Abstract
The objective of this study was to explore the causes of death in Chinese patients with multiple system atrophy (MSA) as well as differences in the cause of death according to sex, subtype, disease onset, and whether the disease was accompanied by nocturnal stridor. A total of 131 MSA patients were enrolled and followed up once every year until their deaths. Clinical information was collected by neurologists, and the cause of death of the MSA patients was obtained from the patients’ relatives or caregivers. The current study included 62 MSA with predominant parkinsonism (MSA-P) and 69 MSA with predominant cerebellar ataxia (MSA-C) patients. Median survival time from disease onset to death of the MSA patients was 5.59 years. The most common cause of death was respiratory infection (65.6%). The second most common cause of death was sudden death (14.5%). Other causes included nutritional disorder due to dysphagia (9.2%), urinary tract infection (3.1%), suicide (2.3%), choking (1.5%), cerebrovascular accident (1.5%), myocardial infarction (1.5%), and lymphoma (0.8%). We found that sudden death was more likely to occur in patients with nocturnal stridor than in those without (P<0.001). There were no significant differences in the cause of death according to subtype, sex, or onset symptoms (autonomic failure or motor symptoms). Sudden death is a relatively common cause of death in MSA patients, second only to respiratory infection, especially in patients with nocturnal stridor. The information provided by our study may help to provide better medical care to MSA patients.
Keywords: multiple system atrophy, cause of death, nocturnal stridor
Multiple system atrophy (MSA) is a sporadic, adult-onset neurodegenerative disease with a heterogeneous combination of autonomic failure, cerebellar ataxia, parkinsonian features, and pyramidal signs [1]. The estimated annual incidence of MSA is about 0.6 per 100 000 per year, reaching 3 per 100 000 per year in the Caucasian population older than 50 years [1]. The crude prevalence rate was 13 per 100 000 for MSA in a rural Japanese district after aging adjustment [2]. There are no epidemiological data of MSA patients in China at present. MSA-P is common in Western countries, while MSA-C is more common in Asian countries [1]. Patients with MSA have a shorter survival time, with a median survival time (from symptom onset to death) of approximately 6~10 years [3-9]. Varied factors have been presented to predict the survival of patients with MSA, such as autonomic failure, the parkinsonian variant of MSA, and older age of onset [8-10]. Patients with MSA have been reported to die of respiratory infection, sudden death, choking, cancer, suicide, and stroke, among other causes [5, 11-18]. The frequency of different causes of death in MSA is inconsistent across studies. Some studies found that the leading cause of death was sudden death (26% ~ 70%) in MSA [11, 13, 14, 16]; others reported that respiratory infection was the most common cause of death in MSA [12, 18]. Only two small studies focused on the relationship between stridor and death, suggesting that stridor increased the risk of death [12, 13]. A few patients with MSA have been reported to die of suicide.
However, the sample sizes of these studies that focused on the causes of death in MSA were relatively small. In addition, some studies used the previous diagnostic criteria and classification of MSA, but there is a new consensus on MSA [19]. Whether the causes of death in MSA patients differ according to sex, subtypes, and onset symptoms remains largely unknown. Whether the causes of death differ between patients with and without nocturnal stridor is also unknown. Better understanding of the causes of death in patients with MSA could help to provide better medical advice and care to the MSA patients at the end of their lives. Thus, exploring the causes of death in MSA patients is a matter of great significance.
MATERIALS AND METHODS
Patients and clinical data
One hundred and thirty-one patients with MSA were recruited from the Department of Neurology, West China Hospital of Sichuan University. These patients were followed up every year by face-to-face interview or by telephone interview (if they could not come to the hospital) until their deaths between 2010 and 2016. All MSA patients met the second consensus criteria for probable MSA [20]. All patients received brain MRI scans and other supplemental tests, such as spinal cerebellar ataxia genetic tests (SCA1, 2, 3, 6, 7), immunological tests, and tumor marker tests to exclude other neurological diseases. All patients were diagnosed in the department by movement specialists (HuiFang Shang, Ying Wu, and Bi Zhao). Neurologists (LingYu Zhang, Bei Cao, QianQian Wei, RuWei Ou, and Jing Yang who passed the consistency check before conducted the study) collected the clinical information of the MSA patients, including age, sex, age of onset, and onset symptoms and so on during face-to face interviews when they first came to our department; these data were input into our MSA patient database. The clinical and demographic information of the MSA patients during follow-up were also recorded in our database. Disease onset was defined as the initial presentation of any motor problems, whether parkinsonian or cerebellar, or autonomic features, with the exception of male erectile dysfunction [20]. Survival time was defined as the time of the disease onset until death. Whether the patients had nocturnal stridor was determined by asking the patients and a family member (bed partner). The causes of deaths of the MSA patients were obtained from the patients’ relatives or caregivers according to the death certification or the latest hospital record.
