Abstract
Introduction
Determination of exercise oscillatory ventilation (EOV) is subjective and the inter-reviewer agreement has not been reported. The purpose of this study was, among patients with heart failure (HF): (1) determine the inter-reviewer agreement for EOV; and (2) describe a novel, objective, and quantifiable measure of EOV.
Methods
This was a secondary analysis of the HEART Camp: Promoting Adherence to Exercise in Patients with Heart Failure study. EOV was determined through a blinded review by 6 individuals based on their interpretation of the EOV literature. Inter-reviewer agreement was assessed with Fleiss kappa (κ). Final determination of EOV was based on agreement by 4 of the 6 reviewers. A new measure (ventilation dispersion index; VDI) was calculated for each test and its ability to predict EOV was assessed with the receiver operator characteristics curve (ROC).
Results
Among 243 patients with HF (age=60±12 years; 45% women) the inter-reviewer agreement for EOV was fair (κ=0.303) with 10-s discrete data averages and significantly better, but only moderate (κ= 0.429) with 30-s rolling data averages. Prevalence of positive and indeterminate EOVs were 18% and 30% with the 10-s discrete averages and 14% and 13% with the 30-s rolling averages, respectively. VDI was strongly associated with EOV with area under the ROC= 0.852 to 0.890.
Conclusions
Inter-reviewer agreement for EOV in patients with HF is fair to moderate which can negatively affect risk stratification. VDI has strong predictive validity with EOV; as such it might be a useful measure of prognosis in patients with HF.
Keywords: cardiopulmonary exercise testing, gas exchange, heart failure, methods
INTRODUCTION
A cardiopulmonary exercise test (CPET) is performed in patients with heart failure (HF) to quantify exercise capacity, stratify risk, and estimate prognosis (1–4). Since the seminal study from Mancini and colleagues (5) in which they showed the prognostic significance of low peak oxygen uptake (VO2) in patients with compensated HF awaiting heart transplant, several other measures from the CPET have been shown to be related to prognosis in these patients (1, 2, 4, 6). Among them, exercise oscillatory ventilation (EOV) is relatively new with increasing scientific interest over the past two decades (7). EOV is an oscillatory pattern of ventilation during exercise that has been associated with worse prognosis in patients with HF (1, 4, 6–13). The mechanisms resulting in EOV are not clear, but may be related to circulatory delay, increased chemosensitivity, pulmonary congestion, or ergoreflex signaling (14).
The reported prevalence of EOV in patients with compensated HF has varied from 7% to 51% (7). This is likely due to heterogeneity (e.g., disease severity, functional capacity) between study cohorts (7, 12) as well as the subjectivity and varying methods (e.g., criteria, data averaging) used to identify EOV (9, 12, 15). Several criteria sets that are based on the amplitude of oscillations and the prevalence during exercise have been proposed to identify EOV (4, 10, 11, 13, 16, 17). As described by Cornelis and colleagues (18), the basis for these criteria are unclear and the subsequent interpretation by scientists is often ambiguous. Even though EOV has been associated with prognosis in several studies of patients with HF (7), the absence of a gold standard method for identifying EOV and the lack of data on the reliability of determining EOV may have hampered widespread clinical adoption (12, 15). The purpose of this study was, among patients with compensated HF: (1) determine the inter-reviewer agreement for the determination of EOV; and (2) describe a novel, objective, and quantifiable measure of EOV. We hypothesized that this quantifiable measure would be positively related to the presence of EOV.
METHODS
Study Overview
This was a secondary analysis of data from the HEART Camp: Promoting Adherence to Exercise in Patients with Heart Failure study. HEART Camp was a randomized, controlled trial in which the effect of a cognitive-behavioral intervention on long-term adherence to exercise training among patients with HF was evaluated. Details of the study design have been published (19). An overview relevant to the present analysis is provided here. Patients ≥19 years-old, with a physician diagnosis of compensated Stage C HF were enrolled from Bryan Health (Lincoln, NE) and Henry Ford Hospital (Detroit, MI) between December 2012 and June 2015. Eligible patients included those with either reduced or preserved ejection fraction and prescribed guideline-based pharmacological therapy for at least 30 days prior to enrollment (19). Exclusion criteria included unstable angina, myocardial infarction, coronary artery bypass graft surgery, or pacemaker implant within 6 weeks of enrollment; regular participation in aerobic exercise 3 days per week within 8 weeks of enrollment; and physical limitations or contraindications to aerobic and resistance exercise training (19). The HEART Camp study was approved by the Institutional Review Boards at the University of Nebraska (approval #608-11-FB) and Henry Ford Health System (approval #7424). Informed consent was obtained from all patients prior to study enrollment.
