Abstract
Plants metabolize polychlorinated biphenyls (PCBs) into hydroxylated derivatives (OH-PCBs), which are sometimes more toxic than the parent PCBs. The objective of this research was to compare the toxicity of a suite of PCBs and OH-PCBs toward the model plant, Arabidopsis thaliana. While parent PCBs and higher-chlorinated OH-PCBs exhibited a low or nondetectable toxicity, lower-chlorinated OH-PCBs significantly inhibited the germination rate and plant growth, with inhibition concentration 50% (IC50) ranging from 1.6 to 12.0 mg L−1. The transcriptomic response of A. thaliana to 2,5-dichlorobiphenyl (2,5-DCB), and its OH metabolite, 4′-OH-2,5-DCB, was then examined using whole-genome expression microarrays (Affymetrix). Exposure to 2,5-DCB and 4′-OH-2,5-DCB resulted in different expression patterns, with the former leading to enrichment of genes involved in response to toxic stress and detoxification functions. Exposure to 2,5-DCB induced multiple xenobiotic response genes, such as cytochrome P-450 and glutathione S-transferases, potentially involved in the PCB metabolism. On the contrary, exposure to both compounds resulted in the down-regulation of genes involved in stresses not directly related to toxicity. Unlike its OH derivative, 2,5-DCB was shown to induce a transcriptomic profile similar to plant safeners, which are nontoxic chemicals stimulating detoxification pathways in plants. The differentiated induction of detoxification enzymes by 2,5-DCB may explain its lower phytotoxicity compared to 4′-OH-2,5-DCB.
Graphical abstract
INTRODUCTION
Polychlorinated biphenyls (PCBs) are persistent and toxic pollutants that have been widely dispersed into the environment. Hydroxylated derivatives of PCBs (OH-PCBs) are produced by the biological and abiotic oxidation of PCBs.1 The first step of the metabolism of PCBs in higher organisms often involves the formation of OH-PCBs, which is catalyzed by oxidative enzymes, such as cytochrome P-450 monooxygenases.2–4 It has also been suggested that OH-PCBs could be formed in the atmosphere through reaction with hydroxyl radicals or could originate from commercial formulations.5,6 As transformation products of PCBs, OH-PCBs are widely dispersed in the environment, and they have been detected in a variety of samples, including air, water, sediments, and animal tissues.6–8 OH-PCBs have raised environmental concerns because of their reported toxicity for a variety of organisms, sometimes even higher than the toxicity of parent PCBs.4,9,10
Higher plants have been shown to take up, accumulate, and, to some extent, metabolize environmental pollutants, including PCBs.11–13 Several plant species were shown to absorb PCBs through the roots and, to some extent, translocate them to aerial parts.14–16 Within plant tissues, PCBs are thought to be metabolized through a three-step process known as the “green liver model”,11 which is typically initiated by their oxidation into OH derivatives.13,17,18 Several studies have shown that PCBs exert generally moderate phytotoxic effect, which are variable depending on the PCB congener and the plant species.19,20 For instance, Weber and Mrozek19 reported that the PCB mixture, Aroclor 1254 (100 mg L−1) resulted in 27% growth inhibition in soybean plants and 3% growth stimulation in fescue plants. However, no information is available about the toxicity of OH-PCBs for plants, even though the reported toxicity of PCBs may be, in part, due to OH metabolites formed following the exposure.
Understanding the effect of PCBs and OH-PCBs on plants is important because plants are the basis of the terrestrial food web and can be used for soil remediation, a process known as phytoremediation. The objective of the present study is to determine and compare the toxicity of a suite of PCBs and their mono-OH congeners for the model plant, Arabidopsis thaliana. To further understand the effects of these compounds on plants, changes in gene expression were examined in A. thaliana exposed to the model PCB, 2,5-dichlorobiphenyl (2,5-DCB) and its major OH-derivative, 4′-OH-2,5-DCB, using whole-genome expression microarrays (Affymetrix). The hypothesis driving this research is that low-molecular-weight OH-PCBs are more toxic than their parent compounds, which is then reflected by distinct gene expression patterns.
MATERIALS AND METHODS
Chemicals
Chlorobiphenyl (CB), dichlorobiphenyl (DCB), trichlorobiphenyl (TCB), tetrachlorobiphenyl (TeCB), and their OH derivatives were purchased from Accustandard (New Haven, CT) in ≥99% purity or custom-synthesized in ≥99% purity by the Superfund Research Program Synthesis Core at The University of Iowa (Iowa City, IA). Phytoagar was obtained from Plant Media (Dublin, OH). Murashige and Skoog (MS) salt base was obtained from Carolina (Burlington, NC).
Germination and Growth Tests
A. thaliana, ecotype Columbia (Col-0/Redei-L211497, The Ohio State University, Columbus, OH) were grown as described in Kaveh et al.21 Surface-sterilized seeds (95% ethanol for 5 min and 0.6% sodium hypochloride for 5 min) were placed in Petri dishes containing sterile semisolid nutrient medium (0.5 strength MS nutrient solution with 0.3% sucrose and 0.7% phytoagar at pH 7.2) supplemented with increasing concentrations of the toxicants. Most PCBs and OH-PCBs were tested above their solubility limit and were added to the medium as an acetone stock solution (5000 mg L−1) to ensure proper dispersion of the toxicant, following the guidelines for testing aquatic toxicity of insoluble substances. Control seeds were grown in the nutrient medium supplemented with the solvent carrier only (acetone, ≤1% v/v). Seeds were incubated at 25 °C under white (cool) fluorescent light (0.38 ± 0.02 W ft−2) with a 16 h light/8 h dark photoperiod. After 7 days, the germination rate was determined by visual observation (plantlets with two visible green leaves were counted as germinated) and expressed as the percentage of germinated seeds – (the number of germinated seeds/initial number of seeds) × 100. After 21 days, surviving plantlets were removed from the medium, washed with water, dried by blotting, and weighed to determine the fresh biomass. The biomass growth was expressed as a percentage relative to the biomass of nonexposed controls – (biomass of exposed plants/biomass of nonexposed plants) × 100. A total of two or three replicate dishes containing at least 20 seeds per plantlets (from 20 to 32) per dish were used for each treatment, representing a minimum of 40 seeds or plantlets per treatment. The phytotoxic effect of PCBs and OH-PCBs was expressed by the inhibition concentration 50% (IC50) obtained by plotting the experimental dose–response curve (percent inhibition versus contaminant concentration) and computing a three-parameter sigmoidal regression (Prism 6.0, GraphPad, La Jolla, CA).
