Abstract
Rationale
Amongst non-smokers, nicotine generally enhances performance on tasks of attention, with limited effect on working memory. In contrast, nicotine has been shown to produce robust enhancements of working memory in non-humans.
Objectives
To address this gap, the present study investigated the effects of nicotine on the performance of non-smokers on a cognitive battery which included a working memory task reverse-translated from use with rodents (the odor span task, OST). Nicotine has been reported to enhance OST performance in rats and the present study assessed whether this effect generalizes to human performance.
Methods
Thirty non-smokers were tested on three occasions after consuming either placebo, 2mg, or 4mg nicotine gum. On each occasion participants completed a battery of clinical and experimental tasks of working memory and attention.
Results
Nicotine was associated with dose dependent enhancements in sustained attention, as evidenced by increased hit accuracy on the rapid visual information processing (RVIP) task. However, nicotine failed to produce main effects on OST performance or on alternative measures of working memory (digit span, spatial span, letter-number sequencing, 2-back) or attention (digits forward, 0-back). Interestingly, enhancement of RVIP performance occurred concomitant to significant reductions in self-reported attention/concentration. Human OST performance was significantly related to N-back performance and, as in rodents, OST accuracy declined with increasing memory load.
Conclusions
Given the similarity of human and rodent OST performance under baseline conditions and the strong association between OST and visual 0-back accuracy, the OST may be particular useful in the study of conditions characterized by inattention.
The integration of clinical research with animal models of behavior is of critical importance for the development of novel therapeutics for psychiatric conditions and for the treatment of substance dependence. As noted by other authors, validated cross-species tasks are needed to provide a framework for translation and to better operationalize cognitive constructs across species (Young and Geyer 2015). The development of the odor span task (OST) represents an effort towards this aim. Developed initially for rats (Dudchenko et al. 2000), and subsequently adapted for use with mice (Young et al. 2007b) and with humans (Levy et al. 2003), the OST allows for analogous assessment of short-term memory capacity (the amount of information to remember) across species. In rodents, this is accomplished by exposing the animal to a variety of odors, to which the animal can provide a response, in a series of trials. In the first trial, the rodent is exposed to a single odor and a response yields a food reward. In the second trial, the rodent is exposed to both a novel odor, and the odor presented in the first trial. Only a response to the novel odor yields a reward. In each subsequent trial, a single novel odor is presented alongside odors which had been used in earlier trials. Again, reinforcement is only provided for a response to the novel odor. As trials progress, accurate performance requires that the subject identify, and inhibit responses to, an ever increasing list of odors.
Given the ethological importance of olfaction to rodents, they reach high levels of accuracy on the OST and continue to perform well when tested with as many as 72 odors (April et al. 2013). Importantly, accuracy declines across trials of the task demonstrating that performance is sensitive to how many odors need to be remembered (capacity-dependent). Several control procedures have been used with rodents to confirm that this effect does not result from reward satiation, or a progressive dulling of olfactory acuity (Galizio et al. 2016; Galizio et al. 2013; MacQueen et al. 2011; MacQueen et al. 2016). In assessing capacity effects, the OST fills a critical gap in the testing of short-term memory across species. Working memory tasks for rodents have largely focused on the maintenance of information across delays using paradigms such as the radial arm maze, water maze, t-maze, or in delayed match/non-match to sample. However in humans, working memory is described in terms of short term memory stores of limited capacity that require controlled attention (Baddeley 2003; Saults and Cowan 2007). Accordingly, assessment of capacity is the primary way in which working memory is evaluated clinical practice (e.g., digit span, spatial span, letter number-sequencing).
The OST has been previously adapted for human testing however, questions remain regarding the construct validity and clinical relevance of the task. Capacity dependence has yet to be demonstrated in humans and at present, it is unclear how human OST performance relates to commonly used assessments of working memory and attention. In addition, the neurobiological substrates of human OST performance remain ambiguous. In a case-control study, patients with brain lesions limited to the hippocampal region were significantly impaired on the OST relative to healthy participants (Levy et al. 2003). This contrasts with findings in rodents in which lesions of the medial prefrontal cortex (mPFC; Davies et al. 2013b) but, not hippocampus (Dudchenko et al. 2000) grossly impair performance. However, it should be noted that patients in the human study still performed reasonably well on the OST (approximately 80% accuracy) and thus, the observed impairment may reflect the contribution of a hippocampus dependent mnemonic process in human OST performance.
In rats, OST performance is selectively impaired by systemic administration of NMDA receptor antagonists (Galizio et al. 2016; Galizio et al. 2013; MacQueen et al. 2011; MacQueen et al. 2016) but, not by opioids or GABA agonists. Cortical glutamate activity appears critical as local administration of NMDA or AMPA receptor antagonists into the medial prefrontal cortex (mPFC) of mice grossly disrupts performance (Davies et al. 2013a). Rodent studies have also demonstrated that OST performance is sensitive to cholinergic manipulations. In rats, performance of the odor span task is transiently disrupted by lesioning of the basal forebrain cholinergic system (Turchi and Sarter 2000). Performance decrements have also been observed in α7 nicotinic subunit knockouts (Young et al. 2007a) and in human amyloid over-expressing mice (Young et al. 2009). Notably, direct facilitation of OST performance after administration of nicotinic agonists has been reported in rats (Rushforth et al. 2010). Taken together, these findings suggest that OST performance is sensitive nicotinic receptor activity in non-human animals.
To advance the translational utility of the OST, the present study established a human OST testing paradigm incorporating control procedures back-translated from rodent designs. As in (Levy et al. 2003), participants sampled odors presented in opaque test tubes which contained household spices. However, a two-comparison procedure was adopted in which the novel odor is always presented with a single comparison odor chosen from the set of odors presented on earlier trials. Limiting the number of comparison odors presented allowed for an unbiased assessment of capacity effects. If participants were to choose the novel odor from an increasing choice set of comparison odors, declining accuracy would be inherently confounded with declining chance performance. In addition, participants were well-trained to discriminate two odors not used in the OST. This simple discrimination was subsequently tested at multiple points during of the OST. High levels of accuracy on the simple discrimination during OST testing demonstrated motivation to respond accurately, ability to discriminate odors, and intact memory of a well-learned odor association.
With the introduction of the aforementioned control procedures, the human OST adaptation is well suited for pharmacological testing and may be useful for bridging gaps in the translation of human and rodent experiments. In particular, the task could aid in clarifying the cognitive effects of nicotine. A meta-analysis of studies with non-smokers, non-deprived smokers, and minimally deprived smokers (less than 2 hours) determined that nicotine produces significant enhancements in fine motor ability, sustained attention accuracy and reaction time, orienting attention reaction time, short-term episodic memory, and working memory reaction time (Heishman et al. 2010). Significant effects were not observed for orienting attention accuracy, long-term episodic memory accuracy, or working memory accuracy. In contrast, nicotine enhancement of working memory is frequently reported in studies of non-human mammals (Buccafusco et al. 2009; Levin et al. 2006). It is unclear whether this discrepancy represents a divergence of neurocognitive processes across species or rather, stems from methodological differences in the administration of nicotine or in the assessment of working memory. Delineating the cognitive effects of nicotine is of critical importance as enhancement of attention has been suggested as a mechanism contributing to tobacco dependence, particularly amongst individuals with attentional impairments, who smoke at elevated rates. Given that the OST is sensitive to cholinergic manipulations and systemic nicotine effects have been observed in rats, the present study evaluated the effects of nicotine on human OST performance. It was expected that nicotine would facilitate OST performance as observed in rodents.
