Abstract
Acclimation of foliage to growth temperature involves both structural and physiological modifications, but the relative importance of these two mechanisms of acclimation is poorly known, especially for isoprene emission responses. We grew hybrid aspen (Populus tremula x P. tremuloides) under control (day/night temperature of 25/20 °C) and high temperature conditions (35/27 °C) to gain insight into the structural and physiological acclimation controls. Growth at high temperature resulted in larger and thinner leaves with smaller and more densely packed chloroplasts and with lower leaf dry mass per area (MA). High growth temperature also led to lower photosynthetic and respiration rates, isoprene emission rate and leaf pigment content and isoprene substrate dimethylallyl diphosphate pool size per unit area, but to greater stomatal conductance. However, the declining characteristics were similar when expressed per unit dry mass, indicating that the area-based differences were primarily driven by MA. Acclimation to high temperature further increased heat stability of photosynthesis, and increased activation energies for isoprene emission and isoprene synthase rate constant. This study demonstrates that temperature acclimation of photosynthetic and isoprene emission characteristics per unit leaf area was primarily driven by structural modifications, and we argue that future studies investigating acclimation to growth temperature must consider structural modifications.
Keywords: dimethylallyl diphosphate, isoprene emission, isoprene synthase, structural controls, photosynthesis rate, physiological controls, temperature acclimation
Introduction
Photosynthesis and respiration are considered the primary biological components regulating the biosphere-atmosphere carbon exchange (Ciais et al. 2001; Mahecha et al. 2010). However, there is a large and commonly ignored flux of biogenic volatile organic compounds (BVOC) generated by pathways of secondary metabolism that can significantly contribute to the carbon balance of the biosphere (Arneth et al. 2008; Guenther et al. 2012; Ashworth et al. 2013). Among BVOC, a central role is played by isoprene that has the greatest worldwide flux attributable to a single volatile compound (Guenther et al. 1993; Li & Sharkey 2013).
Both the primary and the secondary plant metabolism are strongly dependent on temperature, but the temperature sensitivities of different processes differ among plant species, and they often vary with growth conditions (Way & Oren 2010; Centritto et al. 2011; Smith & Dukes 2013; Way & Yamori 2014). In particular, acclimation to growth temperature can importantly alter the shape of the temperature responses of respiration and photosynthesis (Berry & Björkman 1980; Hopkins 1998), and isoprene emission (Wiberley et al. 2008). Although acclimation of physiological processes to long-term temperature is a well-known phenomenon, the degree of acclimation strongly varies among plant species and for different processes for reasons not yet fully understood (Berry & Björkman 1980; Tjoelker et al. 1999; Bunce 2000; Yamasaki et al. 2002; Loveys et al. 2003; Fares et al. 2011). Understanding such acclimation responses and mechanisms of acclimation are important in predicting plant responses to seasonal and year-to-year differences in temperature environments as well as the responses to future climate (Smith & Dukes 2013).
Acclimation of photosynthesis to high temperature is characteristically associated with shifts of the optimum temperature of photosynthesis to higher temperatures (Berry & Björkman 1980; Turnbull et al. 2002; Sage & Kubien 2007). Improved heat stability of photosynthesis is typically associated with changes in the stability of thylakoid membranes as the result of increases in the degree of membrane lipid saturation (Berry & Björkman 1980; Björkman et al. 1980; Devos et al. 1998; Sharkey et al. 2001). Given that the temperature responses of Rubisco and photosynthetic electron transport have different temperature sensitivities, temperature acclimation can also result from changes in the partitioning of rate-limiting photosynthetic proteins among the partial determinants of photosynthesis (Hikosaka 1997; Hikosaka et al. 1999). In addition, growth temperature driven changes in the overall content of rate-limiting components of photosynthesis machinery can result in changes in maximum photosynthetic rates (Björkman et al. 1980; Stitt & Hurry 2002; Yamori et al. 2005; Sage & Kubien 2007).
In isoprene-emitting species, heat-stability can be rapidly altered by isoprene emission that is thought to dissolve in photosynthetic membranes and stabilize membrane lipids (Singsaas & Sharkey 2000; Sharkey & Yeh 2001; Velikova et al. 2011; Velikova et al. 2012) as well as serve as a lipid-soluble antioxidant detoxifying heat stress generated reactive oxygen species and peroxidized membrane lipids (Vickers et al. 2009; Velikova et al. 2012; Possell & Loreto 2013). Thus, isoprene is considered particularly advantageous in responding to rapid increases in leaf temperature such as those occurring during lightflecks (Behnke et al. 2007; Way et al. 2011; Behnke et al. 2013).
Isoprene emission capacity can adapt to temperature environment, typically increasing after changes in leaf temperature environment (Sharkey et al. 1999; Funk et al. 2003; Wiberley et al. 2008) as the result of enhanced expression of isoprene synthase gene and genes of the chloroplastic 2-C-methyl-D-erythritol 4-phosphate pathway responsible for isoprenoid synthesis in chloroplasts (Wiberley et al. 2008; Wiberley et al. 2009; Vickers et al. 2010). However, most work on isoprene emission responses to temperature environment stems from transfer experiments where plants developed at a certain temperature have been transferred to the new temperature environment. The effect of plant growth at different temperature environments per see has been investigated only in a few studies (Wiberley et al. 2008; Hartikainen et al. 2009; Fares et al. 2011) with contrasting results showing either enhanced (Wiberley et al. 2008), similar (Monson et al. 1992; Hartikainen et al. 2009; Centritto et al. 2011; Fares et al. 2011) or reduced emissions (Hartikainen et al. 2009) in plants grown under the higher temperature.
Although most work on temperature acclimation has focused on physiological responses, growth at different temperatures can also importantly modify leaf structure, whole plant leaf area and whole plant sink-source relationships (Starck et al. 1993; Morcuende et al. 1996; Hartikainen et al. 2009; Muhl et al. 2011; Xu et al. 2012). Structural modifications such as changes in chloroplast number, cell size and cell number per unit leaf area are relevant as they can change the amount of rate-limiting enzymes per unit leaf area and result in changes in the process rate per unit leaf area at any given temperature without concomitant changes in physiological potentials of single cells (Niinemets & Sack 2006; Niinemets et al. 2009; Poorter et al. 2009). It has been demonstrated that growth temperature dependent changes in respiratory (Ow et al. 2008) and photosynthetic activities (Yamasaki et al. 2002; Yamori et al. 2005; Hartikainen et al. 2009; Ibrahim et al. 2010) were importantly affected by modifications in leaf dry mass per unit area, which connects area and mass based process rates (Poorter et al. 2009). However, the role of growth temperature driven structural modifications in acclimation of photosynthesis and isoprene emission has not been routinely studied.
The effects of growth temperature on isoprene emission vs. temperature response relationships also vary among the studies with responses ranging from improved thermal sensitivity (Monson et al. 1992) to minor changes or even reduced thermal sensitivity (Fares et al. 2011). Given that the temperature response of isoprene emission is driven by both the temperature effects on isoprene synthase activity and its immediate substrate dimethylallyl diphosphate pool size (Rasulov et al. 2010), gaining insight into such contrasting responses requires information of growth temperature effects on both partial controls of isoprene emission. As isoprene emission and photosynthetic carbon metabolism are strongly linked (Li & Sharkey 2013), improved heat-resistance of leaves grown at higher temperature can result in maintenance of greater DMADP pools at higher temperature, thereby improving the isoprene emission at higher temperatures.
Here we studied the effects of growth temperature on foliage photosynthetic and structural characteristics and isoprene emission in hybrid aspen (Populus tremula x P. tremuloides) with the specific aim to separate the structural and physiological controls on growth temperature acclimation. A recently developed in vivo method for estimation of DMADP pool size and determination of isoprene synthase rate constant and in vivo isoprene synthase kinetic characteristics (Rasulov et al. 2009a; Rasulov et al. 2011) was used to separate the substrate and enzyme level controls on isoprene emission under different conditions. Our results indicate that growth temperature did modify the heat resistance of photosynthesis, and altered the temperature sensitivity of photosynthesis and isoprene emission rate, but the differences in foliage photosynthesis and isoprene emission rates per unit area were primarily driven by structural changes.
Material and methods
Seedlings of hybrid aspen (Populus tremula L. x P. tremuloides Michx.) clone H200 (Rasulov et al. 2009a; Rasulov et al. 2009b; Rasulov et al. 2011) were used in the experiments. Dormant seedlings kept in a cold storage were planted in 4 L plastic pots filled with a mixture of peat and sand (1:1) and maintained at room temperature of 22 °C until dormancy was broken in 2-3 days. Thereafter, the seedlings were moved to Percival AR-95 HIL growth chambers (CLF PlantClimatics) with contrasting temperature regimes (day/night) of 25/20 °C (lower temperature, control) and 35/27 °C (higher temperature). The ambient CO2 concentration was between 380-420 μmol mol-1, the relative humidity was maintained between 60-65% (corresponding to moderate differences in daytime water vapor pressure deficit of ca. 1.1 kPa for the control and 1.9 kPa for the high temperature treatment) and the quantum flux density of 500 μmol m-2 s-1 was provided for 14 h light period. The plants were watered daily to field capacity with the Hoagland nutrient solution for optimum water and nutrient supply. All measurements were conducted with fully-expanded 20-24 days old leaves exhibiting peak physiological activity.
Measurements of gas-exchange rates and isoprene emission
An ultra-fast gas-exchange system (system half-time of approximately 0.15 s) was used for the measurements (Laisk et al. 2002; Rasulov et al. 2010). Briefly, the leaf is enclosed in a clip-on type leaf chamber with a volume of volume 2.4 cm3 and a cross-sectional area of 8.04 cm2. The leaf chamber is thermostatted, and the upper leaf surface is glued to the chamber glass window by a starch paste. Direct measurements by thin thermocouples and energy balance calculations indicated that leaf temperature was maintained within 0.5°C of the leaf chamber water jacket. The system has two identical gas lines where different gas concentrations can be prepared and almost instantaneously, in less than 1 s, switched between the sample and the reference line. Such a setup is particularly appropriate for fast measurement of transient physiological responses, avoiding delays in the detection of leaf gas-exchange response signals due to stabilization of gas concentrations. The enclosed leaf area was illuminated with a Schott KL 1500 light source equipped with a heat-reflecting filter (Optical Coating Laboratory, CA, USA). The air was mixed from pure nitrogen, oxygen, and CO2 using calibrated dynamic mixers, and the air flow rate through the system was kept at 0.5 mmol s-1 (Laisk et al. 2002). A LI-6251 infrared gas analyzer (LI-Cor, Inc., Lincoln, Nebraska, USA) was used to measure the CO2 concentration, a custom-made micropsychrometer was used for the water vapor concentration, and a proton transfer reaction mass spectrometer (PTR-MS, high-sensitivity version; Ionicon Analytik GmbH, Innsbruck, Austria) for the isoprene concentration. The response time of the PTR-MS was approximately 0.1 s and the isoprene detection limit about 10 pmol mol-1. The instrument was calibrated with an isoprene standard mixture of 5.74 μmol mol-1 isoprene in N2 (Hills Scientific, Boulder, Colorado, USA).
The standard conditions used in these experiments were the leaf chamber CO2 concentration of 360 μmol mol-1, O2 concentration of 21%, leaf temperature of 30 °C, light intensity (photosynthetic quantum flux density) of 650 μmol m-2 s-1, and water vapor pressure deficit between the leaf and the atmosphere (VD) of 1.7 kPa. The measurements were conducted with attached leaves, and the protocol for all measurements included leaf enclosure, leaf stabilization under the standard conditions until the steady-state rates of CO2 and H2O vapor exchange and isoprene emission were established, and then changing either CO2 (360 vs. 1200 μmol mol-1), O2 (21 vs. 2%), light (quantum yield measurements) or temperature (temperature response curves) to measure different environmental responses. For the temperature response curves, leaf temperature was changed in steps of 5 °C, and each temperature change was followed by returning to the standard temperature and leaf stabilization as in Rasulov et al. (2010). Chamber air water vapor concentration was adjusted at each temperature to maintain the constant VD and avoid changes in stomatal conductance due to altered VD.
