Abstract
Objective
Ventilator dyssynchrony (VD) is potentially harmful to patients with or at risk for the Acute Respiratory Distress Syndrome (ARDS). Automated detection of VD from ventilator waveforms has been difficult. It is unclear if certain types of VD deliver large tidal volumes and whether levels of sedation alter the frequency of VD.
Design
A prospective observational study.
Setting
A university medical intensive care unit.
Patients
Patients with or at risk for ARDS.
Interventions
Continuous pressure-time, flow-time, and volume-time data were directly obtained from the ventilator. The level of sedation and the use of neuromuscular blockade (NMB) was extracted from the medical record. Machine learning algorithms that incorporate clinical insight were developed and trained to detect four previously described and clinically relevant forms of VD. The association between normalized tidal volume and VD and the association between sedation and the frequency of VD were determined.
Measurements and Main Results
A total of 4.26 million breaths were recorded from 62 ventilated patients. Our algorithm detected three types of VD with an area under the receiver operator curve of greater than 0.89. VD occurred in 34.4%[95% CI=34.41%, 34.49%] of breaths. When compared to synchronous breaths, double-triggered and flow-limited breaths were more likely to deliver tidal volumes greater than 10ml/kg(40% and 11% compared to 0.2%, p<0.001 for both comparisons). Deep sedation reduced but did not eliminate the frequency of all VD breaths (p<0.05). VD was eliminated with NMB (p<0.001).
Conclusion
We developed a computerized algorithm that accurately detects three types of VD. Double-triggered and flow-limited breaths are associated with the frequent delivery of tidal volumes of greater than 10ml/kg. While VD is reduced by deep sedation, potentially deleterious tidal volumes may still be delivered. However, NMB effectively eliminates VD.
Keywords: ventilators, mechanical, machine learning, respiratory distress syndrome, adult, deep sedation, neuromuscular blockade, ventilator induced lung injury
Introduction
Injurious mechanical ventilation with large tidal volumes for 5 to 10 minutes can cause ventilator induced lung injury (VILI), especially in patients with or at risk for the acute respiratory distress syndrome (ARDS).(1) One method to minimize VILI is low-tidal volume mechanical ventilation (LTVV). LTVV reduced mortality in patients with ARDS and reduced pulmonary complications in those at risk for ARDS.(2, 3) However, LTVV alone does not eliminate the development of VILI. One common phenomenon that can induce VILI in the context of LTVV is ventilator dyssynchrony (VD).
Ventilator dyssynchrony, defined as the inappropriate timing and delivery of a ventilator breath in response to a patient’s effort to breathe,(4)(4) can be accurately detected by the analysis of ventilator flow, volume, and pressure waveforms.(4–6) VD may propagate VILI even with the utilization of LTVV and may be potentially harmful in patients with or at risk for ARDS. Several forms of VD have been associated with increased morality and longer durations of mechanical ventilation.(7) Indeed, new pressure-controlled mode of ventilation that target a goal tidal volume have been developed to promote patient-ventilator synchrony. The detection of VD from ventilator waveforms has required a labor-intensive process in which printed pressure, flow, and volume waveforms were manually reviewed. Recent advances in computing have facilitated continuous electronic monitoring of ventilator waveforms to identify VD. However, the current computerized algorithms only detect some forms of VD, do not utilize all waveforms that can be derived from the ventilator, and have limited accuracy.(7)
An accurate computerized algorithm could fill several gaps in our current understanding of VD. First, there are several commonly described types of VD, including double triggered breaths, ineffective triggering, premature ventilator termination, and flow-limited breaths. Though double-triggered breaths have been associated with larger tidal volumes, it is unclear whether ineffective triggering, premature ventilator termination, and flow-limited breaths are also associated with large tidal volumes.(8) Additionally, the association between double-triggered breaths and large tidal volumes in the context of newer modes of ventilation is unknown.
An accurate computerized algorithm could facilitate an understanding of the relationship between VD and subsequent changes in patient management. Sedative medications are commonly used for mechanically ventilated patients to improve patient comfort and the ventilator interface. Currently, sedation is titrated to a measure of agitation, such as the Richmond Agitation and Sedation Scale (RASS) that do not include measures of VD. As a result, the effects of deeper levels of sedation on the frequency of VD is currently unknown. Sedatives may decrease and eliminate certain types of VD. However, over sedation may increase other types of VD – specifically ineffective-triggered breaths.(9) In addition, over sedation is associated with deleterious outcomes including increased duration of mechanical ventilation and ICU length of stay.(8, 10) The development of a real-time, personalized algorithm that utilizes measures of VD to titrate sedation and minimize VD, while accounting for delayed onset of sedation to avoid over sedation is needed.