Given the novelty of the OST, a secondary goal of the study was to examine convergent and divergent validity of the OST with well validated cognitive tasks. Though the OST has been most frequently described as a working memory task (Dudchenko et al. 2000; Young et al. 2007b), it has been suggested that OST performance may more adequately reflect attentional processes (Young et al. 2007a). To evaluate construct validity of the OST, participants were additionally tested on a cognitive battery including self-report, clinical measures (digit span, spatial span, letter-number sequencing), and experimental tasks [rapid visual information processing (RVIP), N-back] of attention and working memory. We expected OST measures to demonstrate convergent validity with the accuracy measures of attention-related tasks such as RVIP, 0-back, and the forward components of clinical working memory tasks. In contrast, odor span measures were not expected to associate with accuracy on complex working memory tasks. As a control measure, it was also expected that simple discrimination performance would show diverge with OST performance and all measures of attention or working memory. In addition to evaluating validity of the OST, the inclusion of a wide cognitive battery allowed for a broad evaluation of nicotine effects. It was expected that nicotine would enhance performance of attention-related tasks, consistent with prior human studies.
Methods
Experimental Design
Participants completed 3 experimental sessions, each spaced at least a week apart, and consumed placebo or one of two doses of nicotine gum before testing in each session. The order of doses was counterbalanced across participants in this double-blind placebo controlled within-subjects design.
Participants
Non-smokers between the ages of 18 and 54 were recruited from the Tampa area through internet, newspaper and radio advertisements. Participants were excluded if they reported more than 5 uses of a nicotine product during their lifetime or any past year use, submitted a breath sample with carbon monoxide greater than 3 ppm, or if recent illicit substance use was reported or identified by urinalysis. Participants were also excluded if they had any history of heart disease, high blood pressure, any blood circulation disorder, phenylketonuria, asthma, food allergies, or a dental condition prohibiting gum chewing. Participants were screened for psychiatric illness using the Structured Clinical Interview for DSM-IV (First et al. 1994) and were excluded if they met criteria for a current mood episode, a psychotic disorder, substance dependence or panic disorder. Female participants were excluded if pregnant or if the possibility of pregnancy during the study was expected.
Procedures
Experimental Sessions
Each experimental session began with the administration of gum delivering 2mg or 4mg nicotine, or placebo gum. Participants then completed a series of self-report questionnaires assessing current physiological and emotional state. Subsequently, participants completed the clinical and experimental tasks. The OST was always administered first followed by the digit span, letter number sequencing, and the spatial span tasks of the WMS-III (Wechsler 1997), the rapid visual information processing task (RVIP), and the N-back task in a quasi-random order (three possible orderings).
Nicotine Administration
Nicotine and placebo gum were prepared by the experimenter and provided to research staff who were blind to the dose. A placebo procedure described by Kleykamp et al. (2005) was used to mask active doses. Nicotine gum (Nicorette® Freshmint™) or a similar style gum (Dentyne Ice®) was wrapped with Wrigley’s sugar-free peppermint gum and received two drops (0.1 ml) of hot sauce. Participants were prompted by a tone to chew their gum at 3 second intervals over 15 minutes (as in Houtsmuller et al. 2002; Kleykamp et al. 2005; Nemeth-Coslett and Henningfield 1986) while compliance was monitored by research staff. This dosing procedure has been demonstrated to produce peak blood plasma levels of 4.6 and 8.5 ng/ml for the 2 and 4 mg dose, respectively (Hindmarch et al. 1990). Peak blood plasma concentration (Tmax) after chewing nicotine gum has been estimated at about 45 minutes to 1 hour for the 2mg and 4mg doses with concentrations remaining substantially elevated at 180 minutes (Dautzenberg et al. 2007; Shiffman et al. 2009).
Measures
Current State Measures
The WSWS (Welsch et al. 1999) concentration subscale provided a self-report measure of attention and concentration. The 20-item PANAS (Watson et al. 1988) was scored to produce positive affect and negative affect scales which are internally reliable, and have been extensively validated (Watson et al. 1999). Six items from the FSQ (irritable, attentive, jittery, nauseous, sick, and dizzy Gilbert et al. 2008) were administered to assess commonly reported aversive effects of nicotine.
Working Memory and Attention Tasks
All clinical assessments (digit span, spatial span, and letter-number sequencing) were conducted as specified by the WMS-III administration and scoring manual (Psychological Corporation 2002). The N-back and RVIP task were completed on a personal computer running E-prime software (Psychology Software Tools, Inc.).
Olfactory Span Task (OST)
The OST was adapted from procedures used by Levy et al. (2003). The task used 22 household spices (High Quality Organics; Reno, NV) individually stored and presented in opaque plastic test tubes with stoppers; each tube 1/3 filled with ground spice. Prior to testing, participants were randomly assigned 2 odors (out of 5 odors not used in the OST) which were subsequently used in all simple discrimination control task testing for that participant.
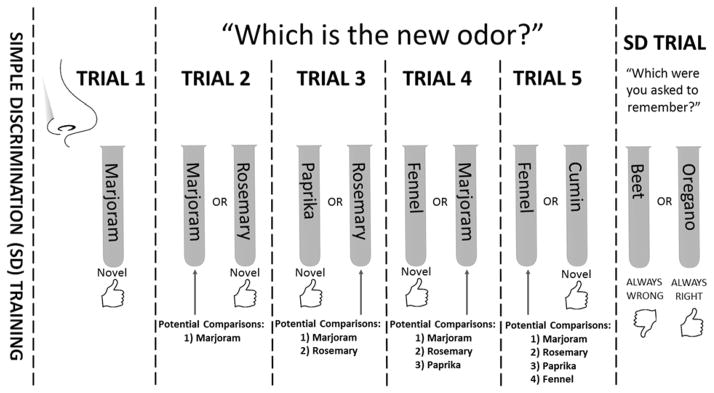
To acquire the simple discrimination task participants received training before each session. This began with the presentation of 2 test tubes containing the simple discrimination odors placed in random order on a rack. Participants were instructed to sample both of the odors from left to right by removing the stopper and breathing in gently with their nose an inch above the tube. Participants were instructed to remember the last odor they had sampled. This served as the target odor in all subsequent simple discrimination testing. They were then presented with these odors repeatedly (10 trials) and asked to identify the target odor. Verbal feedback was given after each choice. The position of stimuli (left/right) were reordered behind a cardboard shield between trials. After simple discrimination training participants were transitioned to the OST. They were instructed that on each new trial they would be asked to either identify (but not name) the odor they had been trained to remember, or to identify an odor that they had not sampled today. The span component, depicted in Figure 1, began with sampling of a single odor. On each subsequent trial, a novel odor was presented alongside a comparison odor, chosen quasi-randomly from the odors presented in previous trials (no longer novel). On each trial participants were asked to identify the novel odor. Every fifth trial, the simple discrimination odors were presented and participants were asked to report which odor they were trained to remember. Accuracy was computed for simple discrimination and OST trials. Span was recorded as the number of consecutive correct choices after the first trial. Longest span was recorded as the longest string of consecutive correct choices at any point of the OST.