The quantum yields for CO2 exchange (αCO2) and isoprene emission (αI) were measured over the incident light range of 20 - 75 μmol m-2 s-1 where the influence of the Kok effect is negligible. In addition, the initial slopes of the net assimilation vs. CO2 response curves over an ambient CO2 range of 0-100 μmol mol-1 at both O2 concentrations of 21% and 2% were also measured at the standard light intensity of 650 μmol m-2 s-1.
All leaf gas-exchange characteristics were calculated according to von Caemmerer and Farquhar (1981). The maximum carboxylase activity or Rubisco (Vcmax) was estimated from the initial slopes of the CO2 response curve at both O2 concentrations and the capacity for photosynthetic electron transport (Jmax) from the high-CO2 concentration (1200 μmol mol-1) measurements according to Niinemets et al. (1999b).
Measurements of chlorophyll fluorescence
A PAM 101 fluorimeter (Walz GmbH, Effeltrich, Germany) was used to measure chlorophyll fluorescence characteristics simultaneously with CO2 and water vapor exchange. A Schott KL 1500 light source provided saturated pulses of white light of 14000 μmol m-2 s-1 for 2 s to measure either the dark-adapted (Fm) or light-adapted (Fm’) maximum fluorescence yields, while another Schott KL 1500 with a 720-nm narrow-pass interference filter (Andover Corp., Salem, NH, USA) was used for far-red light to estimate the dark-adapted minimum fluorescence yield (F0). The fluorimeter operation frequency was 1.6 kHz for the darkened leaves and 100 kHz for the illuminated leaves and during saturated pulses. Fm and F0 were typically measured 20 min. after darkening. The dark-adapted PSII quantum yield was computed as (Fm – F0)/Fm and the quantum yield of illuminated leaves, ΦPSII, as (Fm’ - F)/Fm’, where F is the steady-state fluorescence yield. The rate of photosynthetic electron transport from chlorophyll fluorescence (JETR) was further calculated as (Schreiber et al. 1994):
| (1) |
where Q is the incident quantum flux density, ξ is the leaf absorptance (0.86 for the control and 0.81 for the high temperature grown plants) and β is the fraction of absorbed light used to drive PSII electron transport (taken as 0.5 in this study).
Foliar structural measurements and determination of chlorophylls and carotenoids
Immediately after the gas-exchange measurements, leaf discs of 4 cm2 were taken between the major leaf veins for estimation of leaf fresh and dry mass. For anatomical measurements, leaf samples were taken with a razor blade between the major veins from the central part of the leaves and cut into ca. 1 x 1 mm pieces for fixation.
The fresh mass of the leaf discs was estimated immediately, and the dry mass after oven-drying at 70 °C for at least 48 h. Additional leaf discs for foliar pigment analyses were frozen at -80 °C until the analysis. The frozen discs were pulverized in liquid N2 and the pigments were extracted with 80% ice-cold acetone with some CaCO3. The absorptances of the pigment solutions were measured with a dual-beam Shimadzu UV2550PC spectrophotometer at the wavelengths of 663.2, 646.8 and 470 nm, and the pigment contents were calculated according to the equations of Lichtenthaler and Buschmann (2001).
Anatomical measurements
Preparation of foliar samples for light and transmission electron microscopy (TEM) follows the protocol of Copolovici et al. (2011). Briefly, the samples were fixed by infiltrating with buffered (0.2 M phosphate buffer, pH = 7.2) 2% glutaric aldehyde, dehydrated in ethanol series and embedded in Epoxy Embedding Medium (Sigma-Aldrich Chemie, Steinheim, Germany). Semi-thin (1.5–2 μm) cross- and paradermal sections and ultrathin (70–90 nm) cross-sections were cut with a Leica EM UC7 ultramicrotome (Leica Mikrosysteme GmbH, Austria, Vienna) equipped with a 45° diamond knife (Diatome, Hatfield, PA, USA).
The semi-thin sections were stained for 30 s with Toluidine Blue as described in Burns (1978) and Mercer (1963), viewed at 400-1000x magnification with a Zeiss Axioplan 2 microscope and the digital images were taken with an AxioCam HRc (Carl Zeiss Microscopy, Jena, Germany). Five cross-sections per leaf sample were measured in three locations for total leaf thickness, palisade mesophyll and spongy mesophyll thickness, and chloroplasts and cells were counted in these sections. On the same sections, leaf thickness, palisade mesophyll and spongy mesophyll thickness were measured. In addition, chloroplast and cell numbers were also estimated from 10 paradermal sections per sample, 5 cut through the palisade and 5 through the spongy mesophyll. From these measurements, the chloroplast number per leaf area (nchl) was calculated as:
| (2) |
where nc,p is the number of cells in the palisade and nc,s that in the spongy parenchyma per unit leaf area, nchl,p,p is the chloroplast number per cell in the palisade parenchyma estimated from the paradermal section and nchl,p,c that estimated from the cross-section, and nchl,s,p is the chloroplast number in the spongy parenchyma estimated from the paradermal section and nchl,s,c that estimated from the cross-section.
Ultrathin sections for TEM were contrasted with uranyl acetate and lead citrate (Copolovici et al. 2011 for details) and samples were viewed with a Philips Tecnai 10 TEM (FEI, Eindhoven, Netherlands) with an accelerating voltage of 80 kV. Digital images were taken with Olympus Veleta 2K x 2K sidemounted TEM CCD camera (Olympus Soft Imaging Solutions GmbH, Germany). Magnifications of 7000–10000x were used to measure chloroplast length, thickness and area (ca. 40 chloroplasts were measured in each case). All anatomical characteristics were separately measured for palisade and spongy mesophyll. Digital image analysis was carried out with UTHSCSA Image Tool for Windows 3.00 (UTHSCSA, Texas, USA).
Determination of the dimethylallyl diphosphate (DMADP) pool size (CDMADP)
The postillumination method of Rasulov et al. (2009a; 2010) was used to measure the pool size of the immediate precursor pool responsible for the isoprene emission in the steady state. The method is based on the assumption that postillumination isoprene release continues at the expense of the available precursor pool synthesized prior to leaf darkening (Rasulov et al. 2009a; Rasulov et al. 2010; Li et al. 2011). Thus, the size of this pool (CDMADP) was determined by integrating the fast, 200–400 s after darkening, dark decay of isoprene release (Rasulov et al. 2009a; Rasulov et al. 2010).
In addition to DMADP, the isoprene precursor, isopentenyl diphosphate (IDP) can be converted in the dark to DMADP by IDP isomerase and thus, contribute to the dark release of isoprene (Li et al. 2011). Although there is a variation in chloroplastic IDP/DMADP ratios among studies (Ramos-Valdivia et al. 1997; Zhou et al. 2013) likely indicating differences in the activity of chloroplastic IDP isomerase (Brüggemann & Schnitzler 2002), studies are in agreement that IDP/DMADP ratios are small. Nevertheless, even for relatively high IDP/DMADP ratios of ca. 0.5 (Weise et al. 2013), the method of Rasulov (2009a; 2010) has provided DMADP pool size estimates in excellent agreement with destructive chemical estimations (Weise et al. 2013).
Estimation of the rate constant, the maximum rate (Vmax) and Michaelis-Menten constant (Km) for isoprene synthase
The DMADP pool size (CDMADP) decreases after darkening due to DMADP conversion into isoprene according to a first-order reaction kinetics under strongly substrate-limited conditions (Loreto et al. 2004; Rasulov et al. 2009a; Rasulov et al. 2009b). Given further that the isoprene synthase activation state does not vary during the light-dark transient (as confirmed by the rapid rise of isoprene emission upon illumination of the darkened leaves), isoprene emission rate at any given time t after darkening, I(t), is driven by the CDMADP pool size that has remained at this time, CDMADP(t) (the integral of isoprene emission rate from a given time t until cessation of isoprene emission). Thus, paired values of I(t) vs. CDMADP(t) were derived for the entire dark decay and the linear parts of this relationships were used to estimate the rate constant of isoprene synthase (k, s-1) as in our previous studies (Rasulov et al. 2010; Sun et al. 2012b; Rasulov et al. 2014).
In vivo Vmax and Km for isoprene synthase were estimated from the Hanes-Wolff plots (CDMADP(t)/I(t) vs. CDMADP(t)) using the full dark decay of isoprene emission. The values of both characteristics were expressed per unit leaf area and dry mass.
Data analyses
Considering that fixation of 6 molecules of CO2 is needed for the formation of one molecule of isoprene (e.g., Niinemets et al. 1999c), the fraction of carbon lost due to isoprene emission was estimated as 6I/A, where I is the isoprene emission rate and A the net assimilation rate.
The temperature responses of net assimilation rate, photosynthetic electron transport rate, and isoprene emission characteristics (trait pi) were fitted by an Arrhenius-type temperature response function with an optimum:
| (3) |
where ci is the scaling constant for the trait i, ΔHa,i (J mol–1) is the activation energy, ΔHd,i (J mol–1) is the deactivation energy, ΔSi (J mol–1 K–1) is the entropy term, T (K) is the leaf temperature, and R (8.314 J mol–1 K–1) is the gas constant. The temperature optimum for the trait i, Topt,i, (Niinemets et al. 1999a) was further calculated as:
| (4) |
Dark respiration and stomatal conductance vs. temperature response curves that did not exhibit an optimum were fitted by exponential relationships in the form:
| (5) |
Note that the traditional estimate of temperature responses of chemical and biological processes, Q10, the reaction rate at temperature T+10 relative to the rate at temperature T is given as.
| (6) |
The Q10 concept assumes that the rate of the exponential increase (slope) of the process rate with increasing temperature is independent of temperature. Eq. 5 does not include such an assumption and is thus more flexible than the traditional Q10 analysis. Nevertheless, for rapid comparisons with other reported data, we also calculated the Q10 values. Treatment means for all traits (n = 4-32) were compared by analyses of variance (ANOVA) using Statistica 6.0 (StatSoft, Inc., Tulsa, OK, USA) and the growth temperature effects were considered significant at P < 0.05.
Results
Growth temperature effects on foliage structural characteristics
Increases in growth temperature were associated with about 1.2-fold larger and about 1.4-fold thinner leaves (Fig. 1, Table 1) with 1.6-fold lower leaf dry mass per unit area (MA, Table 1). Decreases in leaf thickness primarily resulted from decreases in the thickness of palisade (1.6-fold) and to a smaller degree in spongy mesophyll thickness (1.3-fold, Fig. 1a, b, Table 1). The ratio of palisade to spongy mesophyll thickness also decreased from an average (± SE) of 1.60 ± 0.08 to 1.22 ± 0.06 with increasing growth temperature (P < 0.001 for the treatment effect). However, leaf density, the ratio of MA to leaf thickness was similar among the treatments (0.34 g cm-3 for the control and 0.30 g cm-3 for plants grown at the higher temperature).
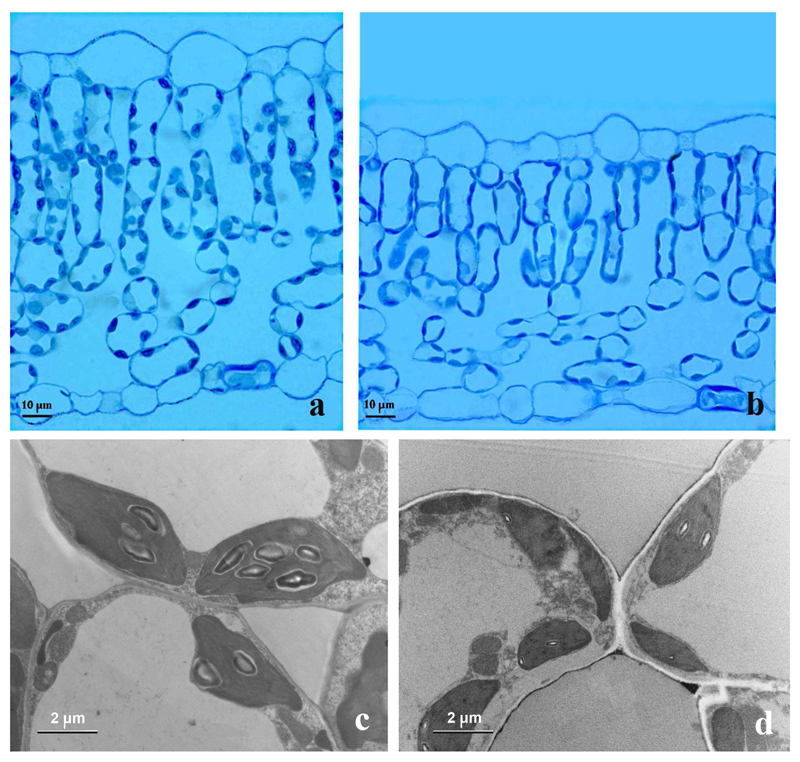
Fig. 1.
