Abstract
ATP-binding cassette transporter A1 (ABCA1) has been found to mediate the transfer of cellular cholesterol across the plasma membrane to apolipoprotein A-I (apoA-I), and is essential for the synthesis of high-density lipoprotein. Mutations of the ABCA1 gene may induce Tangier disease and familial hypoalphalipoproteinemia; they may also lead to loss of cellular cholesterol homeostasis in prostate cancer, and increased intracellular cholesterol levels are frequently found in prostate cancer cells. Recent studies have demonstrated that ABCA1 may exert anticancer effects through cellular cholesterol efflux, which has been attracting increasing attention in association with prostate cancer. The aim of the present review was to focus on the current views on prostate cancer progression and the various functions of ABCA1, in order to provide new therapeutic targets for prostate cancer.
Keywords: ATP-binding cassette transporter A1, prostate cancer, cholesterol
1. Introduction
Prostate cancer is a common malignancy among men in Western countries; however, a sharp increase in its morbidity and mortality was recently observed in several Asian countries, including China (1). Patients with aggressive pathological characteristics are at increased risk for tumor progression and metastasis, even following radical treatment. Furthermore, these characteristics are commonly found in tumors from patients who succumb to prostate cancer. Cholesterol homeostasis is crucial for cell function and survival, whereas dysregulation of cholesterol homeostasis is known to be associated with multiple cancers, including prostate cancer (2), and altered lipid metabolism is increasingly recognized as a hallmark of prostate cancer cells (3). In fact, lethal prostate cancers exhibit higher expression of squalene monooxygenase, which is the second rate-limiting enzyme of cholesterol synthesis (4). Moreover, low serum cholesterol levels have been found in prostate cancer patients, suggesting that cholesterol may accumulate in tumor tissue. Compared with normal prostate cells, prostate tumor cells exhibit increased levels of intracellular cholesterol precursors, with loss of ABCA1-mediated cholesterol efflux (5).
ATP-binding cassette (ABC) transporters are transmembrane proteins responsible for the transfer of various substrates through extracellular and intracellular membranes. The ABC-type transporters act as gatekeepers by allowing or limiting the entrance of a wide variety of substrates across cellular membranes (6). A total of 51 ABC transporter genes have been identified and are grouped into seven subfamilies, namely A-G, based on their phylogenetic distance (7). The physiological importance of ABCA subfamily proteins is underscored by their association with various inherited diseases. Examples of ABC A-subfamily disorders include Tangier disease (ABCA1), Alzheimer's disease (ABCA2/ABCA7), Stargardt's disease (ABCR/ABCA4), harlequin-type ichthyosis (ABCA12) and others (8) (Table I). ABCA1, an ABC subfamily A exporter, mediates the cellular efflux of phospholipids and cholesterol to the extracellular acceptor apolipoprotein A-I (apoA-I) for generation of nascent high-density lipoprotein (HDL) (9). The human ABCA1 has a length of 2,261 amino acids, and was found to contain two transmembrane domains (TMDs) (10). Each TMD is followed by a cytoplasmic region comprising one nucleotide-binding domain (NBD) and one small regulatory domain. The NBDs and regulatory domains are the most highly conserved elements among the ABCA subfamily. The structure of ABCA1 reveals a polar cluster on one side of TMD1 close to the intracellular boundary (11). The structure of the ABCA1 presented herein represents a major step towards the mechanistic understanding of ABCA1-mediated lipid export and nascent HDL biogenesis (12). ABCA1 has been shown to be necessary for the synthesis of HDL by exporting cholesterol out of the cells (13). Functional ABCA1 mediates the expansion of the N-terminus of apoA-I on the cell surface, followed by the release of nascent HDL, which is the only path for elimination of cholesterol from the body (14). Two missense mutations of human ABCA1 proteins linked to Tangier disease and familial HDL deficiency were previously found to be associated with diminished cholesterol efflux activity, and the functionality of these ABCA1 mutants in terms of tumor inhibition and cholesterol efflux was tested in prostate cancer cells lacking endogenous ABCA1 expression (15). Over 50 mutations in the ABCA1 gene were recently identified. The human ABCA1 has been shown to be closely associated with the development of various human cancers, including prostate, colon and breast cancer (16–18). Prostate cancer patients with higher serum low-density lipoprotein (LDL) levels exhibited significantly shorter overall survival. Low ABCA1 expression has also been associated with shorter survival in patients with prostate cancer. This may be explained by the use of lipid as an energy source during growth and metastasis of prostate cancer cells. ABCA1 has been shown to play a critical role in the synthesis of HDL particles by exporting cellular cholesterol (19,20).
Table I.
