Abstract
Background
Parkinson’s disease (PD) patients with 10 years or more survival (PD-10) are not well characterized. The aim of this study was to evaluate the main issues facing PD-10 patients and identify factors that independently contributed to quality of life (QoL).
Methods
A group of 121 PD-10 patients recruited from outpatient clinics participated in this cross-sectional study. Data on demographic and clinical factors were collected. Multiple linear regression analyses were conducted to identify determinants of poor QoL.
Results
The entire PD-10 patients had disease duration ranging from 10 to 23 years, with 84.2% of the total cohort skewed to between 10 and 15 years’ duration. The PD-10 patients had great frequency of left-sided onset, increased motor and non-motor symptoms as well as inferior QoL. The more advanced stage of disease in PD-10 patients was associated with motor phenotype, freezing of gait, higher UPDRS sub-scores and levodopa equivalent dose, less balanced confidence, fatigue, anxiety, depression, reduced quality of life and worse Timed Up & Go performance. Self-reported mood symptoms, decreased balance confidence and reduced daily activities were the three factors most closely associated with poorer QoL, but excessive daytime sleepiness and long disease duration additionally contributed to the explanatory power.
Conclusions
This is the first report to investigate the clinical characteristics of Chinese PD-10 patients. Our study may elucidate an important clue for understanding PD-10 patients in clinical practice and identifying patients with PD at risk for reduced QoL.
Keywords: Parkinson’s disease, longevity, motor and non-motor symptoms, quality of life
Parkinson’s disease (PD) is the second most common neurodegenerative disease after Alzheimer’s disease. Clinically, it is characterized by resting tremor, rigidity, bradykinesia, and postural instability and a variety of poorly treatable non-motor symptoms such as fatigue, anxiety, depression, autonomic dysfunction, sleep disorders and cognitive deficits.
The available data suggests that by five years of PD duration the diagnosis is more reliable and as time progresses, PD patients begin to develop hallmark motor symptoms and increasing disability and mortality [1-3]. Additionally, the motor complications that include wearing off, random oscillations (on-off effect) and delayed "on" effect are also more prominent [4]. Over time, disease burden accumulates and the daily life functioning of patients declines.
To the best of our knowledge, there are a limited number of studies following longer disease duration PD patients [2, 3, 5-8]. Such information acquired from PD patients with 10-year disease duration and beyond will be invaluable in our understanding of the natural course of the disease and for health resources planning in an ageing society.
This study endeavored to identify the main issues facing patients when they reached the 10-year disease duration milestone. We chose 10 years as a point that demarcates clearly a diagnosis of true idiopathic PD firstly. Secondly, PD-10 patients may exhibit some unique clinical characteristics different from the early PD patients according to the literature [2, 3, 5-8]. Additionally, in the PD-10-time frame patients are usually medication dependent, and disease burdens are typically increased. The true nature of clinical characteristics and treatment strategies in Chinese PD-10 patients deserves further investigation.
With increased PD duration, there is an increased difficulty in maintaining a high quality of life (QoL). Compared with the general population, patients with PD reported lower levels of QoL in terms of physical, emotional well-being, and social functioning [9]. Mood symptoms and axial impairment were found to be the main consistent determinants of reduced QoL in PD [10]. A careful investigation of the data obtained from PD-10 cohort may give us important information about the main source of patients’ distress. Additionally, understanding how factors influence the subgroups of patients can help to individualize treatment strategies and relieve the disease burden.
Therefore, the purpose of this investigation was to identify the main issues facing PD patients with 10 years or more survival, and to determine factors that independently contribute to QoL.
MATERIALS AND METHODS
Subjects
In this cross-sectional study, all the PD patients with 10 years of disease duration and beyond were recruited between September 2014 and August 2016 from the Movement Disorder Specialist Clinic at the Department of Neurology, Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China. PD diagnosis was carried out using United Kingdom PD Society Brain Bank criteria [11]. PD patients who were treated with deep brain stimulation and continuous infusion of Levodopa Carbidopa Intestinal Gel were excluded. All the participants provided written informed consent, and the study was approved by the Research Ethics Committee, Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Subject evaluation
