Abstract
Changes in protein glycosylation have been reported in various types of cancer, including cholangiocarcinoma (CCA). Nanospray ionization-linear ion trap mass spectrometry (NSI-MSn) was used in the present study to determine the comparative structural glycomics of the N-linked glycans in the serum of patients with CCA compared with healthy controls. A total of 5 high-mannose and 4 complex N-linked glycans were detected. Mannose7-N-acetyl-glucosamine2 was the most abundant structure among the high-mannose types (control 12.12±2.54 vs. CCA 9.27±2.66%), whereas NeuAc2H2N2M3N2 predominated the complex types (control 61.17±2.55 vs. CCA 64.68±4.23%). The expression of 3 different N-glycans differed significantly between the CCA cases and controls. These included mannose6-N-acetyl-glucosamine2 (P=0.044), mannose9-N-acetyl-glucosamine2 (Ρ=0.030) and NeuAc3H3N3M3N2F (Ρ=0.002). These three glycan structures may therefore be associated with tumor progression in CCA and may be useful for its diagnosis.
Keywords: cholangiocarcinoma, high-mannose N-glycans, Tri-antennary N-glycans
Introduction
Cholangiocarcinoma (CCA), a cancer of the bile duct, is a major health problem in Northeastern Thailand and Southeast Asia. It is associated with infestation by the liver fluke Opisthorchis viverrini (1). The incidence of CCA is high in East and Southeast Asia and its incidence is also increasing in England, the USA and Australia (2,3). Diagnoses of CCA are usually made when the disease is advanced or disseminated, meaning that the prognosis of patients is poor; therefore, novel target biomarkers are required to enable early diagnosis of CCA, as well as increase the therapeutic efficacy of treatments for CCA.
Protein glycosylation is the most common post-translational modification that occurs in human proteins (4,5). It is important in cell and tissue development, host-pathogen interactions, inflammation and malignancy (6). Alterations in protein glycosylation have been reported in various diseases, including different types of cancer (7). Identifying altered cancer-associated glycoproteins may facilitate the development of potential biomarkers of cancer or novel targets for treatment.
A number of in vitro and in vivo molecular studies investigating glycoproteins in CCA have been performed. It has been demonstrated that the expression of sialyl-LewisA in the tissues of patients with CCA is associated with poor prognosis (8). Furthermore, a study using monoclonal antibodies against serum glycoprotein mucin 5AC revealed that levels of serum glycan epitope (S121) are associated with patient prognosis and is specific to CCA (9). This association was investigated further in an animal model. It was demonstrated that the glycan epitope (S121) was expressed in the cytoplasm and apical surface of biliary cells during the early stages of tumor development, and that this expression increased further with tumor progression (10). Immunohistochemical studies have revealed that N-acetylglucosamine (GlcNAc) (11) and O-GlcNAc transferase are overexpressed in CCA (12). Furthermore, the results of ELISA performed on the serum of patients with CCA revealed that the association between glycan epitope CA-S27 and patient prognosis is specific to CCA and may have immunodiagnostic value (13).
It has been demonstrated that the lectin microarray-based sero-biomarker is able to detect O-linked glycosylation in CCA (14). Furthermore, using different CCA cell lines, it has been revealed that different histological types of CCA exhibit differential expression levels of O-glycans (15). In-depth characterization of the glycans expressed in the serum of patients with CCA may facilitate the identification of potential CCA biomarkers.
The present study assessed the structural glycomics of N-glycans in the serum of patients with CCA compared with healthy controls. Three candidate glycan markers were proposed and it was hypothesized that these specific glycans may aid in the development of diagnostic and/or therapeutic markers of CCA.
Patients and methods
Reagents
Sodium borohydride and sodium hydroxide were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Fetuin glycoprotein standard was obtained from Sigma-Aldrich; Merck KGaA. D-galactose, D-mannose and N-acetyl-D-glucosamine were obtained from EMD Millipore (Billerica, MA, USA).