Figure 1.
Diagram of the OST depicting the first 5 of 20 span trials and the 1st of 4 simple discrimination control trials.
N-back (0-back and 2-back)
The N-back task is a computerized assessment of working memory designed to assess performance across increasing memory loads. Participants were presented with sequences of 10 uppercase letters (height = 3 cm) displayed in the center of a computer screen for 250 ms with an inter-trial interval (ITI) of 2 seconds. They were instructed to press the left button of a response box after target stimuli and the right button after non-targets. During the 0-back component of the task, the target stimuli was the letter “X”. During the 2-back component, a target stimulus was any letter which matched the letter displayed 2 presentations prior. There were a total 180 trials for each component (0-back and 2-back) including 54 targets and 126 non-targets, equally distributed across three trial blocks.
Rapid Visual Information Processing Task (RVIP)
The RVIP (Wesnes and Warburton 1983) is a neurocognitive measure of sustained attention. A string of digits were presented on a monitor at a rate of 100 digits per minute (400 trials total) and participants were instructed to press the left button of a response box when three consecutive even or odd digits were presented. Eight target stimuli appeared during each minute, separated by 5–30 foils. Responses within 1,500 milliseconds of a target were scored as a hit.
Statistical Analyses
Two-way repeated measures analysis of variance (ANOVA) tests were conducted to test within-subject effects of nicotine dose by gender on the performance of cognitive tasks and self-report measures. One-way ANOVAs of task performance across testing sessions were used to assess for practice effects. In both cases, a Bonferroni correction was applied to the traditional threshold of significance (p < .05) in the analysis of each task to account for the number of performance measures assessed. For variables in which sphericity was violated, a Greenhouse-Geisser correction was made to degrees of freedom. Main effects and interactions were explored post-hoc, using simple effects analysis with a Bonferroni correction for multiple comparisons. Bivariate correlation was used to determine whether human accuracy on the OST is capacity dependent as is observed in rats (MacQueen et al., 2011) and to evaluate convergent validity of the OST with other measures of working memory and attention.
Results
Participant Characteristics
Thirty participants completed all three experimental sessions of the study. However, two participants were excluded from analyses; one due to failure to follow task instructions, and a second who produced chance level performance on multiple tasks. Participant characteristics are presented in Table 1.
Table 1.
Demographic Characteristics
| n | % | |
|---|---|---|
| Gender | ||
| Male | 16 | 57.14 |
| Female | 12 | 42.86 |
| Ethnicity | ||
| Hispanic or Latino | 9 | 32.14 |
| Not Hispanic of Latino | 19 | 67.86 |
| Race | ||
| Caucasian | 20 | 71.43 |
| African American | 5 | 17.86 |
| Asian | 2 | 7.14 |
| Not reported | 1 | 3.57 |
| Education Level | ||
| Some High School | 1 | 3.57 |
| Completed High School | 2 | 7.14 |
| Some College | 19 | 67.86 |
| Completed College | 4 | 14.29 |
| Some Graduate Work | 1 | 3.4 |
| A Graduate Degree | 1 | 3.57 |
Subjective Effects of Nicotine
As depicted in Figure 2, participants reported feeling significantly more nauseous [F(2,54) = 11.412, p = .001, ▪2 = 0.305], sick [F(2,54) = 11.655, p = .001, ▪2 = 0.310], and dizzy [F(2,54) = 18.147, p < .001, ▪2 = 0.411] after receiving gum containing nicotine. In each case, report of these sensations was significantly greater after the high dose when compared with either the low or placebo doses (p’s < .05). No effect of nicotine was observed on reports of feeling “attentive”, “jittery”, or “irritible”, p > .05 No other effects of nicotine, gender, or their interaction were detected for any measure of the FSQ or the PANAS (p’s > .05).
Figure 2.
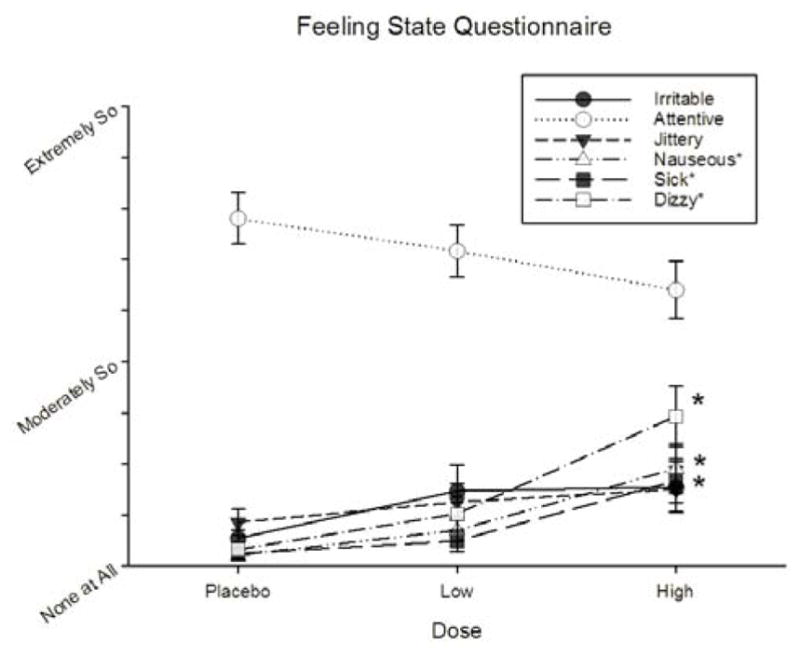
Effects of nicotine dose on the Feeling State Questionnaire items. Asterisks indicate a significant difference relative to placebo.
As presented in Figure 3, nicotine dose produced significant effects on all three WSWS items; “my level of concentration is excellent” [F(2,54) = 6.076, p = .004, ▪2 = 0.189], “It’s difficult to think clearly” [F(2,54) = 8.999, p = .001, ▪2 = 0.257] and “it is hard to pay attention to things” [F(2,54) = 8.965, p = .002, ▪2 = 0.256]. The high dose of nicotine produced a negative effect on all three items relative to placebo (p’s < .05). No effect of, or interaction with, gender was detected on the WSWS.
Figure 3.
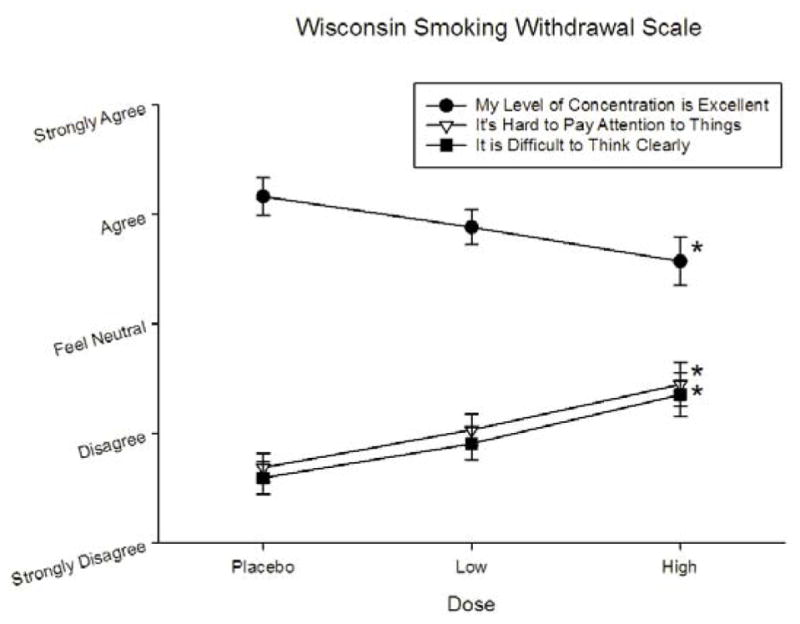
Effects of nicotine dose on selected Wisconsin Smoking Withdrawal Scale items. Asterisks indicate a significant difference relative to placebo.