Structure and function of ABCA subfamily proteins.
| Proteins | Structure | Function | (Refs.) |
|---|---|---|---|
| ABCA1 | Length, 2,261 amino acids; contains two TMDs, and a polar cluster on one side of | Synthesis of HDL, exporting cholesterol out of the cells, lipid efflux activity. Mutation of the | |
| TMD1 close to the intracellular boundary. | ABCA1 gene may induce Tangier disease and familial hypoalphalipoproteinemia. Tumor suppressor function in cancer. | ||
| ABCA2 | Coding region 7.3 kb in size, codes for a 2,436-amino acid polypeptide, comprises 48 exons. | Highly expressed in brain tissue; may play a role in macrophage lipid metabolism and neural development. | (34) |
| ABCA3 | Consists of 33 exons encoding a 1,704-amino acid (~150 kDa) protein. | Mutation of the ABCA3 gene is the most common cause of surfactant deficiency and is associated with cataract-microcornea syndrome. | (35) |
| ABCA4 | Transcribes a large retina-specific protein with two TMDs, two glycosylated ECDs and two NBDs. | An inward-directed retinoid flippase; mutation of ABCA4 induces Stargardt's disease. | |
| ABCA5 | Consists of 1,642 amino acid residues, with two sets of six transmembrane segments and an NBD. | Mutations in ABCA5 cause hair overgrowth and may be associated with a lysosomal disease, particularly in cardiomyocytes and follicular cells. | (36) |
| ABCA7 | Spans a region of ~32 kb and comprises 46 exons. | Predisposes to Alzheimer's disease. | (37) |
| ABAC9 | Consists of 39 exons, genomic region of ~85 kb chromosome 17q24.2. | Involved in monocyte differentiation and macrophage lipid homeostasis. | (38) |
| ABCA10 | The coding sequence of ABCA10 is 4.6 kb in size and codes for a 1,543-amino acid protein. | Involved in macrophage lipid homeostasis. | |
| ABCA12 | Located on the long (q) arm of chromosome 2 between positions 34 and 35. | Active in some types of skin cells, linked to harlequin-type ichthyosis. | |
| ABCA13 | Coding region is 6.7 kb in size and encodes a protein consisting of 2,143 amino acids with a predicted molecular weight of 240 kDa. | Linked to psychiatric disorders such as schizophrenia, bipolar disorder and depression. | (39) |
ABC, ATP-binding cassette; TMD, transmembrane domain; HDL, high-density lipoprotein; ECD, extracellular domain; NBD, nucleotide-binding domain.
Considering these findings, further investigation into ABCA1 as a tumor suppressor in prostate cancer is required. We herein review the role of ABCA1 in prostate cancer and the ongoing research on its association with cancer proliferation, invasion and migration, apoptosis and multidrug resistance. The aim of the present review was to demonstrate that ABCA1 represents a promising new target in the treatment of prostate cancer.
2. Effect of ABCA1 on the progression of prostate cancer
The ABCA1 protein mediates cellular cholesterol transfer to apoA-I through the plasma membrane. ABCA1 has been found to play a crucial role in the etiology of various neurological and cardiovascular diseases, including inflammation and metabolic syndrome (21), and may also be involved in the initiation and development of prostate cancer. A causal association between ABCA1-mediated tumor inhibition and mitochondrial cholesterol depletion has been identified through testing the dependence of ABCA1 anticancer activity on its efflux capacity (22). In addition, transforming growth factor (TGF)-β signaling plays a key role in prostate cancer occurrence and maintenance of cancer stem cell characteristics. The growth inhibitory effect of TGF-β may be due to overexpression of ABCA1 in prostate cancer cells (23). The expression of ABCA1 is associated with nuclear receptor of liver X receptor (LXR) and LXR forms a heterodimer with retinoid X receptor and regulates transcription of ABCA1 by binding to the DR-4 promoter element (24). Furthermore, TGF-β may increase ABCA1 expression through activation of LXR signaling. It has been demonstrated that LXR activation, with accompanying upregulation of ABCA1 expression, may decrease cholesterol levels and reduce the growth of prostate cancer cell xenograft tumors in mice (25). Furthermore, LXR agonists have been shown to induce expression of ABCA1, inhibit tumor growth and reduce progression to androgen independence in a xenograft model of prostate cancer (26). The antitumor effects of these compounds may involve a variety of mechanisms: Statins inhibit GTPases such as Ras and Rho family proteins via blocking protein prenylation and/or farnesylation, and LXR agonists induce cell cycle arrest through upregulation of p27. Conversely, intracellular cholesterol promotes prostate cancer progression as a substrate for de novo androgen synthesis and through regulation of Akt signaling (22).
Hypermethylation of the ABCA1 promoter has been shown to silence ABCA1 expression and is associated with high-grade prostate cancer (27). The interaction of apoA-I with ABCA1 activates the signaling molecule Janus kinase 2 (JAK2), which optimizes the cholesterol efflux activity of ABCA1 by phosphorylation (28). ABCA1-mediated JAK2 activation also activates signal transducer and activator of transcription 3 (STAT3), which significantly attenuates the expression of proinflammatory cytokines in macrophages. The anti-inflammatory effects of the apoA-I/ABCA1/STAT3 pathway, however, is dependent on the activity of suppressor of cytokine signaling 3. Taken together, these findings suggest that the interaction of apoA-I/ABCA1 activates cholesterol efflux and STAT3 branching pathways to synergistically inhibit inflammation in macrophages (29). It was also demonstrated that the apoA-I/ABCA1 interaction with the JAK2/STAT3 pathway to inhibit macrophage inflammatory responses is independent of ABCA1 lipid transport function. ABCA1 mutation carriers also exhibit an increased incidence of systemic and plaque inflammation (30). It has been observed that aberrant regulation of tristetraprolin and human antigen R plays an important role in the progression of prostate cancer and other inflammation-related cancers, as well as in cancer cell proliferation, apoptosis, angiogenesis, invasion and chemoresistance (31).