All patients completed a sign or symptom-limited maximal CPET prior to randomization. Tests were performed based on guidelines published by the American Heart Association (20). Patients were instructed to take their medications as prescribed. Treadmill was the preferred testing mode using a low-level protocol that starts at ~2 metabolic equivalents of task (METs) with increases of ~1 MET every 2 minutes; however, alternate modes or protocols could be considered as indicated for select patients. Respiratory gases were analyzed using a metabolic cart from MGC Diagnostics (Ultima; Minneapolis, MN) at both institutions. The gas and flow analyzers were calibrated immediately prior to each test based on the manufacture’s recommendations. Quality assurance testing (21) was performed by exercise testing laboratories at both institutions at the start of the study and every 6 months until exercise testing for the study was completed.
Quality assurance and subject CPET data were analyzed by a centralized core laboratory at Henry Ford Hospital which was staffed by individuals not otherwise involved with the HEART Camp study. Peak exercise gas exchange measures were identified as the highest value during the final minute of exercise or the first interval in recovery based on data averaged in discrete 20-s intervals. Microsoft Excel (Redmond, WA) was used to calculate the slope of the change in minute ventilation (VE) to the change in carbon dioxide produced (VCO2; ΔVE/ΔVCO2 slope) via least-squares linear regression which included all exercise data averaged in discrete 20-s intervals. Percent predicted maximal VO2 was calculated based on equations from Wasserman et al. (22)
Assessment of Exercise Oscillatory Ventilation
The presence of EOV was assessed through a blinded review by six of the authors (CB, JE, JM, PC, BV, and SK). Each of these individuals are experienced with CPET conduct and analysis, and were asked to base their determination of EOV on their interpretation of the EOV literature. The intent was to provide a real-world evaluation of EOV interpretation. Recommendations from Kremser et al. (16), Leite et al. (10), and Guazzi et al. (4) represented the basis of these interpretations. Reviewers were asked to identify whether a test was positive, negative, or indeterminate for EOV. The final determination of EOV (positive or negative) for a given test required agreement by four of the six reviewers, otherwise it was considered indeterminate.
Reviewers were provided tabular data averaged in discrete 30-s intervals and a graph of exercise time (x-axis) vs. minute VE (y-axis) for each test which was created using Microsoft Excel. To assist reviewers with their determination of EOV, graphs were printed in a large format (17.5 cm × 24.0 cm), constructed with vertical grid lines every 1 minute and horizontal grid lines every 5 L, a continuous line connected each of the data points, and the start and end of exercise were identified with oversized data markers. An example is shown in the supplement Figure 1 (see Figure, SDC 1; example exercise oscillatory ventilation graph). In order to assess the effect data averaging methods might have on the determination of EOV, reviewers knowingly analyzed each test twice: (1) data averaged using a rolling 30-s interval with 10-s increments (i.e., 0–30 s, 10–40 s, 20–50 s, etc.) and (2) data averaged in discrete (non-overlapping) 10-s intervals. The two data averaging formats were provided to reviewers separately. Reviewers completed the evaluation of graphs with rolling 30-s intervals before receiving graphs with 10-s intervals.
An Objective and Quantifiable Measure of Exercise Oscillatory Ventilation
Given the subjective nature by which EOV is currently determined, we developed an objective and quantifiable measure of EOV using the calculations shown below. These calculations were limited to the exercise data (excluding the initial 60 s of exercise) and were based on rolling 30-s interval data. The intent was to quantify the combination of amplitude and frequency of ventilatory oscillations during exercise. First, the mean VE representing 30 s before and after each data interval was calculated. Second, “VE dispersion” was calculated for each data interval as the absolute difference between the VE at a given interval and the mean VE around (±30 s) that interval. Third, the total VE dispersion area (shown graphically in Figure 1) was determined by calculating the area under the curve of exercise time versus the VE dispersion based on the trapezoidal rule using Equation 1. Finally, because the total VE dispersion area would be proportional to exercise test duration, we divided the total VE dispersion area by test duration in minutes and refer to this as the “ventilation dispersion index” (VDI).
Figure 1. Visual example of the ventilation dispersion area.