Effects of nicotine on attention and working memory
Neither nicotine, gender, nor their interaction produced significant effects on any measures derived from the digit span, spatial span, letter-number sequencing, or odor span tasks (all ps > .05). Significant effects of nicotine were however, detected on RVIP target accuracy [F(2,54) = 5.803, p = .005, ▪2 = 0.182]. As presented in Figure 4, RVIP target accuracy was significantly greater after high dose nicotine gum when compared with placebo (p < .05). No effect of nicotine was observed for other RVIP measures (false alarm rate and hit reaction time; p’s > .05). Nicotine also significantly reduced target reaction time on the 0-back component of the N-back task at both doses relative to placebo [F(2,54) = 5.690, p = .006, ▪2 = 0.305]. Effects of nicotine, gender, or their interaction were not detected for any other measure of the 0-back or 2-back tasks (ps < .05).
Figure 4.
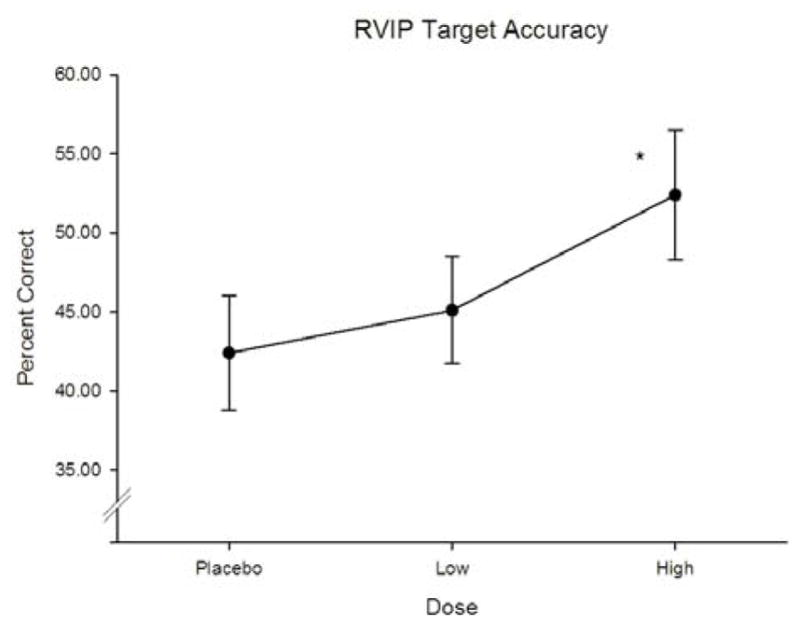
Effects of nicotine dose on RVIP target accuracy. Asterisks indicate a significant difference relative to placebo.
Practice Effects
Given that all cognitive testing was completed within subject, performance measures were also evaluated across the three testing sessions to assess for practice effects. A significant effect of session was detected for the total score measures of both the Spatial Span [F(2,54) = 5.831, p = .005, ▪2 = 0.178] and Letter-number Sequencing [F(2,54) = 4.699, p = .013, ▪2 = 0.148] tasks. In both cases, post-hoc tests revealed that performance was improved at the third session relative to the first (ps < .05). Practice effects were also detected in 2-back reaction time to both target [F(2,54) = 9.968, p = .001, ▪2 = 0.270] and distractor [F(2,54) = 7.270, p = .004, ▪2 = 0.212] stimuli. For both measures, reaction time was significantly reduced in the third session relative to both prior sessions (ps < .05). Lastly, a significant effect of session was detected on the longest backward sequence of the digit span [F(2,54) = 4.539, p = .015, ▪2 = 0.144] in which performance was improved on the third session relative to the second (p < .05).
Validation of the odor span task
After placebo, two thirds of participants provided perfect performances during odor discrimination training and 75% were perfect on subsequent odor discrimination trials (m = 85.71%, std = 27.58%). Span ranged from 0 to 16 (m = 4.71, std = 3.53), and average accuracy was 77.86% (std = 10.58%). . To evaluate the effect of memory load on accuracy, accuracy was computed across participants at each trial of the OST. As reported above, nicotine failed to produce significant effects on odor span accuracy. As such, accuracy for all three sessions (placebo, low dose, and high dose) were averaged for each participant to produce a more reliable estimate of accuracy at each span length. An illustration of Odor span performance by dose is included in supplementary materials (Figure S1). As depicted in Figure 5, a significant negative correlation (r = −.607, p = .006) was detected between accuracy and the number of odors to remember, reflecting a capacity dependent effect on odor span accuracy. A depiction of accuracy on the four odor discrimination trials, collapsed across doses is provided in the supplementary materials (Figure S2). A one-way repeated measures ANOVA of simple discrimination performance found no differences accuracy across trials, [F(3,81) = .808, p > .05].
Figure 5.
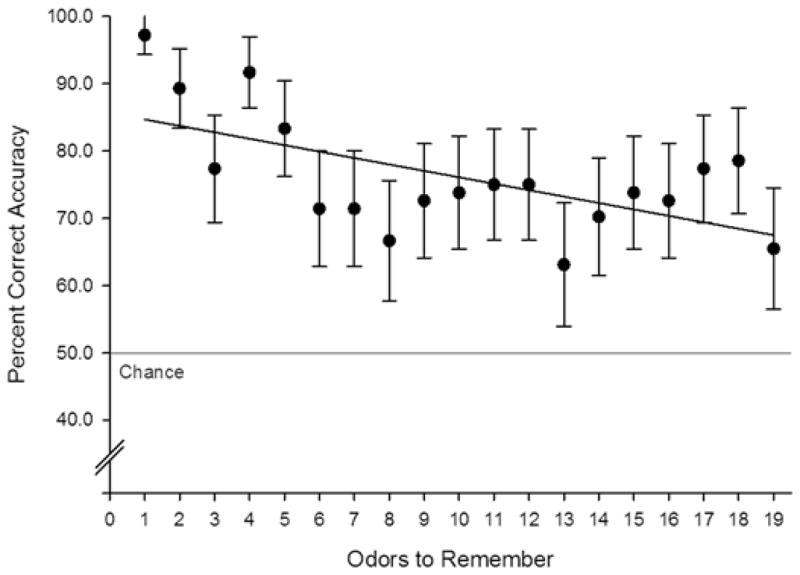
Average accuracy on odor span trials collapsed across dose conditions. Bivariate correlation demonstrated a negative relationship between accuracy and the number of odors participants needed to remember (r = −.607, p = .006).