Epidemiological data have associated a reduced risk of prostate cancer with low serum cholesterol, as well as with the use of statins, demonstrating the role of cholesterol metabolism in the development of aggressive prostate cancer (32). The efflux of intracellular cholesterol is mediated by reverse cholesterol transporters, such as scavenger receptor class B type I, a membrane receptor with extensive tissue distribution and a number of physiological functions (33). A reduction in Akt-dependent survival signaling may also contribute to the anticancer activity of ABCA1. In particular, ~70% of advanced PCa exhibits loss of phosphatase and tensin homolog (PTEN) or consequent activation of the phosphoinositide 3-kinase (PI3K)/Akt pathway, which leads to enhanced cell survival, migration and castration-resistant growth (40).
Intracellular cholesterol promotes prostate cancer progression, as it is a substrate for de novo androgen synthesis, and by regulation of Akt signaling. Akt increased the expression of ABCA1 by delaying its degradation, but does not alter the mRNA levels (41). The protective effect of the ABCA1 protein is mediated by Akt2, possibly by stabilizing ABCA1 resistance to calpain-mediated proteolysis. ABCA1 reduces LDL receptor expression, decreases intracellular cholesterol and relies on LXRα. Of note, the epidermal growth factor receptor (EGFR) signal opposes the effects of LXRα on cholesterol homeostasis, whereas EGFR inhibitors interact with LXRα agonists to destroy cancer cells. Inhibition of activation of LXRα by sterol metabolites may be an effective strategy for targeting EGFR-KRAS signaling against cancer (42).
The promoter region of the ABCA1 gene has been shown to be epigenetically silenced by hypermethylation in the LNCaP human prostate cancer cell line. This suggests that loss of ABCA1 expression may lead to a significant alteration in cholesterol homeostasis and may indicate a fundamental association between high circulating cholesterol levels and prostate cancer growth (43). Epidemiological studies have identified a correlation between high serum cholesterol levels and prostate cancer, as well as a protective effect of statin use (44).
In conclusion, hypermethylation and gene inactivation of ABCA1 promoters leads to accumulation of cholesterol in prostate cancer cells. Thus, this cellular cholesterol efflux pathway may be an important determinant of prostate cancer aggressiveness, as well as a potential therapeutic target.
3. Association of ABCA1 with apoptosis and autophagy in prostate cancer
Overexpression of ABCA1 leads to decreased cellular cholesterol and tumor growth inhibition in prostate cancer cells. ABCA1 antitumor activity requires an efflux function and appears to be mediated by reduced mitochondrial cholesterol combined with an increased potential for release of cell death-promoting molecules, such as cytochrome C, from mitochondria. The loss-of-function phenotype for somatic mutations identified in human colon cancer suggests the role of ABCA1 as a tumor suppressor, albeit in the context of increased cholesterol synthesis sufficient to raise the levels of mitochondrial cholesterol. This antitumor effect may be due to apoptosis, as overexpression of ABCA1 has been shown to increase mitochondrial release of cytochrome C in vitro and in vivo (45).
Furthermore, downregulation of ABCA1 is present in most prostate cancer specimens, and ABCA1 expression levels are inversely associated with Gleason score. DNA hypermethylation on the ABCA1 promoter in LNCaP cells effectively inhibits basal expression and prevents the complete induction of trans-activators. The loss of ABCA1 protein expression directly results in high intracellular cholesterol levels, thereby contributing to an environment conducive to tumor progression. In addition, ABCA1 hypermethylation was exclusively detected in intermediate- and high-grade prostate cancer. Thus, epigenetic inactivation of ABCA1 may be intimately associated with prostate cancer progression, suggesting that dysregulation of cellular cholesterol levels in prostate epithelial cells creates an environment conducive to tumor progression.