Patients with CCA and healthy controls
A total of 8 serum samples from patients with CCA (mean age, 60.25±9.59; 3 females and 5 males) and 4 samples from healthy controls (mean age, 41.75±16.88; 3 females and 1 male) were obtained from participants recruited between January 2014 and May 2014 in the Liver Fluke and Cholangiocarcinoma Research Center, Faculty of Medicine, Khon Kaen University (Khon Kaen, Thailand). Patients were enrolled in the study if they had been diagnosed with intrahepatic CCA and had no apparent chronic inflammatory diseases, including diabetes mellitus or rheumatoid arthritis. The Ethics Committee of Khon Kaen University reviewed and approved the study protocol (registration number HE521209) and patients provided informed consent for the use of their material in the present study. Through peripheral venipuncture, a single blood sample was drawn into a 10 ml BD Vacutainer sterile vacuum tube (BD Biosciences, Franklin Lakes, NJ, USA) in the absence of anticoagulant. Blood was immediately centrifuged at 1,000 × g for 10 min at room temperature. The serum supernatant was collected and centrifuged at 2,500 × g for 10 min at room temperature. Following liquidation, serum was maintained at −80°C until use.
Preparation of protein powder from the serum of patients with CCA and healthy controls
Preparation of protein powder from the serum of patients with CCA and healthy controls was performed following a previously described protocol (16). Briefly, 50 µl serum obtained from patients with CCA and healthy controls were dissolved on ice in cold 50% methanol. The serum mixture was then extracted in a 4:8:3 ratio of chloroform to methanol to water for 2 h at room temperature. Extracts were centrifuged at 2,500 × g for 15 min at room temperature. The resulting pellets were then dried under nitrogen and stored at −20°C until further use.
Preparation of glycopeptides and release of N-glycans
The preparation of glycopeptides and release of N-glycans was performed as previously described (16). Briefly, 1 mg protein powder from the serum of patients with CCA and healthy controls was digested with trypsin and chymotrypsin for 18 h at 37°C in 0.1 M Tris-HCl (pH 8.2) containing 1 mM CaCl2. Digestion products were enriched and freed of contaminants using a 1 ml Sep-Pak C18 cartridge column (Waters Corporation, Milford, MA, USA), as described by Aoki et al (17). Glycopeptides were then digested with 2 µl peptide N-glycosidase F (7.5 U/ml, New England BioLabs, Inc., Ipswich, MA, USA) in 50 µl 20 mM sodium phosphate buffer (pH 7.5) for 18 h at 37°C. Released glycans were separated from peptides and enzymes by passing through a 1 ml Sep-Pak C18 cartridge high-performance liquid chromatography column.
Permethylation of glycans
Released glycan mixtures were permethylated as described by Anumula and Taylor (18). Briefly, released glycan mixtures were permethylated under water-free conditions using 500 µl DMSO, 10 µg NaOH and 200 µl methyliodide (all Sigma-Aldrich; Merck KGaA) for 30 min at room temperature. Permethylated glycans were then extracted with dichloromethane and dried under a stream of nitrogen.
Nanospray ionization-linear ion trap mass spectrometry (NSI-MSn)
NSI-MSn was performed as described by Aoki et al (17). Briefly, permethylated glycans were dissolved in 1 mM NaOH in 50% methanol and infused directly into a linear ion trap mass spectrometer (LTQ Orbitrap Discovery; Thermo Fisher Scientific, Inc., Waltham, MA, USA) using a Thermo Fisher Scientific™ nanospray ion source (Thermo Fisher Scientific, Inc.). MS analysis was performed in a positive ion mode and MS/MS spectra (at 28% collision energy) were obtained using the total ion mapping function of the Xcalibur software (version 2; Thermo Fisher Scientific, Inc.). The fragmentation derived from the MS/MS spectra was identified using the nomenclature described by Domon and Costello (19).
Glycomic analysis of N-glycans in the serum of patients with CCA and healthy controls
The expression of glycans from the serum of patients with CCA and healthy controls were qualitatively and quantitatively compared. The glycans from these sera were enzymatically released, purified and analyzed in their permethylated forms using positive ion NSI-MS/MS. Identification of the glycan structures was based on the i) NSI-MS parent mass ion; ii) NSI-MS/MS fragmentation ion; and iii) similarity to known glycan structures and known biosynthetic limitations. The prevalence of each individual glycan (percentage total profile) in each profile was quantified by comparing its signal intensity to the sum of the signal intensities for all identified glycans.
Statistical analysis
The respective prevalence of glycans (percentage total profile) in the sera of patients with CCA vs. healthy controls was reported as the mean ± standard deviation. The difference in the expression between groups was analyzed using the independent t-test. Cross-tabulations were analyzed using the χ2 test to determine the association between N-glycan expression and the clinicopathological features of CCA. All analyses were performed using SPSS statistical software (version 19.0; SPSS, Inc., Chicago, IL, USA) and P<0.05 was considered to indicate a statistically significant difference.