Gene-Expression Analysis Using Microarrays
A. thaliana was grown in 10 × 10 cm Magenta boxes as described above. Plantlets were exposed to subinhibitory concentrations of 2,5-DCB (50 mg L−1) and 4′-OH-2,5-DCB (5 mg L−1). Control plantlets were grown in the presence of the solvent carrier (acetone) only. A total of six boxes containing five plantlets each were used for each treatment. After 21 days of growth, plantlets were removed from the medium, immediately immersed in RNAlater (Ambion, Foster City, CA), and incubated at 4 °C for 24 h. Plantlets were then washed, dried, and stored at −80 °C until RNA extraction. The five plants from each box were combined, and RNA was extracted from the whole tissue using TRIzol Plus RNA Purification kit with on-column PureLink DNase treatment (Thermofisher, Waltham, MA). Homogenization of plant tissue was performed using bead beating (1 mm glass beads, 4200 rpm, 40 s) after the addition of TRIzol reagent. Purified RNA was kept at −80 °C. RNA was quantified by the OD260 using a NanoDrop ND-2000 spectrophotometer (Vernon Hills, IL). The quality of RNA was assessed by the ratios of OD260 to OD280 and of OD260 to OD230 and using an Agilent 2100 Bioanalyzer (Santa Clara, CA). RNA used for microarray analysis had OD260-to-OD280 ratios of 2.0–2.2, OD260-to-OD230 ratios of 1.8–2.7, and RNA integrity numbers (RIN) of 6.9–8.0. RNA samples were labeled and hybridized to the Arabidopsis Gene 1.0 ST Arrays according to the manufacturer’s instructions (Affymetrix, Santa Clara, CA). A total of three biological replicates per treatment were utilized for microarray experiments. Scanned microarray images were analyzed using the Expression Console and Transcriptome Analysis Console version 3.0 (Affymetrix). Analysis of differential gene expression level was conducted using oneway between-subject ANOVA (unpaired) with a p value of <0.01.
Reverse-Transcription Real-Time PCR
Quantitative analysis of gene expression was performed for selected genes using reverse-transcription real-time PCR (RT-qPCR), as described in Kaveh et al.21 The internal standard was the 18S ribosomal DNA (rDNA) gene. RT-qPCRs were conducted using the same RNA as used for the microarray experiments. RNA was reverse-transcribed into cDNA using SuperScript III First-Strand Synthesis system and oligo-dT primers (Invitrogen, Foster City, CA). Negative controls were generated by running the reactions without reverse-transcriptase. Gene sequences were retrieved from The Arabidopsis Information Resource (TAIR) database (www.arabidopsis.org/) and used to design gene-specific real-time primers using PrimerQuest (IDT, Coralville, IA). When possible (for 9 out of 12 genes), primers were designed with one primer spanning an exon–intron boundary (Table S1). Real-time PCR quantification of cDNA was performed on a StepOnePlus Real-Time PCR System using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). CT (cycle threshold) values were computed by the StepOnePlus Software (version 2.1; Applied Biosystems).
MIAME Compliance
This article is written in compliance with the Minimum Information About a Microarray Experiment (MIAME) guidelines (http://www.mged.org/miame). Microarray data have been submitted to the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo) repository and have been assigned the accession number GSE99345.
RESULTS AND DISCUSSION
Toxicity Testing
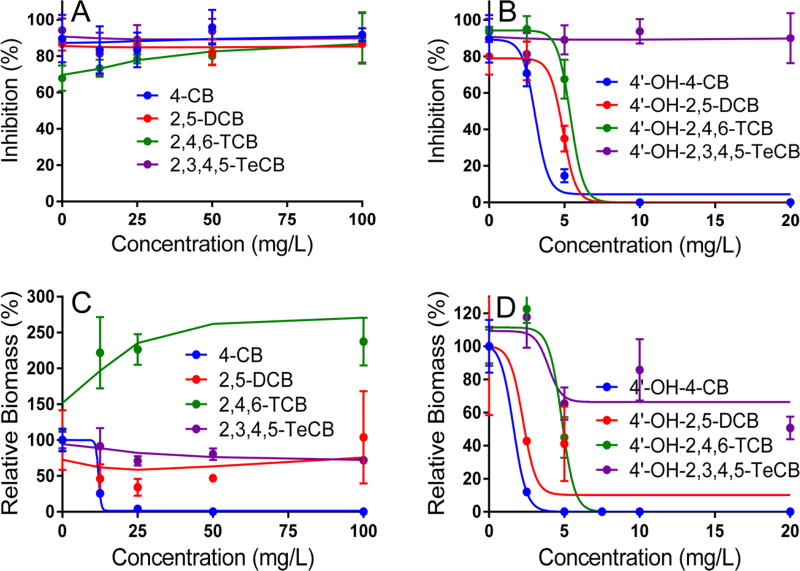
The toxicity of 17 mono–OH PCBs and their 11 parent compounds (including mono-, di-, tri-, and tetrachlorinated congeners) for A. thaliana was determined based on germination and growth tests conducted over 7 and 21 days, respectively (Figure 1 and Table 1).
Figure 1.
Dose–response curves of A. thaliana exposed to PCBs (panels A and C) and OH-PCBs (panels B and D) as determined through germination tests (panels A and B) and growth tests (panels C and D). Solid lines for significantly toxic compounds (e.g., 4-CB, 4′-OH-4-CB, 4′-OH-2,5-DCB, and 4′-OH-2,4,6-TCB) were obtained by fitting to a three-parameter sigmoidal inhibition model (Prism 5.04, Graphpad). Solid lines for other, less toxic compounds were obtained by second-order smoothing of experimental data (Prism 5.04, Graphpad). The error bars represent standard deviations between biological replicates.
Table 1.