The black line with round markers represents the ventilation based on data averaged using 30-s rolling intervals. The red dashed line with square markers represents the mean ventilation for the 30 s before and after each of these intervals. The gray shaded areas marked with “D” represent the difference between the ventilation and the mean ventilation at each interval. The total dispersion area is the sum of D1 + D2 + ⋯ + D10.
| Equation 1 |
Where dVE is the absolute difference between the VE and the mean VE (±30 s) at a given interval; T is the exercise time in seconds; and i is the data interval starting at 70 s of exercise (i= 0) through the second to last exercise interval (i= N-1).
Statistical Analysis
Inter-reviewer agreement for the determination of EOV was assessed with Fleiss kappa (κ) (23). The ability of VDI to predict EOV was assessed with the area under the receiver operator characteristic curve (AUC). The VDI value corresponding to the optimal combination of sensitivity and specificity to predict EOV from the receiver operator characteristic curve data was identified via Youden’s index (24). Continuous data were compared using Student’s T-test or Mann-Whitney U test for normally and non-normally distributed data, respectively. Categorical data were compared using Chi-square. The two-sided alpha level was <.05. Unless noted otherwise, continuous data are reported as mean ± standard deviation. IBM SPSS version 24 (IBM, Somers, NY) with the advanced statistics module was used for all analyses.
RESULTS
Among 246 patients who performed a CPET for enrollment into the HEART Camp study, 243 had complete CPET data for the present analysis. Characteristics of this cohort are shown in Table 1. All but two of the tests were completed on a treadmill. These two alternate modes were a leg (cycle) ergometer and a semi-recumbent stepper. The protocol for each started at ~2 METs with increases of ~2 METs every 3 minutes. Among the patients tested on a treadmill, 92% performed the study-recommended low-level protocol (~1 MET increment every 2 minutes). The remaining patients were tested using another low-level protocol (2–3 MET increment every 3 minutes). Mean exercise duration for all patients was 9.2±3.5 min. In 83% of the patients, the primary reason tests were stopped was general fatigue (n= 121) or shortness of breath (n= 81).
Table 1.
Characteristics of the study cohort
| Characteristic | All Patients | Men | Women |
|---|---|---|---|
| n | 243 | 134 | 109 |
| Age (years) | 60±12 | 60±13 | 59±11 |
| Race, n (%) | |||
| White | 129 (53) | 73 (55) | 56 (51) |
| Black | 106 (44) | 55 (41) | 51 (47) |
| Other | 8 (3) | 6 (5) | 2 (2) |
| Body mass index (kg·m−2) | 35±9 | 33±8 | 36±9 |
| New York Heart Association Class, n (%) | |||
| I | 22 (9) | 13 (10) | 9 (8) |
| II | 130 (54) | 80 (62) | 50 (46) |
| III or IV | 90 (37) | 40 (30) | 50 (46) |
| Months since heart failure diagnosis, median (25th, 75th percentile) | 42 (13, 98) | 40 (15, 101) | 44 (12, 96) |
| Ischemic heart failure etiology, n (%) | 77 (32) | 50 (37) | 27 (25) |
| Ejection fraction (%) | 39±13 | 38±13 | 41±14 |
| BNP, median (25th, 75th percentile) | 102 (35, 255) | 115 (44, 294) | 77 (27, 186) |
| Bi-ventricular pacemaker, n (%) | 54 (22) | 31 (23) | 23 (21) |
| Implanted cardioverter-defibrillator, n (%) | 95 (39) | 51 (38) | 44 (41) |
| Medications, n (%) | |||
| Beta-adrenergic blockade | 236 (97) | 129 (96) | 107 (98) |
| ACE or ARB | 205 (84) | 113 (84) | 92 (84) |
| Diuretic | 193 (79) | 103 (77) | 90 (83) |
| Peak oxygen uptake | |||
| mL·kg−1·min−1 | 15.2±4.4 | 16.5±4.4 | 13.7±3.9 |
| % predicted | 70±19 | 65±18 | 76±19 |
| ΔVE/ΔVCO2 slope | 30.7±6.7 | 30.6±7.0 | 30.8±6.5 |
| Peak respiratory exchange ratio | 1.10±0.10 | 1.11±0.10 | 1.09±0.09 |
Unless noted otherwise, data are mean±sd or n (% of group).
ACE = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; BNP= brain natriuretic peptide; VCO2, carbon dioxide produced; VE, minute ventilation.
