tilg H, Cani PD, Mayer EA. Gut microbiome and liver diseases. Gut. 2016;65:2035–2044. doi: 10.1136/gutjnl-2016-312729. [DOI] [PubMed] [Google Scholar]
- 43.Devereux TR, Anna CH, Foley JF, White CM, Sills RC, Barrett JC. Mutation of beta-catenin is an early event in chemically induced mouse hepatocellular carcinogenesis. Oncogene. 1999;18:4726–4733. doi: 10.1038/sj.onc.1202858. [DOI] [PubMed] [Google Scholar]
- 44.Aydinlik H, Nguyen TD, Moennikes O, Buchmann A, Schwarz M. Selective pressure during tumor promotion by phenobarbital leads to clonal outgrowth of beta-catenin-mutated mouse liver tumors. Oncogene. 2001;20:7812–7816. doi: 10.1038/sj.onc.1204982. [DOI] [PubMed] [Google Scholar]
- 45.Diehl JA. Cycling to cancer with cyclin D1. Cancer Biol Ther. 2002;1:226–231. doi: 10.4161/cbt.72. [DOI] [PubMed] [Google Scholar]
- 46.Hu J, Dong A, Fernandez-Ruiz V, Shan J, Kawa M, Martínez-Ansó E, Prieto J, Qian C. Blockade of Wnt signaling inhibits angiogenesis and tumor growth in hepatocellular carcinoma. Cancer Res. 2009;69:6951–6959. doi: 10.1158/0008-5472.CAN-09-0541. [DOI] [PubMed] [Google Scholar]
- 47.Quan M, Cui J, Xia T, Jia Z, Xie D, Wei D, Huang S, Huang Q, Zheng S, Xie K. Merlin/NF2 suppresses pancreatic tumor growth and metastasis by attenuating the foxm1-mediated wnt/β-catenin signaling. Cancer Res. 2015;75:4778–4789. doi: 10.1158/0008-5472.CAN-14-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su J, Wu S, Wu H, Li L, Guo T. CD44 is functionally crucial for driving lung cancer stem cells metastasis through Wnt/β-catenin-FoxM1-twist signaling. Mol Carcinog. 2016;55:1962–1973. doi: 10.1002/mc.22443. [DOI] [PubMed] [Google Scholar]
- 49.Wang T, Liu Z, Shi F, Wang J. Pin1 modulates chemo-resistance by up-regulating FoxM1 and the involvements of Wnt/β-catenin signaling pathway in cervical cancer. Mol Cell Biochem. 2016;413:179–187. doi: 10.1007/s11010-015-2651-4. [DOI] [PubMed] [Google Scholar]
- 50.Eferl R, Ricci R, Kenner L, Zenz R, David JP, Rath M, Wagner EF. Liver tumor development: c-Jun antagonizes the proapoptotic activity of p53. Cell. 2003;112:181–192. doi: 10.1016/S0092-8674(03)00042-4. [DOI] [PubMed] [Google Scholar]
- 51.Horiguchi N, Takayama H, Toyoda M, Otsuka T, Fukusato T, Merlino G, Takagi H, Mori M. Hepatocyte growth factor promotes hepatocarcinogenesis through c-Met autocrine activation and enhanced angiogenesis in transgenic mice treated with diethylnitrosamine. Oncogene. 2002;21:1791–1799. doi: 10.1038/sj.onc.1205248. [DOI] [PubMed] [Google Scholar]
- 52.Lee GH. Correlation between Bcl-2 expression and histopathology in diethylnitrosamine-induced mouse hepatocellular tumors. Am J Pathol. 1997;151:957–961. [PMC free article] [PubMed] [Google Scholar]
- 53.Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW, Kitajewski J, Wang CY. Wnt-1 signaling inhibits apoptosis by activating β-catenin/T cell factor-mediated transcription. J Cell Biol. 2001;152:87–96. doi: 10.1083/jcb.152.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]