Toxicity of PCBs and OH-PCBs for A. thaliana as Determined through Germination and Growth Testsa
| germination tests
|
growth tests
|
log Kow
|
||||||
|---|---|---|---|---|---|---|---|---|
| PCBs and OH-PCBs | abbreviation | IC50 (mg L−1) | CI 95% (mg L−1) | R2 | IC50 (mg L−1) | CI 95% (mg L−1) | R2 | |
| 3-chlorobiphenyl | 3-CB | NDb | ND | ND | ND | ND | ND | 4.59 |
| 4-hydroxy-3- | 4-OH-3-CB | 5.19 | 4.38–5.99 | 0.98 | 5.08 | 4.22–5.94 | 0.95 | 4.49 |
| 4-chlorobiphenyl | 4-CB | ND | ND | ND | 12.02 | 11.76–12.27 | 1.00 | 4.60 |
| 4′-hydroxy-4- | 4′-OH-4-CB | 3.08 | 1.73–4.42 | 0.98 | 1.62 | 1.62–1.63 | 1.00 | 4.50 |
| 2,4′ -dichlorobiphenyl | 2,4′-DCB | ND | ND | ND | ND | ND | ND | 5.11 |
| 4-hydroxy-2,4′ - | 4-OH-2,4′-DCB | 2.23 | 1.67–2.79 | 0.98 | 3.65 | 2.99–4.31 | 1.00 | 5.11 |
| 2,5-dichlorobiphenyl | 2,5-CDB | ND | ND | ND | ND | ND | ND | 5.20 |
| 2′ -hydroxy-2,5- | 2′-OH-2,5-DCB | 10.74 | 8.90–12.58 | 0.97 | 5.25 | 4.52–5.97 | 0.99 | 4.88 |
| 3′ -hydroxy-2,5- | 3′-OH-2,5-DCB | 10.01 | 8.78–11.24 | 0.95 | 5.44 | 1.45–9.42 | 0.80 | 5.11 |
| 4′ -hydroxy-2,5- | 4′-OH-2,5-DCB | 4.90 | 4.78–5.02 | 1.00 | 2.47 | 0.54–4.40 | 0.89 | 5.11 |
| 3,4-dichlorobiphenyl | 3,4-DCB | ND | ND | ND | ND | ND | ND | 5.19 |
| 4′ -hydroxy-3,4- | 4′-OH-3,4-DCB | 7.31 | 5.34–9.27 | 0.91 | 7.28 | 6.42–8.15 | 0.96 | 5.10 |
| 2,2′,5-trichlorobiphenyl | 2,2′,5-TCB | ND | ND | ND | NTc | NT | 5.65 | |
| 4′-hydroxy-2,2′,5′- | 4′-OH-2,2′,5′-TCB | 8.05 | 0.00–20.84 | 0.98 | NT | NT | 5.44 | |
| 2,4,6-trichlorobiphenyl | 2,4,6-TCB | ND | ND | ND | ND | ND | ND | 5.97 |
| 2′-hydroxy-2,4,6- | 2′-OH-2,4,6-TCB | 9.97 | 8.13–11.81 | 0.90 | 5.40 | 3.26–7.55 | 0.93 | 5.58 |
| 3′-hydroxy-2,4,6- | 3′-OH-2,4,6-TCB | 5.07 | 4.02–6.12 | 0.97 | 4.60 | 3.87–5.33 | 0.99 | 5.87 |
| 4′ -hydroxy-2,4,6- | 4′-OH-2,4,6-TCB | 5.40 | 5.30–5.50 | 1.00 | 4.83 | 3.93–5.73 | 0.98 | 5.88 |
| 3,3′,4-trichlorobiphenyl | 3,3′,4-TCB | ND | ND | ND | ND | ND | ND | 5.66 |
| 4′-hydroxy-3,3′,4- | 4′-OH-3,3′,4-TCB | ND | ND | ND | ND | ND | ND | 5.48 |
| 2,2′,5,5′-tetrachlorobiphenyl | 2,2′,5,5′-TeCB | ND | ND | ND | ND | ND | ND | 6.29 |
| 4′-hydroxy-2,2′,5,5′- | 4′-OH-2,2′,5,5′-TeCB | 5.28 | 3.51–7.06 | 0.86 | 2.27 | 0.89–3.64 | 0.86 | 6.11 |
| 2,3,4,5-tetrachlorobiphenyl | 2,3,4,5-TeCB | ND | ND | ND | ND | ND | ND | 6.55 |
| 2′-hydroxy-2,3,4,5- | 2′-OH-2,3,4,5-TeCB | ND | ND | ND | ND | ND | ND | 6.24 |
| 3′-hydroxy-2,3,4,5- | 3′-OH-2,3,4,5-TeCB | ND | ND | ND | ND | ND | ND | 6.46 |
| 4′ -hydroxy-2,3,4,5- | 4′-OH-2,3,4,5-TeCB | ND | ND | ND | ND | ND | ND | 6.46 |
| 3,3′,4,5′-tetrachlorobiphenyl | 3,3′,4,5′-TeCB | ND | ND | ND | ND | ND | ND | 6.26 |
| 4′-hydroxy-3,3′,4,5′- | 4′-OH-3,3′,4,5′-TeCB | ND | ND | ND | ND | ND | ND | 6.09 |
IC50 were computed from the dose-response curves using a three-parameter sigmoidal model. IC50, 95% confidence interval (CI 95%), coefficients of determination (R2), and octanol–water partition coefficients (Kow) are presented.
ND: Not determined.
NT: Not tested.
Because higher-chlorinated PCBs and OH-PCBs have low water solubility, a homogeneous dispersion was prepared by dissolution in acetone prior to addition to the hydroponic medium.22 The low volume of acetone stock solution added (<1%) was not expected to significantly modify the water solubility of the solutes.23 Based on the germination tests, none of the parent PCBs were shown to exhibit a detectable or significant toxicity (i.e., IC50 non determined, ND) at the concentration of 100 mg L−1, with germination rates ranging from 73 to 94% relative to nonexposed controls (Figure 1 and Table 1). OH-PCBs with one to three chlorine atoms exhibited a recordable toxicity at the concentration of 20 mg L−1 or lower (except 4′-OH-3,3′,4-TCB), with IC50 values ranging from 2.2 to 10.7 mg L−1. Four of the OH-PCBs with four chlorine atoms showed no detectable toxicity at the concentration of 20 mg L−1, with germination rates ranging from 77 to 100% relative to nonexposed controls. 4′-OH-2,2′,5,5′-TeCB was shown to be toxic, with IC50 equal to 5.3 mg L−1 (Figures 1 and S1 and Table 1).
Growth tests based on biomass were overall consistent with germination tests, showing no significant toxicity (and sometimes strong stimulation of the growth) with the parent PCBs (except 4-CB) and tetrachlorinated OH-PCBs (except 4′-OH-2,2′,5,5′-TeCB); biomasses after 21 days ranged from 59 to 237% relative to nonexposed controls (Figure 1 and Table 1). 2,4′-DCB resulted in growth stimulation (227% of nonexposed control) at 25 mg L−1 and complete (100%) inhibition at 50 mg L−1 and higher. As with germination tests, mono-, di-, and trichlorinated OH-PCBs (except 4′-OH-3,3′,4-TCB) and one tetrachlorinated OH-PCBs (4′-OH-2,2′,5,5′-TeCB) resulted in significant growth inhibition with IC50 values ranging from 1.6 to 12.0 mg L−1 (Figures 1 and S2 and Table 1). The data fitted generally well the toxicity model, with coefficients of determination (R2) ≥ 0.9 for most analyses (Table 1).