We conducted this single-center prospective study to address these issues. We developed an accurate computerized algorithm to detect VD. Subsequently, we sought to determine whether dyssynchrony, detected by this algorithm, was associated with large tidal volumes and whether this dyssynchrony improved with deeper sedation or the use of neuromuscular blockade.
Methods
Study Population
Between June 2014 and January 2017, adult patients admitted to the University of Colorado Hospital medical intensive care unit (MICU) at risk for or with ARDS and requiring mechanical ventilation were enrolled within 12 hours of intubation.(11) At risk patients were defined as intubated patients with hypoxemia and a mechanism of lung injury known to cause ARDS, who had not yet met chest x-ray or oxygenation criteria for ARDS. To facilitate the capture of continuous ventilator data, only patients ventilated with a Hamilton G5 ventilator were included. Patients requiring mechanical ventilation only for asthma, COPD, heart failure, or airway protection were excluded. Additionally, patients less than 18 years of age, pregnant, or imprisoned were excluded. The University of Colorado Hospital utilizes a ventilator protocol that incorporated the ARDS network low tidal volume protocol with the low PEEP titration table. The Colorado Multiple Institutional Review Board approved this study and waived the need for informed consent.
Data Collection
Baseline patient information including age, gender, height, and initial P/F ratio were collected. Continuous ventilator data were collected using a laptop connected to the ventilator and using Hamilton DataLogger software (Hamilton, v5.0, 2011) to obtain pressure, flow, and volume measurements. Additionally, the DataLogger software allowed collection of ventilator mode and ventilator settings based on mode (i.e.: set tidal, respiratory rate, positive end-expiratory pressure (PEEP), and fraction inspired oxygen (FIO2)). Richmond Agitation and Sedation Scale (RASS) values and the use of neuromuscular blockade (NMB) were extracted from the medical record at 4-hour intervals. Data were collected until extubation or for up to seven days per patient.
Development of Computerized Algorithm to Detect VD
Our algorithm was developed using Python and the SciPy scientific stack, an open source programing and scientific analysis toolset.(12, 13) The SciPy scientific stack includes machine learning algorithms as well as methods for cross-validation, calculation of accuracy to evaluate distinctive features, and machine learning techniques. We developed a training set of data, calculated characteristics about each breath useful to identifying four types of VD (figure 1), and then iteratively developed machine-learning models to classify breaths as normal or dyssynchronous using the training data (details of algorithm development provided in Supplemental Material).
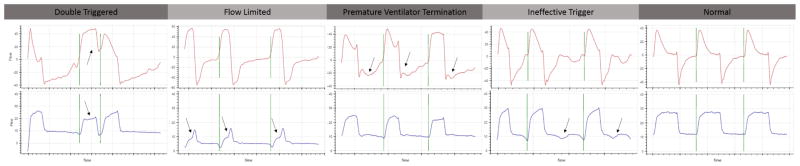
Figure 1. Representative Images of Each Type of Dyssynchrony.
Double-triggered breaths are characterized incomplete exhalation between breaths. Flow-limited breaths are characterized by the scooping of the pressure-time curve (arrow). Premature-ventilator-terminated breaths are characterized by the ventilator terminating the breath and then the patient making an immediate effort to take another breath (arrow). Finally, ineffective-triggered breaths are characterized as the patient making efforts to initiate a breath during the expiratory phase of the breath (arrows).
Determine the Association between VD and Tidal Volume
Only breaths in APVCMV (a proprietary dual triggered mode) or PC mode were included in the final analysis. The delivered inspiratory tidal volumes were normalized to predicted body weight. The mean normalized tidal volumes of double-triggered, flow-limited, premature-ventilator-terminated, or ineffective-triggered breaths were compared to synchronous breaths. Secondly, the percentage of breaths greater than 10ml/kg for each type of dyssynchrony was calculated and compared.
Determine the Association between VD and Sedation
The University of Colorado targets sedations to a RASS score. Sedation strategies were determined by the treating team. RASS scores measured in 4-hour intervals and the presence of NMB during that interval were extracted from the electronic medical record. RASS score range from −5 (comatose) to 5 (highly agitated). RASS scores were stratified as deep sedation (RASS −5 or −4), moderate sedation (RASS −3 or −2), light sedation (RASS of −1 or 0) or agitation (RASS > 0). The frequency of VD averaged over a four-hour window around each recorded RASS score was calculated. Results were analyzed to estimate the difference in the frequency of VD for double-triggered, flow-limited, premature-ventilator-terminated, and ineffective-triggered breaths as stratified using NMB, deep, moderate, or light sedation or agitation. Agitation (RASS>0) was used as the reference group in this regression model.