The following demographic and clinical data were collected from all patients: gender, age, age at onset, disease duration, motor asymmetry and medical history. Patient evaluation was carried out by movement disorder specialists. Disease duration was defined as time since the first motor symptom onset. Disease severity was evaluated by the Unified Parkinson’s Disease Rating Scale (UPDRS) [12] and the modified Hoehn and Yahr (H-Y) scale [13] while patients were on treatment. As the occurrence of motor complications was an important problem in the long-term dopaminergic therapy, we evaluated the prevalence of dyskinesias and motor fluctuations in PD patients using the UPDRS Part IV [14]. Patients were divided into tremor dominant (TD) or postural instability and gait dysfunction dominant (PIGD) subtype, or an intermediate subtype, according to previously described methods [15]. Activities-specific Balance Confidence scale (ABC), the standard “Timed UP-and-Go” (TUG) test and the Freezing of Gait Questionnaire (FOGQ) were used to assess the functional balance and balance confidence to predict the fall risk in people with PD. The ABC [16] was a popular, valid and reliable tool consisting of 16 items with a total score that ranges between 0 and 100, where higher score was indicative of higher balance confidence. The TUG test [17] was a commonly used assessment in older people that involves observing and timing the participant while they rised from an armchair, walked three meters, turned, walked back and sit down. A time of 13.5 seconds or longer to complete the test was considered significant in older adults for discriminating fallers from non-fallers [18]. The FOG-Q was used to assess self-perception of FOG [19]. Patients were divided into “freezers” (FOG-Q item-3 ≥1) or “non-freezers” (FOG-Q item-3 = 0) based on FOG-Q item 3 (“Do you feel that your feet get glued to the floor while walking, making a turn or when trying to initiate walking?”) [20]. The Parkinson’s disease sleep scale (PDSS) [21] and Epworth Sleep Scale (ESS) [22] were used to investigate the quality of sleep in PD patients. The PDSS was a visual analogue scale addressing 15 commonly reported symptoms associated with sleep disturbance in Parkinson’s disease that ranges from 0 to 10, where higher score was indicative of better sleep state [21]. The maximum cumulative score for the PDSS was 150 [21]. Scores of 105 or higher suggested normal sleep, and scores of 90 or less suggested the presence of sleep disturbances [23]. ESS was a simple, self-administered questionnaire intended to measure the subject’s general level of daytime sleepiness. Subjects with an ESS score >10 were considered to have excessive daytime sleepiness [22]. All the participants were evaluated with the rapid eye movement (REM) sleep behavior disorder screening questionnaire (RBDSQ) [24] and a score of at least six positive answers was proposed as the cut-off value for clinical probable RBD [25]. The Fatigue Severity Scale (FSS) was used to evaluate the severity of fatigue symptoms and a score of 36 or more was indicative of fatigue [26, 27]. The 39 item Parkinson’s disease questionnaire (PDQ-39), a 39-item and 8-dimension self-completed questionnaire was used to assess the health-related quality of life in people with PD and the Parkinson’s disease summary index (PDSI) could provide an indication of the global impact of Parkinson’s disease on health status [28]. Odor discrimination was performed with the 16-item odor identification test from extended version of sniffin’ sticks (SS-16; BurghartMesstechnik, Wedel, Germany) as previously described [29] and the cut-off score of SS-16 was 9.5 [30]. The Mini-Mental State Examination (MMSE) [31] was used to evaluate cognitive function. The Hamilton anxiety scale (HAMA) [32] and Hamilton depression scale (HAMD) [33] were used to evaluate the severity of anxiety and depressive symptoms, respectively.
Statistical analysis
All statistical analyses were performed using the SPSS 16.0 software for Windows (SPSS Inc., Chicago, IL, USA). Demographic and disease-related characteristics were summarized with descriptive statistics. The T test, Mann-Whitney U test and chi-square test were used for comparison between groups, when appropriate.
Spearman rank correlation coefficients were calculated to assess the association between explanatory variables and QoL.
All significant (p<0.05) univariately associated explanatory variables were stepwise entered linear multiple regression models with QoL as dependent variable. All statistical tests were two-tailed, and the limit of significance was set at a level of 0.05.
RESULTS
The entire PD-10 population had disease duration ranging from 10 to 23 years since symptom onset and the mean duration was 12.8 years. 84.2% of the total cohort was found skewed to between 10 and 15 years’ duration (Figure 1). The demographic and clinical characteristics were shown in Table 1. The mean age of these patients (55.37% male) was 66.46±8.57 years. Approximately 60.33% had motor signs lateralized predominantly to the left side of body. The incidence of motor fluctuations and dyskinesia was 64.46% and 23.14%, respectively. Approximately two thirds (64.46%) were likely to present PIGD phenotype and almost similar prevalence (61.98%) of FOG. They seemed not to struggle with sleep problems such as insomnia or daytime sleepiness other than RBD. More than half (56.20%, 68/121) of patients were likely to present RBD and nearly nine-in-ten (88.43%, 107/121) of patients had a diminished sense of smell. These PD-10 patients also experienced anxiety and depression. It was found that a high mean daily levodopa equivalent dose of dopaminergic drugs (954.52 ± 359.69 mg) had been used in the PD-10 patients.
Figure 1.
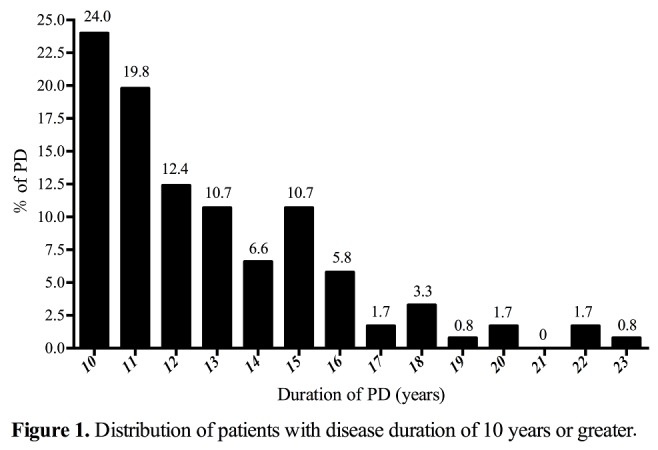
Distribution of patients with disease duration of 10 years or greater.