Autophagy is an intracellular protein-degradation process that is conserved across eukaryotes, including yeast and humans, which responds to cellular stress conditions to maintain a healthy cellular status by degrading and recycling cytoplasmic contents via the lysosomal route (46). Under nutrient starvation conditions, intracellular proteins are transported to lysosomes and vacuoles via membranous structures known as autophagosomes, and are degraded. It has been demonstrated that autophagy participates in the regulation of lipid metabolism and cholesterol homeostasis, with a special emphasis on macrophage-derived foam cells. Autophagy has emerged as an alternative lipid metabolic pathway through the lysosomal degradative pathway, making this process a potential therapeutic target for diseases associated with disorders of lipid metabolism (47). Of note, all these lipoproteins increase autophagic flux in macrophages, and autophagy depletion in these cells results in diminished cholesterol efflux to apoA-I. The autophagy inhibitor 3-methyladenine and an ATG5 siRNA were found to significantly attenuate autophagy, subsequently suppressing the ABCA1-mediated cholesterol efflux (48). Biochemical studies have demonstrated that the accumulation of cholesterol esters in prostate cancer cells is the result of loss of the tumor suppressor PTEN and activation of the PI3K/Akt pathway. AMP-activated protein kinase (AMPK)-dependent ABCA1 expression also involves the mammalian target of rapamycin (mTOR) pathway and inhibits the extracellular-signal regulated kinase (ERK). AMPK-induced ABCA1 expression leads to cholesterol efflux from human macrophages (49). Through the ERK1/2 pathway, intracellular ABCA1 exerts a protective effect against oxidative stress caused by ischemia. The PI3K/Akt/mTOR signaling pathway is a classic autophagy pathway. PI3K and Akt suppression may inhibit mTOR phosphorylation at the Ser2448 site, thereby enhancing the expression of autophagy-related proteins and inducing autophagy (50).
It has been demonstrated that elevated mitochondrial cholesterol promotes resistance to cell death in prostate cancer and may lead to malignant cell transformation (51). Inhibitory RNA (RNAi) knockdown of squalene synthase, the enzyme which catalyzes the first committed step of cholesterol synthesis, leads to decreased cell proliferation and survival of prostate cancer cells in vitro (52). ABCA1 promotes a reduction in mitochondrial cholesterol levels, mitochondrial permeability transition-associated cytochrome C release, and non-apoptotic/necrotic cell death in response to cell stress. Ultimately, ABCA1-mediated cell death depends on cholesterol for modulation of mitochondrial function, while ABCA1 deficiency allows for elevated mitochondrial cholesterol and ultimately promotes cancer cell survival.
4. ABCA1 and multidrug resistance (MDR)
MDR is a major cause of failure of prostate cancer chemotherapy and is associated with an increased mortality risk (53). The PI3K/Akt signaling cascade may be correlated with MDR1/P-glycoprotein (P-gp) and plays a critical role in the MDR phenotype. MDR-associated protein (MRP) 1 is expressed in a variety of human malignancies (54). ABCA1 may export anticancer drugs and decrease intracellular drug concentration, which is associated with limited success of anticancer chemotherapy, attributed to the the cross-resistance of tumor cells and referred to as MDR. The role of ABCA1 in MDR has been well-characterized, and measuring the expression of ABCA1 may be used for predicting the response to anticancer drugs.
Cell viability in prostate cancer may be regulated by modifying critical pathways mediating novel androgen signaling by AR-targeting microRNAs. In this study, androgens stimulating miR-19a, and miR-19a directly repressing ABCA1 mRNA expression, may represent a possible mechanism underlying androgen-mediated repression of ABCA1, promoting PCa cell proliferation (55). The activation of ABCA1 was also shown to inhibit the proliferation of androgen-dependent prostate cancer cells, and androgen treatment in LNCaP cells significantly inhibited the expression of ABCA1 mRNA (56). In vitro studies demonstrated that LXR agonists activate transcription of the MRP2 gene to promote excretion of endogenous and xenobiotic compounds from hepatocytes into the bile (57).
ABCA1 also transfers phospholipids, preferentially phosphatidylcholine and cholesterol, to lipid-free apoA-I to produce pre-β-HDL, which also binds directly to MDR1 and regulates substrate recognition via MDR1 (58). Eukaryotic ABC proteins may retain similar substrate binding pockets and move substrates in an ATP-dependent manner. The prototype of eukaryote ABC proteins may be those involved in membrane lipid transport.
If overexpression of ABCA1 promotes MDR in tumors, ABCA1 gene mutations may lead to drug resistance and alter substrate specificity. Thus, the study of ABCA1 gene mutations and their role in MDR is crucial for individualized patient treatment.
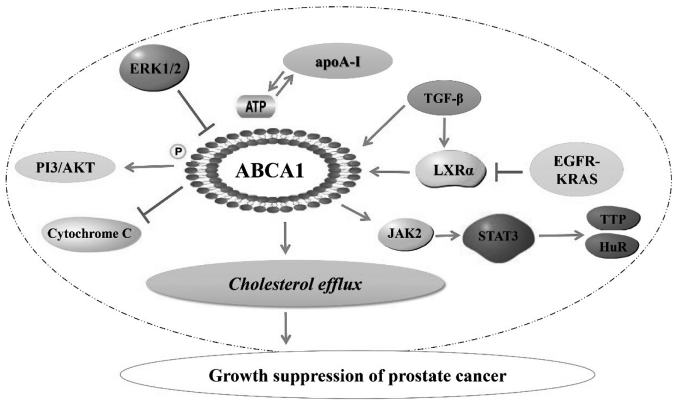
In conclusion, ABCA1 plays an important role in the prevention and treatment of prostate cancer through regulation of cellular cholesterol efflux (Fig. 1). The association of lower ABCA1 expression with shorter survival in prostate cancer prompts ongoing research to further elucidate the mechanism underlying ABCA1 regulation of tumor growth. Various cross-regulatory mechanisms may be the focus of future research. Studies on ABCA1 will contribute to the understanding of drug transport and the role of cholesterol in cancer growth, and may lead to optimization of treatment for prostate and other cancers. Additionally, the study of ABCA1 gene mutations and associated differences in expression and functionality may lay the foundation for future pharmacogenetics in prostate cancer treatment. Ultimately, studies in ABCA1 may provide a novel therapeutic target in cancer.