Statistical Analysis
Continuous data are presented as either mean and standard deviation or as median and 25–75% quartiles when the data were non-normally distributed. Normally distributed data were analyzed with a t-test and non-normally distributed data were analyzed with a Wilcoxon Rank Sum test. Mixed effects linear regression was performed to analyze the association between normalized tidal volume and types of VD. Similarly, generalized linear mixed effects models with a binomial distribution were used to analyze the association between types of VD and RASS score. A binomial distribution was used based on the distribution of dyssynchrony frequency. Mixed-effects modeling was used to account for the repeated measures for each patient. Patient ID was analyzed as a random intercept and random slope. Statistical analysis was performed in MatLab (MathWorks, Inc, R2016b).
Results
Baseline Patient and Ventilator Information
A total of 110 patients met inclusion criteria for enrollment and 62 were enrolled (figure S1). Patients were on average 53.7±15.1 years old, 27(44%) were female, and 33(53%) were Caucasian (table 1). Twenty-two (36%) patients had a primary diagnosis of pneumonia, 19(31%) patients had ARDS, 5(8%) had multiple transfusions, 5(8%) patients were post cardiac arrest, 3(5%) had pancreatitis, and 8(12%) had other diagnoses. A total of 17(27%) patients were treated with NMB. The median duration of mechanical ventilation was 4.7[1.9, 6.4] days. Overall, 24(39%) patients died in the hospital.
Table 1.
Baseline Demographic and Ventilator Data
| CHARACTERISTICS | PATIENT INFORMATION |
|---|---|
|
| |
| AGE (SD) | 53.7 ± 15.0 |
| FEMALE | 27 (44%) |
| CAUCASIAN | 33 (53%) |
| MEDIAN VENTILATOR DAYS [25%, 75% IQR] | 4.7 [1.9, 6.4] |
| NEED FOR NMB | 17 (27%) |
| INITIAL P/F RATIO (SD) | 139 ± 68 |
| MORTALITY | 24 (39%) |
| CHARACTERISTICS | VENTILATOR INFORMATION |
| TOTAL BREATHS | 4,256,323 |
| APVCMV BREATHS | 3,624,109 (85%) |
| PCMV BREATHS | 186,275 (4%) |
| NON-NMB BREATHS IN VC OR PC MODES | 3,021,881 (71%) |
| MEDIAN HOURS OF DATA COLLECTED [25%, 75% IQR] | 37.1 [12.1, 87.0] |
| MEDIAN BREATHS COLLECTED PER PATIENT [25%, 75% IQR] | 40,047 [21,225, 116,834] |
From this cohort of patients, 4.26 million breaths were recorded. Of these breaths, 3.81 million breaths were in APCMV or PC modes of which 3.02 million breaths were in the absence of NMB. A median of 37.1[12.1, 87.0] hours of ventilator data and a median of 40,047[21,225, 116,834] breaths per patient were analyzed.
VD Algorithm Development and Results
Cross-validation and the separate validation set had similar test characteristics for double-triggered, flow-limited, and synchronous breaths, and demonstrated a ROC of greater than 89% for these types of VD. Ineffective triggered breaths had an ROC of 91% and 80% with cross-validation and in a separate validation set, respectively. Premature ventilator terminated breaths had reduced test characteristics in the separate validation set, raising the possibility of overfitting and limiting our ability to draw further conclusions about this type of dyssynchrony (Table 2, Table S2). Of the 3,021,881 breaths recorded while a patient was not on neuromuscular blockade agents and in APVCMC or PC modes, 34.4% [95% CI=34.41%, 34.49%] of breaths were dyssynchronous. When analyzed by patient, the median percentage of dyssynchronous breaths in non-paralyzed patients was 47.3% [95% CI=12.3%, 69.0%]. All patients had some dyssynchronous breaths. Of all breaths not on NMB, 29.2%[95% CI=28.96%, 29.26%] were premature-ventilator-terminated breaths, 24.8% [95% CI=24.2%, 25.04%] were ineffective-triggered breaths, 13.6% [95% CI=13.56%, 13.64%] were flow-limited breaths, and 3.12% [95% CI=3.10%, 3.14%] were double-triggered breaths, noting that different types of VD are not mutually exclusive. Flow-limited and ineffective-triggered breaths (r2=0.18 [95% CI=0.07, 0.26], p<0.001) also showed evidence of correlation in all patients.
Table 2.