As shown in Table 2, based on the Landis and Koch (25) interpretation, the inter-reviewer agreement for EOV was moderate (i.e., κ = 0.41–0.60) and fair (i.e., κ = 0.21–0.40) when data were graphed using 30-s rolling intervals (κ = 0.429) and 10-s discrete intervals (κ = 0.303), respectively. Based on the 95% confidence intervals for kappa that do not overlap, the agreement was significantly better when data were graphed using 30-s rolling intervals compared to the 10-s discrete intervals. In addition, the final determination of EOV based on agreement by four of the six reviewers was indeterminate in less than half as many tests when data were graphed using 30-s rolling intervals (n= 32) compared to the 10-s discrete intervals (n= 73). There were no meaningful variations in the inter-reviewer agreement among select subgroups (Table 3).
Table 2.
Results from a multi-reviewer determination of exercise oscillatory ventilation (EOV) among patients with heart failure (n= 243)
| Statistic 30-s | Data Averaging Method | |
|---|---|---|
| Rolling Intervals | 10-s Discrete Intervals | |
| Fleiss Kappa (95% CI) | ||
| Overall | 0.429 (0.402, 0.456) | 0.303 (0.278, 0.329) |
| Positive EOV | 0.472 (0.439, 0.504) | 0.237 (0.202, 0.270) |
| Negative EOV | 0.514 (0.482, 0.547) | 0.437 (0.405, 0.469) |
| Indeterminate EOV | 0.016 (−0.016, 0.049) | 0.112 (0.080, 0.145) |
| Final EOV Determination, n (%) | ||
| Positive | 35 (14) | 43 (18) |
| Negative | 176 (72) | 127 (52) |
| Indeterminate | 32 (13) | 73 (30) |
CI, confidence interval.
Table 3.
Results from receiver operator characteristics curve analyses of the relationship between ventilation dispersion index (VDI) and the final determination of exercise oscillatory ventilation (EOV) among patients with heart failure
| Statistic | Data Management of the 32 Indeterminate EOVs | ||
|---|---|---|---|
| Excluded from Analysis | Assumed Positive | Assumed Negative | |
| n | 210 | 242 | 242 |
| AUC (95% CI) | 0.890 (0.828, 0.953) | 0.852 (0.798, 0.906) | 0.860 (0.792, 0.928) |
| Optimal Cut-Point | |||
| VDI | ≥0.924 | ≥0.899 | ≥0.924 |
| Sensitivity (%) | 89 | 81 | 89 |
| Specificity (%) | 80 | 78 | 73 |
AUC, area under the receiver operator characteristics curve; CI, confidence interval.
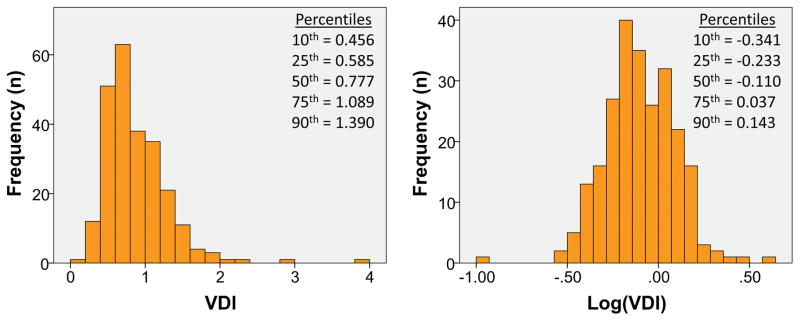
Because the inter-reviewer agreement was significantly better with data averaged using 30-s rolling intervals, we chose to limit the results for VDI to the same data averaging method. As shown in Figure 2, the distribution of VDI was positively skewed. Log transformation of VDI resulted in a nearly normal distribution. There was a strong association between VDI and the final EOV as determined through agreement by four of the six reviewers (Table 3). This was true regardless of whether the indeterminate EOVs were excluded (AUC= 0.890) or assumed to be positive (AUC= 0.852) or negative (AUC= 0.860). Based on the receiver operator characteristics curve data, a VDI ≥0.924 represented the optimal balance between sensitivity and specificity to identify EOV. Patient characteristics below and above a VDI of 0.924 are shown in supplement Table 1 (see Table, SDC 2; patient characteristics by VDI cut-point). Compared to VDI <0.924, a VDI ≥0.924 was associated with a significantly larger proportion of men and tests that were EOV positive.
Figure 2. Distribution of ventilation dispersion index (VDI).