Representative cross-sections of hybrid aspen (Populus tremula X P. tremuloides, clone H200) leaves (a, b) and chloroplasts (c, d) developed under control (day/night temperatures of 25/20 °C) and high temperature (35/27 °C) conditions.
Table 1.
Leaf structural characteristics and pigment contents (mean ± SE) in hybrid aspen (P. tremula x P. tremuloides, clone H200) grown at contrasting temperatures
| Characteristic | Growth temperature (day/night) | |
|---|---|---|
| 25/20 ºC | 35/27 ºC | |
| Leaf area (cm2) | 67.5 ± 2.4a | 82.3 ± 2.7b |
| Leaf dry mass per unit area (g m-2) | 47.6 ± 1.7a | 30.5 ± 1.1b |
| Leaf thickness (μm) | 133.9 ± 3.3a | 93.5 ± 2.8b |
| Palisade mesophyll thickness (μm) | 71.5 ± 3.1a | 40.9 ± 2.6b |
| Spongy mesophyll thickness (μm) | 44.3 ± 1.0a | 34.4 ± 0.8b |
| Chloroplast number per cm2 leaf area (palisade) | (38.8 ± 2.2).106a | (29.3 ± 1.9).106a |
| Chloroplast number per cm2 leaf area (spongy) | (7.5 ± 0.5).106a | (7.1 ± 0.3).106a |
| Total chloroplast number per cm2 leaf area (palisade+spongy) | (46.3 ± 3.7).106a | (36.4 ± 2.3).106a |
| Chloroplast length (μm) | 4.41 ± 0.17a | 4.14 ± 0.18a |
| Chloroplast width (μm) | 1.99 ± 0.09a | 1.52 ± 0.11b |
| Chloroplast area (μm2) | 7.23 ± 0.49a | 4.99 ± 0.51b |
| Starch grain area per chloroplast (μm2) | 1.05 ± 0.11a | 0.047 ± 0.11b |
| Chlorophyll content per area (mg m-2) | 413 ± 16a | 302 ± 12b |
| Chlorophyll content per dry mass (mg g-1) | 9.11 ± 0.27a | 9.20 ± 0.29a |
| Chl a/b ratio | 2.68 ± 0.11b | 2.38 ± 0.08b |
| Carotenoid content (mg m-2) | 88.4 ± 2.9a | 58.9 ± 2.2b |
| Carotenoid content per dry mass (mg g-1) | 1.80 ± 0.05a | 1.90 ± 0.05a |
Means with the same letter are not statistically different (P > 0.05 according to ANOVA, n = 6, for leaf structural measurements and pigment contents and n = 30-42 for leaf anatomical measurements)
Decreased leaf thickness was also associated with reduced number of chloroplasts per unit leaf area, and with reduced chloroplast size (Table 1). In addition, leaf pigment contents per unit leaf area also decreased. Changes in these traits were relatively smaller than in leaf thickness and MA, and the number of chloroplasts per leaf dry mass was 1.2-fold greater in the high-temperature-grown plants (Table 1). Ultrastructural measurements also indicated that leaf chloroplasts of the high-temperature-grown plants had barely visible starch grains, while large starch grains were visible in the chloroplasts in the control treatment (Table 1, Fig. 1c, d).
Effects of growth temperature on foliage photosynthetic and respiratory activities
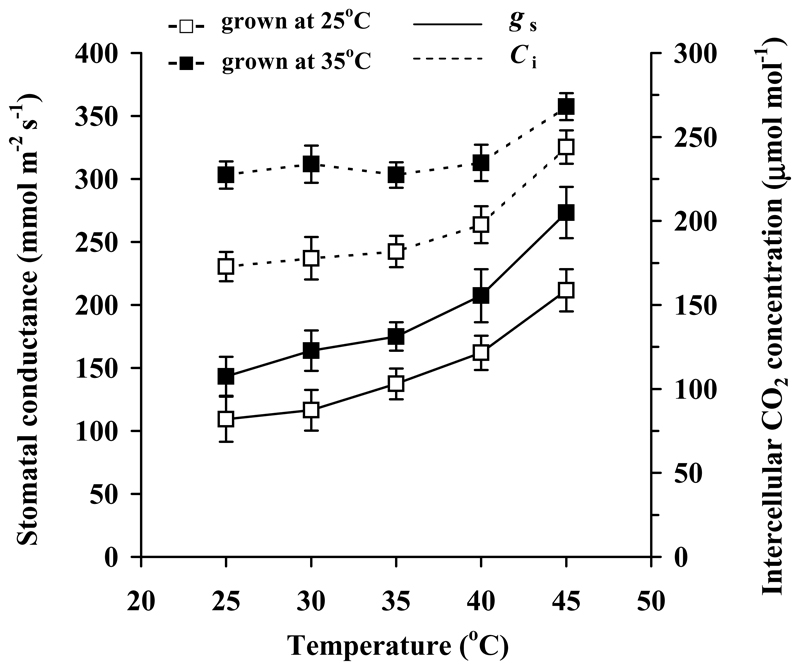
In analyzing the effects of growth temperature on foliage photosynthetic characteristics, we first focus on the effects at the standard measurement temperature of 30 °C, and then consider modifications in the temperature responses. At the standard temperature, acclimation to the higher growth temperature resulted in reductions in net assimilation rate per unit leaf area at the ambient CO2 concentration (Fig. 2a), and this reduction was associated with decreased maximum carboxylase activity of Rubisco (Vcmax, Table 2), decreased photosynthetic electron transport rate estimated from chlorophyll fluorescence at ambient CO2 concentration (JETR, Fig. 2c) and from the CO2-saturated net assimilation rate (Jmax, Table 2) per unit area. Analogously to the net assimilation rate, dark respiration rate per unit area (Fig. 2e, Table 2) and the quantum yield of photosynthesis for the incident light (Table 2) were lower in the warmer treatment. The latter difference reflected lower chlorophyll content per leaf area (Table 1) and leaf absorptance (data not shown) in plants grown at the higher temperature. Conversely, stomatal conductance and intercellular CO2 concentration were greater in plants grown at the higher temperature (Fig. 3, Table 2).
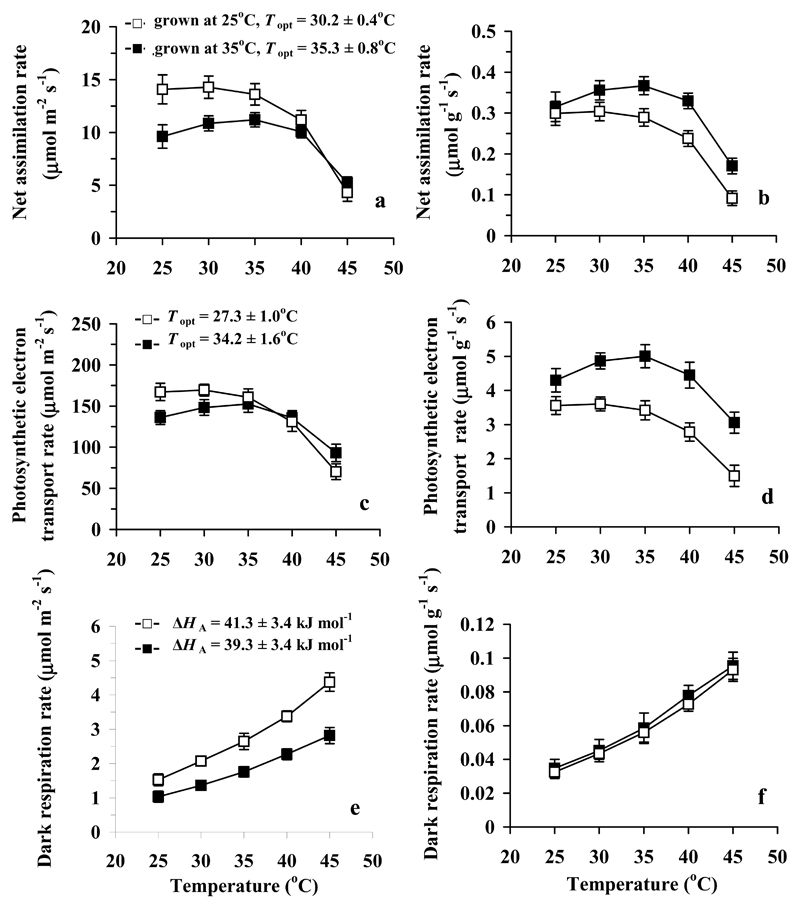
Fig. 2.
Temperature dependencies of light-saturated net assimilation rate (a, b), photosynthetic electron transport rate estimated from chlorophyll fluorescence (Eq. 1, c, d) and dark respiration rate (e, f) per unit leaf area (a, c, e) and per unit dry mass (b, d, f) in hybrid aspen grown at day/night temperatures of 25/20 °C (control) and 35/27 °C (high temperature treatment). Data were fitted by Eq. 3 (a-d) and Eq. 5 (e, f), and the values of the estimated optimum temperature (Topt, Eq. 4) are indicated for (a) and (c) and the values of the activation energy ΔHa (Eq. 5) for (e). The optimum temperatures in (a) and (c) are significantly different among the treatments (P < 0.005 for (a) and P < 0.02 for (c)). The measurements in a-d were conducted at an ambient CO2 concentration of 360 μmol mol-1 and at an incident quantum flux density of 650 μmol m-2 s-1. Data are means ± SE (n = 4).
Table 2.
Effects of growth temperature on average (± SE) foliage photosynthetic and isoprene emission characteristics in hybrid aspen (P. tremula x P. tremuloides, clone H200)
| Trait | Growth temperature (day/night) | |
|---|---|---|
| 25/20 ºC | 35/27 ºC | |
| Photosynthetic characteristics | ||
| An per area (μmol m-2 s-1) | 14.73 ± 0.40a | 11.18 ± 0.35b |
| An per dry mass (μmol g-1 s-1) | 0.310 ± 0.009a | 0.346 ± 0.018a |
| gs (mmol m-2 s-1) | 122 ± 7a | 164 ± 8b |
| Ci (μmol mol-1) | 182.3 ± 4.6a | 247 ± 11b |
| Rd per area (μmol m-2 s-1) | 2.07 ± 0.06b | 1.36 ± 0.07b |
| Rd per dry mass (μmol g-1 s-1) | 0.0436 ± 0.0016a | 0.0421 ± 0.0023a |
| αCO2 (mol mol-1) | 0.0441 ± 0.0026a | 0.0338 ± 0.0025b |
| Vcmax per area (μmol m-2 s-1) | 62.1 ± 1.6a | 32.7 ± 1.4b |
| Vcmax per dry mass (μmol g-1 s-1) | 1.309 ± 0.038a | 1.010 ± 0.043b |
| Jmax per area (μmol m-2 s-1) | 120 ± 6a | 80 ± 6b |
| Jmax per dry mass (μmol g-1 s-1) | 2.04 ± 0.06a | 2.19 ± 0.15a |
| Isoprene emission characteristics | ||
| I per area (nmol m-2 s-1) | 28.8 ± 2.8a | 17.8 ± 1.0b |
| I per dry mass (nmol g-1 s-1) | 0.60 ± 0.05a | 0.547 ± 0.027a |
| CDMADP per area (nmol m-2 s-1) | 1110 ± 90a | 650 ± 60b |
| CDMADP per dry mass (nmol g-1 s-1) | 23.5 ± 2.3a | 20.0 ± 1.8a |
| k (s-1) | 0.0286 ± 0.0021a | 0.0306 ± 0.0023a |
| Vmax per area (nmol m-2 s-1) | 79.5 ± 2.9a | 48.8 ± 3.0b |
| Vmax per dry mass (nmol g-1 s-1) | 1.673 ± 0.037a | 1.51 ± 0.11a |
| Km per area (nmol m-2) | 2640 ± 180a | 1542 ± 30b |
| Km per dry mass (nmol g-1) | 55.6 ± 4.9a | 47.7 ± 1.7a |
| αI (mmol mol-1) | 0.0743 ± 0.0010a | 0.0700 ± 0.0010b |
The photosynthetic characteristics are: An - net assimilation rate; gs - stomatal conductance to water vapor; Ci - intercellular CO2 concentration; Rd - dark respiration rate; αCO2 - quantum yield of photosynthesis for the incident light; Vcmax - apparent (Ci-based) maximum carboxylase activity of Rubisco; Jmax - apparent (Ci-based) capacity for photosynthetic electron transport. The isoprene emission characteristics are: I - isoprene emission rate; CDMADP - dimethylallyl diphosphate (DMADP) pool size; k - rate constant of isoprene synthase; Vmax - apparent maximum activity of isoprene synthase; Km - apparent Michaelis-Menten constant for isoprene synthase; αI - quantum yield of isoprene emission for the incident light.