The present study was approved by the ethics committee of the West China Hospital of Sichuan University. All subjects signed informed consent forms.
Statistical analysis
All continuous data, including the age of onset, disease duration, and age of death, were presented as the mean ± standard deviation. Differences between subgroups were evaluated using the chi-square test or Fisher exact test. Kaplan-Meyer analysis was used to establish the survival curve of our MSA patients. All the data analyses were performed using SPSS 22.0 (IBM, Chicago, IL). A value of P < 0.05 was considered statistically significant.
RESULTS
The demographic and clinical characteristics of the patients with MSA are presented in Table 1. Among the 131 MSA patients, including 73 male and 58 female patients, 62 were MSA-P and 69 were MSA-C patients. The mean age of onset was 56.94 ± 7.50 years, the mean survival time was 5.61 ± 1.77 years, and the mean age of death was 62.55 ± 7.50 years. The median survival from disease onset to death was 5.59 years (Fig. 1).
Table 1.
Demographic and clinical characteristics of the patients with MSA.
| Variables | MSA |
|---|---|
| Diagnosis (MSA-P/MSA-C) | 62/69(47.3%/52.7%) |
| Sex (male/female) | 73/58(55.7%/44.3%) |
| Age of onset | 56.94 ± 7.50 |
| Disease duration | 5.61 ± 1.77 |
| Age of death | 62.55 ± 7.50 |
MSA, multiple system atrophy; MSA-P, multiple system atrophy with predominately parkinsonism; MSA-C, multiple system atrophy with predominately cerebellar ataxia.
Figure 1. Kaplan-Meier survival curve of the 131 MSA patients.
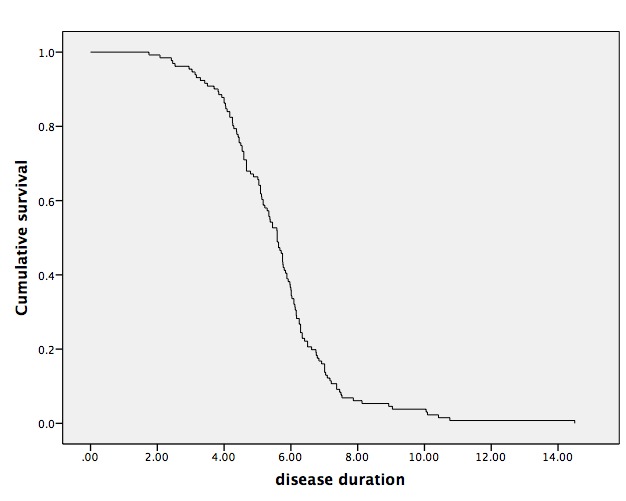
Median survival from symptom onset to death was 5.59 years.
As shown in Table 2, the most common cause of death was respiratory infection (86,65.6%), which included aspiration pneumonia. The second most common cause of death was sudden death (19, 14.5%), which occurred in 12 patients (9.2%) during the day and in 7 patients (5.3%) at night. Twelve patients (9.2%) died of a nutritional disorder due to dysphagia, 4 patients (3.1%) died of a urinary tract infection, 3 patients (2.3%) died of suicide, 2 patients (1.5%) died of choking, 2 patients (1.5%) died of cerebrovascular accident, 2 patients (1.5%) died of myocardial infarction, and one patient (0.8%) died of lymphoma.
Table 2.