Left pane shows the absolute VDI values. Right pane shows the Log10 of VDI.
The inter-reviewer agreement and the receiver operator characteristics curve analyses were repeated in select subgroups (Table 4). The inter-reviewer agreement varied within subgroups by ejection fraction and peak VO2. EOV was more prevalent among men compared to women and among patients with ejection fraction ≤40% compared to >40%. No meaningful variations were noted for the relationship between VDI and EOV as shown by the AUC.
Table 4.
Inter-reviewer agreement of exercise oscillatory ventilation (EOV), final determination of EOV, and AUC for the relationship between ventilation dispersion index and the final EOV among select subgroups of patients with heart failure
| Subgroup | n | Fleiss Kappa (95% CI)* | Final EOV, % (+/−/Indet) | AUC (95% CI)† |
|---|---|---|---|---|
| All Patients | 243 | 0.429 (0.402, 0.456) | 14/72/13 | 0.890 (0.828, 0.953) |
| Sex | ||||
| Men | 134 | 0.424 (0.388, 0.460) | 18/66/16‡ | 0.948 (0.910, 0.987) |
| Women | 109 | 0.419 (0.379, 0.459) | 10/81/9 | 0.789 (0.649, 0.929) |
| Ejection Fraction (%) | ||||
| ≤40 | 144 | 0.477 (0.412, 0.482) | 18/67/15‡ | 0.892 (0.814, 0.970) |
| >40 | 99 | 0.371 (0.330, 0.413) | 9/81/10 | 0.910 (0.836, 0.984) |
| NYHA | ||||
| I or II | 152 | 0.418 (0.384, 0.451) | 14/73/13 | 0.942 (0.901, 0.984) |
| III or IV | 90 | 0.454 (0.409, 0.499) | 16/72/12 | 0.835 (0.707, 0.963) |
| Peak VO2 (mL·kg−1·min−1) | ||||
| <14.9 | 121 | 0.482 (0.443, 0.520) | 13/78/9 | 0.874 (0.762, 0.986) |
| ≥14.9 | 122 | 0.381 (0.344, 0.419) | 16/67/17 | 0.909 (0.840, 0.976) |
AUC, area under the receiver operator characteristics curve; CI, confidence interval; Indet, indeterminate; NYHA, New York Heart Association Class
Based on 30-s rolling interval averaged data.
Excluding indeterminate EOVs.
P<0.05; comparison within subgroup.
DISCUSSION
Using standardized data graphing methods, we showed that the current interpretation of criteria sets for EOV by CPET experts results in fair to moderate inter-reviewer agreement in patients with HF. This agreement was significantly better and resulted in fewer indeterminate EOVs when data were averaged using 30-s rolling intervals (κ = 0.429) versus 10-s discrete intervals (κ = 0.303). We also evaluated a novel and objective mathematical model that quantifies EOV, the “ventilation dispersion index.” In support of our hypothesis, the VDI was strongly associated with the presence of EOV as determined by at least four of six CPET experts. The sensitivity and specificity to predict EOV with VDI ≥0.924 was 89% and 80%, respectively (Table 3). A template to calculate the VDI is provided (see Document, SDC 3; VDI calculator).
Among available measures from the CPET, interest in EOV is relatively recent. There currently are four distinct set of criteria for EOV with variations developed from these (15). There have been at least 20 studies of patients with ambulatory HF in which the association between EOV and clinical outcomes was evaluated (6–8). As discussed by Cornelis and colleagues (15), the criteria set used to determine EOV is typically cited, but the methodological details have been sparse, making replication difficult. Although EOV has been consistently shown to predict risk in patients with HF (6–8), the incomplete description of methods and lack of consistent methodology to determine EOV in previous studies have hindered its broader clinical application (12, 15).
There are two studies in which the methods used to evaluate EOV were assessed. Francis et al. (26) showed that the amplitude of ventilatory oscillations during exercise is 35% smaller when data are averaged using 30 s intervals compared to 10 s intervals. This difference is consistent with the present findings in which the prevalence of positive, negative, and indeterminate EOVs differed between data averaging methods. In another study, Ingle and colleagues (9) evaluated the effect of two different criteria on the identification of EOV evaluated by two reviewers. Among 240 patients, EOV was identified in 47 patients based on both criteria sets and another 40 patients were identified by one, but not both criteria sets (12). Together these results highlight the importance of providing methodological details sufficient for replication and the need for standardization.