VD Detection Algorithm Test Characteristics and Inter-reviewer Agreement
| VD TYPE | ACCURACY ± SD | SENSITIVITY ± SD | SPECIFICITY ± SD | ROC AUC ± SD | KAPPA (95% CI) | PERCENT AGREEMENT |
|---|---|---|---|---|---|---|
| DOUBLE-TRIGGERED | 97% | 90% | 97% | 95% | 0.69 (0.61, 0.77) | 95.5% |
| FLOW-LIMITED | 89% | 84% | 88% | 89% | 0.81 (0.77, 0.85) | 92.4% |
| PREMATURE-VENTILATOR-TERMINATED | 54% | 23% | 47% | 61% | 0.67 (0.55, 0.78) | 97.2% |
| INEFFECTIVE-TRIGGERED | 79% | 63% | 71% | 80% | 0.58 (0.51, 0.66) | 91.1% |
| SYNCHRONOUS BREATHS | 91% | 94% | 98% | 92% | 0.76 (0.73, 0.81) | 88.5% |
Association of VD with Delivered Tidal Volume
The average normalized set tidal volume was 6.33±0.84 ml/kg for APVCMV breaths. Double-triggered and flow-limited breaths were consistently associated with larger mean delivered tidal volumes when compared to synchronous breaths (11.95ml/kg PBW [95% CI=11.06, 12.85] and 7.44ml/kg PBW [95% CI=7.02, 7.86] compared to 6.22ml/kg PBW [95% CI=5.93, 6.51], all p<0.001, figure 2). Ineffective-triggered breaths had slightly smaller average delivered tidal volumes when compared to synchronous breaths (5.90 ml/kg PBW [95% CI=5.52, 6.27], p=0.007).
Figure 2. VD Association with Normalized Tidal Volume.
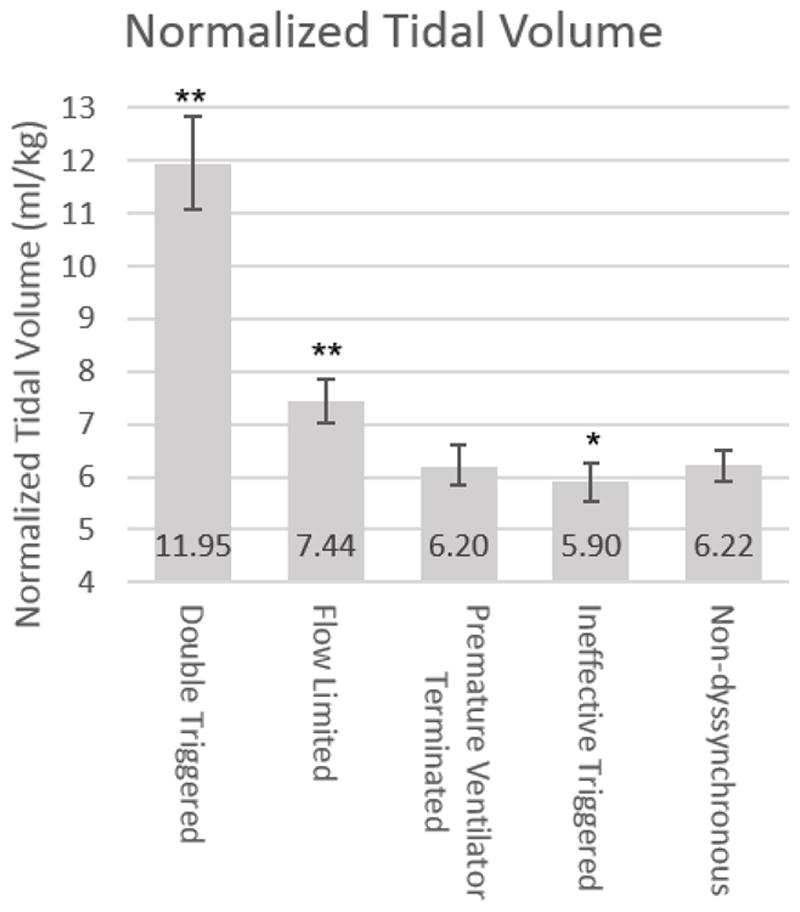
Tidal Volume normalized to predicted body associated with each type of dyssynchrony. Error bars demonstrated 95% confidence intervals. *p=0.007, **p<0.0001.
Double-triggered and flow-limited breaths were associated with the more frequent delivery of large tidal volumes greater than 10ml/kg when compared to synchronous breaths (54%[95%CI=47%, 61%] and 11%[95%CI=7%, 15%] compared to 0.9%[95%CI=0.0%, 1.9%], p<0.001, table S3). Ineffective-triggered breaths were not associated with the more frequent delivery of tidal volumes greater than 10ml/kg(p=0.90). Importantly, because flow-limited breaths were four times more common than double-triggered breaths, flow-limited breaths were responsible for the delivery of more breaths greater than 10ml/kg than double-triggered breaths. Despite the use of low-tidal volume ventilation, over 10% of all breaths recorded were associated with VD and resulted in tidal volumes greater than 10ml/kg.