Cause of death of MSA patients according to gender and subtype.
| Cause of death | Total (131) N (%) |
Female (58) N (%) |
Male (73) N (%) |
P-value# | MSA-C (69) N (%) |
MSA-P (62) N (%) |
P-value# |
|---|---|---|---|---|---|---|---|
| Respiratory infection | 86(65.6) | 38(65.5) | 48(65.8) | 0.977 | 41(59.4) | 45(72.6) | 0.113 |
| Choking | 2(1.5) | 1(1.7) | 1(1.4) | 1.000 | 1(1.4) | 1(1.6) | 1.000 |
| Urinary tract infection | 4(3.1) | 2(3.4) | 2(2.7) | 1.000 | 3(4.3) | 1(1.6) | 0.689 |
| Nutritional disorder | 12(9.2) | 8(13.8) | 4(5.5) | 0.101 | 8(11.6) | 4(6.5) | 0.308 |
| Lymphoma | 1(0.8) | 0(0.0) | 1(1.4) | 1.000 | 1(1.4) | 0(0.0) | 1.000 |
| Cerebrovascular accident | 2(1.5) | 0(0.0) | 2(2.7) | 0.503 | 1(1.4) | 1(1.6) | 1.000 |
| Myocardial infarction | 2(1.5) | 0(0.0) | 2(2.7) | 0.503 | 2(2.9) | 0(0.0) | 0.498 |
| Suicide | 3(2.3) | 0(0.0) | 3(4.1) | 0.330 | 3(4.3) | 0(0.0) | 0.282 |
| Sudden death | 19(14.5) | 9(15.5) | 10(13.7) | 0.769 | 9(13.0) | 10(16.1) | 0.617 |
| Nighttime | 7(5.3) | 1(1.7) | 6(8.2) | 0.211 | 5(7.2) | 2(3.2) | 0.527 |
| Daytime | 12(9.2) | 8(13.8) | 4(5.5) | 0.101 | 4(5.8) | 8(12.9) | 0.159 |
Chi-square or Fischer exact test.1
MSA, multiple system atrophy; MSA-P, multiple system atrophy with predominately parkinsonism; MSA-C, multiple system atrophy with predominately cerebellar ataxia.
There were no significant differences in the causes of death between the MSA-P and MSA-C patients (Table 2), between the male and female patients (Table 2), or between the patients with autonomic failure or motor symptoms (parkinsonism or cerebellar ataxia symptoms) as onset symptoms (Table 3). Sudden death was more likely to occur in patients with nocturnal stridor than in those without (42.9% vs. 9.1%, P < 0.001), regardless of whether death occurred during the day or night. The percentage of respiratory infection was higher in patients without nocturnal stridor than in patients with nocturnal stridor (70.9% vs. 38.1%, P = 0.004). The remaining causes of death did not significantly different between the patients with and without nocturnal stridor.
Table 3.
Cause of death of MSA patients according to disease onset.
| Cause of death | Total (131) N (%) |
Autonomic failure (81) N (%) |
Cerebellar ataxia or parkinsonism (50) N (%) |
P-value# |
|---|---|---|---|---|
| Respiratory infection | 86(65.6) | 50(61.7) | 36(72.0) | 0.229 |
| Choking | 2(1.5) | 1(1.2) | 1(2.0) | 1.000 |
| Urinary tract infection | 4(3.1) | 4(4.9) | 0(0.0) | 0.283 |
| Nutritional disorder | 12(9.2) | 9(11.1) | 3(6.0) | 0.501 |
| Lymphoma | 1(0.8) | 0(0.0) | 1(2.0) | 0.382 |
| Cerebrovascular accident | 2(1.5) | 2(2.5) | 0(0.0) | 0.524 |
| Myocardial infarction | 2(1.5) | 0(0.0) | 2(4.0) | 0.144 |
| Suicide | 3(2.3) | 3(3.7) | 0(0.0) | 0.438 |
| Sudden death | 19(14.5) | 12(14.8) | 7(14.0) | 0.898 |
| Nighttime | 7(5.3) | 4(4.9) | 3(6.0) | 1.000 |
| Daytime | 12(9.2) | 8(9.9) | 4(8.0) | 0.960 |
Chi-square or Fischer exact test.
MSA, multiple system atrophy.
Table 4.
Cause of death of MSA patient according to whether the disease was accompanied by nocturnal stridor.