All measurements were conducted at 30 ºC, and saturating light intensity (Q) of 650 μmol m-2 s-1 was used for all measurements except for the quantum yields. The quantum yields were calculated as the linear slopes of the process rate vs. incident Q over the Q range of 20-75 μmol m-2 s-1 and at the ambient [CO2] of 360 μmol mol-1 and oxygen concentration of 21%. The value of Vcmax is reported as an average of Vcmax estimates determined from measurements at 21% and 2% O2. Use of measurements at different O2 concentrations renders the apparent Vcmax estimates less sensitive to Rubisco kinetic characteristics employed in the calculations.
Each value represents the mean ± SE of four measurements in different plants, and means with different lowercase letters are statistically different at P < 0.05 according to ANOVA.
Fig. 3.
Temperature responses of stomatal conductance to water vapor and intercellular CO2 concentration in hybrid aspen grown at the contrasting temperature regimes, control (day/night temperature 25/20 °C) and high temperature (35/27 °C). Measurement conditions, data presentation and replicates as in Fig. 2.
The growth temperature dependent changes in assimilation rates and foliage biochemical capacities were strongly driven by temperature effects on leaf structure. In fact, dark respiration rate, Jmax and net assimilation rate per dry mass were not significantly different among the treatments (Table 2, Fig. 2), while the CO2-limited photosynthetic electron transport rate per dry mass (electron transport rate when photosynthesis is Rubisco-limited, Fig. 2d) was greater at higher growth temperature. However, Vcmax per dry mass was still greater for the control treatment (Table 2), indicating that the similarity of net assimilation rate per dry mass among the treatments reflected a greater intercellular CO2 concentrations due to higher stomatal conductance (Fig. 3).
Increased growth temperature shifted the optimum temperature of net assimilation to higher temperatures (Fig. 2a, b), and an analogous response was observed for the CO2-limited photosynthetic electron transport rate (Fig. 2c, d). In addition, the activation energies, ΔHA (Eq. 3) were smaller in plants grown at the lower temperature (P < 0.05 for both comparisons). This, together with greater optimum temperatures resulted in greater mass-based net assimilation and electron transport rates at higher temperature (Fig. 2b, d). On the other hand, growth temperature did not affect the shapes of the dark respiration (Fig. 2e, f) and stomatal conductance (Fig. 3) vs. temperature responses (P > 0.3 for the treatment effects on ΔHA for both traits).
Influence of growth temperature on isoprene emission characteristics at the standard measurement temperature of 30 °C
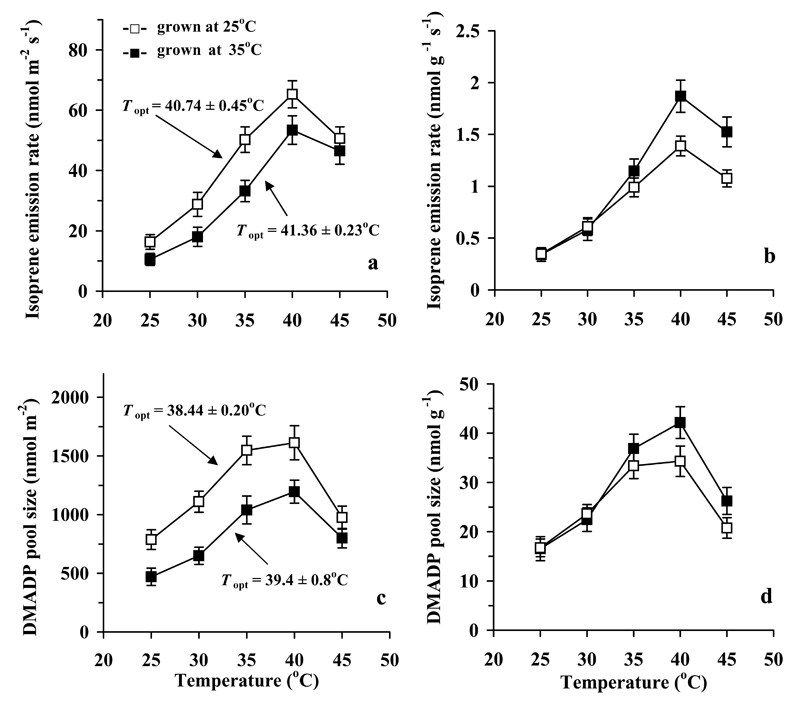
As with the photosynthesis data, in this section, we first analyze the differences in growth treatments at the standard measurement temperature of 30 °C, and then focus on differences in the shape on temperature responses. On a leaf area basis, both the isoprene emission rate (I) and the pool size of the substrate for the isoprene synthase enzyme, dimethylallyl diphosphate (DMADP, CDMADP), were greater in the control than in the high temperature treatment (Fig. 4a, c, Table 2). However, on a leaf dry mass basis, I and CDMADP values were similar among the growth temperature treatments (Fig. 4b,d, Table 2). Furthermore, the rate constant for isoprene synthase (k) was not significantly different between the growth temperature treatments (Fig. 5, Table 2). The percentage of photosynthetic carbon used for isoprene emission was also not significantly different among the treatments at this measurement temperature (Fig. 6). Similarly to the quantum yield for photosynthesis, the quantum yield for isoprene emission for the incident light was greater in the control treatment (Table 2).
Fig. 4.
Temperature dependencies of isoprene emission rate (a, b) and the isoprene synthase substrate dimethylallyl diphosphate (DMADP) pool size (c, d) per unit leaf area (a, c) and per unit leaf dry mass (b, d) in hybrid aspen in dependence on growth temperature conditions, control (day/night temperature 25/20 °C) vs. high temperature (35/27 °C). Measurement conditions and data presentation as in Fig. 2. The optimum temperatures for isoprene emission and DMADP pool size were not significantly different among the treatments (P > 0.1 for both).
Fig. 5.
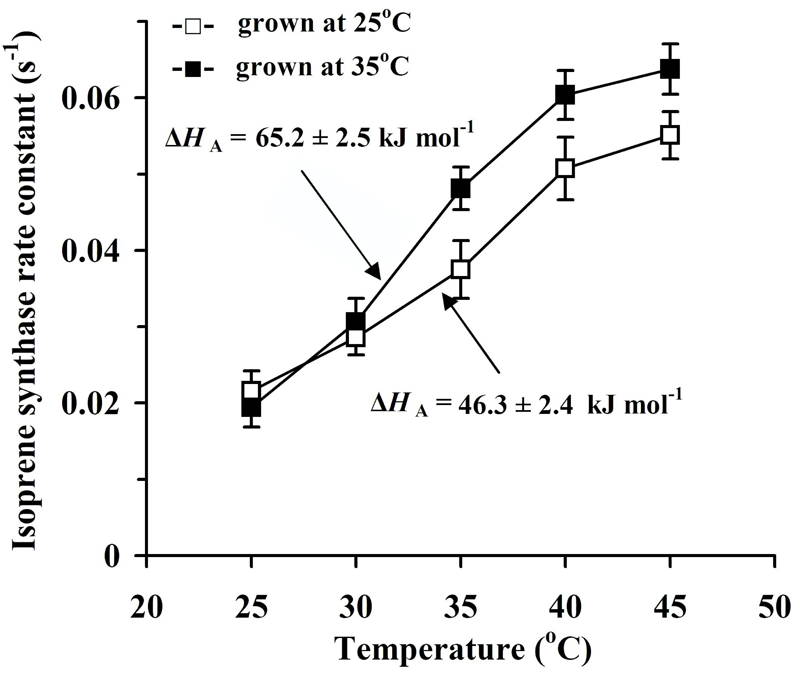
Temperature dependence of the rate constant of isoprene synthase (k) in hybrid aspen grown under control (day/night temperature 25/20 °C) and high temperature (35/27 °C) conditions. The first order reaction rate constant was estimated as the slope of the linear part of dimethylallyl diphosphate pool size vs. isoprene emission rate relationship through the dark decay of isoprene emission and corresponds to the environmental conditions (ambient CO2 concentration of 360 μmol mol-1, O2 concentration of 21% and incident quantum flux density of 650 μmol m-2 s-1) prior to leaf darkening. The number of replicates and data presentation as in Fig. 2. The activation energies for k (ΔHa, kJ mol-1, Eq. 3) are also demonstrated (P < 0.001 for the difference among the treatments).
Fig. 6.
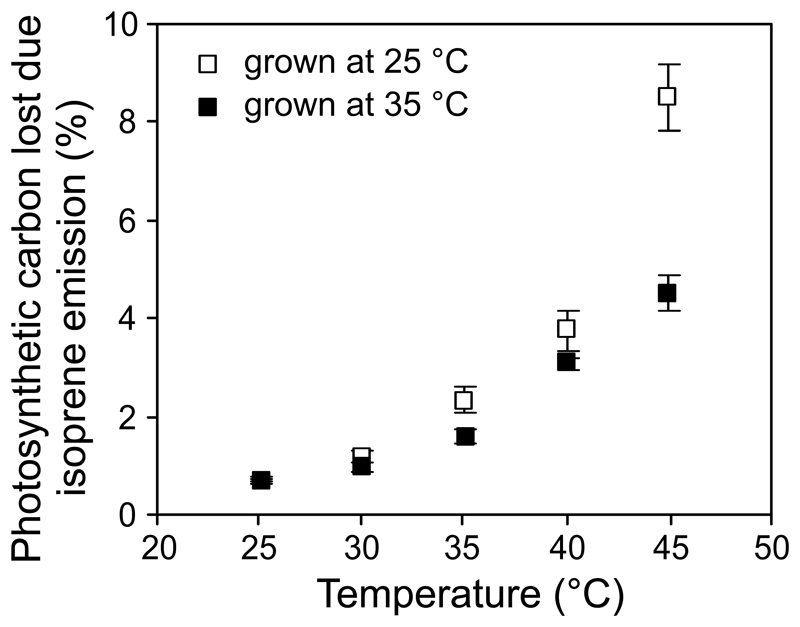
Temperature-dependent changes in the percentage of photosynthetic carbon lost due to isoprene emission in hybrid aspen leaves under control (day/night temperature 25/20 °C) and high temperature (35/27 °C) treatments.