Table 1.
Demographic and motor characteristics for PD patients with 10 years or more disease duration.
| Overall | H-Y ≥ 3.0 n=53 |
H-Y < 3.0 n=68 |
P | |
|---|---|---|---|---|
| Age (years) | 66.46 ± 8.57 | 67.25 ± 8.64 | 65.84 ± 8.52 | 0.37 |
| Age at onset (years) | 53.70 ± 8.53 | 54.21 ± 8.49 | 53.31 ± 8.61 | 0.57 |
| Sex, Male, n (%) | 67 (55.37) | 25 (47.17) | 42 (61.76) | 0.11 |
| Disease duration (years) | 12.84 ± 2.92 | 13.25 ± 2.87 | 12.53 ± 2.93 | 0.07 |
| Asymmetry (left), n (%) | 73 (60.33) | 33 (62.26) | 40 (58.82) | 0.70 |
| Hoehn &Yahr stage | 2.70 ± 0.77 | 3.40 ± 0.57 | 2.16 ± 0.35 | <0.01* |
| UPDRS-I score | 3.95 ± 2.42 | 4.85 ± 2.54 | 3.25 ± 2.08 | <0.01* |
| UPDRS-II score | 17.17 ± 6.25 | 20.15 ± 5.96 | 14.84 ± 5.47 | <0.01* |
| UPDRS-III score | 34.31 ± 11.89 | 38.66 ± 11.71 | 30.93 ± 10.97 | <0.01* |
| Motor fluctuations, n (%) | 78 (64.46) | 39 (73.58) | 39 (57.35) | 0.06 |
| Dyskinesia, n (%) | 28 (23.14) | 13 (24.53) | 15 (22.06) | 0.75 |
| TD phenotype, n (%) | 26 (21.49) | 4 (7.55) | 22 (32.35) | <0.01* |
| PIGD phenotype, n (%) | 78 (64.46) | 39 (73.58) | 39 (57.35) | 0.06 |
| Intermediate, n (%) | 17 (14.05) | 10 (18.87) | 7 (10.29) | 0.18 |
| FOG, n (%) | 75 (61.98) | 44 (83.02) | 31 (45.59) | <0.01* |
| ABC (%) | 64.40 ± 26.64 | 50.25 ± 27.99 | 75.42 ± 19.52 | <0.01* |
| Standardized TUG | 19.98 ± 21.88 | 28.82 ± 30.67 | 13.47 ± 6.83 | <0.01* |
| LED (mg) | 954.52 ± 359.69 | 1033.40 ± 326.46 | 893.05 ± 374.50 | 0.03* |
| PDSS | 98.79 ± 26.83 | 92.64 ± 26.24 | 103.59 ± 26.48 | 0.03* |
| ESS | 8.40 ± 6.24 | 8.21 ± 5.75 | 8.54 ± 6.63 | 0.77 |
| SS-16 | 5.42 ± 3.19 | 4.91 ± 3.36 | 5.82 ± 3.02 | 0.12 |
| RBDSQ | 5.87 ± 2.66 | 6.06 ± 2.53 | 5.72 ± 2.77 | 0.49 |
| HAMA | 14.48 ± 9.28 | 16.66 ± 9.74 | 12.78 ± 8.59 | 0.02* |
| HAMD | 10.79 ± 6.20 | 13.04 ± 6.46 | 9.03 ± 5.42 | <0.01* |
| FSS | 42.32 ± 18.01 | 46.21 ± 16.62 | 39.29 ± 18.57 | 0.03* |
| MMSE | 26.11 ± 4.00 | 25.74 ± 4.00 | 26.40 ± 4.00 | 0.15 |
Table 2.
showed the frequency distribution of medication in PD-10 patients. Although 100% patients were taking levodopa, only 9.09% (11/121) of those were taking levodopa alone and the majority of those were also taking dopamine agonists or any other PD medication. Among PD-10 patients, 43.80% (53/121) of patients were taking three PD medication, 83.02% (44/53) of whom were taking the combination of dopamine agonists, levodopa and other anti-parkinson drugs. Although higher percentage of PD-10 patients with H-Y ≥3.0 was taking COMT inhibitor (39.62% vs. 19.12%), the percentages of taking amantadine (28.30% vs. 48.53%) for patients with H-Y ≥3.0 were significantly lower. We did not observe statistically significant differences in the proportions of levodopa, dopamine agonist, MAO-B inhibitor, anticholinergics and types of PD medication used between PD-10 patients with and without H-Y ≥3.0.
Mean scores of PDQ-39 domains were presented in Table 3. In the PD patients with 10 years or more survival, overall quality of life was reduced in all domains and disease burden was increased. The mean scores of PDQ-39 SI was 29.98 ± 17.80. Cognition (mean score 37.96 ± 23.48) was the most negative quality of life domain, mobility (mean score 34.94 ± 27.85) was the second most negative domain, however, social support (mean score 12.81 ± 22.18) was the least negative domain.
Table 2.