| Cause of death | Total (131) N (%) |
Nocturnal stridor
|
P-value# | |
|---|---|---|---|---|
| No (110) N (%) |
Yes (21) N (%) |
|||
| Respiratory infection | 86(65.6) | 78(70.9) | 8(38.1) | 0.004* |
| Choking | 2(1.5) | 2(1.8) | 0(0.0) | 1.000 |
| Urinary tract infection | 4(3.1) | 4(3.6) | 0(0.0) | 1.000 |
| Nutritional disorder | 12(9.2) | 8(7.3) | 4(19.0) | 0.193 |
| Lymphoma | 1(0.8) | 1(0.9) | 0(0.0) | 1.000 |
| Cerebrovascular accident | 2(1.5) | 2(1.8) | 0(0.0) | 1.000 |
| Myocardial infarction | 2(1.5) | 2(1.8) | 0(0.0) | 1.000 |
| Suicide | 3(2.3) | 3(2.7) | 0(0.0) | 1.000 |
| Sudden death | 19(14.5) | 10(9.1) | 9(42.9) | 0.000* |
| Nighttime | 7(5.3) | 3(2.7) | 4(19.0) | 0.012* |
| Daytime | 12(9.2) | 7(6.4) | 5(23.8) | 0.033* |
Chi-square or Fischer exact test.
Significant difference.
MSA, multiple system atrophy
DISCUSSION
This is a prospective study in that we followed up 131 MSA patients until their deaths. Our present study showed that the leading cause of death in the MSA patients was respiratory infection, followed by sudden death and nutritional disorders. Sudden death was more common in the patients with nocturnal stridor than in those without. The median survival time from symptom onset to death was 5.59 years, which is in line with an Icelandic study that found a median survival time of 5.7 years [6] and a meta-analysis of 433 patients with autopsy-proven MSA that reported a survival time of 6.2 years [3]. However, it was shorter than the findings of other studies that reported a median survival time ranging from 7 to 10 years [4, 5, 7-9]. Several reasons may explain such a discrepancy. It was proved that the early development of autonomic dysfunction is a predictive factor for shorter survival in patients with MSA [5, 21]. The percentage of MSA patients who had autonomic failure (61.8%) as the initial symptom in the current study was higher than that of other studies (23%~50%) [4, 5, 7, 8]. In addition, our patients mainly came from southwest China, where the economy is underdeveloped.
The leading cause of death was respiratory infection (86, 65.6%), which is in accordance with previous studies [12, 18]. Flabeau, et al. found that aspiration pneumonia (9/15, 60%) was the most common cause of death during follow-up, as it was the cause of death of 15 of the 28 enrolled MSA patients [18]. In the late stage of the disease, almost all patients were bedridden at home and their autoimmune function was decreased, which increases the risk of infection. Of the 86 MSA patients who died of respiratory infection, some patients had a history of swallowing aspiration, which increases the risk of respiratory infection.
The second most common cause of death was sudden death (14.5%). In agreement with another study [13], we found that patients with nocturnal stridor were more likely to die of sudden death than those without. Laryngeal dysfunction probably plays an important role in the sudden death of stridor patients [13]. Patients with nocturnal stridor experience an increased risk of developing obstructive sleep apnea (OSA) [22]. Although most of our patients in the current study did not receive the polysomnography (PSG) test, our previous study of MSA patients with PSG investigation found that 70% of MSA patients have OSA [23]. OSA increases the risk of nighttime death in MSA patients [12]. We should advise MSA patients with nocturnal stridor to identify OSA using PSG, and such patients should use a non-invasive ventilator (NIV) during sleep to prevent sleep apnea. Tracheostomy can be proposed to patients with MSA who have stridor to reduce the risk of sudden death [12]. Upper airway dysfunction associated with autonomic failure and dysfunction of the medullary serotonergic system, which regulates the cardiovascular and respiratory systems, could also be responsible for sudden death in patients with MSA [24, 25] since sudden death also occurred during the daytime.
In addition, 9.2% of the MSA patients died of a nutritional disorder. The progression of cerebellar dysfunction and overlapping parkinsonism will worsen tongue movements, and in the late stage of the disease, swallowing function of the oral phase (bolus transport and bolus holding) is remarkably disturbed [26]. Several patients died of a nutritional disorder, with severe weight loss at the late stage of the disease due to dysphagia since they were unable to tolerate the discomfort of a nasogastric tube and did not accept percutaneous endoscopic gastrostomy (PEG) treatment. In addition, choking (1.5%) was one of the causes of death in our patients. Caregivers should pay attention to patients’ dietary traits to reduce the risk of choking. Nasogastric tube or PEG should be recommended to patients when it is necessary. Beyond that, parenteral nutrition can also be taken into consideration.
Overall, 3.1% of the patients died of a urinary tract infection. Neurogenic urinary dysfunction, including urinary incontinence, unexplained urinary urgency or frequency or incomplete bladder emptying, is one of the diagnostic criteria for MSA [20]. Many patients complained of urinary dysfunction, and some suffered a lower urinary tract infection. However, although clean intermittent self-catheterization (CISC) is the recommended treatment for most MSA patients with urinary retention, only a few patients took our advice to perform CISC at home, by either themselves or their family member. Patients who have difficulty in performing CISC can also take urethra-oriented medication and surgery into account [27].