Association of VD with RASS Score and NMB
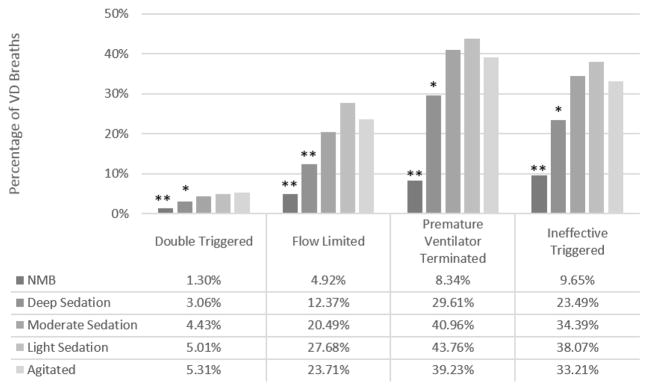
A total of 813 four-hour periods were recorded and analyzed. During these epochs, the patient received deep (n=200, RASS −4 to −5), moderate (n=208, RASS −3 to −2), or light (n=202, RASS −1 to 0) sedation. Patients were agitated (RASS > 1) during 57 epochs and received NMB during 146 epochs. Compared to RASS scores of 1 or greater, all types of VD were less frequent with deep sedation (Figure 3). Double triggered and flow-limited breaths were almost eliminated with NMB. Ineffective triggered breaths were reduced to within the false-positive rate of our detection algorithm. NMB reduced the odds of all types of VD by over 80% (all p<0.001, Table S4).
Figure 3. The Association between Types of Dyssynchrony and RASS Score.
Estimated frequency of each type of VD in a mixed-effects regression model. Error bars are 95% CI. RASS score greater than zero (agitated) as reference group for comparison; *p<0.05, ** p<0.001
Discussion
In this prospective cohort study of mechanically ventilated patients with or at risk for ARDS, we successfully created a computerized algorithm that accurately detects four types of VD. Using this computerized algorithm, we demonstrated that VD is common and that flow-limited and double-triggered breaths may be associated with large tidal volumes in the setting of low tidal volume ventilation. We further demonstrated that VD may be reduced with deep sedation but NMB is needed to eliminate VD.
Our findings expand the recent work by both Blanch and Beitler, and earlier work by Pohlman and Thille. Blanch and others have previously described the ability to accurately detect ineffective-triggered breaths using pressure-time waveforms in the absence of additional monitoring.(6, 14) Subsequently, automated systems to detect VD were created. However the accuracy, sensitivity, and specificity of such algorithms to detect all types of VD remains unclear.(7) Building on this work, we demonstrated the ability of our computerized algorithm to accurately detect a variety of types of VD. Similarly, both Pohlman and Beitler described the association of double-triggered breaths with large delivered tidal volumes.(8, 15) We add to this work by demonstrating that flow-limited breaths may also be associated with higher delivered tidal volumes. The etiology of these large tidal volumes can likely be explained by patient effort and increased work of breathing in dual control modes of ventilation. Indeed, given that flow-limited breaths occur nearly 4-fold more frequently in our patient population than double-triggered breaths, it may play a more significant role in propagating VILI than previously appreciated. The small difference in tidal volumes observed with premature-ventilator-terminated and ineffective-triggered breaths are unlikely to be clinically significant. Importantly, the association between VD and the delivery of large tidal volumes offers a mechanistic pathway to link the association of VD with increased mortality and longer duration of mechanical ventilation that has been described previously.(7, 16) Finally, we described the association between several types of VD and sedation and NMB. De Wit previously demonstrated that increasing sedation was associated with increasing frequency of ineffective-triggered breaths.(9) We build on the work of Chanques, et al. and identified that deep sedation generally decreased but did not eliminate VD.(17) Importantly, sedation alone does not effectively eliminate VD. Elimination of VD required NMB. Consequently, balancing the potential negative consequences of VD with the known negative of consequences of deep sedation and NMB will become critically important to optimizing patient outcomes.