PD medication of H-Y ≥ 3.0 and H-Y < 3.0 patients.
| Overall | H-Y ≥ 3.0 n=53 |
H-Y < 3.0 n=68 |
P | |
|---|---|---|---|---|
| Levodopa | 121 (100%) | 53 (100%) | 68 (100%) | - |
| Dopamine agonist | 79 (65.29%) | 30 (56.60%) | 49 (72.06%) | 0.08 |
| MAO-B inhibitor | 33 (27.27%) | 16 (30.19%) | 17 (25.00%) | 0.53 |
| COMT inhibitor | 34 (28.10%) | 21 (39.62%) | 13 (19.12%) | 0.01* |
| Amantadine | 48 (39.67%) | 15 (28.30%) | 33 (48.53%) | 0.02* |
| Anticholinergics | 26 (21.49%) | 9 (16.98%) | 17 (25.0%) | 0.29 |
| One PD medication alone (Levodopa alone) |
11 (9.09%) | 7 (13.21%) | 4 (5.88%) | 0.21 |
| Two PD medication | 32 (26.45%) | 14 (26.42%) | 18 (26.47%) | 0.99 |
| Three PD medication | 53 (43.80%) | 22 (41.51%) | 31 (45.59%) | 0.65 |
| Four PD medication | 19 (15.70%) | 7 (13.21%) | 12 (17.65%) | 0.51 |
| Five PD medication | 6 (4.96%) | 3 (5.66%) | 3 (4.41%) | 1.00 |
As is well known that H-Y stage was an important predictor of clinical outcome events. Patients were stratified into 2 groups [34]: H-Y score of 2.5 or less (n=68) or H-Y score of 3.0 or higher (n=53). As shown in Table 1, comparison between PD-10 patients with H-Y≥3.0 and patients with H-Y<3.0 revealed significant association of H-Y stage with UPDRS-I score (4.85 ± 2.54 vs. 3.25 ± 2.08 years, p<0.01), UPDRS-II score (20.15 ± 5.96 vs.14.84 ± 5.47 years, p<0.01), UPDRS-III score (38.66 ± 11.71 vs. 30.93 ± 10.97 years, p<0.01), TD phenotype (7.55% vs. 32.35%, p<0.01), FOG (83.02% vs. 45.59%, p<0.01), ABC score (50.25 ± 27.99 % vs.75.42 ± 19.52 %, p<0.01), TUG score (28.82 ± 30.67 vs. 13.47 ± 6.83 seconds, p<0.01), PDSS score (92.64 ± 26.24 vs. 103.59±26.48, p=0.03), LED (1033.40 ± 326.46 vs. 893.05 ± 374.50, p=0.03), FSS score (46.21 ± 16.62 vs. 39.29 ± 18.57, p=0.03), HAMA score (16.66 ± 9.74 vs. 12.78 ± 8.59, p=0.02) and HAMD score (13.04±6.46 vs.9.03 ± 5.42, p<0.01). In addition, there was significant difference in PDQ-39 summary index (37.72 ± 18.96 vs. 23.94 ± 14.28%, p<0.01), including mobility (50.19 ± 27.39 vs. 23.05 ± 21.90 %, p<0.01), activity of daily life (43.71 ± 22.0 vs. 24.14±21.04%, p<0.01), emotional well-being (39.86 ± 29.40 vs. 26.84 ± 23.82, p=0.01), social support (19.81 ± 27.99 vs. 7.35 ± 14.29, p=0.01), and pain (42.77± 24.02 vs. 27.45 ± 27.40, p<0.01) domains (Table 3). Although higher percentages of PD-10 patients with H-Y ≥3.0 had motor fluctuations (73.58% vs. 57.35%), no significant difference was found between the two groups.
Disease duration, UPDRS Parts I-III, H-Y stage, FOG, ABC, TUG, PDSS, ESS, FSS, LED, RBDSQ, HAMA, HAMD, SCOPA-AUT and MMSE were identified as explanatory factors of QoL in the PD-10 patients. Stepwise linear multiple regression analysis was performed on all the explanatory factors and the final model (Table 4) indicated the PDSI was primarily associated with mood symptoms and self-evaluation of balance confidence and activities of daily life. Increasing excessive daytime sleepiness and disease duration contributed additionally to reduced QoL. The full model explained 75% of the variance in the PDSI.
Table 3.
PDQ-39 scores of H-Y ≥ 3.0 and H-Y < 3.0 patients.