Although the current study found that only 3 patients died of suicide, our previous study showed that not only disease severity but also severe non-motor symptoms (especially mood disorder) had a negative impact on the quality of life in MSA [28]. Furthermore, depression has been reported to be common in MSA [29, 30]. We should not only keep an eye on the motor symptoms but also pay attention to mood disorders to avoid the occurrence of suicide.
Furthermore, our present study showed that sex, subtype, and onset symptoms did not influence the cause of death in patients with MSA. However, this study also had limitations. The patients were registered from a single center and may not represent the Chinese MSA patients as a whole, so it needs further multicenter validation in China. Most of our patients did not receive the PSG test, so some patients who had nocturnal stridor could have been missed.
Conclusion
In conclusion, this is the first large study to evaluate the clinical causes of death in Chinese patients with MSA. Although there were several causes of death in MSA, respiratory infection was the major cause. Sudden death was the second most common cause of mortality and was especially common in patients with nocturnal stridor.
Acknowledgments
We would like to thank the patients and their families for their participation in the study. The present study was supported by funding from the Young scholars' scientific research fund of Sichuan University (Grant No. 2016SCU11017) and the National Science Fund of China (Grant No. 81600979).
Footnotes
Conflicts of interest
None
References
- [1].Stefanova N, Bucke P, Duerr S, Wenning GK (2009). Multiple system atrophy: an update. Lancet Neurol, 8: 1172-1178 [DOI] [PubMed] [Google Scholar]
- [2].Osaki Y, Morita Y, Kuwahara T, Miyano I, Doi Y (2011). Prevalence of Parkinson's disease and atypical parkinsonian syndromes in a rural Japanese district. Acta neurologica Scandinavica, 124: 182-187 [DOI] [PubMed] [Google Scholar]
- [3].Ben-Shlomo Y, Wenning GK, Tison F, Quinn NP (1997). Survival of patients with pathologically proven multiple system atrophy: a meta-analysis. Neurology, 48: 384-393 [DOI] [PubMed] [Google Scholar]
- [4].Watanabe H, Saito Y, Terao S, Ando T, Kachi T, Mukai E,et al. (2002). Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain, 125: 1070-1083 [DOI] [PubMed] [Google Scholar]
- [5].Tada M, Onodera O, Tada M, Ozawa T, Piao YS, Kakita A,et al. (2007). Early development of autonomic dysfunction may predict poor prognosis in patients with multiple system atrophy. Arch Neurol, 64: 256-260 [DOI] [PubMed] [Google Scholar]
- [6].Bjornsdottir A, Gudmundsson G, Blondal H, Olafsson E (2013). Incidence and prevalence of multiple system atrophy: a nationwide study in Iceland. J Neurol Neurosurg Psychiatry, 84: 136-140 [DOI] [PubMed] [Google Scholar]
- [7].Figueroa JJ, Singer W, Parsaik A, Benarroch EE, Ahlskog JE, Fealey RD,et al. (2014). Multiple system atrophy: prognostic indicators of survival. Mov Disord, 29: 1151-1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Coon EA, Sletten DM, Suarez MD, Mandrekar JN, Ahlskog JE, Bower JH,et al. (2015). Clinical features and autonomic testing predict survival in multiple system atrophy. Brain, 138: 3623-3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Low PA, Reich SG, Jankovic J, Shults CW, Stern MB, Novak P,et al. (2015). Natural history of multiple system atrophy in the USA: a prospective cohort study. Lancet. Neurol, 14: 710-719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wenning GK, Geser F, Krismer F, Seppi K, Duerr S, Boesch S,et al. (2013). The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol, 12: 264-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Saito Y, Matsuoka Y, Takahashi A, Ohno Y (1994). Survival of patients with multiple system atrophy. Intern Med, 33: 321-325 [DOI] [PubMed] [Google Scholar]
- [12].Silber MH, Levine S (2000). Stridor and death in multiple system atrophy. Mov Disord, 15: 699-704 [DOI] [PubMed] [Google Scholar]