Results
Structural characterization of N-glycans in the serum from patients with CCA and healthy controls
The N-glycan profiles in the serum of patients with CCA and healthy controls are presented in Fig. 1. N-glycans were assigned as high-mannose (M5–9N2; Structures 1, 3, 5, 7 and 8) or complex types (NeuAc1–3Hex2–3HexNAc2–3 + M3N2F0–1; Structures 2, 4, 6 and 9). High-mannose N-glycans were detected from M5N2-M9N2. Complex N-glycans were detected as bi-and tri-antennary structures with the terminal galactose and sialic acid (N-acetylneuraminic acid; NeuAc). A summary of N-glycan structures in the sera of patients with CCA and healthy controls, and their relative abundance is presented in Table I. The representative fragmentation of the high-mannose type and complex type N-glycans are presented in Fig. 2.
Figure 1.
MS spectra of permethylated N-linked glycans in the serum of patients with CCA compared with healthy controls, as detected using NSI-MS. Glycans released from the serum of patients with CCA and healthy controls were permethylated and analyzed. MS spectra present the predominance of the complex type and high-mannose type oligosaccharides in (A) healthy sera vs. (B) CCA sera. The glycan profiles (A vs. B) demonstrate similar glycan patterns, but they differ in their relative quantities. Glycans were detected as doubly [2+] and triply charged species [3+]. The graphical representation of monosaccharide residues are defined in the figure and are consistent with the suggested nomenclature of the Consortium for Functional Glycomics (http://glycomics.scripps.edu/CFGnomenclature.pdf). MS, mass spectrometry; CCA, cholangiocarcinoma; NSI-MS, nanospray ionization-linear ion trap mass spectrometry; m/z, mass/charge ratio; Glc, N-acetylglucosamine.
Table I.
Characteristics and prevalence of N-linked glycans in the serum of patients with CCA compared with healthy controls.
| Structure | Group | Number | Relative abundance, % | P-value |
|---|---|---|---|---|
| M5N2 | N | 4 | 2.45±0.24 | 0.247 |
| T | 8 | 2.19±0.39 | ||
| NeuAc1H2N2M3N2 | N | 4 | 14.21±2.18 | 0.248 |
| T | 8 | 12.58±2.17 | ||
| M6N2 | N | 4 | 3.13±0.56 | 0.044a |
| T | 8 | 3.91±0.55 | ||
| NeuAc2H2N2M3N2 | N | 4 | 61.17±2.55 | 0.162 |
| T | 8 | 64.68±4.23 | ||
| M7N2 | N | 4 | 12.12±2.54 | 0.106 |
| T | 8 | 9.27±2.66 | ||
| NeuAc2H2N3M3N2F | N | 4 | 3.99±0.50 | 0.554 |
| T | 8 | 3.53±1.45 | ||
| M8N2 | N | 4 | 0.89±0.36 | 0.168 |
| T | 8 | 0.66±0.20 | ||
| M9N2 | N | 4 | 1.21±0.25 | 0.030a |
| T | 8 | 0.84±0.24 | ||
| NeuAc3H3N3M3N2F | N | 4 | 0.80±0.30 | 0.002a |
| T | 8 | 2.36±0.68 |
The prevalence of each indicated glycan is expressed as a percentage of the total pool of detected glycans (% total profile, mean ± standard deviation).
P<0.05 vs. N. N, healthy sera; T, CCA sera; CCA, cholangiocarcinoma.