Our study has several limitations. First, the evaluation of ventilator waveforms does not identify all types of VD. Prior studies have demonstrated that waveform analysis is accurate when compared to additional monitoring mechanisms for many types of VD (ie: electrical activity of the diaphragm monitoring or transpulmonary pressure).(5, 6) However further refinement is needed to accurately detect premature ventilator terminated breaths and conclusions regarding premature ventilator terminated breaths cannot be made with this current algorithm. The decrease in detection characteristics in the separate validation set may be from the small samples size of the validation set limiting our ability to draw accurate conclusions or from over-fitting in the cross-validated training-testing set. Second, most of the analyzed breaths were in Hamilton’s APVCMV mode of ventilation. This is a dual control mode of ventilation that is a pressure-controlled mode of ventilation that targets a goal tidal volume. While such modes have potential theoretical benefits, it allows for variation in tidal volumes. Consequently, the higher percentage of delivered tidal volumes greater than 10ml/kg for flow-limited breaths may be a consequence of patient effort in this mode of ventilation. Conversely, in a true volume controlled mode of ventilation, double-triggered breaths should approximate twice the set tidal volume resulting in event higher tidal volumes. Third, the frequency of VD observed in our patient population was higher than previously described. To our knowledge, this is the first study to describe VD in such a dual control mode of ventilation. Thille described a median of 2.1%, Blanche of 3.4%, and De Wit of 11% of breaths had evidence of double-triggered, aborted inspirations, short cycled, prolonged cycled, and ineffective-triggered breaths.(7, 9, 16) These studies did not measure the frequency of flow-limited breaths and only studied breaths in volume or pressure control modes. The use of dual control modes of ventilation was not assessed. Fourth, our assumption that patient’s level of sedation was constant over a four-hour period time may not be valid. Fifth, feature selection was performed by iterative observation of clinically significant features, however, other automated techniques of feature selection exist which may improve reproducibility. While our standardization techniques were preformed to remove interperson variability, this goal may be better achieved in non-normally distributed features which may yield improved results with other means of standardization, such as Box-Cox transformations. Sixth, given the generally high correlation between observers, ground truth for each type of VD was defined by the primary reviewer. However other techniques exist to ‘fuse’ the annotations defined by multiple reviewers, which may improve the overall accuracy of labeling of each type of VD.(18, 19) Given the variation in inter-reviewer agreement, the test-characteristics described in table 1 may overestimate the ability to truly detect each type of VD – particularly for ineffective triggered breaths (κ=0.58), and less so for double-triggered (κ=0.69). Finally, this study does not demonstrate that VD is harmful, rather that VD is associated with the delivery of large tidal volumes. While previous studies have associated continuous high-volume and high-pressure ventilation with increased mortality, it is unknown if the intermittent delivery of high volume breaths associated with VD is sufficient to cause VILI.(2) Indeed, some studies suggests that intermittent delivery of large tidal volumes – so called sigh breaths – or ventilation with variable tidal volumes may be beneficial.(20–22) Moreover, this is a small, single center study describing results from one specific type of ventilator. Conclusions regarding the clinical significance of VD cannot be made without further study.
Conclusion
Our research paves the way for the large-scale study of VD by allowing us to scale analyses to large data sets through the automated the identification of VD. First, it demonstrates that the continuous monitoring and detection of VD is feasible. Second, double-triggered and flow-limited breaths may be associated with large tidal volumes. While double-triggered breaths have been assumed to be the critical type of VD to deliver large tidal volume breaths, we show that flow-limited VD may be equally important. Investigating other mechanism by which VD may propagate VILI, for example through barotrauma caused by high transpulmonary pressures, will yield further insight in the relative potential of each type of each type of VD to propagate VILI. Third, we demonstrated that VD may be eliminated by NMB. However, more research is needed to identify patients where the benefit from the elimination of VD would outweigh the negative consequences of deep sedation needed to administer NMB. To do this, machine learning-decision support systems to measure VD in the context of the underlying physiology and the clinical course of the individual will need to be developed, barriers to its acceptance by providers evaluated, and finally rigorously tested against the current standard of care in large multicentered randomized controlled trials. Additionally, in future research, we can use machine learning methods to phenotype patients, including methods that leverage temporal and waveform information, to identify potential novel VD phenotypes.(23–29)
Supplementary Material
Acknowledgments
Funding Sources: PS and MM were funded by National Institute of Health [K24 HL069223], NIH/NCATS Colorado CTSA [UL1 TR001082]; DA was funded by NLM grant R01 LM06910 and from NHGRI grant 5U01HG008680-02
Footnotes
All research was performed at the University of Colorado – Anschutz Medical Campus
Conflict of Interests: The authors declare that they have no conflict of interest.
Copyright form disclosure: Dr. Sottile, Dr. Albers, Mr. McKeehan, and Dr. Moss received support for article research from the National Institutes of Health (NIH). Dr. Sottile’s institution received funding from the NIH. Ms. Higgins has disclosed that she does not have any potential conflicts of interest.