- [13].Yamaguchi M, Arai K, Asahina M, Hattori T (2003). Laryngeal stridor in multiple system atrophy. Eur Neurol, 49: 154-159 [DOI] [PubMed] [Google Scholar]
- [14].Papapetropoulos S, Tuchman A, Laufer D, Papatsoris AG, Papapetropoulos N, Mash DC (2007). Causes of death in multiple system atrophy. J Neurol Neurosurg Psychiatry, 78: 327-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schrag A, Wenning GK, Quinn N, Ben-Shlomo Y (2008). Survival in multiple system atrophy. Mov Disord, 23: 294-296 [DOI] [PubMed] [Google Scholar]
- [16].Shimohata T, Ozawa T, Nakayama H, Tomita M, Shinoda H, Nishizawa M (2008). Frequency of nocturnal sudden death in patients with multiple system atrophy. J Neurol, 255: 1483-1485 [DOI] [PubMed] [Google Scholar]
- [17].Maule S, Milazzo V, Maule MM, Di Stefano C, Milan A, Veglio F (2012). Mortality and prognosis in patients with neurogenic orthostatic hypotension. Funct Neurol, 27: 101-106 [PMC free article] [PubMed] [Google Scholar]
- [18].Flabeau O, Ghorayeb I, Perez P, Maillard A, Taillard J, Philip P,et al. (2016). Impact of sleep apnea syndrome on survival in patients with multiple system atrophy. Parkinsonism Relat Disord. [DOI] [PubMed] [Google Scholar]
- [19].Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ,et al. (2008). Second consensus statement on the diagnosis of multiple system atrophy. Neurology, 71: 670-676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ,et al. (2008). Second consensus statement on the diagnosis of multiple system atrophy. Neurology, 71: 670-676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].O'Sullivan SS, Massey LA, Williams DR, Silveira-Moriyama L, Kempster PA, Holton JL,et al. (2008). Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain, 131: 1362-1372 [DOI] [PubMed] [Google Scholar]
- [22].Gaig C, Iranzo A (2012). Sleep-disordered breathing in neurodegenerative diseases. Curr Neurol Neurosci Rep, 12: 205-217 [DOI] [PubMed] [Google Scholar]
- [23].Guo XY, Cao B, Lei F, Huang L, Chen K, Song W,et al. (2013). Clinical and polysomnographic features of patients with multiple system atrophy in Southwest China. Sleep Breath, 17: 1301-1307 [DOI] [PubMed] [Google Scholar]
- [24].Munschauer FE, Loh L, Bannister R, Newsom-Davis J (1990). Abnormal respiration and sudden death during sleep in multiple system atrophy with autonomic failure. Neurology, 40: 677-679 [DOI] [PubMed] [Google Scholar]
- [25].Tada M, Kakita A, Toyoshima Y, Onodera O, Ozawa T, Morita T,et al. (2009). Depletion of medullary serotonergic neurons in patients with multiple system atrophy who succumbed to sudden death. Brain, 132: 1810-1819 [DOI] [PubMed] [Google Scholar]
- [26].Higo R, Nito T, Tayama N (2005). Swallowing function in patients with multiple-system atrophy with a clinical predominance of cerebellar symptoms (MSA-C). Eur Arch Otorhinolaryngol, 262: 646-650 [DOI] [PubMed] [Google Scholar]
- [27].Ito T, Sakakibara R, Yasuda K, Yamamoto T, Uchiyama T, Liu Z,et al. (2006). Incomplete emptying and urinary retention in multiple-system atrophy: when does it occur and how do we manage it? Mov Disord, 21: 816-823 [DOI] [PubMed] [Google Scholar]
- [28].Zhang L, Cao B, Ou R, Wei QQ, Zhao B, Yang J,et al. (2017). Non-motor symptoms and the quality of life in multiple system atrophy with different subtypes. Parkinsonism Relat Disord, 35: 63-68 [DOI] [PubMed] [Google Scholar]
- [29].Benrud-Larson LM, Sandroni P, Schrag A, Low PA (2005). Depressive symptoms and life satisfaction in patients with multiple system atrophy. Mov Disord, 20: 951-957 [DOI] [PubMed] [Google Scholar]
- [30].Schrag A, Sheikh S, Quinn NP, Lees AJ, Selai C, Mathias C,et al. (2010). A comparison of depression, anxiety, and health status in patients with progressive supranuclear palsy and multiple system atrophy. Movement disorders : official journal of the Movement Disorder Society, 25: 1077-1081 [DOI] [PubMed] [Google Scholar]