A few investigators have reported mathematical methods to describe aspects of EOV. First among these was Francis and colleagues (27) who reported a method to quantify the relative amplitude of ventilatory oscillation which was later modified by Dall’Ago and colleagues (28). While this measure has been used as a study outcome (27–30), it has not been developed to identify EOV nor quantify total ventilatory dispersion during exercise. Olson and colleagues (31) reported several mathematical methods to quantify the amplitude and periodicity of ventilatory oscillations. They concluded that their methods might be combined in an algorithm to identify EOV. Interestingly, in the Periodic Breathing during Exercise Study, Guazzi and colleagues (8) analyzed tests for EOV by a “rapid manual calculation,” but did not provide details of this method. Finally, Cornelis and colleagues (18) may be the only investigators who have reported an automated system to detect EOV, the VOdEX-tool. While promising, it is proof of concept and has not been tested.
Limitations
The present study is not without limitations. First, reviewers were not trained to assess EOV based on a single set of criteria; therefore our results do not provide insight on the inter-reviewer agreement for any particular EOV criteria. However, our results do shed light on the current state of EOV determination in clinical practice among patients with HF. It is unknown whether these results apply to other patient groups. Second, we did not assess the reliability of a single or multi-reviewer methodology to determine EOV. This remains an opportunity for future study. Third, EOV was indeterminate in 13% (using 30-s rolling intervals) and 30% (using 10-s discrete intervals) of our tests. This was a result of allowing reviewers to identify a test as indeterminate and requiring agreement among the majority (four of six) of the reviewers. This might better reflect real world situations in which CPET data in patients with HF is often noisy and reviewer confidence in the determination of EOV is low. Interestingly, we are not aware of any studies in which indeterminate EOVs were reported. Based on the available studies, an indeterminate EOV does not assist with prognosis estimates. Finally, patients in the present study were enrolled into an exercise training trial. As a result, they may have been healthier and thus had a lower prevalence of EOV than the typical patient with stable HF who might be referred for a CPET.
Conclusions
Inter-reviewer agreement for EOV is fair to moderate in patients with HF and was better when using a 30-s rolling (versus 10-s discrete) interval data averaging method. This level of agreement could negatively affect risk stratification. Ventilation dispersion index is a quantifiable method that shows strong predictive validity with EOV as determined by a majority of CPET experts among patients with compensated HF. Future studies are needed to investigate the association between VDI and outcomes and whether VDI is superior to EOV in predicting risk in these patients. In order to facilitate future research, we have provided a template that others can use to calculate VDI.
Supplementary Material
Acknowledgments
These results do not constitute endorsement by ACSM. The HEART Camp study (clinicaltrials.gov NCT01658670) was supported by a grant from the National, Heart, Blood, and Lung Institute of the National Institutes of Health (award R01HL112979). This agency did not participate in the design, conduct, or analysis of the HEART Camp study. No external financial support was received for the present analysis. Drs. Brawner, Keteyian, and Ehrman operate an exercise testing data core laboratory for multi-site clinical trials. These services are provided based on a fee-for-service agreement between the sponsor and Henry Ford Health System. These include active service agreements with Actelion, Alnylam, and Heart Metabolics. The authors have no other conflicts or financial disclosures.
The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. These results do not constitute endorsement by ACSM.
Disclosure of Funding: The HEART Camp study (clinicaltrials.gov NCT01658670) was supported by a grant from the National, Heart, Blood, and Lung Institute of the National Institutes of Health (award R01HL112979). This agency did not participate in the design, conduct, or analysis of the HEART Camp study. No external financial support was received for the present analysis.
Conflict of Interest: Drs. Brawner, Keteyian, and Ehrman operate an exercise testing data core laboratory for multi-site clinical trials. These services are provided based on a fee-for-service agreement between the sponsor and Henry Ford Health System. These include active service agreements with Actelion, Alnylam, and Heart Metabolics. The authors have no other conflicts or financial disclosures.