The association between measures derived from the odor span task after receiving placebo gum, is presented in Table 2. An adjusted significance threshold (p < .005) was used to control for the number of associations considered (10). As expected, the odor discrimination measures (simple discrimination training and simple discrimination accuracy) demonstrated a strong significant positive association with each other (r = .711, p < .001) but, not with the mnemonic odor span measures (all p’s > .05). Each of the three mnemonic odor span indices demonstrated a significant association with the other mnemonic measures (rs ranging from .584 – .713; all p’s ≤ .002). A positive relationship between simple discrimination training accuracy and span trended towards significance (r = .474, p = .011) but did not reach the adjusted threshold.
Table 2.
Correlations Between Odor Span Measures
| Measure | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Training and Control Measures | |||||
| 1. Simple Discrimination Training | - | ||||
| 2. Simple Discrimination Accuracy | .711* | - | |||
| Mnemonic Measures | |||||
| 3. Percent Correct Accuracy | .352 | .367 | - | ||
| 4. Span | .474† | .280 | .584* | - | |
| 5. Longest Span | .168 | .187 | .713* | .706* | - |
p < .05,
p < .005.
Adjusting for multiple comparisons within each odor span measure, associations meeting a threshold of p < .005 were deemed significant.
The association of odor span performance with measures derived from the cognitive battery are presented in Table 3. As each odor span measure was compared with 21 measures derived from the other tasks, the threshold used to identify significant associations (p < .002) was determined by adjusting for the number of comparisons made with each odor span measure. As expected, neither of the odor discrimination measures produced a significant association with any of the measures from the 6 cognitive tasks (all ps > .05).
Table 3.
Correlations between odor span and cognitive measures
| SD Practice | SD Accuracy | Span | Longest Span | OS Accuracy | |
|---|---|---|---|---|---|
| Digit Span | |||||
| Forward | −.235 | −.046 | −.136 | .251 | −.236 |
| Backward | .054 | .180 | .120 | .345 | .139 |
| Total | −.118 | .062 | −.024 | .328 | −.076 |
| Longest Forward | −.054 | .128 | −.240 | .030 | −.322 |
| Longest Backward | .102 | .167 | .178 | .385† | .250 |
| Spatial Span | |||||
| Forward | −.148 | −.042 | −.186 | −.039 | −.295 |
| Backward | .004 | −.138 | −.022 | .008 | .016 |
| Total | −.092 | −.104 | −.139 | −.032 | −.016 |
| Letter-Number Sequencing | |||||
| Longest Sequence | .045 | .178 | .005 | .099 | .051 |
| Total | −.301 | −.137 | −.116 | .199 | −.069 |
| 0-Back | |||||
| Hit Rate | .058 | .136 | .408† | .434† | .589* |
| False Alarm Rate | −.132 | −.226 | −.278 | −.324 | −.316 |
| Target Reaction Time | −.137 | −.004 | −.163 | −.021 | .181 |
| Distractor Reaction Time | −.219 | −.045 | −.178 | .018 | .173 |
| 2-Back | |||||
| Hit Rate | −.248 | −.023 | .113 | .403† | .335 |
| False Alarm Rate | .272 | .100 | .017 | −.331 | −.145 |
| Target Reaction Time | .167 | .160 | .411† | .300 | .373 |
| Distractor Reaction Time | .129 | .102 | .442† | .264 | .291 |
| RVIP | |||||
| Hit Rate | −.013 | .135 | −.020 | .048 | .239 |
| False Alarm Rate | −.037 | .163 | −.129 | −.163 | −.127 |
| Hit Reaction Time | .198 | .200 | −.020 | −.167 | −.124 |
p < .05,
p < .002.
Adjusting for multiple comparisons within each odor span measure, associations meeting a threshold of p < .002 were deemed statistically significant.
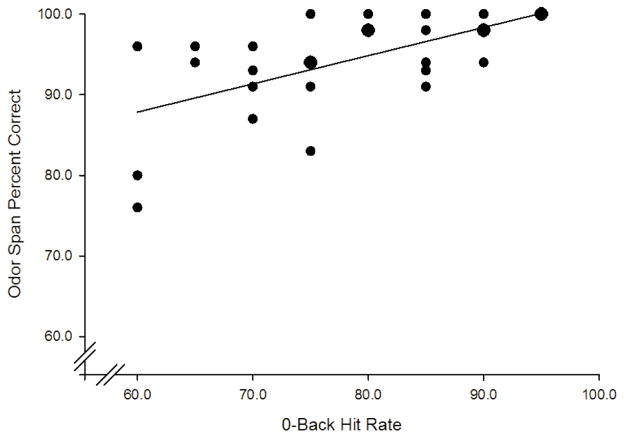
As shown in Figure 6, odor span accuracy demonstrated a moderate significant positive association with 0-back hit rate (r = .589, p = .001) . Similar positive trends were observed between span and 0-back hit rate (r = .408, p = .031) and between longest span and 0-back hit rate (r = .434, p = .021) though, these associations did not reach the adjusted significance threshold. Span demonstrated a trend-level positive association with 2-back target reaction time (r = .411, p = .030) and with 2-back distractor reaction time (r = .442, p = .018). Trends were also observed between longest span and 2-back hit rate (r = .403, p = .033).
Figure 6.
Relationship between OST accuracy and 0-back hit rate during placebo sessions. Enlarged circles represent points in which two participants provided identical performance. Bivariate correlation demonstrated a negative relationship between accuracy and the number of odors participants needed to remember (r = .434, p = .021).
Discussion
A primary aim of the present study was to provide comparative data regarding human performance on the OST. Building upon methods of human OST testing described by Levy et al., (2003) the present study adapted control procedures for the OST which were developed in rodent designs (MacQueen et al. 2011; MacQueen et al. 2016). This included repeated testing of olfactory discrimination during the OST. As in rats, humans achieved higher accuracy on these simple discrimination trials (85.7%) when compared with OST trials (77.9%) after placebo. OST accuracy was lower than was observed in healthy controls (90.6%) in the Levy et al. (2003) study. This was somewhat expected as the present design included more odors in the OST (20 total). The present study also observed lower mean span (4.71) than previously observed. Though, as has been observed in rats (April et al. 2013; Galizio et al. 2013; MacQueen et al. 2011), participants frequently responded with high rates of accuracy even after an initial error. As such, the longest span of the session (longest string of consecutive correct responses) was considerably higher (6.82).
Given that the OST is unique in evaluating capacity effects in rodents, evidencing capacity effects in human performance is particularly pertinent to the translational utility of task. To accommodate an analysis of capacity effects, a two-choice procedure (1 novel and 1 comparison odor) was adapted for the human OST. In a standard OST, the number of odors to be remembered and the number of choices presented increase in tandem. The confound between capacity and chance performance complicates evaluation of capacity effects on performance, especially given that accuracy has been shown to decrease as the number of comparison stimuli presented increases in rats (April et al. 2013). The two-choice procedure avoids this problem as chance performance is equated across all trials after the first at 50%. As is observed with rats, a negative association between accuracy and the number of odors to be remembered was detected in our sample. This trend was not observed across odor discrimination trials suggesting that declining accuracy is not easily accounted for by fatigue, impairment in odor discrimination, or task motivation.