| Overall | H-Y ≥ 3.0 n=53 |
H-Y < 3.0 n=68 |
P | |
|---|---|---|---|---|
| PDQ-39 mobility (%) | 34.94 ± 27.85 | 50.19 ± 27.39 | 23.05 ± 21.90 | <0.01* |
| PDQ-39 ADL (%) | 32.71 ± 23.49 | 43.71 ± 22.00 | 24.14 ± 21.04 | <0.01* |
| PDQ-39 emotion (%) | 32.54 ± 27.08 | 39.86 ± 29.40 | 26.84 ± 23.82 | 0.01* |
| PDQ-39 stigma (%) | 29.49 ± 28.17 | 32.67 ± 27.75 | 27.02 ± 28.46 | 0.20 |
| PDQ-39 social support (%) | 12.81 ± 22.18 | 19.81 ± 27.99 | 7.35 ± 14.29 | 0.01* |
| PDQ-39 cognition (%) | 37.96 ± 23.48 | 42.10 ± 24.58 | 34.74 ± 22.25 | 0.09 |
| PDQ-39 communication (%) | 25.21 ± 25.13 | 30.66 ± 27.92 | 20.96 ± 22.00 | 0.06 |
| PDQ-39 pain (%) | 34.16 ± 26.97 | 42.77 ± 24.02 | 27.45 ± 27.40 | <0.01* |
| PDQ-39 summary index (%) | 29.98 ± 17.80 | 37.72 ± 18.96 | 23.94 ± 14.28 | <0.01* |
DISCUSSION
We explored 121 patients with disease duration of at least 10 years (PD-10) and the majority was between 10 and 15 years’ duration. The data showed that, on average, the majority of patients were in their sixth decade and had motor signs lateralized predominantly to the left side of body. Increasing motor disability appeared to be compounded by increased prevalence of non-motor symptoms and reduced quality of life, which is in accordance with other studies [1-3]. The results of the analysis grouped by H-Y stage (H-Y ≥3.0 vs. H-Y<3.0) revealed that the more advanced stage of disease in PD-10 patients was associated with motor phenotype, FOG, higher UPDRS sub-scores and levodopa equivalent doses, less balanced confidence, reduced quality of life, worse TUG performance, anxiety and depression. Self-reported mood symptoms, decreased balance confidence and reduced daily activities were the factors most closely associated with poorer QoL, but excessive daytime sleepiness and long disease duration additionally contributed to reduced QoL.
Table 4.
Factors associated with QoL in stepwise multiple linear regression model.
| Model | Unstandardized Coefficients | Standardized Coefficients | t | P | |
|---|---|---|---|---|---|
|
| |||||
| B | Std. Error | Beta | |||
| HAMA | 0.643 | 0.125 | 0.339 | 5.148 | <0.001 |
| ABC | -0.175 | 0.037 | - 0.254 | -4.703 | <0.001 |
| UPDRS-II score | 0.665 | 0.154 | 0.225 | 4.310 | <0.001 |
| ESS | 0.562 | 0.139 | 0.196 | 4.044 | <0.001 |
| HAMD | 0.632 | 0.189 | 0.222 | 3.344 | 0.001 |
| Disease duration | 0.716 | 0.296 | 0.116 | 2.417 | 0.017 |
| Adjusted R2 | 0.753 | ||||
Some researchers reported that women had a lower adjusted risk of death than men in PD [35, 36]. However, similar to previous studies [3, 37], our data did not reflect this characteristic. Our study included 67 males and 54 females, and the male to female ratio was approximately 1.2:1.0. This discrepancy, consistent with the controversy [38-40], whether gender could be considered as a potential factor, could be due to small sample size and racial differences. It was worth noting that the proportion of male patients in this Chinese population was lower (55.37 % male vs. 44.63% female; ratio 1.2:1.0) than the reported overall PD sex ratio of 1.5-2.0 times higher in males [41, 42]. Munhoz et al [5] reported that left-sided onset was associated with long disease duration and ambulatory PD survival. Among 121 subjects screened for laterality, approximately two thirds (60.33%) had left-sided onset. It is well known that the motor symptoms of PD are mainly due to progressive asymmetric degeneration of nigral dopaminergic neurons. However, the pathology for the lateralization in the rate of degeneration of nigral dopaminergic neurons in PD remains unclear.
In accordance with previous reports [2, 3], we found that PD-10 patients were likely to manifest the PIGD motor phenotype, which was commonly associated with an increased mortality risk and greater functional disability [2, 15]. Our data showed that PD-10 patients had low ABC score, bad TUG test performance, and high prevalence of FOG. Motor complications, which were closely linked to L-dopa dose [4], were also identified as common symptoms in PD-10 patients. Our results identified and confirmed bad mobility performance, fast disease progression and poor QoL in the long-term PD survivors. The results of the PDQ-39 domains were concordant, as mobility discomfort affected the PD-10 patients greatly, which was in accordance with the study by Kadastik-Eerme et al [43]. In comparison with mobility, cognition was found to be more negative quality of life domain in a cohort of relatively longer duration PD. The prevalence of dementia would dramatically increase after 10 years, as suggested by Reid et al. [44] who demonstrated the evolution of dementia within PD was associated with the age, regardless of the time of PD onset and the stage of disease. Although the PD-10 patients were not likely to suffer from cognitive impairment according to mean MMSE score and prevalence of cognitive decline in this study, more sensitive examinations, such as Frontal Assessment Battery, Stroop test, Verbal Digit Span forward and backward, Phonological verbal fluency would be used to detect cognitive function in PD-10 patients.
To our knowledge, there is little report of an association between long disease duration and non-motor symptoms in PD patients. This study suggested that PD-10 patients were more likely to present RBD, fatigue, anxiety and depression. Moreover, higher HAMA, HAMD, FSS, PDSS scores were associated with advanced H-Y stage. Despite the reported strong correlation of distressing fatigue with depression, anxiety and sleep disturbances [45, 46], most studies agree that all four symptoms are independent factors [47]. There is currently much interest in whether they share pathophysiologic mechanisms.