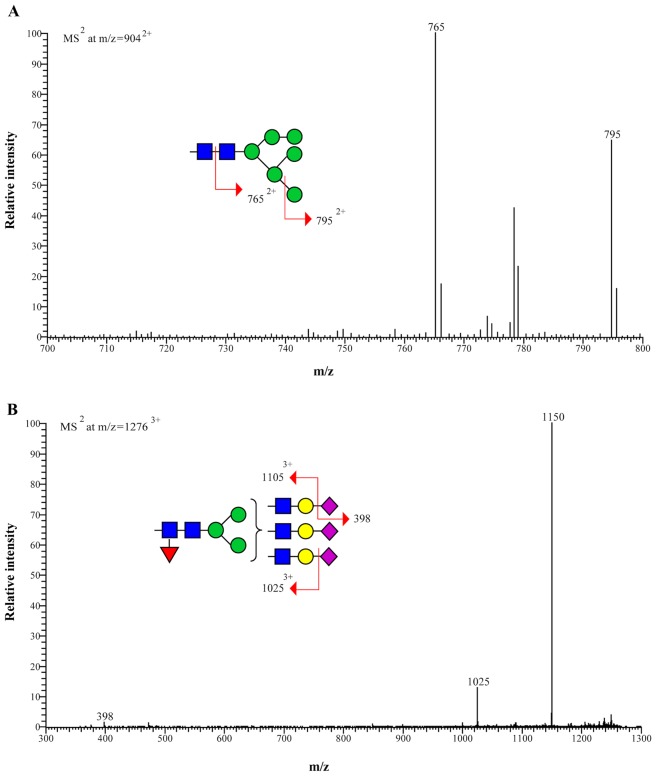
Figure 2.
Representative MS/MS spectra of permethylated N-linked glycans from the sera of patients with cholangiocarcinoma. Fragmentation of (A) parent ion at m/z=9042+ of high-mannose type structure and (B) parent ion at m/z=12763+ of complex type structure are depicted. The parent ion at m/z=9042+ (structure 3) fragments in MS2 to give m/z=7652+ (loss of reducing end GlcNAc; ∆m/z=1392+) and m/z=7952+ (loss of terminal man; ∆m/z=1092+). The parent ion at m/z=12763+ (structure 9) fragments in MS2 to give m/z=11053+ and 10253+ [loss of terminal NeuAc (first) and NeuAc (second) respectively; ∆m/z=1253+] and m/z=398 (terminal NeuAc with Na+). MS, mass spectrometry; m/z, mass/charge ratio; GlcNAc, N-acetylglucosamine; NeuAc, N-acetylneuraminic acid.
Altered expression of N-glycan structures in serum from patients with CCA compared with healthy controls
N-glycans in the serum of patients with CCA and healthy controls were qualitatively and quantitatively assessed using positive ion NSI-MS/MS (Table I). The detected N-glycans were high-mannose-and complex types (bi-and tri-antennary structures) with the terminal galactose and sialic acid (NeuAc). The expression of the high-mannose type N-glycan, M6N2 (structure 3) and the complex tri-antennary N-glycan containing a core fucose and terminal tri-sialic acid, NeuAc3H3N3M3N2F (structure 9), were significantly increased in the serum of patients with CCA compared with healthy controls (P=0.044 and P=0.002, respectively). By contrast, the expression of the high-mannose N-glycan M9N2 (structure 8) was significantly decreased in patients with CCA compared with healthy controls (P=0.030).
Serum N-glycan expression and clinicopathological features of CCA
The association between the expression of the three differentially expressed N-glycans in the serum of patients with CCA and clinicopathological features of CCA were quantitatively analyzed. High expression of M6N2 (structure 3) and NeuAc3H3N3M3N2F (structure 9) were associated with an age <60 years (P=0.028 and P=0.005, respectively). However, there were no significant associations between M9N2 (Structure 8) expression and patient age, sex, histological type, tumor stage, vascular or lymphatic invasion (Table II).
Table II.
Association between N-linked glycan expression and the clinicopathological features of patients with CCA.
| M6N2 expression | M9N2 expression | NeuAc3H3N3M3N2F expression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Low | High | P-value | Low | High | P-value | Low | High | P-value |
| Age, years | |||||||||
| <60 | 1 | 3 | 0.028a | 1 | 3 | 0.157 | 0 | 4 | 0.005a |
| ≥60 | 4 | 0 | 3 | 1 | 4 | 0 | |||
| Sex | |||||||||
| Male | 3 | 2 | 0.850 | 2 | 3 | 0.465 | 3 | 2 | 0.465 |
| Female | 2 | 1 | 2 | 1 | 1 | 2 | |||
| Histological type | |||||||||
| Papillary | 2 | 0 | 0.206 | 1 | 1 | 1.000 | 2 | 0 | 0.102 |
| Non-papillary | 3 | 3 | 3 | 3 | 2 | 4 | |||
| Stage | |||||||||
| III | 2 | 1 | 0.850 | 2 | 1 | 0.465 | 2 | 1 | 0.465 |
| IV | 3 | 2 | 2 | 3 | 2 | 3 | |||
| Lymphatic Invasion | |||||||||
| Present | 3 | 1 | 0.465 | 2 | 2 | 1.000 | 2 | 2 | 1.000 |
| Absent | 2 | 2 | 2 | 2 | 2 | 2 | |||
| Vascular Invasion | |||||||||
| Present | 2 | 0 | 0.206 | 2 | 0 | 0.102 | 2 | 0 | 0.102 |
| Absent | 3 | 3 | 2 | 4 | 2 | 4 | |||
n=8
P<0.05 vs. low expression. CCA, cholangiocarcinoma.