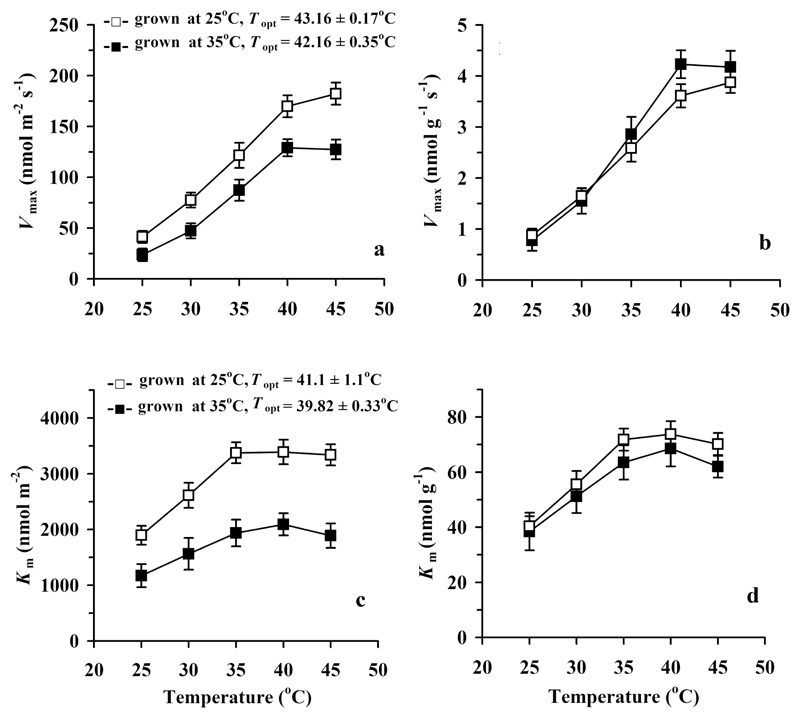
The apparent kinetic characteristics of isoprene synthase, the maximum activity at substrate-saturated conditions, Vmax per unit area (Fig. 7a) and the Michaelis-Menten constant for the area-based CDMADP (Fig. 7c) were both greater in the control treatment. However, similarly to I and CDMADP (Fig. 4b, d), Vmax per unit dry mass (Fig. 7b) and Km for dry-mass based CDMADP (Fig. 7d) were not different among the growth temperatures.
Fig. 7.
Temperature responses of the apparent maximum capacity, Vmax, (a, b) and Michaelis-Menten constant Km (c, d) of isoprene synthase in hybrid aspen grown under control (day/night temperature 25/20 °C) and high temperature (35/27 °C) treatments. Data fitting and presentation as in Fig. 2. The optimum temperatures for Vmax were significantly different among the treatments (P < 0.05), while the optimum temperatures for Km were not (P > 0.3).
Modification of the temperature responses of isoprene emission characteristics by growth temperature
Growth temperature had minor effects on the optimum temperatures (Topt) of isoprene emission characteristics (Figs. 4-5, 7) with only a slightly greater Topt for apparent maximum isoprene synthase activity (Vmax) in the control leaves (Fig. 7a). On the other hand, for the measurement temperature range of 25-35 °C, the average activation energy (ΔHA) for I was 77.9 ± 1.7 kJ mol-1 for the control treatment and 108 ± 5 kJ mol-1 for the high-temperature-grown plants (P < 0.01 for the differences among the treatments). These activation energies correspond to a Q10 of 2.77 ± 0.05 for the control and 4.14 ± 0.29 for the high-temperature treatment. The values of ΔHA for CDMAPD for the same temperature range were 63 ± 7 kJ mol-1 (Q10 = 2.31 ± 0.22) for the control and 76 ± 14 kJ mol-1 (Q10 = 2.9 ± 0.6) for the high temperature treatment (P > 0.4 for the difference among the treatments). Thus, the effects of measurement temperature on and growth temperature sensitivity of I and k were larger than those for CDMADP.
The differences among treatments in activation energies for k and I were ultimately associated with greater k and mass-based I values at higher temperatures (Fig. 4-5). As the result of much lower temperature sensitivity of photosynthesis than isoprene emission, the percentage of photosynthetic carbon lost due to isoprene emission increased with increasing temperature, and this increase was greater for plants grown under the lower temperature (Fig. 6).
Analogously, the temperature sensitivity was greater for the isoprene synthase apparent Vmax than for the apparent Km (Fig. 7). The Q10 values for Vmax were 2.65 ± 0.08 for the control and 3.51 ± 0.15 for the high temperature treatment (P < 0.005 for difference among treatments), while the corresponding Q10 values for Km were 2.16 ± 0.26 for the control and 2.18 ± 0.46 for the high temperature treatment (P > 0.9 for the treatment effect).
Discussion
Modification of leaf structural characteristics and pigment content by growth temperature
Our study demonstrates that acclimation to high growth temperature led to profound changes in anatomy, morphology and chemistry of hybrid aspen leaves (Fig. 1, Table 1). As an integral change, high-temperature-grown leaves were thinner with lower leaf dry mass per unit area (MA). In addition, high growth temperature led to less densely packed mesophyll, with fewer mesophyll cells and fewer chloroplasts per unit leaf area (Fig. 1, Table 1). Despite lower number of chloroplasts per unit leaf area, the chloroplasts were smaller, and chloroplast number per unit leaf dry mass was greater. These results are in a broad agreement in past studies demonstrating significant modifications in leaf anatomy and chloroplast characteristics (Jin et al. 2011), and decreased leaf thickness (Körner et al. 1989; Hartikainen et al. 2009; Muhl et al. 2011) in plants grown in warmer temperatures.
On the other hand, leaf expansion growth was enhanced, resulting in larger leaves (Table 1) and overall greater whole canopy leaf area and mass (data not shown). Such an enhancement of leaf expansion growth is a characteristic response to high growth temperatures in well-watered plants (Wardlaw & Bagnall 1981; Hewitt et al. 1985; Granier et al. 2000) and might reflect both enhanced light interception and enhanced sink activity, i.e. enhanced consumption of photoassimilates, at high temperatures. In fact, chloroplasts in high-temperature-grown leaves were essentially depleted of starch (Table 1, Fig. 1c, d) in agreement with observations in other studies (Buttrose & Hale 1971; Gandin et al. 2011). In addition to a reduction of starch content due to enhanced sugar export from chloroplasts, there is also evidence that high temperatures might inhibit gluconeogenesis and increase sucrose synthesis (Ito et al. 2009; Yamakawa & Hakata 2010). Soluble sugars importantly enhance the thermotolerance of photosynthetic apparatus (Hüve et al. 2006; Hüve et al. 2012; Sun et al. 2013), and thus, a possible elevation of soluble sugar content can be an important mechanism improving the heat resistance of photosynthesis.
Structural control of temperature acclimation of photosynthetic and respiratory characteristics
Our results demonstrate strong reductions in area-based foliage pigment content, biochemical potentials, and net assimilation and dark respiration rates in high temperature grown plants (Tables 1-2, Fig. 2). These results are analogous with previous studies demonstrating enhanced photosynthetic rates and greater photosynthetic electron transport and Rubisco carboxylase activity (Huner 1985; Mawson & Cummins 1989; Yamasaki et al. 2002; Yamori et al. 2005) and number and size of mitochondria and respiratory activity (Jin et al. 2011) per unit leaf area in plants grown at lower temperatures. However, as our study further demonstrates, the mass-based photosynthetic characteristics differed much less among the treatments, indicating that the photosynthetic potentials of single leaf cells were not strongly affected by changes in growth temperature (Table 2, Fig. 2). An increase of MA with increasing growth light availability is well-known (Niinemets & Sack 2006; Niinemets 2007; Niinemets & Anten 2009; Poorter et al. 2009), and this increase has been associated with increases in protein content and photosynthetic rate per area (Niinemets & Sack 2006; Niinemets 2007). Our results further underscore the important role of structural controls in temperature acclimation of photosynthesis.
In addition to the rates of photosynthesis, the quantum yield of photosynthesis for an incident light also decreased with increasing irradiance (Table 2) and this reduction was associated with reduced chlorophyll content per area (Table 1) and reduced leaf absorptance as has also been reported in several other studies (Hikosaka et al. 1999; Yamori et al. 2005). As chlorophyll content per dry mass did not vary with growth temperature (Table 1), modifications in leaf structure were also responsible for the reduction of quantum yields for the incident light in the high-temperature-grown plants (Table 2). Nevertheless, changes in chlorophyll content typically play a much smaller role in whole plant light interception than alterations in total leaf area (Niinemets 2007).
Differently from most of the photosynthetic characteristics, stomatal conductance was strongly enhanced upon growth at high temperatures, leading to enhanced intercellular CO2 concentration (Fig. 3). In fact, as the maximum carboxylase activity of Rubisco per dry mass was somewhat decreased, enhanced intercellular CO2 concentration was responsible for similar mass-based net assimilation rates in different temperature treatments. Enhanced stomatal conductance at higher growth temperature can be a structural response associated with greater stomatal size and/or density as reported in some studies (Ghosh et al. 1996; Pandey et al. 2007) although not always (Crawford et al. 2012). Maintenance of high stomatal conductance at higher temperatures clearly plays an important role in convective cooling of leaves (Crawford et al. 2012), and also importantly compensates for the reduced biochemical capacity of photosynthetic machinery by increasing the internal CO2 concentration.
The maximum electron transport rate per dry mass estimated from the net assimilation rates at high CO2 concentration did not differ among temperature treatments. However, in calculation of the capacity for photosynthetic electron transport rate at high CO2 concentration, we did not consider the possible limitation of photosynthesis by triose phosphates that can lead to underestimation of the true electron transport capacity (Sharkey 1985). Given the overall strong coordination of Rubisco activity and electron transport capacity, the difference in growth temperature effects on Rubisco carboxylase and electron transport capacities might reflect a stronger feedback inhibition of photosynthesis at higher CO2 in plants grown at lower temperature (e.g., Pammenter et al. 1993 for a discussion). Such a possible feedback limitation of maximum electron transport rate is also supported by reduced use of assimilates in plant growth as indicated by accumulation of starch in lower temperatures.
Effects of growth temperature on temperature responses of photosynthesis and respiration rates
In addition to structural modifications, growth at the higher temperature resulted in significantly improved heat resistance of net assimilation rates as evident in greater activation energies and optimum temperatures (Topt) of net assimilation rates and CO2-limited electron transport rates (Fig. 2a-d). Such an improved heat resistance is in line with past observations as discussed in the Introduction and is typically associated with increased lipid saturation in thylakoid membranes (Pearcy 1978; Raison 1986; Kunst et al. 1989; Mawson & Cummins 1989; Shanklin & Cahoon 1998), and can also reflect increased leaf sugar contents as discussed above. In addition, increases in Topt of photosynthesis can partly reflect increased stomatal conductance that reduces the rate of photorespiration at any given temperature and thereby shifts the optimum temperature to higher temperatures (Leegood 1995; Medlyn et al. 2002).
Past studies have indicated that the activation energy for the dark respiration often decreases upon growth at higher temperatures (Atkin & Tjoelker 2003; Atkin et al. 2005). However, there was no evidence of changes in the shape of dark respiration vs. temperature response curve in our study (Fig. 2e, f) suggesting either that the mitochondrial capacity for respiration was unaffected by high growth temperature or that changes in the capacity were obscured by alterations in respiratory substrate availability. The latter possibility is plausible given the altered sink/source relations in plants grown at different temperatures.
Isoprene emission characteristics in relation to growth temperature
As with the area-based photosynthetic characteristics, isoprene emission rate, DMADP pool size (Table 2, Fig. 4) and apparent maximum rate and Km for isoprene synthase at 30 °C (Table 2, Fig. 7) were greater in low-temperature-grown plants. However, none of these characteristics was affected by growth temperature when expressed on a leaf dry mass basis (Fig. 4, 7 and Table 2), indicating that differences in area-based isoprene emission characteristics were primarily driven by leaf structural modifications. Furthermore, the rate constant of isoprene synthase (Fig. 5) and the fraction of carbon lost due to isoprene emission (Fig. 6) also did not depend on growth temperature at the measurement temperature of 30 °C, further indicating that isoprene emission scaled to DMADP pool size and net assimilation rate similarly in both temperature treatments. Contrasting growth temperature effects on the area-based isoprene emission rates have been observed across the studies (see Introduction), and we argue that these contrasting effects primarily result from study-to-study differences in leaf morphological response to growth temperature. We also emphasize that the effects of longer-term temperature on isoprene emission characteristics can be different for transfer experiments where leaves developed at a given temperature are transferred to another temperature. In these experiments, any acclimation to changed conditions is primarily biochemical and physiological (Geron et al. 2000; Mayrhofer et al. 2005; Wiberley et al. 2008; Sun et al. 2012a).