Figure 1.
Association of ABCA1 with prostate cancer. ABCA1, ATP-binding cassette transporter A1; PI3, phosphoinositide 3 kinase; ERK, extracellular signal-regulated kinase; apoA-I, apolipoprotein A-I; TGF, transforming growth factor; LXR, liver X receptor; EGFR, epidermal growth factor receptor; JAK2, Janus kinase 2; STAT3, signal transducer and activator of transcription 3; TTP, tristetraprolin; HuR, human antigen R.
Acknowledgements
The present study was funded by the National Natural Science Foundation of China (grant no. 81541163); the Natural Science Foundation of Hunan Province (grant no. 2015JJ3101); the Open Fund Based on Innovation Platform of Hunan Provincial Education Department (grant no. 15K111); the Hunan Provincial Cooperative Innovation Center for Molecular Target New Drug Study (grant no. 2014-405); and the Innovation Program of Graduate Education in Cooperative Innovation Center for Molecular Target New Drug of University of South China (grant no. 0223-0002-00028).
Glossary
Abbreviations
- ABC
ATP-binding cassette
- LDL
low-density lipoprotein
- apoA-I
apolipoprotein A-I
- TGF-β
transforming growth factor-β
- JAK2
Janus kinase 2
- STAT3
signal transducer and activator of transcription 3
- HDL
high-density lipoprotein
- LXR
liver X receptor
- MDR
multidrug resistance
- PI3K
phosphoinositide 3-kinase
- ERK
extracellular signal-regulated kinase
- P-gp
P-glycoprotein
- MRP
multidrug resistance-associated protein
- EGFR
epidermal growth factor receptor
References
- 1.Zong Y, Goldstein AS, Huang J. The molecular basis for ethnic variation and histological subtype differences in prostate cancer. Sci China Life Sci. 2013;56:780–787. doi: 10.1007/s11427-013-4522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adlakha YK, Khanna S, Singh R, Singh VP, Agrawal A, Saini N. Pro-apoptotic miRNA-128-2 modulates ABCA1, ABCG1 and RXRα expression and cholesterol homeostasis. Cell Death Dis. 2013;4:e780. doi: 10.1038/cddis.2013.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B, Cheng L, Masterson TA, Liu X, Ratliff TL, Cheng JX. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014;19:393–406. doi: 10.1016/j.cmet.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stopsack KH, Gerke TA, Andrén O, Andersson SO, Giovannucci EL, Mucci LA, Rider JR. Cholesterol uptake and regulation in high-grade and lethal prostate cancers. Carcinogenesis. 2017;38:806–811. doi: 10.1093/carcin/bgx058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz PM, Mo H, McConathy WJ, Sabnis N, Lacko AG. The role of cholesterol metabolism and cholesterol transport in carcinogenesis: A review of scientific findings, relevant to future cancer therapeutics. Front Pharmacol. 2013;4:119. doi: 10.3389/fphar.2013.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flores K, Manautou JE, Renfro JL. Gender-specific expression of ATP-binding cassette (Abc) transporters and cytoprotective genes in mouse choroid plexus. Toxicology. 2017;386:84–92. doi: 10.1016/j.tox.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durmus S, Hendrikx JJ, Schinkel AH. Apical ABC transporters and cancer chemotherapeutic drug disposition. Adv Cancer Res. 2015;125:1–41. doi: 10.1016/bs.acr.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Kaminski WE, Piehler A, Wenzel JJ. ABC A-subfamily transporters: Structure, function and disease. Biochim Biophys Acta. 2006;1762:510–524. doi: 10.1016/j.bbadis.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Qian H, Zhao X, Cao P, Lei J, Yan N, Gong X. Structure of the human lipid exporter ABCA1. Cell. 2017;169:1228–1239.e10. doi: 10.1016/j.cell.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald ML, Mendez AJ, Moore KJ, Andersson LP, Panjeton HA, Freeman MW. ATP-binding cassette transporter A1 contains an NH2-terminal signal anchor sequence that translocates the protein's first hydrophilic domain to the exoplasmic space. J Biol Chem. 2001;276:15137–15145. doi: 10.1074/jbc.M100474200. [DOI] [PubMed] [Google Scholar]