Discussion
Aberrant protein glycosylation has been reported in various diseases, including cancer, and certain glycan structures are well-known tumor markers. The present study demonstrated the comparative structural glycomics of the N-glycans in the serum from patients with CCA compared with healthy controls using MS.
The expression of 3 N-glycans, including M6N2 (structure 3; P=0.044), M9N2 (structure 8, P=0.030) and NeuAc3H3N3M3N2F (structure 9; P=0.002), differed significantly between the 4 controls and 8 patients with CCA. The expression of M6N2 and NeuAc3H3N3M3N2F were significantly increased in the serum of patients with CCA, whereas M9N2 expression was significantly decreased. The increased expression of high-mannose N-glycans in patients with CCA is consistent with the results of previous studies investigating N-glycan expression in different types of cancer, including breast (20,21) and colorectal cancer (22). The increased expression of M6N2 high-mannose structures indicates an incomplete maturation of the N-glycans in the glycosylation process and an association with CCA tumor progression.
The significant increase of core fucosylated tri-antennary N-glycans (NeuAc3H3N3M3N2F; structure 9) in the serum of patients with CCA may be an example of the alteration to the glycomic profile observed in different types of cancer, including breast cancer (20), colorectal cancer (22), hepatocellular carcinoma (23) and ovarian cancer (24). Furthermore, core fucosylation has been identified as an important feature in tumor progression and is associated with increased cancer metastasis (25). Tri-antennary N-glycans and core fucose structures have been associated with cancer metastasis and serve as a useful tumor biomarker (26); This suggests that these N-glycans may be associated with tumor progression in patients with CCA.
M9N2 was significantly decreased in CCA, indicating an increase in the glycosylation process that decreases M9N2 expression to produce complex and hybrid oligosaccharides in CCA. Decreased levels of high-mannose type of N-glycans have been detected in ovarian (27) and gastric cancer (28). Furthermore, the alteration of high-mannose glycans, M6N2 and M9N2, may be due to a more complex process that occurs during biosynthetic machinery, involving CCA glycosylation.
Based on the glycan structural analysis, the changes in glycan expression that occur during CCA may reflect specific changes that occur in glycosyltransferase expression. The increase of tri-antennary structures may be attributed to the altered expression of glycosyltransferases, including N-acetylglucosaminyltransferase (GnT)-III, -IV and-V. GnT-V is markedly associated with cancer metastasis, whereas GnT-III is associated with cancer suppression (26). The abundance of terminal sialic acid (NeuAc) in CCA may be attributed to the dominant activity of sialyltransferases, including ST3 β-galactoside α-2,3-sialyltransferase 3 and ST6 β-galactoside α-2,6-sialyltransferase, that represent the majority of glycosyltransferases in CCA and may serve a pivotal role in cancer progression (29).
The present study identified an association between N-glycan expression and age in patients with CCA. The increased expression of M6N2 and NeuAc3H3N3M3N2F is associated with an age of <60 years old in patients with CCA. Age-related changes in the expression of human serum N-glycans have been reported in European (30) and Chinese patients (31). CCA is rarely diagnosed in patients <40 years old; changes in the expression of N-glycans in the serum of patients with CCA may occur due to tumorigenicity and aging.
In conclusion, the altered expression of N-glycans in the serum of patients with CCA indicate that they serve an important role in tumor growth and progression. M6N2 and NeuAc3H3N3M3N2F, which exhibit significantly increased expression in the serum of patients with CCA, may therefore be potentially promising biomarkers for CCA.
Acknowledgements
The present study was supported by the Suranaree University of Technology (grant no. SUT6-606-58-12-04). The authors wish to thank Mr. Bryan Roderick Hamman for assistance with the English-language presentation of the present study.