Temperature response of isoprene emission characteristics
As demonstrated previously (Rasulov et al. 2010; Li et al. 2011), the optimum temperature for isoprene emission (Fig. 4a, b) is co-determined by the temperature effects on the DMADP pool size (Fig. 4c, d) and on the isoprene synthase rate constant (Fig. 5). Thus, the key issue is how the share of different controls is affected by acclimation to different growth temperatures. Our data do demonstrate that the DMADP pool size starts to decrease earlier with increasing temperature (Fig. 4c, d) than the isoprene synthase rate constant or Vmax of isoprene synthase (Fig. 7a, b). Furthermore, our data demonstrate that Km of isoprene synthase increases with increasing temperature, indicating that progressively higher DMADP pools are needed to reach a given value of isoprene emission rate (Fig. 7c, d). Such a temperature-dependent increase of Km is characteristic for many enzymes and indicates weaker associations in the enzyme-substrate complex at higher temperature (Hajdu et al. 2009).
Despite the similarity of mass-based isoprene emission characteristics at 30 °C (Table 2), and similarly high optimum temperatures for isoprene emission between 40-41 °C (Fig. 4a, b), a number of important differences were observed among the growth temperature treatments in responses of isoprene emission characteristics to higher temperatures (Figs. 4-7). In low-temperature-grown plants, the activation energies were almost identical to those observed in hybrid aspen grown in similar conditions (Rasulov et al. 2010). However, in the current study, the activation energy for isoprene emission was greater in leaves grown at greater temperature, and this was associated with greater mass-based emission rates at higher temperatures (Fig. 4b). These effects resulted from a greater activation energy for isoprene synthase rate constant (Fig. 5), while the activation energy for DMADP pool size did not differ among the treatments, suggesting that the rate of substrate production was not affected by growth temperature. This seems surprising given the improved heat tolerance of photosynthetic apparatus in high-temperature-grown plants (Fig. 2). In fact, plants grown at the lower temperature used a greater fraction of photosynthetic carbon for isoprene emission at higher temperatures (Fig. 6). It is plausible that in high-temperature-grown plants with a greater growth rate and reduced sink limitation of photosynthesis, alternative pathways for DMADP consumption for higher molecular mass isoprenoids (Rasulov et al. 2014 for a discussion) were also more strongly activated such that the availability for DMADP for isoprene synthesis was ultimately similar for both treatments.
As isoprene synthase activity is affected by pH and cellular ionic medium characteristics (Sasaki et al. 2005; Köksal et al. 2010) possible growth temperature effects on subcellular environment might also play a role in the observed patterns. Thus, differences in the isoprene synthase rate constant (Fig. 5) and Vmax (Fig. 7a, b) might reflect changes in chloroplastic conditions in plants grown at different temperatures. Such changes are likely given that heat stress gradually leads to leakiness of chloroplastic membranes and reduced proton gradient (Schrader et al. 2004; Zhang & Sharkey 2009) bringing pH in actively photosynthesizing chloroplasts (ca. 7.8-8) closer to the cytosolic values (6-6.5). Given that the pH optimum for isoprene is ca. 8, such a reduction of pH would importantly affect the rate of isoprene synthesis, especially in low-temperature-grown plants with lower heat resistance of membranes.
Apart from subcellular effects, Populus has multiple isoprene synthase genes (Vickers et al. 2010), and expression of a more heat-resistance isoprene synthase might provide a complementary explanation for improved temperature kinetics. However, so far there is no evidence of differences in heat resistance of different isoprene synthase isoforms as well as on the regulation of expression of different isoforms by growth temperature. Finally, smaller chloroplasts (Table 1) with almost missing starch grains might also mean reduced diffusive limitations between the sites of DMADP synthesis and isoprene synthase. Overall, these results are consistent with improved heat stability of isoprene synthesis in high temperature acclimated leaves, but do not support the suggestion of a greater requirement of isoprene synthesis to cope with sustained high temperatures.
Conclusions
Our study demonstrates that increases in growth temperature resulted in major changes in leaf anatomy and morphology and that these structural modifications mainly drove the treatment effects on foliage photosynthetic and isoprene emission characteristics expressed per unit leaf area. While structural acclimation to growth light regime is well-recognized (Niinemets & Anten 2009; Poorter et al. 2009), temperature acclimation studies do not routinely address growth temperature effects on leaf structure. As our study demonstrates, consideration of structural effects clearly provides insight into the mechanisms of temperature-driven photosynthetic acclimation. In fact, structural changes might explain the study-to-study differences in growth temperature effects on foliage area-based physiological characteristics that have been difficult to explain so far.
Higher growth temperature also enhanced the heat stability of photosynthetic characteristics, but had surprisingly minor effects on the heat stability of isoprene emission traits. As noted in the Introduction, there are important study-to-study differences in the effects of growth temperature on the shape of the temperature response of isoprene emission (Monson et al. 1992; Fares et al. 2011). We argue that such contrasting effects most likely result from differences in how growth temperature affects the isoprene precursor DMADP pool size. In our study where a higher growth temperature was associated with enhanced sink activity, growth temperature effects on DMADP pool size were minor. However, much stronger pathway upregulation may occur when primary metabolism is curbed by limited sink activity (Harding et al. 1990; Starck et al. 1993). Although our study demonstrated that growth temperature might also affect the isoprene synthase characteristics, we argue that the overall pathway flux can be potentially more strongly affected by DMADP pool size and isoprene synthase expression (Rasulov et al. 2009b; Rasulov et al. 2010; Li et al. 2011; Li & Sharkey 2013). We conclude that analyses of temperature acclimation of photosynthesis and isoprene emission should consider both structural and physiological adjustments and that more experimental studies are needed to gain insight into the effects of sink strength on expression of isoprenoid synthesis pathway.
Summary statement.
High growth temperature reduced area-based photosynthetic and isoprene emission characteristics in hybrid aspen, and these effects were primarily driven by structural modifications. Acclimation to high temperature also enhanced stomatal conductance, improved heat resistance of photosynthetic apparatus and increased the activation energy of isoprene emission resulting in a greater fraction of photosynthetic carbon emitted as isoprene at higher temperatures. These results emphasize the important roles of both leaf structural and physiological changes in high-temperature acclimation of photosynthesis and isoprene emission.
Acknowledgements
Funding for this research was provided by the Estonian Ministry of Science and Education (institutional grant IUT-8-3), the Estonian Science Foundation (grant 9253), the European Commission through the European Regional Fund (the Center of Excellence in Environmental Adaptation), and the European Research Council (advanced grant 322603, SIP-VOL+).
References
- Arneth A, Monson RK, Schurgers G, Niinemets Ü, Palmer PI. Why are estimates of global isoprene emissions so similar (and why is this not so for monoterpenes)? Atmospheric Chemistry and Physics. 2008;8:4605–4620. [Google Scholar]
- Ashworth K, Boissard C, Folberth G, Lathière J, Schurgers G. Global modeling of volatile organic compound emissions. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 451–487. [Google Scholar]
- Atkin OK, Bruhn D, Hurry VM, Tjoelker M. The hot and the cold: unravelling the variable response of plant respiration to temperature. Evans review no. 2. Functional Plant Biology. 2005;32:87–105. doi: 10.1071/FP03176. [DOI] [PubMed] [Google Scholar]
- Atkin OK, Tjoelker MG. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in Plant Science. 2003;8:343–351. doi: 10.1016/S1360-1385(03)00136-5. [DOI] [PubMed] [Google Scholar]
- Behnke K, Ehlting B, Teuber M, Bauerfeind M, Louis S, Hänsch R, Polle A, Bohlmann J, Schnitzler J-P. Transgenic, non-isoprene emitting poplars don’t like it hot. The Plant Journal. 2007;51:485–499. doi: 10.1111/j.1365-313X.2007.03157.x. [DOI] [PubMed] [Google Scholar]
- Behnke K, Ghirardo A, Janz D, Kanawati B, Esperschütz J, Zimmer I, Schmitt-Kopplin P, Niinemets Ü, Polle A, Schnitzler J-P, Rosenkranz M. Isoprene function in two contrasting poplars under salt and sunflecks. Tree Physiology. 2013;33:562–578. doi: 10.1093/treephys/tpt018. [DOI] [PubMed] [Google Scholar]
- Berry J, Björkman O. Photosynthetic response and adaptation to temperature in higher plants. Annual Review of Plant Physiology. 1980;31:491–543. [Google Scholar]
- Björkman O, Badger MR, Armond PA. Response and adaptation of photosynthesis to high temperatures. In: Turner NC, Kramer PJ, editors. Adaptation of plants to water and high temperature stress. John Wiley & Sons; New York - Chichester - Brisbane - Toronto: 1980. pp. 233–249. [Google Scholar]
- Brüggemann N, Schnitzler J-P. Relationship of isopentenyl diphosphate (IDP) isomerase activity to isoprene emission of oak leaves. Tree Physiology. 2002;22:1011–1018. doi: 10.1093/treephys/22.14.1011. [DOI] [PubMed] [Google Scholar]
- Bunce JA. Acclimation of photosynthesis to temperature in eight cool and warm climate herbaceous C3 species: temperature dependence of parameters of a biochemical photosynthesis model. Photosynthesis Research. 2000;63:59–67. doi: 10.1023/A:1006325724086. [DOI] [PubMed] [Google Scholar]
- Burns WA. Thick sections: technique and applications. In: Trump BF, Jones RJ, editors. Diagnostic electron microscopy. Wiley; New York: 1978. p. 146-. [Google Scholar]
- Buttrose MS, Hale CR. Effects of temperature on accumulation of starch or lipid in chloroplasts of grapevine. Planta. 1971;101:166–170. doi: 10.1007/BF00387627. [DOI] [PubMed] [Google Scholar]
- Centritto M, Brilli F, Fodale R, Loreto F. Different sensitivity of isoprene emission, respiration and photosynthesis to high growth temperature coupled with drought stress in black poplar (Populus nigra) saplings. Tree Physiology. 2011;31:275–286. doi: 10.1093/treephys/tpq112. [DOI] [PubMed] [Google Scholar]
- Ciais P, Friedlingstein P, Friend A, Schimel DS. Integrating global models of terrestrial primary productivity. In: Mooney HA, Saugier B, Roy J, editors. Terrestrial global productivity. Academic Press,Inc; San Diego: 2001. pp. 449–478. [Google Scholar]
- Copolovici L, Kännaste A, Remmel T, Vislap V, Niinemets Ü. Volatile emissions from Alnus glutinosa induced by herbivory are quantitatively related to the extent of damage. Journal of Chemical Ecology. 2011;37:18–28. doi: 10.1007/s10886-010-9897-9. [DOI] [PubMed] [Google Scholar]
- Crawford AJ, McLachlan DH, Hetherington AM, Franklin KA. High temperature exposure increases plant cooling capacity. Current Biology. 2012;22:R396–R397. doi: 10.1016/j.cub.2012.03.044. [DOI] [PubMed] [Google Scholar]