Consistently with our results, several studies have shown that PCBs exert low to moderate toxicity toward terrestrial plants except when exposed to high concentrations.19,20 For instance, Weber and Mrozek19 observed that exposure of soybean plants to Aroclor 1254 for 26 days resulted in approximately 11%, 27%, and 22% growth inhibition (based on fresh weight) at the concentration of 10, 100, and 1000 mg L−1, respectively. In fescue plants, exposure to Aroclor 1254 for 42 days resulted in growth stimulation at a low concentration (10–100 mg L−1) and 16% inhibition at 1000 mg L−1. Examining the phytoremediation capabilities of nine plant species growing in various soils contaminated with Aroclor 1260, Zeeb et al.14 reported biomass reduction and toxic stress symptoms (e.g., chlorosis and leaf yellowing) in most plants growing in the highest contaminated soil (4200 mg kg−1), while plants growing in lower contaminated soils (90 and 250 mg kg−1) showed no toxic effects. However, several studies have shown more severe effects of PCBs on plants at concentrations well below the maximum concentration (100 mg L−1) used in our study. Jin et al.24 exposed Arabidopsis plants, growing on agar plates and in soil, to increasing concentration of 2,2′,3,3′-TeCB and observed that low concentrations (below 10 mg L−1) resulted in growth stimulation (e.g., increase of root length and plant height), while higher concentrations (40 to 100 mg L−1) resulted in growth inhibition. Wang et al.25 recently showed that exposure of alfalfa seedlings to a low concentration of 3,3′,4,4′-TeCB (3 mg L−1) had a significant impact on plant growth (reduction of root and shoot biomass dry weight by 17.6 and 20.6%, respectively). In addition, PCB exposure caused reduction of the chlorophyll content and oxidative stress. Ahammed et al.26 investigated the effects of foliar application of Aroclor 1242 on tomato plants and observed that low concentrations (0.4, 2, and 10 µL L−1) resulted in significant reduction of the plant biomass (reduction of root and shoot dry weight by 8.0, 33.0, and 38.4%, respectively). The authors also reported a negative effect on photosynthesis (e.g., chlorophyll content and CO2 assimilation) and increase of lipid peroxidation and reactive oxygen species (ROS). Using hairy root culture of Solanum nigrum, Kucerova et al.18 showed that exposure to 3 mg L−1 of various lower-chlorinated PCBs resulted in 21 to 53% inhibition of the biomass growth after 14 days. This rather high toxicity may be explained by the higher susceptibility of plant cell cultures as compared with whole plants.
Our study also showed that exposure to 3,3′,4,4′-TeCB, which is a coplanar, dioxin-like PCB, did not result in noticeable toxicity. This result was expected because plants do not possess the aryl hydrocarbon receptor (AHR), which is the specific target of coplanar PCBs in mammals.27 In several cases, hormesis (i.e., biphasic dose–response, reflected by metabolic stimulation at a low exposure level and inhibition at a higher level) was observed, as was the case in our study. Hormesis has been described in plants as well as in other organisms exposed to various types of stressors, including toxic chemicals.21,24,28 It is believed that a low level of stress triggered cellular signaling pathways and defense mechanisms involving transcription factors, protective proteins, and antioxidant molecules, which have beneficial effects for the organisms.29
Although not conducted with the model plant, A. thaliana, prior studies have shown that plants were capable to take up and translocate PCBs, which were then susceptible to induce phytotoxicity. For instance, in their study on PCB uptake and translocation by nine plant species (see above), Zeeb et al.14 reported that the PCB concentration in plant tissues varied widely depending on the species and the concentration in soil (from 90 to 4200 mg kg−1) and were higher in root tissues (from 47 to 6500 mg kg−1) than in shoot tissues (from 0.32 to 470 mg kg−1). In a pilot-scale field study conducted at a PCB-contaminated site (Aroclor 1254/1260), Aslund et al.16 reported root concentrations of 66, 40, and 15 mg kg−1 and shoot concentrations of 13, 6.7, and 4.7 mg kg−1 in sedge, pumpkin, and tall fescue, respectively. Shoot bioaccumlation factors (BAFs) ranged from 0.1 (tall fescue) to 0.29 (sedge). Liu and Schnoor15 have reported that hybrid poplar trees (Populus deltoides × nigra) were capable to take up various PCBs with one to four chlorine atoms from the hydroponic solution. Concentrations of PCBs in root tissues decreased when the degree of chlorination increased (from 1.1 mg kg−1 fresh weight with monochlorinated PCB to 0.17 mg kg−1 with tetrachlorinated PCB). Root concentration factors (RCFs) increased with the degree of chlorination (from 185 with monochlorinated PCB to 1965 with tetrachlorinated PCB), likely reflecting different dosages in the hydroponic solution and differential sorption of PCBs to root tissues. As in other studies, no PCBs or a low level of PCBs were detected in aerial parts (stems and leaves), showing limited translocation of PCBs in plants. Results published in the literature suggest that the uptake and toxic effects of PCBs are highly variable depending on the congeners and the plant species. Not only are different PCBs characterized by different hydrophobicities and bioavailabilities, but different congeners also have different substitution patterns, potentially conferring distinct biological activities (e.g., dioxin-like versus nondioxin-like PCBs). As generally observed in experimental toxicology, the different toxicities reported in the literature likely reflect different experimental conditions, including the route of exposure (e.g., medium), duration of the experiment, and detected end points (e.g., germination, biomass).
To the best of our knowledge, the toxicity of OH-PCBs to plants has not been previously investigated. Consistently with our observations, studies conducted in other organisms, including animals and bacteria, have also reported that OH-PCBs can be more toxic than the parent PCBs.4,9,10 Even though hydroxylation of PCBs is generally regarded as a detoxification mechanism, in vitro studies have shown that OH-PCBs can be further hydroxylated, generating toxic species, such as quinones and reactive oxygen species (ROS), which can damage DNA and form adducts with proteins, lipids, and nucleic acids.4 In fact, the toxicity of many halogenated contaminants, including PCBs, has been attributed to the formation of OH metabolites.4,9 The higher toxicity of OH-PCBs (expressed by the IC50) as compared with their parent PCB could be explained partially by the higher solubility (expressed by the log Kow) and bioavailability of the former (Table 1).10,30 Although well-studied in humans and other animals,4,9 the mechanisms of toxicity of PCBs and OH-PCBs in plants have received little attention.