References
- 1.Sripa B. Pathobiology of opisthorchiasis: An update. Acta Trop. 2003;88:209–220. doi: 10.1016/j.actatropica.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 3.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: A true increase? J Hepatol. 2004;40:472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 4.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473:4–8. doi: 10.1016/S0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 5.Wong CH. Protein glycosylation: New challenges and opportunities. J Org Chem. 2005;70:4219–4225. doi: 10.1021/jo050278f. [DOI] [PubMed] [Google Scholar]
- 6.Biological Functions of Glycans. In: Varki A, Gagneux P, editors; Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH, editors. Essentials of Glycobiology. 3rd. Cold Spring Harbor (NY): 2015. [DOI] [PubMed] [Google Scholar]
- 7.Kim EH, Misek DE. Glycoproteomics-based identification of cancer biomarkers. Int J Proteomics. 2011;2011:601937. doi: 10.1155/2011/601937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juntavee A, Sripa B, Pugkhem A, Khuntikeo N, Wongkham S. Expression of sialyl Lewis(a) relates to poor prognosis in cholangiocarcinoma. World J Gastroenterol. 2005;11:249–254. doi: 10.3748/wjg.v11.i2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silsirivanit A, Araki N, Wongkham C, Pairojkul C, Narimatsu Y, Kuwahara K, Narimatsu H, Wongkham S, Sakaguchi N. A novel serum carbohydrate marker on mucin 5AC: Values for diagnostic and prognostic indicators for cholangiocarcinoma. Cancer. 2011;117:3393–3403. doi: 10.1002/cncr.25912. [DOI] [PubMed] [Google Scholar]
- 10.Sawanyawisuth K, Silsirivanit A, Kunlabut K, Tantapotinan N, Vaeteewoottacharn K, Wongkham S. A novel carbohydrate antigen expression during development of opisthorchis viverrini-associated cholangiocarcinoma in golden hamster: A potential marker for early diagnosis. Parasitol Int. 2012;61:151–154. doi: 10.1016/j.parint.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Indramanee S, Silsirivanit A, Pairojkul C, Wongkham C, Wongkham S. Aberrant glycosylation in cholangiocarcinoma demonstrated by lectin-histochemistry. Asian Pac J Cancer Prev. 2012;13(Suppl):S119–S124. [PubMed] [Google Scholar]
- 12.Phoomak C, Silsirivanit A, Wongkham C, Sripa B, Puapairoj A, Wongkham S. Overexpression of O-GlcNAc-transferase associates with aggressiveness of mass-forming cholangiocarcinoma. Asian Pac J Cancer Prev. 2012;13(Suppl):S101–S105. [PubMed] [Google Scholar]
- 13.Silsirivanit A, Araki N, Wongkham C, Vaeteewoottacharn K, Pairojkul C, Kuwahara K, Narimatsu Y, Sawaki H, Narimatsu H, Okada S, et al. CA-S27: A novel Lewis a associated carbohydrate epitope is diagnostic and prognostic for cholangiocarcinoma. Cancer Sci. 2013;104:1278–1284. doi: 10.1111/cas.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuda A, Kuno A, Nakagawa T, Ikehara Y, Irimura T, Yamamoto M, Nakanuma Y, Miyoshi E, Nakamori S, Nakanishi H, et al. Lectin microarray-based sero-biomarker verification targeting aberrant O-Linked glycosylation on mucin 1. Anal Chem. 2015;87:7274–7281. doi: 10.1021/acs.analchem.5b01329. [DOI] [PubMed] [Google Scholar]
- 15.Talabnin K, Talabnin C, Ishihara M, Azadi P, Wongkham S, Sripa B. Differential expression of O-glycoprotein glycans in cholangiocarcinoma cell lines. Asian Pac J Cancer Prev. 2016;17:691–695. doi: 10.7314/APJCP.2016.17.2.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talabnin K, Aoki K, Saichua P, Wongkham S, Kaewkes S, Boons GJ, Sripa B, Tiemeyer M. Stage-specific expression and antigenicity of glycoprotein glycans isolated from the human liver fluke, Opisthorchis viverrini. Int J Parasitol. 2013;43:37–50. doi: 10.1016/j.ijpara.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aoki K, Perlman M, Lim JM, Cantu R, Wells L, Tiemeyer M. Dynamic developmental elaboration of N-linked glycan complexity in the Drosophila melanogaster embryo. J Biol Chem. 2007;282:9127–9142. doi: 10.1074/jbc.M606711200. [DOI] [PubMed] [Google Scholar]