- Devos N, Ingouff M, Loppes R, Matagne RF. Rubisco adaptation to low temperatures: a comparative study in psychrophlilic and mesophilic unicellular algae. Journal of Phycology. 1998;34:655–660. [Google Scholar]
- Fares S, Mahmood T, Liu S, Loreto F, Centritto M. Influence of growth temperature and measuring temperature on isoprene emission, diffusive limitations of photosynthesis and respiration in hybrid poplars. Atmospheric Environment. 2011;45:155–161. [Google Scholar]
- Funk JL, Jones CG, Baker CJ, Fuller HM, Giardina CP, Lerdau MT. Diurnal variation in the basal emission rate of isoprene. Ecological Applications. 2003;13:269–278. [Google Scholar]
- Gandin A, Gutjahr S, Dizengremel P, Lapointe L. Source-sink imbalance increases with growth temperature in the spring geophyte Erythronium americanum. Journal of Experimental Botany. 2011;62:3467–3479. doi: 10.1093/jxb/err020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geron C, Guenther A, Sharkey T, Arnts RR. Temporal variability in basal isoprene emission factor. Tree Physiology. 2000;20:799–805. doi: 10.1093/treephys/20.12.799. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Ichii M, Asanuma K, Kusutani A. Optimum and sub-optimal temperature effects on stomata and photosynthesis rate of determinate soybeans. Acta Horticulturae. 1996;440:81–86. doi: 10.17660/actahortic.1996.440.15. [DOI] [PubMed] [Google Scholar]
- Granier C, Turc O, Tardieu F. Co-ordination of cell division and tissue expansion in sunflower, tobacco, and pea leaves: Dependence or independence of both processes? Journal of Plant Growth Regulation. 2000;19:45–54. doi: 10.1007/s003440000006. [DOI] [PubMed] [Google Scholar]
- Guenther AB, Jiang X, Heald CL, Sakulyanontvittaya T, Duhl T, Emmons LK, Wang X. The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions. Geosci Model Dev. 2012;5:1471–1492. [Google Scholar]
- Guenther AB, Zimmerman PR, Harley PC, Monson RK, Fall R. Isoprene and monoterpene emission rate variability: model evaluations and sensitivity analyses. Journal of Geophysical Research. 1993;98:12609–12617. [Google Scholar]
- Hajdu I, Szilagyi A, Kardos J, Zavodszky P. A link between hinge-bending domain motions and the temperature dependence of catalysis in 3-isopropylmalate dehydrogenase. Biophysical Journal. 2009;96:5003–5012. doi: 10.1016/j.bpj.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SA, Guikema JA, Paulsen GM. Photosynthetic decline from high temperature stress during maturation of wheat.II. Interaction with source and sink processes. Plant Physiology. 1990;92:654–658. doi: 10.1104/pp.92.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartikainen K, Nerg A-M, Kivimäenpää M, Kontunen-Soppela S, Mäenpää M, Oksanen E, Rousi M, Holopainen T. Emissions of volatile organic compounds and leaf structural characteristics of European aspen (Populus tremula) grown under elevated ozone and temperature. Tree Physiology. 2009;29:1163–1173. doi: 10.1093/treephys/tpp033. [DOI] [PubMed] [Google Scholar]
- Hewitt JD, Casey LL, Zobel RW. Effect of day length and night temperature on starch accumulation and degradation in soybean. Annals of Botany. 1985;56:513–522. [Google Scholar]
- Hikosaka K. Modelling optimal temperature acclimation of the photosynthetic apparatus in C3 plants with respect to nitrogen use. Annals of Botany. 1997;80:721–730. [Google Scholar]
- Hikosaka K, Murakami A, Hirose T. Balancing carboxylation and regeneration of ribulose-1,5-bisphosphate in leaf photosynthesis: temperature acclimation of an evergreen tree, Quercus myrsinaefolia. Plant, Cell and Environment. 1999;22:841–849. [Google Scholar]
- Hopkins WG. Introduction to plant physiology. 2nd ed. John Wiley; New York: 1998. [Google Scholar]
- Huner NPA. Acclimation of winter rye to cold hardening temperatures results in an increased capacity for photosynthetic electron transport. Canadian Journal of Botany. 1985;63:506–511. [Google Scholar]
- Hüve K, Bichele I, Ivanova H, Keerberg O, Pärnik T, Rasulov B, Tobias M, Niinemets Ü. Temperature responses of dark respiration in relation to leaf sugar concentration. Physiologia Plantarum. 2012;144:320–334. doi: 10.1111/j.1399-3054.2011.01562.x. [DOI] [PubMed] [Google Scholar]
- Hüve K, Bichele I, Tobias M, Niinemets Ü. Heat sensitivity of photosynthetic electron transport varies during the day due to changes in sugars and osmotic potential. Plant, Cell and Environment. 2006;29:212–228. doi: 10.1111/j.1365-3040.2005.01414.x. [DOI] [PubMed] [Google Scholar]
- Ibrahim MA, Mäenpää M, Hassinen V, Kontunen-Soppela S, Malec L, Rousi M, Pietikäinen L, Tervahauta A, Kärenlampi S, Holopainen JK, Oksanen EJ. Elevation of night-time temperature increases terpenoid emissions from Betula pendula and Populus tremula. Journal of Experimental Botany. 2010;61:1583–1595. doi: 10.1093/jxb/erq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Hara T, Kawanami Y, Watanabe T, Thiraporn K, Ohtake N, Sueyoshi K, Mitsui T, Fukuyama T, Takahashi Y, Sato T, et al. Carbon and nitrogen transport during grain filling in rice under high-temperature conditions. Journal of Agronomy and Crop Science. 2009;195:368–376. [Google Scholar]
- Jin B, Wang L, Wang J, Jiang KZ, Wang Y, Jiang XX, Ni CY, Wang YL, Teng NJ. The effect of experimental warming on leaf functional traits, leaf structure and leaf biochemistry in Arabidopsis thaliana. BMC Plant Biology. 2011;11:35. doi: 10.1186/1471-2229-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köksal M, Zimmer I, Schnitzler J-P, Christianson DW. Structure of isoprene synthase illuminates the chemical mechanism of teragram atmospheric carbon emission. Journal of Molecular Biology. 2010;402:363–373. doi: 10.1016/j.jmb.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner C, Neumayer M, Pelaez Menendez-Riedl S, Smeets-Scheel A. Functional morphology of mountain plants. Flora. 1989;182:353–383. [Google Scholar]
- Kunst L, Browse J, Somerville C. A mutant of Arabidopsis deficient in desaturation of palmitic acid in leaf lipids. Plant Physiology. 1989;90:943–947. doi: 10.1104/pp.90.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laisk A, Oja V, Rasulov B, Rämma H, Eichelmann H, Kasparova I, Pettai H, Padu E, Vapaavuori E. A computer-operated routine of gas exchange and optical measurements to diagnose photosynthetic apparatus in leaves. Plant, Cell and Environment. 2002;25:923–943. [Google Scholar]
- Leegood RC. Effects of temperature on photosynthesis and photorespiration. In: Smirnoff N, editor. Environment and plant metabolism: flexibility and acclimation. Bios Scientific Publishers Ltd; Oxford: 1995. pp. 45–62. [Google Scholar]
- Li Z, Ratliff EA, Sharkey TD. Effect of temperature on postillumination isoprene emission in oak and poplar. Plant Physiology. 2011;155:1037–1046. doi: 10.1104/pp.110.167551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Sharkey TD. Molecular and pathway controls on biogenic volatile organic compound emissions. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 119–151. [Google Scholar]
- Lichtenthaler HK, Buschmann C. Chlorophylls and carotenoids: measurement and characterisation by UV-VIS. In: Lichtenthaler HK, Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Smith DM, Sporns P, editors. Current protocols in food analytical chemistry. John Wiley & Sons; Madison: 2001. pp. F4.3.1–F4.3.8. [Google Scholar]
- Loreto F, Pinelli P, Brancaleoni E, Ciccioli P. 13C labelling reveals chloroplastic and extra-chloroplastic pools of dimethylallyl pyrophosphate and their contribution to isoprene formation. Plant Physiology. 2004;135:1903–1907. doi: 10.1104/pp.104.039537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveys BR, Atkinson LJ, Sherlock DJ, Roberts RL, Fitter AH, Atkin OK. Thermal acclimation of leaf and root respiration: an investigation comparing inherently fast- and slow-growing plant species. Global Change Biology. 2003;9:895–910. [Google Scholar]
- Mahecha MD, Reichstein M, Carvalhais N, Lasslop G, Lange H, Seneviratne SI, Vargas R, Ammann C, Arain MA, Cescatti A, Janssens IA, et al. Global convergence in the temperature sensitivity of respiration at ecosystem level. Science. 2010;329:838–840. doi: 10.1126/science.1189587. [DOI] [PubMed] [Google Scholar]
- Mawson BT, Cummins WR. Thermal acclimation of photosynthetic electron transport activity by thylakoids of Saxifraga cernua. Plant Physiology. 1989;89:325–332. doi: 10.1104/pp.89.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer S, Teuber M, Zimmer I, Louis S, Fischbach RJ, Schnitzler J-P. Diurnal and seasonal variation of isoprene biosynthesis-related genes in grey poplar leaves. Plant Physiology. 2005;139:474–484. doi: 10.1104/pp.105.066373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlyn BE, Dreyer E, Ellsworth D, Forstreuter M, Harley PC, Kirschbaum MUF, Le Roux X, Montpied P, Strassemeyer J, Walcroft A, Wang K, et al. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant, Cell and Environment. 2002;25:1167–1179. [Google Scholar]
- Mercer EH. A scheme for section staining in electron microscopy. Journal of the Royal Microscopical Society. 1963;81:179–186. [Google Scholar]
- Monson RK, Jaeger CH, Adams WW, III, Driggers EM, Silver GM, Fall R. Relationships among isoprene emission rate, photosynthesis, and isoprene synthase activity as influenced by temperature. Plant Physiology. 1992;98:1175–1180. doi: 10.1104/pp.98.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcuende R, Pérez P, Martínez-Carrasco R, Martín del Molino I, Sanchez de la Puente L. Long- and short-term responses of leaf carbohydrate levels and photosynthesis to decreased sink demand in soybean. Plant, Cell and Environment. 1996;19:976–982. [Google Scholar]
- Muhl QE, Du Toit ES, Robbertse PJ. Leaf adaptation to temperature regimes in Moringa oleifera (horseradish tree) International Journal of Agriculture and Biology. 2011;13:1021–1024. [Google Scholar]
- Niinemets Ü. Photosynthesis and resource distribution through plant canopies. Plant, Cell and Environment. 2007;30:1052–1071. doi: 10.1111/j.1365-3040.2007.01683.x. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Anten NPR. Packing the photosynthesis machinery: from leaf to canopy. In: Laisk A, Nedbal L, Govindjee, editors. Photosynthesis in silico: understanding complexity from molecules to ecosystems. Springer Verlag; Berlin: 2009. pp. 363–399. [Google Scholar]
- Niinemets Ü, Oja V, Kull O. Shape of leaf photosynthetic electron transport versus temperature response curve is not constant along canopy light gradients in temperate deciduous trees. Plant, Cell and Environment. 1999a;22:1497–1514. [Google Scholar]
- Niinemets Ü, Sack L. Structural determinants of leaf light-harvesting capacity and photosynthetic potentials. In: Esser K, Lüttge UE, Beyschlag W, Murata J, editors. Progress in Botany. Springer Verlag; Berlin: 2006. pp. 385–419. [Google Scholar]
- Niinemets Ü, Tenhunen JD, Canta NR, Chaves MM, Faria T, Pereira JS, Reynolds JF. Interactive effects of nitrogen and phosphorus on the acclimation potential of foliage photosynthetic properties of cork oak, Quercus suber, to elevated atmospheric CO2 concentrations. Global Change Biology. 1999b;5:455–470. [Google Scholar]
- Niinemets Ü, Tenhunen JD, Harley PC, Steinbrecher R. A model of isoprene emission based on energetic requirements for isoprene synthesis and leaf photosynthetic properties for Liquidambar and Quercus. Plant, Cell and Environment. 1999c;22:1319–1336. [Google Scholar]
- Niinemets Ü, Wright IJ, Evans JR. Leaf mesophyll diffusion conductance in 35 Australian sclerophylls covering a broad range of foliage structural and physiological variation. Journal of Experimental Botany. 2009;60:2433–2449. doi: 10.1093/jxb/erp045. [DOI] [PubMed] [Google Scholar]