References
- 1.Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. [Internet] Am Rev Respir Dis. 1974;110:556–65. doi: 10.1164/arrd.1974.110.5.556. Available from: http://www.ncbi.nlm.nih.gov/pubmed/4611290. [DOI] [PubMed] [Google Scholar]
- 2.Acute Respiratory Distress Syndrome Network. Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. [Internet] N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [cited 2012 Oct 28] Available from: http://www.ncbi.nlm.nih.gov/pubmed/10793162. [DOI] [PubMed] [Google Scholar]
- 3.Futier E, Constantin J-M, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. [Internet] N Engl J Med. 2013;369:428–37. doi: 10.1056/NEJMoa1301082. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23902482. [DOI] [PubMed] [Google Scholar]
- 4.Gilstrap D, MacIntyre N. Patient-ventilator interactions. Implications for clinical management. [Internet] Am J Respir Crit Care Med. 2013;188:1058–68. doi: 10.1164/rccm.201212-2214CI. [cited 2014 Jan 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/24070493%5Cnhttp://www.atsjournals.org/doi/pdf/10.1164/rccm.201212-2214CI. [DOI] [PubMed] [Google Scholar]
- 5.Mulqueeny Q, Ceriana P, Carlucci A, et al. Automatic detection of ineffective triggering and double triggering during mechanical ventilation [Internet] Intensive Care Med. 2007;33:2014–2018. doi: 10.1007/s00134-007-0767-z. [cited 2013 Oct 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/17611736. [DOI] [PubMed] [Google Scholar]
- 6.Blanch L, Sales B, Montanya J, et al. Validation of the Better Care® system to detect ineffective efforts during expiration in mechanically ventilated patients: A pilot study [Internet] Intensive Care Med. 2012;38:772–780. doi: 10.1007/s00134-012-2493-4. [cited 2013 Oct 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/22297667. [DOI] [PubMed] [Google Scholar]
- 7.Blanch L, Villagra A, Sales B, et al. Asynchronies during mechanical ventilation are associated with mortality. [Internet] Intensive Care Med. 2015;41:633–41. doi: 10.1007/s00134-015-3692-6. [cited 2015 May 1] Available from: http://link.springer.com/10.1007/s00134-015-3692-6. [DOI] [PubMed] [Google Scholar]
- 8.Pohlman MC, McCallister KE, Schweickert WD, et al. Excessive tidal volume from breath stacking during lung-protective ventilation for acute lung injury. [Internet] Crit Care Med. 2008;36:3019–23. doi: 10.1097/CCM.0b013e31818b308b. [cited 2013 Nov 15] Available from: http://www.ncbi.nlm.nih.gov/pubmed/18824913. [DOI] [PubMed] [Google Scholar]
- 9.de Wit M, Pedram S, Best AM, et al. Observational study of patient-ventilator asynchrony and relationship to sedation level. [Internet] J Crit Care. 2009;24:74–80. doi: 10.1016/j.jcrc.2008.08.011. [cited 2013 Nov 14] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2676917&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kress JP, Pohlman AS, O’Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. [Internet] N Engl J Med. 2000;342:1471–7. doi: 10.1056/NEJM200005183422002. [cited 2013 Jan 22] Available from: http://www.ncbi.nlm.nih.gov/pubmed/10816184. [DOI] [PubMed] [Google Scholar]
- 11.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. [Internet] Lancet. 2007;369:1553–64. doi: 10.1016/S0140-6736(07)60604-7. [cited 2012 Nov 7] Available from: http://www.ncbi.nlm.nih.gov/pubmed/17482987. [DOI] [PubMed] [Google Scholar]
- 12.Michel V, Gramfort A, Varoquaux G, et al. A supervised clustering approach for fMRI-based inference of brain states [Internet] Pattern Recognit. 2012;45:2041–2049. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0031320311001439. [Google Scholar]
- 13.Yi Q-X, Huang J-F, Wang F-M, et al. Monitoring rice nitrogen status using hyperspectral reflectance and artificial neural network. [Internet] Environ Sci Technol. 2007;41:6770–5. doi: 10.1021/es070144e. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17969693. [DOI] [PubMed] [Google Scholar]
- 14.Chen C-W, Lin W-C, Hsu C-H, et al. Detecting ineffective triggering in the expiratory phase in mechanically ventilated patients based on airway flow and pressure deflection: feasibility of using a computer algorithm. [Internet] Crit Care Med. 2008;36:455–461. doi: 10.1097/01.CCM.0000299734.34469.D9. [cited 2013 Oct 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/18091543. [DOI] [PubMed] [Google Scholar]