- 18.Anumula KR, Taylor PB. A comprehensive procedure for preparation of partially methylated alditol acetates from glycoprotein carbohydrates. Anal Biochem. 1992;203:101–108. doi: 10.1016/0003-2697(92)90048-C. [DOI] [PubMed] [Google Scholar]
- 19.Domon B, Costello CE. Structure elucidation of glycosphingolipids and gangliosides using high-performance tandem mass spectrometry. Biochemistry. 1988;27:1534–1543. doi: 10.1021/bi00405a021. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Nie H, Zhang Y, Yao Y, Maitikabili A, Qu Y, Shi S, Chen C, Li Y. Cell surface-specific N-glycan profiling in breast cancer. PLoS One. 2013;8:e72704. doi: 10.1371/journal.pone.0072704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Leoz ML, Young LJ, An HJ, Kronewitter SR, Kim J, Miyamoto S, Borowsky AD, Chew HK, Lebrilla CB. High-mannose glycans are elevated during breast cancer progression. Mol Cell Proteomics. 2011;10:M110.002717. doi: 10.1074/mcp.M110.002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sethi MK, Thaysen-Andersen M, Smith JT, Baker MS, Packer NH, Hancock WS, Fanayan S. Comparative N-glycan profiling of colorectal cancer cell lines reveals unique bisecting GlcNAc and alpha-2,3-linked sialic acid determinants are associated with membrane proteins of the more metastatic/aggressive cell lines. J Proteome Res. 2014;13:277–288. doi: 10.1021/pr400861m. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa T, Miyoshi E, Yakushijin T, Hiramatsu N, Igura T, Hayashi N, Taniguchi N, Kondo A. Glycomic analysis of alpha-fetoprotein L3 in hepatoma cell lines and hepatocellular carcinoma patients. J Proteome Res. 2008;7:2222–2233. doi: 10.1021/pr700841q. [DOI] [PubMed] [Google Scholar]
- 24.Schwedler C, Kaup M, Weiz S, Hoppe M, Braicu EI, Sehouli J, Hoppe B, Tauber R, Berger M, Blanchard V. Identification of 34 N-glycan isomers in human serum by capillary electrophoresis coupled with laser-induced fluorescence allows improving glycan biomarker discovery. Anal Bioanal Chem. 2014;406:7185–7193. doi: 10.1007/s00216-014-8168-y. [DOI] [PubMed] [Google Scholar]
- 25.Dennis JW, Laferté S, Waghorne C, Breitman ML, Kerbel RS. Beta 1–6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987;236:582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- 26.Taniguchi N, Kizuka Y. Glycans and cancer: Role of N-glycans in cancer biomarker, progression and metastasis and therapeutics. Adv Cancer Res. 2015;126:11–51. doi: 10.1016/bs.acr.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Kronewitter SR, De Leoz ML, Strum JS, An HJ, Dimapasoc LM, Guerrero A, Miyamoto S, Lebrilla CB, Leiserowitz GS. The glycolyzer: Automated glycan annotation software for high performance mass spectrometry and its application to ovarian cancer glycan biomarker discovery. Proteomics. 2012;12:2523–2538. doi: 10.1002/pmic.201100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozcan S, Barkauskas DA, Renee Ruhaak L, Torres J, Cooke CL, An HJ, Hua S, Williams CC, Dimapasoc LM, Han Kim J, et al. Serum glycan signatures of gastric cancer. Cancer Prev Res (Phila) 2014;7:226–235. doi: 10.1158/1940-6207.CAPR-13-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dall'Olio F, Malagolini N, Trinchera M, Chiricolo M. Sialosignaling: Sialyltransferases as engines of self-fueling loops in cancer progression. Biochim Biophys Acta. 2014;1840:2752–2764. doi: 10.1016/j.bbagen.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Vanhooren V, Laroy W, Libert C, Chen C. N-glycan profiling in the study of human aging. Biogerontology. 2008;9:351–356. doi: 10.1007/s10522-008-9140-z. [DOI] [PubMed] [Google Scholar]
- 31.Ding N, Nie H, Sun X, Sun W, Qu Y, Liu X, Yao Y, Liang X, Chen CC, Li Y. Human serum N-glycan profiles are age and sex dependent. Age Ageing. 2011;40:568–575. doi: 10.1093/ageing/afr084. [DOI] [PubMed] [Google Scholar]