- Ow LF, Griffin KL, Whitehead D, Walcroft AS, Turnbull MH. Thermal acclimation of leaf respiration but not photosynthesis in Populus deltoides x nigra. The New Phytologist. 2008;178:123–134. doi: 10.1111/j.1469-8137.2007.02357.x. [DOI] [PubMed] [Google Scholar]
- Pammenter NW, Loreto F, Sharkey TD. End product feedback effects on photosynthetic electron transport. Photosynthesis Research. 1993;35:5–14. doi: 10.1007/BF02185407. [DOI] [PubMed] [Google Scholar]
- Pandey R, Chacko PM, Choudhary ML, Prasad KV, Pal M. Higher than optimum temperature under CO2 enrichment influences stomata anatomical characters in rose (Rosa hybrida) Scientia Horticulturae. 2007;113:74–81. [Google Scholar]
- Pearcy RW. Effect of growth temperature on the fatty acid composition of the leaf lipids in Atriplex lentiformis (Torr.) Wats. Plant Physiology. 1978;61:484–486. doi: 10.1104/pp.61.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R. Tansley review. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. The New Phytologist. 2009;182:565–588. doi: 10.1111/j.1469-8137.2009.02830.x. [DOI] [PubMed] [Google Scholar]
- Possell M, Loreto F. The role of volatile organic compounds in plant resistance to abiotic stresses: responses and mechanisms. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 209–235. [Google Scholar]
- Raison JK. Alterations in the physical properties and thermal response of membrane lipids: correlations with acclimation to chilly and high temperature. In: St John JB, Berlin E, Jackson PC, editors. Frontiers of membrane research in agriculture. Rowman and Allanheld; Totoma, NJ, USA: 1986. pp. 383–401. [Google Scholar]
- Ramos-Valdivia AC, Heijden R, Verpoorte R. Isopentenyl diphosphate isomerase: a core enzyme in isoprenoid biosynthesis. A review of its biochemistry and function. Nat Prod Rep. 1997;14:591–603. doi: 10.1039/np9971400591. [DOI] [PubMed] [Google Scholar]
- Rasulov B, Bichele I, Laisk A, Niinemets Ü. Competition between isoprene emission and pigment synthesis during leaf development in aspen. Plant, Cell and Environment. 2014;37:724–741. doi: 10.1111/pce.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Copolovici L, Laisk A, Niinemets Ü. Postillumination isoprene emission: in vivo measurements of dimethylallyldiphosphate pool size and isoprene synthase kinetics in aspen leaves. Plant Physiology. 2009a;149:1609–1618. doi: 10.1104/pp.108.133512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Bichele I, Laisk A, Niinemets Ü. Temperature response of isoprene emission in vivo reflects a combined effect of substrate limitations and isoprene synthase activity: a kinetic analysis. Plant Physiology. 2010;154:1558–1570. doi: 10.1104/pp.110.162081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Laisk A, Niinemets Ü. Induction of a longer-term component of isoprene release in darkened aspen leaves: origin and regulation under different environmental conditions. Plant Physiology. 2011;156:816–831. doi: 10.1104/pp.111.176222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Välbe M, Laisk A, Niinemets Ü. Evidence that light, carbon dioxide and oxygen dependencies of leaf isoprene emission are driven by energy status in hybrid aspen. Plant Physiology. 2009b;151:448–460. doi: 10.1104/pp.109.141978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Kubien DS. The temperature response of C3 and C4 photosynthesis. Plant, Cell and Environment. 2007;30:1086–1106. doi: 10.1111/j.1365-3040.2007.01682.x. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Ohara K, Yazaki K. Gene expression and characterization of isoprene synthase from Populus alba. FEBS Letters. 2005;579:2514–2518. doi: 10.1016/j.febslet.2005.03.066. [DOI] [PubMed] [Google Scholar]
- Schrader SM, Wise RR, Wacholtz WF, Ort DR, Sharkey TD. Thylakoid membrane responses to moderately high leaf temperature in Pima cotton. Plant, Cell and Environment. 2004;27:725–735. [Google Scholar]
- Schreiber U, Bilger W, Neubauer C. Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze E-D, Caldwell MM, editors. Ecophysiology of photosynthesis. Springer Verlag; Berlin - Heidelberg - New York - London - Paris - Tokyo - Hong Kong - Barcelona - Budapest: 1994. pp. 49–70. [Google Scholar]
- Shanklin J, Cahoon EB. Desaturation and related modifications of fatty acids. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:611–641. doi: 10.1146/annurev.arplant.49.1.611. [DOI] [PubMed] [Google Scholar]
- Sharkey TD. Photosynthesis in intact leaves of C3 plants: physics, physiology and rate limitations. The Botanical Review. 1985;51:53–105. [Google Scholar]
- Sharkey TD, Badger MR, von Caemmerer S, Andrews TJ. Increased heat sensitivity of photosynthesis in tobacco plants with reduced Rubisco activase. Photosynthesis Research. 2001;67:147–156. doi: 10.1023/A:1010633823747. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Singsaas EL, Lerdau MT, Geron CD. Weather effects on isoprene emission capacity and applications in emissions algorithms. Ecological Applications. 1999;9:1132–1137. [Google Scholar]
- Sharkey TD, Yeh SS. Isoprene emission from plants. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:407–436. doi: 10.1146/annurev.arplant.52.1.407. [DOI] [PubMed] [Google Scholar]
- Singsaas EL, Sharkey TD. The effects of high temperature on isoprene synthesis in oak leaves. Plant, Cell and Environment. 2000;23:751–757. [Google Scholar]
- Smith NG, Dukes JS. Plant respiration and photosynthesis in global-scale models: incorporating acclimation to temperature and CO2. Global Change Biology. 2013;19:45–63. doi: 10.1111/j.1365-2486.2012.02797.x. [DOI] [PubMed] [Google Scholar]
- Starck Z, Wazynska Z, Kucewicz O. Comparative effects of heat stress on photosynthesis and chloroplast ultrastructure in tomato plants with source-sink modulated by growth regulators. Acta Physiologiae Plantarum. 1993;15:125–133. [Google Scholar]
- Stitt M, Hurry VM. A plant for all seasons: alteration in photosynthetic carbon metabolism during cold acclimation in Arabidopsis. Current Opinion in Plant Biology. 2002;5:199–206. doi: 10.1016/s1369-5266(02)00258-3. [DOI] [PubMed] [Google Scholar]
- Sun Z, Copolovici L, Niinemets Ü. Can the capacity for isoprene emissions acclimate to environmental modifications during autumn senescence in temperate deciduous tree species Populus tremula? Journal of Plant Research. 2012a;125:263–274. doi: 10.1007/s10265-011-0429-7. [DOI] [PubMed] [Google Scholar]
- Sun Z, Hüve K, Vislap V, Niinemets Ü. Elevated [CO2] magnifies isoprene emissions under heat and improves thermal resistance in hybrid aspen. Journal of Experimental Botany. 2013;64:5509–5523. doi: 10.1093/jxb/ert318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Niinemets Ü,, Hüve K, Noe SM, Rasulov B, Copolovici L, Vislap V. Enhanced isoprene emission capacity and altered light responsiveness in aspen grown under elevated atmospheric CO2 concentration. Global Change Biology. 2012b;18:3423–3440. [Google Scholar]
- Tjoelker MG, Oleksyn J, Reich PB. Acclimation of respiration to temperature and CO2 in seedlings of boreal tree species in relation to plant size and relative growth rate. Global Change Biology. 1999;5:679–691. [Google Scholar]
- Turnbull MH, Murthy R, Griffin KL. The relative impacts of daytime and night-time warming on photosynthetic capacity in Populus deltoides. Plant, Cell & Environment. 2002;25:1729–1737. [Google Scholar]
- Velikova V, Sharkey TD, Loreto F. Stabilization of thylakoid membranes in isoprene-emitting plants reduces formation of reactive oxygen species. Plant Signaling & Behavior. 2012;7:139–141. doi: 10.4161/psb.7.1.18521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velikova V, Várkonyi Z, Szabó M, Maslenkova L, Nogues I, Kovács L, Peeva V, Busheva M, Garab G, Sharkey TD, Loreto F. Increased thermostability of thylakoid membranes in isoprene-emitting leaves probed with three biophysical techniques. Plant Physiology. 2011;157:905–916. doi: 10.1104/pp.111.182519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers CE, Gershenzon J, Lerdau MT, Loreto F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nature Chemical Biology. 2009;5:283–291. doi: 10.1038/nchembio.158. [DOI] [PubMed] [Google Scholar]
- Vickers CE, Possell M, Hewitt CN, Mullineaux PM. Genetic structure and regulation of isoprene synthase in poplar (Populus spp.) Plant Molecular Biology. 2010;73:547–558. doi: 10.1007/s11103-010-9642-3. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Wardlaw IF, Bagnall D. Phloem transport and the regulation of growth of Sorghum bicolor (Moench) at low temperature. Plant Physiology. 1981;68:411–414. doi: 10.1104/pp.68.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way DA, Oren R. Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data. Tree Physiology. 2010;30:669–688. doi: 10.1093/treephys/tpq015. [DOI] [PubMed] [Google Scholar]
- Way DA, Schnitzler J-P, Monson RK, Jackson RB. Enhanced isoprene-related tolerance of heat- and light-stressed photosynthesis at low but not high, CO2 concentrations. Oecologia. 2011;166:273–282. doi: 10.1007/s00442-011-1947-7. [DOI] [PubMed] [Google Scholar]
- Way DA, Yamori W. Thermal acclimation of photosynthesis: on the importance of adjusting our definitions and accounting for thermal acclimation of respiration. Photosynthesis Research. 2014;119:89–100. doi: 10.1007/s11120-013-9873-7. [DOI] [PubMed] [Google Scholar]
- Weise SE, Li Z, Sutter AE, Corrion A, Banerjee A, Sharkey TD. Measuring dimethylallyl diphosphate available for isoprene synthesis. Analytical Biochemistry. 2013;435:27–34. doi: 10.1016/j.ab.2012.11.031. [DOI] [PubMed] [Google Scholar]
- Wiberley AE, Donohue AR, Meier ME, Westphal MM, Sharkey TD. Regulation of isoprene emission in Populus trichocarpa leaves subjected to changing growth temperature. Plant, Cell and Environment. 2008;31:258–267. doi: 10.1111/j.1365-3040.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- Wiberley AE, Donohue AR, Westphal MM, Sharkey TD. Regulation of isoprene emission from poplar leaves throughout a day. Plant Cell and Environment. 2009;32:939–947. doi: 10.1111/j.1365-3040.2009.01980.x. [DOI] [PubMed] [Google Scholar]
- Xu C-Y, Salih A, Ghannoum O, Tissue DT. Leaf structural characteristics are less important than leaf chemical properties in determining the response of leaf mass per area and photosynthesis of Eucalyptus saligna to industrial-age changes in [CO2] and temperature. Journal of Experimental Botany. 2012;63:5829–5841. doi: 10.1093/jxb/ers231. [DOI] [PubMed] [Google Scholar]
- Yamakawa H, Hakata M. Atlas of rice grain filling-related metabolism under high temperature: joint analysis of metabolome and transcriptome demonstrated inhibition of starch accumulation and induction of amino acid accumulation. Plant and Cell Physiology. 2010;51:795–809. doi: 10.1093/pcp/pcq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T, Yamakawa T, Yamane Y, Koike H, Satoh K, Katoh S. Temperature acclimation of photosynthesis and related changes in photosystem II electron transport in Winter wheat. Plant Physiology. 2002;128:1087–1097. doi: 10.1104/pp.010919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamori W, Noguchi K, Terashima I. Temperature acclimation of photosynthesis in spinach leaves: analyses of photosynthetic components and temperature dependencies of photosynthetic partial reactions. Plant, Cell and Environment. 2005;28:536–547. [Google Scholar]
- Zhang R, Sharkey TD. Photosynthetic electron transport and proton flux under moderate heat stress. Photosynthesis Research. 2009;100:29–43. doi: 10.1007/s11120-009-9420-8. [DOI] [PubMed] [Google Scholar]
- Zhou C, Li Z, Wiberley-Bradford AE, Weise SE, Sharkey TD. Isopentenyl diphosphate and dimethylallyl diphosphate ratio measured with recombinant isopentenyl diphosphate isomerase and isoprene synthase. Analytical Biochemistry. 2013;440:130–136. doi: 10.1016/j.ab.2013.05.028. [DOI] [PubMed] [Google Scholar]