References
- 1.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al. Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 2.Task Force of the Italian Working Group on Cardiac R Prevention, Working Group on Cardiac R, Exercise Physiology of the European Society of C. Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction: recommendations for performance and interpretation Part III: Interpretation of cardiopulmonary exercise testing in chronic heart failure and future applications. Eur J Cardiovasc Prev Rehabil. 2006;13(4):485–94. doi: 10.1097/01.hjr.0000201518.43837.bc. Epub 2006/07/29. [DOI] [PubMed] [Google Scholar]
- 3.Force ERST, Palange P, Ward SA, Carlsen KH, Casaburi R, Gallagher CG, et al. Recommendations on the use of exercise testing in clinical practice. Eur Respir J. 2007;29(1):185–209. doi: 10.1183/09031936.00046906. Epub 2007/01/02. [DOI] [PubMed] [Google Scholar]
- 4.Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126(18):2261–74. doi: 10.1161/CIR.0b013e31826fb946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83(3):778–86. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 6.Brawner CA, Shafiq A, Aldred HA, Ehrman JK, Leifer ES, Selektor Y, et al. Comprehensive analysis of cardiopulmonary exercise testing and mortality in patients with systolic heart failure: the Henry Ford Hospital cardiopulmonary exercise testing (FIT-CPX) project. J Card Fail. 2015;21(9):710–8. doi: 10.1016/j.cardfail.2015.06.001. Epub 2015/06/13. [DOI] [PubMed] [Google Scholar]
- 7.Cornelis J, Taeymans J, Hens W, Beckers P, Vrints C, Vissers D. Prognostic respiratory parameters in heart failure patients with and without exercise oscillatory ventilation - a systematic review and descriptive meta-analysis. Int J Cardiol. 2015;182:476–86. doi: 10.1016/j.ijcard.2015.01.029. Epub 2015/01/24. [DOI] [PubMed] [Google Scholar]
- 8.Guazzi M, Boracchi P, Arena R, Myers J, Vicenzi M, Peberdy MA, et al. Development of a cardiopulmonary exercise prognostic score for optimizing risk stratification in heart failure: the (P)e(R)i(O)dic (B)reathing during (E)xercise (PROBE) study. J Card Fail. 2010;16(10):799–805. doi: 10.1016/j.cardfail.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Ingle L, Isted A, Witte KK, Cleland JG, Clark AL. Impact of different diagnostic criteria on the prevalence and prognostic significance of exertional oscillatory ventilation in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2009;16(4):451–6. doi: 10.1097/HJR.0b013e32832a4f54. [DOI] [PubMed] [Google Scholar]
- 10.Leite JJ, Mansur AJ, de Freitas HF, Chizola PR, Bocchi EA, Terra-Filho M, et al. Periodic breathing during incremental exercise predicts mortality in patients with chronic heart failure evaluated for cardiac transplantation. J Am Coll Cardiol. 2003;41(12):2175–81. doi: 10.1016/s0735-1097(03)00460-1. [DOI] [PubMed] [Google Scholar]
- 11.Sun XG, Hansen JE, Beshai JF, Wasserman K. Oscillatory breathing and exercise gas exchange abnormalities prognosticate early mortality and morbidity in heart failure. J Am Coll Cardiol. 2010;55(17):1814–23. doi: 10.1016/j.jacc.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 12.Corra U. Exercise oscillatory ventilation in heart failure. Int J Cardiol. 2016;206(Suppl):S13–5. doi: 10.1016/j.ijcard.2016.02.122. Epub 2016/03/05. [DOI] [PubMed] [Google Scholar]
- 13.Corra U, Giordano A, Bosimini E, Mezzani A, Piepoli M, Coats AJ, et al. Oscillatory ventilation during exercise in patients with chronic heart failure: clinical correlates and prognostic implications. Chest. 2002;121(5):1572–80. doi: 10.1378/chest.121.5.1572. Epub 2002/05/15. [DOI] [PubMed] [Google Scholar]
- 14.Dhakal BP, Lewis GD. Exercise oscillatory ventilation: Mechanisms and prognostic significance. World J Cardiol. 2016;8(3):258–66. doi: 10.4330/wjc.v8.i3.258. Epub 2016/03/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornelis J, Beckers P, Vanroy C, Volckaerts T, Vrints C, Vissers D. An overview of the applied definitions and diagnostic methods to assess exercise oscillatory ventilation--a systematic review. Int J Cardiol. 2015;190:161–9. doi: 10.1016/j.ijcard.2015.04.111. Epub 2015/04/29. [DOI] [PubMed] [Google Scholar]