Gene-Expression Microarrays
The transcriptional response of Arabidopsis plantlets exposed to 2,5-DCB and 4′-OH-2,5-DCB was investigated using Affymetrix whole-genome expression microarrays. Concentration of 50 mg 2,5-DCB L−1 and 5 mg 4′-OH-2,5-DCB L−1, which resulted in subinhibitory effects based on germination and growth tests, were chosen for microarray experiments. After the filtering out of low-quality and insignificant expression results, based on one-way ANOVA (p < 0.01), 958 genes were shown to be differentially expressed by exposure to 2,5-DCB compared to the nonexposed control, including 323 (33.7%) up-regulated genes (change fold >2.0) and 635 (66.3%) down-regulated genes (change fold <0.5). Exposure to 4′-OH-2,5-DCB resulted in differential expression of 508 genes, including 167 (32.9%) up-regulated genes and 341 (67.1%) down-regulated genes. A total of 259 genes were differentially expressed by exposure to both 2,5-DCB and 4′-OH-2,5-DCB, including 35 up-regulated genes (10.8% of total up-regulated genes) and 224 down-regulated genes (35.3% of total down-regulated genes). Many more genes were down-regulated than up-regulated in response to 2,5-DCB and 4′-OH-2,5-DCB, suggesting that gene expressions (and many related biological functions) were inhibited by the two compounds. Hierarchical clustering revealed that exposure to both 2,5-DCB and 4′-OH-2,5-DCB induced rather similar gene expression profiles compared to control plants (Figure S3), which may be explained by the structural similarities between the two compounds or by the partial in vivo transformation of 2,5-DCB into 4′-OH-2,5-DCB.
To validate the microarray results, quantitative analysis of gene expression was performed using RT-qPCR on 12 selected genes, including genes down- and up-regulated upon exposure to both compounds. Expression levels obtained with microarrays have been plotted against expression levels obtained by RT-qPCR (Figure S4), showing satisfactory correlations (Pearson’s correlation coefficient of 0.95 and 0.96 for exposure to 2,5-DCB and 4′-OH-2,5-DCB, respectively).
Functional Categories of Differentially Expressed Genes
The enrichment of transcripts in different Gene Ontology (GO) categories was interrogated using the Remote Term Enrichment tool with Bonferroni correction (http://amigo.geneontology.org/rte).31 To avoid redundancy, only the most-specific categories (i.e., at the lowest hierarchical level) were considered in the analysis (Table 2). Genes up-regulated in response to 2,5-DCB showed strong enrichment in several biological processes related to the metabolism of toxic substances, including toxin catabolism, response to toxic substance, and response to oxidative stress, which likely reflect the phytotoxicity of 2,5-DCB. Exposure to 2,5-DCB also induced genes not directly related to toxic chemicals, such as genes involved in response to oomycetes and bacteria. Many biotic and abiotic stresses result in similar cellular damages and, hence, similar plant responses, potentially explaining PCB-mediated induction of genes involved in different types of stresses.32 Plants possess various mechanisms of xenobiotic sensing, and it is difficult to distinguish between responses directly induced by the toxic compounds and indirectly induced by their deleterious effects.33 Prior studies focusing on transcription factors in Arabidopsis showed overlap of genes expressed in response to different types of stresses.34 Toxic compounds have been reported to induce genes involved in response to oxidative stress and pathogens, which frequently produce toxins inside the host cells.33,35 Genes up-regulated in response to 2,5-DCB showed also enrichment in molecular functions potentially involved in the PCB metabolism, including glutathione transferase activity, alkyl/aryl transferase activity, and transmembrane transporter activity (Table 2). Genes up-regulated upon exposure to 4′-OH-2,5-DCB did not show significant enrichment in specific GO terms. However, genes down-regulated upon exposure to both 2,5-DCB and 4′-OH-2,5-DCB showed enrichment in several biological processes involved in jasmonic acid (JA) and abscisic acid (ABA) signaling, along with genes involved in response to a range of biotic and abiotic factors not directly related to toxicity, such as cold, wounding, water deprivation, chitin, drought, and salt (Table 2). Stress response in plants involves complex signaling pathways, which can act synergistically as shown above or antagonistically. For instance, response to flooding has been shown to reduce plant resistance to herbivores through negative cross-talk between ethylene and JA signaling.36 Other examples of the negative effect of a type of stress on tolerance to other stresses have been reported, such as the impact of drought or nanoparticles on resistance against pathogens.28,37 ABA and JA are plant hormones involved in many plant processes, including response to biotic (JA) and abiotic (ABA) stresses.38 The down-regulation of JA and ABA signaling pathways by 2,5-DCB and 4′-OH-2,5-DCB is therefore consistent with the repression of genes involved in response to a variety of stresses and it can be interpreted as a plant attempt to prioritize protection against toxic compounds. Genes down-regulated upon exposure to both compounds showed also enrichment in the molecular functions involved in transcription, including transcription co-repressor activity and transcription cofactor activity (Table 2). Inhibition of transcription is a common response to toxic stress and it is consistent with the observation that a larger number of genes were down-regulated than up-regulated upon exposure to 2,5-DCB and 4′-OH-2,5-DCB. A remarkable difference between the two compounds is that exposure to 2,5-DCB, but not 4′-OH-2,5-DCB, resulted in a large enrichment of up-regulated genes involved toxic response and detoxification reactions (see below).
Table 2.
Enrichment Analysis of Genes Up- and Down-regulated upon Exposure to 2,5-DCB and 4′-OH-2,5-DCB Based on Biological Processes and Molecular Functionsa
| response to 2,5-DCB
| |||||
|---|---|---|---|---|---|
| up-regulated genes
|
down-regulated genes
|
||||
| biological processes | enrichment | p value | biological processes | enrichment | p value |
| toxin catabolic process | 17.89 | 6.92 × 10−06 | regulation of jasmonic acid signaling | 12.18 | 5.13 × 10−03 |
| defense response to oomycetes | 17.68 | 3.22 × 10−03 | cold acclimation | 11.44 | 6.46 × 10−05 |
| response to toxic substance | 14.25 | 1.62 × 10−10 | response to wounding | 6.11 | 1.37 × 10−09 |
| response to bacterium | 4.15 | 2.54 × 10−03 | response to chitin | 5.98 | 1.35 × 10−04 |
| response to oxidative stress | 4.10 | 2.95 × 10−03 | response to water deprivation | 4.95 | 5.49 × 10−08 |
| response to inorganic | 2.97 | 6.14 × 10−03 | response to jasmonic acid | 4.18 | 2.66 × 10−03 |
| oxidation-reduction process | 2.88 | 1.39 × 10−06 | response to abscisic acid | 3.26 | 4.04 × 10−05 |
| hormone-mediated signaling | 2.80 | 3.38 × 10−05 | |||
| transcription, DNA-templated | 1.85 | 3.31 × 10−03 | |||
| regulation of transcription | 1.71 | 8.57 × 10−03 | |||
| molecular function | enrichment | p value | molecular function | enrichment | p value |
|---|---|---|---|---|---|
| glutathione transferase activity | 16.72 | 8.03 × 10−06 | transcription co-repressor activity | 11.71 | 4.32 × 10−03 |
| alkyl or aryl transferase activity | 8.22 | 8.06 × 10−04 | transcription factor activity | 1.90 | 3.92 × 10−03 |
| secondary transmembrane transporter activity | 5.32 | 5.42 × 10−05 | |||
| ion transmembrane transporter activity | 3.19 | 3.43 × 10−03 | |||
| oxidoreductase activity | 2.81 | 4.75 × 10−06 |
| response to 4′-OH-2,5-DCB | |||||
|---|---|---|---|---|---|
|
| |||||
| biological process | enrichment | p value | biological process | enrichment | p value |
| no significant enrichment | regulation of jasmonic acid signaling | 23.78 | 5.84 × 10−05 | ||
| cold acclimation | 20.12 | 2.53 × 10−06 | |||
| response to wounding | 10.10 | 3.54 × 10−12 | |||
| response to water deprivation | 9.68 | 5.75 × 10−15 | |||
| response to chitin | 7.79 | 1.99 × 10−03 | |||
| response to jasmonic acid | 6.72 | 8.27 × 10−05 | |||
| regulation of defense response | 5.97 | 9.51 × 10−04 | |||
| response to abscisic acid | 4.72 | 3.13 × 10−06 | |||
| cellular response to acid | 4.07 | 3.34 × 10−03 | |||
| response to salt stress | 3.74 | 5.31 × 10−03 | |||
| defense response | 2.55 | 3.58 × 10−04 | |||
| signal transduction | 2.29 | 2.45 × 10−03 | |||
| molecular function | enrichment | p value | molecular function | enrichment | p value |
|---|---|---|---|---|---|
| no significant enrichment | transcription co-repressor activity | 22.87 | 4.96 × 10−05 |
GO terms with enrichment p values of <0.01 are presented. Only the most-specific GO terms (lowest hierarchical level) are shown. GO terms of special interest are underlined.