Though odor span performance appears to be capacity dependent, the task has not been demonstrated to index a limited capacity memory process of the kind proposed in contemporary models of human working memory. As noted in rats (see April et al. 2013) and observed presently in humans, performance does not fail entirely after an initial error, as is seen in traditional span tasks (i.e., digit span). We observed accuracy well above chance at the highest memory load (19 odors) and rats perform above chance with at least 71 odors (April et al. 2013). A capacity limit for either species has yet to be identified and it has been suggested that the OST may assess function of a near limitless form of recognition memory akin to picture recognition in humans (April et al. 2013).
The control procedures introduced into the human OST also improve the suitability of the task for pharmacological testing. The effect of nicotine on human OST performance was of particular interest in that it is a reputed cognitive enhancer, and cholinergic manipulations have been found to impact OST performance in rodents (Rushforth et al. 2010; Turchi and Sarter 2000; Young et al. 2007a). No effect of nicotine was detected however, on any of the measures derived from the human OST. nicotine has been reported to improve span on the OST in rats (Rushforth et al. 2011). While this may reflect a species difference, methodological considerations warrant consideration. In rats, nicotine enhancement of OST performance was demonstrated in a procedure in which the task was terminated after the first error. This OST variant yields a span measure but, does not provide an overall accuracy measure as subjects vary with regard to trials completed. In the present study, increases in span were observed across doses of nicotine but, this effect did not reach significance. The effect of nicotine on overall accuracy has yet to be assessed in rodents. It is also worth noting that nicotine did not reverse NMDA antagonist induced OST accuracy deficits (MacQueen et al. 2016), the nicotinic antagonist scopolamine did not selectively impair OST accuracy (Galizio 2016; Galizio et al. 2013), and other reputed cognitive enhancing stimulant drugs, such as methylphenidate, have not been found to improve accuracy in rats (Galizio 2016; Galizio et al. 2016).
The effects of nicotine were also tested on a battery of attention and working memory tasks. Delineating cognitive processes enhanced by nicotine is important as it has been suggested that cognitive enhancement may serve to motivate and reinforce tobacco smoking in some individuals (Evans and Drobes 2009). Amongst non-smokers, nicotine dose-dependently improved RVIP accuracy. This corroborates earlier findings of enhanced RVIP accuracy after nicotine gum (4mg) in non-smokers (Knott et al. 2011). Increased “fast hits” (hits occurring in ≤ 450 ms) have also been observed on the RVIP task in non-smokers receiving subcutaneous injections of 0.3 and 0.6 mg of nicotine (Foulds et al. 1996) and RVIP hits tended to improve in non-smokers after nicotine delivered by an inhaler (File et al. 2001). However, in contrast with Foulds et al. (1996) and Knott et al., (2011) significant reductions in RVIP hit reaction time were not observed presently. The RVIP is a target detection task measuring sustained attention. That is, detection of stimulus events which occur infrequently and unpredictably over extended periods of time (Sarter et al. 2001). Alternative continuous performance tests have also been used to assess the effects of nicotine on sustained attention, revealing robust enhancements amongst smokers in deprivation and more limited evidence of enhancement in non-smokers (Heishman et al. 2010; Heishman et al. 1994). Interestingly, the enhancement of RVIP performance reported presently occurred in the context of self-reported impairments in attention. That participants reported increased difficulty with attention after the high dose nicotine gum suggests that the enhancement of RVIP accuracy is not easily accounted for by participant expectancies regarding nicotine effects. That nicotine did not impact RVIP reaction time suggests that RVIP effects do not simply reflect enhancement of sensorimotor abilities.
The present study also included alternative measures of attention, most notably the 0-back component of the N-back task, which requires participants to indicate whether sequentially presented stimuli match a predefined target. In contrast to the RVIP, which requires responses only when three even or odd digits are presented in a row (requiring memory of the last two stimuli presented), the 0-back task has no mnemonic demand. As such, one might expect 0-back to be more sensitive to the attention facilitating effects of nicotine. However, in the present study, RVIP hit rate was 43% (median = 42%) during placebo sessions, compared with 93% (median = 95%) for the 0-back. Thus, a ceiling effect may have limited the ability to observe an enhancement effect of nicotine on 0-back accuracy. It is also worth noting that the tasks also differed with regard to the speed of stimulus presentation (1:600ms for RVIP; 1:2250ms for 0-back), and response requirement (after targets for RVIP; every trial for 0-back). Across a range of delivery methods, nicotine generally has not produced significant effects in N-back accuracy amongst non-smokers (Heishman et al. 2010; Kleykamp et al. 2005). A notable exception is reported by Kumari et al. (2003) who observed improved 0-back accuracy after subcutaneous doses of nicotine (12 μg/kg body weight) in 11 male non-smokers.
Nicotine did not impact any of the clinical measures of working memory in the present sample. While these tasks receive widespread use in clinical neuropsychology, they have been less commonly used to evaluate the effects of nicotine/smoking. In limited study, impairments in digit span performance have not been observed amongst nicotine deprived smokers or non-smokers (Jones et al. 1992; Merritt et al. 2012; Merritt et al. 2010). To our knowledge, the present study represents the first test of the effects of nicotine on the spatial span and letter number-sequencing tasks conducted in healthy non-smokers. It should be noted however, that significant practice effects were detected on all three clinical tasks (digit span, spatial span, letter-number sequencing) and reaction time within the 2-back task, which may have interfered with detection of nicotine effects. Thus, between-group testing may be more fruitful for detecting drug effects with these tasks.
The association of measures from cognitive battery were also assessed to evaluate validity of the OST. As expected, the odor discrimination training and performance measures correlated strongly with each other but, did not associate with primary odor span measures or correlate with any other measure of human attention or working memory. In contrast, significant relationships were detected between OST and N-back measures. In general, odor span performance appears to be most related to 0-back performance, somewhat associated with 2-back performance, and demonstrated no relationship with other span tasks.
The relationship between odor span accuracy and hit rate on a visual/symbolic N-back task suggests that odor span measures may index neurocognitive processes of attention and recognition which are shared across tasks employing disparate stimulus modalities. Several psychiatric conditions are characterized by inattention, as evidenced by deficits in N-back performance, including ADHD (Karatekin et al. 2009; Klein et al. 2006; Shallice et al. 2002) and schizophrenia/psychosis (Haatveit et al. 2010; Jansma et al. 2004; Karatekin et al. 2009). While these conditions are also associated with working memory deficits, inattention may underlie deficits observed in these groups on more complex working memory tasks (Karatekin et al. 2009).
The OST has been acknowledged as a promising tool for preclinical models of schizophrenia (Dudchenko et al. 2013) and the present study lends credibility to the translational merits of the task. While other cross-species tasks of attention and recognition have been developed, this OST variant is unique in that in includes control procedures for assessing effects on stimulus discrimination and motivation. The availability of a validated task utilizing olfactory stimuli also provides a means by which to distinguish shared and distinct neurocognitive processes involved in attention across stimulus/sensory modalities. Further, the odor span task may be particularly useful for evaluating the neurocognitive pathology of conditions associated with changes in olfaction such as Alzheimer’s disease (Devanand et al. 2000; Gilbert et al. 2004; Gilbert and Murphy 2004a; b) and PTSD (Croy et al. 2010; Dileo et al. 2008).