- 16.Kremser CB, O’Toole MF, Leff AR. Oscillatory hyperventilation in severe congestive heart failure secondary to idiopathic dilated cardiomyopathy or to ischemic cardiomyopathy. Am J Cardiol. 1987;59(8):900–5. doi: 10.1016/0002-9149(87)91116-7. Epub 1987/04/01. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Dov I, Sietsema KE, Casaburi R, Wasserman K. Evidence that circulatory oscillations accompany ventilatory oscillations during exercise in patients with heart failure. Am Rev Respir Dis. 1992;145(4 Pt 1):776–81. doi: 10.1164/ajrccm/145.4_Pt_1.776. Epub 1992/04/01. [DOI] [PubMed] [Google Scholar]
- 18.Cornelis J, Denis T, Beckers P, Vrints C, Vissers D, Goossens M. Development of a clinical applicable graphical user interface to automatically detect exercise oscillatory ventilation: The VOdEX-tool. Int J Cardiol. 2017;240:291–6. doi: 10.1016/j.ijcard.2016.12.159. Epub 2017/01/09. [DOI] [PubMed] [Google Scholar]
- 19.Pozehl BJ, Duncan K, Hertzog M, McGuire R, Norman JF, Artinian NT, et al. Study of adherence to exercise in heart failure: the HEART camp trial protocol. BMC Cardiovasc Disord. 2014;14:172. doi: 10.1186/1471-2261-14-172. Epub 2014/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128(8):873–934. doi: 10.1161/CIR.0b013e31829b5b44. Epub 2013/07/24. [DOI] [PubMed] [Google Scholar]
- 21.Brawner CA, Ehrman JK, Aldred H, Schairer JR, Keteyian SJ. Quality assurance and cardiopulmonary exercise testing in clinical trials. J Card Fail. 2008;14(4):283–9. doi: 10.1016/j.cardfail.2008.01.001. Epub 2008/05/14. [DOI] [PubMed] [Google Scholar]
- 22.Wasserman K, Hansen D, Sue DY, Whipp BJ, Casaburi R. Principles of Exercise Testing and Interpretation. Philadelphia, PA: Lippincott Williams and Wilkins; 1999. [Google Scholar]
- 23.Fleiss JL. Measuring nomical scale agreement among many raters. Psychol Bull. 1971;76(5):378–82. [Google Scholar]
- 24.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–5. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. Epub 1950/01/01. [DOI] [PubMed] [Google Scholar]
- 25.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. Epub 1977/03/01. [PubMed] [Google Scholar]
- 26.Francis DP, Davies LC, Willson K, Wensel R, Ponikowski P, Coats AJ, et al. Impact of periodic breathing on measurement of oxygen uptake and respiratory exchange ratio during cardiopulmonary exercise testing. Clin Sci (Lond) 2002;103(6):543–52. doi: 10.1042/cs1030543. Epub 2002/11/26. doi:10.1042/ [DOI] [PubMed] [Google Scholar]
- 27.Francis DP, Davies LC, Piepoli M, Rauchhaus M, Ponikowski P, Coats AJ. Origin of oscillatory kinetics of respiratory gas exchange in chronic heart failure. Circulation. 1999;100(10):1065–70. doi: 10.1161/01.cir.100.10.1065. Epub 1999/09/08. [DOI] [PubMed] [Google Scholar]
- 28.Dall’Ago P, Chiappa GR, Guths H, Stein R, Ribeiro JP. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: a randomized trial. J Am Coll Cardiol. 2006;47(4):757–63. doi: 10.1016/j.jacc.2005.09.052. Epub 2006/02/21. [DOI] [PubMed] [Google Scholar]
- 29.Callegaro CC, Martinez D, Ribeiro PA, Brod M, Ribeiro JP. Augmented peripheral chemoreflex in patients with heart failure and inspiratory muscle weakness. Respir Physiol Neurobiol. 2010;171(1):31–5. doi: 10.1016/j.resp.2010.01.009. Epub 2010/01/26. [DOI] [PubMed] [Google Scholar]
- 30.Winkelmann ER, Chiappa GR, Lima CO, Viecili PR, Stein R, Ribeiro JP. Addition of inspiratory muscle training to aerobic training improves cardiorespiratory responses to exercise in patients with heart failure and inspiratory muscle weakness. Am Heart J. 2009;158(5):768.e1–7. doi: 10.1016/j.ahj.2009.09.005. Epub 2009/10/27. [DOI] [PubMed] [Google Scholar]
- 31.Olson TP, Johnson BD. Quantifying oscillatory ventilation during exercise in patients with heart failure. Respir Physiol Neurobiol. 2014;190:25–32. doi: 10.1016/j.resp.2013.09.008. Epub 2013/10/15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.