We found significant differences between H-Y stage groups in mobility, balance, motor phenotype, quality of life, fatigue, sleep problems, LED and mental problems; however, we did not find the difference between disease stage groups was significant for age, age at onset, gender and laterality in this study. As has been reported [1-3], patients with PD-10 in general, but particularly in patients with H-Y score ≥3.0, were unable to move around confidently, keep mental health and enjoy better life quality.
As hypothesized, mood problems would contribute more to reduced QoL than other features of PD, consistent with recent literature [48-50]. In our study, subjective complaints about mood symptoms, as measured with the HAMA and HAMD, accounted for the largest proportion of variance in the PDSI. Along with mood complaints, decreased balance confidence and reduced daily activities were the factors most closely associated with poorer QoL, but excessive daytime sleepiness and long disease duration were identified as important determinants of poor QoL, which was in accordance with other studies [50-53]. Our findings were consistent with previous reports showing that non-motor symptoms including depression, anxiety, sleep dysfunction may play a more important role than motor symptoms in PD patients’ QoL [43, 49, 54].
There are another two issues need to be addressed. Firstly, our study is cross-sectional and descriptive in nature. This is similar to other reports that have attempted to investigate this issue [3, 5, 6]. The confirmation of the interconnected relationship of QoL and clinical events in long duration PD patients would require a long-term large sample randomized prospective trial. Secondly, we evaluated all the PD-10 patients during the on state. With increasing motor disability, it was significantly difficult for PD patients with 10 years or greater to come to hospital during the off state. This study suggested that, during the on state, PD-10 patients tended to present increased motor and non-motor symptoms and reduced QoL. Not to mention the worse QoL and more serious symptoms PD-10 patients would suffer from during the off state. It is of great significance to identify the main issues facing PD-10 patients, and to determine factors that contribute to QoL.
In conclusion, this observational study suggested that a large proportion of PD-10 patients are suffering from both increasing clinical symptoms and worsening of quality of life. Self-report indices of mood status, balance confidence and daily activities were identified as the main determinants of poor quality of life (QoL). Improvement in access to health care quality and in tools to relieve the disease burden is an issue of crucial importance to these long duration PD patients.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81430022, 91332107, 81371407). We thank all the patients who participated in this study.
Contributor Information
Qian Sun, Department of Neurology & Collaborative Innovation Center for Brain Science, Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, 200025 China.
Tian Wang, Department of Neurology & Collaborative Innovation Center for Brain Science, Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, 200025 China.
Tian-Fang Jiang, Department of Neurology & Collaborative Innovation Center for Brain Science, Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, 200025 China.
Pei Huang, Department of Neurology & Collaborative Innovation Center for Brain Science, Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, 200025 China.
Ying Wang, Department of Neurology & Collaborative Innovation Center for Brain Science, Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, 200025 China.
Qin Xiao, Department of Neurology & Collaborative Innovation Center for Brain Science, Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, 200025 China.
Jun Liu, Department of Neurology & Collaborative Innovation Center for Brain Science, Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, 200025 China.
Sheng-Di Chen, Department of Neurology & Collaborative Innovation Center for Brain Science, Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, 200025 China.
References
- [1].Alves G, Wentzel-Larsen T, Aarsland D, Larsen JP (2005). Progression of motor impairment and disability in Parkinson disease: a population-based study. Neurology, 65:1436-41. [DOI] [PubMed] [Google Scholar]
- [2].Auyeung M, Tsoi TH, Mok V, Cheung CM, Lee CN, Li R,et al. (2012). Ten year survival and outcomes in a prospective cohort of new onset Chinese Parkinson’s disease patients. J Neurol Neurosurg Psychiatry, 83:607-11. [DOI] [PubMed] [Google Scholar]
- [3].Hassan A, Wu SS, Schmidt P, Malaty IA, Dai YF, Miyasaki JM,et al. (2012). What are the issues facing Parkinson’s disease patients at ten years of disease and beyond? Data from the NPF-QII study. Parkinsonism Relat Disord, 18 Suppl 3:S10-4. [DOI] [PubMed] [Google Scholar]
- [4].Warren Olanow C, Kieburtz K, Rascol O, Poewe W, Schapira AH, Emre M,et al. (2013). Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson’s disease. Mov Disord, 28:1064-71. [DOI] [PubMed] [Google Scholar]