- 11.Lamping E, Baret PV, Holmes AR, Monk BC, Goffeau A, Cannon RD. Fungal PDR transporters: Phylogeny, topology, motifs and function. Fungal Genet Biol. 2010;47:127–142. doi: 10.1016/j.fgb.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lund-Katz S, Phillips MC. High density lipoprotein structure-function and role in reverse cholesterol transport. Subcell Biochem. 2010;51:183–227. doi: 10.1007/978-90-481-8622-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sene A, Khan AA, Cox D, Nakamura RE, Santeford A, Kim BM, Sidhu R, Onken MD, Harbour JW, Hagbi-Levi S, et al. Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell Metab. 2013;17:549–561. doi: 10.1016/j.cmet.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S, Smith JD. ABCA1 and nascent HDL biogenesis. Biofactors. 2014;40:547–554. doi: 10.1002/biof.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murano T, Yamaguchi T, Tatsuno I, Suzuki M, Noike H, Takanami T, Yoshida T, Suzuki M, Hashimoto R, Maeno T, et al. Subfraction analysis of circulating lipoproteins in a patient with Tangier disease due to a novel ABCA1 mutation. Clin Chim Acta. 2016;452:167–172. doi: 10.1016/j.cca.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Bi DP, Yin CH, Zhang XY, Yang NN, Xu JY. MiR-183 functions as an oncogene by targeting ABCA1 in colon cancer. Oncol Rep. 2016;35:2873–2879. doi: 10.3892/or.2016.4631. [DOI] [PubMed] [Google Scholar]
- 17.Sharma M, Tuaine J, McLaren B, Waters DL, Black K, Jones LM, McCormick SP. Chemotherapy agents alter plasma lipids in breast cancer patients and show differential effects on lipid metabolism genes in liver cells. PLoS One. 2016;11:e0148049. doi: 10.1371/journal.pone.0148049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneko T, Kanno C, Ichikawa-Tomikawa N, Kashiwagi K, Yaginuma N, Ohkoshi C, Tanaka M, Sugino T, Imura T, Hasegawa H, Chiba H. Liver X receptor reduces proliferation of human oral cancer cells by promoting cholesterol efflux via up-regulation of ABCA1 expression. Oncotarget. 2015;6:33345–33357. doi: 10.18632/oncotarget.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sviridov DO, Drake SK, Freeman LA, Remaley AT. Amphipathic polyproline peptides stimulate cholesterol efflux by the ABCA1 transporter. Biochem Biophys Res Commun. 2016;471:560–565. doi: 10.1016/j.bbrc.2016.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang M, Li L, Xie W, Wu JF, Yao F, Tan YL, Xia XD, Liu XY, Liu D, Lan G, et al. Apolipoprotein A-1 binding protein promotes macrophage cholesterol efflux by facilitating apolipoprotein A-1 binding to ABCA1 and preventing ABCA1 degradation. Atherosclerosis. 2016;248:149–159. doi: 10.1016/j.atherosclerosis.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Sultana A, Cochran BJ, Tabet F, Patel M, Torres LC, Barter PJ, Rye KA. Inhibition of inflammatory signaling pathways in 3T3-L1 adipocytes by apolipoprotein A-I. FASEB J. 2016;30:2324–2335. doi: 10.1096/fj.201500026R. [DOI] [PubMed] [Google Scholar]
- 22.Smith B, Land H. Anticancer activity of the cholesterol exporter ABCA1 gene. Cell Rep. 2012;2:580–590. doi: 10.1016/j.celrep.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Her NG, Jeong SI, Cho K, Ha TK, Han J, Ko KP, Park SK, Lee JH, Lee MG, Ryu BK, Chi SG. PPARδ promotes oncogenic redirection of TGF-β1 signaling through the activation of the ABCA1-Cav1 pathway. Cell Cycle. 2013;12:1521–1535. doi: 10.4161/cc.24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tazoe F, Yagyu H, Okazaki H, Igarashi M, Eto K, Nagashima S, Inaba T, Shimano H, Osuga J, Ishibashi S. Induction of ABCA1 by overexpression of hormone-sensitive lipase in macrophages. Biochem Biophys Res Commun. 2008;376:111–115. doi: 10.1016/j.bbrc.2008.08.101. [DOI] [PubMed] [Google Scholar]
- 25.Dufour J, Viennois E, De Boussac H, Baron S, Lobaccaro JM. Oxysterol receptors, AKT and prostate cancer. Curr Opin Pharmacol. 2012;12:724–728. doi: 10.1016/j.coph.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Chuu CP, Hiipakka RA, Kokontis JM, Fukuchi J, Chen RY, Liao S. Inhibition of tumor growth and progression of LNCaP prostate cancer cells in athymic mice by androgen and liver X receptor agonist. Cancer Res. 2006;66:6482–6486. doi: 10.1158/0008-5472.CAN-06-0632. [DOI] [PubMed] [Google Scholar]