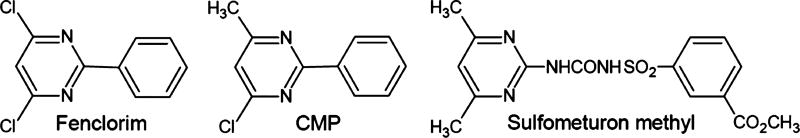
The transcriptomic profile of Arabidopsis exposed to 2,5-DCB and 4′-OH-2,5-DCB was then searched against the Arabidopsis expression microarray database, Genevestigator (http://genevestigator.com/gv/).39 The analysis was restricted to expression profiles recorded with wild-type A. thaliana and the Perturbation Category, Chemicals. The expression profile induced by 2,5-DCB (based on the 300 most-up-regulated genes), was most similar to the ones induced by various toxic organic and inorganic chemicals, including pesticides, metals, and ozone (Figure S5). A total of 5 out of the 10 expression profiles most similar to the one induced by 2,5-DCB, but not by 4′-OH-2,5-DCB, were associated with 5-(3,4-dichlorophenyl)furan-2-yl) (piperidin-1-yl)methanethione (DFPM), 4-chloro-6-methyl-2-phenylpyrimidine (CMP), fenclorim, and sulfomethuron methyl. Intriguingly, fenclorim, sulfomethuron methyl, and CMP are structurally related compounds involving a pyrimidine moiety with rather similar substitution patterns, suggesting that they act by binding the same receptor(s) (Figure 2). In addition, CMP and fenclorim are compounds known as plant safeners, which are nontoxic chemicals commonly applied to crops together with pesticides because of their protective effect on the plants.32,40 Plant safeners are thought to act through the induction of detoxification enzymes, known as xenobiotic response genes (XRGs). Best-characterized XRGs are the cytochrome P-450 monooxygenases, glucosyltransferases, glutathione S-transferases, and ABC transporter proteins.41 For instance, fenclorim was found to be a strong inducer of glutathione S-transferases in Arabidopsis.42 DFPM, although not structurally related to CMP, fenclorim, or sulfomethuron methyl, is a signaling molecule synthesized in plants in response to safeners, such as fenclorim and CMP.43 On the contrary, the 4′-OH-2,5-DCB expression profile was most similar to the ones induced by the herbicides, norflurazon and benzothiadiazole, and the phytotoxic antibiotic, sulfamethoxazole (data not presented).
Figure 2.
Chemical structure of fenclorim, 4-chloro-6-methyl-2-phenylpyrimidine (CMP), and sulfomethuron methyl.
Consistent with these observations, exposure to 2,5-DCB resulted in up-regulation (fold-change >2.0) of 38 genes encoding potential XRGs, and exposure to 4′-OH-2,5-DCB induced only 11 potential XRGs (Table S2). XRGs overexpressed in response to 2,5-DCB include genes possibly involved in the three phases of the “green liver model”,11 including initial activation, conjugation with plant molecules, and sequestration in plant organelles (e.g., vacuole) or structures (e.g., cell wall). Cytochrome P450 monooxygenases and peroxidases have been suggested to be involved in the initial hydroxylation of PCBs (Phase I)3 glutathione S-transferases and glycosyltransferases are known to be involved in the conjugation of several chlorinated xenobiotic compounds (Phase II),11 and ABC transporter proteins are known to be involved in vacuolar excretion of xenobiotic conjugates (Phase III).44 A prior study focusing on the transcriptomic response of Arabidopsis exposed to 2,2′,3,3′-TeCB similarly reported overexpression of genes potentially involved in the three phases of the green liver model, including cytochrome P-450 monooxygenases, UDP-glucuronosyltransferases, and transporter proteins.24 Based on the observations above, we hypothesize that the differentiated induction of detoxification enzymes in response to the parent PCB, 2,5-DCB, explains its lower toxicity as compared to its OH stmetabolite, 4′-OH-2,5-DCB.
It is noteworthy that our gene expression study only focused on a single PCB congener (out of 208) and one single mono-OH-PCB congener (out of 837). Other PCBs and OH-PCBs (even isomers) are likely to induce different plant responses and gene expression profiles. The goal of this study was to show that hydroxylation of a model PCB molecule, which is part of a detoxification sequence, could result in dramatic increase of toxicity, partially explained at the molecular level (for instance, by changing the interaction with specific receptors and subsequent expression of specific transcription factors). As mentioned earlier, coplanar, dioxin-like PCBs exhibit high toxicity toward mammals because of their defined stereochemistry, allowing the binding of the aryl hydrocarbon receptor (AHR).27 Similarly, selected para-OH PCBs exert higher endocrine disruption activity, as compared with ortho-or meta-OH isomers, because of their capability to specifically bind estrogen receptor.45 Many studies consider PCBs in mixtures because they have been released in the environment as complex commercial formulations (e.g., Aroclor). However, one must keep in mind that each PCB and OH-PCB congener is a distinct compound, with specific chemical and physical properties, potentially resulting in different biological activities and toxicities.