- 15.Beitler JR, Sands SA, Loring SH, et al. Quantifying unintended exposure to high tidal volumes from breath stacking dyssynchrony in ARDS: the BREATHE criteria [Internet] Intensive Care Med. 2016;42:1427–1436. doi: 10.1007/s00134-016-4423-3. Available from: http://link.springer.com/10.1007/s00134-016-4423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thille AW, Rodriguez P, Cabello B, et al. Patient-ventilator asynchrony during assisted mechanical ventilation [Internet] Intensive Care Med. 2006;32:1515–1522. doi: 10.1007/s00134-006-0301-8. [cited 2013 Nov 14] Available from: http://www.ncbi.nlm.nih.gov/pubmed/16896854. [DOI] [PubMed] [Google Scholar]
- 17.Chanques G, Kress JP, Pohlman A, et al. Impact of ventilator adjustment and sedation-analgesia practices on severe asynchrony in patients ventilated in assist-control mode. [Internet] Crit Care Med. 2013;41:2177–87. doi: 10.1097/CCM.0b013e31828c2d7a. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23782972. [DOI] [PubMed] [Google Scholar]
- 18.Raykar VC, Yu S, Zhao LH, et al. Learning From Crowds. J Mach Learn Res. 2010;11:1297–1322. [Google Scholar]
- 19.Zhu T, Dunkley N, Behar J, et al. Fusing Continuous-Valued Medical Labels Using a Bayesian Model. [Internet] Ann Biomed Eng. 2015;43:2892–902. doi: 10.1007/s10439-015-1344-1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26036335. [DOI] [PubMed] [Google Scholar]
- 20.Pelosi P, Bottino N, Chiumello D, et al. Sigh in supine and prone position during acute respiratory distress syndrome. [Internet] Am J Respir Crit Care Med. 2003;167:521–7. doi: 10.1164/rccm.200203-198OC. Available from: http://www.atsjournals.org/doi/abs/10.1164/rccm.200203-198OC. [DOI] [PubMed] [Google Scholar]
- 21.Mauri T, Eronia N, Abbruzzese C, et al. Effects of Sigh on Regional Lung Strain and Ventilation Heterogeneity in Acute Respiratory Failure Patients Undergoing Assisted Mechanical Ventilation* [Internet] Crit Care Med. 2015;43:1823–1831. doi: 10.1097/CCM.0000000000001083. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00003246-201509000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Spieth PM, Carvalho AR, Pelosi P, et al. Variable tidal volumes improve lung protective ventilation strategies in experimental lung injury. [Internet] Am J Respir Crit Care Med. 2009;179:684–93. doi: 10.1164/rccm.200806-975OC. Available from: http://www.atsjournals.org/doi/abs/10.1164/rccm.200806-975OC. [DOI] [PubMed] [Google Scholar]
- 23.Lasko TA, Denny JC, Levy MA. Computational phenotype discovery using unsupervised feature learning over noisy, sparse, and irregular clinical data. [Internet] PLoS One. 2013;8:e66341. doi: 10.1371/journal.pone.0066341. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23826094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hripcsak G, Albers DJ. Next-generation phenotyping of electronic health records. [Internet] J Am Med Inform Assoc. 2013;20:117–21. doi: 10.1136/amiajnl-2012-001145. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3555337&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hripcsak G, Albers DJ, Perotte A. Parameterizing time in electronic health record studies. [Internet] J Am Med Inform Assoc. 2015;22:794–804. doi: 10.1093/jamia/ocu051. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25725004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine ME, Albers DJ, Hripcsak G. Comparing lagged linear correlation, lagged regression, Granger causality, and vector autoregression for uncovering associations in EHR data. [Internet] AMIA Annu Symp proceedings AMIA Symp. 2016;2016:779–788. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28269874. [PMC free article] [PubMed] [Google Scholar]
- 27.Hripcsak G, Albers DJ. Correlating electronic health record concepts with healthcare process events. [Internet] J Am Med Inform Assoc. 2013;20:e311–8. doi: 10.1136/amiajnl-2013-001922. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23975625%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3861922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albers DJ, Elhadad N, Tabak E, et al. Dynamical phenotyping: using temporal analysis of clinically collected physiologic data to stratify populations. [Internet] PLoS One. 2014;9:e96443. doi: 10.1371/journal.pone.0096443. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24933368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claassen J, Perotte A, Albers D, et al. Nonconvulsive seizures after subarachnoid hemorrhage: Multimodal detection and outcomes [Internet] Ann Neurol. 2013;74:53–64. doi: 10.1002/ana.23859. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23813945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.