- 27.Lee BH, Taylor MG, Robinet P, Smith JD, Schweitzer J, Sehayek E, Falzarano SM, Magi-Galluzzi C, Klein EA, Ting AH. Dysregulation of cholesterol homeostasis in human prostate cancer through loss of ABCA1. Cancer Res. 2013;73:1211–1218. doi: 10.1158/0008-5472.CAN-12-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao GJ, Yin K, Fu YC, Tang CK. The interaction of ApoA-I and ABCA1 triggers signal transduction pathways to mediate efflux of cellular lipids. Mol Med. 2012;18:149–158. doi: 10.2119/molmed.2011.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang C, Houston BA, Storey C, LeBoeuf RC. Both STAT3 activation and cholesterol efflux contribute to the anti-inflammatory effect of apoA-I/ABCA1 interaction in macrophages. J Lipid Res. 2016;57:848–857. doi: 10.1194/jlr.M065797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bochem AE, van der Valk FM, Tolani S, Stroes ES, Westerterp M, Tall AR. Increased systemic and plaque inflammation in ABCA1 mutation carriers with attenuation by statins. Arterioscler Thromb Vasc Biol. 2015;35:1663–1669. doi: 10.1161/ATVBAHA.114.304959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramírez CM, Lin CS, Abdelmohsen K, Goedeke L, Yoon JH, Madrigal-Matute J, Martin-Ventura JL, Vo DT, Uren PJ, Penalva LO, et al. RNA binding protein HuR regulates the expression of ABCA1. J Lipid Res. 2014;55:1066–1076. doi: 10.1194/jlr.M044925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohno Y, Ohori M, Nakashima J, Okubo H, Satake N, Hashimoto T, Tachibana M. Association between preoperative serum total cholesterol level and biochemical recurrence in prostate cancer patients who underwent radical prostatectomy. Mol Clin Oncol. 2016;4:1073–1077. doi: 10.3892/mco.2016.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cardenas E, Ghosh R. Vitamin E: A dark horse at the crossroad of cancer management. Biochem Pharmacol. 2013;86:845–852. doi: 10.1016/j.bcp.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaminski WE, Piehler A, Püllmann K, Porsch-Ozcürümez M, Duong C, Bared GM, Büchler C, Schmitz G. Complete coding sequence, promoter region, and genomic structure of the human ABCA2 gene and evidence for sterol-dependent regulation in macrophages. Biochem Biophys Res Commun. 2001;281:249–258. doi: 10.1006/bbrc.2001.4305. [DOI] [PubMed] [Google Scholar]
- 35.Paolini A, Baldassarre A, Del Gaudio I, Masotti A. Structural features of the ATP-Binding Cassette (ABC) transporter ABCA3. Int J Mol Sci. 2015;16:19631–19644. doi: 10.3390/ijms160819631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kubo Y, Sekiya S, Ohigashi M, Takenaka C, Tamura K, Nada S, Nishi T, Yamamoto A, Yamaguchi A. ABCA5 resides in lysosomes, and ABCA5 knockout mice develop lysosomal disease-like symptoms. Mol Cell Biol. 2005;25:4138–4149. doi: 10.1128/MCB.25.10.4138-4149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broccardo C, Osorio J, Luciani MF, Schriml LM, Prades C, Shulenin S, Arnould I, Naudin L, Lafargue C, Rosier M, et al. Comparative analysis of the promoter structure and genomic organization of the human and mouse ABCA7 gene encoding a novel ABCA transporter. Cytogenet Cell Genet. 2001;92:264–270. doi: 10.1159/000056914. [DOI] [PubMed] [Google Scholar]
- 38.Piehler A, Kaminski WE, Wenzel JJ, Langmann T, Schmitz G. Molecular structure of a novel cholesterol-responsive A subclass ABC transporter, ABCA9. Biochem Biophys Res Commun. 2002;295:408–416. doi: 10.1016/S0006-291X(02)00659-9. [DOI] [PubMed] [Google Scholar]
- 39.Maeß MB, Stolle K, Cullen P, Lorkowski S. Evidence for an alternative genomic structure, mRNA and protein sequence of human ABCA13. Gene. 2013;515:298–307. doi: 10.1016/j.gene.2012.11.072. [DOI] [PubMed] [Google Scholar]
- 40.Marques RB, Aghai A, de Ridder CMA, Stuurman D, Hoeben S, Boer A, Ellston RP, Barry ST, Davies BR, Trapman J, van Weerden WM. High efficacy of combination therapy Using PI3K/AKT inhibitors with androgen deprivation in prostate cancer preclinical models. Eur Urol. 2015;67:1177–1185. doi: 10.1016/j.eururo.2014.08.053. [DOI] [PubMed] [Google Scholar]
- 41.Okoro EU, Guo Z, Yang H. Akt isoform-dependent regulation of ATP-Binding cassette A1 expression by apolipoprotein E. Biochem Biophys Res Commun. 2016;477:123–128. doi: 10.1016/j.bbrc.2016.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gabitova L, Restifo D, Gorin A, Manocha K, Handorf E, Yang DH, Cai KQ, Klein-Szanto AJ, Cunningham D, Kratz LE, et al. Endogenous sterol metabolites regulate growth of EGFR/KRAS-dependent tumors via LXR. Cell Rep. 2015;12:1927–1938. doi: 10.1016/j.celrep.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solomon KR, Allott EH, Freeman MR, Freedland SJ. Words of wisdom. Re: Dysregulation of cholesterol homeostasis in human prostate cancer through loss of ABCA1. Eur Urol. 2013;63:1128–1129. doi: 10.1016/j.eururo.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayashi N, Matsushima M, Yamamoto T, Sasaki H, Takahashi H, Egawa S. The impact of hypertriglyceridemia on prostate cancer development in patients aged ≥60 years. BJU Int. 2012;109:515–519. doi: 10.1111/j.1464-410X.2011.10358.x. [DOI] [PubMed] [Google Scholar]