Further understanding of the metabolism of PCBs in plants, which may have important implications for human health and the environment, will require additional research to identify the xenobiotic receptors and transcription factors involved in the plant response to PCBs and their metabolites. Studying the transcriptomic profiles developing in response to various PCBs and their derivatives will help identify the structural elements necessary to activate specific receptors and trigger the detoxification sequence (i.e., comparative toxicogenomics).
Supplementary Material
Acknowledgments
This work was funded by the Iowa Superfund Basic Research Program, National Institute of Environmental Health Sciences, grant no. P42ES013661. The authors thank Hans-Joachim Lehmler (University of Iowa) for providing some PCBs and OH-PCBs used in this study and Sasha Eisenman (Temple University) for valuable discussion on plant hormones.
Footnotes
ASSOCIATED CONTENT
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.7b01538.
Figures showing dose–response curves of A. thaliana, hierarchical clustering of gene expression profiles, and comparisons between gene expression levels and profiles. Tables showing a list of primers used in RT-qPCR and expression levels of potential xenobiotic response genes (XRGs) in A. thaliana exposed to 2,5-DCB and 4′-OH-2,5-DCB. (PDF)
The authors declare no competing financial interest.
References
- 1.Tehrani R, Van Aken B. Hydroxylated polychlorinated biphenyls in the environment: sources, fate, and toxicities. Environ. Sci. Pollut. Res. 2014;21:6334–6345. doi: 10.1007/s11356-013-1742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckman A, Wong C, Chow E, Brown S, Solomon K, Fisk A. Biotransformation of polychlorinated biphenyls (PCBs) and bioformation of hydroxylated PCBs in fish. Aquat. Toxicol. 2006;78:176–185. doi: 10.1016/j.aquatox.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 3.Chroma L, Moeder M, Kucerova P, Macek T, Mackova M. Plant enzymes in metabolism of polychlorinated biphenyls. Fresen. Environ. Bull. 2003;12:291–295. [Google Scholar]
- 4.Grimm F, Hu D, Kania-Korwel I, Lehmler H, Ludewig G, Hornbuckle K, Duffel M, Bergman A, Robertson L. Metabolism and metabolites of polychlorinated biphenyls. Crit. Rev. Toxicol. 2015;45:245–272. doi: 10.3109/10408444.2014.999365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson P, Hites R. OH radical reactions: The major removal pathway for polychlorinated biphenyls from the atmosphere. Environ. Sci. Technol. 1996;30:1756–1763. [Google Scholar]
- 6.Marek R, Martinez A, Hornbuckle K. Discovery of Hydroxylated Polychlorinated Biphenyls (OH-PCBs) in Sediment from a Lake Michigan Waterway and Original Commercial Aroclors. Environ. Sci. Technol. 2013;47:8204–8210. doi: 10.1021/es402323c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawano M, Hasegawa J, Enomoto T, Onishi H, Nishio Y, Matsuda M, Wakimoto T. Hydroxylated polychlorinated biphenyls (OH-PCBs): Recent advances in wildlife contamination study. Environ. Sci. 2005;12:315–324. [PubMed] [Google Scholar]
- 8.Awad A, Martinez A, Marek R, Hornbuckle K. Occurrence and Distribution of Two Hydroxylated Polychlorinated Biphenyl Congeners in Chicago Air. Environ. Sci. Technol. Lett. 2016;3:47–51. doi: 10.1021/acs.estlett.5b00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montano M, Gutleb A, Murk A. Persistent toxic burdens of halogenated phenolic compounds in humans and wildlife. Environ. Sci. Technol. 2013;47:6071–6081. doi: 10.1021/es400478k. [DOI] [PubMed] [Google Scholar]
- 10.Bhalla R, Tehrani R, Van Aken B. Toxicity of hydroxylated polychlorinated biphenyls (HO-PCBs) using the bioluminescent assay Microtox®. Ecotoxicology. 2016;25:1438–1444. doi: 10.1007/s10646-016-1693-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandermann H., Jr Higher plant metabolism of xenobiotics: The ”green liver” concept. Pharmacogenetics. 1994;4:225–241. doi: 10.1097/00008571-199410000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Schnoor JL, Licht LA, McCutcheon SC, Wolfe NL, Carreira LH. Phytoremediation of organic and nutrient contaminants. Environ. Sci. Technol. 1995;29:318A–323A. doi: 10.1021/es00007a747. [DOI] [PubMed] [Google Scholar]
- 13.Van Aken B, Correa P, Schnoor J. Phytoremediation of Polychlorinated Biphenyls: New Trends and Promises. Environ. Sci. Technol. 2010;44:2767–2776. doi: 10.1021/es902514d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeeb BA, Amphlett JS, Rutter A, Reimer KJ. Potential for phytoremediation of polychlorinated biphenyl-(PCB-)-contaminated soil. Int. J. Phytorem. 2006;8:199–221. doi: 10.1080/15226510600846749. [DOI] [PubMed] [Google Scholar]
- 15.Liu JY, Schnoor JL. Uptake and translocation of lesser-chlorinated polychlorinated biphenyls (PCBs) in whole hybrid poplar plants after hydroponic exposure. Chemosphere. 2008;73:1608–1616. doi: 10.1016/j.chemosphere.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aslund MLW, Rutter A, Reimer KJ, Zeeb BA. The effects of repeated planting, planting density, and specific transfer pathways on PCB uptake by Cucurbita pepo grown in field conditions. Sci. Total Environ. 2008;405:14–25. doi: 10.1016/j.scitotenv.2008.07.066. [DOI] [PubMed] [Google Scholar]
- 17.Lee I, Fletcher JS. Involvement of mixed-function oxidase systems in polychlorinated biphenyl metabolism by plant-cells. Plant Cell Rep. 1992;11:97–100. doi: 10.1007/BF00235262. [DOI] [PubMed] [Google Scholar]
- 18.Kucerova P, Mackova M, Chroma L, Burkhard J, Triska J, Demnerova K, Macek T. Metabolism of polychlorinated biphenyls by Solanum nigrum hairy root clone SNC-90 and analysis of transformation products. Plant Soil. 2000;225:109–115. [Google Scholar]
- 19.Weber J, Mrozek E. Polychlorinated biphenyls - phytotoxicity, absorption and translocation by plants, and inactivation by activated carbon. Bull. Environ. Contam. Toxicol. 1979;23:412–417. doi: 10.1007/BF01769980. [DOI] [PubMed] [Google Scholar]
- 20.Mahanty HK. Polychlorinated biphenyls: Accumulation and effects upon plants. In: Waid JS, editor. PCBs and the Environment. Vol. 2. CRC Press; Boca Raton, FL: 1986. pp. 1–8. [Google Scholar]