- [5].Munhoz RP, Espay AJ, Morgante F, Li JY, Teive HA, Dunn E,et al. (2013). Long-duration Parkinson’s disease: role of lateralization of motor features. Parkinsonism Relat Disord, 19:77-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hassan A, Wu SS, Schmidt P, Simuni T, Giladi N, Miyasaki JM,et al. (2015). The Profile of Long-term Parkinson’s Disease Survivors with 20 Years of Disease Duration and Beyond. J Parkinsons Dis, 5:313-9. [DOI] [PubMed] [Google Scholar]
- [7].Vu TC, Nutt JG, Holford NH (2012). Disease progress and response to treatment as predictors of survival, disability, cognitive impairment and depression in Parkinson’s disease. Br J Clin Pharmacol, 74:284-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vu TC, Nutt JG, Holford NH (2012). Progression of motor and nonmotor features of Parkinson’s disease and their response to treatment. Br J Clin Pharmacol, 74:267-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schrag A, Jahanshahi M, Quinn N (2000). How does Parkinson’s disease affect quality of life? A comparison with quality of life in the general population. Mov Disord, 15:1112-8. [DOI] [PubMed] [Google Scholar]
- [10].Muslimovic D, Post B, Speelman JD, Schmand B, de Haan RJ (2008). Determinants of disability and quality of life in mild to moderate Parkinson disease. Neurology, 70:2241-7. [DOI] [PubMed] [Google Scholar]
- [11].Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992). Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry, 55:181-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ramaker C, Marinus J, Stiggelbout AM, Van Hilten BJ (2002). Systematic evaluation of rating scales for impairment and disability in Parkinson’s disease. Mov Disord, 17:867-76. [DOI] [PubMed] [Google Scholar]
- [13].Hoehn MM, Yahr MD (1976). Parkinsonism: onset, progression and mortality. Neurology, 17:427-42. [DOI] [PubMed] [Google Scholar]
- [14].Schrag A, Quinn N (2000). Dyskinesias and motor fluctuations in Parkinson’s disease. A community-based study. Brain, 123 (Pt 11):2297-305. [DOI] [PubMed] [Google Scholar]
- [15].Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L,et al. (1990). Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology, 40:1529-34. [DOI] [PubMed] [Google Scholar]
- [16].Powell LE, Myers AM (1995). The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci, 50a:M28-34. [DOI] [PubMed] [Google Scholar]
- [17].Podsiadlo D, Richardson S (1991). The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc, 39:142-8. [DOI] [PubMed] [Google Scholar]
- [18].Shumway-Cook A, Brauer S, Woollacott M (2000). Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther, 80:896-903. [PubMed] [Google Scholar]
- [19].Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD (2000). Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism Relat Disord, 6:165-70. [DOI] [PubMed] [Google Scholar]
- [20].Giladi N, Tal J, Azulay T, Rascol O, Brooks DJ, Melamed E,et al. (2009). Validation of the freezing of gait questionnaire in patients with Parkinson’s disease. Mov Disord, 24:655-61. [DOI] [PubMed] [Google Scholar]
- [21].Chaudhuri KR, Pal S, DiMarco A, Whately-Smith C, Bridgman K, Mathew R,et al. (2002). The Parkinson’s disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson’s disease. J Neurol Neurosurg Psychiatry, 73:629-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Johns MW (1991). A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep, 14:540-5. [DOI] [PubMed] [Google Scholar]
- [23].Porter B, Macfarlane R, Walker R (2008). The frequency and nature of sleep disorders in a community-based population of patients with Parkinson’s disease. Eur J Neurol, 15:50-4. [DOI] [PubMed] [Google Scholar]
- [24].Stiasny-Kolster K, Mayer G, Schafer S, Moller JC, Heinzel-Gutenbrunner M, Oertel WH (2007). The REM sleep behavior disorder screening questionnaire--a new diagnostic instrument. Mov Disord, 22:2386-93. [DOI] [PubMed] [Google Scholar]
- [25].Nomura T, Inoue Y, Kagimura T, Uemura Y, Nakashima K (2011). Utility of the REM sleep behavior disorder screening questionnaire (RBDSQ) in Parkinson’s disease patients. Sleep Med, 12:711-3. [DOI] [PubMed] [Google Scholar]
- [26].Friedman JH, Alves G, Hagell P, Marinus J, Marsh L, Martinez-Martin P,et al. (2010). Fatigue rating scales critique and recommendations by the Movement Disorders Society task force on rating scales for Parkinson’s disease. Mov Disord, 25:805-22. [DOI] [PubMed] [Google Scholar]
- [27].Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD (1989). The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol, 46:1121-3. [DOI] [PubMed] [Google Scholar]
- [28].Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N (1997). The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age Ageing, 26:353-7. [DOI] [PubMed] [Google Scholar]
- [29].Chen W, Tan YY, Hu YY, Zhan WW, Wu L, Lou Y,et al. (2012). Combination of olfactory test and substantia nigra transcranial sonopraphy in the differential diagnosis of Parkinson’s disease: a pilot study from China. Transl Neurodegener, 1:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen W, Chen S, Kang WY, Li B, Xu ZM, Xiao Q,et al. (2012). Application of odor identification test in Parkinson’s disease in China: a matched case-control study. J Neurol Sci, 316:47-50. [DOI] [PubMed] [Google Scholar]