- 45.Chou JL, Huang RL, Shay J, Chen LY, Lin SJ, Yan PS, Chao WT, Lai YH, Lai YL, Chao TK, et al. Hypermethylation of the TGF-β target, ABCA1 is associated with poor prognosis in ovarian cancer patients. Clin Epigenetics. 2015;7:1. doi: 10.1186/s13148-014-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puri P, Chandra A. Autophagy modulation as a potential therapeutic target for liver diseases. J Clin Exp Hepatol. 2014;4:51–59. doi: 10.1016/j.jceh.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han XB, Li HX, Jiang YQ, Wang H, Li XS, Kou JY, Zheng YH, Liu ZN, Li H, Li J, et al. Upconversion nanoparticle-mediated photodynamic therapy induces autophagy and cholesterol efflux of macrophage-derived foam cells via ROS generation. Cell Death Dis. 2017;8:e2864. doi: 10.1038/cddis.2017.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin XL, Liu MH, Hu HJ, Feng HR, Fan XJ, Zou WW, Pan YQ, Hu XM, Wang Z. Curcumin enhanced cholesterol efflux by upregulating ABCA1 expression through AMPK-SIRT1-LXRα signaling in THP-1 macrophage-derived foam cells. DNA Cell Biol. 2015;34:561–572. doi: 10.1089/dna.2015.2866. [DOI] [PubMed] [Google Scholar]
- 50.Jiang Y, Kou J, Han X, Li X, Zhong Z, Liu Z, Zheng Y, Tian Y, Yang L. ROS-dependent activation of autophagy through the PI3K/Akt/mTOR pathway is induced by hydroxysafflor yellow A-sonodynamic therapy in THP-1 macrophages. Oxid Med Cell Longev. 2017;2017:8519169. doi: 10.1155/2017/8519169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu X, Daniels G, Lee P, Monaco ME. Lipid metabolism in prostate cancer. Am J Clin Exp Urol. 2014;2:111–120. [PMC free article] [PubMed] [Google Scholar]
- 52.Brusselmans K, Timmermans L, Van de Sande T, Van Veldhoven PP, Guan G, Shechter I, Claessens F, Verhoeven G, Swinnen JV. Squalene synthase, a determinant of Raft-associated cholesterol and modulator of cancer cell proliferation. J Biol Chem. 2007;282:18777–18785. doi: 10.1074/jbc.M611763200. [DOI] [PubMed] [Google Scholar]
- 53.Wang H, Jia XH, Chen JR, Yi YJ, Wang JY, Li YJ, Xie SY. HOXB4 knockdown reverses multidrug resistance of human myelogenous leukemia K562/ADM cells by downregulating P-gp, MRP1 and BCRP expression via PI3K/Akt signaling pathway. Int J Oncol. 2016;49:2529–2537. doi: 10.3892/ijo.2016.3738. [DOI] [PubMed] [Google Scholar]
- 54.Chen JR, Jia XH, Wang H, Yi YJ, Wang JY, Li YJ. Timosaponin A-III reverses multi-drug resistance in human chronic myelogenous leukemia K562/ADM cells via downregulation of MDR1 and MRP1 expression by inhibiting PI3K/Akt signaling pathway. Int J Oncol. 2016;48:2063–2070. doi: 10.3892/ijo.2016.3423. [DOI] [PubMed] [Google Scholar]
- 55.Mo W, Zhang J, Li X, Meng D, Gao Y, Yang S, Wan X, Zhou C, Guo F, Huang Y, et al. Identification of novel AR-targeted microRNAs mediating androgen signalling through critical pathways to regulate cell viability in prostate cancer. PLoS One. 2013;8:e56592. doi: 10.1371/journal.pone.0056592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sekine Y, Demosky SJ, Stonik JA, Furuya Y, Koike H, Suzuki K, Remaley AT. High-density lipoprotein induces proliferation and migration of human prostate androgen-independent cancer cells by an ABCA1-dependent mechanism. Mol Cancer Res. 2010;8:1284–1294. doi: 10.1158/1541-7786.MCR-10-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chisaki I, Kobayashi M, Itagaki S, Hirano T, Iseki K. Liver X receptor regulates expression of MRP2 but not that of MDR1 and BCRP in the liver. Biochim Biophys Acta. 2009;1788:2396–2403. doi: 10.1016/j.bbamem.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 58.Kimura Y, Morita SY, Matsuo M, Ueda K. Mechanism of multidrug recognition by MDR1/ABCB1. Cancer Sci. 2007;98:1303–1310. doi: 10.1111/j.1349-7006.2007.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]