With regard to nicotine effects, a strength of the present study is that interpretation of nicotine effects are not complicated by a history of nicotine exposure given that the sample was constrained to participants with a very limited history of nicotine use. However, it is plausible that this criteria biased the sample towards individuals who experience only limited positive effects of nicotine or are especially sensitive to the aversive effects of nicotine. Participants did indeed report dose dependent increases in aversive nicotine effects which may have counteracted cognitive enhancing effects. Future studies might utilize a younger sample to avoid individuals who have opted out of initiating regular smoking as a result of aversive reactions to nicotine. It is also worth noting that the present sample was screened for medical and psychiatric conditions, as well as substance use. This was done for participant safety and to avoid potential confounding cognitive effects of these variables. However, this also limits generalizability of the findings to non-smokers free of the aforementioned conditions. It has been suggested that cognitive enhancing effects of nicotine may be more pronounced in individuals who have relative deficits in certain cognitive domains (Evans and Drobes 2009). To the extent that deficits are associated with the excluded conditions the sample may have been biased towards individuals less likely to show nicotine-induced cognitive enhancement. As a whole the inclusion/exclusion criteria used presently allowed for timely recruitment of a non-smoking sample larger than is typical of experimental studies of nicotine (See Heishman et al. 2010).
In sum, the odor span task was successfully adapted for humans with the inclusion of control procedures reverse-translated from non-human designs. Human odor span performance demonstrated capacity effects, as are observed in rats, and was positively associated with accuracy on a visual/symbolic 0-back task. Thus, the odor span task may be best conceptualized as an attention or recognition memory task which is sensitive to capacity effects. As such, it may be particularly useful in preclinical models of disorders characterized by impaired attention or selective olfactory deficits.
Supplementary Material
Acknowledgments
This study was funded by National Institute of Drug Abuse grant F31 DA033114-01A1. The authors would like to thank Madeline Brown, Eric Brotherton, Samantha Gonzalez, and Emily Cutolo for their work on the project.
References
- April LB, Bruce K, Galizio M. The Magic Number 70 (plus or minus 20): Variables Determining Performance in the Rodent Odor Span Task. Learn Motiv. 2013;44:143–158. doi: 10.1016/j.lmot.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–39. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Beach JW, Terry AV., Jr Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. J Pharmacol Exp Ther. 2009;328:364–70. doi: 10.1124/jpet.108.145292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy I, Schellong J, Joraschky P, Hummel T. PTSD, but not childhood maltreatment, modifies responses to unpleasant odors. Int J Psychophysiol. 2010;75:326–31. doi: 10.1016/j.ijpsycho.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Dautzenberg B, Nides M, Kienzler JL, Callens A. Pharmacokinetics, safety and efficacy from randomized controlled trials of 1 and 2 mg nicotine bitartrate lozenges (Nicotinell) BMC Clin Pharmacol. 2007;7:11. doi: 10.1186/1472-6904-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DA, Greba Q, Howland JG. GluN2B-containing NMDA receptors and AMPA receptors in medial prefrontal cortex are necessary for odor span in rats. Front Behav Neurosci. 2013a;7:183. doi: 10.3389/fnbeh.2013.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DA, Molder JJ, Greba Q, Howland JG. Inactivation of medial prefrontal cortex or acute stress impairs odor span in rats. Learn Mem. 2013b;20:665–9. doi: 10.1101/lm.032243.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanand DP, Michaels-Marston KS, Liu X, Pelton GH, Padilla M, Marder K, Bell K, Stern Y, Mayeux R. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer’s disease at follow-up. Am J Psychiatry. 2000;157:1399–405. doi: 10.1176/appi.ajp.157.9.1399. [DOI] [PubMed] [Google Scholar]
- Dileo JF, Brewer WJ, Hopwood M, Anderson V, Creamer M. Olfactory identification dysfunction, aggression and impulsivity in war veterans with post-traumatic stress disorder. Psychol Med. 2008;38:523–31. doi: 10.1017/S0033291707001456. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Talpos J, Young J, Baxter MG. Animal models of working memory: a review of tasks that might be used in screening drug treatments for the memory impairments found in schizophrenia. Neurosci Biobehav Rev. 2013;37:2111–24. doi: 10.1016/j.neubiorev.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Wood ER, Eichenbaum H. Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation. J Neurosci. 2000;20:2964–77. doi: 10.1523/JNEUROSCI.20-08-02964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Drobes DJ. Nicotine self-medication of cognitive-attentional processing. Addict Biol. 2009;14:32–42. doi: 10.1111/j.1369-1600.2008.00130.x. [DOI] [PubMed] [Google Scholar]
- File SE, Fluck E, Leahy A. Nicotine has calming effects on stress-induced mood changes in females, but enhances aggressive mood in males. Int J Neuropsychopharmacol. 2001;4:371–6. doi: 10.1017/S1461145701002577. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders - Patient Edition (SCID-I/P, version 2.0) Biometrics Research, New York State Psychiatric Institute; 1994. [Google Scholar]
- Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell MA. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology (Berl) 1996;127:31–8. doi: 10.1007/BF02805972. [DOI] [PubMed] [Google Scholar]
- Galizio M. Olfactory Stimulus Control and the Behavioral Pharmacology of Remembering. Behav Anal (Wash D C) 2016;16:169–178. doi: 10.1037/bar0000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizio M, April B, Deal M, Hawkey A, Panoz-Brown D, Prichard A, Bruce K. Behavioral pharmacology of the odor span task: Effects of flunitrazepam, ketamine, methamphetamine and methylphenidate. J Exp Anal Behav. 2016;106:173–194. doi: 10.1002/jeab.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizio M, Deal M, Hawkey A, April B. Working memory in the odor span task: effects of chlordiazepoxide, dizocilpine (MK801), morphine, and scopolamine. Psychopharmacology (Berl) 2013;225:397–406. doi: 10.1007/s00213-012-2825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DG, Rabinovich NE, Malpass D, Mrnak J, Riise H, Adams L, Sugai C, DevlescHoward M. Effects of nicotine on affect are moderated by stressor proximity and frequency, positive alternatives, and smoker status. Nicotine Tob Res. 2008;10:1171–83. doi: 10.1080/14622200802163092. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Barr PJ, Murphy C. Differences in olfactory and visual memory in patients with pathologically confirmed Alzheimer’s disease and the Lewy body variant of Alzheimer’s disease. J Int Neuropsychol Soc. 2004;10:835–42. doi: 10.1017/s1355617704106024. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Murphy C. Differences between recognition memory and remote memory for olfactory and visual stimuli in nondemented elderly individuals genetically at risk for Alzheimer’s disease. Exp Gerontol. 2004a;39:433–41. doi: 10.1016/j.exger.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Murphy C. The effect of the ApoE epsilon4 allele on recognition memory for olfactory and visual stimuli in patients with pathologically confirmed Alzheimer’s disease, probable Alzheimer’s disease, and healthy elderly controls. J Clin Exp Neuropsychol. 2004b;26:779–94. doi: 10.1080/13803390490509439. [DOI] [PubMed] [Google Scholar]