- 21.Kaveh R, Li YS, Ranjbar S, Tehrani R, Brueck CL, Van Aken B. Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ. Sci. Technol. 2013;47:10637–10644. doi: 10.1021/es402209w. [DOI] [PubMed] [Google Scholar]
- 22.Organisation for Economic Co-operation and Development–OECD/OCDE. Lemna sp. Growth Inhibition Test: OECD Guidelines for the Testing of Chemicals. OECD; Paris, France: 2006. [Google Scholar]
- 23.Schwarzenbach RP, Gschwend PM, Imboden DM. Environmental Organic Chemistry. 3. John Wiley & Sons; Hoboken, NJ: 2016. [Google Scholar]
- 24.Jin X-F, Shuai J-J, Peng R-H, Zhu B, Fu X-Y, Tian Y-S, Zhao W, Han H-J, Chen C, Xu J, Yao Q-H, Qu S-C, Xiong A-S. Identification of candidate genes involved in responses of Arabidopsis to polychlorinated biphenyls based on microarray analysis. Plant Growth Regul. 2011;65:127–135. [Google Scholar]
- 25.Wang XM, Teng Y, Zhang N, Christie P, Li ZC, Luo YM, Wang J. Rhizobial symbiosis alleviates polychlorinated biphenyls-induced systematic oxidative stress via brassinosteroids signaling in alfalfa. Sci. Total Environ. 2017;592:68–77. doi: 10.1016/j.scitotenv.2017.03.066. [DOI] [PubMed] [Google Scholar]
- 26.Ahammed G, Ruan Y, Zhou J, Xia X, Shi K, Zhou Y, Yu J. Brassinosteroid alleviates polychlorinated biphenyls-induced oxidative stress by enhancing antioxidant enzymes activity in tomato. Chemosphere. 2013;90:2645–2653. doi: 10.1016/j.chemosphere.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 27.Hahn M. The aryl hydrocarbon receptor: A comparative perspective. Comp. Biochem. Physiol, Part C: Pharmacol., Toxicol. Endocrinol. 1998;121:23–53. doi: 10.1016/s0742-8413(98)10028-2. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Sanchez S, Bernales I, Cristobal S. Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling. BMC Genomics. 2015;16:341. doi: 10.1186/s12864-015-1530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balasubramaniyam A. Hormetic dose response as the paradigm of plant response to stress. Int. J. Plant Biol. Res. 2015;3:1037. [Google Scholar]
- 30.Camara B, Herrera C, Gonzalez M, Couve E, Hofer B, Seeger M. From PCBs to highly toxic metabolites by the biphenyl pathway. Environ. Microbiol. 2004;6:842–850. doi: 10.1111/j.1462-2920.2004.00630.x. [DOI] [PubMed] [Google Scholar]
- 31.Carbon S, Ireland A, Mungall CJ, Shu S, Marshall B, Lewis S, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JG, Richardson JE, Ringwald M, Rubin GM, Sherlock G AmiGO: Online access to ontology and annotation data. AmiGO Hub, Web Presence Working Group. AmiGO: Online access to ontology and annotation data. Bioinformatics. 2009;25:288–9. doi: 10.1093/bio-informatics/btn615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skipsey M, Knight K, Brazier-Hicks M, Dixon D, Steel P, Edwards R. Xenobiotic responsiveness of Arabidopsis thaliana to a chemical series derived from a herbicide safener. J. Biol. Chem. 2011;286:32268–32276. doi: 10.1074/jbc.M111.252726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramel F, Sulmon G, Serra A, Gouesbet G, Couee I. Xenobiotic sensing and signalling in higher plants. J. Exp. Bot. 2012;63:3999–4014. doi: 10.1093/jxb/ers102. [DOI] [PubMed] [Google Scholar]
- 34.Singh K, Foley R, Onate-Sanchez L. Transcription factors in plant defense and stress responses. Curr. Opin. Plant Biol. 2002;5:430–436. doi: 10.1016/s1369-5266(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 35.Jiang R, Tyler B, VanAlfen N, Leach J, Lindow S. Mechanisms and evolution of virulence in oomycetes. Annu. Rev. Phytopathol. 2012;50:295–318. doi: 10.1146/annurev-phyto-081211-172912. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen D, Rieu I, Mariani G, van Dam N. How plants handle multiple stresses: Hormonal interactions underlying responses to abiotic stress and insect herbivory. Plant Mol. Biol. 2016;91:727–740. doi: 10.1007/s11103-016-0481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramegowda V, Senthil-Kumar M. The interactive effects of simultaneous biotic and abiotic stresses on plants: Mechanistic understanding from drought and pathogen combination. J. Plant Physiol. 2015;176:47–54. doi: 10.1016/j.jplph.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki N, Shinozaki K. Crosstalk between abiotic and biotic stress responses: A current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 2006;9:436–442. doi: 10.1016/j.pbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P. Genevestigator v3: A reference expression database for the meta-analysis of transcriptomes. Adv. Bioinf. 2008;2008:420747. doi: 10.1155/2008/420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riechers DE, Kreuz K, Zhang Q. Detoxification without Intoxication: herbicide safeners activate plant defense gene expression. Plant Physiol. 2010;153:3–13. doi: 10.1104/pp.110.153601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Behringer G, Bartsch K, Schaller A. Safeners recruit multiple signalling pathways for the orchestrated induction of the cellular xenobiotic detoxification machinery in Arabidopsis. Plant, Cell Environ. 2011;34:1970–1985. doi: 10.1111/j.1365-3040.2011.02392.x. [DOI] [PubMed] [Google Scholar]
- 42.DeRidder B, Dixon D, Beussman D, Edwards R, Goldsbrough P. Induction of glutathione S-transferases in Arabidopsis by herbicide safeners. Plant Physiol. 2002;130:1497–1505. doi: 10.1104/pp.010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rigal A, Ma Q, Robert S. Unraveling plant hormone signaling through the use of small molecules. Front. Plant Sci. 2014;5:373. doi: 10.3389/fpls.2014.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tommasini R, Vogt E, Fromenteau M, Hortensteiner S, Matile P, Amrhein N, Martinoia E. An ABC-transporter of Arabidopsis thaliana has both glutathione-conjugate and chlorophyll catabolite transport activity. Plant J. 1998;13:773–780. doi: 10.1046/j.1365-313x.1998.00076.x. [DOI] [PubMed] [Google Scholar]
- 45.Arulmozhiraja S, Shiraishi F, Okumura T, Iida M, Takigami H, Edmonds J, Morita M. Structural requirements for the interaction of 91 hydroxylated polychlorinated biphenyls with estrogen and thyroid hormone receptors. Toxicol. Sci. 2005;84:49–62. doi: 10.1093/toxsci/kfi063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.