- [31].Folstein MF, Folstein SE, McHugh PR (1975). "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res, 12:189-98. [DOI] [PubMed] [Google Scholar]
- [32].Maier W, Buller R, Philipp M, Heuser I (1988). The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord, 14:61-8. [DOI] [PubMed] [Google Scholar]
- [33].Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW,et al. (1991). Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry, 48:851-5. [DOI] [PubMed] [Google Scholar]
- [34].Lee HK, Altmann LJ, McFarland N, Hass CJ (2016). The relationship between balance confidence and control in individuals with Parkinson’s disease. Parkinsonism Relat Disord, 26:24-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Diem-Zangerl A, Seppi K, Wenning GK, Trinka E, Ransmayr G, Oberaigner W,et al. (2009). Mortality in Parkinson’s disease: a 20-year follow-up study. Mov Disord, 24:819-25. [DOI] [PubMed] [Google Scholar]
- [36].Willis AW, Schootman M, Kung N, Evanoff BA, Perlmutter JS, Racette BA (2012). Predictors of survival in patients with Parkinson disease. Arch Neurol, 69:601-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lo RY, Tanner CM, Albers KB, Leimpeter AD, Fross RD, Bernstein AL,et al. (2009). Clinical features in early Parkinson disease and survival. Arch Neurol, 66:1353-8. [DOI] [PubMed] [Google Scholar]
- [38].Diamond SG, Markham CH, Hoehn MM, McDowell FH, Muenter MD (1990). An examination of male-female differences in progression and mortality of Parkinson’s disease. Neurology, 40:763-6. [DOI] [PubMed] [Google Scholar]
- [39].Ebmeier KP, Calder SA, Crawford JR, Stewart L, Besson JA, Mutch WJ (1990). Parkinson’s disease in Aberdeen: survival after 3.5 years. Acta Neurol Scand, 81:294-9. [DOI] [PubMed] [Google Scholar]
- [40].D’Amelio M, Ragonese P, Morgante L, Reggio A, Callari G, Salemi G,et al. (2006). Long-term survival of Parkinson’s disease: a population-based study. J Neurol, 253:33-7. [DOI] [PubMed] [Google Scholar]
- [41].Baldereschi M, Di Carlo A, Rocca WA, Vanni P, Maggi S, Perissinotto E,et al. (2000). Parkinson’s disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. ILSA Working Group. Italian Longitudinal Study on Aging. Neurology, 55:1358-63. [DOI] [PubMed] [Google Scholar]
- [42].Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J (2004). Are men at greater risk for Parkinson’s disease than women? J Neurol Neurosurg Psychiatry, 75:637-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kadastik-Eerme L, Rosenthal M, Paju T, Muldmaa M, Taba P (2015). Health-related quality of life in Parkinson’s disease: a cross-sectional study focusing on non-motor symptoms. Health Qual Life Outcomes, 13: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Reid WG, Hely MA, Morris JG, Loy C, Halliday GM (2011). Dementia in Parkinson’s disease: a 20-year neuropsychological study (Sydney Multicentre Study). J Neurol Neurosurg Psychiatry, 82:1033-7. [DOI] [PubMed] [Google Scholar]
- [45].Beiske AG, Loge JH, Hjermstad MJ, Svensson E (2010). Fatigue in Parkinson’s disease: prevalence and associated factors. Mov Disord, 25:2456-60. [DOI] [PubMed] [Google Scholar]
- [46].Zuo LJ, Yu SY, Wang F, Hu Y, Piao YS, Du Y,et al. (2016). Parkinson’s Disease with Fatigue: Clinical Characteristics and Potential Mechanisms Relevant to alpha-Synuclein Oligomer. J Clin Neurol, 12:172-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Friedman JH, Brown RG, Comella C, Garber CE, Krupp LB, Lou JS,et al. (2007). Fatigue in Parkinson’s disease: a review. Mov Disord, 22:297-308. [DOI] [PubMed] [Google Scholar]
- [48].Jones JD, Butterfield LC, Song W, Lafo J, Mangal P, Okun MS,et al. (2015). Anxiety and Depression Are Better Correlates of Parkinson’s Disease Quality of Life Than Apathy. J Neuropsychiatry Clin Neurosci, 27:213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Karlsen KH, Larsen JP, Tandberg E, Maeland JG (1999). Influence of clinical and demographic variables on quality of life in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry, 66:431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Fan JY, Chang BL, Wu YR (2016). Relationships among Depression, Anxiety, Sleep, and Quality of Life in Patients with Parkinson’s Disease in Taiwan. Parkinsons Dis, 2016:4040185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Grimbergen YA, Schrag A, Mazibrada G, Borm GF, Bloem BR (2013). Impact of falls and fear of falling on health-related quality of life in patients with Parkinson’s disease. J Parkinsons Dis, 3:409-13. [DOI] [PubMed] [Google Scholar]
- [52].Havlikova E, van Dijk JP, Nagyova I, Rosenberger J, Middel B, Dubayova T,et al. (2011). The impact of sleep and mood disorders on quality of life in Parkinson’s disease patients. J Neurol, 2011;258:2222-9. [DOI] [PubMed] [Google Scholar]
- [53].Benge JF, Kekecs Z, Encarnacion E, Ainslie M, Herff C, Elkins G,et al. (2016). Duration of disease does not equally influence all aspects of quality of life in Parkinson’s disease. J Clin Neurosci, 28:102-6. [DOI] [PubMed] [Google Scholar]
- [54].Duncan GW, Khoo TK, Yarnall AJ, O’Brien JT, Coleman SY, Brooks DJ,et al. (2014). Health-related quality of life in early Parkinson’s disease: the impact of nonmotor symptoms. Mov Disord, 29:195-202. [DOI] [PubMed] [Google Scholar]