- Haatveit BC, Sundet K, Hugdahl K, Ueland T, Melle I, Andreassen OA. The validity of d prime as a working memory index: results from the “Bergen n-back task”. J Clin Exp Neuropsychol. 2010;32:871–80. doi: 10.1080/13803391003596421. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 2010;210:453–69. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Taylor RC, Henningfield JE. Nicotine and Smoking: A Review of Effects on Human Performance. Exp Clin Psychopharmacol. 1994;2:345–395. [Google Scholar]
- Hindmarch I, Kerr JS, Sherwood N. Effects of nicotine gum on psychomotor performance in smokers and non-smokers. Psychopharmacology (Berl) 1990;100:535–41. doi: 10.1007/BF02244008. [DOI] [PubMed] [Google Scholar]
- Houtsmuller EJ, Fant RV, Eissenberg TE, Henningfield JE, Stitzer ML. Flavor improvement does not increase abuse liability of nicotine chewing gum. Pharmacol Biochem Behav. 2002;72:559–568. doi: 10.1016/s0091-3057(02)00723-2. [DOI] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, van der Wee NJ, Kahn RS. Working memory capacity in schizophrenia: a parametric fMRI study. Schizophr Res. 2004;68:159–71. doi: 10.1016/S0920-9964(03)00127-0. [DOI] [PubMed] [Google Scholar]
- Jones GM, Sahakian BJ, Levy R, Warburton DM, Gray JA. Effects of acute subcutaneous nicotine on attention, information processing and short-term memory in Alzheimer’s disease. Psychopharmacology (Berl) 1992;108:485–94. doi: 10.1007/BF02247426. [DOI] [PubMed] [Google Scholar]
- Karatekin C, Bingham C, White T. Regulation of cognitive resources during an n-back task in youth-onset psychosis and attention-deficit/hyperactivity disorder (ADHD) Int J Psychophysiol. 2009;73:294–307. doi: 10.1016/j.ijpsycho.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biol Psychiatry. 2006;60:1088–97. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Kleykamp BA, Jennings JM, Blank MD, Eissenberg T. The Effects of Nicotine on Attention and Working Memory in Never-Smokers. Psychol Addict Behav. 2005;19:433–438. doi: 10.1037/0893-164X.19.4.433. [DOI] [PubMed] [Google Scholar]
- Knott VJ, Millar AM, McIntosh JF, Shah DK, Fisher DJ, Blais CM, Ilivitsky V, Horn E. Separate and combined effects of low dose ketamine and nicotine on behavioural and neural correlates of sustained attention. Biol Psychol. 2011;88:83–93. doi: 10.1016/j.biopsycho.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, ffytche DH, Mitterschiffthaler MT, Das M, Zachariah E, Vythelingum GN, Williams SC, Simmons A, Sharma T. Cognitive effects of nicotine in humans: an fMRI study. Neuroimage. 2003;19:1002–13. doi: 10.1016/s1053-8119(03)00110-1. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184:523–39. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Levy DA, Manns JR, Hopkins RO, Gold JJ, Broadbent NJ, Squire LR. Impaired visual and odor recognition memory span in patients with hippocampal lesions. Learn Mem. 2003;10:531–6. doi: 10.1101/lm.66703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen DA, Bullard L, Galizio M. Effects of dizocilpine (MK801) on olfactory span in rats. Neurobiol Learn Mem. 2011;95:57–63. doi: 10.1016/j.nlm.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen DA, Dalrymple SR, Drobes DJ, Diamond DM. Influence of pharmacological manipulations of NMDA and cholinergic receptors on working versus reference memory in a dual component odor span task. Learn Mem. 2016;23:270–7. doi: 10.1101/lm.041251.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt PS, Cobb AR, Cook GI. Sex differences in the cognitive effects of tobacco abstinence: a pilot study. Exp Clin Psychopharmacol. 2012;20:258–63. doi: 10.1037/a0027414. [DOI] [PubMed] [Google Scholar]
- Merritt PS, Cobb AR, Moissinac L, Hirshman E. Evidence that episodic memory impairment during tobacco abstinence is independent of attentional mechanisms. J Gen Psychol. 2010;137:331–42. doi: 10.1080/00221309.2010.499395. [DOI] [PubMed] [Google Scholar]
- Nemeth-Coslett R, Henningfield JE. Effects of nicotine chewing gum on cigarette smoking and subjective and physiologic effects. Clin Pharmacol Ther. 1986;39:625–30. doi: 10.1038/clpt.1986.110. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation; Corporation P, editor. WAIS III/WMS III Technical Manual Update. Psychological Corporation; San Antonio, TX: 2002. [Google Scholar]
- Rushforth SL, Allison C, Wonnacott S, Shoaib M. Subtype-selective nicotinic agonists enhance olfactory working memory in normal rats: a novel use of the odour span task. Neurosci Lett. 2010;471:114–8. doi: 10.1016/j.neulet.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Rushforth SL, Steckler T, Shoaib M. Nicotine improves working memory span capacity in rats following sub-chronic ketamine exposure. Neuropsychopharmacology. 2011;36:2774–81. doi: 10.1038/npp.2011.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35:146–60. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Saults JS, Cowan N. A central capacity limit to the simultaneous storage of visual and auditory arrays in working memory. J Exp Psychol Gen. 2007;136:663–84. doi: 10.1037/0096-3445.136.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Marzocchi GM, Coser S, Del Savio M, Meuter RF, Rumiati RI. Executive function profile of children with attention deficit hyperactivity disorder. Developmental neuropsychology. 2002;21:43–71. doi: 10.1207/S15326942DN2101_3. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Cone EJ, Buchhalter AR, Henningfield JE, Rohay JM, Gitchell JG, Pinney JM, Chau T. Rapid absorption of nicotine from new nicotine gum formulations. Pharmacol Biochem Behav. 2009;91:380–4. doi: 10.1016/j.pbb.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Turchi J, Sarter M. Cortical cholinergic inputs mediate processing capacity: effects of 192 IgG-saporin-induced lesions on olfactory span performance. Eur J Neurosci. 2000;12:4505–14. [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Watson D, Wiese D, Vaidya J, Tellegen A. The two general activation systems of affect: Structural findings, evolutionary considerations, and psychobiological evidence. J Pers Soc Psychol. 1999;76:820–838. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol. 1999;7:354–61. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Warburton DM. Effects of smoking on rapid information processing performance. Neuropsychobiology. 1983;9:223–9. doi: 10.1159/000117969. [DOI] [PubMed] [Google Scholar]
- Young JW, Crawford N, Kelly JS, Kerr LE, Marston HM, Spratt C, Finlayson K, Sharkey J. Impaired attention is central to the cognitive deficits observed in alpha 7 deficient mice. Eur Neuropsychopharmacol. 2007a;17:145–155. doi: 10.1016/j.euroneuro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Young JW, Geyer MA. Developing treatments for cognitive deficits in schizophrenia: the challenge of translation. J Psychopharmacol. 2015;29:178–96. doi: 10.1177/0269881114555252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Kerr LE, Kelly JS, Marston HM, Spratt C, Finlayson K, Sharkey J. The odour span task: a novel paradigm for assessing working memory in mice. Neuropharmacology. 2007b;52:634–45. doi: 10.1016/j.neuropharm.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Young JW, Sharkey J, Finlayson K. Progressive impairment in olfactory working memory in a mouse model of Mild Cognitive Impairment. Neurobiol Aging. 2009;30:1430–43. doi: 10.1016/j.neurobiolaging.2007.11.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.